Abstract
Background
Change in hormone receptor (estrogen [ER] and progesterone [PR]) and/or human epidermal growth factor receptor type 2 (HER2) status during the evolutionary course of metastatic breast cancer and the effect of tumor classification subtype switching remain understudied and underappreciated in brain metastasis patients.
Methods
Using preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, a systematic review of series published prior to April 2020 obtained from the Medline database of biopsied or resected breast cancer brain metastasis (BCBM) was performed. Weighted random effects models were used to calculate pooled estimates.
Results
15 full-text articles were included with receptor expression analyses on 1373 patients who underwent biopsy or resection of at least one intracranial lesion to compare to the primary tumor. Primary tumor receptor expression immunophenotypes were 45.0% ER+, 41.0% ER−, 31.0% PR+, 51.0% PR−, 35% HER2+, and 47.0% HER2−. Corresponding BCBM immunophenotypes were 19.0% ER+, 31.0% ER−, 13.0% PR+, 40.0% PR−, 21.0% HER2+, and 26.0% HER2−. On primary/BCBM comparison, 540 patients (42.6%) exhibited discordance in any receptor with 17.0% (95% CI: 13.0%–23.0%) discordant on ER, 23.0% (95% CI: 18.0%–30.0%) discordant on PR, and 12.0% (95% CI: 8.0%–16.0%) discordant on HER2 status. The most common receptor conversions found in BCBM were ER loss 11.0% (95% CI: 8.0%–16.0%), PR loss 15.0% (95% CI: 11.0%–21.0%), and HER2 gain 9.0% (95% CI: 7.0%–11.0%).
Conclusions
BCBM exhibits significant receptor expression discordance in comparison to primary tumors in approximately 40% of patients. Classification patterns need to be analyzed to determine factors predictive of BCBM/primary tumor discordance. Overall, tumor subtype switching and its effect on clinical management remains underappreciated.
Keywords: breast cancer, discordance, estrogen receptor, HER2, progesterone receptor
Key Points.
40% of brain metastasis exhibit receptor discordance from primary tumors.
Approximately 12% had discordance in HER2 between the primary and brain metastasis.
ER loss, PR loss, and HER2 gain were the most common conversions observed.
Importance of the Study.
Management guidelines for relapsed or metastatic breast cancer, newly diagnosed or progressing on systemic therapy, support the reassessment of hormone receptor and HER2 expression during the disease course of breast cancer. However, given the challenges in routinely obtaining intracranial tissue, patients with brain metastasis are underrepresented or excluded entirely from series evaluating subtype switching. These findings, combined with the recent revelation that extracranial metastatic sites may not be reliable genomic surrogates for intracranial metastatic disease, underscore the need for a systematic analysis of receptor expression discordance rates specifically among breast cancer brain metastasis compared to paired primary tumor immunophenotypes.
Advances in the management of locally-advanced and metastatic breast cancer, increased central nervous system (CNS) screening, and improved access to MRI have collectively contributed to an increase in the detection and diagnosis of breast cancer brain metastases (BCBM).1,2 The incidence, prognosis, and management of BCBM differs based on hormone receptor expression status (estrogen receptor [ER] and progesterone receptor [PR]) and human epidermal growth factor receptor type 2 (HER2). Importantly, treatment paradigms for patients with metastatic breast cancer consist of cytotoxic chemotherapy, hormone therapy, and targeted therapy, all of which depend on the receptor expression profile of the patient's disease.
Large series of matched primary tumor and metastatic site tissue samples have demonstrated that receptor expression profiles can change during a disease course, due to biological changes in the tumor, as a result of selective pressure of systemic therapy, or because metastatic lesions at a specific location might result from clones with a molecular pattern for homing and successfully growing in those particular organs.3,4 However, given the challenges in routinely obtaining intracranial tissue, patients with BCBM were underrepresented5 or excluded entirely from these series.6 These findings, combined with the recent revelation that extracranial metastatic sites may not be reliable genomic surrogates for intracranial metastatic disease,7 underscore the need for a systematic analysis of receptor expression discordance rates specifically among BCBM compared to primary tumor immunophenotypes.
Methods
Selection of Articles
This systematic review was performed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) criteria.8 Initial article selection was performed by searching the MEDLINE (PubMed) and Cochrane electronic bibliographic databases. To ensure a comprehensive initial search strategy, generic keywords were used in the initial article screen: “breast cancer” and “brain metastasis” combined with “estrogen receptor/ER,” “progesterone receptor/PR,” “HER2/neu,” and “receptor conversion/dis- or concordance.” Full text articles published in the English language were considered, and no publishing date restrictions were used through April 2020.
The initial query identified 3141 articles that were subsequently screened by a thorough review of the article titles, abstracts, and manuscripts, as necessary. Specific inclusion criteria included: retrospective or prospective case series of >10 adult patients, original full-text research articles directly describing ER, PR, or HER2 statuses in primary breast tumors compared with BCBM, and receptor conversion or discordance. Exclusion criteria included: nonclinical reports, expert opinion, commentary or review studies which did not provide unique data on >10 patients; abstract only publications; and studies on receptor comparisons to extracranial metastasis only (ie, lung, liver, or bone). The search strategy used for this report and the methodology for study inclusion is illustrated in Supplementary Figure 1.
Outcome measures and statistical analysis
The hormonal subtypes data included the techniques used to define a positive ER, PR, and HER2 status. The individual receptor statuses at the initial diagnosis of the primary tumor and BCBM were recorded. The receptor discordance data included the BCBM to primary tumor discordances by individual receptor expression and overall tumor subtype. Gain or loss of each individual receptor, also termed receptor “conversion,” was also recorded. The primary outcomes consisted of the incidence of individual receptor expression, overall tumor subtypes, and individual receptor discordances among primary tumors and BCBM. R Studio (version 1.1.423, Boston, Massachusetts) was used for statistical analyses and R package “metafor” (version 2.0–0)9 was used for meta-analyses, tests for heterogeneity, and analysis of publication bias. Study variances for overall estimates were calculated using the DerSimonian-Laird method.10,11 Weighted random effects models were used to calculate pooled estimates for each of the outcome variables. Given the types of studies included in this meta-analysis, spanning numerous years in a number of different populations and varied geographic locations, the random effects model was considered superior to the fixed effects model when calculating pooled estimates.12,13 Since some studies had missing data, we conducted a sensitivity analysis by excluding studies with missing data. We compared the ER, PR, and HER2 discordance rates between studies with and without missing data to see assess for potential statistically significant differences in the pooled estimates. I2 statistic was used for identifying heterogeneity: I2 of 0%, 25%, 50%, and 75% were interpreted as absent, low, moderate, and high heterogeneity, respectively.14 Funnel plots and the Egger test (P value < .05 indicating presence of bias) were used for identification of publication bias.
Results
The initial search strategy used for this study yielded 201 unique reports, that were further reviewed based on the strict inclusion criteria. Detailed individual study review revealed that a majority of reports (n = 92, 46% of evaluated studies) only described receptor comparisons among extra-cranial metastasis (or included <10 patients with brain metastasis), resulting in 15 reports that met all inclusion and exclusion criteria for this meta-analysis (Supplementary Figure 1). There was no presence of publication bias detected across the included reports regarding the primary tumor receptor immunophenotype, the BCBM receptor immunophenotype, or discordances in ER, PR, or HER2 status (P > .05). A majority of studies (n = 9, 60%) represented single-institution reports and 6 studies (40%) were multi-institutional collaborations. All were retrospective in nature.
Key patient characteristics, demographics, and treatment information were not uniformly or consistently reported across the literature (Supplementary Table 1). In total, 1368 patients were included in this meta-analysis and the median number of patients in each study was 41 (range: 18–316 patients). The median age was 52 years (range: 40–56 years). The median time between primary tumor diagnosis and development of BCBM was 33 months (11 studies, range 2–46 months). Ten studies included information on systemic therapy prior to BCBM diagnosis, of which 38% (n = 370 patients) received hormonal therapy and 31% (n = 301 patients) received HER2-directed therapy.
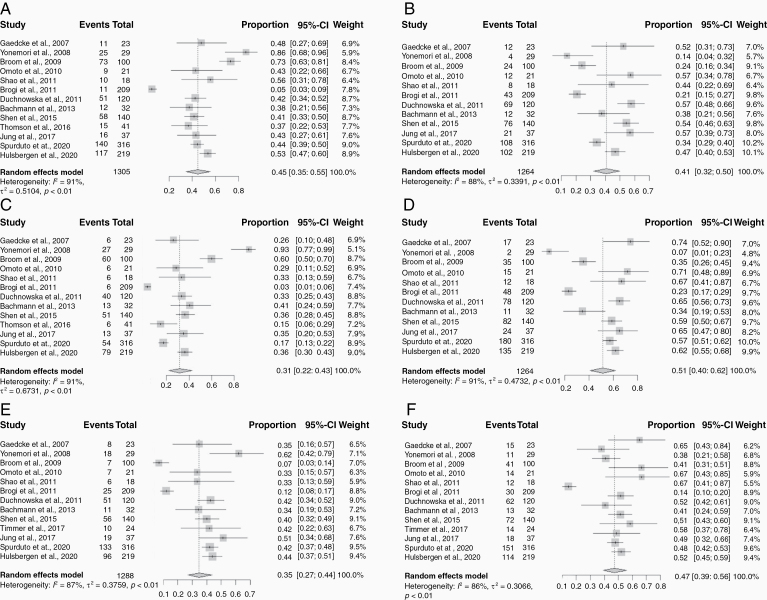
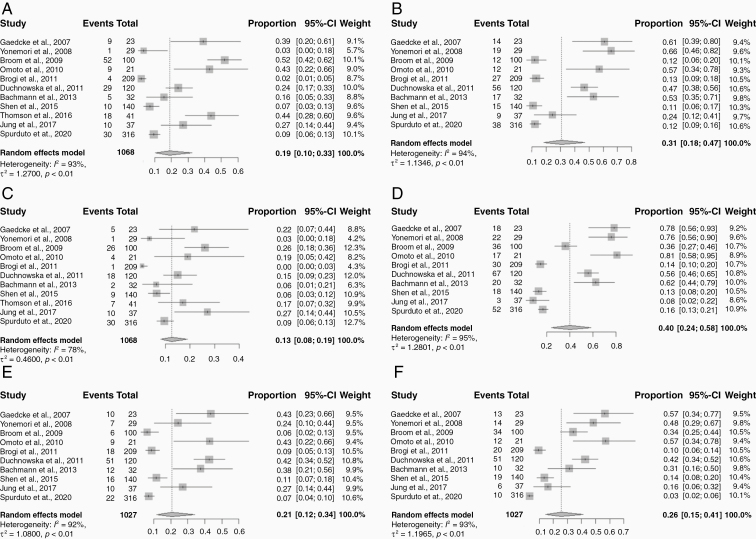
Details regarding ER, PR, and HER2 positivity cutoffs, proportions at initial diagnosis and at time of brain metastasis are presented in Table 1. The ER/PR positive threshold varied across the included studies: >1% of cells staining positive was used in 6 studies; >10% by immunohistochemistry (IHC) in 8 studies, and an Allred score of >3 in 1 study. The HER2 positive cutoff methodology used was 3+ overexpression by IHC or 2+ with fluorescence in-situ hybridization (FISH) in 13 studies, and >30% IHC in 2 studies. Since only one study reported the total number of hormone positive or negative cases and 6 studies reported subtype grouping (with 2 studies having missing information in these sections), all random effects meta-analyses were performed by individual receptor expression only. Weighted pooled estimates of the tumor receptor expression immunophenotypes from the primary tumors were 45.0% ER+ (95% CI: 35.0–55.0%), 41.0% ER− (95% CI: 32.0–50.0%), 31.0% PR+ (95% CI: 22.0–43.0%), 51.0% PR− (95% CI: 40.0–62.0%), 35.0% HER2+ (95% CI: 27.0–44.0%), and 47.0% HER2− (95% CI: 39.0–56.0%) (Figure 1). Weighted pooled estimates of the paired receptor expression immunophenotypes from the corresponding BCBM were 19.0% ER+ (95% CI: 10.0–33.0%), 31.0% ER− (95% CI: 18.0–47.0%), 13.0% PR+ (95% CI: 8.0–19.0%), 40.0% PR− (95% CI: 24.0–58.0%), 21.0% HER2+ (95% CI: 12.0–34.0%), and 26.0% HER2− (95% CI: 15.0–41.0%) (Figure 2). Sensitivity analysis showed that there was no significant difference in pooled estimates of ER discordance rates between studies without any missing data and studies with incomplete data (0.15 [95% CI: 0.10–0.23] versus 0.17 [95% CI: 0.13–0.23]). Similarly, estimates of PR discordance rates (0.17 [95% CI: 0.12–0.26] versus 0.23 [95% CI: 0.18–0.30]) and HER2 discordance rates (0.05 [95% CI: 0.05–0.14] versus 0.12 [95% CI: 0.08–0.16]) between studies without any missing data and studies with incomplete data did not show significant differences in pooled estimates.
Table 1.
Estrogen, Progesterone, and HER2 Receptor Statuses at Initial Diagnosis of the Primary Tumor and in the Brain Metastasis
| Author | N | ER/PR Positive Cutoff | HER2 Positive Cutoff | ER+ | ER− | PR+ | PR− | HR+ | HR− | HER2+ | HER2− | HR+/HER2+ | HR+/HER2− | HR-/HER2+ | HR-/HER2− |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gaedcke et al., 200715 | 23 | >10% IHC | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 11 | 12 | 6 | 17 | NA | NA | 14 | 30 | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 29 | 56 | 15 | 70 | NA | NA | 29 | 56 | NA | NA | NA | NA | ||||
| Yonemori et al., 200816 | 24 | >10% IHC | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 25 | 4 | 27 | 2 | NA | NA | 18 | 11 | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 1 | 19 | 1 | 22 | NA | NA | 7 | 14 | NA | NA | NA | NA | ||||
| Broom et al., 200917 | 100 | >10% IHC | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 73 | 24 | 60 | 35 | NA | NA | 7 | 41 | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 52 | 12 | 26 | 36 | NA | NA | 6 | 34 | NA | NA | NA | NA | ||||
| Hoefnagel et al., 201018 | 44 | >10% IHC | >6 HER2 copies/ tumor cell nucleus | Initial diagnosis | Initial diagnosis | ||||||||||
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||
| Omoto et al., 201019 | 21 | >10% IHC | >30% IHC | Initial diagnosis | Initial diagnosis | ||||||||||
| 9 | 12 | 6 | 15 | NA | NA | 7 | 14 | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 9 | 12 | 4 | 17 | NA | NA | 9 | 12 | NA | NA | NA | NA | ||||
| Shao et al., 201120 | 18 | >1% of cells stained positive | >30% IHC | Initial diagnosis | Initial diagnosis | ||||||||||
| 10 | 8 | 6 | 12 | NA | NA | 6 | 12 | NA | NA | NA | 6 | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 9 | 9 | 6 | 12 | NA | NA | 7 | 11 | NA | NA | NA | NA | ||||
| Brogi et al., 201121 | 209 | >10% IHC | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 11 | 43 | 6 | 48 | NA | NA | 25 | 30 | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 4 | 27 | 1 | 30 | NA | NA | 18 | 20 | NA | NA | NA | NA | ||||
| Duchnowska et al., 201222 | 120 | >1% of cells stained positive | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 51 | 69 | 40 | 78 | NA | NA | 51 | 62 | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 29 | 56 | 18 | 67 | NA | NA | 51 | 51 | NA | NA | NA | NA | ||||
| Bachmann et al., 201323 | 32 | >10% IHC | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 12 | 12 | 13 | 11 | NA | NA | 11 | 13 | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 5 | 17 | 2 | 20 | NA | NA | 12 | 10 | NA | NA | NA | NA | ||||
| Shen et al., 201524 | 140 | >1% of cells stained positive | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 58 | 76 | 51 | 82 | NA | NA | 56 | 72 | 26 | 34 | 29 | 37 | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 10 | 15 | 9 | 18 | NA | NA | 16 | 19 | NA | NA | NA | NA | ||||
| Thomson et al., 201625 | 41 | Allred score of >3 | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 15 | NA | 6 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 18 | NA | 7 | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||
| Timmer et al., 201726 | 24 | >1% of cells stained positive | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 10 | 14 | 10 | 14 | NA | NA | 10 | 14 | NA | NA | NA | 5 | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 3 | 21 | 4 | 20 | NA | NA | 15 | 8 | NA | NA | NA | NA | ||||
| Jung et al., 201827 | 37 | >1% of cells stained positive | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 16 | 21 | 13 | 24 | NA | NA | 19 | 18 | 3 | 13 | 16 | 5 | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 10 | 9 | 10 | 3 | NA | NA | 10 | 6 | NA | NA | NA | NA | ||||
| Sperduto et al., 202028 | 316 | >1% of cells stained positive | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 140 | 108 | 54 | 180 | 118 | 120 | 133 | 151 | 68 | 88 | 75 | 85 | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| 30 | 38 | 30 | 52 | 40 | 38 | 22 | 10 | 84 | 74 | 71 | 87 | ||||
| Hulsbergen et al., 202029 | 219 | >10% IHC | 3+ overexpression IHC or 2+ with FISH | Initial diagnosis | Initial diagnosis | ||||||||||
| 117 | 102 | 79 | 135 | 114 | NA | 96 | 114 | 53 | 61 | 42 | 53 | ||||
| Brain metastasis | Brain metastasis | ||||||||||||||
| NA | NA | NA | NA | NA | NA | NA | NA | 40 | 47 | 64 | 55 |
BM, brain metastasis; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER2, human epidermal growth factor receptor type 2; HR, hormonal receptor; IHC, immunohistochemistry; NA, not available; PR, progesterone receptor.
Figure 1.
Forest plots of primary tumor receptor expression profiles for each receptor expression subtype: (A) ER+; (B) ER−; (C) PR+; (D) PR−; (E) HER2+; (F) HER2−. Squares indicate the proportions from individual studies and horizontal lines indicate the 95% confidence interval. The size of the data marker corresponds to the relative weight assigned in the pooled analysis using the random effects model. Diamond indicates the pooled proportion with 95% confidence interval.
Figure 2.
Forest plots of breast cancer brain metastasis tumor receptor expression profiles for each receptor expression subtype: (A) ER+; (B) ER−; (C) PR+; (D) PR−; (E) HER2+; (F) HER2−. Squares indicate the proportions from individual studies and horizontal lines indicate the 95% confidence interval. The size of the data marker corresponds to the relative weight assigned in the pooled analysis using the random effects model. Diamond indicates the pooled proportion with 95% confidence interval.
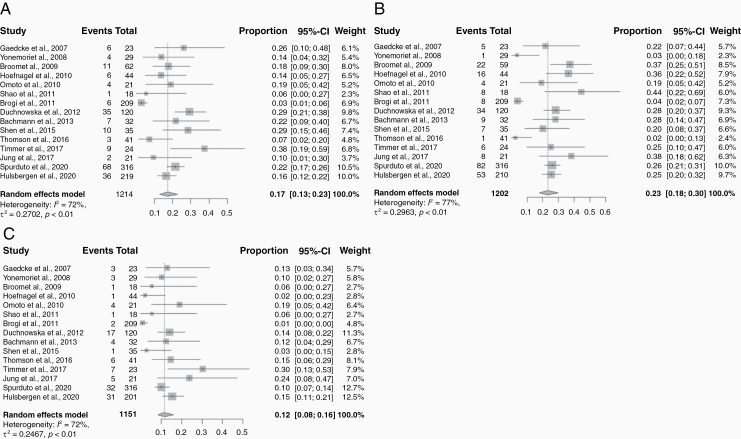
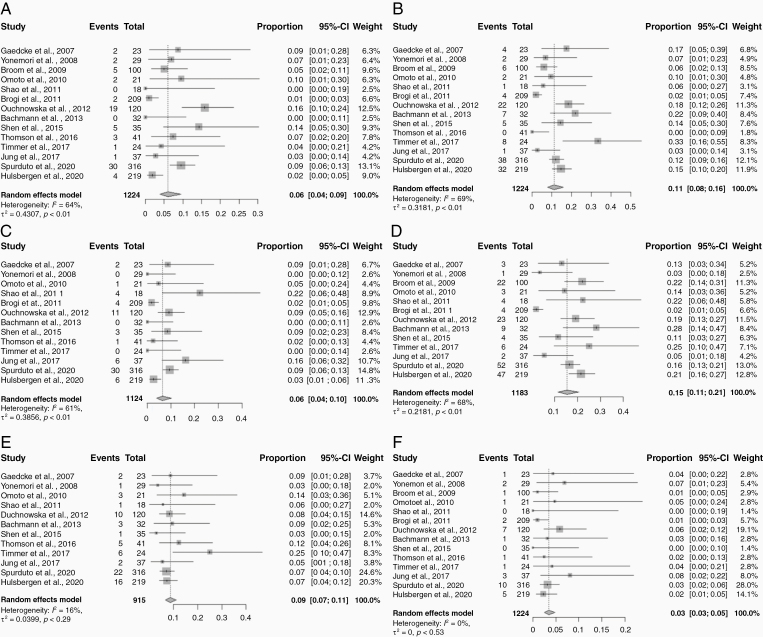
When compared to the primary tumor receptor expression immunophenotypes, 540 patients (42.6%) exhibited discordance in any receptor status (Table 2). When evaluated by each receptor individually, 17.0% (95% CI: 13.0%–23.0%) were discordant for ER status, 23.0% (95% CI: 18.0%–30.0%) discordant for PR status, and 12.0% (95% CI: 8.0%–16.0%) discordant for HER2 status (Figure 3). ER conversions found in BCBM compared to primary tumors were ER gain in 6.0% (95% CI: 4.0–9.0%) and ER loss in 11.0% (95% CI: 8.0–16.0%). Similarly, PR gain occurred in 6.0% (95% CI: 4.0–10.0%) and PR loss in 15.0% (95% CI: 11.0–21.0%). HER2 gain occurred in 9.0% (95% CI: 7.0–11.0%) and HER2 loss in 3.0% (95% CI: 3.0–5.0%) (Figure 4).
Table 2.
Estrogen, Progesterone, and HER2 Receptor Discordances and Gain/loss in Brain Metastases Compared to Paired Primary Tumors
| Author and Year | N | Breast/Brain ER Discordance | BM ER Gain | BM ER Loss | Breast/Brain PR Discordance | BM PR Gain | BM PR Loss | Breast/Brain HER2 Discordance | BM HER2 Gain | BM HER2 Loss |
|---|---|---|---|---|---|---|---|---|---|---|
| Gaedcke et al., 200715 | 23 | 6 | 2 | 4 | 5 | 2 | 3 | 3 | 2 | 1 |
| Yonemori et al., 200816 | 24 | 4 | 2 | 2 | 1 | 0 | 1 | 3 | 1 | 2 |
| Broom et al., 200917 | 100 | 11 | 5 | 6 | 22 | NA | 22 | 1 | NA | 1 |
| Hoefnagel et al., 201018 | 44 | 6 | NA | NA | 16 | NA | NA | 1 | NA | NA |
| Omoto et al., 201019 | 21 | 4 | 2 | 2 | 4 | 1 | 3 | 4 | 3 | 1 |
| Shao et al., 201120 | 18 | 1 | 0 | 1 | 8 | 4 | 4 | 1 | 1 | 0 |
| Brogi et al., 201121 | 209 | 6 | 2 | 4 | 8 | 4 | 4 | 2 | NA | 2 |
| Duchnowska et al., 201222 | 120 | 35 | 19 | 22 | 34 | 11 | 23 | 17 | 10 | 7 |
| Bachmann et al., 201323 | 32 | 7 | 0 | 7 | 9 | 0 | 9 | 4 | 3 | 1 |
| Shen et al., 201524 | 35 | 10 | 5 | 5 | 7 | 3 | 4 | 1 | 1 | 0 |
| Thomson et al., 201625 | 41 | 3 | 3 | 0 | 1 | 1 | NA | 6 | 5 | 1 |
| Timmer et al., 201726 | 24 | 9 | 1 | 8 | 6 | 0 | 6 | 7 | 6 | 1 |
| Jung et al., 201827 | 37 | 2 | 1 | 1 | 8 | 6 | 2 | 5 | 2 | 3 |
| Sperduto et al., 202028 | 316 | 68 | 30 | 38 | 82 | 30 | 52 | 32 | 22 | 10 |
| Hulsbergen et al., 202029 | 219 | 36 | 4 | 32 | 53 | 6 | 47 | 31 | 16 | 5 |
BM, brain metastasis; ER, estrogen receptor; HER2, human epidermal growth factor receptor type 2; NA—not available; PR, progesterone receptor.
Figure 3.
Forest plots of discordances for each receptor expression subtype in the brain metastasis compared to the primary tumor: (A) ER status; (B) PR status; (C) HER2 status. Squares indicate the proportions from individual studies and horizontal lines indicate the 95% confidence interval. The size of the data marker corresponds to the relative weight assigned in the pooled analysis using the random effects model. Diamond indicates the pooled proportion with 95% confidence interval.
Figure 4.
Forest plots of receptor conversions for each receptor expression subtype in the brain metastasis compared to the primary tumor: (A) ER gain; (B) ER loss; (C) PR gain; (D) PR loss; (E) HER2 gain; (F) HER2 loss. Squares indicate the proportions from individual studies and horizontal lines indicate the 95% confidence interval. The size of the data marker corresponds to the relative weight assigned in the pooled analysis using the random effects model. Diamond indicates the pooled proportion with 95% confidence interval.
Discussion
Management guidelines for relapsed or metastatic breast cancer support the reassessment of hormone receptor and HER2 expression during the disease course of breast cancer.30 Historically, this practice originated from case series of patients with ER+ disease who developed recurrent disease despite hormonal therapy and, on re-biopsy, were determined to exhibit ER-receptor conversion.31 These discoveries have been bolstered by recent large retrospective and prospective studies; however, the application of these findings to a brain metastasis population has remained relatively understudied until recently. Most commonly, patients are treated based on the primary tumor immunophenotype, or the most recent biopsy from a metastatic site, assuming no change had occurred in the brain metastasis. The present meta-analysis provides key insights into the tumor biology of BCBM and offers important implications for clinical practice and clinical trial design, by suggesting that receptor discordance is in fact very common, >40%.
The breast cancer subtype has been correlated with the incidence, kinetics, and prognosis of BCBM patients.32 One of the most commonly utilized tools used in clinical practice and trial stratification, the diagnosis-specific Graded Prognostic Assessment, uses tumor subtype (in addition to performance status and age), to estimate survival after diagnosis of BCBM.33 The most recent iteration defined 5 criteria for estimating prognosis, with subtype continuing to be an important variable for prognosis estimation.34 Incorporation of receptor expression status at the time of brain metastasis development may lead to further refinements of current prognostic estimates. However, to-date, the influence of receptor expression conversion at the time of disease relapse or development of metastatic disease on survival remains controversial. For example, one large retrospective analysis failed to demonstrate any difference in survival in patients exhibiting receptor discordances,35 while a prospective study demonstrated significant differences in survival in patients exhibiting receptor conversion.36 The studies included in this BCBM meta-analysis also yielded conflicting results in this regard. Therefore, updated analysis and comparison of prognostic estimates specific to BCBM patients using primary tumor subtype (as is classically used) and tumor subtype of the brain metastasis, is clearly warranted.
The most common receptor discordances between primary tumors and BCBM were PR status (23%) and ER status (17%). The most common receptor conversions in BCBM compared to paired primary tumors were PR loss in 15% and ER loss in 11%. Whether these conversions occur as result of changes in tumor biology to a more malignant phenotype with increased potential for metastatic disease spread to the brain or from selective pressure of systemic therapy is controversial. Conversion to hormone receptor negative status has been associated with upregulation of growth factor expression, a more aggressive disease course, and reduced survival.17 Moreover, genomic sequencing of small subsets of BCBM and paired primary tumor samples have revealed multiple shared mutations as well as de novo mutations, suggesting that BCBM arises from a minority of cells present in the original tumor.37 On the other hand, selective pressure of hormonal therapies can also result in the development of dominant metastatic clones that lack the hormone receptors of the original primary tumor.38 To some degree, both processes are likely at play. To provide further etiological insight, large-scale, massively-paralleled DNA sequencing studies are needed to screen the entire genomes of primary tumors, extracranial metastases, and intracranial metastases at initial diagnosis and throughout the disease course.
One of the key actionable findings from the present study was the gain of HER2 observed in approximately 10% of BCBM. This observation differs from the largest series of comparisons between primary tumors and extracranial metastases which have demonstrated discordance in ER and PR status in up to 40% of patients, but relatively unchanged levels of HER2 expression in metastatic sites, whether synchronous or metachronous.5,39 Smaller series of extracranial metastases which focused on HER2 conversions verified by IHC and FISH have, in fact, revealed an opposite trend: HER2 loss in extracranial metastatic sites.17 The effect of tumor discordance on the selection of systemic therapy is difficult to assess from retrospective studies. However, a prospective cohort of 40 patients with metastatic breast cancer who underwent biopsy of a metastatic site for the purpose of treatment modification demonstrated a 20% rate of change in the treatment plan, the most common of which was treatment with HER2 directed therapy or enrollment on a clinical trial with a novel anti-HER2 agent.6 For HER2− breast cancer patients with brain metastasis whose BCBM-specific HER2 status is unestablished, HER2-targeted therapy would obviously not be selected. The data from this study suggest that perhaps up to 10% of these patients could have been found to be HER2+ and could potentially have benefitted from a change in the therapeutic regimen.28 Given the increasing availability of HER-2 directed therapeutic options for patients with BCBM with intracranial response rates of 50–66%,40–43 as well as the evidence of the importance of timing these agents with SRS,44 tumor receptor expression analysis is recommended to be performed on all biopsied or resected BCBM, with specific inclusion of retesting HER2. Whether this practice change translates into an actual survival benefit could only be determined by a large randomized prospective trial.
A number of factors are hypothesized to correlate with receptor expression discordance, including age, tumor grade, number and location of sites of metastatic disease, the interval between primary tumor diagnosis and development of the intracranial metastatic disease, and use of systemic therapy. However, consistent observations of key factors correlated with tumor subtype switching in BCBM were not observed in any of the series in this meta-analysis. Yet, it is important to note the considerable variability in time to development of brain metastasis from primary diagnoses across series ranging from months to years, depending on the series. Therefore, the influence of timing and associated systemic therapy interventions on subtype switching remains prone to retrospective selection bias. To date, no model has been developed with enough diagnostic accuracy to predict the BCBM immunophenotype based on patient characteristics and treatment details alone. Adding to this complexity, a small series of rapid autopsies on 10 patients with metastatic breast cancer, 5 of whom had brain metastases, not only demonstrated extensive heterogeneity between the primary breast tumor and metastatic sites but also among distinct metastatic sites when compared to each other.45 Whole-exome sequencing studies have also supported the principle that extracranial metastatic sites may not be reliable genetic surrogates for brain metastasis.7 Given the challenges in routinely obtaining intracranial tissue for patient management, the development of minimally invasive approaches of analyzing the biology and tumor immunophenotype are clearly needed. To this end, advanced imaging studies and radiomics research aimed at noninvasively predicting tumor immunophenotype are needed.46,47
Clinical trial enrollment criteria for brain metastasis patients cannot mandate re-biopsy of intracranial disease. Mandatory tumor biopsy, with an estimated 5–10% risk of procedure-related complication, for correlative studies and selection of novel cancer-directed therapies in clinical trials, was found to be acceptable to only 22% of patients, 1% of oncologists, and 1% of academic medical center IRBs in a recent survey.48 However, the implications to clinical trial design and the reported outcomes of these studies can be severely affected without considering subtype switching. Brain metastasis trials are often powered to show small differences, and eligibility criteria are based on the tumor immunophenotype, generally determined from the primary tumor.17 A 40% discordance rate between the primary tumor and BCBM, as observed in this meta-analysis, may lead to (1) underpowered studies given the unknown true BCBM immunophenotype in each of the study cohorts and/or (2) suboptimal treatment in the absence of hormone and HER2 receptor expression information from the BCBM. From this study, the most common conversions were PR loss (15%), ER loss (11%), and HER2 gain (9%), which may lead to the inclusion or exclusion of hormonal agents or HER2-directed therapies into tested therapeutic combinations. Taken together, one could consider biopsy in patients who are to be enrolled onto clinical trials where no local therapy (such as radiation therapy) would be offered, with a trial of targeted systemic therapy alone. Moreover, a biopsy can be considered for patients with multiply recurrent brain metastasis in the setting of targeted therapy to determine mechanisms of resistance or in those with discordant responses between extracranial and intracranial sites. Highly sensitive and targeted methods of evaluating the immunophenotype of cerebrospinal fluid cell-free DNA, such as next-generation sequencing and digital PCR,49 should be integrated in future trials of BCBM to promote minimally invasive efforts at guiding precision therapy.
Although there was no publication bias detected in this meta-analysis, there is a potential for selection bias for higher proportions of metastatic-prone immunophenotypes in each of the individual series in this BCBM-specific meta-analysis. Patients undergo biopsy or resection of brain metastasis for a specific and finite number of reasons: (1) to confirm a diagnosis of cancer (ie, in patients with suspicion of another pathological abnormality or atypical presentation of disease), (2) to confirm a diagnosis of metastatic cancer spread (ie, presence of a solitary metastasis or prolonged interval from the primary tumor), or (3) to aid in tumor control or improve tumor-related symptomatology (ie, large brain metastasis or in those with tumor-related symptoms expected to benefit from resection).5,6 This inherent selection bias may result in a cohort of BCBM at higher risk for subtype switching being reported in the literature, and consequently, in this meta-analysis. A comparison of patient, tumor, and treatment-related characteristics between patients who undergo biopsy or resection for BCBM and those diagnosed radiographically may provide more information on the impact of this selection bias.
There are several limitations to the present study. Given the study periods and retrospective nature of the data collection, it is possible that technical differences in tumor sample analysis or variations in staining methodology over time contributed to a reporting of “pseudo-discordance” between the primary tumor and BCBM.17 However, given that intracranial metastasis, unlike commonly biopsied osseous sites, do not have to pre-treated with agents that may alter receptor expression rates,50 we consider receptor conversion in BCBM to reflect true biological events. Although each study reported strict definitions for ER, PR, and HER2 status, these likely remain at risk for inter-laboratory differences (ie, initial diagnosis at one center and relapsed diagnosis at a different center).51 Moreover, 3 different definitions of ER/PR status were considered “ER positive” for this study, as were the 3 different definitions for “HER2 positive.” Each of these definitions is consistent with the clinical practice guidelines.52 However, it is possible that the percent discordances would vary across studies based on these different positive thresholds,53 especially when considered with the possibility of heterogeneity of receptor expression in the sampled tumors. Finally, known technical limitations with current testing methodologies (ie, different lengths of antigen retrieval and tissue fixation across institutions54 or the subjectivity associated with the immunostaining approaches used across centers), are known errors in the determination of receptor expression status.55 Together, these findings, in weighted summation, may lead to individual study variances, but given the methodology of the meta-analysis, the overall conclusions from this study would likely be reasonably consistent.
Conclusion
This meta-analysis of BCBM compared with paired primary tumors in over 1300 patients with metastatic breast cancer demonstrated a high-rate (40%) of receptor expression discordance. These findings could help to better refine our current prognostic models, treatment paradigms, clinical trial design, and stratification criteria. Most intriguingly, a proportion of patients may be eligible for an increasing selection of emerging CNS-active targeted therapies, while others may be able to discontinue ineffective therapies, and therefore be spared treatment-related toxicities. Further analysis of receptor conversion dynamics, studied in a prospective manner with sensitive and standardized profiling tests, is clearly needed in the modern era of precision medicine.
Supplementary Material
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
This article was presented in Society for Neuro-Oncology Virtual Conference on Brain Metastases, August 14, 2020, held in association with the AANS/CNS Section on Tumors.
Conflict of interest statement. R.K.: Honorarium from Elsevier, Elekta AB, Accuray Inc, Novocure Inc. Research support from Novocure Inc. and Medtronic Inc. R.T.: None. M.R.: None. H.A.: None. M.W.M.: Consulting for Stryker. Y.O.: Advisory Board for Novocure Inc. and Abbvie Inc. Research support from Novocure Inc. and BMS. M.P.M.: Consulting for Karyopharm, Tocagen, Astra-Zeneca, Blue Earth Diagnostics, Celgene, Abbvie. Board of Directors: Oncoceutics.
Authorship Statement Conception and Design: R.K., M.P.M. Analysis: R.K., R.T., M.R. Critical Review of Manuscript: R.K., R.T., M.R., H.A., M.W.M., Y.O., M.P.M.
References
- 1. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol. 2020;17(5):279–299. [DOI] [PubMed] [Google Scholar]
- 2. Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. [DOI] [PubMed] [Google Scholar]
- 3. Thompson AM, Jordan LB, Quinlan P, et al. ; Breast Recurrence in Tissues Study Group . Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010;12(6):R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gong Y, Han EY, Guo M, Pusztai L, Sneige N. Stability of estrogen receptor status in breast carcinoma: a comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer. 2011;117(4):705–713. [DOI] [PubMed] [Google Scholar]
- 5. Curtit E, Nerich V, Mansi L, et al. Discordances in estrogen receptor status, progesterone receptor status, and HER2 status between primary breast cancer and metastasis. Oncologist. 2013;18(6):667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20(9):1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):48. [Google Scholar]
- 10. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 11. Raudenbush SW. Analyzing effect sizes: random-effects models. In: The Handbook of Research Synthesis and Meta-analysis, 2nd ed. New York, NY: Russell Sage Foundation; 2009:295–315. [Google Scholar]
- 12. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25(6):646–654. [DOI] [PubMed] [Google Scholar]
- 13. Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44(2):127–139. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. [DOI] [PubMed] [Google Scholar]
- 15. Gaedcke J, Traub F, Milde S, et al. Predominance of the basal type and HER-2/neu type in brain metastasis from breast cancer. Mod Pathol. 2007;20(8):864–870. [DOI] [PubMed] [Google Scholar]
- 16. Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical profiles of brain metastases from breast cancer. J Neurooncol. 2008;90(2):223–228. [DOI] [PubMed] [Google Scholar]
- 17. Broom RJ, Tang PA, Simmons C, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29(5):1557–1562. [PubMed] [Google Scholar]
- 18. Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12(5):R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Omoto Y, Kurosumi M, Hozumi Y, et al. Immunohistochemical assessment of primary breast tumors and metachronous brain metastases, with particular regard to differences in the expression of biological markers and prognosis. Exp Ther Med. 2010;1(4):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shao MM, Liu J, Vong JS, et al. A subset of breast cancer predisposes to brain metastasis. Med Mol Morphol. 2011;44(1):15–20. [DOI] [PubMed] [Google Scholar]
- 21. Brogi E, Murphy CG, Johnson ML, et al. Breast carcinoma with brain metastases: clinical analysis and immunoprofile on tissue microarrays. Ann Oncol. 2011;22(12):2597–2603. [DOI] [PubMed] [Google Scholar]
- 22. Duchnowska R, Dziadziuszko R, Trojanowski T, et al. ; Polish Brain Metastasis Consortium . Conversion of epidermal growth factor receptor 2 and hormone receptor expression in breast cancer metastases to the brain. Breast Cancer Res. 2012;14(4):R119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bachmann C, Grischke EM, Staebler A, Schittenhelm J, Wallwiener D. Receptor change-clinicopathologic analysis of matched pairs of primary and cerebral metastatic breast cancer. J Cancer Res Clin Oncol. 2013;139(11):1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen Q, Sahin AA, Hess KR, et al. Breast cancer with brain metastases: clinicopathologic features, survival, and paired biomarker analysis. Oncologist. 2015;20(5):466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thomson AH, McGrane J, Mathew J, et al. Changing molecular profile of brain metastases compared with matched breast primary cancers and impact on clinical outcomes. Br J Cancer. 2016;114(7):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Timmer M, Werner JM, Röhn G, et al. Discordance and conversion rates of progesterone-, estrogen-, and HER2/neu-receptor status in primary breast cancer and brain metastasis mainly triggered by hormone therapy. Anticancer Res. 2017;37(9):4859–4865. [DOI] [PubMed] [Google Scholar]
- 27. Jung J, Lee SH, Park M, et al. Discordances in ER, PR, and HER2 between primary breast cancer and brain metastasis. J Neurooncol. 2018;137(2):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sperduto PW, Mesko S, Li J, et al. Estrogen/progesterone receptor and HER2 discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival. Neuro Oncol. 2020;22(9):1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hulsbergen AFC, Claes A, Kavouridis VK, et al. Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol. 2020;22(8):1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma A, Crook T, Thompson A, Palmieri C. Surgical oncology: why biopsying metastatic breast cancer should be routine. Nat Rev Clin Oncol. 2010;7(2):72–74. [DOI] [PubMed] [Google Scholar]
- 31. Kuukasjärvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14(9):2584–2589. [DOI] [PubMed] [Google Scholar]
- 32. Darlix A, Louvel G, Fraisse J, et al. Impact of breast cancer molecular subtypes on the incidence, kinetics and prognosis of central nervous system metastases in a large multicentre real-life cohort. Br J Cancer. 2019;121(12):991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30(4):419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sperduto PW, Mesko S, Li J, et al. Beyond an updated graded prognostic assessment (Breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int J Radiat Oncol Biol Phys. 2020;107(2):334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30(21):2601–2608. [DOI] [PubMed] [Google Scholar]
- 36. Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30(6):587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464(7291):999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kurbel S. Selective reduction of estrogen receptor (ER) positive breast cancer occurrence by estrogen receptor modulators supports etiological distinction between ER positive and ER negative breast cancers. Med Hypotheses. 2005;64(6):1182–1187. [DOI] [PubMed] [Google Scholar]
- 39. Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: discordances during tumor progression. Breast Cancer Res Treat. 2011;125(2):553–561. [DOI] [PubMed] [Google Scholar]
- 40. Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. [DOI] [PubMed] [Google Scholar]
- 41. Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 43. Freedman R, Gelman R, Melisko M, et al. TBCRC 022: phase II trial of neratinib + capecitabine for patients (Pts) with human epidermal growth factor receptor 2 (HER2+) breast cancer brain metastases (BCBM). J Clin Oncol. 2019;37(13):1081–1089. doi: 10.1200/JCO.18.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Parsai S, Miller JA, Juloori A, et al. Stereotactic radiosurgery with concurrent lapatinib is associated with improved local control for HER2-positive breast cancer brain metastases. J Neurosurg. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 45. Wu JM, Fackler MJ, Halushka MK, et al. Heterogeneity of breast cancer metastases: comparison of therapeutic target expression and promoter methylation between primary tumors and their multifocal metastases. Clin Cancer Res. 2008;14(7):1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Venema CM, Mammatas LH, Schröder CP, et al. Androgen and estrogen receptor imaging in metastatic breast cancer patients as a surrogate for tissue biopsies. J Nucl Med. 2017;58(12):1906–1912. [DOI] [PubMed] [Google Scholar]
- 47. Perik PJ, Lub-De Hooge MN, Gietema JA, et al. Indium-111-labeled trastuzumab scintigraphy in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2006;24(15):2276–2282. [DOI] [PubMed] [Google Scholar]
- 48. Agulnik M, Oza AM, Pond GR, Siu LL. Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol. 2006;24(30):4801–4807. [DOI] [PubMed] [Google Scholar]
- 49. McEwen AE, Leary SES, Lockwood CM. Beyond the blood: CSF-derived cfDNA for diagnosis and characterization of CNS tumors. Front Cell Dev Biol. 2020;8:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gruchy JR, Barnes PJ, Dakin Haché KA. CytoLyt® fixation and decalcification pretreatments alter antigenicity in normal tissues compared with standard formalin fixation. Appl Immunohistochem Mol Morphol. 2015;23(4):297–302. [DOI] [PubMed] [Google Scholar]
- 51. Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24(19):3032–3038. [DOI] [PubMed] [Google Scholar]
- 52. Cardoso F, Kyriakides S, Ohno S, et al. ; ESMO Guidelines Committee. clinicalguidelines@esmo.org. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(8):1194–1220. [DOI] [PubMed] [Google Scholar]
- 53. Zhang H, Moisini I, Ajabnoor RM, Turner BM, Hicks DG. Applying the new guidelines of HER2 testing in breast cancer. Curr Oncol Rep. 2020;22(5):51. [DOI] [PubMed] [Google Scholar]
- 54. Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol. 2008;21(Suppl 2):S8–S15. [DOI] [PubMed] [Google Scholar]
- 55. Gong Y, Yan K, Lin F, et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. Lancet Oncol. 2007;8(3):203–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.