Abstract
More men died of coronavirus disease 2019 (COVID-19) than women, suggesting estrogens may protect women. However, COVID-19 deaths among men and women were inconsistent among countries throughout the world. Genetics, epigenetics, and inborn errors of immunity may account for the disparity in mortality among men and women with COVID-19 more than sex steroid hormones.
Keywords: SARS-CoV-2, COVID-19, sex hormones, estradiol, progesterone
Introduction
Sex steroid hormones were implicated in the outcomes of COVID-19 infection, disease severity, and mortality in men and women, albeit without credible biochemical or physiological evidence. Pinna [1] suggested that ‘evidence shows coronavirus disease 2019 (COVID-19)-induced symptom severity and mortality [are] more frequent in men than in women, suggesting estrogens and progestins may play a protective role in women but not in men.’ Yet, no credible biochemical, physiological, or clinical evidence was provided to support such a broad and fundamentally important scientific supposition [1]. It is worth emphasizing that men do synthesize and secrete estrogens, albeit at reduced concentrations compared to women, which would be expected to confer similar physiological action in men [2]. Similarly, men produce progesterone in significant amounts [3]. Thus, the hypothesis that gender disparity in COVID-19 infection and disease severity is attributed, mainly, to estrogens and progesterone is, at best, simplistic and profoundly unsubstantiated [1]. If the advanced hypothesis that ‘Estradiol and progesterone are potent immune-modulators and confer protective advantages in women compared to men against COVID-19 disease severity and outcomes’ is correct, then the following predictions should be confirmed.
(i) Males and females prior to adrenarche would exhibit similar death rates, since circulating levels of sex steroid hormones are relatively low. (ii) Severity of COVID-19 and related mortality would remain consistently higher among men compared to women, irrespective of geographical location, populations, or cultural or socioeconomic factors. (iii) Premenopausal women, irrespective of age, would exhibit similar disease severity and outcome, since they produce physiological levels of estrogens and progestins. (iv) Pregnancy is associated with elevated circulating levels of estradiol, estriol, and progesterone and therefore would be expected to confer reduced COVID-19-related disease severity and mortality among pregnant women. (v) Postmenopausal women on hormone replacement therapy would be expected to experience greatly reduced disease severity and mortality compared to postmenopausal women who remained untreated. However, a careful review of the literature on sex steroids and COVID-19 together with data from the Center for Disease Control (https://www.cdc.gov/), GlobalHealth5050 (https://globalhealth5050.org/the-sex-gender-and-covid-19-project/), and the Harvard GenderSci Lab (Table 1 ) did not support the aforementioned predictions, suggesting other underlying biological factors play a role in the disparity of COVID-19 severity and deaths among men and women.
Table 1.
US gender/sex COVID-19 dataa
| State | Date | Confirmed cases |
Deaths |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Count |
Percentage |
Crude rate |
Count |
Percentage |
Crude rate |
||||||||||
| Total | Male | Female | Male | Female | Male | Female | Total | Male | Female | Male | Female | Male | Female | ||
| CT | 11 January 2021 | 205 994 | 96 661 | 108 516 | 46.92 | 52.68 | 5532.56 | 5915.70 | 6324 | 3066 | 3254 | 48.48 | 51.45 | 175.49 | 177.39 |
| DE | 11 January 2021 | 65 273 | 30 512 | 34 670 | 46.75 | 53.12 | 6639.25 | 7076.59 | 972 | 475 | 497 | 48.87 | 51.13 | 103.36 | 101.44 |
| KY | 11 January 2021 | 303 625 | 140 548 | 159 980 | 46.29 | 52.69 | 6427.36 | 7099.21 | 2901 | 1424 | 1477 | 49.07 | 50.90 | 65.10 | 65.53 |
| ME | 11 January 2021 | 29 298 | 13 824 | 15 474 | 47.18 | 52.82 | 2119.27 | 2273.87 | 432 | 211 | 221 | 48.84 | 51.16 | 32.35 | 32.48 |
| MA | 11 January 2021 | 413 329 | 12 875 | 5995 | 6836 | 46.56 | 53.10 | 180.90 | 194.41 | ||||||
| NH | 11 January 2021 | 51 600 | 24 264 | 26 474 | 47.02 | 51.31 | 3648.82 | 3901.04 | 869 | 427 | 441 | 49.14 | 50.75 | 64.21 | 64.98 |
| PA | 11 January 2021 | 720 816 | 331 084 | 386 085 | 45.93 | 53.56 | 5286.19 | 5914.30 | 17 770 | 8617 | 8777 | 48.49 | 49.39 | 137.58 | 134.45 |
| RI | 11 January 2021 | 97 614 | 44 902 | 52 712 | 46.00 | 54.00 | 8747.25 | 9702.48 | 1916 | 901 | 937 | 47.03 | 48.90 | 175.52 | 172.47 |
| VT | 11 January 2021 | 87 324 | 41 392 | 45 234 | 47.40 | 51.80 | 13 440.00 | 14 269.17 | 156 | 70 | 78 | 44.87 | 50.00 | 22.73 | 24.61 |
Confirmed cases and deaths in several states in which more women died than men. US Gender/Sex COVID-19 Data Tracker (2020) Harvard GenderSci La (www.genderscilab.org/gender-and-sex-in-covid19).
To date, the exact underlying pathophysiological mechanisms contributing to COVID-19 susceptibility to infection, increased disease presentation and severity, and increased mortality in men and women remains incompletely understood. Nevertheless, viral and host genetic variants are likely key factors impacting the immune response, disease severity, and mortality. Surprisingly, clinical trials on use of estrogens in men and women were commenced to determine their effectiveness against COVID-19, without biochemical or physiological data to establish a proof of concept or proof of relevance [1,4]. Use of estrogens as therapeutic modalities against COVID-19 has been proposed, even in the absence of clinical trials. In our view, such propositions are, at best, premature. Furthermore, there is no credible evidence to suggest that sex hormones per se are responsible for the disparity in COVID-19 infections, disease severity and death among men and women.
Moreover, considerable clinical variability was encountered among individuals infected with COVID-19 ranging from asymptomatic, mild to moderate diseases to severe and life-threatening disease that results in mortality [5., 6., 7.]. Type-I interferons (IFNs) are thought to be essential for sustained antiviral immunity, and type-I IFNs deficiency is a hallmark of severe COVID-19 [8]. IFN activity in serum of patients with severe or critical COVID-19 was significantly reduced compared to those with mild to moderate COVID-19 [8]. Thus, it is plausible that COVID-19 severity is associated with markedly impaired IFN type I response with no IFN-β and low IFN-α production and activity. Patients with impaired type I IFN response in severe and critical COVID-19 patients exhibited high blood viral load and an excessive NF-κB-driven inflammatory response associated with increased TNF-α and IL-6 [8]. Thus, the role of genetics and inborn errors of immunity may contribute to greater COVID19-related mortality among men compared to women rather than circulating sex steroid hormone levels do [5., 6., 7., 8., 9.].
Bastard et al. [6] proposed that inborn errors of cytokine immunity may, in part, contribute to the observed disparity in disease severity among individuals, and in particular among men and women. It was suggested that inborn errors of type-I IFNs and neutralizing autoantibodies (auto-Abs) against type-I IFNs possibly underlie the greater variability of severe adverse respiratory disease. For example, neutralizing IgG auto-Abs against IFN-ω and IFN-α were detected in patients with onset of critical COVID-19. However, no auto-Abs were detected in individuals with asymptomatic or mild COVID-19 infections. The excess of auto-Abs was greater in men with critical COVID-19 pneumonia and neutralizing auto-Abs against type-I IFNs and was equally higher than that observed in patients with critical COVID-19 without auto-Abs in men with asymptomatic disease. Interestingly, inborn errors of type-I IFN immunity, which accounts for life-threatening COVID-19 pneumonia was detected in at least 2.6% of women and 12.5% of men. This observation suggests that more men than women exhibit inborn errors of immunity. These findings provide an alternative explanation for the major sex bias seen in patients with life-threatening COVID-19 among men and women.
Zhang et al. [5] reported that at least 3.5% of patients with life threatening COVID-19 pneumonia had known autosomal recessive IFN regulatory factor 7 (IRF7) and IFNAR1 deficiencies or autosomal dominant Toll-like receptor 3 (TLR3), TICAM1, TBK1, and IRF3 deficiencies or new autosomal dominant UNC93B1, IRF7, IFNAR1, and IFNAR2 deficiencies.
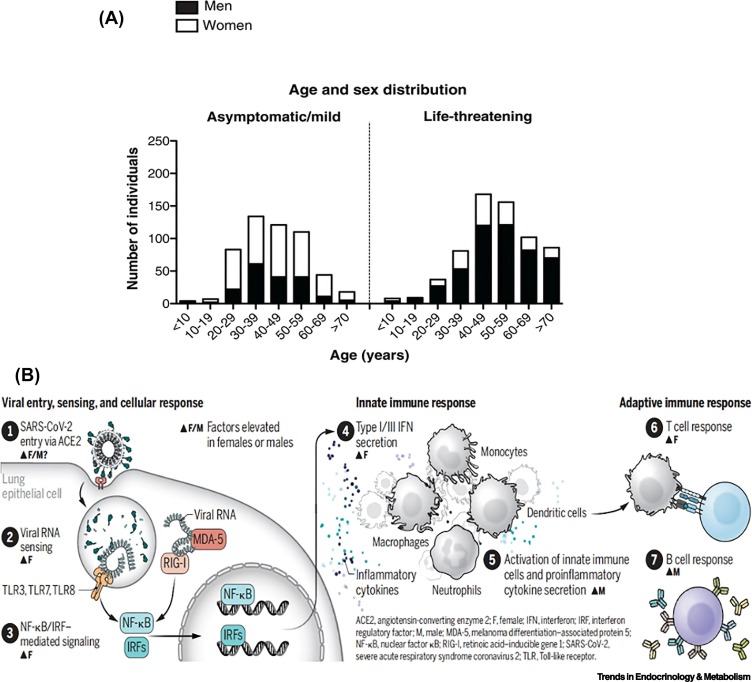
In patients with life-threatening COVID-19, there was a higher proportion of men than women with inborn errors of type 1IFN immunity at all ages (Figure 1A). This, however, was not the case in the asymptomatic patients [5,6]. Takahashi and Iwasaki [9] proposed a potential mechanism that may explain the sex differences pertaining to biochemical factors that modulate infection and immunity in COVID-19 in men and women (Figure 1B) and further suggested that each of these steps may shape the antiviral immune response in men and women.
Figure 1.
(A) Demographic and genetic data for the COVID-19 cohort.
Age and sex distribution of patients with life-threatening COVID-19. Reproduced, with permission, from [5]. (B) Sex differences in factors that affect infection and immunity in COVID-19. SARS-CoV-2 binds to ACE2 to initiate host cell entry. This activates the viral RNA sensors TLR3/7/8 and RIG-I–MDA-5, which induce secretion of IFNs and other inflammatory cytokines, leading to innate and adaptive immune responses. In each of these steps, sex differences may shape the antiviral immune response. Reproduced, with permission, from [9]. Abbreviations: ACE2, angiotensin-converting enzyme 2; IFN, interferon; IRF, interferon regulatory factor; MDA-5, melanoma differentiation–associated protein 5; NF-kB, nuclear factor kB; RIG-I, retinoic acid–inducible gene 1; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TLR, Toll-like receptor.
It is important to marshal sound scientific evidence based on biochemical and physiological data in order to propose clinical trials or advocate for the use of sex steroid hormones in COVID-19 patients. The sex disparity of COVID-19-related morbidity and mortality is likely explained by a combination of biological sex differences, such as inborn errors of immunity and gender-specific factors. Griffith et al. [10] noted that the mechanisms underlying sex differences in mortality remain unknown and a combination of biological, behavioral, and psychosocial factors may account for individuals’ health patterns. Krieger et al. [11] raised skepticism regarding the notion that increased deaths in men are entirely due to sex or gender.
In summary, none of the predictions set forth related to sex steroid hormones and disparity of COVID-19 severity and mortality among men and women could be confirmed. We suggest that inborn errors of immunity and genetics appear to be more critical in life-threatening COVID-19 than circulating sex steroid hormones are. Advocating for use of estrogens and progesterone to treat COVID-19 patients is not supported by biochemical, physiological, or clinically credible evidence. Caution should be exercised regarding the claims that sex steroid hormones account for the gender disparity in COVID-19 infection severity and mortality.
References
- 1.Pinna G. Sex and COVID-19: a protective role for reproductive steroids. Trends Endocrinol. Metab. 2020;32:3–6. doi: 10.1016/j.tem.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell N., Grossmann M. Mechanisms in endocrinology: estradiol as a male hormone. Eur. J. Endocrinol. 2019;181:R23–R43. doi: 10.1530/EJE-18-1000. [DOI] [PubMed] [Google Scholar]
- 3.Oettel M., Mukhopadhyay A.K. Progesterone: the forgotten hormone in men? Aging Male. 2004;7:236–257. doi: 10.1080/13685530400004199. [DOI] [PubMed] [Google Scholar]
- 4.Seeland U., et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18:369. doi: 10.1186/s12916-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bastard P., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton P.T. Is susceptibility to severe COVID-19 disease an inborn error of metabolism? J. Inherit. Metab. Dis. 2020;43:906–907. doi: 10.1002/jimd.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjadj J., et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T., Iwasaki A. Sex differences in immune responses. Science. 2021;371:347–348. doi: 10.1126/science.abe7199. [DOI] [PubMed] [Google Scholar]
- 10.Griffith D.M., et al. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Prev. Chronic Dis. 2020;17 doi: 10.5888/pcd17.200247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krieger N., et al. Excess mortality in men and women in Massachusetts during the COVID-19 pandemic. Lancet. 2020;395:1829. doi: 10.1016/S0140-6736(20)31234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]