Abstract
One of the first events of mammalian sperm capacitation is the activation of the soluble adenyl cyclase/cAMP/protein kinase A (SACY/cAMP/PKA) pathway. Here, we evaluated whether the increase in PKA activity at the onset of human sperm capacitation is responsible for the activation of the sperm proteasome and whether this activation is required for capacitation progress. Viable human sperm were incubated with inhibitors of the SACY/cAMP/PKA pathway. The chymotrypsin-like activity of the sperm proteasome was evaluated using a fluorogenic substrate. Sperm capacitation status was evaluated using the chlortetracycline assay and tyrosine phosphorylation. To determine whether proteasomal subunits were phosphorylated by PKA, the proteasome was immunoprecipitated and tested on a western blot using an antibody against phosphorylated PKA substrates. Immunofluorescence microscopy analysis and co-immunoprecipitation (IPP) were used to investigate an association between the catalytic subunit alpha of PKA (PKA-Cα) and the proteasome. The chymotrypsin-like activity of the sperm proteasome significantly increased after 5 min of capacitation (P < 0.001) and remained high for the remaining incubation time. Treatment with H89, KT5720 or KH7 significantly decreased the chymotrypsin-like activity of the proteasome (P < 0.001). IPP experiments indicated that PKA inhibition significantly modified phosphorylation of proteasome subunits. In addition, PKA-Cα colocalized with the proteasome in the equatorial segment and in the connecting piece, and co-immunoprecipitated with the proteasome. This is the first demonstration of sperm proteasome activity being directly regulated by SACY/PKA-Cα. This novel discovery extends our current knowledge of sperm physiology and may be used to manage sperm capacitation during assisted reproductive technology procedures.
Keywords: sperm, capacitation, proteasome, protein kinase A, SACY/cAMP/PKA, human
Introduction
Mammalian spermatozoa are unable to fertilize an oocyte immediately after ejaculation. They require a period of priming in the female reproductive tract to acquire fertilizing capacity. During this time, the spermatozoa undergo a series of physiological and functional changes known as capacitation. Sperm capacitation was first reported over six decades ago by Austin (1952) and Chang (1951). In vitro, this phenomenon can be mimicked by incubating the spermatozoa in an appropriated culture medium (Harrison, 1996). It appears that certain components of the capacitation medium, such as bicarbonate, serum albumin and calcium play important regulatory roles in promoting sperm capacitation. Bicarbonate activates the spermatozoa soluble adenyl cyclase (SACY), which results in increased levels of sperm cAMP (Visconti et al., 1995b). Many studies have shown that cAMP plays an important role as a second messenger in the initiation of signalling cascade of sperm capacitation, acting on a cyclic AMP (cAMP)-dependent protein kinase (PKA) (Visconti et al., 1995a; Leclerc et al., 1996; Galantino-Homer et al., 1997; Wennemuth 2003). The PKA is a ubiquitous tetrameric serine/threonine (Ser/Thr) kinase holoenzyme composed of two regulatory (R) and two catalytic subunits (C). Its activity depends on cAMP. The binding of four cAMP molecules to the regulatory subunits (two molecules per subunit) allows tetramer dissociation and activation of the catalytic subunit (Nolan et al., 2004). Once free, the catalytic subunits are active to phosphorylate a wide variety of substrates in the Arg-X-X-Ser/Thr motif of their substrates (Shabb, 2001; Bruce et al., 2002). Indirectly, this increase in cAMP/PKA regulates the phosphorylation of proteins in Tyr residues (P-Tyr) later during capacitation. In human spermatozoa, P-Tyr rises significantly only after 2.5 h of incubation (Leclerc et al., 1996).
Mice that lack the unique sperm PKA catalytic subunit Cα2 are infertile despite normal mating behaviour, and do not show the increase in tyrosine phosphorylation after sperm incubation in capacitating medium (Nolan et al., 2004), suggesting the involvement of PKA in this process. PKA is one of the most prevalent Ser/Thr kinases present in the flagellum of mammalian spermatozoa (Vijayaraghavan et al., 1997). However, little is known about the phosphorylation of proteins at Ser/Thr residues in mammalian spermatozoa.
Both the mammalian and non-mammalian models show the involvement of the ubiquitin-proteasome system (UPS) in the regulation of multiple steps in the cascade of fertilization events (reviewed in Kerns et al., 2016). The UPS degrades most long-and short-lived normal and defective/misfolded intracellular proteins (Goldberg, 2003). In the UPS, substrates are typically marked for degradation by covalent linkages to multiple ubiquitin molecules. Ubiquitin is a monomeric protein of 76 amino acid residues and a molecular weight of 8.5 kDa (Schlesinger et al., 1975; Bebington et al., 2001), and is the essential element of UPS (Ciechanover, 2005). Ubiquitination is one of the most common post-translational protein modifications. Three conserved enzymes, E1 (UBA1), E2 (UBE2) and E3 (UBE3), carry out protein ubiquitination in a sequential manner, first activating (E1), then transporting (E2) and ligating (E3) ubiquitin molecules to their substrates. Their activity culminates with the tandem-binding of multiple ubiquitin molecules to the lysine residues of proteins flagged for elimination/recycling (Hershko and Ciechanover, 1998). Once marked by polyubiquitin chains, proteins are rapidly degraded by the 26S proteasome, typically composed of a 20S core capped with a 19S regulatory particle.
The 20S core is formed by two pairs of homologous rings, each containing seven subunits. The two outer rings contain α-type subunits (PSMA1–7), the function of which is to operate a ‘gate’ through which proteins enter the catalytic sites (Jung et al., 2009). The β-subunits (PSMB1–7) form the two inner rings. Three of the β-subunits, β1 (PSMB6), β2 (PSMB7) and β5 (PSMB5), are catalytically active and possess the caspase-like (or peptidylglutamyl-peptide), trypsin-like and chymotrypsin-like activities, respectively (Adams, 2004; Jung and Grune, 2012). Given its proteolytic action, the proteasome degrades proteins synthesized with errors or altered post-translationally (Oberdorf et al., 2001; Jung et al., 2009; Bedford et al., 2010; Xie, 2010). The activity of the proteasome can be regulated at several levels. However, its most common post-translational modification is phosphorylation (Konstantinova et al., 2008). Studies in mouse cardiac myocytes indicate that PKA co-immunoprecipitates with the proteasome and that this association increases the chymotrypsin-like and Peptidyl-Glutamyl Peptide-Hydrolyzing (PGPH) activities of the proteasome (Zong et al., 2006). In addition, at least six different proteasomal subunits are phosphorylated on Tyr residues to influence its intracellular localization (Tanaka et al., 1990; Benedict et al., 1995).
In spermatozoa from mouse, boar and rat, phosphoproteome studies have identified several proteins of the UPS undergoing phosphorylation during capacitation. These proteins include the ubiquitin activating enzyme UBE1 (Baker et al., 2010) and multiple proteasome subunits (Arcelay et al., 2008; Baker et al., 2010). In addition, there are differences in the two-dimensional electrophoretic migration pattern of several 19S and 20S proteasome subunits, between capacitated versus non-capacitated sperm from boars (Choi et al., 2008; Sutovsky, 2011; Zigo et al., 2018). This suggests that during capacitation, changes occur in the structure of the sperm proteasome.
Various reports indicate that the activation of PKA is one of the first intracellular events of capacitation since spermatozoa incubated in a capacitating medium show a rapid (≤1 min) increase in PKA activity (Moseley et al., 2005; Martínez-León et al., 2015). Ser/Thr protein phosphorylation rapidly ensued.
To date, there are no studies evaluating the role and the regulation of the proteasome at the onset of sperm capacitation. Thus, the aim of the present work was to characterize the role of the sperm proteasome during the early events of human sperm capacitation and to determine whether the PKA pathway regulates the enzymatic activity of this multicatalytic protease. In the context of this work, capacitation refers to this process in vitro.
Methods
Ethics statement
The research presented in this manuscript was approved by the Ethics Committee on Scientific Research of the University of Antofagasta, and the Institutional Review Board of The University of Missouri. The institutional review board approved the use of all human semen samples described in this study and followed the current guidelines for human semen studies (Björndahl et al., 2016). All donors, anonymous to researchers, signed a consent form for the use of their spermatozoa for research purpose.
Chemicals and reagents
The following reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA): Nα-tosyl-L-lysine chloromethyl ketone hydrochloride (TLCK); 7-chlorotetracycline (CTC) hydrochloride; (E)-2-(1H-Benzo [d]imidazol-2-ylthio)-N′-(5-bromo-2-hydroxybenzylidene) propane hydrazide (KH7); bovine serum albumin (BSA; A7030); Ponceau Red; HEPES; EDTA; Hoechst 33258 (H258); 1,4-diazabicyclo [2.2.2.] octane (DABCO); dimethyl sulfoxide (DMSO); Na3VO4; NaF; phenylmethylsulfonyl fluoride; aprotinin; and leupeptin. The following compounds were purchased from Enzo Life Sciences (Farmingdale, NY, USA): (9R,10S,12S)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i] [1,6]benzodiazocine-10-carboxylic acid hexyl ester (KT5720); N- [2-(p bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide 2HCl (H89); N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC); Sp-Cyclic 3′,5′-hydrogen phosphorothioate adenosine hydrate (Sp-cAMPS); 3-isobutyl-1-methylxanthine (IBMX); N-Acetyl-N-methyl-L-isoleucyl-L-isoleucyl-N-[(1S)-3-methyl-1-[[(2R)-2-methyloxiranyl]carbonyl]butyl]-L-threoninamide (epoxomicin). Dako Fluorescent Mounting Medium was purchased from Dako North America.
The following antibodies were used: anti-phospho PKA substrates (RRXS*/T*) rabbit monoclonal antibody (mAb #9624, Cell Signaling Technology, Inc., USA); anti-α-4 proteasome subunit antibody and agarose-immobilized anti-α-4 proteasome subunit antibody (BML-PW8120 and BML-PW005, respectively, Enzo Life Sciences; Farmingdale, NY, USA); anti-PKA catalytic subunit antibody (#4782, Cell Signaling Technology, Danvers, MA, USA); anti β-tubulin antibody (E7, Developmental Studies Hybridoma Bank, Iowa, USA); and R-phycoerythrin-labeled mAb, clone pY20 (#558008 BD Phosflow™). The following secondary antibodies were used: biotinylated goat anti-rabbit and goat anti-mouse (AP132B and AP124B, respectively, Chemicon International, Temecula, CA, USA), Alexa 594-conjugated chicken anti-rabbit (Thermo Fisher Scientific, Waltham, MA USA) and Alexa 488-conjugated goat anti-mouse (A-21442 and A-10680, respectively, Thermo Fisher Scientific). The chemiluminescence detection system and Immobilon P transfer membranes were purchased from Millipore Corporation (Bedford, MA, USA). The DC protein assay kit was purchased from Bio-Rad Laboratories Inc. (Hercules, CA, USA).
None of the inhibitors or solvents used in this study affected negatively sperm viability or motility (data not shown). The deionized water used in these experiments was purified to 18-megohms with an EASYpure UV/UF ion-exchange system (Barnstead/Thermolyne, Dubuque, IA, USA). All other chemicals were of analytical grade and obtained from standard sources.
Culture media
The basic medium used for all the experiments was a modified Tyrode’s medium (117 mM NaCl, 19 mM sodium lactate, 2.5 mM glucose, 8.6 mM KCl, 2.4 mM CaCl2, 0.25 mM sodium pyruvate, 0.49 mM MgCl2, 0.36 mM NaH2PO4, 70 μg/ml of penicillin and streptomycin and phenol red). This medium was designated non-capacitating medium (NCM). The capacitating medium (CM) was similar to NCM, except that it was supplemented with 2.6% BSA (w/v) and 25 mM NaHCO3 (Signorelli et al., 2013). The pH of all media was adjusted to 7.4–7.45 before use.
Semen collection
Semen samples were provided by healthy donors aged between 20 and 30 years; all donors provided more than one sample. Freshly ejaculated spermatozoa were obtained by masturbation after 2–3 days of sexual abstinence. Samples obtained from the same donor were requested with at least a 2-week interval. Semen was collected in a sterile vessel for which each lot was tested for sperm toxicity. All semen samples used were normospermic according to World Health Organization criteria (WHO, 2010). Semen samples were allowed to liquefy for 30–60 min at 37°C. All semen samples were processed by the same person using compatible equipment, and analyses of volume, pH, sperm concentration and percentages of motile and viable spermatozoa were performed. The mean values for semen parameters are summarized in Supplementary Table SI.
Sperm selection
Motile spermatozoa were obtained using a dual Percoll gradient (40/80%), as described previously (Morales et al., 2003). Briefly, aliquots of semen were layered over the upper layer of the Percoll gradient and centrifuged at 300 g for 20 min. The pellet was then resuspended in 10 ml of NCM and centrifuged again at 300 g for 10 min. Finally, the sperm pellet was resuspended in the appropriate medium at the required concentration.
Approximately 10 × 106 spermatozoa/ml were incubated for different times 0 (NCM), 1, 5, 10 15, 30 and 60 min (CM) at 37°C and 5% CO2 in air, in the presence or absence of inhibitors. None of the treatments had any detrimental effect on sperm motility or viability.
CTC assay
The sperm capacitation status was assessed using the CTC fluorescence assay method, as described previously (Lee et al., 1987; Kong et al., 2009). CTC is a fluorescent antibiotic whose distribution in the spermatozoa changes during the transition from non-capacitated to capacitated and then to acrosome-reacted state, thereby allowing to differentiation various steps of the sperm capacitation process (Fraser et al., 1993; Varner et al., 1993; Neild et al., 2005). Briefly, the CTC solution was prepared on the day of use and contained 750 μM CTC in a buffer of 130 mM NaCl, 5 mM cysteine, and 20 mM Tris-HCl, pH adjusted to 7.8. This solution was kept wrapped in foil at 4°C until use. A 10 μl aliquot of a sperm suspension supplemented with 10 μg/ml supravital stain Hoechst 33258 (H258) was incubated at 37°C for 10 min; 10 μl of CTC stock solution was then rapidly added, followed within 30 sec by 2 μl of 2% (v/v) glutaraldehyde in 1 M Tris buffer (pH 7.8). A 20 μl aliquot of this suspension was placed on a slide: once it was dried, a drop of DABCO mounting medium was carefully mixed in, to retard fading of the fluorescence, and a cover slip was placed on top. Cells were assessed for their viability using H258, as described previously (Cross et al., 1986). In each sample, 200 live cells were assessed for CTC staining patterns, and in all cases, the proportion of dead cells was very low.
Three distinct patterns of CTC fluorescence were classified: the F pattern, with uniform fluorescence over the entire sperm head, was characteristic of non-capacitated, acrosome intact cells; the B pattern, with a fluorescence-free band in the post-acrosomal region, was characteristic of capacitated, acrosome-intact cells; and the AR pattern, with weak or absent fluorescence over the sperm head, was characteristic of capacitated, acrosome-reacted cells (Lee et al., 1987).
Flow cytometric analysis
To corroborate CTC, sperm capacitation status was assessed using the pattern of tyrosine phosphorylation. Aliquots of motile sperm suspensions (2 × 106 sperm/ml) were fixed and permeabilized with BD Cytofix/Cytoperm kit (San Jose, CA, USA) for 20 min at 4°C. Permeabilization of spermatozoa is a critical step in the staining, as the tyrosine-phosphorylated sperm proteins are mainly intracellular. Then, the spermatozoa were centrifuged at 14000 g for 2 min and washed with BD Perm/Wash™ buffer (San Jose, CA, USA). The pellet was resuspended in 100 μl of phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 4.3 mM Na2PO4, pH 7.4) supplemented with 2% (w/v) BSA and incubated with 10 μl of antibody. Tyrosine phosphoproteins were detected using the mAb clone pY20. The optimal working concentration of the anti-phosphotyrosine mAb and the time of coincubation with spermatozoa (2 h) were chosen by titration tests in preliminary experiments. Stained samples were analysed using flow cytometry (FACSJazz Cell Sorter, BD Biosciences, San Jose, CA, USA).
Preparation of sperm extracts
After capacitation, aliquots of spermatozoa were washed twice in cold PBS and centrifuged at 9000 g for 30 sec. To measure proteasome enzymatic activity, the pellet was resuspended in homogenization buffer (50 mM Hepes, 10% (v/v) glycerol, 200 μM TLCK, pH 7.4), sonicated (Virsonic, Gardiner, NY, USA) with seven 60-W bursts of 30 sec each, and then centrifuged for 30 sec at 5000 g in a Beckman microfuge to remove nuclear and flagellar material (Morales et al., 1994). The supernatant was used as the enzyme stock preparation.
For the immunoprecipitation (IPP) analysis, the sperm pellet was resuspended in 200 μl IPP buffer (20 mM Tris HCl pH 7.5, 137 mM NaCl, 2 mM ethylenediaminetetraacetic acid (EDTA), 0.1% sodium dodecyl sulfate (SDS) (w/v), 0.5% C24H39O4Na (sodium deoxycholate, w/v), 1% Triton X-100 v/v). The buffer was supplemented with protease and phosphatase inhibitors. According to previous work from our laboratory, none of the protease inhibitors used have a significant effect on the chymotrypsin-like activity of the sperm proteasome (Pizarro et al., 2004). The protein concentration in each sperm extract preparation was measured using the RC DC protein assay. All procedures were performed at 4°C.
IPP
Control sperm aliquots were obtained at the beginning (0 min) and after 15 min of incubation, and sperm aliquots treated with 50 μM H89 were obtained after 15 min. To immunoprecipitate the proteasome these aliquots were then treated with an anti-PSMA7 proteasomal subunit antibody, as described (Diaz et al., 2007; Kong et al., 2009). Briefly, 400 μl of the sample containing 400 μg of protein were incubated with 7 μl of the anti-proteasome antibody, immobilized on agarose (2 mg/ml). The reaction mixture was incubated on an orbital shaker overnight at 4°C. Antigen-antibody-A/G-agarose protein complexes were concentrated to the bottom of the tube by centrifugation at 14250 g for 30 sec at 4°C. The supernatants were discarded, and the pellets were washed twice with IPP buffer and once with PBS. The washed pellets were mixed with SDS sample buffer and heated in a boiling water bath for 5 min, and the supernatant was subjected to SDS-PAGE.
SDS-PAGE and immunoblotting
SDS-PAGE was performed using 12% gels according to the method of Laemmli (1970). Briefly, sperm extract aliquots were boiled for 5 min with sample buffer (500 μM Tris-HCl, 10% SDS (w/v), 30% glycerol v/v), 0.5% β-mercaptoethanol (v/v) and 0.5% bromophenol blue, pH 6.8 (w/v)) and then immediately stored on ice. Isolated proteins were separated on SDS-PAGE (60 mA, 120 min). Afterwards, proteins were transferred to polyvinylidene fluoride (PVDF) membranes at 250 mA for 60 min at 4°C. Transfer was monitored by Ponceau red stain. The membranes were blocked in TRIS-buffer saline with 0.1% Tween 20 (T-TBS, v/v) and 5% milk (w/v). Then, they were washed six times in T-TBS and incubated with primary antibodies at 4°C overnight. After that, membranes were washed six additional times and incubated with the appropriate biotinylated secondary antibody for 1 h at room temperature. Next, the membranes were washed with T-TBS for the last time and blots were visualized by chemiluminescence (Amersham Corp., Sydney, Australia) according to the manufacturer’s instructions. Finally, the signal was imaged using an In Vivo F Pro molecular imaging system (Bruker Corporation, Billerica, MA, USA).
Stripping the PVDF membranes
To confirm an equal protein load, blots were stripped and re-probed with an antibody against β-tubulin and PSMA7 proteasome subunit (IPP control). For this procedure, 15 ml of stripping buffer, consisting of 2% (w/v) SDS, 62.5 mM Tris, pH 6.7, 100 mM 2-mercaptoethanol, was added to the membrane for 1 h with constant shaking at 60°C. The membrane was then washed six times for 10 min in TBS, blocked and probed with the primary antibody, as described above.
Measurement of proteasomal enzyme activity
To determine the influence of the SACY/cAMP/PKA pathway on the enzymatic activity of the sperm proteasome, motile spermatozoa were incubated with 50 μM H89, 100 nM KT5720, 25 μM KH7, 100 μM IBMX or 100 μM Sp-cAMPS for 0, 1, 15, 30 or 60 min at 37°C, 5% CO2. Control aliquots were treated with the inhibitor solvent/vehicle solutions at equivalent volumes. H89, KH7 and IBMX were dissolved in 0.1% (v/v) DMSO. Sp-cAMPS was dissolved in water. KT5720 was dissolved in 0.01% (v/v) methanol. The chymotrypsin-like activity of the sperm proteasome was assayed using the fluorogenic substrate N-Succinyl-Leu-Leu-Val-Tyr-7-amido-4-trifluoromethylcoumarin (Suc-LLVY-AMC) (Morales et al., 2003). Briefly, 50 μl aliquots were incubated in a final volume of 995 μl of homogenization buffer for 15 min at 37°C, 5% CO2, before adding 10 μM substrate. The assay was run at 37°C, and the fluorescence was monitored with excitation at 380 nm and emission at 460 in a Shimadzu 5301 spectrofluorometer (Kyoto, Japan).
Indirect immunofluorescence
Spermatozoa were fixed in 4% (v/v) paraformaldehyde for 15 min and washed twice by centrifugation with 0.1 M glycine in PBS. Then, the cells were permeabilized for 10 min with 0.1% Triton X-100 diluted in PBS and washed twice with PBS at 14000 g for 2 min. The final pellet was resuspended in PBS supplemented with 1% BSA (BSA-PBS, w/v) for 30 min. The samples were incubated overnight with rabbit polyclonal anti-PKA catalytic subunit antibody (dilution 1:50) and, after three washes with PBS, incubated overnight with mouse monoclonal anti-proteasome PSMA7 subunit antibody (dilution1:50). Samples were washed three times with PBS and incubated with chicken anti-rabbit conjugated to Alexa 594 antibody diluted 1:200 in BSA-PBS for 2 h at room temperature. Samples were washed three times with PBS and incubated with goat anti-mouse conjugated to Alexa 488 antibody diluted 1:500 in BSA-PBS for 2 h at room temperature. Finally, the samples were washed three times for 5 min with PBS and the coverslips were mounted with Dako Fluorescent Mounting Medium and examined under a confocal microscope (Leica TCS SP8 SMD, Mannheim, Germany). A minimum of 400 cells were analysed in each sample. Controls using a secondary antibody alone were performed to assure specificity.
Statistical analyses
Data were assessed by the Kolmogorov–Smirnov test and were normally distributed. They were analysed by a one-way analysis of variance with Tukey’s post hoc test. Densitometry analysis was performed using the image 2.0j software and normalized with regard to the internal control tubulin.
Quantitative results are presented as mean values+SEM. In all cases, results with P < 0.05 were considered as values with significant differences.
Results
The chymotrypsin-like activity of the sperm proteasome increases during capacitation
The chymotrypsin-like activity of the sperm proteasome started to rise after 5 min of capacitation (Fig. 1, P < 0.01), plateaued at 10 min and remained high for the remaining incubation time (Fig. 1, P < 0.001). Furthermore, spermatozoa incubated during different times in NCM medium did not show an increase in proteasome chymotrypsin-like activity (Supplementary Fig. S1).
Figure 1.
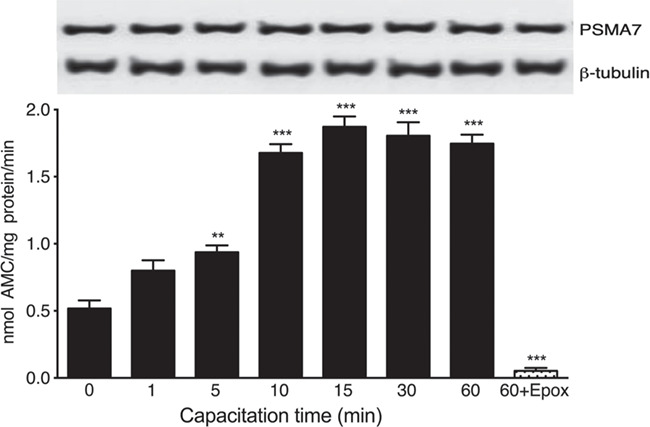
The chymotrypsin-like activity of the sperm proteasome increases at the onset of human sperm capacitation. Spermatozoa were incubated under capacitating conditions for the indicated time periods. The chymotrypsin-like activity of the sperm proteasome was evaluated using the fluorogenic substrate N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC). An additional sperm aliquot was incubated under capacitating conditions for 60 min in the presence of 10 μM epoxomicin (60 + Epox). Data are expressed as mean ± SEM of five experiments. **P < 0.01; ***P < 0.001 compared to time 0. In the insert, the upper panel represents the abundance of the PSMA7 subunit of the sperm proteasome at each capacitation time; the lower panel corresponds to the load control evaluated with an anti ß-tubulin antibody.
Simultaneously, we evaluated changes in the sperm content of the proteasome during capacitation by western blotting (WB). The results indicate that there were no changes in the density of PSMA7 subunit band (Fig. 1), suggesting that the increase in proteasome activity was not due to changes in the sperm content of proteasomes.
The increase in proteasome activity is required for sperm capacitation
We evaluated the capacitation status of human spermatozoa using the CTC and tyrosine phosphorylation assays by flow cytometry and WB. Non-capacitated spermatozoa (0) exhibited a basal level of cells with the B pattern (11 ± 1.1%) (Fig. 2A). When the spermatozoa were incubated under capacitating conditions for 60 min (60), a significant increase in pattern B was observed (34.4 ± 2.0%). In contrast, spermatozoa capacitated for 60 min in the presence of 10 μM epoxomicin (60 + Epox) did not exhibit an increase in capacitation B pattern (P < 0.001 versus capacitated control) (Fig. 2A). In regard to the dynamics of global sperm tyrosine phosphorylation (pY) during capacitation, the flow cytometry results show that the percentage of pY increased during capacitation in a time-dependent manner, from 10% after 60 min to 44% pY-positive spermatozoa at 300 min (Fig. 2B). However, spermatozoa capacitated in the presence of 10 μM epoxomicin exhibited a significant decrease in the percentage of pY during capacitation (24%). The mean ± SEM percentage of pY for six experiments is given in the Supplementary materials (Supplementary Fig. S2). In addition, we investigated the capacitation-induced changes in tyrosine phosphorylation of proteins by WB (Leclerc et al., 1996; Lefièvre et al., 2000; Kirkman-Brown et al., 2002; Moseley et al., 2005). Similar to what was observed by flow cytometry, protein tyrosine phosphorylation levels were low in spermatozoa incubated in the presence of a proteasome inhibitor (Supplementary Fig. S3). These results strongly suggest that the sperm proteasome has a role in early capacitation events.
Figure 2.
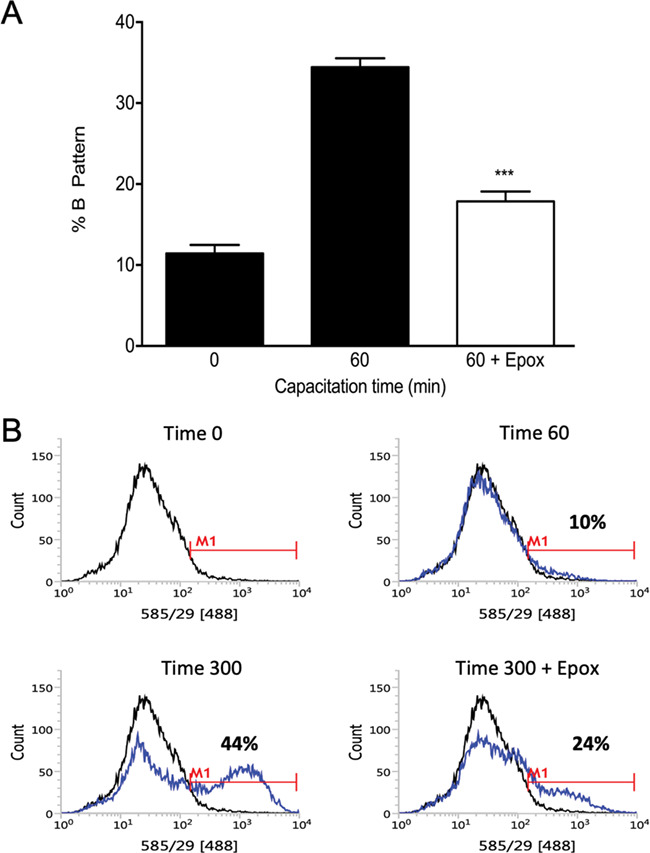
Effect of proteasome inhibition on human sperm capacitation. (A) Evaluation of sperm capacitation by the CTC assay. Spermatozoa were incubated for 0 (0) or 60 (60) min with 0.1% v/v DMSO or for 60 min with 10 μM epoxomicin (60 + Epox). Data are expressed as mean ± SEM, N = 7. ***P < 0.001 compared to 60 min without inhibitor. (B) Evaluation of sperm capacitation by tyrosine phosphorylation, assayed by flow cytometry. A representative histogram out of six replicates with consistent results is shown. Black lines represent the fluorescence distribution pattern of non-capacitated sperm suspensions (Time 0) and the blues lines represent the fluorescence distribution pattern of spermatozoa capacitated for 60 min (Time 60), 300 min (Time 300) and 300 min in the presence of 10 μM epoxomicin (Time 300 + Epox).
The increase in sperm proteasome activity during capacitation is mediated by the SACY/cAMP/PKA pathway
It is known that proteasomal subunits undergo post-translational modifications, the most common being phosphorylation (Konstantinova et al., 2008). Considering that activation of PKA is one of the first intracellular events of capacitation (Visconti, 2009), the next step was to evaluate if PKA was involved in the regulation of proteasomal activity. The results show that the treatment with 50 μM H89 (Fig. 3A) significantly blocked the increase in chymotrypsin-like activity of the sperm proteasome at all incubation time points (P < 0.001). Spermatozoa treated with a different PKA inhibitor, KT520, also failed to increase their proteasomal activity during capacitation (Fig. 3B, P < 0.001). These results suggest that PKA regulates the enzymatic activity of the sperm proteasome during capacitation.
Figure 3.
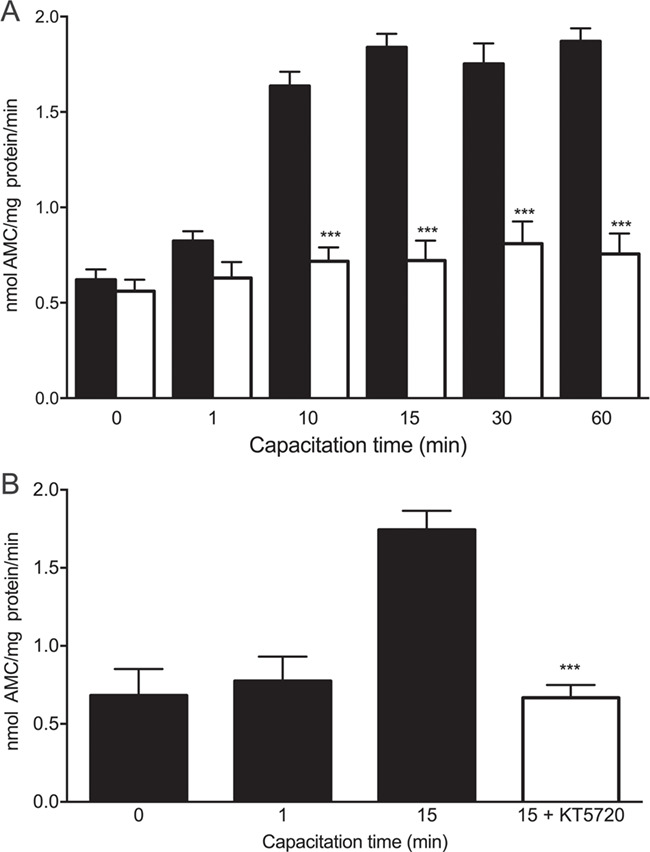
Effect of protein kinase A (PKA) inhibitors on the chymotrypsin-like activity of the sperm proteasome at the onset of human sperm capacitation. (A) Sperm aliquots were incubated under capacitating conditions for 0, 1, 10, 15, 30 or 60 min with 0.1% v/v DMSO (black bars) or with 50 μM H89 (white bars). Then, the chymotrypsin-like activity was evaluated using the fluorogenic substrate N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC). ***P < 0.001 versus corresponding control, N = 8. (B) Other sperm aliquots were incubated under capacitating conditions for 0, 1 or 15 min with 0.1% v/v DMSO (black bars) or for 15 min with 100 nM KT520 (white bar). Chymotrypsin-like activity was evaluated using Suc-Leu-Leu-Val-Tyr-AMC. ***P < 0.001 versus corresponding control. N = 5. Data are expressed as mean ± SEM.
Additional experiments were designed to determine if the upstream elements of PKA pathway are involved in increasing the chymotrypsin-like activity of the human sperm proteasome. The activation of PKA requires the second messenger cAMP (Nolan et al., 2004) and the intracellular cAMP concentration is regulated by two opposite enzyme systems: SACY, which generates cAMP and phosphodiesterases (PDE), which degrade cAMP (Leclerc et al., 1996). To test directly whether SACY modulates proteasome activity, spermatozoa were incubated with KH7, a specific small molecule inhibitor of SACY (Hess et al., 2005). When spermatozoa were incubated for 15 min with 25 μM KH7, the increase in proteasome activity was prevented (Fig. 4A; P < 0.001). In contrast, when spermatozoa were incubated for 15 min with the broadly specific PDE inhibitor IBMX, the chymotrypsin-like activity of the sperm proteasome was significantly enhanced (Fig. 4B; P < 0.01). These results suggest that the increase in chymotrypsin-like activity during early capacitation is mediated by an increase in sperm intracellular cAMP levels.
Figure 4.
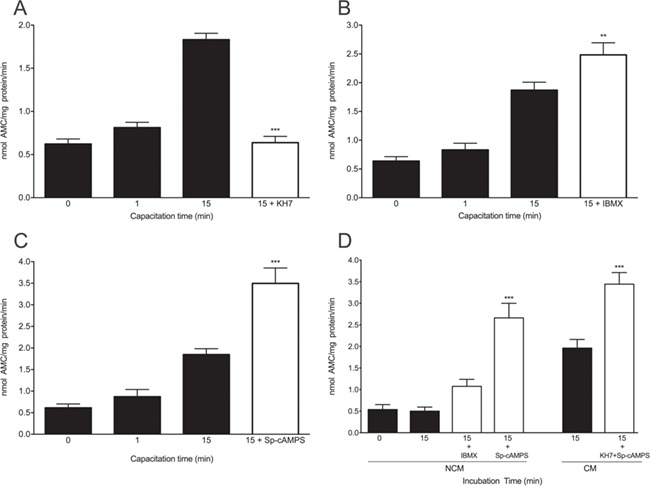
Effect of interference with the soluble adenyl cyclase/cAMP/protein kinase A (SACY/cAMP/PKA) pathway on the chymotrypsin-like activity of the sperm proteasome at the onset of human sperm capacitation. Spermatozoa were incubated under capacitating conditions for 0, 1 or 15 min with 0.1% v/v DMSO (black bars) or with (A) 25 μM KH7; (B, D) 100 μM IBMX; or (C, D) 100 μM Sp-cAMPS (white bars). (D) Other sperm aliquots were incubated simultaneously with 25 μM KH7 and 100 μM Sp-cAMPS for 15 min. The chymotrypsin-like activity of the sperm proteasome was evaluated using the fluorogenic substrate N N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC). Data are expressed as mean ± SEM. **P < 0.01; ***P < 0.001 versus control.
The importance of PKA in the modulation of proteasome activity during capacitation was corroborated using the PKA activator Sp-cAMPS. When spermatozoa were incubated for 15 min in the presence of Sp-cAMPS, the chymotrypsin-like activity of the sperm proteasome was significantly increased (Fig. 4C; P < 0.001). Finally, we carry out experiments designed to recover PKA activity in conditions that the SACY/cAMP/PKA pathway was inhibited (Fig. 4D). When spermatozoa were incubated in the presence of SP-cAMPS in NCM for 15 min, proteasome activity increased (P < 0.001). Likewise, when the spermatozoa were incubated in CM in the presence of SP-cAMPS and KH7, we observed significant increases in proteasome activity (P < 0.001).
Proteasome subunits are phosphorylated by PKA during human sperm capacitation
The next experiment was designed to determine whether proteasomal subunits are phosphorylated by PKA at the onset of capacitation. Spermatozoa were incubated under capacitating conditions for 0 and 15 min with 0.1% DMSO or for 15 min with 50 μM H89. To immunoprecipitate the proteasome, the sperm extracts were treated with an anti-PSMA7 proteasomal subunit antibody, as described in Materials and Methods. The precipitated proteins were tested on a western blot using an antibody against phosphorylated PKA substrates. Spermatozoa incubated for 15 min exhibited an increase in the band density of proteasomal subunits phosphorylated by PKA (Fig. 5A; P < 0.01). However, spermatozoa treated with H89 did not increase their content of PKA phosphorylated proteasome subunits (P < 0.01). There were no differences between non-capacitated spermatozoa (0 min) and those capacitated for 15 min in the presence of H89. These changes were not due to an artefact or unequal protein loading, as demonstrated by the α4 proteasome subunit control (Fig. 5, bottom). As a negative control, lysis buffer and the antibody attached to the agarose used for IPP were incubated. As shown in Fig. 5, there was no artefactual cross-reaction.
Figure 5.
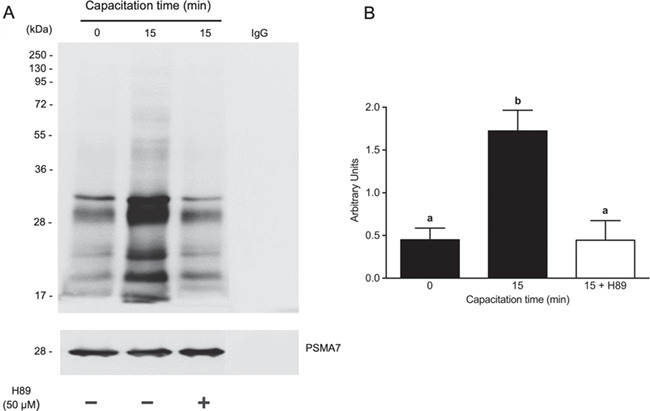
Protein kinase A (PKA)-dependent phosphorylation of proteasomal subunits at the onset of human sperm capacitation. (B) Spermatozoa were incubated under capacitating conditions for 0 or 15 min with 0.1% v/v DMSO or for 15 min with 50 μM H89. Then, the proteasome was immunoprecipitated with an anti-PSMA7 proteasome subunit antibody. The phosphorylation of proteasomal subunits by PKA was evaluated by western blots using an antibody against phosphorylated PKA substrates. (B) For the densitometric analysis, the PSMA7 subunit was used as a control. The bars represent the mean ± SEM of total signal from phosphorylated proteosome components. N = 4. Different letters indicate statistically significant differences (P < 0.01) between the groups.
Catalytic alpha subunit of PKA colocalizes with the proteasome complexes during sperm capacitation
Finally, we investigated if there is an association between the catalytic subunit alpha of the PKA (PKA-Cα) and the proteasome during human sperm capacitation. Immunofluorescence revealed that PKA-Cα was present in all spermatozoa analysed. The labelling of PKA-Cα was homogeneously localized in the principal piece, strong in the midpiece and absent from the sperm tail end piece (Fig. 6B). However, differences in the localization of PKA-Cα were observed in the sperm head. The average percentage of spermatozoa that showed PKA-Cα labelling in the head is shown in Supplementary Table SII. More than half of the spermatozoa presented labelling in the connecting piece of the tail and about a quarter of them presented labelling in the head equatorial segment. In addition, 20% presented labelling in the connecting piece of tail and in the equatorial segment (Fig. 6B). The antibody PKA-Cα specificity was demonstrated by western blot analysis (Supplementary Fig. S4). A single band is observed of an approximate molecular weight of 42 kDa.
Figure 6.
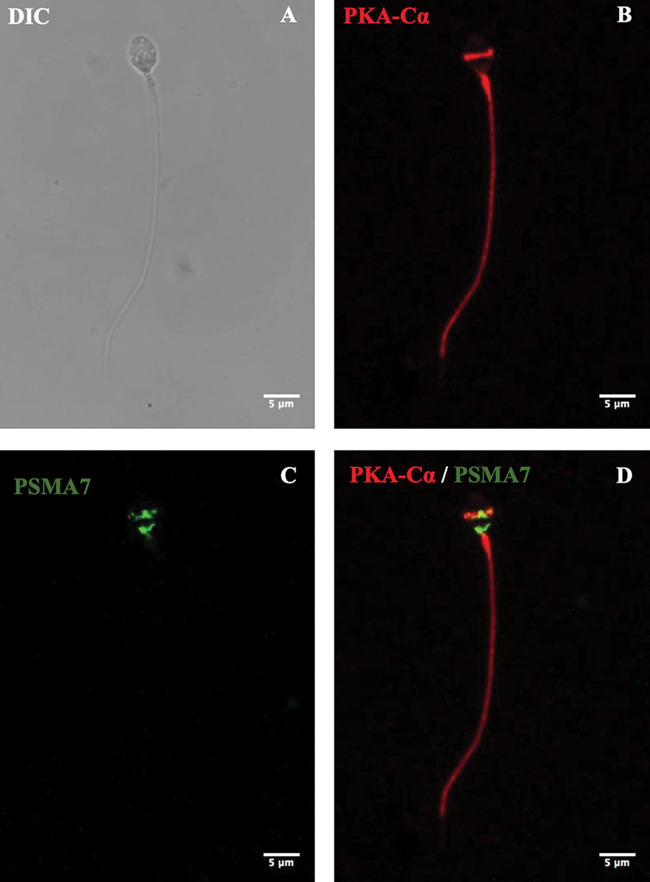
Subcellular localization of the catalytic subunit of protein kinase A (PKA) and its colocalization with the proteasome in human spermatozoa. Spermatozoa were incubated under capacitating conditions for 15 min. The cells were fixed and labelled with a PKA catalytic subunit primary antibody (red; [B]) and primary antibody against proteasomal subunit PSMA7 (green; [C]). DIC: differential interference contrast. Scale bar is 5 μm in all photos.
Next, we evaluated the subcellular localization of the proteasomal subunit PSMA7. Immunofluorescence revealed that the said subunit and by extrapolation proteasomes were not present in the flagella of all spermatozoa evaluated. In the sperm head, differences in the localization of the proteasomes were observed (Supplementary Table SIII). Labelling was observed in the connecting-piece/implantation fossa, equatorial segment, acrosome and post acrosomal sheath. Finally, immunofluorescence revealed that PKA-Cα colocalized with the proteasome in the connecting piece and equatorial segment. However, only 21% of the spermatozoa evaluated after 15 min of capacitation showed evidence of colocalization between PKA-Cα and proteasome. As a negative control, trials with secondary antibody only were carried out (Supplementary Fig. S5).
Because PKA-Cα and the proteasome partially overlap in human spermatozoa, the next experiment was designed to examine possible molecular interaction between PKA-Cα and the proteasome. A co-IPP experiment revealed that PKA-Cα was successfully co-precipitated with the proteasome (Fig. 7A). The co-precipitation of PKA-Cα with the proteasome was significantly higher in spermatozoa capacitated for 15 min (P < 0.01) versus non-capacitated spermatozoa (time 0). It can be seen in the total cell extract that the content of PKA-Cα is constant during the time points evaluated. To rule out non-specific IPP, the blots were stripped and reprobed with an antibody against β-actin (Supplementary Fig. S6). The β-actin was detected only in the extracts and not in the immunoprecipites.
Figure 7.
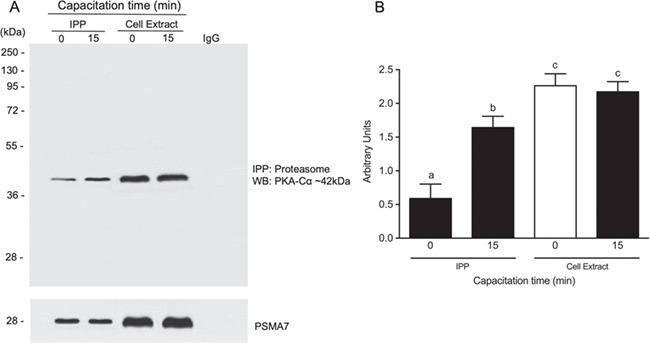
Association of the catalytic protein kinase A (PKA) subunit with proteasome during human sperm capacitation. (A) Co-immunoprecipitation (IPP) of the proteasome and the catalytic subunit of PKA. Spermatozoa were incubated for 0 or 15 min. After incubation, the spermatozoa were lysed in lysis buffer and immunoprecipitated overnight with an anti-PSMA7 proteasome subunit antibody. The PKA catalytic subunit was detected by western blotting (WB). Cell extracts correspond to the lysates of individual treatment groups. (B) For densitometry, the α4 subunit was used as a control. Bars represent the mean ± SEM of three different experiments. Different letters indicate statistically significant differences (P < 0.01) between the groups.
Discussion
In the present study, we report that the enzymatic activity of the sperm proteasome increases as early as 5 min after the onset of capacitation. This increase seems to be required for capacitation to occur since proteasome inhibition with the specific inhibitor epoxomicin significantly blocked capacitation. When we evaluated capacitation using the CTC assay, the percentage of spermatozoa with the capacitated B pattern significantly increased after 60 min of in vitro capacitation. These results are consistent with published work (Signorelli et al., 2013; Martínez-León et al., 2015). In contrast, spermatozoa capacitated in the presence of epoxomicin did not exhibit this increase, a pattern similar to that described by Kong et al. (2009). Protein tyrosine phosphorylation has been associated with capacitation and the acquisition of mouse sperm ability to undergo acrosomal exocytosis (Visconti et al., 1995a, 1999a). Thus, we also evaluated sperm capacitation by measuring the level of protein tyrosine phosphorylation and detected a significant increase in the global phosphotyrosine content. These results, obtained by flow cytometry, were similar to those reported by Barbonetti et al. (2008). We also found that sperm incubation with epoxomicin significantly blocked this increase in tyrosine phosphorylation during capacitation. The observation that proteasome inhibition prevents sperm capacitation strongly suggests that the sperm proteasome has a role early in the capacitation process. Our preliminary evidence indicates that epoxomicin does not have a direct effect on PKA activity (Zapata-Carmona et al., unpublished results).
Proteasomes are present in spermatozoa of numerous species and several studies have shown that human spermatozoa have all components/subunits of the 26S proteasome (Tipler et al., 1997; Wojcik et al., 2000; Morales et al., 2003). Additionally, multiple ubiquitin specific proteases (Baker et al., 2008) and ubiquitin conjugating enzymes E2 (Sutovsky et al., 2000; Fischer et al., 2005) and E3-ligases (Rodriguez and Stewart, 2007; Rivkin et al., 2009; Zimmerman et al., 2014) have been identified in mammalian spermatozoa. It is known that reproductive success of mammalian species requires precise orchestration of multiple events, all regulated through the cAMP dependent protein kinase A (PKA) pathway. It has been shown that PKA activity peaks at 1 min after the beginning of capacitation (Moseley et al., 2005; Martínez-León et al., 2015) and it has been referred as an ‘earlier’ event of capacitation. In addition, PKA inhibitors effectively prevent sperm capacitation in vitro (Kong et al., 2009; Battistone et al., 2013).
The present results indicate that the increase in proteasome activity takes place after 5 min of capacitation, giving support to the concept that proteasome activation is also an early event of sperm capacitation. In addition, the stable expression of the proteasome during capacitation suggests that the initial increase is related to a regulatory mechanism and not to the amount of proteasomes present. One possible mechanism involves post-translational modification of the proteasomal subunits. Phosphorylation is a well-studied post-translational modification (reviewed by Guo et al., 2017). Under physiological conditions, the proteasome typically consists of two multi-subunit complexes, a 20S proteasome capped with one or two 19S regulatory particles. Both 20S and 19S multi-subunit complexes undergo phosphorylation to become active (Iwafune et al., 2002; Kikuchi et al., 2010). Here, we present evidence that two validated PKA inhibitors, H89 and KT5720, prevented the increase in proteasome activity at the beginning of capacitation. These results suggested that an increase in proteasome activity is necessary for capacitation to occur and that this increase seems to be mediated by PKA and its downstream elements. As a precedent, proteasome function is regulated by the PKA pathway in other cellular systems. It has been shown that PKA is capable of stimulating the proteolytic activity of the proteasome by phosphorylation of Ser120 of the Rpt6 subunit in NRK cell line. This phosphorylation of Rpt6 by PKA directly stimulates the chymotrypsin and trypsin-like activities of the 20S proteasome (Zhang et al., 2007). Asai et al. (2009) showed that the exogenous or endogenous stimulation of PKA speeds up and enhances the assembly of the 26S proteasome and its enzymatic activity in canine heart cells. Metcalfe et al. (2012) reported a dibutyryl-cAMP stimulated proteasome activity and reduced prostaglandin-induced proteasome inhibition in cultured cortical neurons. They proposed that targeting cAMP/PKA to boost proteasome activity in a sustainable manner could offer an effective approach to avoid early accumulation of ubiquitinated proteins, possibly preventing/delaying Alzheimer’s disease-associated neurodegeneration (Metcalfe et al., 2012).
In this study, proteasome phosphorylation by PKA during sperm capacitation has been confirmed by activating PKA with Sp-cAMPS (direct effect) and IBMX (indirect effect through inhibition of PDE), or by using PKA inhibitors such as H89 and KT5720. Furthermore, we used the SACY inhibitor KH7. The results strongly suggest that SACY/cAMP/PKA pathway stimulates the chymotrypsin-like activity of the sperm proteasome at the onset of capacitation. To our knowledge, this is the first report that suggests that subunits of the sperm proteasome are phosphorylated by PKA during capacitation. The results obtained with the antibody that recognizes the PKA-phosphorylated substrates, showing that the subunits of the core 20S increased their phosphorylation level at the beginning of capacitation, support this conclusion. When the spermatozoa were incubated in the presence of H89, there was a decrease in the degree of PKA substrate phosphorylation. This observation suggests that PKA phosphorylation positively regulates the 20S proteasome. Kong et al. (2009) showed that the sperm proteasome was phosphorylated on Thr and Tyr residues in spermatozoa incubated for 18 h (late capacitation events) and that the observed phosphorylation was not confined to the 20S core, but to subunits of the proteasomal 19S regulatory complexes. These phosphorylations may also be related to acrosomal exocytosis or the initial acrosomal remodelling during sperm capacitation. Our results agree with reports in other cell types, where the activation of the proteasome is related to the phosphorylation of the 20S core subunits. Zong et al. (2006) reported that PKA phosphorylates in cardiomyocytes the α1 (PSMA6), α2 (PSMA2), α3 (PSMA4), β2 (PSMB7), β3 (PSMB3) and β7 (PSMB4) subunits of the 20S proteasome in vitro, increasing its chymotrypsin and PGPH activities. This has also been demonstrated in human kidney cells, where purified PKA phosphorylated some 28–30 kDa subunits in vitro, leading to evident up-regulation of peptidase activity (Marambaud et al., 1996).
The increase of cAMP levels and activation of PKA are considered early events of sperm capacitation. Upon activation, PKA induces the phosphorylation of target proteins on Ser/Thr residues. Our results indicate that the proteasome is a substrate for PKA at the beginning of the capacitation. The role of cAMP in the pathway conducive to sperm capacitation has been elucidated over the years by both biochemical and pharmacological approaches; increased cAMP levels promote the release of the PKA-Cα from the regulatory subunits, stimulating the activity of the kinase (Nolan et al., 2004). It has been shown that PKA activation is a necessary element for sperm capacitation; animals lacking the PKA-Cα subunit are infertile, despite of normal mating behaviour (Nolan et al., 2004). In the present work, we also report the localization of PKA-Cα in the subcellular compartments in human spermatozoa during capacitation. The subcellular localization of the PKA-Cα is consistent with published work in human sperm (Neuhaus et al., 2006; Mitchell et al., 2008). We show that PKA-Cα is readily detected in the spermatozoa and that there is a strong labelling in the midpiece part of the flagellum and in the equatorial segment of the sperm head. The subcellular localization of the proteasome that we report here agrees with observations of human spermatozoa reported by others (Wojcik et al., 2000; Biały et al., 2001; Morales et al., 2004). The sperm proteasome is mainly located in the acrosomal and post-acrosomal head regions and in the tail connecting piece. We also show for the first time that PKA-Cα and the proteasome overlap in the same subcellular compartment. In addition, PKA-Cα interacts with the proteasome and this interaction may induce the increase in chymotrypsin-like proteasomal core activity at the beginning of capacitation. These results suggest a strong interaction between PKA-Cα and the proteasome during the early events of sperm capacitation. These findings agree with other cell models. For instance, it has been reported that the 20S proteasomes isolated from murine heart also contain PKA (catalytic) subunit as confirmed by WB and mass spectrometry, and the PKA-mediated in vitro phosphorylation enhances peptidase activities of 20S proteasomes isolated from both heart and liver (Zong et al., 2006; Lu et al., 2008).
In conclusion, our results indicate that PKA phosphorylation positively regulates the 20S proteasome during early stages of capacitation. Our current model (Fig. 8) indicates that at the onset of capacitation, the entry of HCO3− activates soluble adenyl cyclase (SACY) and increases the intracellular pH. The activation of SACY produces an increase in cAMP levels. The binding of cAMP to the regulatory subunits of PKA allows the dissociation of the tetramer and the activation of the catalytic subunit. Once free, the catalytic subunits remain active to phosphorylate a wide variety of substrates on Ser/Thr residues. Once active, PKA is responsible for phosphorylating multiple subunit of the proteasome, thus increasing its enzymatic activity. Finally, this proteasome activation by PKA is necessary for regulation in the capacitation process.
Figure 8.
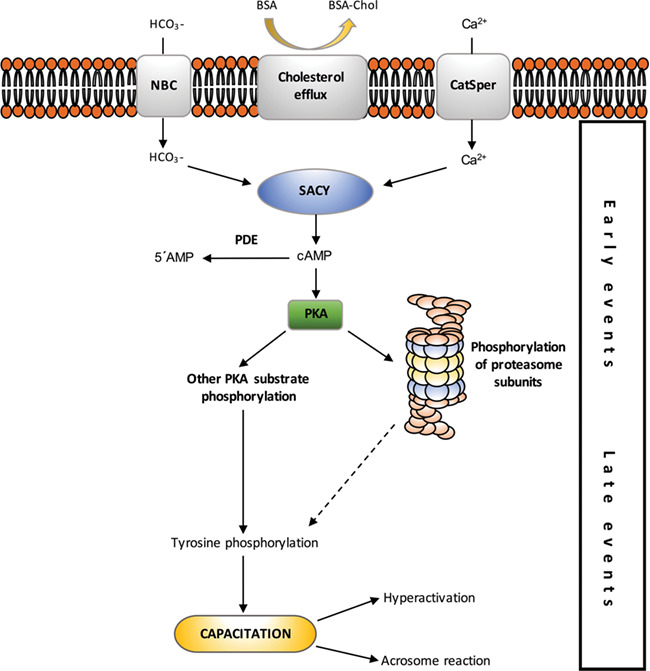
Proposed model of protein kinase A (PKA) and proteasome participation in sperm capacitation-signalling. The arrows represent stimulation. Dashed line indicates that the presence of a signal activating tyrosine phosphorylation by the proteasome has been proposed but the mechanism remains to be proven.
Authors’ roles
HZ-C performed the experiments, analysed the data, and wrote the manuscript. LB and EZD processed sperm samples, and LB performed the experiments and analysed the data. LZ and MK drafted the manuscript and prepared the figures. ESD designed the experiments. PM conceived and designed the experiments, performed the experiments, analysed the data and wrote the manuscript. PS advised LB in experimental design and co-wrote the manuscript. All authors read and approved the final manuscript.
Funding
P.S. is funded by Agriculture and Food Research Initiative Competitive Grant no. 2015-67015-23231 from the USDA National Institute of Food and Agriculture, grant number 5 R01 HD084353-02 from NIH National Institute of Child and Human Development, and by seed funding from the Food for The 21st Century Program of the University of Missouri. P.M. is funded by Fondo Puente de lnvestigación de Excelencia of the University of Antofagasta.
Conflict of interest
The authors have no conflicts of interest to disclose.
Supplementary Material
References
- Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell 2004;5:417–421. [DOI] [PubMed] [Google Scholar]
- Arcelay E, Salicioni AM, Wertheimer E, Visconti PE. Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int J Dev Biol 2008;52:463–472. [DOI] [PubMed] [Google Scholar]
- Asai M, Tsukamoto O, Minamino T, Asanuma H, Fujita M, Asano Y, Takahama H, Sasaki H, Higo S, Asakura Met al. PKA rapidly enhances proteasome assembly and activity in in vivo canine hearts. J Mol Cell Cardiol 2009;46:452–462. [DOI] [PubMed] [Google Scholar]
- Austin CR. The capacitation of the mammalian sperm. Nature 1952;170:326. [DOI] [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Reeves G, Müller J, Aitken RJ. The rat sperm proteome characterized via IPG strip prefractionation and LC-MS/MS identification. Proteomics 2008;8:2312–2321. [DOI] [PubMed] [Google Scholar]
- Baker MA, Smith ND, Hetherington L, Taubman K, Graham ME, Robinson PJ, Aitken RJ. Label-free quantitation of phosphopeptide changes during rat sperm capacitation. J Proteome Res 2010;9:718–729. [DOI] [PubMed] [Google Scholar]
- Barbonetti A, Vassallo MRC, Cinque B, Antonangelo C, Sciarretta F, Santucci R, D’Angeli A, Francavilla S, Francavilla F. Dynamics of the global tyrosine phosphorylation during capacitation and acquisition of the ability to fuse with oocytes in human spermatozoa. Biol Reprod 2008;79:649–656. [DOI] [PubMed] [Google Scholar]
- Battistone MA, Da Ros VG, Salicioni AM, Navarrete FA, Krapf D, Visconti PE, Cuasnicú PS. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol Hum Reprod 2013;19:570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebington C, Doherty FJ, Fleming SD. The possible biological and reproductive functions of ubiquitin. Hum Reprod Update 2001;7:102–111. [DOI] [PubMed] [Google Scholar]
- Bedford L, Paine S, Sheppard PW, Mayer RJ, Roelofs J. Assembly, structure, and function of the 26S proteasome. Trends Cell Biol 2010;20:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict CM, Ren L, Clawson GA. Nuclear multicatalytic proteinase alpha subunit RRC3: differential size, tyrosine phosphorylation, and susceptibility to antisense oligonucleotide treatment. Biochemistry 1995;34:9587–9598. [DOI] [PubMed] [Google Scholar]
- Biały LP, Ziemba HT, Marianowski P, Fracki S, Bury M, Wójcik C. Localization of a proteasomal antigen in human spermatozoa: immunohistochemical electron microscopic study. Folia Histochem Cytobiol 2001;39:129–130. [PubMed] [Google Scholar]
- Björndahl L, Barratt CLR, Mortimer D, Jouannet P. “How to count sperm properly”: checklist for acceptability of studies based on human semen analysis. Hum Reprod 2016;31:227–232. [DOI] [PubMed] [Google Scholar]
- Bruce JIE, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells: a mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem 2002;277:1340–1348. [DOI] [PubMed] [Google Scholar]
- Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951;168:697–698. [DOI] [PubMed] [Google Scholar]
- Choi Y-J, Uhm S-J, Song S-J, Song H, Park J-K, Kim T, Park C, Kim J-H. Cytochrome c upregulation during capacitation and spontaneous acrosome reaction determines the fate of pig sperm cells: linking proteome analysis. J Reprod Dev 2008;54:68–83. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ 2005;12:1178–1190. [DOI] [PubMed] [Google Scholar]
- Cross NL, Morales P, Overstreet JW, Hanson FW. Two simple methods for detecting acrosome-reacted human sperm. Gamete Res 1986;15:213–226. [Google Scholar]
- Diaz ES, Kong M, Morales P. Effect of fibronectin on proteasome activity, acrosome reaction, tyrosine phosphorylation and intracellular calcium concentrations of human sperm. Hum Reprod 2007;22:1420–1430. [DOI] [PubMed] [Google Scholar]
- Fischer KA, Van Leyen K, Lovercamp KW, Manandhar G, Sutovsky M, Feng D, Safranski T, Sutovsky P. 15-lipoxygenase is a component of the mammalian sperm cytoplasmic droplet. Reproduction 2005;130:213–222. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Umar G, Sayed S. Na(+)-requiring mechanisms modulate capacitation and acrosomal exocytosis in mouse spermatozoa. J Reprod Fertil 1993;97:539–549. [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Visconti PE, Kopf GS. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol Reprod 1997;56:707–719. [DOI] [PubMed] [Google Scholar]
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature 2003;426:895–899. [DOI] [PubMed] [Google Scholar]
- Guo X, Huang X, Chen MJ. Reversible phosphorylation of the 26S proteasome. Protein Cell 2017;8:255–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RA. Capacitation mechanisms, and the role of capacitation as seen in eutherian mammals. Reprod Fertil Dev 1996;8:581–594. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998;67:425–479. [DOI] [PubMed] [Google Scholar]
- Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, Miyamoto C, Zippin JH, Kopf GS, Suarez SSet al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell 2005;9:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafune Y, Kawasaki H, Hirano H. Electrophoretic analysis of phosphorylation of the yeast 20S proteasome. Electrophoresis 2002;23:329–338. [DOI] [PubMed] [Google Scholar]
- Jung T, Catalgol B, Grune T. The proteasomal system. Mol Aspects Med 2009;30:191–296. [DOI] [PubMed] [Google Scholar]
- Jung T, Grune T. Structure of the proteasome. Prog Mol Biol Transl Sci 2012;109:1–39. [DOI] [PubMed] [Google Scholar]
- Kerns K, Morales P, Sutovsky P. Regulation of sperm capacitation by the 26S proteasome: an emerging new paradigm in spermatology. Biol Reprod 2016;94:117. [DOI] [PubMed] [Google Scholar]
- Kikuchi J, Iwafune Y, Akiyama T, Okayama A, Nakamura H, Arakawa N, Kimura Y, Hirano H. Co- and post-translational modifications of the 26S proteasome in yeast. Proteomics 2010;10:2769–2779. [DOI] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Lefièvre L, Bray C, Stewart PM, Barratt CLR, Publicover SJ. Inhibitors of receptor tyrosine kinases do not suppress progesterone-induced [Ca2+]i signalling in human spermatozoa. Mol Hum Reprod 2002;8:326–332. [DOI] [PubMed] [Google Scholar]
- Kong M, Diaz ES, Morales P. Participation of the human sperm proteasome in the capacitation process and its regulation by protein kinase A and tyrosine kinase. Biol Reprod 2009;80:1026–1035. [DOI] [PubMed] [Google Scholar]
- Konstantinova IM, Tsimokha AS, Mittenberg AG. Role of proteasomes in cellular regulation. Int Rev Cell Mol Biol 2008;267:59–124. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685. [DOI] [PubMed] [Google Scholar]
- Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3′,5’monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod 1996;55:684–692. [DOI] [PubMed] [Google Scholar]
- Lee MA, Trucco GS, Bechtol KB, Wummer N, Kopf GS, Blasco L, Storey BT. Capacitation and acrosome reactions in human spermatozoa monitored by a chlortetracycline fluorescence assay. Fertil Steril 1987;48:649–658. [DOI] [PubMed] [Google Scholar]
- Lefièvre L, De Lamirande E, Gagnon C. The cyclic GMP-specific phosphodiesterase inhibitor, sildenafil, stimulates human sperm motility and capacitation but not acrosome reaction. J Androl 2000;21:929–937. [PubMed] [Google Scholar]
- Lu H, Zong C, Wang Y, Young GW, Deng N, Souda P, Li X, Whitelegge J, Drews O, Yang P-Yet al. Revealing the dynamics of the 20 S proteasome phosphoproteome: a combined CID and electron transfer dissociation approach. Mol Cell Proteomics 2008;7:2073–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Wilk S, Checler F. Protein kinase A phosphorylation of the proteasome: a contribution to the alpha-secretase pathway in human cells. J Neurochem 1996;67:2616–2619. [DOI] [PubMed] [Google Scholar]
- Martínez-León E, Osycka-Salut C, Signorelli J, Pozo P, Pérez B, Kong M, Morales P, Pérez-Martínez S, Díaz ES. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Hum Reprod 2015;30:2138–2151. [DOI] [PubMed] [Google Scholar]
- Metcalfe MJ, Huang Q, Figueiredo-Pereira ME. Coordination between proteasome impairment and caspase activation leading to TAU pathology: neuroprotection by cAMP. Cell Death Dis 2012;3: e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, Nixon B, Baker MA, Aitken RJ. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. MHR Basic Sci Reprod Med 2008;14:235–243. [DOI] [PubMed] [Google Scholar]
- Morales P, Kong M, Pizarro E, Pasten C. Participation of the sperm proteasome in human fertilization. Hum Reprod 2003;18:1010–1017. [DOI] [PubMed] [Google Scholar]
- Morales P, Pizarro E, Kong M, Jara M. Extracellular localization of proteasomes in human sperm. Mol Reprod Dev 2004;68:115–124. [DOI] [PubMed] [Google Scholar]
- Morales P, Socias T, Cortez J, Llanos MN. Evidences for the presence of chymotrypsin-like activity in human spermatozoa with a role in the acrosome reaction. Mol Reprod Dev 1994;38:222–230. [DOI] [PubMed] [Google Scholar]
- Moseley FLC, Jha KN, Björndahl L, Brewis IA, Publicover SJ, Barratt CLR, Lefièvre L. Protein tyrosine phosphorylation, hyperactivation and progesterone-induced acrosome reaction are enhanced in IVF media: an effect that is not associated with an increase in protein kinase A activation. Mol Hum Reprod 2005;11:523–529. [DOI] [PubMed] [Google Scholar]
- Neild DN, Gadella BM, Agüero A, Stout TAE, Colenbrander B. Capacitation, acrosome function and chromatin structure in stallion sperm. Anim Reprod Sci 2005;89:47–56. [DOI] [PubMed] [Google Scholar]
- Neuhaus EM, Mashukova A, Barbour J, Wolters D, Hatt H. Novel function of beta-arrestin2 in the nucleus of mature spermatozoa. J Cell Sci 2006;119:3047–3056. [DOI] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase a catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A 2004;101:13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdorf J, Carlson EJ, Skach WR. Redundancy of mammalian proteasome beta subunit function during endoplasmic reticulum associated degradation. Biochemistry 2001;40:13397–13405. [DOI] [PubMed] [Google Scholar]
- Pizarro E, Pastén C, Kong M, Morales P. Proteasomal activity in mammalian spermatozoa. Mol Reprod Dev 2004;69:87–93. [DOI] [PubMed] [Google Scholar]
- Rivkin E, Kierszenbaum AL, Gil M, Tres LL. Rnf19a, a ubiquitin protein ligase, and Psmc3, a component of the 26S proteasome, tether to the acrosome membranes and the head-tail coupling apparatus during rat spermatid development. Dev Dyn 2009;238:1851–1861. [DOI] [PubMed] [Google Scholar]
- Rodriguez CI, Stewart CL. Disruption of the ubiquitin ligase HERC4 causes defects in spermatozoon maturation and impaired fertility. Dev Biol 2007;312:501–508. [DOI] [PubMed] [Google Scholar]
- Schlesinger DH, Goldstein G, Niall HD. The complete amino acid sequence of ubiquitin, an adenylate cyclase stimulating polypeptide probably universal in living cells. Biochemistry 1975;14:2214–2218. [DOI] [PubMed] [Google Scholar]
- Shabb JB. Physiological substrates of cAMP-dependent protein kinase. Chem Rev 2001;101:2381–2411. [DOI] [PubMed] [Google Scholar]
- Signorelli JR, Díaz ES, Fara K, Barón L, Morales P. Protein phosphatases decrease their activity during capacitation: a new requirement for this event. PLoS One 2013;8: e81286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutovsky P. Sperm proteasome and fertilization. Reproduction 2011;142:1–14. [DOI] [PubMed] [Google Scholar]
- Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Ubiquitinated sperm mitochondria, selective proteolysis, and the regulation of mitochondrial inheritance in mammalian embryos. Biol Reprod 2000;63:582–590. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Yoshimura T, Tamura T, Fujiwara T, Kumatori A, Ichihara A. Possible mechanism of nuclear translocation of proteasomes. FEBS Lett 1990;271:41–46. [DOI] [PubMed] [Google Scholar]
- Tipler CP, Hutchon SP, Hendil K, Tanaka K, Fishel S, Mayer RJ. Purification and characterization of 26S proteasomes from human and mouse spermatozoa. Mol Hum Reprod 1997;3:1053–1060. [DOI] [PubMed] [Google Scholar]
- Varner DD, Bowen JA, Johnson L. Effect of heparin on capacitation/acrosome reaction of equine sperm. Arch Androl 1993;31:199–207. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Olson GE, NagDas S, Winfrey VP, Carr DW. Subcellular localization of the regulatory subunits of cyclic adenosine 3′,5′-monophosphate-dependent protein kinase in bovine spermatozoa. Biol Reprod 1997;57:1517–1523. [DOI] [PubMed] [Google Scholar]
- Visconti PE. Understanding the molecular basis of sperm capacitation through kinase design. Proc Natl Acad Sci U S A 2009;106:667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995a;121:1129–1137. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995b;121:1139–1150. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning XP, Fornés MW, Alvarez JG, Stein P, Connors SA, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm: cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 1999a;214:429–443. [DOI] [PubMed] [Google Scholar]
- Wennemuth G. Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development 2003;130:1317–1326. [DOI] [PubMed] [Google Scholar]
- Wojcik C, Benchaib M, Lornage J, Czyba JC, Guerin JF. Proteasomes in human spermatozoa. Int J Androl 2000;23:169–177. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO laboratory manual for the Examination and processing of human semen. Geneva: Switzerland, 2010. [Google Scholar]
- Xie Y. Structure, assembly and homeostatic regulation of the 26S proteasome. J Mol Cell Biol 2010;2:308–317. [DOI] [PubMed] [Google Scholar]
- Zhang F, Hu Y, Huang P, Toleman CA, Paterson AJ, Kudlow JE. Proteasome function is regulated by cyclic AMP-dependent protein kinase through phosphorylation of Rpt6. J Biol Chem 2007;282:22460–22471. [DOI] [PubMed] [Google Scholar]
- Zigo M, Kerns K, Sutovsky M, Sutovsky P. Modifications of the 26S proteasome during boar sperm capacitation. Cell Tissue Res 2018;372:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman SW, Yi YJ, Sutovsky M, Van Leeuwen FW, Conant G, Sutovsky P. Identification and characterization of RING-finger ubiquitin ligase UBR7 in mammalian spermatozoa. Cell Tissue Res 2014;356:261–278. [DOI] [PubMed] [Google Scholar]
- Zong C, Gomes AV, Drews O, Li X, Young GW, Berhane B, Qiao X, French SW, Bardag-Gorce F, Ping P. Regulation of murine cardiac 20S proteasomes: role of associating partners. Circ Res 2006;99:372–380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


