Summary
Data suggest that interleukin (IL)-6 blockade could reduce mortality in severe COVID-19, yet IL-6 is only modestly elevated in most patients. Chen et al. describe the role of soluble interleukin-6 receptor (sIL-6R) in IL-6 trans-signaling and how understanding the IL-6:sIL-6R axis might help define and treat COVID-19 cytokine storm syndrome.
Keywords: cytokine storm, COVID-19, interleukin-6, interleukin-6 receptor, tocilizumab, threshold concept
Data suggest that interleukin (IL)-6 blockade could reduce mortality in severe COVID-19, yet IL-6 is only modestly elevated in most patients. Chen et al. describe the role of soluble interleukin-6 receptor (sIL-6R) in IL-6 trans-signaling and how understanding the IL-6:sIL-6R axis might help define and treat COVID-19 cytokine storm syndrome.
Main text
The comprehensive immunological study of 85 Australian patients with coronavirus disease of 2019 (COVID-19) by Dr. Koutsakos and colleagues provides a fresh perspective on the immune dysregulation caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 Their cohort included patients with a broad spectrum of severities, including 12 critically ill, 20 ward, 1 discharged, and 52 ambulatory patients with a mix of acute and chronic phase samples. In this commentary, we elaborate on their findings regarding the soluble interleukin (IL)-6 receptor and how further exploration of the complex checks and balances involved in IL-6 signaling may be key to diagnosing and treating COVID-19 immunopathology.
COVID-19 is associated with remarkably heterogeneous presentations and outcomes in humans. The majority of those infected are asymptomatic or have mild ambulatory disease, but approximately 2%–10% develop severe disease leading to hospitalization and 25%–30% of hospitalized patients will require intensive care.2 Surges of severe and critical patients have overwhelmed health care systems in many countries. Some risk factors for critical illness are older age, male gender, and co-morbid conditions, yet a substantial proportion of severe cases are driven by a maladaptive, pathological immune response known as COVID-19 cytokine storm (COVID-CSS) which can afflict young, otherwise healthy adults.3 Early in the pandemic, numerous studies reported an association between elevated inflammatory cytokine levels, particularly IL-6, and poor outcomes, including respiratory failure and death. The typical immune response to a viral illness such as influenza is a short incubation time followed by a vigorous type I/III interferon response (IFN), resulting in physiological elevation of inflammatory cytokines that facilitates viral clearance. However, COVID-19 has an unusually long latency period of 2–14 days, after which a defective type I/III IFN response unleashes persistent, maladaptive hypercytokinemia.2
Early reports describing COVID-CSS generated great enthusiasm for immunomodulatory therapies, including corticosteroids, blockade of specific cytokines (e.g., IL-1, IL-6, TNF), interferon therapy, and modulation of intra-cellular pathways (e.g., JAK inhibition). In studies of prognostic biomarkers, IL-6 and C-reactive protein (which is produced by hepatocytes in response to IL-6) consistently emerged as the most sensitive and specific for predicting adverse outcomes such as respiratory failure and death.3 Autopsy studies also pointed toward cytokine storm as a major contributor to COVID-related morbidity and mortality. While some patients died with a high viral load evident in their tissues, others had profound inflammation in lungs, heart, brain, and other organs but little to no evidence of SARS-CoV-2, suggesting that these patients died with pathologic hyper-immune activation despite effective viral clearance.4
In June 2020, the RECOVERY dexamethasone study provided the first evidence in favor of immunomodulation in severe COVID-19, showing that corticosteroids reduce mortality in hospitalized patients. In contrast, early randomized controlled trials (RCTs) of IL-6 blockade, reported in the autumn of 2020, were largely disappointing. These initial studies of tocilizumab primarily examined patients with moderate disease, most of whom did not receive corticosteroids, and demonstrated little or no improvement in endpoints such as progression to critical illness or death. However, in early 2021, the large, pragmatic REMAP-CAP and RECOVERY trials demonstrated that IL-6 inhibition improves mortality and other key outcomes in a broad range of hospitalized patients. REMAP-CAP examined IL-6 blockade with tocilizumab (n = 353) or sarilumab (n = 48) in patients on high flow oxygen or mechanical ventilation.5 In-hospital mortality was reduced from 35.8% to 28%. The RECOVERY trial was released to a pre-print server on February 11, 2021. In this study, tocilizumab reduced 28-day mortality in hospitalized patients (n = 2,022) with hypoxemia (SaO2 < 92%) and C-reactive protein > 75 mg/L from 33.1% to 29.5%. Concomitant corticosteroids were given to 88% of patients in REMAP-CAP and 92% of patients in RECOVERY, and the benefit of IL-6 blockade with steroids was at least additive, if not synergistic. A Cochrane living systematic review of the first 10 RCTs of IL-6 blockade confirmed a high certainty of improvement in mortality by day 28, relative risk 0.89 (95% confidence interval [CI] 0.82–0.97); however, there was uncertainty as to whether IL-6 blockade improves other endpoints such as improvement by day 28 and progression to WHO Clinical Progression Score of 7 (i.e., mechanical ventilation ± other organ support or death).6 Taken together, these recent reports suggest that the negative results from earlier trials were most likely due to lack of concomitant steroid therapy, small sample sizes, and low event rates in less severely ill patients. The positive impact of corticosteroids and IL-6 blockade is even more important in the context of negative results from the antiviral domains of large international trials.
Despite impressive prognostic and therapeutic discoveries related to COVID-CSS, the concept of pathological immune activation has received considerable criticism. Opponents of the cytokine storm concept have argued that the IL-6 elevation in COVID is relatively modest compared to conditions such as sepsis, acute respiratory distress syndrome (ARDS), and chimeric antigen receptor T cell-associated cytokine release syndrome (CAR-T CRS). For example, a pooled analysis of nearly 1,000 COVID-19 patients reported a mean IL-6 level of only 36.7 pg/mL in severe or critical COVID-19 compared to 983.6 pg/mL in sepsis, 460 pg/mL in non-COVID ARDS, and 3,110.5 pg/mL in CAR-T CRS.7 While this analysis provides an important frame of reference, the clear efficacy of IL-6 blockade in severe COVID-19 suggests that comparison with other inflammatory conditions that respond to IL-6 blockade is more appropriate.
COVID-CSS is distinct from other cytokine storm syndromes such as hemophagocytic lymphohistiocytosis (HLH) and CAR-T CRS. In the Temple cytokine storm study, most COVID-CSS patients did not meet diagnostic criteria for HLH such as HLH-2004 and the HScore.8 Patients with COVID-CSS had an average IL-6 of 93 pg/mL (±162 standard deviation) compared to 35 pg/mL (±35) in COVID patients without CSS. The serum IL-6 levels of patients with severe COVID-19 are in fact similar to IL-6 levels observed in inflammatory diseases that are approved indications for IL-6 blockade. In rheumatoid arthritis (RA), giant cell arteritis (GCA), and Castleman disease (CD), median serum IL-6 levels are typically in the 20–200 pg/mL range. Further, although levels of serum IL-6 in CAR-T CRS are often an order of magnitude higher than in RA and CD, the dose of tocilizumab used is the same (8 mg/kg up to 800 mg IV). Thus, markedly elevated serum IL-6 levels are clearly not a pre-requisite for response to IL-6 blockade, and similar doses of IL-6 blockade are sufficient to treat patients with widely varying baseline IL-6 levels. However, these observations do beg the question of why inflammatory diseases such as COVID-CSS and Castleman disease exhibit serum IL-6 levels that are generally lower than sepsis and non-COVID ARDS. The role of the soluble IL-6 receptor, which is an essential agonist for IL-6 in trans-signaling, may be key to elucidating this apparent paradox.
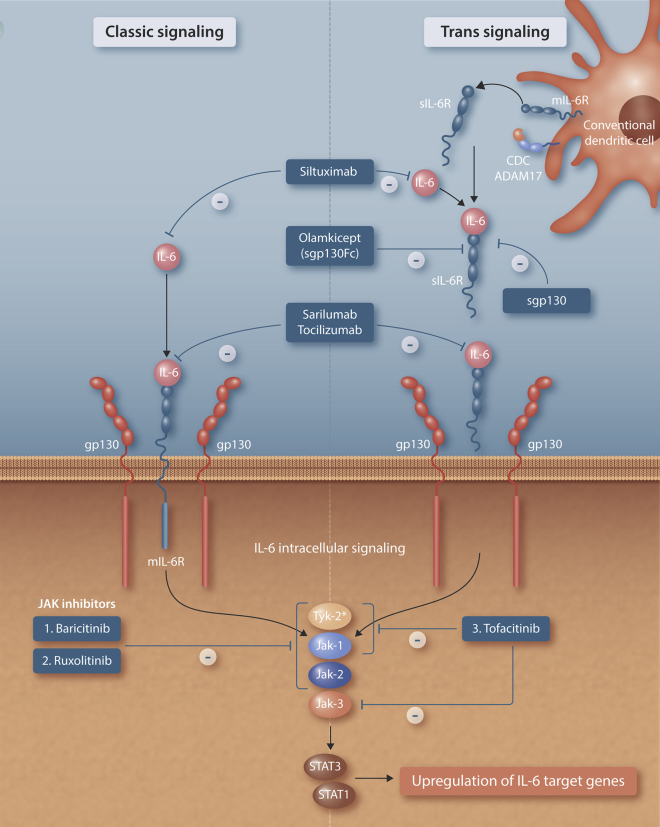
IL-6 is unique among cytokines in that it uses two signaling pathways: classic and trans (Figure 1).9 Classic signaling is restricted to cells with membrane-bound IL-6 receptor (mIL-6R), co-displaying its signaling subunit, glycoprotein 130 (gp130). These include immune cells (macrophages, lymphocytes), liver, and gut epithelium. The IL-6:mIL-6R complex associates with gp130, inducing dimerization and leading to intracellular signaling via the JAK/STAT pathway as well as activation of the RAS-MAPK pathway. All other tissues, including lungs, central nervous system, and myocardium, where COVID inflammation is particularly exuberant, rely on trans-signaling mediated by soluble interleukin-6 receptor (sIL-6R). IL-6 has pleiotropic effects, and classic signaling is in general thought to be anti-inflammatory, while trans-signaling is pro-inflammatory. trans-signaling is inhibited by soluble glycoprotein 130 (sgp130), which is present at levels of 100–200 ng/mL and binds with picomolar affinity to the IL-6:sIL-6R complex. While the dual signaling pathway is unique to IL-6, the presence of an inhibitor (sgp130) is common to many other cytokine signaling pathways. Most aspects of innate and adaptive cell-mediated and humoral immunity are subject to checks and balances; for example, IL-18 is inhibited by IL-18-blocking protein (IL-18 BP) and likewise with IL-22 and IL-22BP. In pro-inflammatory states, there is increased expression of sIL-6R, with relatively constant production of sgp130. This imbalance facilitates IL-6 trans-signaling through IL6: sIL-6R complexes that are formed but not cleared. The contribution of conventional dendritic cells (cDCs), which can secrete sIL-6R to overcome sgp130 buffering, and ADAM17, which cleaves membrane-bound IL-6R into sIL-6R, to drive inflammatory trans-signaling has been very recently elucidated by Yousif et al. (Figure 1).10 The soluble IL-6 receptor produced by these cDC-mediated pathways acts as a chaperone to circulating IL-6, prolonging its half-life and overcoming sgp130 inhibition to induce trans-signaling in a wide range of cells, even with relatively low IL-6 levels present. IL-6-mediated inflammation is therefore a dynamic and complex process involving interactions between free IL-6 and both membrane and soluble forms of IL-6R and gp130. Thus, circulating serum IL-6 levels in isolation provide an incomplete picture of the amount of biologically active IL-6 in the body, which may explain the apparent discrepancy between relatively modest elevations in IL-6 levels in tocilizumab-responsive conditions, such as COVID-CSS, RA, and CD, versus markedly elevated IL-6 levels in sepsis and ARDS, which are not treated with IL-6 blockade.
Figure 1.
IL-6 classic and trans-signaling and blockade
Classic signaling via membrane-bound IL-6 receptor is restricted to immune cells (macrophages, lymphocytes, dendritic cells), hepatocytes, and gut epithelium. Other organs, such as lungs, myocardium, and nervous system, require soluble IL-6 receptor to initiate trans-signaling. The trans-signaling system is buffered by soluble glycoprotein 130, which binds and inhibits the IL-6:sIL-6R complex with picomolar affinity. Conventional dendritic cells overcome this buffering system by secreting sIL-6R directly as well as facilitating cleavage of mIL-6R to produce sIL-6R via the membrane-bound sheddase ADAM17. Siltuximab (antibody against IL-6), sarilumab, and tocilizumab (antibodies against IL-6:IL-6R) block both classic and trans-signaling. Olamkicept (sgp130Fc) specifically blocks trans-signaling. The intracellular TYK2/JAK1/JAK2/JAK3 system leads to upregulation of IL-6 target genes and is inhibited by Jak inhibitors such as baricitinib, ruxolitinib, and tofacitinib. The inhibition of TYK-2 (∗) is relatively weak relative to JAK inhibition by these molecules.
Studies from CAR-T cell therapeutic trials suggest that sIL-6R levels may serve as a bottleneck in driving CAR-T CRS and support evaluating the IL-6:sIL-6R ratio as a parameter for CRS-mediated toxicity. In a study of 63 patients with RA, sIL-6R levels were a better predictor of response to tocilizumab than IL-6.11 Patients with high sIL-6R (mean ± SD 752.7 ± 243 ng/mL) responded poorly to tocilizumab, whereas patients with low sIL-6R (250.5 ± 72 ng/mL) responded well to tocilizumab irrespective of their IL-6 levels.11 Whether sIL-6R is predictive of response to IL-6 blockade in COVID-19 remains to be seen. In the study by Koutsakos et al.,1 sIL-6R was higher in ICU versus ward patients (57.9 ng/mL ICU versus 40.37 ng/mL ward, p = 0.002); likewise, ICU patients had higher IL-6 levels than ward patients (median 28.8 pg/mL versus 11.95 pg/mL, p = 0.025). However, levels of sIL-6R did not correlate with IL-6 levels; some patients with very high IL-6 had low sIL-6R and vice versa. In this study, sIL-6R was more predictive of critical illness (area under the receiver operator curve [AUROC] = 0.77, 95% CI 0.65–0.88) than IL-6 (AUROC = 0.70, 95% CI 0.57–0.63). These observations provide impetus to further study the IL-6 trans-signaling pathway and, in particular, the potential utility of sIL-6R and sgp130 for treatment decisions.
Although great progress has been made in understanding the biology and treatment of COVID-CSS, important questions remain. Mortality in the IL-6 treatment arms of RECOVERY and REMAP-CAP remain high, nearly 30%, and investigating other therapies for those patients who fail to respond to IL-6 blockade is crucial. Both trials were pragmatic, and there is little data available on biomarkers beyond simple clinical and laboratory data. Further investigation into who benefits most, and why, is needed. The immune response to COVID is clearly heterogeneous, and it is quite likely that the IL6-sIL-6R-sgp130 axis plays a vital role in some patients, whereas in others, it has little effect. Baseline serum IL-6 predicted response to tocilizumab in some retrospective cohorts, and further work is needed to determine whether sIL-6R and sgp130 may provide additional prognostic information. A common polymorphism in the IL-6 receptor gene (IL6R Asp358Ala; rs2228145 A>C), which leads to increased serum sIL-6R, has been implicated in several inflammatory diseases, including coronary artery disease, RA, and CD, and examination of this polymorphism in COVID-19 patients is warranted.12
Beyond corticosteroids and IL-6 blockade, other immunomodulatory therapies may have a role in treatment of COVID-CSS. A large genome-wide association study (GWAS) of critically ill COVID-19 patients identified the interferon receptor gene IFNAR2 and tyrosine kinase 2 (TYK2) as potential targets for repurposing of licensed medications.13 TYK2 is part of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway, which is implicated in IL-6 intracellular signaling (Figure 1).13 The JAK inhibitor baricitinib has been added as the current backbone of the RECOVERY immunomodulation domain, and interferon and other JAK inhibitors are being investigated in other trials. The study by Koutsakos et al.1 also highlights the prognostic value of IL-18, which was higher in critical care versus ward patients (700.9 pg/mL ICU versus 238.8 pg/mL ward), although it is not clear if the measured cytokine was free IL-18 or free plus IL-18BP-bound IL-18. IL-18 levels in this study were lower than in HLH/MAS, where they are typically >1,000 pg/mL. IL-18 is a key “helper” cytokine for IFN-γ and both of these cytokines are amenable to targeted therapy.
The recognition of pathological immune activation in a pandemic illness, in the form of COVID cytokine storm, represents a “threshold concept,” analogous to the discovery of germ theory in the 19th century.4 While the IL-6-sIL-6R-sgp130 trans-signaling axis does not explain all the myriad immunopathology caused by COVID-19, it does provide a crucial prognostic and therapeutic framework, and discoveries from COVID-CSS may be transferable to other diseases. For example, although critics of COVID-CSS present ARDS and bacterial sepsis as examples where IL-6 blockade is not helpful, this may in fact be premature because pre-clinical models show tocilizumab may in fact improve the vascular dysfunction in these conditions. Further, while the focus to date has been blockade of both classic and trans-signaling with tocilizumab and sarilumab in COVID-19, selective blockade of trans-signaling is an area for further investigation (Figure 1). Olamkicept, a novel inhibitor of IL-6/sIL-6R and therefore selective inhibitor of trans-signaling, has shown promising activity in a phase II trial of inflammatory bowel disease,14 as well as a murine sepsis model.15 Thinking ahead to future pandemic viruses, improving our ability to identify and treat maladaptive viral immune responses through improved understanding of IL-6 signaling and blockade is imperative.
Acknowledgments
L.Y.C.C. is supported by the Hal Kettleson Hematology Research Fund and the UBC Hematology Research Program. C.M.B. is supported by the Michael Smith Foundation for Health Research Health Professional-Investigator Award. C.L.W. is supported by grants from the Canadian Institutes for Health Research, National Institutes of Health, Weston Brain Institute, and Cure Alzheimer Fund. M.S.S. is supported by grants from the Canadian Institutes for Health Research and the Michael Smith Foundation for Health Research Health Professional-Investigator Award.
Author contributions
All authors were involved in the conceptualization, writing the original draft, and revising/editing of the final version of this commentary.
References
- 1.Koutsakos M., Rowntree L.C., Hensen L., Chua B.Y., van de Sandt C.E., Habel J.R., Zhang W., Jia X., Kedzierski L., Ashhurst T.M. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep. Med. 2021;2:100208. doi: 10.1016/j.xcrm.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Q., Bastard P., Bolze A., Jouanguy E., Zhang S.Y., Cobat A., Notarangelo L.D., Su H.C., Abel L., Casanova J.L., COVID Human Genetic Effort Life-Threatening COVID-19: Defective Interferons Unleash Excessive Inflammation. Med. (N Y) 2020;1:14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L.Y.C., Hoiland R.L., Stukas S., Wellington C.L., Sekhon M.S. Confronting the controversy: interleukin-6 and the COVID-19 cytokine storm syndrome. Eur. Respir. J. 2020;56:2003006. doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L.Y.C., Quach T.T.T. COVID-19 cytokine storm syndrome: a threshold concept. Lancet Microbe. 2021;2:e49–e50. doi: 10.1016/S2666-5247(20)30223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M., Annane D., Beane A., van Bentum-Puijk W., Berry L.R., REMAP-CAP Investigators Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2100433. Published online February 25, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosn L., Chaimani A., Evrenoglou T., Davidson M., Graña C., Schmucker C., Bollig C., Henschke N., Sguassero Y., Nejstgaard C.H. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst. Rev. 2021 doi: 10.1002/14651858.CD013881/full. Published online March 18, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., Hirayama A.V., Mastroiani F., Turtle C.J., Harhay M.O. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8:1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caricchio R., Gallucci M., Dass C., Zhang X., Gallucci S., Fleece D., Bromberg M., Criner G.J., Temple University COVID-19 Research Group Preliminary predictive criteria for COVID-19 cytokine storm. Ann. Rheum. Dis. 2021;80:88–95. doi: 10.1136/annrheumdis-2020-219720. [DOI] [PubMed] [Google Scholar]
- 9.Jones S.A., Jenkins B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018;18:773–789. doi: 10.1038/s41577-018-0066-7. [DOI] [PubMed] [Google Scholar]
- 10.Yousif A.S., Ronsard L., Shah P., Omatsu T., Sangesland M., Bracamonte Moreno T., Lam E.C., Vrbanac V.D., Balazs A.B., Reinecker H.C. The persistence of interleukin-6 is regulated by a blood buffer system derived from dendritic cells. Immunity. 2021;54:235–246.e5. doi: 10.1016/j.immuni.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Torne C., Ortiz M.D.A., Moya P., Hernandez M.V., Reina D., Castellvi I., De Agustin J.J., Fuente D., Corominas H., Sanmarti R. The combination of IL-6 and its soluble receptor is associated with the response of rheumatoid arthritis patients to tocilizumab. Semin. Arthritis Rheum. 2018;47:757–764. doi: 10.1016/j.semarthrit.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira R.C., Freitag D.F., Cutler A.J., Howson J.M., Rainbow D.B., Smyth D.J., Kaptoge S., Clarke P., Boreham C., Coulson R.M. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pairo-Castineira E., Clohisey S., Klaric L., Bretherick A.D., Rawlik K., Pasko D., Walker S., Parkinson N., Fourman M.H., Russell C.D., GenOMICC Investigators. ISARIC4C Investigators. COVID-19 Human Genetics Initiative. 23andMe Investigators. BRACOVID Investigators. Gen-COVID Investigators Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber S., Aden K., Bernardes J.P., Conrad C., Tran F., Höper H., Volk V., Mishra N., Blase J.I., Nikolaus S. Therapeutic IL-6 trans-signalling inhibition by olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology. 2021 doi: 10.1053/j.gastro.2021.02.062. Published online March 2, 2021. [DOI] [PubMed] [Google Scholar]
- 15.Barkhausen T., Tschernig T., Rosenstiel P., van Griensven M., Vonberg R.P., Dorsch M., Mueller-Heine A., Chalaris A., Scheller J., Rose-John S. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 2011;39:1407–1413. doi: 10.1097/CCM.0b013e318211ff56. [DOI] [PubMed] [Google Scholar]