Abstract
Objective
To determine the non-inferiority of nurse-led care (NLC) in patients with anticitrullinated protein antibody (ACPA)-positive and/or rheumatoid factor (RF)-positive rheumatoid arthritis (RA) with active disease who are starting disease-modifying antirheumatic drug therapy, following treat-to-target (T2T) recommendations.
Methods
A multicentre, pragmatic randomised controlled trial was conducted to assess clinical effectiveness, anxiety, depression and patient satisfaction following a non-inferiority design. The participants were 224 adults with ACPA/RF-positive RA who were randomly assigned to either NLC or rheumatologist-led care (RLC). The primary outcome was the Disease Activity Score in 28 Joints measured with C reactive protein (DAS28-CRP) assessed at baseline and after 3, 6, 9 and 12 months. A DAS28-CRP difference of 0.6 was set as the non-inferiority margin. Mean differences between the groups were assessed following per-protocol and intention-to-treat strategies.
Results
Demographic data and baseline characteristics of patients in the NLC group (n=111) were comparable to those of patients in the RLC group (n=113). The improvement in disease activity (change in DAS28-CRP, primary outcome) over the course of 12 months was significant in both groups (p<0.001). No significant differences were observed between the NLC and RLC groups (p=0.317). Non-inferiority of NLC was shown for the primary outcome and all secondary outcomes.
Conclusion
This study supported the non-inferiority of NLC in managing T2T and follow-up care of patients with RA with moderate to high disease activity and poor prognostic factors in addition to RLC.
Trial registration number
DRKS00013055.
Keywords: nursing, health services research, outcomes research, rheumatoid arthritis
Key messages.
What is already known about this subject?
There is evidence of non-inferiority of nurse-led care (NLC) in comparison to rheumatologist-led care, which focuses mainly on patients with low risk of disease progression.
What does this study add?
This study supports the non-inferiority of NLC in managing treat-to-target (T2T) and follow-up care of patients with rheumatoid arthritis with moderate to high disease activity and poor prognostic factors in addition to rheumatologist-led care.
How might this impact on clinical practice or further developments?
T2T in the early stages of the disease and/or after modification of therapy can include NLC as a part of the rheumatology team.
Adding nurses to the multidisciplinary team may not only close the gap of an unmet demand for rheumatologists but also provide an important added value of care.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease that can have a significant impact on patients’ physical, psychological and social functioning. The prevalence of RA in Germany is estimated to be 1%.1 Increased life expectancy will lead to an even higher prevalence of RA in the future, creating an increased need for treatment and continuous medical care.
The primary treatment goal is to achieve remission of disease in order to prevent structural joint damage and to improve patients’ quality of life.2 RA is a disease that progresses over time, frequently resulting in impairment in everyday life, leading to fatigue and psychological distress.3 In addition, up to 70% of patients show at least one poor prognostic factor,4 such as the presence of rheumatoid factor (RF) and/or anticitrullinated protein antibodies (ACPAs), and 20%–30% of patients do not respond favourably to current treatment options.5
To improve the outcomes of patients with RA, especially those with poor prognostic factors, early diagnosis and initiation of pharmacological treatment, along with quick therapy adjustments according to disease activity, are crucial.2 The increasing availability of highly effective therapies and the optimisation of current standards of care have led to a better achievement of treatment goals.6 7 The current EULAR RA management recommendations suggest a frequent monitoring in patients with active disease every 1–3 months.2 However, management strategies such as treat-to-target (T2T) require frequent patient visits, at least in the early stages of the disease and/or after modification of therapy.
To ensure adequate care, multidisciplinary teams that include clinical nurse specialists (CNSs) are required to successfully manage these patients to provide alternatives to conventional outpatient-based rheumatologist-led care (RLC). The current EULAR recommendations for the treatment of RA include the involvement of specialist nurses in the follow-up of patients with RA.8 Nurse-led care (NLC) has already been successfully established in some countries (eg, the UK, the Netherlands, Denmark and Sweden),9–14showing evidence of non-inferiority of NLC in comparison to RLC in different clinical settings.11 Furthermore, evidence suggests an added value of a holistic perspective and an increased focus on individual patients needs, and this has been shown to be appreciated by patients.15–19 However, the majority of previous trials either focused on patients in remission or have included a substantial number of patients without poor prognostic factors. The under-representation of patients with active disease and poor prognostic factors has raised the concern that these trials on NLC might have been underpowered to detect significant differences regarding the safety and efficacy of NLC in patients with higher demands.20 In addition, EULAR has recently updated the recommendations for the role of nurses in the treatment of inflammatory arthritis,21 22 raising the need for further scientific evidence in this challenging patient group.
Although previous trials have shown encouraging results for NLC, these results cannot be readily transferred to all countries on account of differences in the health systems and regulatory frameworks. Ambulatory specialist care in Germany is delivered by physicians in outpatient clinics. Outpatient services in this context refer to specialist care, mostly in private practice. However, the role of hospitals in this sector is limited. Registration with a primary care physician is not required, and general practitioners currently have no formal gatekeeper functions. Patients have a free choice of ambulatory care physicians and hospitals. Physicians in ambulatory care are generally reimbursed on a fee-for-service basis. Health insurance is compulsory and provides nearly universal healthcare coverage. The statutory health insurance system currently consists of 103 sickness funds, which are autonomous, not-for-profit, non-governmental bodies covered by law, and cover 87% of the population (11% private health insurance and 2% special regimens). They are funded by compulsory contributions that stem from calculated percentages of gross wages, equally shared by employers and employees.23 24
The aim of this study was to compare the 1-year treatment outcomes in patients with ACPA/RF-positive RA with RLC and NLC using a non-inferiority design.
Methods
Study design
This study was conducted as a multicentre pragmatic randomised controlled trial (RCT). The duration of the intervention was 12 months. The participants had five follow-up visits (baseline and weeks 6 (week 8 optional), 12, 24, 36 and 52) after their recruitment. The methods were in line with current guidelines regarding the design, conduct and analysis of pragmatic RCTs.25 26 The assessment of primary and secondary outcomes followed a non-inferiority design. The study was conducted in eight rheumatology outpatient clinics in Germany.
Patient involvement
Patients and members of the public were involved in the planning and conduction of the trial. Before the trial started, members of the German self-support patient group in rheumatology, the Rheuma-Liga Niedersachsen e.V.,27 identified this research as being very important for improving the care of patients with chronic inflammatory arthritis. In addition, useful advice was received from patients of various outpatient clinics, including advice about the design and management of the trial. In the early stages of the trial, patients and members of the public received a questionnaire regarding the acceptance of NLC as a general idea. The results of this trial will be shared with the participants, other patients and the public through the website and journal of the Rheuma-Liga Niedersachsen e.V.27 once it has been published.
Participants and randomisation
From January to August 2018, patients with RA were recruited from eight centres across Germany. All centres had rheumatologists providing outpatient specialist care, reflecting the current care of patients with RA in Germany. The study centres differed in size (range one-eight rheumatologists). Patients were recruited at their regular care visit with their rheumatologist at the outpatient clinic. Due to the requirement of introduction, change or escalation of therapy, a preselection was not possible. If patients met the inclusion criteria, the rheumatologist informed the patient about the study at this visit. After obtaining written informed consent, the patients were randomised 1:1 to either the NLC group (intervention) or the RLC group (standard of care/control), repectively. This visit was the baseline visit for both groups. Each centre was asked to recruit 30 patients to avoid centre bias. Each centre received 30 envelopes (intervention group: 15, control group: 15) that were opened randomly by the nurse to include the patients in either the NLC or the RLC group. The inclusion criteria were as follows: written consent to participate in the study, a diagnosis of ACPA/RF-positive RA (American College of Rheumatology (ACR)/EULAR criteria)28 and being at least 18 years of age. There was no distinction between early and established RA. Patients were eligible if therapy was initiated because of a new diagnosis of ACPA/RF-positive RA. In addition, patients with a former diagnosis of ACPA/RF-positive RA met the inclusion criteria if their current therapy was escalated or changed. The exclusion criteria were as follows: presence of severe comorbidities, insufficient ability to speak the German language, inability to give informed consent and foreseeability of patient unavailability for the full study duration.
NLC (intervention group)
Per centre, one CNS was assigned to participate in this study. Each of the eight participating CNSs had more than 10 years of experience in rheumatology. In addition to their basic nursing training of 3 years, all CNSs were required to have participated in an advanced 60-hour training that resulted in the certification ‘CNS in Rheumatology’.29 Important sessions, among others, included theoretical and practical exercises to practice joint assessments in healthy and sick individuals. Furthermore, a training at their outpatient clinic was required to participate in this study. The training focused on taking the patients’ history and performing joint assessments and had to be applied in the outpatient setting at least 15 times for each chosen centre.
The allocated time slot for NLC was 30 min. Patients were seen by their CNS at weeks 6 (T2T visit), 12 and 36, respectively. At weeks 24 and 52, the patients attended a regular visit with the rheumatologist. During the allocated time slot, the CNS took the patient’s history, performed the physical examination and joint assessments, evaluated blood tests, evaluated the Disease Activity Score in 28 Joints measured with C reactive protein (DAS28-CRP), screened for comorbidities, and monitored the medication in terms of therapeutic effect and side effects. However, the main focus of the patient visit was on overall well-being in everyday life, addressing mental health issues and the potential need for support. This included assessment of work participation, assessment of the need for rehabilitation, and physical or occupational therapy. Patient education, especially about the newly introduced therapy, was performed by the CNS to increase knowledge about the medication, to impart training on the application of the medication, and to assess anxiety and overall concerns regarding the therapy.
After the T2T visit, the CNS discussed the results with the rheumatologist on the same day. The CNS consulted the rheumatologist while the patient was still in the clinic in case of side effects or questions. At weeks 12 and 36, a brief contact with a rheumatologist was obligatory due to the current legal requirements.30 If no further questions or problems arose, this contact was only a formality. The necessity for this requirement was also assessed accordingly. Since the CNSs were formally not permitted to make dose adjustments and drug changes or to prescribe referrals to other health professionals, such as physiotherapists or occupational therapists, the rheumatologist needed to sign the corresponding prescriptions. However, the nurse could offer her assessment on whether a change in therapy or dose adjustment was required and whether other members of the multidisciplinary team should be included in the treatment, based on her preparatory work during the consultation.
RLC (control group)
The patients randomised to the RLC continued the follow-up according to the usual standard of care with planned appointments every 3 months for 15–20 min with a senior rheumatologist. In addition, a short T2T was required to follow the study protocol. The usual RLC is a medical approach that includes taking history, physical examination, joint assessment, drug monitoring and evaluation of blood tests. The participants in this group were seen by the same rheumatologist during the study period.
In both groups, the patients could get additional appointments between planned consultations in case of flare-ups, and both groups were offered an additional T2T visit 8 weeks after baseline.
Outcome measures
Primary and secondary measures
The primary outcome measure was the change in DAS28-CRP over the course of 12 months. The data were collected at each visit.
The secondary outcome measures were patient-reported outcomes (PROs), including work ability, functionality, satisfaction, health-related quality of life, depression and anxiety. The Rheumatoid Arthritis Disease Activity Index (RADAI) was administered, in addition to the DAS28-CRP, to obtain information about the patients’ assessment of disease activity. The cut-off score for safety alerts for the RADAI was set at ≥5.6 (high disease activity).31 The ‘Funktionsfragebogen Hannover’ (FFbH) was used to assess functionality when carrying out everyday activities.32 Health-related quality of life was measured using the Rheumatoid Arthritis Impact of Disease Index, which included physical, psychological and social dimensions.33 34 Depression and anxiety were assessed using the Hospital Anxiety and Depression Scale (HADS).35 36 The cut-off scores for both, HADS—anxiety and HADS—depression were set at ≥8. The threshold for safety alerts was set at ≥11 (abnormal depression).37 38
Patient satisfaction with outpatient care was measured using the German questionnaire ‘Patient Satisfaction with Outpatient Care’ ('Fragebogen zur Zufriedenheit in der ambulanten Versorgung', satisfaction about outpatient care (ZAP)).39 40 After contacting the authors, the wording ‘physician’ was changed to a general term to include nurses. Secondary outcome measures were collected at baseline, after 6 months and after 12 months. The patients were asked to fill out the questionnaires during their visits of 6 and 12 months visits before their appointment with the physician.
Sample size calculation
The G*Power41 programme was used to calculate the required sample size. Based on a one-sided repeated-measures analysis of variance (ANOVA), an α of 0.025, a power of 90, an SD of 1.5, an intraclass correlation of 0.5,42 an effect size of d=0.4,43 1:1 randomization, five time points and a 10% dropout, 204 patients had to be included in the study. Because NLC was not common in Germany, a higher dropout rate was anticipated for the study. It was planned to include 240 patients in total, with 120 patients in each group.
Additional power calculations were performed for the secondary outcome measures. Based on the Wilcoxon signed-rank test for matched pairs, a total sample size of 73 was needed, allowing a dropout rate of 10%, considering 95% power, two-sided testing with a 5% significance level, and an effect size of 0.4.44
Statistical analysis
Statistical analyses were performed using the IBM SPSS V.25. The analysis followed per-protocol (PP) and intention-to-treat (ITT) strategies.25 For the PP approach, all participants who completed all visits were evaluated, providing a complete case analysis. For the ITT strategy, multiple imputations using chained equations were used. Missing data were tested at random, and 10 imputed datasets were computed.45 A repeated-measures mixed ANOVA model was used to measure changes in the primary outcome. Summary estimates were computed to show the average pooled differences between the two groups over the follow-up period at all time points. It was tested whether the time of measurement (within-subject effects), randomisation, study centre (between-group effects) and the interaction between the randomization and time of measurement (randomization×time), and the interaction of randomisation and centre (randomization×centre) influenced the outcome. Bonferroni correction was used to adjust for multiple comparisons.
For the DAS28-CRP, a value of 0.6 was set as the margin for inferiority/non-inferiority. A change of 1.2 points was considered to be clinically important.46 The null hypothesis (inferiority) stated: mean ΔDAS28RLC−mean ΔDAS28NLC≥0.6, where Δ equals the change from the baseline value.
The results of the Shapiro-Wilk test showed that the secondary outcomes are not normally distributed. The Wilcoxon signed-rank test and the Mann-Whitney U test were conducted to study the change over time and to test for non-inferiority based on a margin of 0.4.
Differences in proportion over time were explored using the McNemar test.
Results
A total of 272 patients out of 891 were eligible and were invited to participate in the study. Thirty-six patients declined to participate (reasons were wishing to remain in regular care, living far away and declining the additional T2T visit, not wanting to complete questionnaires, and not wanting to prolong the visit in the outpatient clinic due to their family or work situation). A total of 236 patients were screened, but after verification of the inclusion criterion ‘seropositivity’, 12 patients did not meet this requirement. A total of 224 patients finally were randomly assigned to either the NLC or the RLC group (figure 1).
Figure 1.
Trial profile. #, patients seronegative; ##, difference not statistically significant; *, PP analysis based on patients attending all study visits and having completed DAS28-CRP; **, ITT analysis based on multiple imputation of the missing DAS28-CRP. DAS28-CRP, Disease Activity Score in 28 Joints measured with C reactive protein; ITT, intention to treat; NLC, nurse-led care, PP, per protocol; RLC, rheumatologist-led care.
Of these, 89.2% (99/111) of patients receiving NLC and 94.7% (107/113) of patients receiving RLC completed the study. The dropout rate was found to be 8%. Categories of dropouts were withdrawal of consent, death, moving away, non-compliance and not attending for other reasons (figure 1). Withdrawal of consent in the NLC group was not related to the intervention. Two patients in the NLC group died during the study period. An 82-year-old patient treated with 10 mg leflunomide died of acute renal failure, presumably caused by an infection, and a 72-year-old patient with several cardiovascular risk factors died suddenly. Demographic and baseline characteristics of the patients who did not complete the study did not differ significantly from those of the study group, with the exception that the participants who dropped out were less educated (p=0.03).
Demographic and baseline characteristics did not differ between the study groups. A total of 81% (90/111) of patients in the NLC group and 77% (87/113) of patients in the RLC group attended all five visits. Complete data of all five visits were available for 173 patients (77 %) (PP analysis). The study population resembled the general population of patients with RA, with 74% being female and a mean age of 58 years.47 The baseline characteristics of the participants are shown in table 1.
Table 1.
Baseline characteristics of study population stratified by study group
| RLC (n=113) | NLC (n=111) | Total (224) | |
| Women, n (%) | 86 (76.8) | 80 (72.1) | 166 (74.4) |
| Age (years), mean (SD) | 58.10 (11.54) | 58.81 (12.03) | 58.57 (11.88) |
| RF-positive (n) | 105 | 101 | 206 |
| ACPA-positve (n) | 96 | 96 | 192 |
| Employed, n (%) (112, 110)* | 51 (45.5) | 54 (49.1) | 105 (47.3) |
| Education (92, 94)* | |||
| No secondary school, n (%) | 89 (96.7) | 83 (88.3) | 172 (77.10) |
| Secondary school, n (%) | 3 (3.3) | 11 (11.7) | 14 (6.30) |
| Professional training (111,109)* | |||
| None, n (%) | 18 (16.2) | 16 (14.7) | 34 (15.2) |
| Vocational, n (%) | 85 (76.6) | 79 (72.41) | 164 (73.5) |
| University degree, n (%) | 8 (7.2) | 14 (12.8) | 22 (9.9) |
| Therapy regimen | |||
| New therapy, n (%) | 41 (36.6) | 28 (25.2) | 69 (30.9) |
| Change of therapy, n (%) | 40 (35.7) | 49 (44.1) | 89 (39.9) |
| Dose escalation, n (%) | 31 (27.7) | 34 (30.6) | 65 (29.1) |
| Disease duration (years), median (IQR) (111, 109) | 5.83 (2.5–13) | 7.67 (2.63–18.79) | 6.24 (2.6–26.19) |
| Baseline RA regimen, n (%) | |||
| Glucocorticoids (103,104)* | 39 (37.8) | 39 (37.5) | 78 (37.7) |
| Methotrexate (103, 105) | 35 (34.0) | 41 (39.0) | 76 (36.5) |
| Leflunomide (103, 104)* | 14 (13.6) | 11 (10.6) | 25 (12.1) |
| Sulfasalazine (103, 104)* | 4 (3.9) | 4 (3.8) | 8 (3.9) |
| Hydroxychloroquine (103, 104)* | 4 (3.9) | 2 (1.9) | 6 (2.9) |
| JAK inhibitors | 2 (1.8) | 0 (0) | 2 (0.9) |
| Biological DMARD | 22 (21.4) | 27 (25.9) | 49 (21.97) |
| Outcomes, median (IQR) | |||
| DAS28-CRP (110, 111) | 4.41 (3.48–5.07) | 4.51 (3.42–5.18) | 4.42 (3.48–5.16) |
| Tender joints | 6 (2–10) | 6 (2–12) | 6 (2–11) |
| Swollen joints | 3 (1–6) | 3 (1–6) | 3 (1–6) |
| Patient Global Health | 60 (43.5–74.75) | 60 (40–79) | 60 (42–75) |
| FFbH (111, 109)* | 77.78 (61.11–88.89) | 75 (52.78–88.89) | 77.78 (56.25–88.89) |
| RADAI (110, 110)* | 4.75 (3.14–5.82) | 4.70 (3.36–6.10) | 4.73 (3.23–5.99) |
| RAID total (112,110)* | 5.30 (3.67–6.90) | 5.32 (2.84–7.21) | 5.30 (3.34–7.07) |
| Pain (112, 110)* | 6 (4–8) | 6 (4–8) | 6 (4–8) |
| Fatigue (112, 110)* | 5 (3–7) | 5 (3–8) | 5 (3–8) |
| HADS (112, 109)* | |||
| Depression | 6 (3–9) | 4 (2–8) | 5 (2–8) |
| Anxiety | 6 (3–9) | 6 (3–10) | 6 (3–9) |
| ZAP | |||
| Trust (112, 110) | 4 (3–4) | 4 (4–4) | 4 (3–4) |
| Quality (110, 109) | 2 (2–3) | 3 (2–3) | 3 (2–3) |
| Satisfaction (110, 109) | 2 (2–3) | 3 (2–3) | 3 (2–3) |
*Numbers of available data were as per randomised allocation, that is, 113 for RLC and 111 for NLC unless otherwise stated in parentheses.
ACPA, anticitrullinated protein antibody; DAS28-CRP, Disease Activity Score in 28 Joints measured with C reactive protein; DMARD, disease-modifying antirheumatic drug; FFbH, Funktionsfragebogen Hannover; HADS, Hospital Anxiety and Depression Scale; NLC, nurse-led care; RA, rheumatoid arthritis; RADAI, Rheumatoid Arthritis Disease Activity Index; RAID, Rheumatoid Arthritis Impact of Disease; RF, rheumatoid factor; RLC, rheumatologist-led care; ZAP, 'Fragebogen zur Zufriedenheit in der ambulanten Versorgung', satisfaction about outpatient care.
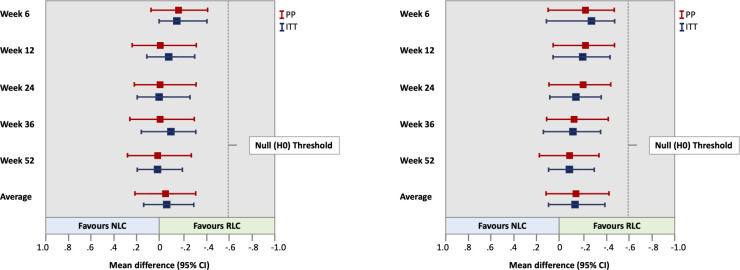
The improvement in disease activity (change in DAS28-CRP, primary outcome) over the course of 12 months was significant in both groups (p<0.001). No significant differences were observed between the NLC and RLC groups (p=0.317). The improvement in disease activity at week 6 was slighty better in the RLC group. At all other time points, the improvements were similar, with a very small mean difference. Non-inferiority was reached at all time points. Table 2 shows the summary estimates of the changes in DAS28-CRP over 12 months. Figure 2A illustrates the difference between the groups for PP and ITT for patients, according to the criteria for seropositive RA (ACR/EULAR criteria).28 Figure 2B shows the difference between the groups for PP and ITT for patients with RA having two or more factors linked to poor prognosis.2
Table 2.
Summary estimates for change in DAS28-CRP (primary outcome measure) over 12 months
| RLC | NLC | Difference* | |
| Mean (SD) | Mean (SD) | Mean (95% CI) | |
| Week 6 | |||
| PP | −1.37 (1.27) | −0.95 (1.25) | −0.15 (−0.41 to 0.11) |
| ITT | −1.35 (1.21) | −0.97 (1.22) | −0.17 (−0.41 to 0.06) |
| Week 12 | |||
| PP | −1.54 (1.21) | −1.31 (1.40) | −0.03 (−0.29 to 0.23) |
| ITT | −1.53 (1.53) | −1.34 (1.40) | −0.09 (−0.31 to 0.13) |
| Week 24 | |||
| PP | −1.58 (1.4) | −1.46 (1.59) | −0.04 (−0.29 to 0.21) |
| ITT | −1.56 (1.37) | −1.48 (1.57) | −0.02 (−0.23 to 0.19) |
| Week 36 | |||
| PP | −1.71 (1.34) | −1.58 (1.50) | −0.03 (−0.30 to 0.24) |
| ITT | −1.44 (1.20) | −1.26 (1.27) | −0.08 (−0.30 to 0.15) |
| Week 52 | |||
| PP | −1.84 (1.28) | −1.87 (1.35) | 0.02 (−0.23 to 0.27) |
| ITT | −1.86 (1.20) | −1.87 (1.32) | 0.02 (−0.19 to 0.19) |
| Average | |||
| PP | −1.61 (1.30) | −1.43 (1.42) | −0.05 (−0.30 to 0.21) |
| ITT | −1.55 (1.30) | −1.38 (1.36) | −0.07 (−0.29 to 0.14) |
Analysis of complete cases (number of DAS28-CRP responders: RLC group and NLC group).
Primary endpoint evaluation.
*Difference in mean DAS28-CRP change scores for the RLC group minus the NLC group (adjusted for centre).
DAS28-CRP, Disease Activity Score in 28 Joints measured with C reactive protein; ITT, intention to treat; NLC, nurse-led care; PP, per protocol; RLC, rheumatologist-led care.
Figure 2.
(A) Summary estimates for change in DAS28-CRP over 12 months. Mean difference is for RLC group minus NLC group. (B) Summary estimates for change in DAS-CRP over 12 months with two or more poor prognostic factors. Mean difference is for RLC group minus NLC group. DAS28-CRP, Disease Activity Score in 28 Joints measured with C reactive protein; ITT, intention to treat; NLC, nurse-led care; PP, per protocol; RLC, rheumatologist-led care.
The secondary outcomes are presented in table 3. NLC was associated with significant improvements in anxiety as measured by the HADS after 12 months (p=0.036, r=0.15). The proportion of patients with no anxiety in the overall study population changed significantly from 63.3% at baseline to 68.8% after 12 months (p=0.010). The proportion of patients in the RLC group did not change significantly (from 63.4% to 61.8%, p=0.500). In the NLC group, the proportion of patients with no anxiety changed significantly, from 63.3% at baseline to 76.3% after 12 months (p=0.001).
Table 3.
Summary estimates for secondary outcome measures over 12 months
| RLC | NLC | Difference | Effect size | |||||
| Baseline | Month 12 | Baseline | Month 12 | U | z | P value† | r | |
| Median (IQR)* | Median (IQR)* | |||||||
| FFbH (n)‡ |
77.78 (61.11–88.89) (111) |
80.56 (65.28–97.22) (101) |
75 (52.78–88.89) (109) |
83.33 (54.17–97.22) (97) |
4442.000 |
−0.905 | 0.365 | |
| HADS-D (n)‡ |
6 (3–9) | 5 (2.0–9.50) (101) |
4 (2–8) (109) |
3 (1–6) (97) |
4781.000 | −0.169 | 0.866 | |
| HADS-A (n)‡ |
6 (3–9) | 6 (2–9) (102) |
6 (3–10) (109) |
6 (3–10) (97) |
4056.000 | −2.101 | 0.036 | 0.15 |
| RAID, pain (n)‡ |
6 (4–8) | 3 (1, 5) (105) |
6 (4, 8) (110) |
3 (2, 5) (97) |
4829.500 | −0.639 | 0.523 | |
| RAID, fatigue (n‡) |
5 (3, 7) | 3 (1, 6) (106) |
5 (3, 8) (110) |
3 (1, 5) (97) |
4939.000 | −0.488 | 0.626 | |
| ZAP trust (n)‡ |
4 (3, 4) | 4 (3, 4) (102) |
4 (4, 4) (110) |
4 (4, 4) (97) |
4856.500 | −0.285 | 0.775 | |
| ZAP quality (n)‡ |
2 (2, 3) (110) |
3 (2, 3) (102) |
3 (2, 3) (109) |
3 (3, 3) (97) |
4506.500 | −1.074 | 0.283 | |
| ZAP satisfaction (n)‡ | 2 (2, 3) (110) |
3 (2, 3) (102) |
3 (2, 3) (109) |
3 (3, 3) (97) |
4722.000 | −0.399 | 0.690 | |
*Medians of observed values (not change).
†P values based on non-inferiority testing of change scores (at corresponding standardised effect size margin of 0.4).
‡Numbers of available data were as per randomised allocation, that is, 112 for RLC and 111 for NLC unless otherwise stated in parentheses.
FFbH, Funktionsfragebogen Hannover; HADS-A, Hospital Anxiety and Depression Scale—Anxiety; HADS-D, Hospital Anxiety and Depression Scale—Depression; NLC, nurse-led care; r, effect size; RAID, Rheumatoid Arthritis Impact of Disease; RLC, rheumatologist-led care; U, Mann-Whitney U statistic; z, z-score; ZAP, 'Fragebogen zur Zufriedenheit in der ambulanten Versorgung', satisfaction about outpatient care.;
The HADS-D scores did not differ between the groups (p=0.866). The proportion of patients with no depression did not change significantly in the overall population (p=0.092), the RLC group (p=0.324) or the NLC group (p=0.090).
Activities of daily living were measured using the FFbH. There were no statistically significant differences in the mean change after 12 months between the two groups (p=0.365). In addition, there were no statistically significant differences between the groups after 12 months with regard to pain (p=0.523) or fatigue (p=0.626). Three global questions of the ZAP regarding trust, quality of care and satisfaction were considered in this analysis. The change in the scores of the three global questions did not differ significantly between the NLC and RLC over 12 months (trust, p=0.775; quality of care, p=0.283; satisfaction, p=0.690).
The evaluation of the consultation time of the rheumatologist in addition to NLC showed a median of 5 min (mean 7.1 min, SD ±4.4). Considering a 20 min time slot according to regular care, 65% of the consultation time for the rheumatologist was saved. Of the consultations of the rheumatologist in addition to NLC, 64% were considered not necessary and considered only as a formality.
Patient safety issues
Data regarding patient safety measures are reported in table 4. In the NLC group, a significantly higher number of patients had DAS28-CRP alerts due to an increase of 0.646 or more compared with the previous visit (χ2=7.277, p=0.043, OR=1.49). In the NLC group, a significantly higher number of patients made use of the optional visit 8 weeks after baseline (χ2=5.368, p=0.023, OR=2.17). No significant differences were observed between the groups in any of the other safety measures.
Table 4.
Patient safety measures
| Type of intervention | RLC | NLC | Total |
| DAS28 alert | 47 | 66 | 113 |
| HADS-D alerts | 57 | 34 | 91 |
| RADAI alerts | 96 | 99 | 195 |
| Initiation of biologicals | 15 | 18 | 33 |
| Additional visit (8 weeks) | 17 | 31 | 48 |
| Died | 0 | 2 | 2 |
DAS28 alerts: total number of times patients increased in DAS28>0.6 compared to the previous visit. Alerts: number of times of increased HADS-D or RADAI score above the defined levels. Initiation of biologicals: number of patients who started receiving biological treatment during the 12-month study period. Additional visit: number of patients who made use of the optional visit.
DAS28, Disease Activity Score in 28 Joints; HADS-D, Hospital Anxiety and Depression Scale—Depression; NLC, nurse-led care; RADAI, Rheumatoid Arthritis Disease Activity Index; RLC, rheumatologist-led care.
Consultations of the rheumatologist marked necessary were centred on change of therapy (20%), side effects (33%), other aspects of the rheumatic disease (21%) and other aspects not related to RA (26%).
Discussion
To the best of our knowledge, this is the first study demonstrating the non-inferiority of NLC in patients with RA having high disease activity in combination with poor prognostic factors. The results indicate that additional care in T2T can include NLC, in addition to appointments with rheumatologists, to ensure more frequent monitoring in patients with active disease every 1–3 months, as recommended by EULAR.2
Our results are in line with those of other studies, indicating that NLC is a meaningful addition to RLC.9 10 12 13 However, other studies have predominantly included patients with low disease activity,10 13 and only two studies included patients with RA with different disease activity levels.9 14
A meta-analysis showed that involving nurses in the care of patients with RA with low disease activity or in remission was a feasible addition to follow-up care.11 To date, there is limited evidence on whether NLC is applicable to patients with high disease activity and poor prognostic factors. Our study is powered to demonstrate that, even in ACPA/RF-positive patients with active disease, quality of care is non-inferior with the addition of NLC; thus, it provides evidence that it is safe to combine RLC with NLC for these patients. In addition to the inclusion criteria of ACPA/RF positivity, a subgroup analysis focusing on patients with one or more poor prognostic factors2 confirmed the initial result.
Despite improved pharmacological treatment options, approximately 30% of patients still respond poorly to medical therapy.5 Patients with a complicated, severe disease course may require closer psychological care. Research has shown that rheumatology teams can play an important role in improving psychological well-being.48 Evidence suggests that patient preferences for psychological support state nurses as one of the ideal sources,49 and European guidelines recommend that nurses provide psychological and self-management support.22 This is especially true for patients with active disease. Consequently, adding nurses to the multidisciplinary team not only may close the gap of an unmet demand for rheumatologists, but, more importantly, may also provide an important added value of care in enabling more frequent monitoring of patients with active disease. It also allows the rheumatologist to focus on non-delegable tasks in this challenging patient population and more complicated cases, such as the increasing number of patients with RA with significant comorbidities.
Secondary endpoints showed non-inferiority in all measures. Psychological well-being was measured using the HADS. While the outcome of depression showed no difference between the groups, the outcome of anxiety significantly favoured NLC. In addition, the proportion of patients with no anxiety changed significantly, while there was no change in the RLC group. This may be explained by the fact that, according to research regarding experiences of NLC, the patients experienced security as well as familiarity. In addition, increasing knowledge leads to a feeling of participation. Patients found it easier to share information with a nurse than with a rheumatologist.15 Focusing on satisfaction with care, previous studies showed greater satisfaction with NLC across one or more Leeds Satisfaction Questionnaire (LSQ) subscales.9 19 50 51 Since there is no validated German version of the LSQ available, the equivalent ZAP39 40 was used. Patient satisfaction with follow-up care increased significantly in both groups but did not favour NLC. This was different from the results of other studies9 10 19 and might be due to the fact that patients in Germany were not used to being seen by a nurse. Non-inferiority indicated that the patients did not feel abandoned and accepted this new form of care.
NLC included a brief contact with the rheumatologist due to legal requirements at the quarterly visits. T2T visits are excluded from this requirement because these visits are in addition to quarterly visits. An optional second T2T visit was offered to both groups. Significantly more patients in the NLC group took advantage of this offer compared with the RLC group. Research has shown that patients value the encounter with a nurse, for example, because of additional perspectives provided by nurses and the perception of being more on the same level.15 52 This might explain the difference and might imply that the patients valued the advantage of being offered more time with a healthcare professional with a different perspective. Even though nurses in Germany do not have the legal competencies to prescribe the medication, they do have the competencies to assess the best option for a new therapy in terms of shared decision-making with the patient. Taking part in decision-making regarding treatment was shown to be important to the patients.15
The primary endpoint change in disease activity was met at all time points but was slightly better in the RLC group at the T2T visit. Additionally, patient safety measures showed a significantly higher number of alerts in the NLC group. This may be explained by considering that the patients might have been more nervous at the beginning, as they were not familiar with NLC. In addition, although only nurses with experience in rheumatology participated in the trial and were trained before the start of the study, there was a learning curve. The differences subsided over time.
This study has some limitations. First, we were unable to include an independent masked assessor performing joint counts for DAS28-CRP due to financial constraints. However, we included several PROs, such as the RADAI, which indicated no significant difference in disease activity from the patients’ perspective. Second, as a randomisation method, sealed envelopes were used, which might have led to a selection bias, especially in the later phases of the trial. This method was used to aim for an equal allocation of patients in both groups in each centre to account for centre influence. In addition, having balanced groups by time, especially at the beginning of the trial, was considered important for safety measures. While selection bias cannot be ruled out, the likelihood of occurrence is considered low, because the rheumatologists invited the patients to participate in the study during their normal practice, but randomisation was done later by the nurse. Third, for the PP analysis, only 79% of the patients had complete datasets for all five visits for the primary endpoint. However, due to the inclusion of more patients than the estimated 10% dropout rate, the study still had sufficient power according to the initial power calculation. Finally, in Germany, NLC is currently implemented with a brief contact with the rheumatologist. Thus, the results of this trial might not be generalisable to a setting where the involvement of the rheumatologist only requires either a chart review or discussion after the visit or a consultation on demand in case of problems. However, T2T visits were excluded from this requirement, and 64% of the consultations of the rheumatologist in addition to NLC were considered only as a formality. Nevertheless, generalisability across Europe may be limited, as the implementation of NLC depends on the healthcare system and regulatory framework in each country.
Conclusion
This study supported the non-inferiority of NLC in managing T2T and follow-up care of patients with RA with moderate to high disease activity and poor prognostic factors in addition to RLC. The patients accepted this concept of care, and there might be some benefits in terms of psychological well-being. Hence, the implementation of NLC should be considered independent of the available resources of rheumatologists or restrictions due to legal requirements. Further studies are required to examine the added value of NLC.
Acknowledgments
We especially thank the participating patients for their willingness to contribute to the study. We thank the multicentre study team: Dr Sven Dubbert, Silke Dubbert, Nadine Lowald, Dr Karin Rockwitz, Anne Hohaus, Dr Carsten Stille, Martina Fellner, Dr Jochen Walter, Andrea Schwager, Birthe König, Ramona Merkle and Lissy Sandner. Thank you to the trial support team: data management, Marianne Richter; research secretary, Marion Wiegand; and trial logistics, Paul Olson and Florian Wiegand. Thank you to Dr Inge Ehlebracht-König and Hans Metzig, Rheuma-Liga Niedersachsen e.V. for supporting the study. We also thank Editage (www.editage.com) for English language editing. Thank you very much to the EULAR Office to Ursula Aring and Team for the support during the EULAR 2020 press conference.
Footnotes
Presented at: The study was introduced and discussed at the EULAR 2020 International Virtual Press Conference and has been presented at the EULAR 2020 Conference. Hoeper JR, Gauler G, Meyer-Olson D et al. OP0154. Effect of nurse-led-care on patient outcomes in rheumatoid arthritis in Germany: a multicentre randomised controlled trial. Annals of the Rheumatic Diseases 2020; 79:97–98
Contributors: JRH, GG, PS-K, MW, JW, FS, UvH and KH conceptualised and designed the study. AS was the main grant holder. KH and JRH were responsible for the study protocol development. KH was responsible for study set-up, recruiting sites and ethics application, and oversaw the study conduct. SEM conducted the data management. JRH and JZ conducted the statistical analyses. GG, PS-K, MW, JW, FS and DM-O recruited patients and collected data. JRH, KH, DM-O, TW, SEM, AS and JZ participated in the analysis and drafting or revision of the manuscript. All coauthors edited and reviewed the final version of the manuscript.
Funding: The study was conducted as a subproject of the 'Rheuma-VOR' project and received funding from the Federal Joint Committee (G-BA). Funding number: 01NVF16029.
Competing interests: JH, JZ, SEM, TW and UvH: none declared; GG has undertaken consultancies and speakers’ bureau for Abbvie, Lilly, Gilead, Celgene and Novartis; PSK has undertaken consultancies and speakers’ bureau for Abbvie, Chugai, Novartis, Sanofi, Mylan and Lilly. MW has undertaken consultancies and speakers’ bureau for Abbvie, Actelion, Aescu, Amgen, Biogen, BMS, Berlin Chemie, Celgene, Gilead, GSK, Hexal, Janssen, Medac, MSD, Mundipharma, Mylan, Novartis, Pfizer, Riemser, Roche, Sanofi, SOBI and UCB. JW has undertaken consultancies and speakers’ bureau for Janssen, Abbvie, Roche, Chugai and Novartis. FS has undertaken consultancies and speakers’ bureau for Novartis, Abbvie and Gilead. AS has received grants from Pfizer, GSK and Novartis, and has undertaken consultancies and speakers’ bureau for GSK and Roche. DMO has received grants from Novartis and Sandoz Hexal and has undertaken consultancies and speakers’ bureau for Abbvie, Amgen, BMS, Chugai, Lilly, Mylan, Novartis, Pfizer, Sandoz Hexal and Sanofi. KH has undertaken consultancies and speakers’ bureau for Abbvie, Chugai, Novartis, Lilly, Celgene, UCB, Sandoz Hexal, Sanofi and Gilead.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Multicentre ethics approval was obtained from the the ethical committee of Hannover Medical School (number 3638–2017).
References
- 1.Zink A, Braun J, Gromnica-Ihle E, et al. [Memorandum of the German Society for Rheumatology on the quality of treatment in rheumatology - Update 2016]. Z Rheumatol 2017;76:195–207. 10.1007/s00393-017-0297-1 [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 3.Kristiansen TM, Primdahl J, Antoft R, et al. Everyday life with rheumatoid arthritis and implications for patient education and clinical practice: a focus group study. Musculoskeletal Care 2012;10:29–38. 10.1002/msc.224 [DOI] [PubMed] [Google Scholar]
- 4.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 5.Bécède M, Alasti F, Gessl I, et al. Risk profiling for a refractory course of rheumatoid arthritis. Semin Arthritis Rheum 2019;49:211–7. 10.1016/j.semarthrit.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Gullick NJ, Ibrahim F, Scott IC, et al. Real world long-term impact of intensive treatment on disease activity, disability and health-related quality of life in rheumatoid arthritis. BMC Rheumatol 2019;3:6. 10.1186/s41927-019-0054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mian AN, Ibrahim F, Scott IC, et al. Changing clinical patterns in rheumatoid arthritis management over two decades: sequential observational studies. BMC Musculoskelet Disord 2016;17:44. 10.1186/s12891-016-0897-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international Task force. Ann Rheum Dis 2016;75:3–15. 10.1136/annrheumdis-2015-207524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ndosi M, Lewis M, Hale C, et al. The outcome and cost-effectiveness of nurse-led care in people with rheumatoid arthritis: a multicentre randomised controlled trial. Ann Rheum Dis 2014;73:1975–82. 10.1136/annrheumdis-2013-203403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Primdahl J, Sørensen J, Horn HC, et al. Shared care or nursing consultations as an alternative to rheumatologist follow-up for rheumatoid arthritis outpatients with low disease activity--patient outcomes from a 2-year, randomised controlled trial. Ann Rheum Dis 2014;73:357–64. 10.1136/annrheumdis-2012-202695 [DOI] [PubMed] [Google Scholar]
- 11.de Thurah A, Esbensen BA, Roelsgaard IK, et al. Efficacy of embedded nurse-led versus conventional physician-led follow-up in rheumatoid arthritis: a systematic review and meta-analysis. RMD Open 2017;3:e000481. 10.1136/rmdopen-2017-000481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner S, Lopatina E, Rankin JA, et al. Nurse-Led care for patients with rheumatoid arthritis: a systematic review of the effect on quality of care. J Rheumatol 2017;44:757–65. 10.3899/jrheum.160535 [DOI] [PubMed] [Google Scholar]
- 13.Larsson I, Fridlund B, Arvidsson B, et al. Randomized controlled trial of a nurse‐led rheumatology clinic for monitoring biological therapy. J Adv Nurs 2014;70:164–75. 10.1111/jan.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergsten U, Almehed K, Baigi A, et al. A randomized study comparing regular care with a nurse-led clinic based on tight disease activity control and person-centred care in patients with rheumatoid arthritis with moderate/high disease activity: a 6-month evaluation. Musculoskeletal Care 2019;17:215–25. 10.1002/msc.1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsson I, Bergman S, Fridlund B, et al. Patients' experiences of a nurse-led rheumatology clinic in Sweden: a qualitative study. Nurs Health Sci 2012;14:501–7. 10.1111/j.1442-2018.2012.00723.x [DOI] [PubMed] [Google Scholar]
- 16.Primdahl J, Wagner L, Holst R, et al. The impact on self-efficacy of different types of follow-up care and disease status in patients with rheumatoid arthritis--a randomized trial. Patient Educ Couns 2012;88:121–8. 10.1016/j.pec.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 17.Arvidsson SB, Petersson A, Nilsson I, et al. A nurse-led rheumatology clinic's impact on empowering patients with rheumatoid arthritis: a qualitative study. Nurs Health Sci 2006;8:133–9. 10.1111/j.1442-2018.2006.00269.x [DOI] [PubMed] [Google Scholar]
- 18.Bala S-V, Samuelson K, Hagell P, et al. The experience of care at nurse-led rheumatology clinics. Musculoskeletal Care 2012;10:202–11. 10.1002/msc.1021 [DOI] [PubMed] [Google Scholar]
- 19.Koksvik HS, Hagen KB, Rødevand E, et al. Patient satisfaction with nursing consultations in a rheumatology outpatient clinic: a 21-month randomised controlled trial in patients with inflammatory arthritides. Ann Rheum Dis 2013;72:836–43. 10.1136/annrheumdis-2012-202296 [DOI] [PubMed] [Google Scholar]
- 20.Albrecht K, Zink A. Poor prognostic factors guiding treatment decisions in rheumatoid arthritis patients: a review of data from randomized clinical trials and cohort studies. Arthritis Res Ther 2017;19:68. 10.1186/s13075-017-1266-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Eijk-Hustings Y, van Tubergen A, Boström C, et al. EULAR recommendations for the role of the nurse in the management of chronic inflammatory arthritis. Ann Rheum Dis 2012;71:13–19. 10.1136/annrheumdis-2011-200185 [DOI] [PubMed] [Google Scholar]
- 22.Bech B, Primdahl J, van Tubergen A, et al. 2018 update of the EULAR recommendations for the role of the nurse in the management of chronic inflammatory arthritis. Ann Rheum Dis 2020;79:61–8. 10.1136/annrheumdis-2019-215458 [DOI] [PubMed] [Google Scholar]
- 23.OECD . European Observatory on health systems and policies. Germany: Country Health Profile 2019: OECD Publishing, Paris / European Observatory on Health Systems and Policies, Brussels, 2019. [Google Scholar]
- 24.Busse R, Blümel M, Stock S. The German health care system, 2011. [Google Scholar]
- 25.Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308:2594–604. 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 26.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rheuma-Liga Niedersachsen e.V . Rheuma-Liga Niedersachsen e.V. (German League against rheumatism), 2020. Available: https://www.rheuma-liga-nds.de [Accessed 28 Oct 2020].
- 28.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. 10.1002/art.27584 [DOI] [PubMed] [Google Scholar]
- 29.Rheumaakademie . Rheumatologische Fachassistenz, 2021. Available: https://wwwrheumaakademiede/fileadmin/user_upload/rheumatologische_fachassistenz/Rh_Fass_Grundkurspdf [Accessed 08 Mar 2021].
- 30.Bundesmantelvertrag-Ärzte . Vereinbarung über die delegation ärztlicher Leistungen an nichtärztliches personal in Der ambulanten vertragsärztlichen Versorgung gemäß § 28 Abs. 1 S. 3 SGB V. GKV-Spitzenverband KB, ed, 2015. [Google Scholar]
- 31.Anderson JK, Zimmerman L, Caplan L. Measures of rheumatoid arthritis disease activity: patient (PtGA) and provider (PrGA) global assessment of disease activity, disease activity score (DAS) and disease activity score with 28-Joint counts (DAS28), simplified disease activity index (SDAI), clinical disease activity index (CDAI), patient activity score (PAS) and patient activity Score-II (PASII), routine assessment of patient index data (rapid), rheumatoid arthritis disease activity index (radai) and rheumatoid arthritis disease activity Index-5 (RADAI-5), chronic arthritis systemic index (CASI), patient-based disease activity score with ESR (PDAS1) and patient-based disease activity score without ESR (PDAS2), and mean overall index for rheumatoid arthritis (MOI-RA). Arthritis Care Res 2011;63 Suppl 11:S14–36. 10.1002/acr.20621 [DOI] [PubMed] [Google Scholar]
- 32.Lautenschläger J, Mau W, Kohlmann T. [Comparative evaluation of a German version of the Health Assessment Questionnaire and the Hannover Functional Capacity Questionnaire]. Z Rheumatol 1997;56:144–55. 10.1007/s003930050030 [DOI] [PubMed] [Google Scholar]
- 33.Gossec L, Dougados M, Rincheval N, et al. Elaboration of the preliminary rheumatoid arthritis impact of disease (raid) score: a EULAR initiative. Ann Rheum Dis 2009;68:1680–5. 10.1136/ard.2008.100271 [DOI] [PubMed] [Google Scholar]
- 34.Gossec L, Paternotte S, Aanerud GJ, et al. Finalisation and validation of the rheumatoid arthritis impact of disease score, a patient-derived composite measure of impact of rheumatoid arthritis: a EULAR initiative. Ann Rheum Dis 2011;70:935–42. 10.1136/ard.2010.142901 [DOI] [PubMed] [Google Scholar]
- 35.Herrmann C, Buss U, Lingen R, et al. [The screening for anxiety and depression in routine medical care]. Dtsch Med Wochenschr 1994;119:1283–6. 10.1055/s-2008-1058834 [DOI] [PubMed] [Google Scholar]
- 36.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 37.Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--a review of validation data and clinical results. J Psychosom Res 1997;42:17–41. 10.1016/s0022-3999(96)00216-4 [DOI] [PubMed] [Google Scholar]
- 38.Bjelland I, Dahl AA, Haug TT, et al. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res 2002;52:69–77. 10.1016/s0022-3999(01)00296-3 [DOI] [PubMed] [Google Scholar]
- 39.Bitzer EM, Dierks ML, Dörning H. Zufriedenheit in Der Arztpraxis AUS Patientenperspektive – Psychometrische Prüfung eines standardisierten Erhebungsinstrumentes. Zeitschrift für Gesundheitswissenschaften 1999;7:196–209. [Google Scholar]
- 40.Kbv . Kassenärztliche Bundesvereinigung. Available: https://www.kbv.de/media/sp/ZAP_Fragebogen_Englisch.pdf [Accessed 23 Nov 2020].
- 41.Faul F, Erdfelder E, Lang A-G, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 42.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum 1998;41:1845–50. [DOI] [PubMed] [Google Scholar]
- 43.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–92. 10.1097/01.MLR.0000062554.74615.4C [DOI] [PubMed] [Google Scholar]
- 44.Cohen J. Statistical power analysis for the behavioral sciences. New York: Lawrence Erlbaum Associates, 1988. [Google Scholar]
- 45.White IR, Horton NJ, Carpenter J, et al. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342:d40. 10.1136/bmj.d40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fransen J, van Riel PLCM. The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 2005;23:S93–9. [PubMed] [Google Scholar]
- 47.Hense S, Luque Ramos A, Callhoff J. [Prevalence of rheumatoid arthritis in Germany based on health insurance data : Regional differences and first results of the PROCLAIR study]. Zeitschrift fur Rheumatologie 2016. [DOI] [PubMed] [Google Scholar]
- 48.Dures E, Hewlett S. Cognitive-Behavioural approaches to self-management in rheumatic disease. Nat Rev Rheumatol 2012;8:553–9. 10.1038/nrrheum.2012.108 [DOI] [PubMed] [Google Scholar]
- 49.Dures E, Almeida C, Caesley J, et al. Patient preferences for psychological support in inflammatory arthritis: a multicentre survey. Ann Rheum Dis 2016;75:142–7. 10.1136/annrheumdis-2014-205636 [DOI] [PubMed] [Google Scholar]
- 50.Hill J, Thorpe R, Bird H. Outcomes for patients with RA: a rheumatology nurse practitioner clinic compared to standard outpatient care. Musculoskeletal Care 2003;1:5–20. 10.1002/msc.35 [DOI] [PubMed] [Google Scholar]
- 51.Hill J, Lewis M, Bird H. Do OA patients gain additional benefit from care from a clinical nurse specialist?--a randomized clinical trial. Rheumatology 2009;48:658–64. 10.1093/rheumatology/kep049 [DOI] [PubMed] [Google Scholar]
- 52.Sjö A-S, Bergsten U. Patients' experiences of frequent encounters with a rheumatology nurse-A tight control study including patients with rheumatoid arthritis. Musculoskeletal Care 2018;16:305–12. 10.1002/msc.1348 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.