Abstract
Deltamethrin (DLM) is a commonly used pesticide that helps to control crop destruction, disease, and nuisance insects. In rodents DLM can produce choreoathetosis, salivation, and decreased acoustic startle responses (ASR). Herein, adult Sprague Dawley rats were assessed for ASR 2 h after DLM delivered in 5 ml/kg corn oil, however no decrease was observed. Therefore, a test-retest protocol was used to reduce variability, and the effects on ASR on postnatal day 15 (P15) and adult rats were assessed 2, 4, 6, and 8 h after DLM administration (0, 1, 2, or 4 mg/kg for P15 rats and 0, 2, 8, or 25 mg/kg for adults). In a separate set of rats identically treated, DLM levels were determined in blood and brain. DLM (8 or 25 mg/kg) in adult rats decreased ASR up to 4 h, whereas in P15 rats decreases were observed between 2 and 8 h. The adult 25 mg/kg group showed consistent signs of salivation and tremor, whereas in P15 rats salivation was observed in the 2 and 4 mg/kg groups and tremor was observed at all doses over the 8-h period. Mortality was observed in all P15 dose groups but not in adults. Dose-dependent increases of DLM in blood and brain regardless of age were observed. At approximately equivalent whole brain concentrations, effects were more pronounced in P15 rats than in adult rats. Comparable brain levels of DLM do not explain differences in ASR and tremor between the P15 and adult rats. These data indicate age-dependent differences in sensitivity to DLM.
Keywords: deltamethrin, acoustic startle, tremor, pyrethroid
AQ5Pyrethroids are some of the most widely used insecticides in agriculture, industry, residential, and public areas. Pyrethroids are structurally derived from natural pyrethrins and act at voltage-sensitive sodium channels (Wolansky and Harrill, 2008). During brain development, the expression of voltage-sensitive sodium channels changes and may impact the effect of pyrethroids (Shafer et al., 2005). Depending on the structure and mode of toxic action, pyrethroids are classified as Type I or Type II (Soderlund, 2012 ; Soderlund et al., 2002). Type I pyrethroids such as allethrin, permethrin, pyrethrin I, and resmethrin, lack an α-cyano moiety. Type I pyrethroids cause whole body tremors (T syndrome) at toxic doses in rodents (Verschoyle and Aldridge, 1980). Type II pyrethroids have an α-cyano-3-phenoxybenzyl moiety and include cypermethrin and deltamethrin (DLM). In contrast to Type I pyrethroids, Type II pyrethroids produce a CS syndrome, ie, choreoathetosis (writhing) and salivation at toxic doses in rats and mice (Verschoyle and Aldridge, 1980). Some pyrethroids produce signs that are a mixture of T and CS syndromes and are referred to as mixed or Type I/II (Soderlund, 2012 ; Soderlund et al., 2002). Examples of Type I/II pyrethroids include bifenthrin, fenpropathrin, and fenvalerate (Soderlund et al., 2002). Both the Type I and Type II pyrethroids prolong voltage-sensitive sodium channel opening, with the Type I inducing repetitive neuronal firing, whereas no repetitive firing is observed with the Type II pyrethroids that hold the voltage-sensitive sodium channels open for a much longer period of time (Clark and Brooks, 1989). A number of considerations are required for assessing the LD50 and the ED50 for pyrethroids such as the age of the animal, route of administration, vehicle, as well as dose volume (Wolansky and Harrill, 2008; Wolansky and Tornero-Velez, 2013).
Together with the T or CS syndrome (Verschoyle and Aldridge, 1980), quantifiable acute toxic effects of Type I and Type II pyrethroids are also known. For example, Type I and Type II pyrethroids produce decreased locomotor activity after administration by gavage (Crofton and Reiter, 1984, 1987, 1988) and decreased operant response after intraperitoneal or oral administration (Bloom et al., 1983; Peele and Crofton, 1987). However, the acoustic startle response (ASR) differentiates between the Type I and Type II pyrethroids. In adult rats, the Type I pyrethroid permethrin increases ASR 1.5–3 h after acute oral administration (Crofton and Reiter, 1988; Hijzen and Slangen, 1988), whereas the Type II pyrethroid DLM decreases ASR (Crofton and Reiter, 1984, 1987, 1988). Age at the time of exposure also affects ASR. For example, after permethrin (30, 60, or 120 mg/kg) was given to postnatal day (P)21 rats, there was no effect on ASR when examined 2 h later (Sheets, 2000), but after DLM (1, 2, and 4 mg/kg) was given at this age, ASR was decreased (Sheets et al., 1994). Comparative studies between adult and developing rodents are limited. In P15 rats, permethrin had differential effects on ASR that were dependent on dose and time of assessment, whereas adult rats had increased ASR up to 8 h later (Williams et al., 2018). For DLM, Sheets et al. (1994) showed that ASR was reduced in P21 and adult rats 2 h after administration with the P21 rats showing more signs of salivation and burrowing at a given dose compared with adults. Concentrations of DLM in brain were determined in a separate group of rats 2 h after administration, but the dosing volumes between the rats tested for ASR and those used to determine brain concentrations were different. Furthermore, the P21 rats came from litters shipped while dams were pregnant, an influence with unknown effects. Therefore, within the literature, there is a lack of consistency in comparing the acute neurotoxicity as manifested by ASR changes in young rats and adult rats under identical procedures. ASR can be detected in many species, including humans, and is used as a marker for acute neurotoxicity (Tilson, 1990).
The current study was undertaken to examine the acute neurotoxicity of DLM in P15 and adult rats using identical procedures to test ASR and to determine plasma and brain levels of DLM using a consistent dosing volume of 5 ml/kg of corn oil (CO) as used by Weiner et al. (2009). P15 rats were obtained from dams bred in-house. Because there are steep age-dependent differences in acute toxicity for DLM (Sheets et al., 1994), P15 rats were dosed with 0–4 mg/kg whereas adult rats were dosed with 0–25 mg/kg. ASR was first tested in adult rats at 2 h, but in subsequent experiments ASR was tested over an 8-h interval, rather than 2 h based on data obtained recently with permethrin (Williams et al., 2018). Another group of rats was used to determine DLM levels in brain and plasma at the same time points used to assess ASR. These experiments tested whether there are age-dependent differences in ASR and clinical signs in relation to internal DLM concentrations over time and across ages.
MATERIALS AND METHODS
Animals
For the experiments with adult rats, Sprague Dawley CD, IGS rats (strain no. 001, Charles River, Raleigh, North Carolina) were purchased from the supplier and pair housed the day of arrival and for the duration of the study. For the experiments with P15 rats, male and female rats were obtained and breed in-house from rats of the same strain and supplier. All purchased rats were habituated to the vivarium in polysulfone cages (46 cm × 24 cm × 20 cm) for a minimum of 1 week prior to use. The cages contained woodchip bedding and a stainless steel hut for enrichment (Vorhees et al., 2008). For breeding, male and nulliparous female rats were housed together in cages with a wire subfloor, and cage floors were examined daily for presence of a sperm plug. The day a sperm plug was found was embryonic day 0 (E0). On E1 gravid females were individually housed. Date of birth was designated P0, and on P1 litters were culled using a random number table to 4 males and 4 females. A split litter design was used because it provides control over metagenome and microbiome, because littermates share the same intestinal flora and fauna (Stappenbeck and Virgin, 2016).
The vivarium is AAALAC International accredited, pathogen free, and uses the Modular Animal Caging System (Alternative Design, Siloam Spring, Arkansas) with HEPA filtered air that is supplied via the Flex-Air System (Alternative Design, Siloam Spring, Arkansas) at 30 air changes/hour. Rats had ad libitum access to NIH-07 rat chow (LabDiet no. 5018, Richmond, Indiana) and reverse osmosis filtered, UV purified water from a Lixit automated system (SE Lab Group, Napa, California). Temperature (19°C ± 1°C), humidity (50% ± 10%), and light-dark cycle (14:10 h, lights on at 600 h) were automatically regulated. Rats were treated in accordance with protocols approved by the Cincinnati Children’s Research Foundation’s Institutional Animal Care and Use Committee.
Deltamethrin
DLM was provided by Bayer CropScience AG, Frankfurt, Germany (CAS no. 52918‐63‐5) > 99.9% pure and stored at 4°C prior to mixing. DLM was dissolved in CO (Acros Organics, Geel, Belgium) and was administered by gavage (5 ml/kg) via a stainless steel 20-gauge gavage needle with a 2.25-mm diameter ball-tip (Cadence Science, Cranston, Rhode Island) that was 2.5 cm long for P15 rats and 7.6 cm long for adult rats. The solution was kept at room temperature on the day of administration. The 5 ml/kg volume was determined by the Sponsor (Council for the Advancement of Pyrethroid Human Risk Assessment, LLC [CAPHRA]) and designed similarly to related studies (Weiner et al., 2009).
Acoustic startle response
ASR was measured in 8 identical SR-LAB test chambers (San Diego Instruments [SDI], San Diego, California). Rats were placed in acrylic cylindrical holders (SDI large enclosure for adult rats and SDI small enclosure for P15 rats) mounted on a platform with a piezoelectric accelerometer attached to the underside; the platforms were located inside sound-attenuating chambers with a house light and fan. The sensitivity setting of the accelerometers was adjusted by age to avoid exceeding the maximum capacity of the device for adult rats and to increase detection sensitivity in P15 rats. A 5-min adaptation period preceded trials. Each ASR session consisted of 50 trials. The pulse was a 20 ms, 120 dB SPL mixed frequency white noise burst (rise time 1.5 ms). The recording window lasted 100 ms from the onset of the pulse. Maximum startle amplitude (V max) was measured in millivolts. A baseline was obtained at the beginning of each trial and this was subtracted from V max. V max is reported because the average response (V avg) is highly correlated with V max (r > 0.95), see Williams et al. (2018).
Experiment 1
Experiment 1 was to test that DLM decreased ASR in adult rats when delivered in 5 ml CO/kg, because published data more commonly used volumes of 1 ml/kg or less (Crofton and Reiter, 1984; Hijzen and Slangen, 1988; Sheets et al., 1994). There were 4 dose levels: 0, 2, 4, or 8 mg/kg of DLM with 12 adult males/dose matched for body weight ± standard error of the mean (SEM) (mean across groups 422.0 ± 2.5 g, range 388.8–461.6 g). Food was removed from cages 2 h prior to DLM administration. Observations for tremor and salivation were noted and body weights recorded on the day of exposure. Rats were counterbalanced for dose and test chamber and were tested within a 4-day period. Testing began 2 h after DLM administration. For all experiments, researchers were blinded to group membership and inter-rater reliability was >90%.
Experiment 2
In this experiment the dose was increased, as was the number of rats per dose, number of time points assessed, and this time rats were matched on ASR based on responses to CO alone the day before the main experiment (Williams et al., 2018). On the pretest day, all 64 rats were gavaged with CO and 2 h later given 50 ASR trials. The average V max ASR was determined for each rat and used to assign rats to one of the 4 DLM groups (16 adult male rats/dose [mean weight ± SEM: 277.2 ± 2.0 g]), such that all groups had comparable V max values. Rats were then tested in cohorts of 16/day over 4 days. Rats remained with the littermate originally assigned to that cage, regardless of what DLM group assignment each rat had. On the test day, rats were gavaged with 0, 2, 8, or 25 mg/kg of DLM and tested at 2, 4, 6, and 8 h later. Rats were given 50 ASR trials per test interval. Incidences of salivation and tremor were rated on a scale from 0 to 3. For salivation 0 = not present, 1 = mild and was characterized as slight evidence of wetness immediately adjacent to the area around the mouth, 2 = moderate and was characterized by the fur around the mouth as being wet and potentially matted, and 3 = severe that was characterized by a broad area of fur around the mouth that was obviously wet and may have had active salivation, blood on fur or around mouth, and bubbles around mouth. For tremor, 0 = not present, 1 = mild that was characterized as evidence of tremors but barely perceptible, usually within the head or tail region, 2 = moderate that was characterized as body tremor of select muscle groups with the rat still upright, and 3 = severe that was characterized as extensive whole body tremor with the rat on its side. Rats were scored prior to and after each ASR test.
Experiment 3
The effect of DLM on the ASR of P15 male and female rats was assessed in 17 litters. The doses were 0, 1, 2, or 4 mg/kg of DLM. Within each litter, 1 female and 1 male were used per dose. The mean female weight ± SEM was 33.7 ± 0.8 g, and the mean male weight ± SEM was 34.5 ± 0.8 g. On P14, pups were gavaged with CO and ASR tested starting 2 h later. Pups were matched on average V max response within litter and assigned to one of 4 DLM dose groups. On P15, pups were gavaged with DLM at the designated dose and tested 2, 4, 6, and 8 h later. Rats were given 50 ASR trials per time interval, and incidences of salivation and tremor were rated on a scale from 0 to 3 as above. The total time to gavage all 8 rat pups in a litter was <5 min and total duration away from the dam for ASR testing was <25 min per test period.
Experiment 4
Separate groups of P15 (8 females and 8 males per dose per time point from 32 litters) and adult rats (8 males per dose per time point) were used for plasma and brain tissue collection. Adult rats were dosed with 2, 8, or 25 mg/kg DLM and P15 rats with 1, 2, or 4 mg/kg DLM. Trunk blood and whole brain were collected at 2, 4, 6, or 8 h after dosing. Trunk blood was collected in Vacutainer plus plastic PST blood collection tubes with polymer gel and lithium heparin (76 USP: BD Medical, Franklin Lakes, New Jersey). A 0.64 M concentration of sodium fluoride (∼100 µl/ml of blood) was added to each blood sample in order to inhibit carboxylesterase activity. Blood was centrifuged at 1500 g for 15 min to separate plasma from red blood cells. Plasma was transferred to individual, polyethylene tubes, frozen, and stored at −80°C. Brain was rapidly removed and snap-frozen in isopentane, placed in tubes, and stored at −80°C. Brain and plasma samples were sent overnight on dry ice to Frontage Laboratories, Inc. (Exton, Pennsylvania) for DLM determination by liquid chromatography tandem mass spectrometry (LC/MS/MS).
For DLM determination in plasma, each of the standards and assay samples had 20 µl of the internal standard of DLM-d5 (Toronto Research Chemicals, Inc.) added to 250 ng/ml and 400 µl of 0.1% formic acid in methanol, and the solution was vortexed for 1 min. The mixtures were centrifuged for 5 min at 2300 RCF. After centrifugation, 20 µl of the resulting supernatant was transferred into high-pressure liquid chromatography vials containing 200 µl of reconstitution solution (50:50 methanol/water) and vortexed for 1 min. About 10 µl of the vortexed solution were injected into an API5000 LC/MS/MS system. The limit of quantification was 1 ng/ml in 50 µl aliquots of plasma. No values fell below the limit of quantification.
For adult and P15 brain, tissues were weighed and a 50:50 mixture of H2O/acetonitrile (containing 0.64 M sodium fluoride) was added at a ratio of 5:1. Brains were then homogenized for 60 s at 6 m/s on a MP fast prep-24TM 5g with two ceramic beads (1/4″ ceramic sphere, MP Biomedicals). The homogenates were vortexed and then stored at −20°C until assayed. For brain, 50 µl of 50:50 methanol/water was added to 150 µl rat brain tissue homogenates. Then, 20 µl of DLM-d5 (250 ng/ml) and 400 µl of acetonitrile were added, and samples were vortexed for 20 s. The samples were then centrifuged for 2 min at 14 000 g. The supernatant (500 µl) was transferred to 12 × 75 mm plastic tubes containing 950 µl of water and vortexed for 20 s. Oasis SPE HLB cartridges (30 mg/1 cc) were conditioned with 1 ml of acetonitrile and equilibrated with 1 ml of purified water. The samples were loaded in the cartridges and washed with 1 ml of 0.1% formic acid in purified water and eluted with 1 ml of 0.1% formic acid in acetonitrile. The solutions were collected in clean test tubes and evaporated to dryness (∼20 min) under nitrogen gas at 40°C. Finally, 200 µl of reconstitution solution (50:50 methanol/water) was added to each test tube and vortexed for 60 s. About 20 µl of solution was injected into an API5500 LC/MS/MS system. The limit of quantification was 2 ng/g in 150 µl aliquots of brain homogenate. No values fell below the limit of quantification. For the internal standards, the % coefficient of variation for the low (6 ng/g), mid (120 ng/g), and high (750 ng/g) controls were 15%, 8.2%, and 3.2%, respectively. Values greater than 2 SD from the group mean for brain or plasma were not included in the analyzes. For the adult rats, no more than 1 value was excluded per group per tissue type per time point, with the exception of the brain for the DLM 2 mg/kg group (a total of n = 4 exclusions for brain and n = 3 exclusions for plasma). For the P15 rats, no more than 1 value was excluded per group per tissue type per sex per time point (a total of n = 4 exclusions for brain and n = 7 exclusions for plasma). Final numbers are provided in the figure legends for both adults and P15 rats.
Statistical procedures
Data were analyzed using mixed linear analyses of variance (ANOVA) models (SAS Proc Mixed, SAS Institute, Cary, North Carolina, version 9.3) with an autoregressive moving average (ARMA) covariance structure when repeated measures were used. Kenward-Rogers first-order method was used to calculate degrees of freedom. Significant interactions were analyzed using slice-effect ANOVAs at each level of the repeated-measure factor. For post hoc tests, Dunnett’s method was used to determine significant differences between DLM groups versus CO or Tukey-Kramer for comparisons between DLM groups. The P15 experiment used a split-litter design; therefore, in order to ensure that litter effects were controlled, litter was a block (random) factor in a completely randomized block ANOVA. DLM dose and sex were between factors within block and litter was the block factor (Williams et al., 2017). For ASR, test interval was the within-subject factor. Significance was set at p ≤ .05 (2-tailed, where no a priori prediction was made). For cases where predictions were made (eg, reduced ASR), directional tests were used. Because salivation and tremor are ordinal data, an ordinal logistic regression analysis was done for each dependent variable using Proc Logistic in SAS to determine if there was an effect of dose. Pairwise comparisons were done with the Dwass, Steel, Critchlow-Fligner (DSCF) method in Proc Npar1way. Data are presented as least square (LS) mean ± SEM.
RESULTS
Experiment 1
ASR was examined in adult male rats 2 h after 0, 2, 4, or 8 mg/kg DLM in 5 ml/kg CO. Because rats were matched on body weight, no group differences were detected for body weight, p > .97. Body weight means ± SEM were 0 mg/kg = 422.6 ± 5.5 g; 2 mg/kg = 422.8 ± 5.4 g; 4 mg/kg = 419.8 ± 4.3 g; and 8 mg/kg = 422.6 ± 5.5 g. There were no differences in V max, although the effect of dose showed a trend (p < .08). The V max means ± SEM were: 0 mg/kg = 869.5 ± 144.3 mV; 2 mg/kg = 661.2 ± 156.9 mV; 4 mg/kg = 1025.1 ± 156.9 mV; and 8 mg/kg = 600.7 ± 156.5 mV. The correlation between V max and V avg was r = 0.99, p < .001, whereas the correlation between weight and V max was r = −0.22, ns; a finding similar to our previous study (Williams et al., 2018). No salivation or tremor was seen.
Experiment 2
ASR was tested in adult male rats at four 2 h intervals after 0, 2, 8, or 25 mg/kg DLM in 5 ml/kg CO. There were no effects of dose or hour, however, there was a dose × hour interaction, F(9, 148) = 3.75, p < .0003. Examination of the interaction showed that at 2 and 4 h, the 8 mg/kg (p < .03 and 0.04, respectively) and 25 mg/kg (p < .0007 and .002, respectively) groups had decreased ASR compared with CO controls (Figure 1 A). No differences were found at 6 or 8 h. There was no difference between the 2 mg/kg group compared with CO controls. The correlation between V max and V avg was r = 0.99, p < .0001. There was no mortality.
Figure 1.
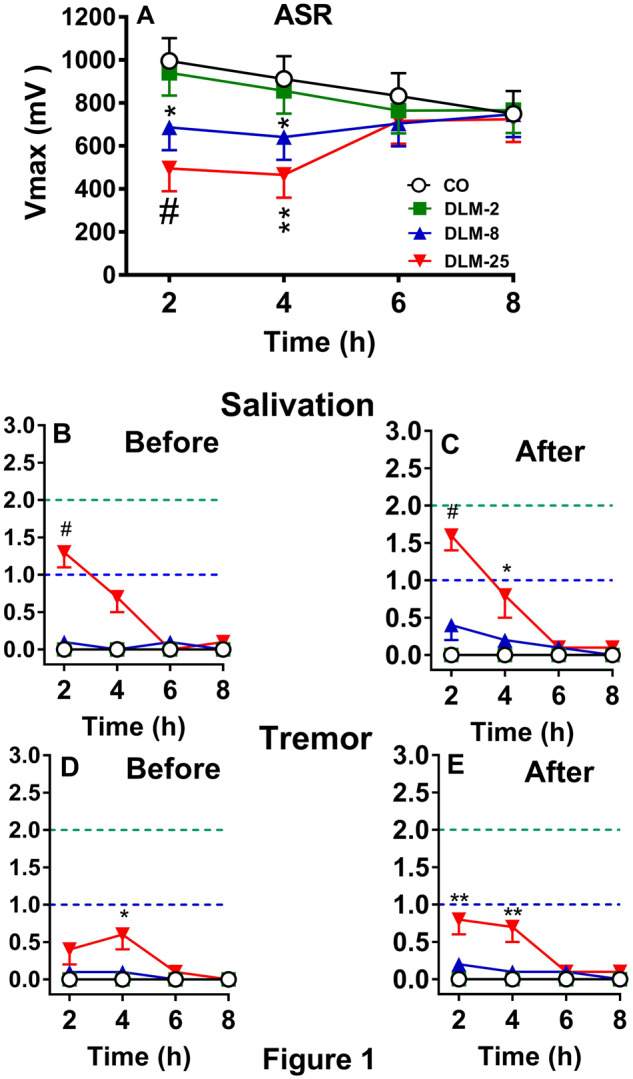
Effects of DLM in adult rats (mean ± SEM). A, ASR after 0, 2, 8, or 25 mg/kg DLM. B, Salivation score before ASR. C, Salivation score after ASR. D, Tremor score before ASR. E, Tremor score after ASR. Dashed line (bottom): mild effect; dashed line (top): moderate effect. n = 16/group, *p < .05, **p < .01, #p < .001 versus CO.
Salivation is shown in Figures 1B and 1C and tremor in Figures 1D and 1E before and after ASR. CO controls and DLM 2 mg/kg groups showed no symptoms. For the DLM 8 mg/kg group, 2/16 (12%) rats showed salivation before and 5/16 (31%) rats showed salivation after ASR testing. For the DLM 25 mg/kg group, 14/16 (87%) rats showed salivation at one or more time points before and/or after ASR. As can be seen in the figures by the average response, most of the symptoms were mild to moderate. For salivation before ASR testing, there was a significant effect of dose at 2 h, χ2 (3) = 21.2, p < .0001 with the DLM 25 mg/kg group showing more salivation than the CO group (Figure 1B). No other differences were noted before ASR testing. After ASR testing, there were effects of dose at 2 and 4 h, χ2 (3) = 16.0 and 8.8, p < .002 and .05, respectively. The DLM 25 mg/kg group had more salivation than the CO group at both time points (Figure 1C). No other differences were found after ASR testing.
For tremor in the DLM 8 mg/kg group, 3/16 (18%) rats before and 2/16 (12%) rats after ASR displayed tremors. In the DLM 25 mg/kg group, tremor was seen in 10/16 (62%) rats before and 11/16 (68%) rats after ASR. Most of the tremors were mild. For tremor before ASR testing, there was a significant effect of dose at 4 h, χ2 (3) = 7.8, p < .05 with the DLM 25 mg/kg group showing more tremor than the CO group (Figure 1C). For tremor after ASR testing, there were significant effects of dose at 2 and 4 h, χ2 (3) = 8.2 and 11.7, p < .05 and .009, respectively. The DLM 25 mg/kg group had more tremor than the CO group (Figure 1D).
Experiment 3
ASR was tested in P15 rats 4 times at 2 h intervals from 2 to 8 h after being dosed with 0, 1, 2, or 4 mg/kg DLM in 5 ml/kg CO. For V max, there were significant main effects of dose, F(3, 112) = 63.5, p < .0001 and hour (p < .0001), as well as a dose × hour interaction, F(9, 308) = 12.4, p < .0001. Examination of the interaction showed that rats dosed with 1, 2, or 4 mg/kg DLM, regardless of sex, had decreased V max compared with CO controls at each time interval (p’s < .0001, with the DLM 1 mg/kg group at 8 h, p < .002: Figure 2 A). There was no effect of sex or other interactions. The correlation between V max and V avg was r = 0.95, p < .0001. Unlike in adult rats, DLM induced mortality in the P15 rats. Two males dosed with 2 mg/kg died (12%) (one died at 4 h and one died at 8 h). Four females in the DLM 4 mg/kg group died (24%); 2 at 6 h and 2 at 8 h, and 5 males at DLM 4 mg/kg died at 8 h (29%).
Figure 2.
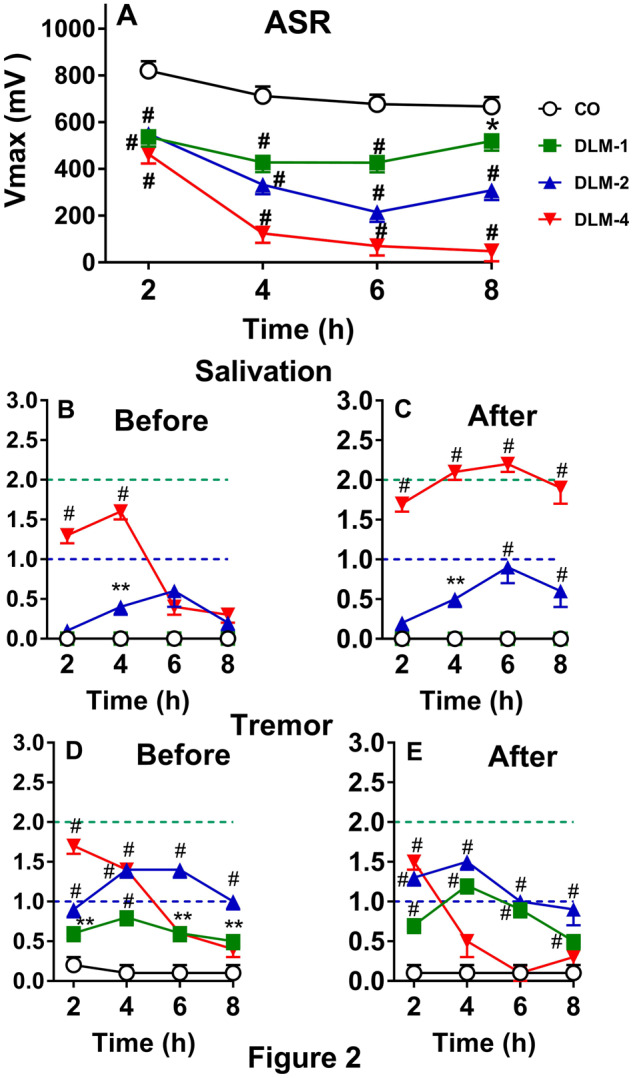
Effect of DLM in P15 rats (mean ± SEM). A, ASR after 0, 1, 2, or 4 mg/kg DLM. B, Salivation score before ASR. C, Salivation score after ASR. D, Tremor score before ASR. E, Tremor score after ASR. Dashed line (bottom): mild effect; dashed line (top): moderate effect. Starting group sizes (please see text for mortality details): 0 mg/kg, n = 34; 1 mg/kg, n = 33; 2 mg/kg, n = 34; and 4 mg/kg, n = 34. *p < .05, **p < .01, #p < .001 versus CO.
For salivation before ASR, none of the CO control or DLM 1 mg/kg rats showed salivation, whereas 18/34 (53%) in the DLM 2 mg/kg group and 32/34 (94%) in the DLM 4 mg/kg group showed salivation at least at one time interval (Figure 2B). After ASR testing, 3/33 (9%) of the DLM 1 mg/kg group, 21/34 (62%) of the DLM 2 mg/kg group, and 33/34 (97%) of the DLM 4 mg/kg group showed salivation (Figure 2C); none of the CO controls showed salivation. For salivation before ASR testing, there was a significant effect of dose at 2 and 4 h, χ2 (3) = 40.7 and 49.0, p’s < .0001, respectively. At 2 h, only the 4 mg/kg group showed more salivation than the CO group. At 4 h, the 2 and 4 mg/kg groups had more salivation than the CO group (Figure 2B). No other differences were noted before ASR testing for salivation. After ASR testing there were effects of dose at 2 h (χ2 (3) = 56.8, p < .0001), 4 h (χ2 (3) = 77.6, p < .001), 6 h (χ2 (3) = 61.0, p < .0001), and 8 h (χ2 (3) = 55.5, p < .0001). The DLM 4 mg/kg group had more salivation than the CO group at all time points, whereas the DLM 2 mg/kg group had more salivation at 4, 6, and 8 h compared with the CO group (Figure 2C). No other differences were found after ASR testing.
For tremor before ASR testing, 11/34 (32%) of the CO controls, 29/33 (88%) of the DLM 1 mg/kg, and 100% of the DLM 2 and 4 mg/kg groups had signs of tremor at one or more time intervals (Figure 2D). Before ASR testing, there were effects of dose at 2 h (χ2 (3) = 121.0, p < .0001), 4 h (χ2 (3) = 105.1, p < .001), 6 h (χ2 (3) = 61.6, p < .0001), and 8 h (χ2 (3) = 51.5, p < .0001). All of the DLM groups had more tremor before ASR testing compared with the CO group. After ASR, 11/34 (32%) of CO controls (as before), 32/33 (97%) of the DLM 1 mg/kg, 34/34 (100%) of the DLM 2 mg/kg, and 27/34 (79%) of the DLM 4 mg/kg rats showed tremor at one or more test intervals (Figure 2E). After ASR testing, there were effects of dose at 2 h (χ2 (3) = 113.9, p < .0001), 4 h (χ2 (3) = 72.2, p < .001), 6 h (χ2 (3) = 64.2, p < .0001), and 8 h (χ2 (3) = 40.1, p < .0001). After ASR testing, all DLM groups at all time points, with the exception of the DLM 4 mg/kg group at 6 and 8 h had more tremor than the CO group. Unlike adult rats, most of the salivation and tremors were moderate; they were severe in only a few cases, as can be seen from the increase in mean scores across intervals. The “tremor” seen in CO controls was mild and appeared to be endogenous, a typical head wobble seen in immature rats lifting their heads before neck musculature is fully developed.
Experiment 4
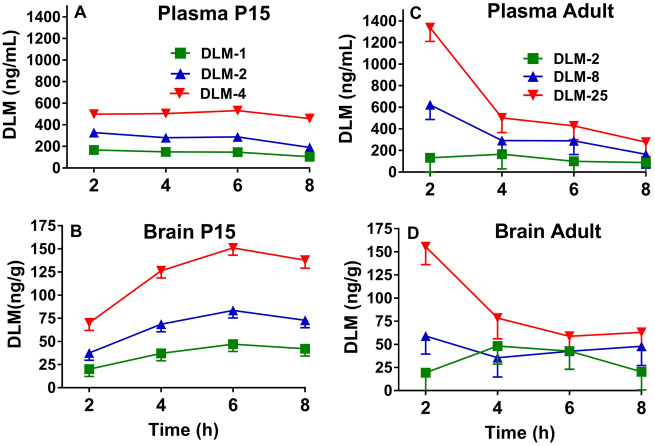
Rats were dosed on P15 with 1, 2, or 4 mg/kg DLM in 5 ml/kg CO or adults with 2, 8, or 25 mg/kg DLM in 5 ml/kg CO, and levels of DLM were determined in plasma and brain at the same 2, 4, 6, or 8 h intervals as for ASR in the previous experiments. For plasma concentrations of DLM in the P15 rats (both sexes), there were effects of dose, F(2, 131) = 387.9, p < .0001, hour (p < .004), a trend for sex (p < .06), but no interactions. All doses were significantly different from one another (Figure 3 A; p < .0001; asterisks not used for clarity of presentation). The mean ± SEM for each dose of DLM combined over 8 h was: 1 mg/kg = 141.6 ± 10.4 ng/ml; 2 mg/kg = 271.7 ± 10.3 ng/ml; and 4 mg/kg = 498.2 ± 10.7 ng/ml.
Figure 3.
Plasma and brain DLM concentrations as a function of time (mean ± SEM). P15 DLM levels in plasma (A) and brain (B) after doses of 1, 2, or 4 mg/kg DLM from 2 to 8 h post-treatment. Adult DLM levels in plasma (C) and brain (D) after doses of 2, 8, or 25 mg/kg DLM from 2 to 8 h post-treatment. In plasma for P15 rats (females, males), n = (8, 8) except at 1 mg/kg at 4 h, 2 mg/kg at 6 h, and 4 mg/kg at 2 h, n = (7, 8) and at 4 mg/kg at 8 h, n = (6, 7). In brain for P15 rats (females, males), n = (8, 8) except at 1 mg/kg at 4, 6, and 8 h, 2 mg/kg at 6 h, and 4 mg/kg at 2 h, n = (7, 8), n = (8, 7) for 4 mg/kg at 6 h, and n = (6, 6) at 8 h. In blood for adult rats, n = 8, except at 8 mg/kg at 4 and 8 h, n = 7, and n = 6 for 2 mg/kg at 2 h and 25 mg/kg at 4 h. In brain for adult rats, n = 8, except at 2 mg/kg at 4 and 8 h, 8 mg/kg at 2 and 4 h, and 25 mg/kg at 2 h, n = 7.
For brain, there were effects of dose, F(2, 134) = 186.1, p < .0001, hour (p < .0001), and dose × hour, F(6, 134) = 3.8, p < .002 (Figure 3B). No effect of sex or other interactions were found. Examination of dose × hour showed that at 2 h, there was no difference in brain concentrations in rats that received 1 or 2 mg/kg DLM, however all other comparisons at all other hours showed significant differences (p’s < .002–.0001; asterisks not used for clarity of presentation). The mean ± SEM for each DLM dose averaged across all intervals was: 1 mg/kg = 36.6 ± 4.0 ng/g; 2 mg/kg = 65.6 ± 4.0 ng/g; and 4 mg/kg = 121.3 ± 4.1 ng/g.
For plasma levels in adult male rats, there were effects of dose, F(2, 79) = 15.29, p < .0001, hour (p < .0001), and dose × hour, F(6, 79) = 3.19, p < .008 (Figure 3C). All doses were significantly different at 2 h (p’s < .05–.0001). The mean ± SEM for each DLM dose averaged across intervals was: 2 mg/kg = 121.1 ± 66.1 ng/ml; 8 mg/kg = 342.4 ± 65.0 ng/ml; and 25 mg/kg = 636.1 ± 66.1 ng/ml. For brain levels of DLM in adult rats, there was an effect of dose, F(2, 78) = 8.36, p < .0005 (Figure 3D) but not of hour. The dose × hour interaction approached significance (p < .08). Across time intervals, the DLM 2 mg/kg and 8 mg/kg groups differed from the DLM 25 mg/kg group (p < .0006 and p < .02, respectively), with no difference between the 2 and 8 mg/kg groups. The mean ± SEM for each DLM dose averaged across intervals was: 2 mg/kg = 32.6 ± 10.2 ng/g; 8 mg/kg = 46.2 ± 10.1 ng/g; and 25 mg/kg = 88.9 ± 10.1 ng/g.
DISCUSSION
In the first experiment, no effects of DLM on ASR in adult rats at doses up to 8 mg/kg in a dosing volume of 5 ml/kg CO were seen, nor were there differences in salivation or tremor. Compared with these results, no effects of DLM on ASR with doses up to 4 mg/kg in 0.2 ml/kg in adult rats were seen 2 h after dosing in other studies (Crofton and Reiter, 1984; Hijzen and Slangen, 1988). However, decreased ASR in adult rats was found 1.5 h after dosing with 6 mg/kg DLM in 0.2 ml/kg CO (Crofton and Reiter, 1984, 1987; Hijzen and Slangen, 1988) and at 2 h after dosing with 2, 4, or 6 mg/kg DLM in 1 ml/kg CO (Sheets et al., 1994). The ED50 for DLM in CO (0.2 ml/kg) in adult rats for ASR was determined to be 6 mg/kg in one study (Crofton and Reiter, 1987). There are a number of differences that might account for the lack of effect of DLM in experiment 1 here. One is that the dosing volume was 5 ml/kg, whereas it was 0.2–1 ml/kg in previous studies, thus potentially affecting the uptake of DLM from the digestive tract (Mortuza et al., 2018; Wolansky et al., 2007). Another difference is the strain of rats: Sprague Dawley versus Wistar versus Long-Evans. However, the dosing volume may be the biggest factor between studies because Hijzen and Slangen (1988) saw increased salivation, pawing, and burrowing in Wistar rats dosed with 4 or 6 mg/kg DLM in 0.2 ml/kg CO, whereas no differences in salivation were seen in Experiment 1 with up to 8 mg/kg in 5 ml/kg CO. The use of a 2-day ASR method decreased variability and increased the sensitivity on the test day. For example, when we gave adult rats a day of ASR testing with CO the day before testing them with 8 or 25 mg/kg DLM, ASR was decreased for up to 4 h with return to control levels by 6 h. Thus, even with a greater dosing volume, DLM produced the expected decrease in ASR when rats were first matched for ASR responsiveness and the effect was observed at a later interval.
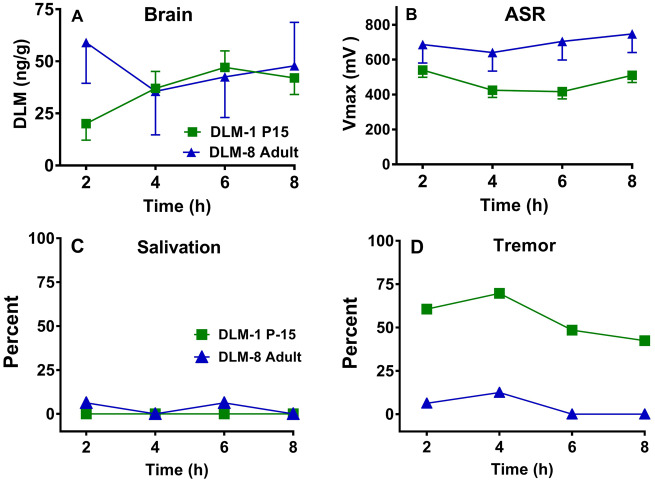
For the P15 rats, the effects of DLM were protracted in that decreases in ASR were observed even 8 h post-administration at all dose levels. This is the first study to show that DLM has persistent effects on ASR in young rats. In comparison with adults, P15 rats clearly had a greater response to DLM. Examination of the brain levels of DLM (cf. Figs. 3B and 3D) shows that at the same administered dose of 2 mg/kg, brain levels were greater at P15 compared with adults. However, brain levels of DLM alone do not entirely explain the differences across age. For example, Figure 4 A shows the brain levels of DLM over 8 h after administration of 1 mg/kg in the P15 or 8 mg/kg in adult rats. Levels were comparable from 4 to 8 h, but in rats tested for ASR (Figure 4B) the 1 mg/kg P15 group had reduced ASR at 6 and 8 h, whereas the 8 mg/kg adult male rats had no differences compared with CO controls. In contrast, for salivation, the 1 mg/kg P15 rats had comparable responses as the 8 mg/kg adult rats (Figure 4C). At the same time, very little tremor was observed in the 8 mg/kg adults, but tremor was observed in ∼40%–70% of the 1 mg/kg P15 rats, dependent upon time interval (Figure 4D). Taken together, at comparable whole-brain tissue concentrations of DLM, P15 pups showed greater responses than adults for tremor and ASR.
Figure 4.
Comparisons of DLM effects at comparable brain concentrations as a function of age and time after treatment. A, P15 DLM brain levels in the 1 mg/kg group compared with similar levels in adults in the 8 mg/kg group. B, Based on panel A, ASR in the DLM P15 1 mg/kg group compared with the adult 8 mg/kg group. C, Comparison of percent of DLM 1 mg/kg P15 versus DLM 8 mg/kg adult rats showing salivation (same groups as in panels A and B). D, Comparison of percent of DLM 1 mg/kg P15 versus DLM 8 mg/kg adult rats showing tremor (same groups as in panel A, B, and C). Number of rats/group as above.
The greatest peak brain level of DLM in neonates was ∼151 ng/g, 6 h after 4 mg/kg, whereas the peak in adult rats was ∼156 ng/g, 2 h after 25 mg/kg DLM. In a previous study, brain levels of DLM were measured at the first sign of salivation, tremor, or choreoathetosis. After an intraperitoneal injection of 8 mg/kg DLM in adult rats, salivation occurred when brain levels reached ∼60 ng/g, tremor at ∼114 ng/g, and choreoathetosis/writhing at ∼191 ng/g (Rickard and Brodie, 1985). Furthermore, latency to each symptom was: salivation: ∼29 min, tremor: ∼65 min, and writhing: ∼85 min after 8 mg/kg DLM. In agreement with Rickard and Brodie (1985), no rats showed writhing/choreoathetosis at the brain levels obtained herein. Also in agreement with Rickard and Brodie (1985), adult rats in the 25 mg/kg group had the most salivation when brain levels were over 60 ng/g and the most tremor when levels were over or near 100 ng/g. P15 rats also showed salivation in the 2 and 4 mg/g DLM groups when brain levels exceeded 60 ng/g; however, in the P15 rats tremor was observed after DLM at brain levels that were less than 100 ng/g. Some tremor was noted in the CO group. P15 pups may have shivered or had head wobbling, given that almost 20% of CO controls showed head movement in the first 6 h after CO gavage. At P15 rats exhibit shivering to maintain temperature homeostasis when removed from the dam (Alberts, 1978). Although not a factor in the CO group, it is known that body temperature can be reduced by DLM in adults (Weiner et al., 2009) and in young rats it can be reduced by cypermethrin, another Type II pyrethroid (Bardullas et al., 2015). Perhaps body temperatures were lower in the P15 pups, such that shivering combined with the temperature lowering may have contributed to higher tremor responses at this age especially in the DLM groups compared with CO pups. Future studies should assess body temperatures to resolve this issue or maintain rats in a normothermic environment to alleviate this concern.
One limitation of the experiment is not having levels of DLM in the same rats assessed for ASR so that correlations could be made between brain levels and ASR. However, although that type of study design would permit correlations at each interval, it would require separate rats at each of the intervals. The serial ASR data show that little intersession habituation to the stimulus occurs for up to 8 h, making this a valuable way of studying time-course effects. Furthermore, rats used for plasma/tissue levels were not pretreated with CO the day prior to dosing, as was done in the rats given ASR. CO pretreatment 24 h prior is unlikely to have any effects on plasma or brain levels of DLM, therefore, we did not include this control procedure. Another consideration is that rats were tested only up to 8 h after DLM exposure. Therefore, we were not able to determine how long the effects in the P15 rats lasted. Investigation of the full time course of DLM on ASR in P15 rats may be informative. Because there is a steep LD50 for DLM in young rats (Sheets et al., 1994), rats of other ages should also be evaluated. Another limitation is the acute exposure in rats compared with the prolonged exposure that occurs in humans. The exposure routes of DLM for humans are predominately through ingestion (diet or other ingestion), dermal, or inhalation and occurs on a chronic basis. DLM detected in hair samples was not different between occupational workers exposed directly to DLM compared with a control population not directly exposed, suggesting that DLM exposure is pervasive (Lehmann et al., 2018). There is some concern for the long-term effects of DLM during early childhood development on cognitive ability (Viel et al., 2015), and therefore chronic studies in rats should be considered in the future. There are many factors that must be considered to extrapolate data from P15 rats to children. An important factor is the difference in brain development. Neurogenesis in rats continues during the first 3 weeks after birth at rates higher than later in life, whereas in humans most neurogenesis occurs before birth (Bayer et al., 1993; Clancy et al., 2001, 2007). Determining the effect of DLM at different ages in rats should be encouraged. Another factor to consider is that only whole brain levels of DLM were assessed here and it is known that DLM has regional effects in the brain (Beghoul et al., 2017; Gasmi et al., 2017; Yadav et al., 2006). Therefore, regional DLM determinations should be done. In the case of ASR, the caudal pontine reticular nucleus that mediates this reflex (Koch, 1999) should be assessed.
These experiments show that P15 rats have protracted ASR changes and tremor after oral DLM compared with adult rats. These age differences are not the result of differences in brain concentrations of DLM because differences remain when brain concentrations are comparable. These differences in tremor and ASR lead to the conclusion that there are age-dependent differences in sensitivity after acute oral exposure to DLM that remain to be identified. Methodologically, ASR pretesting, with a day of acclimation before the test compound is given, made it possible to assign rats to groups matched for ASR. This, in turn, reduced variability and increased ASR sensitivity as shown for permethrin (Williams et al., 2018). The detection of ASR changes after known neurotoxins at various ages of rats is important and demonstrates why ASR has been used in test batteries to determine neurotoxicity for a variety of chemicals (Tilson, 1990). The ability to identify chemicals that produce neurotoxicity to a greater extent based upon age is important to protect the most vulnerable from exposure to these chemicals.
ACKNOWLEDGMENTS
We thank Adam Fritz and Chiho Sugimoto for dosing and testing the rats used in this research. We also appreciate the input of Drs Larry Sheets, Dan Minnema, and Thomas Osimitz. These experiments were funded by the Council for the Advancement of Pyrethroid Human Risk Assessment, LLC (CAPHRA).
FUNDING
This project was supported by a Research Agreement between the Council for the Advancement of Pyrethroid Human Risk Assessment, LLC (CAPHRA) and Cincinnati Children’s Research Foundation that granted Drs Williams and Vorhees intellectual freedom to publish the data as they deemed most appropriate.
REFERENCES
- Alberts J. R. (1978). Huddling by rat pups: Group behavioral mechanisms of temperature regulation and energy conservation. J. Comp. Physiol. Psychol. 92 , 231–245. [DOI] [PubMed] [Google Scholar]
- Bardullas U., Sosa-Holt C. S., Pato A. M., Nemirovsky S. I., Wolansky M. J. (2015). Evidence for effects on thermoregulation after acute oral exposure to type I and type II pyrethroids in infant rats. Neurotoxicol. Teratol. 52 , 1–10. [DOI] [PubMed] [Google Scholar]
- Bayer S. A., Altman J., Russo R. J., Zhang X. (1993). Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology 14 , 83–144. [PubMed] [Google Scholar]
- Beghoul A., Kebieche M., Gasmi S., Chouit Z., Amiour C., Lahouel A., Lakroun Z., Rouabhi R., Fetoui H., Soulimani R. et al. (2017). Impairment of mitochondrial integrity and redox status in brain regions during a low-dose long-term exposition of rats to pyrethrinoids: The preventive effect of quercetin. Environ. Sci. Pollut. Res. Int. 24 , 19714–19722. [DOI] [PubMed] [Google Scholar]
- Bloom A. S., Staatz C. G., Dieringer T. (1983). Pyrethroid effects on operant responding and feeding. Neurobehav. Toxicol. Teratol. 5 , 321–324. [PubMed] [Google Scholar]
- Clancy B., Darlington R. B., Finlay B. L. (2001). Translating developmental time across mammalian species. Neuroscience 105 , 7–17. [DOI] [PubMed] [Google Scholar]
- Clancy B., Finlay B. L., Darlington R. B., Anand K. J. S. (2007). Extrapolating brain development from experimental species to humans. Neurotoxicology 28 , 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. J., Brooks M. W. (1989). Aquatic toxicology of the pyrethroid insecticides: Neurotoxicology of pyrethroids: Single or multiple mechanisms of action? Environ . Toxicol. Chem. 8 , 361–372. [Google Scholar]
- Crofton K. M., Reiter L. W. (1984). Effects of two pyrethroid insecticides on motor activity and the acoustic startle response in the rat. Toxicol. Appl. Pharmacol. 75 , 318–328. [DOI] [PubMed] [Google Scholar]
- Crofton K. M., Reiter L. W. (1988). The effects of type I and II pyrethroids on motor activity and the acoustic startle response in the rat. Fundam. Appl. Toxicol. 10 , 624–634. [DOI] [PubMed] [Google Scholar]
- Crofton K. M., Reiter L. W. (1987) Pyrethroid insecticides and the gamma-aminobutyric acidA receptor complex: Motor activity and the acoustic startle response in the rat. J. Pharmacol. Exp. Ther. 243 , 946–954. [PubMed] [Google Scholar]
- Gasmi S., Rouabhi R., Kebieche M., Boussekine S., Salmi A., Toualbia N., Taib C., Bouteraa Z., Chenikher H., Henine S. et al. (2017). Effects of deltamethrin on striatum and hippocampus mitochondrial integrity and the protective role of quercetin in rats. Environ. Sci. Pollut. Res. Int. 24 , 16440–16457. [DOI] [PubMed] [Google Scholar]
- Hijzen T. H., Slangen J. L. (1988). Effects of type I and type II pyrethroids on the startle response in rats. Toxicol. Lett. 40 , 141–152. [DOI] [PubMed] [Google Scholar]
- Koch M. (1999). The neurobiology of startle. Prog. Neurobiol. 59 , 107–128. [DOI] [PubMed] [Google Scholar]
- Lehmann E., Oltramare C., Nfon Dibié J.-J., Konaté Y., de Alencastro L. F. (2018). Assessment of human exposure to pesticides by hair analysis: The case of vegetable-producing areas in Burkina Faso. Environ. Int. 111 , 317–331. [DOI] [PubMed] [Google Scholar]
- Mortuza T. et al. (2018). Toxicokinetics of deltamethrin: Dosage dependency, vehicle effects, and low-dose age-equivalent dosimetry in rats. Toxicol. Sci. 162 , 327–336. [DOI] [PubMed] [Google Scholar]
- Peele D. B., Crofton K. M. (1987). Pyrethroid effects on schedule-controlled behavior: Time and dosage relationships. Neurotoxicol. Teratol. 9 , 387–394. [DOI] [PubMed] [Google Scholar]
- Rickard J., Brodie M. E. (1985). Correlation of blood and brain levels on the neurotoxic pyrethroid deltamethrin with the onset of symptoms in rats. Pesticide Biochem. Physiol. 23 , 143–156. [Google Scholar]
- Shafer T. J., Meyer D. A., Crofton K. M. (2005). Developmental neurotoxicity of pyrethroid insecticides: Critical review and future research needs. Environ. Health Perspect. 113 , 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets L. P. (2000). A consideration of age-dependent differences in susceptibility to organophosphorus and pyrethroid insecticides. Neurotoxicology 21, 57–63. [PubMed] [Google Scholar]
- Sheets L. P., Doherty J. D., Law M. W., Reiter L. W., Crofton K. M. (1994). Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol. Appl. Pharmacol. 126 , 186–190. [DOI] [PubMed] [Google Scholar]
- Soderlund D. M. (2012). Molecular mechanisms of pyrethroid insecticide neurotoxicity: Recent advances. Arch. Toxicol. 86 , 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund D. M., Clark J. M., Sheets L. P., Mullin L. S., Piccirillo V. J., Sargent D., Stevens J. T., Weiner M. L. (2002). Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology 171 , 3–59. [DOI] [PubMed] [Google Scholar]
- Stappenbeck T. S., Virgin H. W. (2016). Accounting for reciprocal host-microbiome interactions in experimental science. Nature 534 , 191–199. [DOI] [PubMed] [Google Scholar]
- Tilson H. A. (1990). Behavioral indices of neurotoxicity. Toxicol. Pathol. 18 , 96–104. [DOI] [PubMed] [Google Scholar]
- Verschoyle R. D., Aldridge W. N. (1980). Structure-activity relationships of some pyrethroids in rats. Arch. Toxicol. 45 , 325–329. [DOI] [PubMed] [Google Scholar]
- Viel J.-F., Warembourg C., Le Maner-Idrissi G., Lacroix A., Limon G., Rouget F., Monfort C., Durand G., Cordier S., Chevrier C. et al. (2015). Pyrethroid insecticide exposure and cognitive developmental disabilities in children: The PELAGIE mother-child cohort. Environ. Int. 82 , 69–75. [DOI] [PubMed] [Google Scholar]
- Vorhees C. V., Herring N. R., Schaefer T. L., Grace C. E., Skelton M. R., Johnson H. L., Williams M. T. (2008). Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: Effects of dose and rearing conditions. Int. J. Dev. Neurosci. 26 , 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. L., Nemec M., Sheets L., Sargent D., Breckenridge C. (2009). Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. Neurotoxicology 30 , S1–16. [DOI] [PubMed] [Google Scholar]
- Williams D. R., Carlsson R., Burkner P. C. (2017). Between-litter variation in developmental studies of hormones and behavior: Inflated false positives and diminished power. Front. Neuroendocrinol. 47 , 154–166. [DOI] [PubMed] [Google Scholar]
- Williams M. T., Gutierrez A., Vorhees C. V. (2018). Effects of acute exposure of permethrin in adult and developing Sprague-Dawley rats on acoustic startle response and brain and plasma concentrations. Toxicol. Sci. 165 , 361–371. [DOI] [PubMed] [Google Scholar]
- Wolansky M. J., Harrill J. A. (2008). Neurobehavioral toxicology of pyrethroid insecticides in adult animals: A critical review. Neurotoxicol. Teratol. 30 , 55–78. [DOI] [PubMed] [Google Scholar]
- Wolansky M. J., Tornero-Velez R. (2013). Critical consideration of the multiplicity of experimental and organismic determinants of pyrethroid neurotoxicity: A proof of concept. J. Toxicol. Environ. Health B 16 , 453–490. [DOI] [PubMed] [Google Scholar]
- Wolansky M. J., McDaniel K. L., Moser V. C., Crofton K. M. (2007). Influence of dosing volume on the neurotoxicity of bifenthrin. Neurotoxicol. Teratol. 29 , 377–384. [DOI] [PubMed] [Google Scholar]
- Yadav S., Johri A., Dhawan A., Seth P. K., Parmar D. (2006). Regional specificity in deltamethrin induced cytochrome P450 expression in rat brain. Toxicol. Appl. Pharmacol. 217 , 15–24. [DOI] [PubMed] [Google Scholar]