Abstract
Purpose
Numerous studies have demonstrated that a variety of systemic inflammatory markers were associated with the survival of different tumors. However, the association between elevated postoperative neutrophil-lymphocyte ratio (postNLR) and long-term outcomes, including overall survival (OS), disease-free survival (DFS), in patients with solid tumors remains controversial. A systematic review was conducted to explore the association between the postNLR and long-term outcomes in solid tumors.
Materials and methods
Relevant literature was identified using PubMed, Embase, Web of Science, and the Cochrane Library from the initiation of the databases to October 2020. Data were extracted from included studies reporting hazard ratio (HR) and 95% confidence intervals (CI), and were pooled using generic inverse-variance and random-effects modeling. 25 studies reporting on7539 patients were included in the analysis.
Results
Elevated postNLR was associated with poor OS (HR 1.87, 95% CI = 1.53–2.28; P < 0.00001), and worse DFS (HR 1.69, 95% CI = 1.28–2.22; P = 0.0002). Subgroup analyses showed that the trend of the pooled HR for most of the subgroups was not changed, and the heterogeneity of the same tumor type was not obvious. However, there was no correlation between high postNLR obtained within 7days and poor DFS (n = 3, HR 1.25, 95CI% = 0.54–2.88; P = 0.60).
Conclusions
Elevated postNLR might be a readily available and inexpensive biomarker for long-term outcomes in solid tumors. Multicenter and prospective studies are needed to explore the impact of the postNLR on the prognosis of solid tumors.
Introduction
Cancer is becoming the leading cause of morbidity and mortality in every region of the world, and the global incidence of cancer is expected to increase from 12.7 million new cases in 2008 to 22.2 million by 2030 [1]. Surgery is critical to the treatment of cancer, and it is estimated that annually, 45 million surgical operations will be needed worldwide by 2030 [2]. Therefore, reliable and inexpensive biomarkers are needed to predict the survival of tumor patients and to distinguish subgroups of patients who will benefit from aggressive surgical treatment.
The mechanism of tumorigenesis is complex. The inflammatory response plays an important role in tumorigenesis, progression and metastasis [3,4]. Numerous studies have demonstrated that a variety of systemic inflammatory markers, such as preoperative neutrophil-lymphocyte ratio (NLR), were associated with the survival of different tumors [5,6]. As we all know, neutrophils promote tumorigenesis, progression and metastasis in multiple ways [7], while lymphocytes inhibit tumor growth, higher lymphocytes indicate a better prognosis for patients with cancer [8]. A high NLR indicates a decrease in the number of lymphocytes and an increase in the number of neutrophils. Changes in NLR may represent the balance of promoting or anti-tumor progression, and has prognostic value. Recently, a few studies have begun to focus on the impact of the postoperative NLR (postNLR) on tumor prognosis [9,10]. However, the prognostic value of the postNLR in solid tumors is controversial. A previous study showed that high postNLR was an independent prognostic factor for worse overall survival (OS) in tumor patients [9], but subsequent studies failed to demonstrate the prognostic value of the postNLR [10]. Therefore, the purpose of this meta-analysis is to investigate the association between the postNLR and long-term outcomes for solid tumors.
Methods
The meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11].
Search strategy
A systematic literature search of PubMed, EMBASE, Web of Science and the Cochrane Library was performed to select relevant articles from the initiation of the databases to October 09, 2019. No additional restrictions were applied to the searches. The following search strategies and keywords were used: (Neoplasm OR Neoplasia OR Tumor OR Cancer OR Carcinoma OR Malignancy) AND (Neutrophil OR lymphocyte) AND (postoperative).
Selection criteria
Endnote X9 was used to screen duplicate documents. The literature was screened by title and abstract, and the full text was further reviewed to obtain qualified literature. Citation lists of the retrieved articles were screened manually to obtain relevant articles. The inclusion criteria were as follows: (1) patients with a pathologically confirmed diagnosis of cancer, and treated with surgery; (2) the postNLR cutoff was clearly defined; (3) postNLR was a categorical variable; (4) postNLR was included as a variable in outcome analysis. The exclusion criteria were as follows: (1) animal experiments; (2) review, letters, comments, and articles not related to the topic; (3) non-English articles.
Quality assessment
Newcastle–Ottawa scale (NOS) were used to assess the quality of nonrandomized studies [12]. A score of 6–9 indicates that the quality of the article is high, while a score of 0–5 indicates that the quality of the article is low.
Data extraction
The following data were extracted from the literature included in the study: first author, year of publication, recruitment period and region, tumor type, sample size, time of obtaining the postNLR, postNLR cutoff, hazard ratio (HR) and corresponding 95% confidence interval (CI) for OS and disease-free survival (DFS). The HR was preferentially extracted from multivariate analysis, otherwise from univariate analysis, or obtained from Kaplan–Meier curves using Engauge Digitizer 4.1 [13].
Data analysis
RevMan 5.3 analysis software was used to conduct the meta-analysis. The estimates for HR were pooled and weighted by generic inverse variance and then computed by random-effects modeling. Odds ratio (OR) was synthesized to explore the relationship between high postNLR and clinicopathological indicators. Heterogeneity was assessed using I2 statistics. If I2 > 50%, the heterogeneity was considered statistically significant [14]. Publication bias was assessed by visually inspecting funnel plots. All statistical tests were two-sided, and statistical significance was defined as P < 0.05.
Results
Study selection and patient characteristics
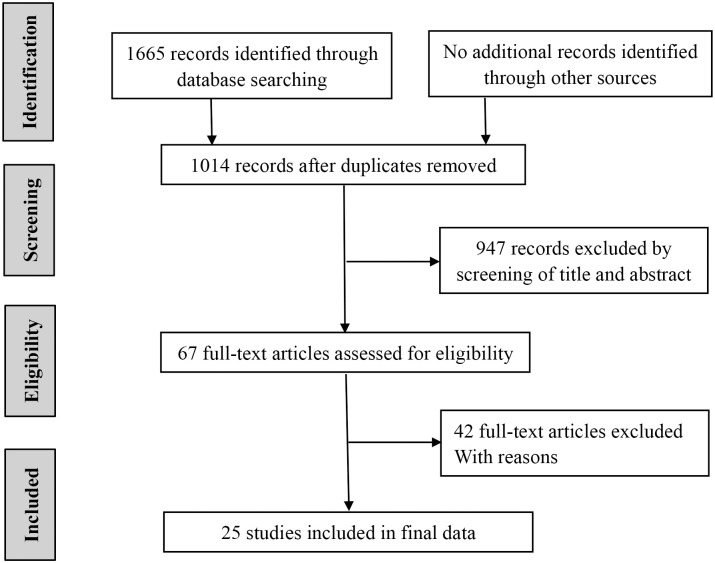
Fig 1 shows the detailed steps of the literature search. A total of 1665 articles were retrieved. No additional records were identified through other sources. 651 duplicate records were excluded. Then, 947 references were eliminated by screening the titles and abstracts. Of the remaining 67 potentially relevant studies, 42 studies were excluded because they fulfilled one of the exclusion criteria. Finally, 25 studies (all retrospective) reporting on 7539 patients were eligible to be included in the present meta-analysis [9,10,15–37]. As shown in Table 1, the studies were conducted in Belgium (1 study), Japan (8 studies), Switzerland (1study), China (7 studies), Poland (1 study), Korea (5 studies), USA (2 studies). The 25 studies were published between 2012 and 2020. 19 of them studied OS, 15 discussed DFS. The surgery in 11 studies was radical surgical treatment, two of which also performed chemoradiation or chemotherapy. The remaining 14 studies underwent resection, and 5 of them received chemotherapy or radiotherapy for some patients. The median of NLR was 3.28 (1.8–14.10). The median of sample size was 176 (93–2302). The NOS score ranged from 6 to 8 points, indicating that the quality of the literature included in the study is high.
Fig 1. Selection of studies included in the analysis.
Table 1. Baseline characteristics of the included studies.
| studies | tumor type | treatment | region | sample | Inclusion period | NLR/time obtained | outcomes | NOS |
|---|---|---|---|---|---|---|---|---|
| Albisinni 2019 [15] | bladder cancer | CR | Belgium | 134 | 2013–2018 | 5.58/ 1 month | OS, DFS | 8 |
| Bojaxhiu 2018 [16] | head and neck cancer | R, RC | Switzerland | 186 | 2007–2010 | 3.28/ <10 days | OS, DFS | 6 |
| Cui 2020 [17] | colorectal cancer | R | China | 146 | 2011–2014 | 2.96/≥7 days | DFS | 7 |
| Guo 2018 [18] | colorectal cancer | R | China | 135 | 2009–2014 | 3.64/>1 month | OS, DFS | 7 |
| Hayama 2020 [19] | colorectal cancer | CR | Japan | 176 | 2012–2016 | 3.1/ 7th day | DFS | 6 |
| He 2017 [20] | synchronous colorectal cancer | CR | China | 114 | 2009–2013 | 10.5/≤3 days | OS, DFS | 6 |
| Hoshimoto 2020 [21] | pancreatic cancer | R | Japan | 211 | 1995–2016 | 1.8/ 18–86 days | OS | 7 |
| Jakubowska 2020 [22] | colorectal cancer | R | Poland | 144 | 2014–2016 | 3.3/ 3 days | DFS | 6 |
| Jang 2017 [23] | prostate cancer | CR | Korea | 2302 | 2000–2010 | 3.5/ 2–3 months | OS | 7 |
| Jin 2016 [24] | non-small cell lung cancer | R | China | 123 | 2007–2010 | 3.9/ >1 month | OS, DFS | 8 |
| Kang 2016 [9] | bladder cancer | CR | Korea | 385 | 1999–2012 | 2/ 3–4 month | OS | 7 |
| Kim 2019 [25] | pancreas adenocarcinoma | CRC | Korea | 178 | 2010–2018 | 2.535/ 1 months | OS, DFS | 6 |
| Kim 2012 [26] | stomach cancer | R | Korea | 93 | 2004–2009 | 7.7/ third day | DFS | 8 |
| Li 2018 [27] | colon cancer | CR | China | 344 | 2012–2015 | 3/ 1–3 months | OS | 6 |
| Lin 2019 [28] | oropharyngeal cancer | R/RR | USA | 108 | 1997–2017 | 6.2/ 3 months | OS, DFS | 6 |
| Lin 2018 [29] | palatine tonsil cancer | RR | USA | 99 | 1997–2013 | 11.875/<2.5 months | OS | 6 |
| Miyatani 2018 [30] | gastric cancer | CR | Japan | 280 | 2001–2013 | 1.8/ 1 month | OS | 6 |
| Nishihara 2019 [31] | upper tract urothelial carcinoma | CR | Japan | 134 | 2004–2015 | 2.5/ 1–2 month | OS | 8 |
| Ohno 2012 [32] | clear cell renal cell carcinoma | R | Japan | 250 | 1990–2008 | 2.7/ 3 months | DFS | 6 |
| Paik 2014 [33] | colorectal cancer | R | Korea | 600 | 2006–2009 | 5/ NA | OS | 7 |
| Pu 2019 [34] | pancreatic ductal adenocarcinoma | R | China | 97 | 2012–2016 | 14.1/ NA | OS, DFS | 7 |
| Shibutani 2015 [35] | colorectal cancer | CR | Japan | 254 | 2006–2011 | 3/ 29 (23–36) days | OS | 7 |
| Tanaka 2018 [36] | gastric cancer | RC | Japan | 170 | 2006–2015 | 1.99/ NA | DFS | 6 |
| Zhou 2016 [10] | gastric cancer | CR/CRC | China | 360 | 2006–2008 | 6.19/≥5 days | OS | 8 |
| Zhou 2018 [37] | colorectal cancer | R | China | 516 | 2007–2015 | 2.41/ 1 month | OS, DFS | 7 |
CR, curative resection; CRC, curative resection and chemotherapy; R, resection; RR, resection and radiotherapy; RC, resection and chemotherapy; NLR, neutrophil-lymphocyte ratio; OS, overall survival; DFS, disease-free survival; NOS, Newcastle–Ottawa scale; NA, not available.
PostNLR and OS
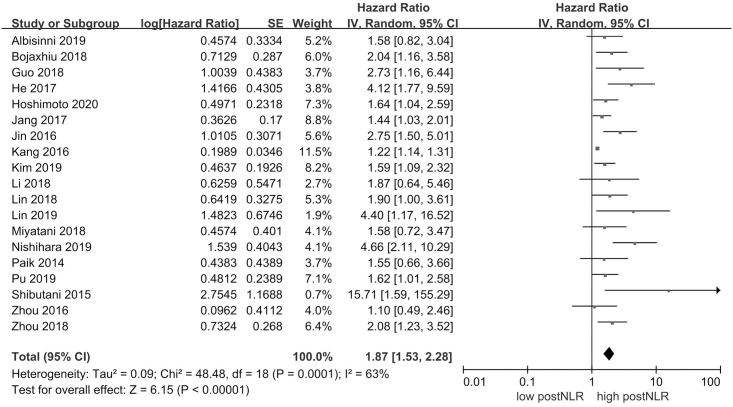
19 studies comprising a total of 6560 patients reported HR for OS. Fig 2 shows that high postNLR was associated with poor OS (HR 1.87, 95% CI = 1.53–2.28, P < 0.00001). There was statistically significant heterogeneity (I2 = 63%, P = 0.0001).
Fig 2. Results of the meta-analysis on pooled hazard ratio values for overall survival.
PostNLR and DFS
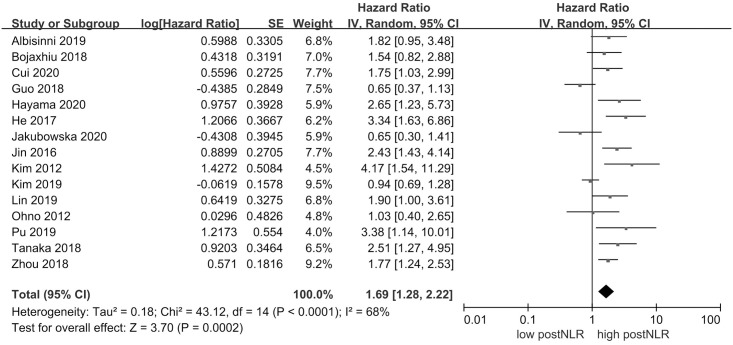
15 studies with 2570 individuals were included in the analysis of postNLR and DFS. Fig 3 demonstrated that high postNLR was associated with worse DFS (HR 1.69, 95% CI = 1.28–2.22, P = 0.0002). There was evidence of significant heterogeneity across the included studies (I2 = 68%, P < 0.0001).
Fig 3. Results of the meta-analysis on pooled hazard ratio values for disease-free survival.
Subgroup analysis
A subgroup analysis of the tumor type, treatment and time of obtaining postNLR was used to investigate the correlation between high postNLR and OS or DFS. Subgroup analyses showed that the trend of the pooled HR for most of the subgroups was not changed (Table 2). Stratified analysis by tumor type showed that studies conducted in colorectal cancer (n = 4, HR 2.25, 95% CI = 1.40–3.61, P = 0.008), bladder cancer (n = 2, HR 1.22, 95% CI = 1.14–1.31, P<0.00001), and pancreatic cancer (n = 3, HR 1.61, 95C%CI = 1.26–2.07, P = 0.0001) were significantly associated with reduced OS. A similar result indicated that high post NLR was associated with worse DFS of colorectal cancer. Subgroup analysis revealed that the heterogeneity of the same tumor type was small. However, there was no significant difference in gastric cancer between high postNLR and poor OS (n = 2, HR 1.32,95%CI = 0.75–2.33, P = 0.33), and in colorectal cancer between high postNLR and worse DFS (n = 5, HR 1.30, 95%CI = 0.78–2.16, P = 0.32).
Table 2. Subgroup analysis of the association between high postNLR and the long-term outcomes of solid tumors.
| Overall survival | Disease-free survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| subgroup | studies | patients | HR (95CI%) | I2 | P | studies | patients | HR 95CI% | I2 | P |
| Tumor type | ||||||||||
| Colorectal cancer | 4 | 1505 | 2.25 (1.40–3.61) | 20% | 0.0008 | 5 | 1117 | 1.30 (0.78–2.16) | 75% | 0.32 |
| Gastric cancer | 2 | 640 | 1.32 (0.75–2.33) | 0% | 0.33 | 2 | 263 | 2.95 (1.68–5.17) | 0% | 0.0002 |
| Bladder cancer | 2 | 519 | 1.22 (1.14–1.31) | 0% | <0.00001 | - | - | - | - | - |
| Pancreatic cancer | 3 | 486 | 1.61 (1.26–2.07) | 0% | 0.0001 | - | - | - | - | - |
| Treatment | ||||||||||
| Curative resection | 8 | 3947 | 1.95 (1.35–2.81) | 73% | 0.0004 | 3 | 424 | 2.46 (1.64–3.70) | 0% | <0.0001 |
| resection | 6 | 1682 | 1.91 (1.52–2.41) | 0% | <0.00001 | 8 | 1504 | 1.55 (1.01–2.37) | 71% | 0.04 |
| NLR time | ||||||||||
| <7 days | - | - | - | - | - | 3 | 351 | 1.25 (0.54–2.88) | 83% | 0.60 |
| ≥7 days | 13 | 5104 | 1.83 (1.45–2.30) | 65% | <0.00001 | 9 | 1766 | 1.50 (1.09–2.05) | 68% | 0.01 |
NLR, neutrophil-lymphocyte ratio; HR, hazard ratio; CI, confidence interval.
As shown in Table 1, 8 studies of curative resection studied OS and 3 researches studied DFS. 6 of the 14 resection studies studied OS, and 8 studies studied DFS. The included studies are divided into 2 subgroups based on treatment methods (Table 2). Subgroup analysis showed that the high postNLR group had adverse effects on OS (curative resection: n = 8, HR 1.95, 95%CI = 1.35–2.81, P = 0.0004; resection: n = 6, HR 1.91, 95CI% = 1.52–2.41, P<0.00001) and DFS (curative resection: n = 3, HR 2.46, 95%CI = 1.64–3.70, P<0.0001; resection: n = 8, HR 1.55, 95CI% = 1.01–2.37, P = 0.04) compared to the low postNLR group.
Stratified by the time to obtain postNLR showed that high postNLR in the group greater than 7 days has adverse effects on OS and DFS (Table 2). However, there was no correlation between high postNLR obtained within 7days and poor DFS (n = 3, HR 1.25, 95CI% = 0.54–2.88, P = 0.60).
Correlation between postNLR and Clinicopathological indicators
As shown in Table 3, five studies reported the relationship between age (≥60 years and <60years) and high postNLR. There was no correlation between high postNLR and age (OR 1.04, 95CI% = 0.65–1.67, P = 0.87). Similar results showed that there was no significant correlation between gender and high postNLR (P = 0.16).
Table 3. Correlation between high postNLR and clinicopathological indicators.
| Clinicopathological indicators | studies | patients | OR (95CI%) | I2 | P |
|---|---|---|---|---|---|
| Age≥60 years VS Age<60 years | 5 | 840 | 1.04 (0.65–1.67) | 59% | 0.87 |
| Male VS female | 10 | 1672 | 0.78 (0.56–1.10) | 59% | 0.16 |
| CEA normal VS elevated | 4 | 576 | 0.67 (0.41–1.09) | 28% | 0.11 |
| AJCC (7th) III+IV VS I+II | 2 | 281 | 7.67 (0.08–709.58) | 94% | 0.38 |
| AJCC (7th) T III+IV VS T I+II | 3 | 601 | 0.29 (0.06–1.31) | 91% | 0.11 |
NLR, neutrophil-lymphocyte ratio; OR, odds ratio; CEA, carcinoembryonic antigen; AJCC (7th), 7th edition of the American Joint Committee on Cancer staging system.
Four studies were pooled to explore the relationship between high postNLR and tumor marker (carcinoembryonic antigen, CEA) (Table 3). The result suggested that there was no significant difference between elevated CEA and high postNLR (OR 0.67, 95CI% = 0.41–1.09, P = 0.1).
The clinical staging systems and T stage were synthesized to explore the correlation between tumor stage and high postNLR (Table 3). Different clinical staging systems were used in the original research, which limited the number of studies that could quantitatively compare the relationship between postNLR and clinical staging systems. Finally, 4 studies [17,18,20,27] of the 7th edition of the American Joint Committee on Cancer (AJCC 7th) staging system were included in quantitative synthesis to explore the relationship between AJCC (7th) staging system and high postNLR. The results indicated that there was no significant difference between high postNLR and AJCC (7th) staging system (OR 7.67, 95CI% = 0.08–709.58, P = 0.38), and T stage (OR 0.29, 95CI% = 0.06–1.31, P = 0.11).
Publication bias
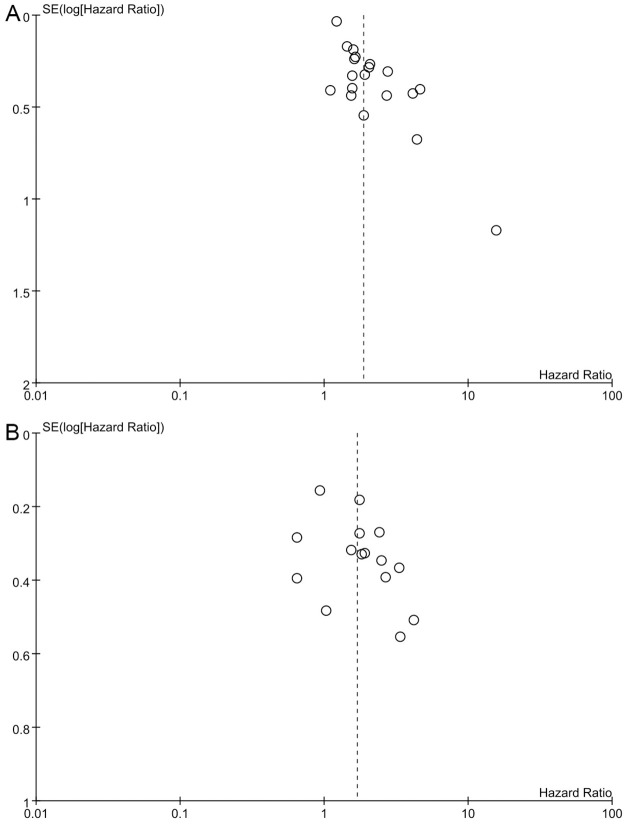
As shown in Fig 4, the included studies were not symmetrically distributed, indicating that there was evidence of publication bias for OS, and DFS.
Fig 4. Funnel plot of the hazard ratio for (A) overall survival; (B) disease-free survival.
Discussion
To the best of our knowledge, this is the first meta-analysis investigating the association between the postNLR and long-term outcomes (OS and DFS) in solid tumors. The current systematic review and meta-analysis of 25 studies including 7539 patients provided solid evidence of an association between a high postNLR and reduced long-term outcomes. This result was further confirmed by subgroup analysis. Subgroup analysis of different types of solid tumors showed that high postNLR was associated with poor long-term outcomes, and the heterogeneities within the subgroup were not obvious. Further analysis found that there was no correlation between clinicopathological indicators including tumor stage and postNLR. Our previous study found that the preoperative inflammatory indicators were related to tumor burden such as tumor size, while the preoperative tumor burden was not correlated with postoperative inflammatory indicators [38]. The possible reason was that after the primary tumor burden was removed, the host’s immune response has changed [39]. This meta-analysis further confirmed that there was no significant correlation between postNLR and preoperative tumor burden such as tumor size and tumor stage. The results from the present study demonstrated that the postNLR could serve as a readily available and inexpensive biomarker to predict the long-term outcomes of patients with solid tumors after surgery. It is worth noting that this study found that there was no significant difference between postNLR obtained within 7 days after surgery and poor DFS. The possible reason is that surgical stress and wound healing have an impact on inflammatory indicators [40]. Therefore, the prognostic value of postNLR in the early postoperative period may be limited.
The mechanism for the correlation between increased postNLR and worse survival outcomes in patients with solid tumors is complex. Recent studies have demonstrated that the NLR represents the balance between antitumor immune function and the inflammatory response [41]. Increased preoperative NLR was associated with lymph node metastasis and distant metastasis [42] as well as treatment resistance [43]. We suspect that the postNLR may play an important role in the activation of micrometastases and resistance to treatment. A high NLR indicates a decrease in the number of lymphocytes and an increase in the number of neutrophils. Generally, neutrophils play a critical role in tumorigenesis, progression and metastasis in multiple ways, including both direct effects on cancer cells and indirect effects on the tumor microenvironment [7]. Tumor growth can be directly enhanced by neutrophil elastase [44]. Cools et al [45] reported that tumor cell trapping within neutrophil extracellular traps was associated with increased micrometastases. As reported, a high NLR correlates with increased interleukin (IL)-6, IL-8 and regulatory T cell expression [46]. IL-6 increases vascular endothelial growth factor release, stimulates defective angiogenesis [47], and enhances chemoresistance, resistance to apoptosis and invasion [48]. Comparatively, the importance of lymphocytes has been highlighted in systematic review in which high tumor-infiltrating lymphocyte densities were associated with improved survival outcomes in cancer [8]. Moreover, the apoptosis of tumor-infiltrating lymphocytes mediates resistance to cancer immunotherapy [49]. One study showed that an early decline in the NLR at 6 weeks in patients with metastatic renal cell carcinoma receiving immune checkpoint blockade was associated with improved survival outcomes [50]. Therefore, an elevated NLR marked the suppression of host immunity. Our findings support this result that high postNLR indicates poor prognosis for solid tumors.
Our study has some strengths that need to be addressed. First, to the best of our knowledge, this is the first meta-analysis to explore the association between the postNLR and long-term outcomes in solid tumor patients. Second, heterogeneities in most of the subgroup analyses for OS were not obvious. Third, we developed a reproducible and systematic search strategy for the major medical databases, and the references of the initially selected studies were screened for additional studies that were not included in the database search. Finally, this study suggests that during the entire treatment process, we should not only focus on the preoperative immune or inflammatory status but also on the impact of the postoperative inflammatory status on tumor progression and treatment resistance.
There are several limitations in our study. First, all studies are retrospective with immeasurable deviations. Second, the limited number of studies for subgroup analysis may affect the reliability of the results. Third, HR and corresponding 95% CI of some included studies were extracted by Kaplan–Meier curves, affecting the accuracy of the results. Furthermore, we found evidence of publication bias, as shown in Fig 4. Unpublished gray literature, mainly with negative results, may have affected the pooled estimates. Forth, the NLR of different tumors may vary. NLR values may be affected by tumor type, and especially postoperative detection time because surgical stress and wound healing have an impact on inflammatory indicators. At the same time, most studies come from East Asia, and there may be ethnic differences that restrict the generalization and reliability of the results. Fifth, confounding factors such as neoadjuvant therapy may have a potentially unclear influence on this result. In addition, 14 of the 25 studies did not state in the original text whether it was a radical resection, which may affect the oncologic outcome itself. Finally, due to the limitation of the number of studies and the different tumor staging systems, we were unable to explore the relationship between AJCC (7th) I-IV, T I-IV and high postNLR, which may have unknown effects.
Conclusion
Elevated postNLR might be a promising biomarker for OS and DFS in solid tumors. Routine monitoring of postNLR during postoperative follow-up may be helpful in predicting the prognosis of patients with tumors Multicenter and prospective studies are needed to explore the impact of the postNLR on the prognosis of solid tumors.
Supporting information
(DOCX)
(ZIP)
Acknowledgments
We thank the authors of the studies included in this meta-analysis.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The work is supported by the National Major Project for Infectious Diseases of China (no.2017ZX100203205005), National Natural Science Foundation of China (no. 81930119), Natural Science Foundation of Beijing Municipality (no. Z190024), and the Medical-engineering project of Tsinghua University School of Software (no. MESR201912-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol. 2012;13(8):790–801. Epub 2012/06/05. 10.1016/S1470-2045(12)70211-5 . [DOI] [PubMed] [Google Scholar]
- 2.Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16(11):1193–224. Epub 2015/10/03. 10.1016/S1470-2045(15)00223-5 . [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. Epub 2008/07/25. 10.1038/nature07205 . [DOI] [PubMed] [Google Scholar]
- 4.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. Epub 2014/10/05. 10.1016/S1470-2045(14)70263-3 . [DOI] [PubMed] [Google Scholar]
- 5.Semeniuk-Wojtas A, Lubas A, Stec R, Syrylo T, Niemczyk S, Szczylik C. Neutrophil-to-lymphocyte Ratio, Platelet-to-lymphocyte Ratio, and C-reactive Protein as New and Simple Prognostic Factors in Patients With Metastatic Renal Cell Cancer Treated With Tyrosine Kinase Inhibitors: A Systemic Review and Meta-analysis. Clin Genitourin Cancer. 2018;16(3):e685–e93. Epub 2018/02/20. 10.1016/j.clgc.2018.01.010 . [DOI] [PubMed] [Google Scholar]
- 6.Kumarasamy C, Sabarimurugan S, Madurantakam RM, Lakhotiya K, Samiappan S, Baxi S, et al. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2019;98(24):e14834. Epub 2019/06/14. 10.1097/MD.0000000000014834 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016;37(1):41–52. Epub 2015/12/25. 10.1016/j.it.2015.11.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105(1):93–103. Epub 2011/06/02. 10.1038/bjc.2011.189 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang M, Jeong CW, Kwak C, Kim HH, Ku JH. The Prognostic Significance of the Early Postoperative Neutrophil-to-Lymphocyte Ratio in Patients with Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Annals of Surgical Oncology. 2016;23(1):335–42. 10.1245/s10434-015-4708-8 [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, Xu L, Huang Z, Zhang L, Zhang H, Zhu W, et al. The hematologic markers as prognostic factors in patients with resectable gastric cancer. Cancer Biomark. 2016;17(3):359–67. Epub 2016/11/02. 10.3233/CBM-160648 . [DOI] [PubMed] [Google Scholar]
- 11.Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313(16):1657–65. Epub 2015/04/29. 10.1001/jama.2015.3656 . [DOI] [PubMed] [Google Scholar]
- 12.Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii–x, 1–173. Epub 2003/09/23. 10.3310/hta7270 . [DOI] [PubMed] [Google Scholar]
- 13.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. Epub 2007/06/09. 10.1186/1745-6215-8-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. Epub 2002/07/12. 10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 15.Albisinni S, Moussa I, Aoun F, Quackels T, Assenmacher G, Peltier A, et al. The impact of postoperative inflammatory biomarkers on oncologic outcomes of bladder cancer. Prog Urol. 2019;29(5):270–81. Epub 2019/04/08. 10.1016/j.purol.2019.02.008 . [DOI] [PubMed] [Google Scholar]
- 16.Bojaxhiu B, Templeton AJ, Elicin O, Shelan M, Zaugg K, Walser M, et al. Relation of baseline neutrophil-to-lymphocyte ratio to survival and toxicity in head and neck cancer patients treated with (chemo-) radiation. Radiat Oncol. 2018;13(1):216. Epub 2018/11/08. 10.1186/s13014-018-1159-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui M, Xu R, Yan B. A persistent high neutrophil-to-lymphocyte ratio predicts poor prognosis in patients with colorectal cancer undergoing resection. Molecular and clinical oncology. 2020;13(5):63-. 10.3892/mco.2020.2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo D, Han A, Jing W, Chen D, Jin F, Li M, et al. Preoperative to postoperative change in neutrophil-to-lymphocyte ratio predict survival in colorectal cancer patients. Future Oncology. 2018;14(12):1187–96. 10.2217/fon-2017-0659 [DOI] [PubMed] [Google Scholar]
- 19.Hayama T, Hashiguchi Y, Okada Y, Ono K, Nemoto K, Shimada R, et al. Significance of the 7th postoperative day neutrophil-to-lymphocyte ratio in colorectal cancer. International Journal of Colorectal Disease. 2020;35(1):119–24. 10.1007/s00384-019-03463-3 [DOI] [PubMed] [Google Scholar]
- 20.He W, Wei M, Yang X, Chen B, Wu Q, Zheng E, et al. Do inflammatory markers predict prognosis in patients with synchronous colorectal cancer? Medicine. 2017;96(17). 10.1097/MD.0000000000006607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoshimoto S, Hishinuma S, Shirakawa H, Tomikawa M, Ozawa I, Ogata Y. Validation and clinical usefulness of pre- and postoperative systemic inflammatory parameters as prognostic markers in patients with potentially resectable pancreatic cancer. Pancreatology. 2020;20(2):239–46. 10.1016/j.pan.2019.12.004 [DOI] [PubMed] [Google Scholar]
- 22.Jakubowska K, Koda M, Kisielewski W, Kanczuga-Koda L, Grudzinska M, Famulski W. Pre- and postoperative neutrophil and lymphocyte count and neutrophil-to-lymphocyte ratio in patients with colorectal cancer. Molecular and clinical oncology. 2020;13(5):56-. 10.3892/mco.2020.2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang WS, Cho KS, Kim MS, Yoon CY, Kang DH, Kang YJ, et al. The prognostic significance of postoperative neutrophil-to-lymphocyte ratio after radical prostatectomy for localized prostate cancer. Oncotarget. 2017;8(7):11778–87. 10.18632/oncotarget.14349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin F, Han A, Shi F, Kong L, Yu J. The postoperative neutrophil-to-lymphocyte ratio and changes in this ratio predict survival after the complete resection of stage I non-small cell lung cancer. Oncotargets and Therapy. 2016;9:6529–37. 10.2147/OTT.S117290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EY, Hong TH. Changes in total lymphocyte count and neutrophil-to-lymphocyte ratio after curative pancreatectomy in patients with pancreas adenocarcinoma and their prognostic role. Journal of Surgical Oncology. 2019;120(7):1102–11. 10.1002/jso.25725 [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, Choi WJ. The effectiveness of postoperative neutrophils to lymphocytes ratio in predicting long-term recurrence after stomach cancer surgery. Journal of the Korean Surgical Society. 2012;83(6):352–9. 10.4174/jkss.2012.83.6.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Zhao R, Cui Y, Zhou Y, Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Scientific Reports. 2018;8. 10.1038/s41598-018-27896-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin AJ, Gang M, Rao YJ, Campian J, Daly M, Gay H, et al. Association of Posttreatment Lymphopenia and Elevated Neutrophil-to-Lymphocyte Ratio With Poor Clinical Outcomes in Patients With Human Papillomavirus-Negative Oropharyngeal Cancers. Jama Otolaryngology-Head & Neck Surgery. 2019;145(5):413–21. 10.1001/jamaoto.2019.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin AJ, Rao YJ, Chin RI, Campian J, Mullen D, Thotala D, et al. Post-operative radiation effects on lymphopenia, neutrophil to lymphocyte ratio, and clinical outcomes in palatine tonsil cancers. Oral Oncol. 2018;86:1–7. Epub 2018/11/10. 10.1016/j.oraloncology.2018.08.008 . [DOI] [PubMed] [Google Scholar]
- 30.Miyatani K, Saito H, Kono Y, Murakami Y, Kuroda H, Matsunaga T, et al. Combined analysis of the pre- and postoperative neutrophil-lymphocyte ratio predicts the outcomes of patients with gastric cancer. Surgery Today. 2018;48(3):300–7. 10.1007/s00595-017-1587-6 [DOI] [PubMed] [Google Scholar]
- 31.Nishihara K, Suekane S, Ueda K, Nakiri M, Matsuo M, Igawa T. High postoperative neutrophil-to-lymphocyte ratio as a poor prognostic marker in patients with upper tract urothelial carcinoma. Oncology Letters. 2019;17(6):5241–50. 10.3892/ol.2019.10178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of Neutrophil-to-Lymphocyte Ratio and Recurrence of Clear Cell Renal Cell Carcinoma. Journal of Urology. 2012;187(2):411–7. 10.1016/j.juro.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 33.Paik KY, Lee IK, Lee YS, Sung NY, Kwon TS. Clinical Implications of Systemic Inflammatory Response Markers as Independent Prognostic Factors in Colorectal Cancer Patients. Cancer Research and Treatment. 2014;46(1):65–73. 10.4143/crt.2014.46.1.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu N, Yin H, Zhao G, Nuerxiati A, Wang D, Xu X, et al. Independent effect of postoperative neutrophil-to-lymphocyte ratio on the survival of pancreatic ductal adenocarcinoma with open distal pancreatosplenectomy and its nomogram-based prediction. Journal of Cancer. 2019;10(24):5935–43. 10.7150/jca.35856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, et al. The prognostic significance of a postoperative systemic inflammatory response in patients with colorectal cancer. World Journal of Surgical Oncology. 2015;13. 10.1186/s12957-015-0609-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka H, Tamura T, Toyokawa T, Muguruma K, Miki Y, Kubo N, et al. Clinical Relevance of Postoperative Neutrophil-Lymphocyte Ratio (NLR) to Recurrence After Adjuvant Chemotherapy of S-1 for Gastric Cancer. Anticancer Res. 2018;38(6):3745–51. Epub 2018/06/01. 10.21873/anticanres.12655 . [DOI] [PubMed] [Google Scholar]
- 37.Zhou ZQ, Pang S, Yu XC, Xue Q, Jiang HY, Liang XJ, et al. Predictive Values of Postoperative and Dynamic Changes of Inflammation Indexes in Survival of Patients with Resected Colorectal Cancer. Curr Med Sci. 2018;38(5):798–808. Epub 2018/10/21. 10.1007/s11596-018-1946-6 . [DOI] [PubMed] [Google Scholar]
- 38.Wu M, Yang S, Feng X, Yu F, Liu X, Dong J. Preoperative plus postoperative neutrophil-lymphocyte ratio for predicting overall survival following partial hepatectomy for hepatocellular carcinoma. Oncol Lett. 2020;20(6):375. Epub 2020/11/07. 10.3892/ol.2020.12238 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashid OM, Nagahashi M, Ramachandran S, Graham L, Yamada A, Spiegel S, et al. Resection of the primary tumor improves survival in metastatic breast cancer by reducing overall tumor burden. Surgery. 2013;153(6):771–8. Epub 2013/03/16. 10.1016/j.surg.2013.02.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16(6):378–91. Epub 2016/05/28. 10.1038/nri.2016.49 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang H, Li B, Zhang A, Lu W, Xiang C, Dong J. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio in Colorectal Liver Metastasis: A Systematic Review and Meta-Analysis. PLoS One. 2016;11(7):e0159447. Epub 2016/07/20. 10.1371/journal.pone.0159447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu N, Jian Y, Wang Y, Tian W. Evaluation of neutrophil-to-lymphocyte ratio and calcitonin concentration for predicting lymph node metastasis and distant metastasis in patients with medullary thyroid cancer. Mol Clin Oncol. 2018;9(6):629–34. Epub 2018/12/14. 10.3892/mco.2018.1727 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong YS, Tan J, Zhou XL, Song YQ, Song YJ. Systemic immune-inflammation index predicting chemoradiation resistance and poor outcome in patients with stage III non-small cell lung cancer. J Transl Med. 2017;15(1):221. Epub 2017/11/02. 10.1186/s12967-017-1326-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lerman I, Hammes SR. Neutrophil elastase in the tumor microenvironment. Steroids. 2018;133:96–101. Epub 2017/11/21. 10.1016/j.steroids.2017.11.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013. Epub 2013/07/19. 10.1172/JCI67484 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morizawa Y, Miyake M, Shimada K, Hori S, Tatsumi Y, Nakai Y, et al. Correlation of Immune Cells and Cytokines in the Tumor Microenvironment with Elevated Neutrophil-To-Lymphocyte Ratio in Blood: An Analysis of Muscle-Invasive Bladder Cancer. Cancer Invest. 2018;36(7):395–405. Epub 2018/09/11. 10.1080/07357907.2018.1506800 . [DOI] [PubMed] [Google Scholar]
- 47.Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA, et al. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015;75(15):3098–107. Epub 2015/06/18. 10.1158/0008-5472.CAN-15-1227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res. 2018;10:6685–93. Epub 2018/12/26. 10.2147/CMAR.S179189 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J, Powis de Tenbossche CG, Cane S, Colau D, van Baren N, Lurquin C, et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat Commun. 2017;8(1):1404. Epub 2017/11/11. 10.1038/s41467-017-00784-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lalani AA, Xie W, Martini DJ, Steinharter JA, Norton CK, Krajewski KM, et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. Epub 2018/01/23. 10.1186/s40425-018-0315-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(ZIP)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.