Abstract
Calcium is a major intracellular signaling messenger in innate immune cells. Similar to other immune cell subsets, the majority of calcium entry into innate immune cells is induced by cell surface receptors that stimulate store-operated calcium entry through calcium-release activated calcium (CRAC) channels. Since the molecular description of the STIM family of calcium sensors and the ORAI family of CRAC channel proteins, the majority of studies support a dominant role for these proteins in calcium signaling in innate cells. In reviewing the literature on CRAC channel function in innate cells, several general themes emerge. All innate cells express multiple members of the STIM and ORAI family members, however the ratio and relative contribution of individual isoforms changes depending on the cell type and activation state of the cell. It is evident that study of functional roles for STIM molecules is clearly ahead of studies of specific ORAI family members in all innate cell types, and that studies of CRAC channels in innate cells are not nearly as advanced as studies in lymphocytes. However, taken together, evidence from both STIM calcium sensors and ORAI channels in innate cells indicates that deficiency of STIM and ORAI proteins tends not to affect the development of any innate cell lineage, but certainly affects their function, in particular activation of the neutrophil oxidase and mast cell activation via IgE receptors. Furthermore, there are clearly hints that therapeutic targeting of CRAC channels in innate cells offers a new approach to various inflammatory and allergic diseases.
Keywords: Calcium, Store-operated calcium entry, CRAC channel, ORAI, STIM, Neutrophil, Mast cell, Monocyte, Dendritic cell, Inflammation, Orai1, Orai2, Orai3, Stim1, Stim2
1. Introduction
1.1. A brief introduction to store-operated calcium entry
The innate immune system is a complex network of defenses designed to protect the host in the setting of injury or infection. Evolutionary pressures have designed a system that is capable of sensing and responding to a range of viral, bacterial, fungal and parasitic threats. The ability to fine tune the immune response to adapt to this multitude of organisms requires complex signaling mechanisms, many of which utilize calcium as a central signaling mediator. In non-excitable cells including immune cells, the primary mechanism of calcium entry is via store-operated calcium entry (SOCE). Resting cells maintain low intracellular calcium levels by sequestering calcium outside of the cell and within “store” organelles, particularly the endoplasmic reticulum (ER). Upon receptor activation, subsequent signaling steps ultimately release calcium from the ER. Classically, this process is driven by phospholipases which generate inositol-trisphosphate (IP3) from membrane bound phosphatidylinositol 4,5-bisphosphate (PIP2). The ER-resident IP3 receptor is a receptor-operated calcium channel that liberates calcium from the ER. ER calcium depletion is sensed by dissociation of calcium from the EF-hand of STIM calcium sensors, which undergo oligomerization and conformational change to gate the plasma membrane calcium-release activated calcium (CRAC) channels. CRAC channel opening allows sustained calcium entry and activation of calcium-dependent signaling [1–3]. After much debate over the molecular identity of the CRAC channel, ORAI family members were identified over 10 years ago as the primary component of the CRAC channel pore [4–6]. Patients with mutations in ORAI1 display a SCID-like immunodeficiency, suggesting that ORAI1 is the dominant channel member in many immune cells, particularly lymphocytes [7]. However, there are three ORAI family members, ORAI1, ORAI2 and ORAI3, and other channel proteins that have been proposed to comprise the CRAC channel in immune cells [8]. It is likely that the composition of the CRAC channel differs depending on cell type, localization, differentiation and activation state.
Here we discuss the evidence for the composition of the CRAC channel in innate immune cells, the role of CRAC channels in innate immune cell function, as well as the contribution, demonstrated or anticipated, of innate cell CRAC channels to disease. We focus on neutrophils, monocytes, macrophages, mast cells and dendritic cells since, while this in no way includes all innate immune cells, evidence regarding CRAC channel function in other cells including innate lymphoid cells is exceedingly limited.
2. Neutrophils
Neutrophils are highly motile cells that efficiently track, engulf and eliminate microorganisms through production of toxic mediators. In addition to their classically recognized bactericidal role, more recent studies have demonstrated that neutrophils are more complex than previously perceived. Reverse transmigration, neutrophil subtypes, production of resolving mediators, and coordination of adaptive immune responses are examples of neutrophil functions described in the last several years [9–13]. Although the ability of neutrophils to phagocytose and kill pathogens has been known for over 100 years, our knowledge of the molecular mechanisms guiding neutrophil activation lags significantly behind that of other immune cells, at least in part due to the challenges of studying such a short lived and sensitive cell. Since the early 1980’s, calcium has been recognized as a signaling molecule that is induced through ligation of multiple neutrophil receptors, such as tyrosine kinase-associated receptors (integrins, FcγR, Dectin-1) and G-protein coupled receptors (GPCRs), which mediate neutrophil interactions with both the host and pathogens [14,15].
Ample evidence supports a role for CRAC channels and SOCE in human and mouse neutrophil function, in particular for activation of the NADPH oxidase. Early studies using [45] Ca and patch clamp techniques demonstrated that calcium entry stimulated by GPCRs is mediated through SOCE [14,15]. Subsequent inhibitor studies supported these findings [16,17]. Since the identification of STIM and ORAI family members, more targeted studies using siRNA and genetic approaches have begun to characterize the composition of CRAC channels in human and mouse neutrophils, defining how CRAC channels and SOCE are required for neutrophil responses.
2.1. Composition of the neutrophil CRAC channel
Neutrophils in both mouse and human express all five Stim and ORAI family members, although the relative expression of each isoform varies across species and cellular context. In the mouse, data from the Immgen microarray database (www.immgen.org/databrowser/index.html) reports a predominance of ORAI2 in resting bone marrow neutrophils (relative values 480 (Orai1), 700 (Orai2), 400 (Orai3)), with the ratio changing to favor ORAI in activated neutrophils from inflamed joints (relative values 400 (Orai1), 275 (Orai2), 400 (Orai3)). However, in our hands we find a predominance of ORAI1 expression by qPCR even in resting cells (unpublished data). This discrepancy may be the result of ORAI2 signal from the ORAI2 pseudogene, which is nearly identical to the ORAI2 mRNA [18]. RNAseq data from the BLUEPRINT Epigenome project (www.ebi.ac.uk/gxa/experiments?experimentSet=BLUEPRINT) suggests that human neutrophils express predominantly ORAI1 and ORAI2 in bone marrow (14TPM and 60TPM) and blood (27TPM and 38TPM). Expression levels of ORAI3 in both human and mouse is reported to be lower (11TPM in human bone marrow). TRPC channels, in particular TRPC1 and TRPC6 have been reported to contribute to SOCE in mouse neutrophils [19,20], however the expression level of these channel proteins is quite low (relative values 30 (TRPC1), 30 (TRPC6), Immgen microarray database and unpublished data). Expression of TRPC’s in human neutrophils is not detected per the BLUEPRINT RNAseq project, however TRPC3 and TRPC6 expression along with low level expression of TRPC 1 and 4 has been reported [21]. Validation by qPCR of relative expression of ORAI1, 2, and 3 in primary human and mouse neutrophils has yet to be published.
Similar to other immune cells, several studies support a role for ORAI1 as a primary component of neutrophil CRAC channels. In differentiated HL-60 cells, ORAI1 knockdown impairs calcium influx in response to thapsigargin, fMLF and FcγR cross-linking [16,22]. Schaff et al. extended these results in finding that both ORAI1 knockdown in HL-60 cells and mice heterozygous for ORAI1 demonstrate decreased calcium flux induced by thapsigargin or shear stress in a microfluidics chamber [23]. Interestingly a report analyzing neutrophils from a single patient with a loss of function ORAI1 R91W mutation demonstrated mildly reduced SOCE, corroborating the mouse and cell line data [24]. In contrast, a conflicting report by Sogkas et al. found that thapsigargin-induced SOCE was normal in neutrophils from hematopoietic chimera Orai1−/− mice [25]. ORAI1 was required for C5a-dependent calcium influx and neutrophil migration, but via a non-SOCE mechanism. The reason for these conflicting results is unclear but could result from differing methods of neutrophil purification (density gradient versus FACS sorting), which could alter cellular responses in these sensitive cells. Notably, in all these studies the decrease in SOCE with knockdown of ORAI1 alone is modest, implying that other channel components such as ORAI2, ORAI3 or TRPC family members can contribute to SOCE in neutrophils. This question has been addressed in a handful studies. Knock down of TRPC1, TRPC6, in differentiated HL-60 cells demonstrated modestly impaired calcium influx in response to thapsigargin and fMLF suggesting that these channels participate in neutrophil SOCE [21]. A role for TRPC6 has also been suggested by mouse studies showing decreased calcium influx in response to CXCR2 agonists in Trpc6−/− neutrophils [20]. Studies of ORAI2 or ORAI3 in neutrophils are similarly limited. Steinckwich et al. found no significant contribution of ORAI3 and a non-significant trend towards decreased FcγR-mediated calcium flux in differentiated HL-60 cells22]. A recent report in undifferentiated HL-60 cells demonstrated that knock down of ORAI1 and ORAI2 in combination significantly impaired SOCE and cell proliferation [26]. Whether this finding will translate into similar results in differentiated HL-60 cells or primary neutrophils remains to be seen. Additionally, the majority of these studies were performed in resting cells, however gene expression data suggests that the dominant channel component may change during inflammation. Receptor localization may also mean that CRAC channel composition may vary depending on the receptor used. Considerable work remains to determine receptor and context-dependent requirements for the neutrophil CRAC channel.
2.2. CRAC channels in neutrophil function
Several studies indicate that CRAC channels are critical for activation of the neutrophil NADPH oxidase leading to radical oxygen species (ROS) production (Fig. 1). In cell lines CRAC channel inhibition with BTP2 or knock down of TRPC1, TRPC6 or ORAI1 decreases ROS production in response to several ligands [16,21,22]. In addition to these studies that directly investigate the channel components, numerous studies of STIM1 and STIM2 deficient cells, both in cell lines and primary murine neutrophils indirectly support a role for CRAC channels in activation of the oxidase. The observation that neutrophils from a patient with ORAI1 deficiency display normal respiratory burst is seemingly at odds with the majority of other reports [24]. However, in this patient SOCE is only mildly reduced, either due to developmental alterations after bone marrow transplantation, or perhaps in humans there is sufficient redundancy from other CRAC channel components that loss of only ORAI1 has minimal impact.
Fig. 1. CRAC channel function in neutrophils.
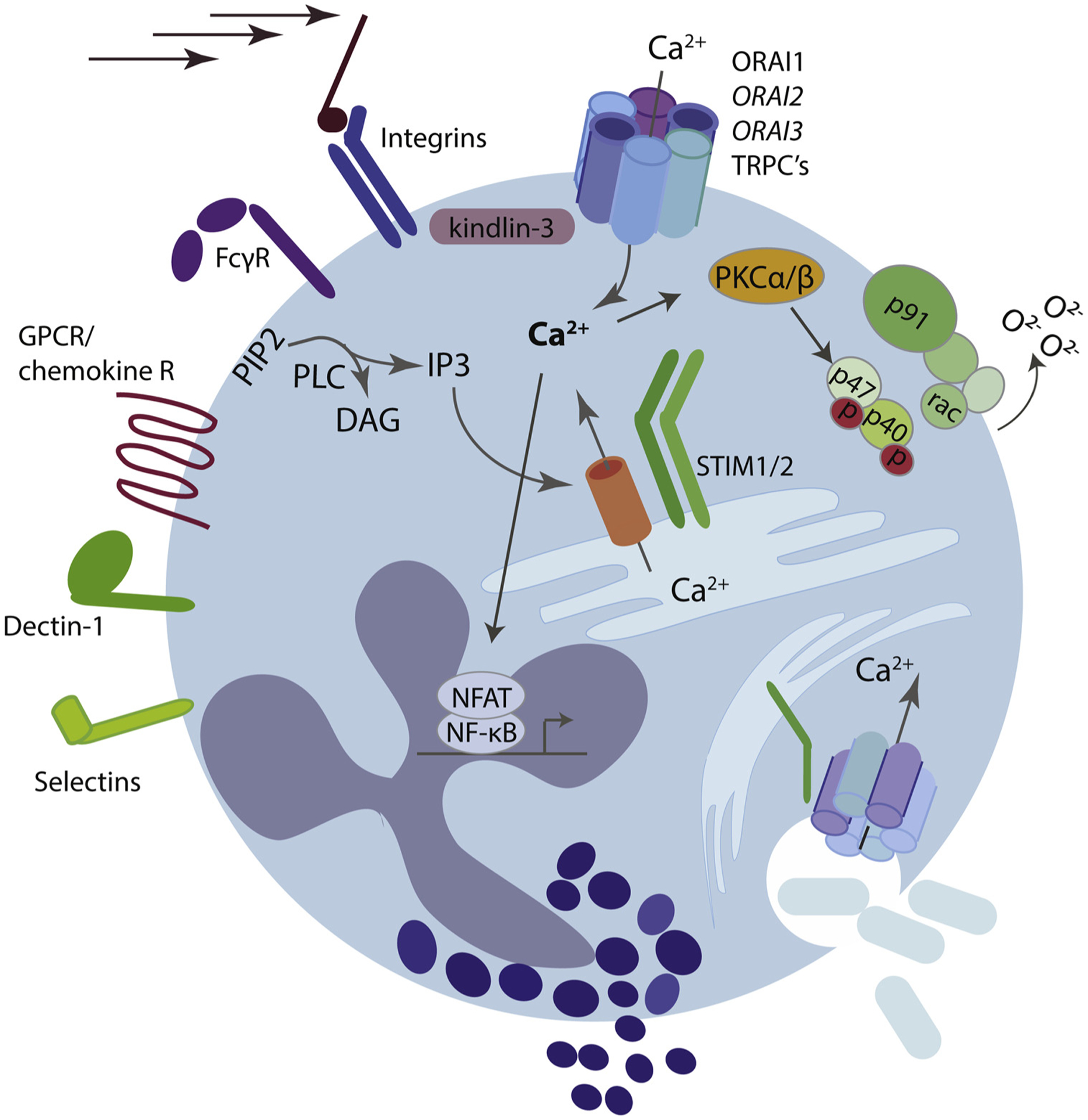
Multiple neutrophil receptors including G-protein coupled chemokine receptors and tyrosine-kinase associated receptors such as Fc-receptors, integrins, Dectin, and selectins induce calcium entry via store-operated calcium entry (SOCE). Activation of these receptors induces a signaling cascade that activates phospholipase C (PLC) family members, an enzyme that liberates IP3 from membrane bound PIP2. IP3 then releases calcium from the endoplasmic reticulum (ER) through binding to the IP3R. Depletion of ER calcium is sensed by ER resident STIM molecules, STIM1 and STIM2. Calcium-disassociation from the ER luminal EF-hand induces STIM conformational change, oligomerization, binding and opening of the plasma membrane CRAC-channels. This allows sustained calcium entry into the cytoplasm which is important for multiple neutrophil functions, including phagocytosis, ROS production, degranulation, adhesion under shear stress, and cytokine production.
The role of oxidative stress on neutrophil CRAC channels is particularly relevant given the sheer capacity of neutrophils for generating oxygen radicals. Oxidative stress may have direct effects on channel function as has been shown for human monocytes. Additionally, phagocyte (particularly neutrophil) ROS production creates large ion fluxes generated via activation of the NADPH oxidase. The oxidase machinery creates an uncompensated flux of electrons across the plasma or phagosomal membrane and also generates an intracellular proton, both of which depolarize the cell [27]. Alone, this depolarization would decrease both the electrochemical gradient driving calcium entry through CRAC channels and ultimately ROS production as well. Several groups recognized years ago that SOCE is strongly inhibited by PMA-induced ROS and that SOCE is enhanced in neutrophils from patients with chronic granulomatous disease [28,29]. Neutrophils have developed compensatory mechanisms that counteract these effects including high expression of the voltage gated proton channel (Hcvn1) which allows efflux of protons and serves to temper ROS-induced depolarization [30,31]. However, it is likely that the interaction of these large ionic fluxes and direct STIM/ORAI oxidative modifications has generated compensatory mechanisms that uniquely modulate CRAC channel function in neutrophils.
Neutrophil migration requires chemokine receptors, selectins and integrins, which mediate shape polarization, chemokinesis, neutrophil rolling, cell arrest, adhesion and transmigration. Calcium flux initiated by chemokine receptors or selectins promotes integrin activation. Integrin ligation further induces SOCE, thus it seems obvious that neutrophil migration should rely on CRAC channel function [32,33]. That said, the data supporting a role for SOCE in neutrophil migration is mixed. Schaff and Dixit nicely demonstrated in two studies that ORAI1 is required for cell arrest and shape polarization mediated through LFA-1, both through inside-out signaling from a GPCR (CXCR1 or FPR1) to initiate integrin clustering and change to higher affinity conformation, and via integrin-induced SOCE that mediates cell arrest [23,34]. These studies demonstrate that ORAI1 physically associates with high affinity LFA-1 clusters facilitated by the adaptor molecule Kindlin-3, thereby allowing the neutrophil to translate tensile forces into spatially regulated calcium signals that promote cytoskeletal reorganization and adhesion strengthening. These findings demonstrate an important role for ORAI1 in integrin-mediated mechanosignaling (Fig. 1). It will be interesting to see how other ORAI isoforms modulate these mechanically-driven signals. Genetic deletion of TRPC6 also decreases neutrophil directional migration in response to CXCR2 ligands in vitro [20]. How this impacts neutrophil migration in vivo is less clear. Sogkas et al. used hematopoietic chimeras to demonstrate that ORAI1 is required for C5a and CXCL2-dependent migration in vitro and in immune complex pneumonitis and LPS-induced peritonitis models [25]. Interestingly, they concluded that this was via a STIM1 and SOCE-independent mechanism since they observed normal migration of Stim1−/− neutrophils to C5a in vitro and enhanced migration in LPS-induced inflammation in vivo. Confirmation of these findings and further mechanistic studies will be required to understand whether this departure from the typical STIM1-ORAI1-SOCE relationship is generalizable to other neutrophil responses and receptors, or specific to C5a-mediated neutrophil activation. Corroborative evidence from STIM-deficient neutrophils is similarly mixed. We have seen intact neutrophil migration to sites of inflammation in STIM1 and STIM1/2 deficient mice, while Steinckwich et al. observed impaired migration of Stim1−/− neutrophils in vitro and in a psoriasis model in vivo [35–37]. Neutrophil migration is a complex process and the requirements for calcium and CRAC channels likely vary depending on the context and activation state of the neutrophil. Additional studies will be necessary to clearly establish the requirements for CRAC channels in different steps of neutrophil migration, and during more complex migratory patterns such as neutrophil swarming. The role of CRAC channels in synchronizing neutrophil polarization and migration is nicely discussed by Dixit and Simon in a previous review [38] and more recently by Immler et al. [39].
Studies of STIM1 and STIM2-deficient neutrophils also suggest a role for CRAC channels in degranulation [35,37]. Additionally, STIM1 is required for local calcium elevations required for phagocytosis [40], however direct investigation to determine the relative contribution of individual CRAC channel proteins to granule release and phagocytosis as well as other neutrophil functions such as NETosis and production of lipid mediators remains to be done. The role of STIM proteins in neutrophil functions has also recently been reviewed [41].
3. Monocytes and macrophages
3.1. Channel composition
Monocytes and macrophages and are a diverse group of circulating and tissue resident cells that are critical for modulation of the immune response. In disease, macrophages play a multitude of roles that vary depending on disease context and cell location. Tissue resident macrophages such as alveolar macrophages play a critical role as a first line of anti-bacterial defense. Macrophages produce robust amounts of cytokines and can shape the immune response during allergy and inflammation. Additionally, these phagocytic cells are responsible for clearance of auto-antibody coated cells during autoimmune thrombocytopenia, anemia or neutropenia.
Although signaling mechanisms in macrophages bear many similarities to other phagocytes including neutrophils, a role for CRAC channels and SOCE is less clear. Resting blood monocytes express ORAI1, ORAI2, and ORAI3 in approximately even proportions as determined by qPCR [42], while bone marrow and peritoneal cavity macrophages have a predominance of ORAI3 (microarray, www.immgen.org/databrowser/index.html). In humans, the BLUEPRINT RNAseq database reports predominantly ORAI1 expression with lower levels of ORAI2 and ORAI3 (www.ebi.ac.uk/gxa/experiments?experimentSet=BLUEPRINT). Interestingly, inflammatory macrophages have nearly double the expression of ORAI1 compared with alternatively activated macrophages or classical monocytes (29TPM vs 14TPM or 17TPM), suggesting that perhaps this isoform is more important in this context.
3.2. CRAC channels in monocyte/macrophage function
There is limited data addressing the role of CRAC channel proteins in monocyte or macrophage activation. An intriguing study in human monocytes demonstrated that CRAC channels are important for monocyte ROS production and bacterial killing. This study reported that ORAI1-mediated the majority of the CRAC current under resting conditions, however ORAI3 was critical for preventing oxidative inactivation of the CRAC channel during ROS production in vitro [42]. Staphylococcal infection in mice or treatment of human monocytes with bacterial peptides increased the ORAI3/ORAI1 ratio, suggesting that this mechanism enhances calcium signaling in monocytes during inflammation (Fig. 2). Very little is known about the role of CRAC channels in macrophages. Reports from STIM-deficient mice provide conflicting data on the role of SOCE in macrophage function [43–45]. Sogkas et al. observed in peritoneal macrophages from STIM1-deficient chimeric mice that STIM1 was required for IgG-mediated phagocytosis, while STIM2-deficient cells displayed decreased migration and cytokine production [43,45]. In contrast Vaeth et al. reported that while STIM1 and STIM2 doubly deficient macrophages have absent SOCE, they found no discernable defects in phagocytosis, lysosomal trafficking, cytokine production, inflammasome activation or antigen presentation [44]. Different animal models (Cre-lox deletion vs chimera) and macrophage source (BMDM vs thioglycolate-elicited macrophage vs peritoneal resident macrophage) could contribute to these discrepancies. One lone report suggests a role for ORAI1 in LDL-induced SOCE in macrophage-like THP-1 cells [46].
Fig. 2. Monocyte CRAC channels are modulated during infection.
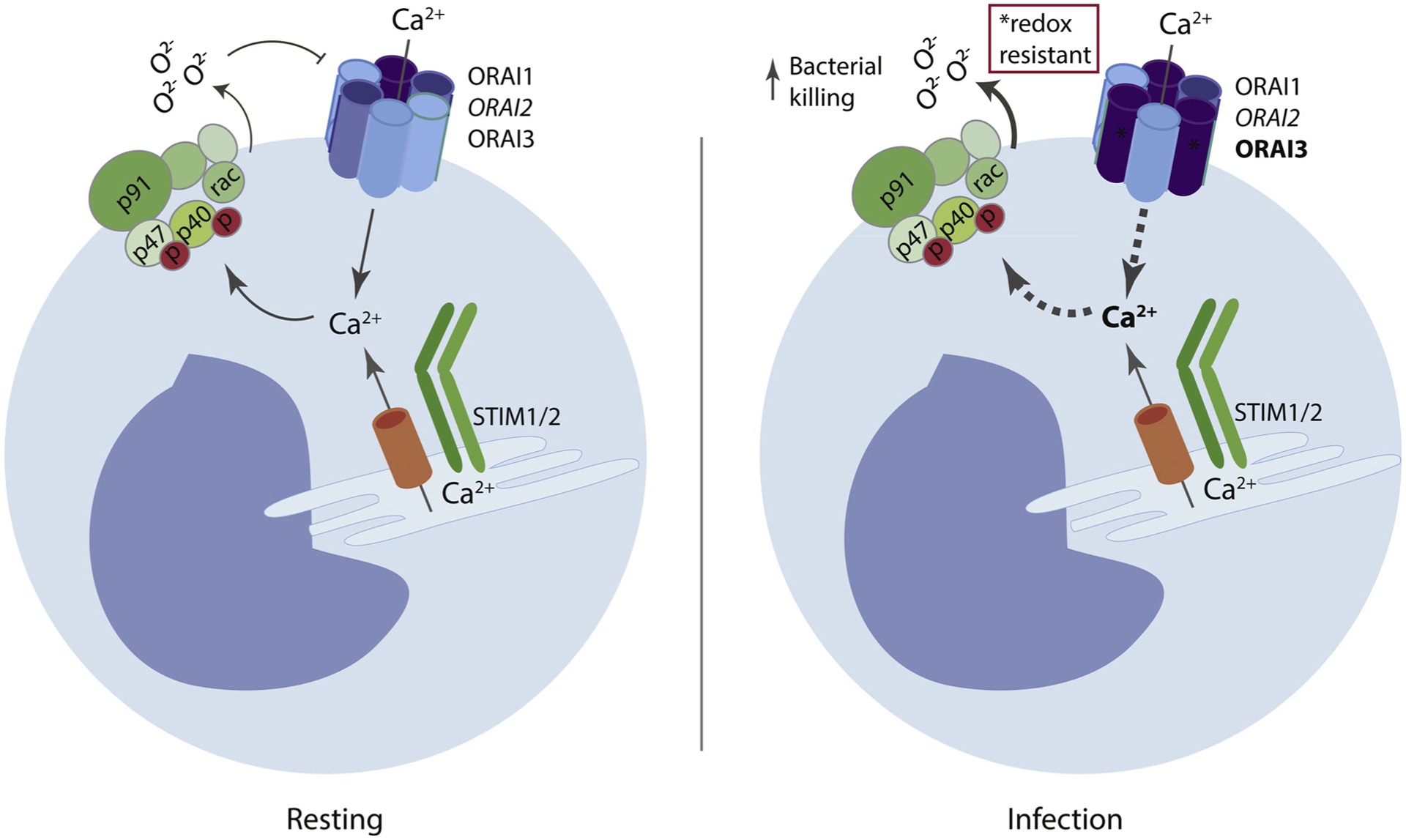
SOCE is induced in monocytes in response to bacterial pathogens. In naïve mice or human blood monocytes, the redox sensitive ORAI1 is the predominant isoform. However, during infection, monocytes upregulate expression of the redox-insensitive isoform ORAI3, lending the CRAC-channel more resistant to ROS-mediated inactivation. This switch is important for calcium entry, ROS production and bacterial killing.
A role for macrophage CRAC channels in disease is suggested by studies using Stim1−/− chimeras or the CRAC channel inhibitor BTP2 which were protective in models of autoimmune hemolytic anemia, LPS sepsis, and immune complex disease [43,47]. However, the attribution of these effects to macrophages is not certain and will require confirmation with macrophage/myeloid specific conditional mutants. Given the vast array of macrophage phenotypes, additional work is required to detail the requirements for CRAC channels in development and function of these cells.
4. Mast cells
Mast cells are the major drivers of acute allergic reactions. Mast cells are localized primarily at serosal sites throughout the body, sensing foreign antigens through engagement of their high-affinity receptors for IgE (FcεRI). This leads to robust secretion of proinflammatory cytokines, histamines and leukotrienes that mediate acute allergic reactions [48]. Not all mast cells are the same; those residing the peritoneum or lung manifest different functional responses than those cultured from bone marrow. CRAC channel activity during mast cell FcεRI activation was first discovered in mast cells more than 25 years ago [49]. Since then, the role of calcium signaling in FcεRI-mediated mast cell activation has been extensively studied, using both inhibitor approaches and more recently through functional studies of mast cells isolated from STIM and ORAI-deficient mice [50,51].
4.1. Channel composition
Murine and human mast cells express both STIM1 and STIM2 as well as all three ORAI family members in varying ratios. In mouse, skin, lung, gut and peritoneal mast cells express ~2 fold more STIM1 than STIM2, at the RNA level. ORAI1 and 2 expression is approximately the same in these cell types, while mRNA encoding ORAI3 is 3-fold higher (based on microarray data, www.immgen.org/databrowser/index.html). Similar results have been reported in human lung mast cells, with STIM1 expression being significantly higher than STIM2, but ORAI1 and 3 roughly 3-fold over ORAI2 as determined by qPCR [52]. Whether these ratios of expression change during chronic allergic inflammation remains to be investigated.
4.2. CRAC channels in mast cell function
Many studies have demonstrated the critical role of STIM and ORAI proteins in FcεRI signaling in mast cells and mast cell lines using inhibitor approaches with the relatively non-specific CRAC channel inhibitors 2-APB and SKF96365 [53]. More recently, using the more ORAI-selective compound Synta66, Ashmole et al. described an important role for CRAC channel signaling in human lung mast cell activation through FcεRI crosslinking [54]. These observations have been extended by Wajdner et al., showing that Synta66 reduced SOCE entry in human lung mast cells and mast cell lines, leading to reduced cytokine and histamine release [52]. By contrast, treatment of cells with the TRPC3/6 specific inhibitors (GSK3503A and 2934A) had no effect on mast cell activation. Nor did expression of the STIM1-KK684–685EE-TRPC1 gating-deficient mutant, suggesting that ORAI family members are the major calcium channel proteins mediating mast cell activation (Fig. 3). Many knockdown siRNA experiments have been conducted in the rat basophilic leukemia mast cell line (RBL-2H3), showing a dominant role for STIM1 and ORAI1 in mediating SOCE [55]. Interestingly, while overexpression of ORAI1 amplified SOCE in RBL cells, expression of ORAI2 actually restricted calcium signaling [56]. Similarly, siRNA knockdown of ORAI2 in OUMS-27 cells (a human chondrocyte line) lead to increased SOCE [57]. These are among the first observations that suggest that ORAI2 may limit calcium flux, in a cell line dependent manner, and that the ratios of various channel proteins are important in determining the overall level of SOCE in cells. Likewise, siRNA knockdown studies in RBL mast cells demonstrated the role of STIM2 in detecting low-level activating signaling, through its ability to sense milder changes in ER calcium levels [58]. This too was one of the early studies demonstrating cell type specific function for STIM1 versus STIM2.
Fig. 3. CRAC channel function in mast cells.
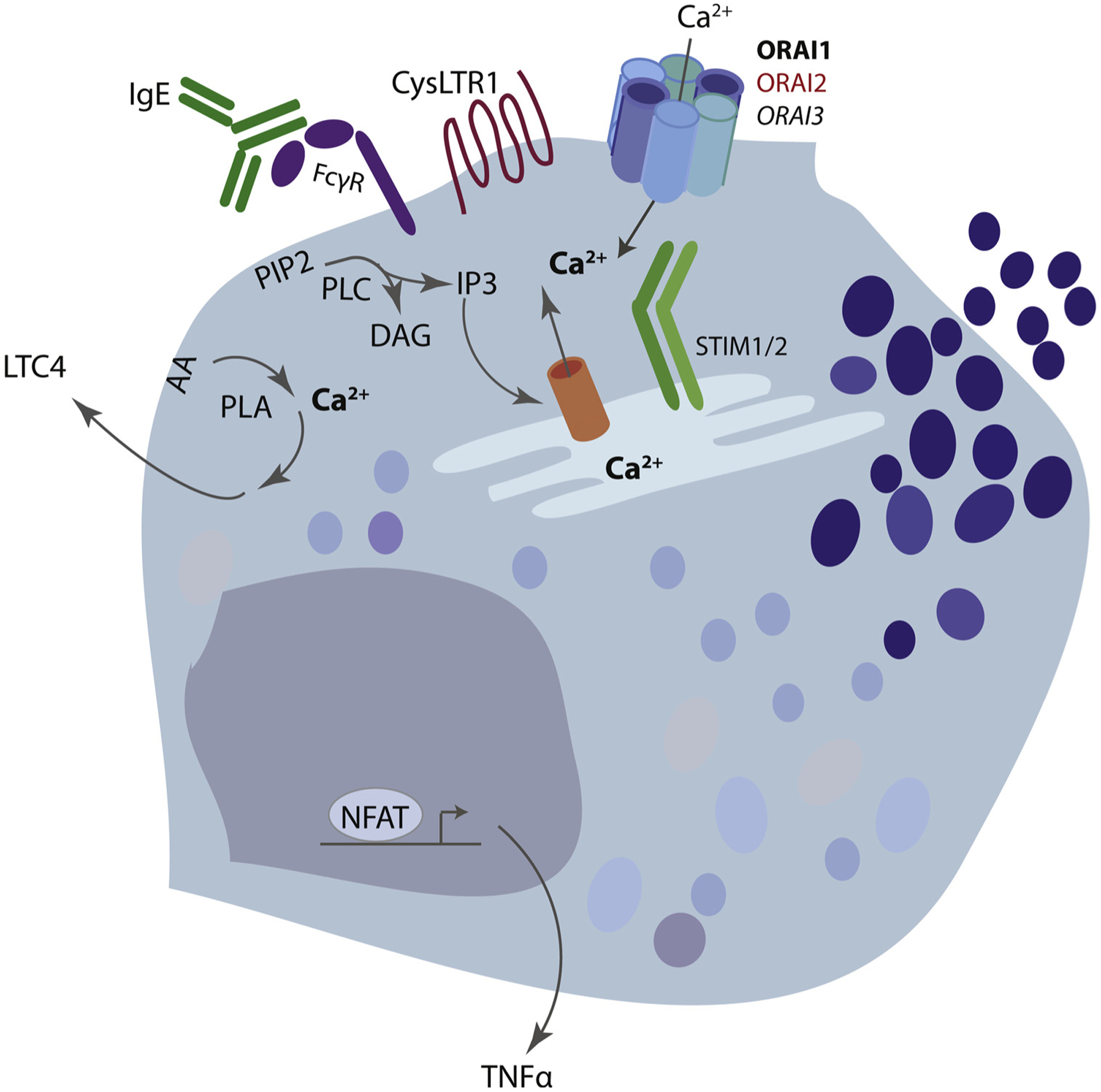
Mast cell Fcε-receptors and GPCR’s such as the cysteinyl leukotriene receptor 1 (CysLTR1) induce calcium entry via store-operated calcium entry (SOCE). Activation of these receptors induces a signaling cascade that activates phospholipase C (PLC) family members, an enzyme that liberates IP3 from membrane bound PIP2. IP3 then releases calcium from the endoplasmic reticulum (ER) through binding to the IP3R. Depletion of ER calcium is sensed by ER resident STIM molecules, STIM1 and STIM2. Calcium-disassociation from the ER luminal EF-hand induces STIM conformational change, oligomerization, binding and opening of the plasma membrane CRAC-channels. This allows sustained calcium entry into the cytoplasm which is important for multiple mast cell functions, including degranulation, leukotriene production and cytokine production.
The most definitive work has been with gene knockout mice. Mast cells derived from mice lacking either ORAI1 or STIM1 produce weak calcium signals in response to FcεRI cross-linking [50,51,59]. As a result, these knockout mice also have severely impaired histamine release, leukotriene production, reduced TNFα secretion, and fail to mount a subcutaneous anaphylactic response. However, it is important to note that these in vivo experiments were conducted with Stim1−/− fetal liver chimeric mice (or Stim1+/− heterozygous animals), since STIM1 deletion in mice results in late fetal lethality. Studies of conditional mast cell-specific STIM1 or STIM2 deficient mice have yet to be reported. Indeed, studies of primary mast cells from Stim2−/− mice are also lacking. By contrast, Tsvilovskyy et al. have very recently reported studies of an Orai2f/f mouse, which when crossed to the Mcpt5-Cre, results in ORAI2 loss in connective tissue and mucosal mast cells [60]. Peritoneal mast cells from these mice show enhanced SOCE responses to both FcεRI and G-protein receptor signaling, primarily at high levels (2 mM) of extracellular calcium. As a result, ORAI2-deficient peritoneal mast cells showed increased degranulation responses and the mast cell specific mutant animals displayed enhanced systemic anaphylaxis responses in vivo (determined only by body temperature). These results support the concept that ORAI2 is a negative regulator of SOCE in peritoneal mast cells, similar to findings in T-lymphocytes [61]. Future studies investigating immune function in these important mice will certainly follow.
5. Dendritic cells
Dendritic cells (DCs) play a major role in linking the innate and adaptive immune systems. Under steady state, DCs reside in various tissues, where they ingest pathogen and host-derived material, processing it for presentation to naïve T cells to initiate adaptive immune responses. DC-T cell interactions occur primarily in draining lymph nodes and the spleen, and less frequently in the tissues themselves. The migration of DCs from tissue sites to secondary lymphoid organs is accompanied by changes in expression level of various cell surface proteins that enhance their ability to present antigens or otherwise interact with T cells. This process is referred to as DC maturation. The maturation state of DCs is dramatically influenced by a number of inflammatory cytokines and chemokines, usually produced at sites of tissue infection or injury, as well as various PAMPs and DAMPs that act on TLRs (similar to macrophages). On top of this complexity, there are significant baseline differences in DCs found at different tissue sites, with at least 5 different subsets of DCs being recognized. Hence studies of calcium signaling kinetics in one DC type may not be representative of all DC types.
Murine and human DCs express both STIM1 and 2 as well as all three ORAI family members in various ratios. In mouse, at the RNA level, STIM2 is more highly expressed (~2-fold) than STIM1 in both tissue resident and lymphoid resident DC subtypes, while all three ORAI family members are expressed at relatively the same level, save for plasmacytoid DCs (pDCs) which contain nearly 3-fold higher levels of ORAI2 and ORAI3 mRNA (microarray data, www.immgen.org/databrowser/index.html). Human blood-derived DCs also express more STIM2 than STIM1, however ORAI expression levels vary between databases (RIKEN (https://gexc.riken.jp/) and BLUEPRINT (www.ebi.ac.uk/gxa/experiments?experimentSet=BLUEPRINT) databases. At the protein level, murine bone marrow derived DCs (BMDCs), which are cultured from bone marrow precursors, express much more STIM2 and ORAI2 than other family members [62].
Early studies with inhibitors (SKF-96365 and 2APB) demonstrated that CRAC channel function is required for murine BMDC and human blood-derived DC maturation (assessed by cell surface expression of MHC II molecules as well as migratory capacity in response to CCL21) in response to TLR stimulation [63,64]. Likewise, treatment of human blood-derived DCs with the calcium ionophore A23187 leads to cell maturation in culture. A23187 also synergizes with a number of TLR agonists to promote robust maturation and IL12 secretion of murine BMDCs [65]. The ability of eicosanoids to induce maturation of murine BMDCs was also found to be mediated by calcium store-depletion [66]. Knockdown of STIM1 or ORAI1 in human blood derived DCs similarly inhibits maturation, though the profile of responses varied with the maturation stimulus (LPS versus TNF)64]. These observations would suggest that SOCE signaling has a direct impact on DC maturation/function.
Contrasting with these studies, Vaeth et al. found that murine BMDCs derived from double mutant Stim1fl/flStim2fl/flVav-Cre mice showed no major functional defects in ex vivo cell culture experiments [44]. Though SOCE in response to thapsigargin was completely abrogated in these cells, no defects in DC maturation, cytokine production, inflammasome activation or antigen presentation to CD4+ OTII cells were observed. in vivo, the Stim1fl/flStim2fl/flVav-Cre mice had normal levels of splenic DCs. By contrast, many of these DC functions (such as phagocytosis, inflammasome activation and antigen presentation) were significantly reduced by intracellular calcium chelation (using BAPTA), suggesting that other calcium signaling pathways (potentially mediated through TRP channels) are critical for DC function. More recent studies using BMDCs derived from Stim1fl/flLysM-Cre mice confirm these findings: STIM1 is the dominant calcium sensor in BMDCs and its deletion does not affect DC maturation, phagocytosis, or phagosomal pH changes. However, these authors made the clever observation that antigen cross-presentation to CD8+ OTI cells was reduced both in vivo and in culture. This was due to defects in phago-lysosomal fusion, leading to poor processing of phagocytosed (and endocytosed) proteins into peptides to be loaded on MHC class I molecules (Fig. 4). Thus, SOCE regulates vesicle trafficking to some degree, at least in BMDCs [67]. In a related study, Maschalidi et al. found that mutation of the ER membrane protein UNC93B1, which regulates endosomal trafficking leading to defective antigen cross-presentation, resulted in a block in STIM1 aggregation [68]. This group also found that knockdown of STIM1 also blocked cross-presentation to CD8+ T cells, while over expression of an activated version of STIM1 was able to restore cross presentation to UNC93B1 mutant cells. Nunes-Hasler et al. also reported that STIM1-deficient BMDCs had reduced migration in response to chemokine stimulation (CXCL12 and CCL21). This may be a consequence of reduced STIM1 coupling to TRP channels, since deficiency of TRPML1 and TRPM2 leads to poor lysosomal calcium release and reduced actin-myosin contraction, leading to altered DC migration [69,70]. Whether these same defects are seen in other DC subsets, and what the immune consequences of defective SOCE in DCs, remains to be seen. As in other innate immune cell types, there have been fewer (or no) reported genetic studies involving ORAI family members in DCs. Likewise, the apparent discrepancy between effects of loss of SOCE signaling in human blood-derived DCs versus murine BMDCs remains to be validated.
Fig. 4. SOCE regulates dendritic cell cross presentation.
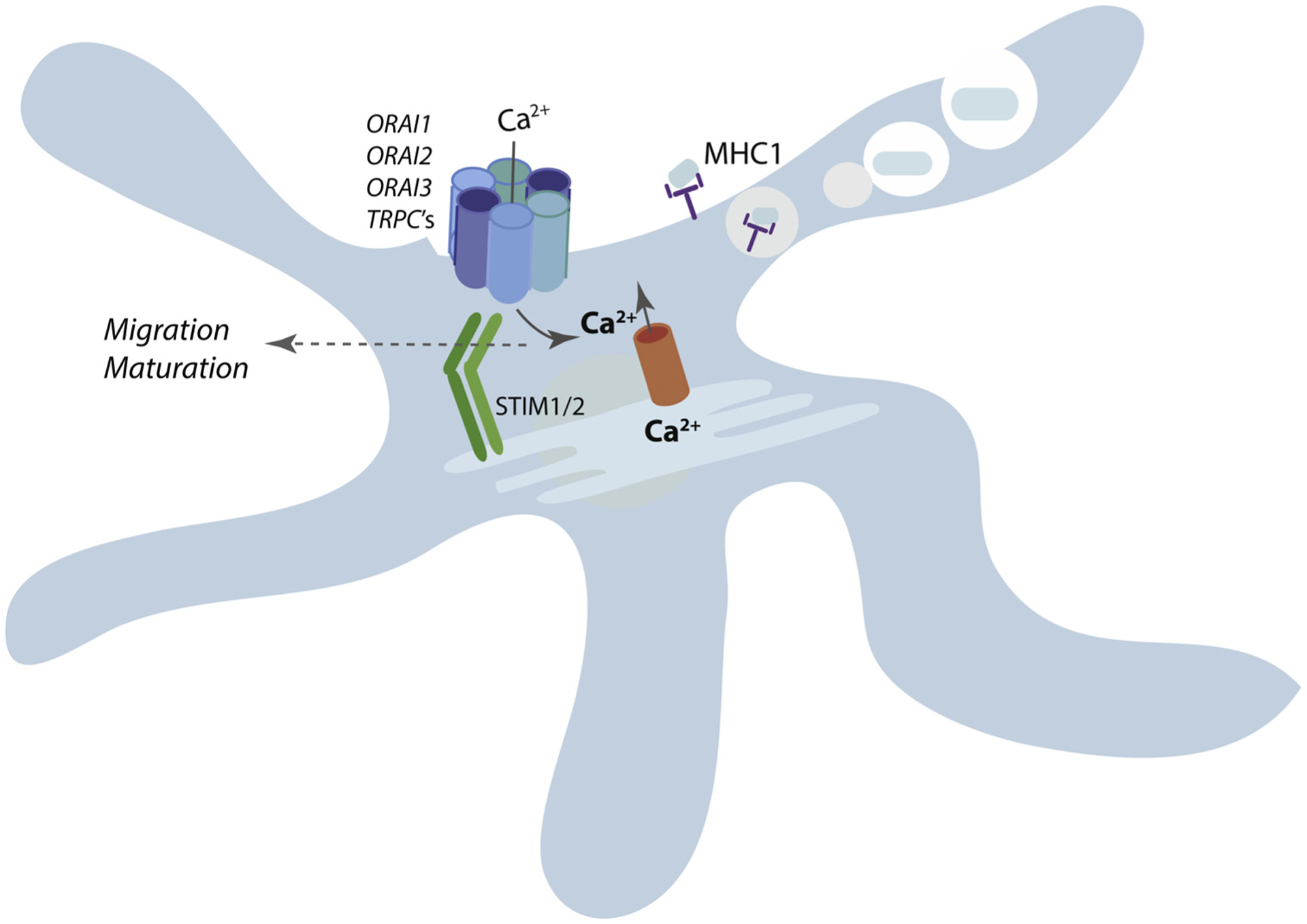
STIM1-deficient dendritic cells display defects in phago-lysosomal fusion, leading to poor processing of phagocytosed (and endocytosed) proteins into peptides to be loaded on MHC class I molecules, and thus impaired cross-presentation. Evidence regarding the role of SOCE in DC migration and maturation is mixed.
6. CRAC channels in disease and as therapeutic targets
6.1. Infection
Innate immune cells are equipped to respond to a dizzying array of viral, bacterial, fungal and parasitic pathogens. Given the paucity of studies directly assessing the role of CRAC channels in innate immune cells and disease, most of our current knowledge is inferred from studies of STIM1 and STIM2. Stim1−/− chimeras demonstrate that neutrophil-dependent bacterial killing in a Staphylococcus aureus pneumonia model requires SOCE, likely due to combined deficits in ROS production, phagocytosis and degranulation [37]. Neutrophil recruitment to the infection site in this study was normal, suggesting that neutrophil migration is not grossly affected by STIM1 deficiency. Studies are needed to extend these findings to other pathogens, and to directly determine the relative contributions of ORAI1 and ORAI2.
Very few studies have addressed the role of innate immune cells in detection and defense against viruses or parasites. Children with ORAI1 mutations suffer from recurrent viral infections; although lymphocyte defects are presumed to be the primary contributor to this phenotype, innate immune cells may play a role as well. Neutrophils are the predominant leukocyte recruited to the airways during respiratory syncytial virus (RSV) infection, an infection found in several patients with loss-of-function ORAI1 mutations [71]. Neutrophils play a role in both the antiviral response and disease pathogenesis suggesting neutrophil CRAC channels may contribute to RSV disease [72]. Given the role of STIM1 in DC cross-presentation to CD8+ T cells, it will be important to determine how this affects viral disease [67,68].
Pathogens may also coopt innate immune SOCE as a mechanism of immune evasion or to enhance pathogen survival. For instance, helminths have been shown to secrete soluble ligands that inhibit macrophage SOCE and dampen the inflammatory response [73]. Ebstein barr virus (EBV) can enhance SOCE in B cells through upregulation of ORAI1 and enhanced calcium flux is suggested to play a role in the viral life-cycle, as well as immortalization of the B cell [74]. Additionally, viral and bacterial toxins can form calcium permeable ion pores that can either induce or modulate SOCE [75,76]. Whether similar phenomena occur in virally infected myeloid cells remains to be seen.
6.2. Inflammation
Similarly, our understanding of how innate immune CRAC channels modulate inflammatory disease relies predominantly on studies of STIM molecules. Deletion of STIM1 and/or STIM2 is protective in models of hepatic ischemia-reperfusion injury, zymosan-induced peritonitis, and psoriatic skin inflammation, due to dampening of neutrophil oxidative damage and cytokine release [35,37]. The relevance of these results to neutrophil CRAC channels is supported by an earlier study that demonstrated that the CRAC channel inhibitor MRS1845 decreased post-hemorrhagic shock acute lung injury in a rat model [77], although the neutrophil-specificity of these effects is not clear.
While data from disease models are limited, in vitro studies and understanding of calcium signaling in other systems projects an even broader role of CRAC channels in neutrophil-mediated disease. Evidence from STIM1-deficient neutrophils suggests that SOCE and CRAC channels are required for phagocytosis, degranulation and cytokine production, although these findings need to be directly assessed in ORAI-deficient cells. Neutrophil extracellular traps (NETs) have been implicated in the pathophysiology of lupus and sterile acute lung injury [78–82] and likely contribute to tissue injury during severe bacterial infection as well. Although the molecular mechanisms of NET formation are still a source of debate, calcium signaling would be predicted to contribute to several aspects of NETosis, including ROS-dependent NETosis, and activation of cell-cycle pathways that allow nuclear decondensation [83,84]. Neutrophils are also abundant sources of eicosanoids such as PGE2 and LTB4. These lipid mediators are potent inflammatory agents and chemoattractants whose production strongly relies on calcium-dependent liberation of arachidonic acid via phospholipase A2 [85]. The GPCRs for these mediators also induce SOCE thus placing CRAC channels at the center of an important feed forward loop. Neutrophil LTB4 production is critical for allergic dermatitis, arthritis, Candida-induced vascular inflammation, and neutrophil swarming behavior, making CRAC channels a potential target for several neutrophil-dependent inflammatory diseases [86–89]. Alternatively, lipid resolving mediators such as lipoxins and resolvins also require calcium-dependent pathways for their synthesis and receptor-dependent responses [90]. Thus, much work remains to explore the role of CRAC channels in neutrophil-dependent initiation, progression and resolution of inflammatory disease.
6.3. Allergy and autoimmunity
Since mast cells were one of the first immune cells targeted by studies of CRAC channels, our knowledge of mast cell-mediated disease, predominantly allergy, exceeds other areas. Vig et al. demonstrated in 2008 that ORAI1 is required in a mouse model of mast cell-mediated anaphylaxis [51]. The role of CRAC channels in allergic disease are further supported by several other studies. A rat model of food allergy demonstrated increased expression of ORAI1 under allergic conditions [91], and anti-allergic properties of glycyrrhizic acid have been linked to decreased expression of ORAI1 and STIM1 on mast cells, and subsequently, decreased degranulation [92]. CRAC channel inhibitors have also been proposed to complement leukotriene inhibitors as a treatment option for allergic nasal polyposis and a newly developed oligonucleotide aptamer shown to inhibit ORAI1 offer a new drug class with potential to inhibit mast cell disease [93,94].
Data derived from studies of STIM1 and/or STIM2 deficient mice suggest a role for myeloid CRAC channels in autoimmune immune complex diseases such as autoimmune hemolytic anemia [43,45]. These studies are also corroborated by a similar study by Sogkas et al. demonstrating that the CRAC channel inhibitor BTP2 also attenuated immune complex disease [47]. Additionally, multiple genetic analyses in humans have shown association of ORAI1 SNPs in allergic and autoimmune diseases including atopic dermatitis, rheumatoid arthritis, Kawasaki’s disease and chronic spontaneous urticaria [95–98]. Together these studies identify the need for additional studies to detail the cell types and molecular pathways underlying these disease susceptibilities.
7. Conclusions and future directions
Significant progress has been made over the past 10–15 years in understanding the role of CRAC channels and SOCE in innate immune cells and disease, however additional work, in particular with ORAI conditional mutants, is required to better understand the true scope of CRAC channel contribution in disease.
7.1. Molecular regulation of CRAC channels in innate immune cells
An open question within neutrophil CRAC channel biology is how the composition and activation of CRAC channels is modulated in homeostasis and during inflammatory responses. As discussed above, gene expression data demonstrate that ORAI1:ORAI2:ORAI3 ratios change markedly with different inflammatory stimuli (Immgen microarray database and unpublished data). Recent work into CRAC channel structure argues that CRAC channels function as heteromeric hexamers [99–101]. Studies both in innate cells and other lineages detail differences in molecular structure and function of ORAI isoforms (e.g. redox resistance of ORAI3 [42], and enhanced calcium-dependent inactivation of ORAI2 [61]) thus relative abundance of ORAI isoforms and their incorporation into heteromeric channels creates a system with great potential for context-specific modulation. CRAC channel components may also interact cooperatively as described for TRPC3, which promotes SOCE via promoting IP3R association with ORAI1 in a RACK1 dependent manner [102]. In addition to the composition of the channel itself, the ratio of STIM:ORAI proteins also dose-dependently controls SOCE [103]. Furthermore, an additional level of regulation is conferred by the splice and translational STIM and ORAI variants. An inhibitory STIM2 splice variant (STIM2.1 or STIM2β) was reported to be a dominant negative in cell lines and primary CD4 T cells [104,105] and ORAI1 variants generated by alternative initiation of translation at Met 64 or 71 (Orai1α and Orai1β) were reported to have distinct membrane mobility, calcium-dependent inactivation, and ability to regulate CRAC and arachidonic acid regulated calcium (ARC) channels [106,107]. While it is beyond the scope of this review to discuss fully, STIM1 and STIM2 also have distinct features including differences in activation thresholds and CRAC channel gating characteristics that adds an addition layer to the regulation of SOCE. Finally, there are several identified regulators of SOCE that participate in STIM-ORAI complex formation (Junctate, Stimate, septins) and disassociation (SARAF) [108–111].
Certainly, while the gene expression databases referenced here offer clues of the relative expression levels in different cell types, given the complexity inherent in combinatorial factors and post-translational regulation described above, much work remains to be done to understand the dynamic regulation of CRAC channels in innate immune cells.
7.2. Local (tissue-specific) alterations in CRAC channel function during inflammation
Additionally, inflammatory tissue creates a unique milieu where oxygen tension, calcium bioavailability, oxidative stress and other local mediators may impact CRAC channel function. In the blood, the majority of calcium is bound to proteins or other anions while the bioavailable (ionized) calcium concentration in blood is approximately 1 mM. In tissues however, particularly inflamed tissues, liberation of extracellular matrix proteins provides an additional calcium “binder”, thus the actual available calcium concentration within this environment could be quite limited, thus changes in CRAC channel ratios on the cell surface may drastically alter calcium-dependent neutrophil responses in the tissues. Chronic hypoxia markedly increases ORAI family member expression in pulmonary vasculature due to HIF-dependent regulation [112]. Inflammatory tissues are often relatively hypoxic environments and evidence in HL-60 cells suggests that hypoxia may also alter SOCE in neutrophils. The mechanism and biological significance of this observation remains to be seen. Future studies focusing on ORAI expression and function throughout the course of disease, in situ tissue immune responses, and in vivo calcium imaging will be required to fully understand the complexity of CRAC channel function in innate immune cells.
Acknowledgements
Funding for this project was provided by the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (R.C.), NIH AI119134 (R.C.), AI65495 and AI68150 (C.A.L.).
References
- [1].Liou J, Kim ML, Heo WD, et al. , STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx, Curr. Biol 15 (13) (2005) 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Picard C, McCarl CA, Papolos A, et al. , STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity, New Engl. J. Med 360 (19) (2009) 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roos J, DiGregorio PJ, Yeromin AV, et al. , STIM1, an essential and conserved component of store-operated Ca2+ channel function, J. Cell Biol 169 (3) (2005) 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG, Orai1 is an essential pore subunit of the CRAC channel, Nature 443 (7108) (2006) 230–233. [DOI] [PubMed] [Google Scholar]
- [5].Vig M, Beck A, Billingsley JM, et al. , CRACM1 multimers form the ion-selective pore of the CRAC channel, Curr. Biol 16 (20) (2006) 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vig M, Peinelt C, Beck A, et al. , CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry, Science 312 (5777) (2006) 1220–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Feske S, Gwack Y, Prakriya M, et al. , A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function, Nature 441 (7090) (2006) 179–185. [DOI] [PubMed] [Google Scholar]
- [8].Lis A, Peinelt C, Beck A, et al. , CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties, Curr. Biol 17 (9) (2007) 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Oliveira S, Rosowski EE, Huttenlocher A, Neutrophil migration in infection and wound repair: going forward in reverse, Nat. Rev. Immunol 16 (6) (2016) 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fridlender ZG, Sun J, Mishalian I, et al. , Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils, PLoS One 7 (2) (2012) e31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN, Lipid mediator class switching during acute inflammation: signals in resolution, Nat. Immunol 2 (7) (2001) 612–619. [DOI] [PubMed] [Google Scholar]
- [12].Mantovani A, Cassatella MA, Costantini C, Jaillon S, Neutrophils in the activation and regulation of innate and adaptive immunity, Nat. Rev. Immunol 11 (8) (2011) 519–531. [DOI] [PubMed] [Google Scholar]
- [13].Silvestre-Roig C, Hidalgo A, Soehnlein O, Neutrophil heterogeneity: implications for homeostasis and pathogenesis, Blood 127 (18) (2016) 2173–2181. [DOI] [PubMed] [Google Scholar]
- [14].Boucek MM, Snyderman R, Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride, Science 193 (4256) (1976) 905–907. [DOI] [PubMed] [Google Scholar]
- [15].Korchak HM, Rutherford LE, Weissmann G, Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability, J. Biol. Chem 259 (7) (1984) 4070–4075. [PubMed] [Google Scholar]
- [16].Steinckwich N, Frippiat JP, Stasia MJ, et al. , Potent inhibition of store-operated Ca2+ influx and superoxide production in HL60 cells and polymorphonuclear neutrophils by the pyrazole derivative BTP2, J. Leukoc. Biol 81 (4) (2007) 1054–1064. [DOI] [PubMed] [Google Scholar]
- [17].Itagaki K, Kannan KB, Livingston DH, Deitch EA, Fekete Z, Hauser CJ, Store-operated calcium entry in human neutrophils reflects multiple contributions from independently regulated pathways, J. Immunol 168 (8) (2002) 4063–4069. [DOI] [PubMed] [Google Scholar]
- [18].Wissenbach U, Philipp SE, Gross SA, Cavalie A, Flockerzi V, Primary structure, chromosomal localization and expression in immune cells of the murine ORAI and STIM genes, Cell Calcium 42 (4–5) (2007) 439–446. [DOI] [PubMed] [Google Scholar]
- [19].Lindemann O, Strodthoff C, Horstmann M, et al. , TRPC1 regulates fMLP-stimulated migration and chemotaxis of neutrophil granulocytes, Biochim. Biophys. Acta 1853 (9) (2015) 2122–2130. [DOI] [PubMed] [Google Scholar]
- [20].Lindemann O, Umlauf D, Frank S, et al. , TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils, J. Immunol 190 (11) (2013) 5496–5505. [DOI] [PubMed] [Google Scholar]
- [21].Brechard S, Melchior C, Plancon S, Schenten V, Tschirhart EJ, Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes, Cell Calcium 44 (5) (2008) 492–506. [DOI] [PubMed] [Google Scholar]
- [22].Steinckwich N, Schenten V, Melchior C, Brechard S, Tschirhart EJ, An essential role of STIM1, Orai1, and S100A8-A9 proteins for Ca2+ signaling and FcgammaR-mediated phagosomal oxidative activity, J. Immunol 186 (4) (2011) 2182–2191. [DOI] [PubMed] [Google Scholar]
- [23].Schaff UY, Dixit N, Procyk E, Yamayoshi I, Tse T, Simon SI, Orai1 regulates intracellular calcium, arrest, and shape polarization during neutrophil recruitment in shear flow, Blood 115 (3) (2010) 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Elling R, Keller B, Weidinger C, et al. , Preserved effector functions of human ORAI1- and STIM1-deficient neutrophils, J. Allergy Clin. Immunol 137 (5) (2016) 1587–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sogkas G, Vogtle T, Rau E, et al. , Orai1 controls C5a-induced neutrophil recruitment in inflammation, Eur. J. Immunol 45 (7) (2015) 2143–2153. [DOI] [PubMed] [Google Scholar]
- [26].Diez-Bello R, Jardin I, Salido GM, Rosado JA, Orai1 and Orai2 mediate store-operated calcium entry that regulates HL60 cell migration and FAK phosphorylation, Biochim. Biophys. Acta—Mol. Cell Res 1864 (6) (2017) 1064–1070. [DOI] [PubMed] [Google Scholar]
- [27].Murphy R, DeCoursey TE, Charge compensation during the phagocyte respiratory burst, Biochim. Biophys. Acta 1757 (8) (2006) 996–1011. [DOI] [PubMed] [Google Scholar]
- [28].Geiszt M, Kapus A, Nemet K, Farkas L, Ligeti E, Regulation of capacitative Ca2+ influx in human neutrophil granulocytes. Alterations in chronic granulomatous disease, J. Biol. Chem 272 (42) (1997) 26471–26478. [DOI] [PubMed] [Google Scholar]
- [29].Tintinger GR, Theron AJ, Steel HC, Anderson R, Accelerated calcium influx and hyperactivation of neutrophils in chronic granulomatous disease, Clin. Exp. Immunol 123 (2) (2001) 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N, VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification, J. Exp. Med 207 (1) (2010) 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Femling JK, Cherny VV, Morgan D, et al. , The antibacterial activity of human neutrophils and eosinophils requires proton channels but not BK channels, J. Gen. Physiol 127 (6) (2006) 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stadtmann A, Brinkhaus L, Mueller H, et al. , Rap1a activation by CalDAG-GEFI and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling, Eur. J. Immunol 41 (7) (2011) 2074–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stadtmann A, Germena G, Block H, et al. , The PSGL-1-l-selectin signaling complex regulates neutrophil adhesion under flow, J. Exp. Med 210 (11) (2013) 2171–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dixit N, Kim MH, Rossaint J, Yamayoshi I, Zarbock A, Simon SI, Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation, J. Immunol 189 (12) (2012) 5954–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Clemens RA, Chong J, Grimes D, Hu Y, Lowell CA, STIM1 and STIM2 cooperatively regulate mouse neutrophil store-operated calcium entry and cytokine production, Blood 130 (13) (2017) 1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Steinckwich N, Myers P, Janardhan KS, et al. , Role of the store-operated calcium entry protein, STIM1, in neutrophil chemotaxis and infiltration into a murine model of psoriasis-inflamed skin, FASEB J 29 (7) (2015) 3003–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang H, Clemens RA, Liu F, et al. , STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense, Blood 123 (14) (2014) 2238–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dixit N, Simon SI, Chemokines, selectins and intracellular calcium flux: temporal and spatial cues for leukocyte arrest, Front. Immunol 3 (2012) 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Immler R, Simon SI, Sperandio M, Calcium signalling and related ion channels in neutrophil recruitment and function, Eur. J. Clin. Invest 48 (Suppl. 2) (2018) e12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nunes P, Cornut D, Bochet V, et al. , STIM1 juxtaposes ER to phagosomes, generating Ca(2)(+) hotspots that boost phagocytosis, Curr. Biol.: CB 22 (21) (2012) 1990–1997. [DOI] [PubMed] [Google Scholar]
- [41].Demaurex N, Saul S, The role of STIM proteins in neutrophil functions, J. Physiol 596 (14) (2018) 2699–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Saul S, Gibhardt CS, Schmidt B, et al. , A calcium-redox feedback loop controls human monocyte immune responses: the role of ORAI Ca2+ channels, Sci. Signal 9 (418) (2016) ra26. [DOI] [PubMed] [Google Scholar]
- [43].Sogkas G, Stegner D, Syed SN, et al. , Cooperative and alternate functions for STIM1 and STIM2 in macrophage activation and in the context of inflammation, Immun. Inflamm. Dis 3 (3) (2015) 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vaeth M, Zee I, Concepcion AR, et al. , Ca2+ signaling but not store-operated Ca2+ entry is required for the function of macrophages and dendritic cells, J. Immunol 195 (3) (2015) 1202–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Braun A, Gessner JE, Varga-Szabo D, et al. , STIM1 is essential for Fcgamma receptor activation and autoimmune inflammation, Blood 113 (5) (2009) 1097–1104. [DOI] [PubMed] [Google Scholar]
- [46].Liang SJ, Zeng DY, Mai XY, et al. , Inhibition of Orai1 store-operated calcium channel prevents foam cell formation and atherosclerosis, Arterioscler. Thromb. Vasc. Biol 36 (4) (2016) 618–628. [DOI] [PubMed] [Google Scholar]
- [47].Sogkas G, Rau E, Atschekzei F, Syed SN, Schmidt RE, The pyrazole derivative BTP2 attenuates IgG immune complex-induced inflammation, Inflammation 41 (1) (2018) 42–49. [DOI] [PubMed] [Google Scholar]
- [48].Olivera A, Rivera J, Paradigm shifts in mast cell and basophil biology and function: an emerging view of immune regulation in health and disease, Methods Mol. Biol 1192 (2014) 3–31. [DOI] [PubMed] [Google Scholar]
- [49].Hoth M, Penner R, Depletion of intracellular calcium stores activates a calcium current in mast cells, Nature 355 (6358) (1992) 353–356. [DOI] [PubMed] [Google Scholar]
- [50].Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T, Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses, Nat. Immunol 9 (1) (2008) 81–88. [DOI] [PubMed] [Google Scholar]
- [51].Vig M, DeHaven WI, Bird GS, et al. , Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels, Nat. Immunol 9 (1) (2008) 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wajdner HE, Farrington J, Barnard C, et al. , Orai and TRPC channel characterization in FcepsilonRI-mediated calcium signaling and mediator secretion in human mast cells, Physiol. Rep 5 (5) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Di Capite J, Parekh AB, CRAC channels and Ca2+ signaling in mast cells, Immunol. Rev 231 (1) (2009) 45–58. [DOI] [PubMed] [Google Scholar]
- [54].Ashmole I, Duffy SM, Leyland ML, Morrison VS, Begg M, Bradding P, CRACM/Orai ion channel expression and function in human lung mast cells, J. Allergy Clin. Immunol 129 (6) (2012) 1628–1635 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ma HT, Peng Z, Hiragun T, Iwaki S, Gilfillan AM, Beaven MA, Canonical transient receptor potential 5 channel in conjunction with Orai1 and STIM1 allows Sr2+ entry, optimal influx of Ca2+, and degranulation in a rat mast cell line, J. Immunol 180 (4) (2008) 2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gross SA, Wissenbach U, Philipp SE, Freichel M, Cavalie A, Flockerzi V, Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels, J. Biol. Chem 282 (27) (2007) 19375–19384. [DOI] [PubMed] [Google Scholar]
- [57].Inayama M, Suzuki Y, Yamada S, et al. , Orai1-Orai2 complex is involved in store-operated calcium entry in chondrocyte cell lines, Cell Calcium 57 (5–6) (2015) 337–347. [DOI] [PubMed] [Google Scholar]
- [58].Thiel M, Lis A, Penner R, STIM2 drives Ca2+ oscillations through store-operated Ca2+ entry caused by mild store depletion, J. Physiol 591 (6) (2013) 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gwack Y, Srikanth S, Oh-Hora M, et al. , Hair loss and defective T- and B-cell function in mice lacking ORAI1, Mol. Cell. Biol 28 (17) (2008) 5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Tsvilovskyy V, Solis-Lopez A, Schumacher D, et al. , Deletion of Orai2 augments endogenous CRAC currents and degranulation in mast cells leading to enhanced anaphylaxis, Cell Calcium 71 (2018) 24–33. [DOI] [PubMed] [Google Scholar]
- [61].Vaeth M, Yang J, Yamashita M, et al. , ORAI2 modulates store-operated calcium entry and T cell-mediated immunity, Nat. Commun 8 (2017) 14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bandyopadhyay BC, Pingle SC, Ahern GP, Store-operated Ca2+ signaling in dendritic cells occurs independently of STIM1, J. Leukoc. Biol 89 (1) (2011) 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Matzner N, Zemtsova IM, Nguyen TX, Duszenko M, Shumilina E, Lang F, Ion channels modulating mouse dendritic cell functions, J. Immunol 181 (10) (2008) 6803–6809. [DOI] [PubMed] [Google Scholar]
- [64].Felix R, Crottes D, Delalande A, et al. , The Orai-1 and STIM-1 complex controls human dendritic cell maturation, PLoS One 8 (5) (2013) e61595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Huang E, Showalter L, Xu S, Czernliecki BJ, Koski GK, Calcium mobilizing treatment acts as a co-signal for TLR-mediated induction of interleukin-12 (IL-12p70) secretion by murine bone marrow-derived dendritic cells, Cell. Immunol 314 (2017) 26–35. [DOI] [PubMed] [Google Scholar]
- [66].Itagaki K, Barton BE, Murphy TF, et al. , Eicosanoid-induced store-operated calcium entry in dendritic cells, J. Surg. Res 169 (2) (2011) 301–310. [DOI] [PubMed] [Google Scholar]
- [67].Nunes-Hasler P, Maschalidi S, Lippens C, et al. , STIM1 promotes migration, phagosomal maturation and antigen cross-presentation in dendritic cells, Nat. Commun 8 (1) (2017) 1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Maschalidi S, Nunes-Hasler P, Nascimento CR, et al. , UNC93B1 interacts with the calcium sensor STIM1 for efficient antigen cross-presentation in dendritic cells, Nat. Commun 8 (1) (2017) 1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Bretou M, Saez PJ, Sanseau D, et al. , Lysosome signaling controls the migration of dendritic cells, Sci. Immunol 2 (16) (2017). [DOI] [PubMed] [Google Scholar]
- [70].Saez PJ, Saez JC, Lennon-Dumenil AM, Vargas P, Role of calcium permeable channels in dendritic cell migration, Curr. Opin. Immunol 52 (2018) 74–80. [DOI] [PubMed] [Google Scholar]
- [71].Lian J, Cuk M, Kahlfuss S, et al. , ORAI1 mutations abolishing store-operated Ca (2+) entry cause anhidrotic ectodermal dysplasia with immunodeficiency, J. Allergy Clin. Immunol 142 (4) (2018) 1297–1310 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Stokes KL, Currier MG, Sakamoto K, et al. , The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection, J. Virol 87 (18) (2013) 10070–10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chauhan A, Sun Y, Pani B, et al. , Helminth induced suppression of macrophage activation is correlated with inhibition of calcium channel activity, PLoS One 9 (7) (2014) e101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dellis O, Arbabian A, Papp B, Rowe M, Joab I, Chomienne C, Epstein-Barr virus latent membrane protein 1 increases calcium influx through store-operated channels in B lymphoid cells, J. Biol. Chem 286 (21) (2011) 18583–18592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hyser JM, Utama B, Crawford SE, Broughman JR, Estes MK, Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity, J. Virol 87 (24) (2013) 13579–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jover E, Tawk MY, Laventie BJ, Poulain B, Prevost G, Staphylococcal leukotoxins trigger free intracellular Ca(2+) rise in neurones, signalling through acidic stores and activation of store-operated channels, Cell. Microbiol 15 (5) (2013) 742–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lee C, Xu D-ZZ, Feketeova E, et al. , Store-operated calcium channel inhibition attenuates neutrophil function and postshock acute lung injury, J. Trauma 59 (1) (2005) 56. [DOI] [PubMed] [Google Scholar]
- [78].Caudrillier A, Kessenbrock K, Gilliss BM, et al. , Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury, J. Clin. Invest 122 (7) (2012) 2661–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lefrancais E, Mallavia B, Zhuo H, Calfee CS, Looney MR, Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury, JCI Insight 3 (3) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Looney MR, Matthay MA, Neutrophil sandwiches injure the microcirculation, Nat. Med 15 (4) (2009) 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ortiz-Munoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR, Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice, Blood 124 (17) (2014) 2625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Sayah DM, Mallavia B, Liu F, et al. , Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation, Am. J. Respir. Crit. Care Med 191 (4) (2015) 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Amulic B, Knackstedt SL, Abu Abed U, et al. , Cell-cycle proteins control production of neutrophil extracellular traps, Dev. Cell 43 (4) (2017) 449–462 e5. [DOI] [PubMed] [Google Scholar]
- [84].Kaplan MJ, Radic M, Neutrophil extracellular traps: double-edged swords of innate immunity, J. Immunol 189 (6) (2012) 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dennis EA, Norris PC, Eicosanoid storm in infection and inflammation, Nat. Rev. Immunol 15 (8) (2015) 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Oyoshi MK, He R, Li Y, et al. , Leukotriene B4-driven neutrophil recruitment to the skin is essential for allergic skin inflammation, Immunity 37 (4) (2012) 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lee EKS, Gillrie MR, Li L, et al. , Leukotriene B4-mediated neutrophil recruitment causes pulmonary capillaritis during lethal fungal sepsis, Cell Host Microbe 23 (1) (2018) 121–133 e4. [DOI] [PubMed] [Google Scholar]
- [88].Lammermann T, Afonso PV, Angermann BR, et al. , Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo, Nature 498 (7454) (2013) 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kim ND, Chou RC, Seung E, Tager AM, Luster AD, A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis, J. Exp. Med 203 (4) (2006) 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Serhan CN, Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol 177 (4) (2010) 1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Yang B, Yang C, Wang P, et al. , Food allergen–induced mast cell degranulation is dependent on PI3K-mediated reactive oxygen species production and upregulation of store-operated calcium channel subunits, Scand. J. Immunol 78 (1) (2013) 35–43. [DOI] [PubMed] [Google Scholar]
- [92].Han S, Sun L, He F, Che H, Anti-allergic activity of glycyrrhizic acid on IgE-mediated allergic reaction by regulation of allergy-related immune cells, Sci. Rep 7 (1) (2017) 7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Di Capite J, Nelson C, Bates G, Parekh AB, Targeting Ca2+ release-activated Ca2+ channel channels and leukotriene receptors provides a novel combination strategy for treating nasal polyposis, J. Allergy Clin. Immunol 124 (5) (2009) 1014–1021 e1–3. [DOI] [PubMed] [Google Scholar]
- [94].Sun R, Yang Y, Ran X, Yang T, Calcium influx of mast cells is inhibited by aptamers targeting the first extracellular domain of Orai1, PLoS One 11 (7) (2016) e0158223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Onouchi Y, Fukazawa R, Yamamura K, et al. , Variations in ORAI1 gene associated with kawasaki disease, PLoS One 11 (1) (2016) e0145486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Li J, Guo A, Chen W, et al. , Association of ORAI1 gene polymorphisms with chronic spontaneous urticaria and the efficacy of the nonsedating H1 anti-histamine desloratadine, J. Allergy Clin. Immunol 139 (4) (2017) 1386–1388 e9. [DOI] [PubMed] [Google Scholar]
- [97].Yen JH, Chang CM, Hsu YW, et al. , A polymorphism of ORAI1 rs7135617, is associated with susceptibility to rheumatoid arthritis, Mediators Inflamm 2014 (2014) 834831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Chang WC, Lee CH, Hirota T, et al. , ORAI1 genetic polymorphisms associated with the susceptibility of atopic dermatitis in Japanese and Taiwanese populations, PLoS One 7 (1) (2012) e29387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Cai X, Nwokonko RM, Loktionova NA, et al. , Pore properties of Orai1 calcium channel dimers and their activation by the STIM1 ER calcium sensor, J. Biol. Chem 293 (33) (2018) 12962–12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Cai X, Zhou Y, Nwokonko RM, et al. , The Orai1 store-operated calcium channel functions as a hexamer, J. Biol. Chem 291 (50) (2016) 25764–25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhou Y, Nwokonko RM, Cai X, et al. , Cross-linking of Orai1 channels by STIM proteins, Proc. Natl. Acad. Sci. U. S. A 115 (15) (2018) E3398–E3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Woodard GE, Lopez JJ, Jardin I, Salido GM, Rosado JA, TRPC3 regulates agonist-stimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5-trisphosphate receptor, RACK1, and Orai1, J. Biol. Chem 285 (11) (2010) 8045–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Hoover PJ, Lewis RS, Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1), Proc. Natl. Acad. Sci. U. S. A 108 (32) (2011) 13299–13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Miederer AM, Alansary D, Schwar G, et al. , A STIM2 splice variant negatively regulates store-operated calcium entry, Nat. Commun 6 (2015) 6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rana A, Yen M, Sadaghiani AM, et al. , Alternative splicing converts STIM2 from an activator to an inhibitor of store-operated calcium channels, J. Cell Biol 209 (5) (2015) 653–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Desai PN, Zhang X, Wu S, et al. , Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message, Sci. Signal 8 (387) (2015) ra74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Fukushima M, Tomita T, Janoshazi A, Putney JW, Alternative translation initiation gives rise to two isoforms of Orai1 with distinct plasma membrane mobilities, J. Cell. Sci 125 (Pt. 18) (2012) 4354–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jing J, He L, Sun A, et al. , Proteomic mapping of ER-PM junctions identifies STIMATE as a regulator of Ca(2)(+) influx, Nat. Cell Biol 17 (10) (2015) 1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Srikanth S, Jew M, Kim KD, Yee MK, Abramson J, Gwack Y, Junctate is a Ca2+-sensing structural component of Orai1 and stromal interaction molecule 1 (STIM1), Proc. Natl. Acad. Sci. U. S. A 109 (22) (2012) 8682–8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sharma S, Quintana A, Findlay GM, et al. , An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry, Nature 499 (7457) (2013) 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E, SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling, Cell 149 (2) (2012) 425–438. [DOI] [PubMed] [Google Scholar]
- [112].Ma S, Cai C, Ma Y, et al. , Store-operated Ca(2)(+) entry mediated regulation of polarization in differentiated human neutrophil-like HL-60 cells under hypoxia, Mol. Med. Rep 9 (3) (2014) 819–824. [DOI] [PubMed] [Google Scholar]


