Abstract
BACKGROUND
Limited US clinical data are available on the use of aesthetic products in patients with skin of color (SOC).
OBJECTIVE
To compare the efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in patients with and without SOC.
METHODS AND MATERIALS
Post hoc analyses were performed on the pooled population of all 492 patients treated with 20U prabotulinumtoxinA in the 2 US single-dose Phase III glabellar line clinical studies. Patients were grouped by Fitzpatrick skin Type: IV + V + VI (with SOC) versus I + II + III (without SOC). The primary efficacy end point was the proportion of responders with a ≥1-point improvement from baseline at maximum frown on the 4-point Glabellar Line Scale. Adverse events (AEs) were also summarized.
RESULTS
Responder rates among patients with SOC (n = 140) were lower than those without SOC (n = 352), by 5.9% on average across all visits; at no time point were differences statistically significant. At Day 30, responder rates were 94.0% and 96.0%, respectively (p = .401). Headache was the most common treatment-related AE, occurring in 12.1% and 8.2% of patients with and without SOC, respectively.
CONCLUSION
A single dose of 20U prabotulinumtoxinA was well tolerated and similar in effectiveness in patients with and without SOC for the treatment of glabellar lines.
Treatment with botulinum toxin Type A has been the leading aesthetic procedure in the United States since 1999; in 2018, more than 1.8 million procedures were performed.1 Although 70% of all aesthetic procedures were performed in Caucasians in that year, a significant and growing percentage of procedures were performed in non-Caucasians, including Hispanics (13%), African Americans (9%), and Asians (6%). Collectively, these and other non-Caucasian groups are identified as people with skin of color (SOC); by 2044, it is projected that people with SOC will comprise more than 50% of the US population.2 People with SOC typically have Fitzpatrick skin Types IV, V, or VI; those without SOC have Types I, II, or III.3
In recent years, the importance of understanding facial aesthetic considerations and aging differences as they pertain to patients in the US with SOC has been recognized.4–7 However, the US clinical trial data in facial aesthetic procedures in this population are limited. In the case of botulinum toxins, despite the widespread use of these products, only 2 clinical reports have been published in the US patients with SOC.8,9
PrabotulinumtoxinA (Jeuveau, Evolus, Inc, Newport Beach, CA) is a 900 kDa botulinum toxin Type A preparation produced by Clostridium botulinum that was approved for use in the United States for the treatment of glabellar lines in adult patients in 2019. Five multicenter clinical studies were undertaken to establish the efficacy and safety of 20U prabotulinumtoxinA for this indication.10–13 Of these, the studies conducted in the United States with the highest degree of rigor were the 2 identical 150-day, randomized, double-blind, placebo-controlled, single-dose Phase III studies (EV-001 and EV-002).10 The current post hoc analyses of data from these pivotal studies were undertaken to better understand the efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in the US patients with SOC, examining both physiologically based and race-based definitions of color.
Methods
Conduct of the Original Studies
All patients in the EV-001 and EV-002 studies were adults, at least aged 18 years, who had moderate-to-severe glabellar lines at maximum frown, as independently agreed by both investigator and patient assessment using the 4-point photonumeric Glabellar Line Scale (GLS; 0 = no lines, 1 = mild, 2 = moderate, and 3 = severe).10 A total of 492 patients were treated with a single dose of 20U prabotulinumtoxinA in these studies; 246 patients in each. Patients were followed for 150 days. Efficacy outcomes evaluated included glabellar lines at maximum frown on the GLS, aesthetics on the 5-point Global Aesthetic Improvement Scale (GAIS, 2 = much improved, 1 = improved, 0 = no change, −1 = worse, and −2 = much worse), and satisfaction on the 5-point Subject Satisfaction Scale (SSS, 2 = very satisfied, 1 = satisfied, 0 = indifferent, −1 = unsatisfied, and −2 = very unsatisfied).10 Key safety outcomes included investigator assessment of adverse events (AEs). PrabotulinumtoxinA-treated patients in these studies were largely similar in their baseline characteristics (e.g., age and sex).10
Statistical Methods of the Post hoc Analyses
Data were pooled from all prabotulinumtoxinA-treated patients who participated in these US single-dose Phase III studies. Efficacy and safety outcomes were compared between those with and without SOC, as defined by Fitzpatrick skin Types IV + V + VI and I + II + III, respectively, where Type I = always burns, never tans (pale white skin), Type II = usually burns, tans minimally (white skin), Type III = sometimes burns, tans uniformly (cream/light brown skin), Type IV = rarely burns, always tans well (moderate brown skin), Type V = very rarely burns, tans very easily (dark brown skin), and Type VI = never burns, deeply pigmented (dark brown to black skin). Outcomes were also compared between race-based subsets of each population: those with SOC who self-identified as Black/African American and those without SOC who self-identified as White.
Analyses were primarily descriptive in nature; data were summarized by numbers and percentages of patients. The primary efficacy end point was defined as the proportion of responders with a ≥1-point improvement from baseline on the GLS at maximum frown by investigator assessment. For this end point alone, the Fisher exact test was used to compare the proportion of responders between groups; two-sided exact 95% confidence intervals and associated p-values were calculated for the absolute differences in the proportions of responders at each visit, based on inverting 2 one-sided tests. Secondary efficacy end points included the proportions of responders on the GAIS (i.e., those with a score of improved or much improved) and on the SSS (i.e., those with a score of satisfied or very satisfied). All treatment-related AEs (all events assessed by the investigator as possibly, probably, or definitely treatment related) were summarized, including those of particular interest for this type of treatment and indication—for example, headache and eye disorders.
Results
Patient Disposition and Demographics
Of the 492 prabotulinumtoxinA-treated patients who participated in the 2 US single-dose Phase III studies, 140 (28.5%) had Fitzpatrick skin Types IV, V, or VI and, accordingly, were identified as patients with SOC; 352 (71.5%) had Fitzpatrick skin Types I, II, or III and, as such, were identified as patients without SOC (See Supplemental Digital Content 1, Table 1, http://links.lww.com/DSS/A616). Most patients were women and were younger than age 65 years, with severe glabellar lines at maximum frown. Similar percentages of patients with and without SOC were women (88.6% and 91.8%, respectively) and had severe glabellar lines (76.4% and 75.3%, respectively). A higher percentage of patients with SOC were younger than 65 years old (95.7% vs 86.6% of those without); a lower percentage had received previous treatment with a botulinum toxin (30.0% vs 43.2% of those without). Based on self-reported race/ethnicity data, most patients with SOC identified as White (53.6%) or Black/African American (26.4%); most patients without SOC identified as White (98.0%).
The 37 patients with SOC who self-identified as Black/African American (7.5%) and the 345 patients without SOC who self-identified as White (70.1%) were included in the subset analyses. All Blacks/African Americans were included in these analyses; the 75 Whites who were assessed as having SOC (17.9% of all Whites) were excluded (See Supplemental Digital Content 1, Table 1, http://links.lww.com/DSS/A616). Similarities and differences in demographic data between the 2 subsets largely paralleled those reported for patients with and without SOC. Of note, compared with 75.1% of Whites without SOC, approximately 10% fewer Blacks/African Americans (64.9%) had severe glabellar lines at maximum frown at baseline—a difference not seen between those with and without SOC. Also, differences in the history of botulinum toxin use were more pronounced; compared with 43.2% of Whites without SOC, 27.2% fewer Blacks/African Americans (16.2%) reported a history of previous botulinum toxin use.
Efficacy
Representative photographs of a patient with SOC's glabellar lines at maximum frown taken at baseline and at 2 days, 7 days, 30 days, 90 days, 120 days, and 150 days after treatment with 20U prabotulinumtoxinA are presented in Figure 1A–G.
Figure 1.
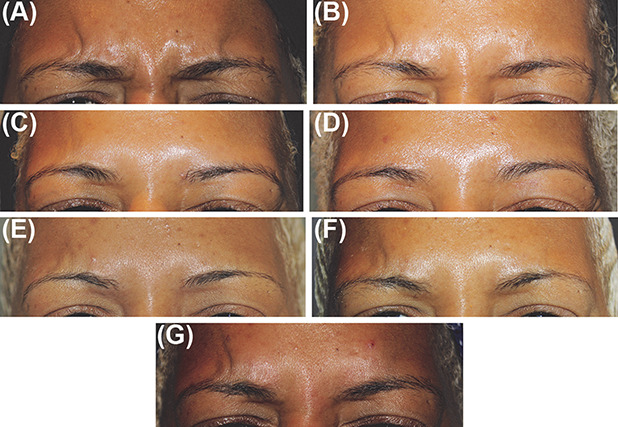
(A–G) Photographs of glabellar lines at maximum frown at baseline (A) and at each of Day 2 (B), Day 7 (C), Day 30 (D), Day 90 (E), Day 120 (F), and Day 150 (G) after treatment with 20U PrabotulinumtoxinA in a 36-year-old female patient with skin of color. This representative patient with skin of color was assessed as having Fitzpatrick skin Type VI and moderate glabellar lines at maximum frown at baseline by investigator assessment.
Responders on the Glabellar Line Scale
For the primary efficacy end point of the post hoc analyses, the percentages of responders were slightly lower at all time points (by an absolute mean difference of 5.9% across all visits) for those with SOC than that of those without SOC; at no time point were the differences statistically significant (all p > .05) (See Supplemental Digital Content 1, Table 2, http://links.lww.com/DSS/A616 and Figure 2). By Day 2, approximately half of all patients had achieved a ≥1-point improvement on the GLS at maximum frown by investigator assessment: 47.0% and 52.8% with and without SOC, respectively. By Day 7, near maximal responder rates had been reached in both groups; by Day 30, 94.0% and 96.0% of those with and without SOC, respectively, had achieved the primary end point. At the end of study on Day 150, more than 30% of patients continued to show a ≥1-point improvement on the GLS at maximum frown: 31.5% and 40.1% with and without SOC, respectively.
Figure 2.
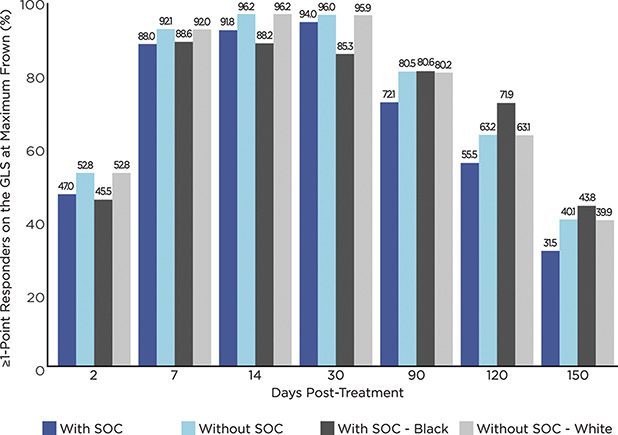
Percentage of responders based on a ≥1-Point improvement on the GLS at maximum frown from Day 0, by Fitzpatrick skin type and race as well as by visit (N = 492). GLS = Glabellar line scale (0 = no lines, 1 = mild, 2 = moderate, and 3 = severe); SOC = skin of color; with SOC = Fitzpatrick skin Types IV + V + VI; without SOC = Fitzpatrick skin Types I + II + III.
A somewhat different pattern of response was observed between the subsets of Blacks/African Americans and Whites without SOC (See Supplemental Digital Content 1, Table 2, http://links.lww.com/DSS/A616 and Figure 2). Compared with Whites without SOC, the percentage of responders among Blacks/African Americans was less at each of Days 2 through 30 (by an absolute mean difference of 7.3% across these visits). At Day 90, the percentages of responders in both subsets were similar. After Day 90, the percentage of responders among Blacks/African Americans was greater at each of Days 120 and 150 (by an absolute mean difference of 6.2% across these visits). As was seen among those with and without SOC, at no time point were the differences between these subsets statistically significant (all p > .05).
Responders on the Global Aesthetic Improvement Scale
Data on the percentages of responders based on the GAIS (those assessed by the investigator as either improved or much improved) tended to parallel that of responders based on the GLS (Figures 2 and 3). That is, the percentages of responders on the GAIS were lower at all time points (by an absolute mean difference of 7.6% across all visits) for those with SOC than that of those without SOC. Similarly, the percentages of responders on the GAIS were lower at all time points (by an absolute mean difference of 9.1% across all visits) for Blacks/African Americans than that of Whites without SOC. Overall, by Day 2 and throughout the study including study end at Day 150, more than 40% of patients were assessed as responders on the GAIS, regardless of skin color (Figure 3); at each of Days 7, 14, and 30, more than 85% of patients were assessed as responders on the GAIS, regardless of skin color or race.
Figure 3.
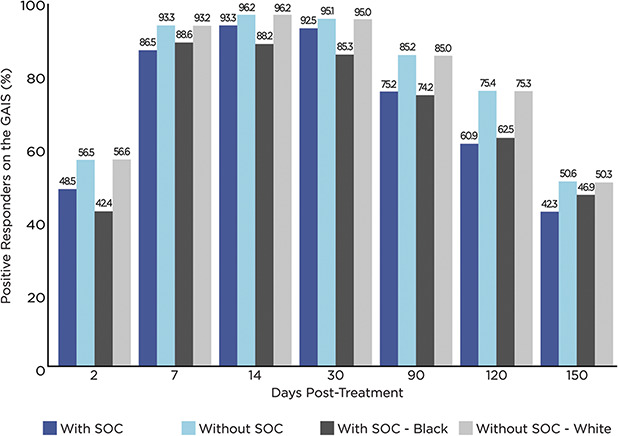
Percentage of positive responders (improved + much improved) on the GAIS, by Fitzpatrick skin type and race as well as by visit (N = 492). GAIS = Global Aesthetic Improvement Scale (2 = much improved, 1 = improved, 0 = no change, −1 = worse, and −2 = much worse); SOC = skin of color; with SOC = Fitzpatrick skin Types IV + V + VI; without SOC = Fitzpatrick skin Types I + II + III.
Responders on the Subject Satisfaction Scale
Patient satisfaction also remained high throughout the study (Figure 4). At each of Days 7 through 150, the percentage of responders based on the SSS (those who rated their level of satisfaction as satisfied or very satisfied) exceeded 70%, regardless of skin color. The percentages of responders were similar at all time points between those with and without SOC. Of note, compared with Whites without SOC, the percentages of responders among Blacks/African Americans were markedly higher at each of Days 2, 7, 120, and 150 (by an absolute mean difference of 10% across these visits).
Figure 4.
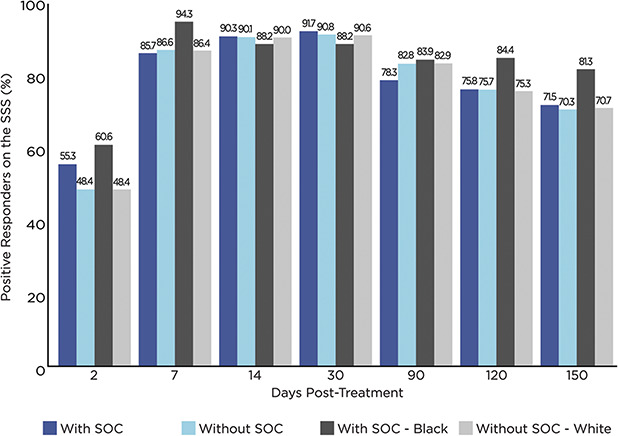
Percentage of positive responders (satisfied + very satisfied) on the SSS, by Fitzpatrick skin type and race as well as by visit (N = 492). SSS = Subject Satisfaction Scale (2 = very satisfied, 1 = satisfied, 0 = indifferent, −1 = unsatisfied, and −2 = very unsatisfied); SOC = skin of color; with SOC = Fitzpatrick skin Types IV + V + VI; without SOC = Fitzpatrick skin Types I + II + III.
Safety
The incidences of AEs assessed by the investigator as treatment related were similar among those with and without SOC: 14.3% versus 11.9%, respectively (See Supplemental Digital Content 1, Table 3, http://links.lww.com/DSS/A616). The most common treatment-related event was headache, which was reported in 12.1% and 8.2% of patients with and without SOC, respectively, an absolute difference of 3.9%. Treatment-related AEs of particular interest were uncommon. No patients with SOC experienced this type of event compared with 2.0% without SOC. The treatment-related AEs of particular interest that were reported in patients without SOC included eyelid ptosis (1.4%), brow ptosis (0.6%), blurred vision (0.6%), and diplopia (0.3%). Parallel trends were observed in the incidence of AEs among Blacks/African Americans and Whites without SOC. As was observed for all patients with SOC, no Black/African American experienced a treatment-related AE of particular interest.
Discussion
There are many reasons to suspect that patients with SOC might experience different outcomes from facial aesthetic procedures than patients without SOC. Most notably, SOC is associated with greater melanin content; the stratum corneum of Black/African American skin is more compact, and the dermis is thicker with more cornified cell layers, more active fibroblasts, and greater lipid content.3,5,14 As a result, SOC is more robust to the extrinsic factors of aging. The formation of fine lines and wrinkles are typically delayed by several years and are overall less common in patients with SOC than that in those without SOC.5 At the same time, SOC is susceptible to a number of intrinsic structural changes that include fat atrophy, loss of facial volume, and sagging skin of the lower face and neck.3
Despite these potential sources of disparity, this post hoc analysis establishes that the effectiveness of 20U prabotulinumtoxinA for the treatment of moderate-to-severe glabellar lines was similar in patients with and without SOC. Although the percentages of responders based on the GLS were consistently lower among patients with SOC, the mean absolute difference from those without SOC was 5.9% across all visits. At no time point were differences in the percentages of responders with and without SOC statistically significant. The authors postulate that these small but consistent differences in response between the 2 groups are explained, at least in part, by differences in the degree of previous botulinum toxin exposure recorded at baseline. In our study, compared with 30.0% of patients with SOC, 43.2% of patients without SOC reported previous exposure. In a separate post hoc analysis of this same population conducted for the purposes of regulatory approval, those without previous toxin exposure had a lower responder rate (by a mean of approximately 10%) than did those with previous toxin exposure (data on file, Evolus Inc.). In any case, based on the current analyses, response to treatment in these studies was robust in both patient groups—that is, with or without SOC, almost 50% of patients had a ≥1-point improvement on the GLS at maximum frown at Day 2, between 88.0% and 96.2% of patients did at Days 7, 14, and 30, and more than 30% of patients did at Day 150.
Following the observation that approximately half of those with SOC also self-identified as White (see final paragraph on study limitations), efficacy and safety outcomes were further compared between race-based subsets of those with and without SOC, in an effort to mitigate the impact of this potentially confounding factor. Unexpectedly, the percentages of responders on the GLS among the subsets of Blacks/African Americans and Whites without SOC did not directly parallel those observed among the larger populations of patients with and without SOC. This inconsistency between the subsets and the larger populations studied may simply be a by-product of the small sample size of Black/African American patients. In any case, although a similarly lower percentage (by a mean of 7.3%) of Blacks/African Americans achieved a ≥1-point improvement on the GLS at maximum frown from Days 2 through 30 compared with Whites without SOC, a greater percentage (by a mean of 6.2%) of Blacks/African Americans achieved this degree of response at Days 120 and 150. These results are particularly noteworthy given that approximately 10% fewer Blacks/African Americans than Whites without SOC had severe glabellar lines at maximum frown at baseline—that is, it might have been expected that a lower—not a higher—percentage of Black/African Americans would sustain a prolonged response of a ≥1-point improvement of the GLS at maximum frown. Furthermore, given that 27% fewer Blacks/African Americans had a history of previous botulinum toxin use, it might have been expected that a lower percentage of Blacks/African Americans would have been responders on the GLS at all time points assessed—not just during the first month post-treatment.
Data based on the investigator's assessment of the patients' overall aesthetic improvement mostly paralleled findings based on the GLS in the case of patients with and without SOC. Whereas the percentages of Blacks/African Americans assessed as being improved or much improved in their aesthetic appearance were consistently lower (by a mean of 9.1%) than that of Whites without SOC, despite better outcomes on the GLS at some time points. Patients with and without SOC were more similar in the assessment of their level of satisfaction, which remained high from Day 7 on. Of interest, at 4 of the 7 visits, a higher percentage of Blacks/African Americans were satisfied or very satisfied with their treatment.
Although various differences and inconsistencies in efficacy outcomes were observed between the populations studied, importantly, none of the differences noted in the percentages of responders for the primary efficacy end point were statistically significant at any study visit between those with and without SOC and between the race-based subsets of these populations. Accordingly, these efficacy data support the conclusion that no dose adjustments based on skin color are required in the administration of prabotulinumtoxinA therapy. Similarities in the percentages of patients with and without SOC who experienced treatment-related AEs and most common events support this conclusion. Of note, treatment-related headache was slightly more common in patients with SOC: 12.1% versus 8.2% in patients without SOC. Importantly, unlike those without SOC, none of the 140 patients with SOC, including the 37 Blacks/African Americans, experienced any treatment-related AEs of particular interest, such as eyelid ptosis, brow ptosis, diplopia, or blurred vision. At the same time, it is acknowledged that this observation may be a reflection of the difficulty in detecting rare events among a smaller patient population.
Limited data comparing outcomes with and without SOC have been published with other botulinum toxins. Our findings differ somewhat from an earlier report of a post hoc analysis of pooled data from 3 placebo-controlled Phase III studies investigating the effectiveness of 50U abobotulinumtoxinA (Dysport, Medicis Aesthetics, Inc., Scottsdale, AZ) in patients with glabellar lines.9 In that analysis, compared with Whites (n = 216), a significantly greater percentage of patients with SOC (n = 117) had a ≥1-point improvement on the GLS at maximum frown at Day 30 (p = .03). Yet, at all other time points, differences for this end point between those with and without SOC were not statistically significantly. Of interest, patients with SOC in that analysis included those who self-identified as Black, non-black Hispanic, Asian, Native American, or as other ethnicities self-reported as non-White; Fitzpatrick skin types were not reported. Furthermore, in one of the 3 trials, in which responder rates were compared only between Whites and Blacks, similar response rates were found at all time point for outcomes based on a ≥1-point improvement on the GLS at maximum frown.9
In the case of botulinum toxin studies, patients with SOC have often been under-represented. Post hoc analyses of pooled data from similar clinical studies, such as this one, are particularly useful in examining outcomes in subpopulations where there is limited representation in any one study. Still, there are limitations inherent to these types of analyses, particularly those attempting to distinguish patients based on SOC. In this case, it was necessary to broadly pool study patients into 2 dichotomous groups where all patients with SOC formed a single cohort, regardless of differences in ethnicity (e.g., African American, Native or East Indian, Asian, non-White Hispanic, etc.) or degree of skin pigmentation (i.e., skin Types IV vs V vs VI). Even so, only 140 of the 492 prabotulinumtoxinA-treated patients were assessed as having SOC. Although the authors also sought to restrict the group with SOC to only Blacks/African Americans, this further limited the data set available for evaluation to 37 patients. It should also be acknowledged that, in keeping with the FDA guidance on the collection of race and ethnicity data in clinical trials,15 the category of “White” broadly included all people who have their origins in any of the original peoples of Europe, the Middle East, or North Africa. No doubt, this mix of ethnicities is why many patients who self-identified as White were also categorized as having SOC based on their Fitzpatrick skin type. Even the category of Whites without SOC may still represent a broad group of ethnicities. It was an oversight in the original studies that data capturing Hispanic/Latino ethnicity were not collected and a shortcoming that more patients who self-identified as Black/African American were not enrolled.
Conclusions
Patients with SOC differ from those without SOC in a number of important ways, including skin structure and the pathophysiology of aging. Yet, limited data are available on comparative outcomes of aesthetic procedures in these 2 populations. Based on post hoc analyses of pooled data from the 492 prabotulinumtoxinA-treated patients who participated in the 2 US multicenter, randomized, double-blind, placebo-controlled, single-dose phase III clinical studies, a single dose of 20U prabotulinumtoxinA was well tolerated and similar in effectiveness for the treatment of glabellar lines in patients with and without SOC and in Blacks/African Americans and Whites without SOC. None of the differences in responder rates that were observed, based on the primary efficacy outcome of achieving a ≥1-point improvement on the 4-point GLS at maximum frown, reached a statistical significance. Accordingly, no dose adjustment based on skin color is believed to be necessary with this therapy. Patient satisfaction with their treatment remained high throughout the 150 days of follow-up.
Acknowledgments
The authors acknowledge the dedicated work of all investigators in the EV-001 and EV-002 studies, as follows:
EV-001: Jeffrey S. Dover and Lara Kruter, Chestnut Hill, MA; Steven Fagien, Boca Raton, FL; Dee Anna Glaser, Joseph Kallini, and Timur Galperin, St. Louis, MO; Miles Graivier, Alpharetta, GA; Pearl E. Grimes, Los Angeles, CA; Derek H. Jones and Naissan Wesley, Los Angeles, CA; Neil Sadick, Zeena Al-Dujaili, and Dina Began, New York, NY; Ava Theresa Shamban and Soheil Simzar, Santa Monica, CA; Stacy R. Smith and Sharon Boothe-Kepple, Encinitas, CA; and Robert A. Weiss, Margaret Weiss, Karen Beasley, and Christian Halvorson, Hunt Valley, MD.
EV-002: Kenneth R. Beer and Hillary Julius, West Palm Beach, FL; Sue Ellen Cox and Chris G. Adigun, Chapel Hill, NC; John H. Joseph, Beverly Hills, CA; Z. Paul Lorenc, New York, NY; Gary D. Monheit, James Highsmith, and Heidi Neugent, Birmingham, AL; Ronald L. Moy, Beverly Hills, CA; Alexander Rivkin and Robert Cohen, Los Angeles, CA; Harry H. Sharata and Blaine Jensen, Madison, WI; Susan C. Taylor, Philadelphia, PA; and, Vernon Leroy Young, Lisa Backues, Cyndi Buchanan, Ann-Elizabeth Mohart, Michelle Schaning, and Donna Straatmann, Washington, MO. The authors also gratefully acknowledge the contributions of the following: the study sponsor, Evolus, Inc. of Newport Beach, CA, with special mention of Gregg Peterson and Rose Monroe; and Song Wang at Pharmaceutical Product Development (PPD) of Wilmington, NC, who served as senior statistical consultant.
Footnotes
S.C. Taylor and J.H. Joseph served as principal investigators for the EV-002 clinical study; P.E. Grimes served as a principal investigator for the EV-001 clinical study. J.H. Joseph holds stock in Evolus, Inc., provided funding, study materials, equipment, and medications to all investigational sites. Evolus also provided funding to contract organizations involved in data collection, analysis, and reporting of the results, including these post hoc analyses. Anneke Jonker of Medical Writing Associates, West Vancouver, BC, Canada, provided technical assistance with manuscript preparation and submission; she holds stock in Evolus, Inc. R.L. Avelar is the Head of R&D and Chief Medical Officer for Evolus, Inc. and receives compensation in salary, stock, and stock options.
References
- 1.American Society of Aesthetic Plastic Surgery. Cosmetic (Aesthetic) Surgery National Data Bank Statistics. 2018. Available from: https://www.surgery.org/sites/default/files/ASAPS-Stats2018_0.pdf. Accessed January 21, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. In: Current Population Reports. Washington, DC. Mar: U.S. Census Bureau; 2015; pp. 25–1143. [Google Scholar]
- 3.Henry M, Sadick N. Aesthetic considerations in female skin of color: what you need to know. Semin Cutan Med Surg 2018;37:210–6. [DOI] [PubMed] [Google Scholar]
- 4.Burgess C, Awosika O. Ethnic and gender considerations in the use of facial injectables: African-American patients. Plast Reconstr Surg 2015;136:28S–31S. [DOI] [PubMed] [Google Scholar]
- 5.Vashi NA, Buainain de Castro Maymone M, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol 2016;9:31–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Alexis AF, Few J, Callender VD, Grimes P, et al. Myths and knowledge gaps in the aesthetic treatment of patients with skin of color. J Drugs Dermatol 2019;18:616–22. [PubMed] [Google Scholar]
- 7.Alexis A, Boyd C, Callender V, Downie J, et al. Understanding the female African American facial aesthetic patient. J Drugs Dermatol 2019;18:858–66. [PubMed] [Google Scholar]
- 8.Grimes PE, Shabazz D. A four-month randomized, double-blind evaluation of the efficacy of botulinum toxin type A for the treatment of glabellar lines in women with skin types V and VI. Dermatol Surg 2009;35:429–36. [DOI] [PubMed] [Google Scholar]
- 9.Taylor SC, Callender VD, Albright CD, Coleman J, et al. AbobotulinumtoixnA for reduction of glabellar lines in patients with skin of color: post hoc analysis of pooled clinical trial data. Dermatol Surg 2012;38:1804–11. [DOI] [PubMed] [Google Scholar]
- 10.Beer KR, Shamban AT, Avelar RL, Gross JE, et al. On behalf of the EV-001/EV-002 Study Group. Efficacy and safety of prabotulinumtoxinA for the treatment of glabellar lines in adult subjects: results from 2 identical Phase III studies. Dermatol Surg 2019;45:1381–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rzany BJ, Ascher B, Avelar RL, Bergdahl J, et al. A multicenter, randomized, double-blind, placebo-controlled, single-dose, Phase III, non-inferiority study comparing prabotulinumtoxinA and onabotulinumtoxinA for the treatment of moderate to severe glabellar lines in adult subjects. Aesthet Surg J 2020;40:413–29. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman-Janette J, Avelar RL, Biesman BS, Draelos ZD, et al. The first of two 1-year, multicenter, open-label, repeat-dose, Phase II safety studies of prabotulinumtoxinA for the treatment of moderate to severe glabellar lines in adult patients. Aesthet Surg J. [DOI] [PMC free article] [PubMed]
- 13.Lorenc ZP, Adelglass JM, Avelar RL, Baumann L, et al. The second of two 1-year, multicenter, open-label, repeat-dose, Phase II safety studies of prabotulinumtoxinA for the treatment of moderate to severe glabellar lines in adult patients. Aesthet Surg J. [DOI] [PMC free article] [PubMed]
- 14.Taylor SC. Skin of color: biology, structure, function and implications for dermatologic disease. J Am Acad Dermatol 2002;46(2 Suppl):S41–S62. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services Food and Drug Administration. Collection of Race and Ethnicity Data in Clinical Trials. Guidance for Industry and Food and Drug Administration Staff. Washington, DC: U.S. Department of Health and Human Services; 2016. [Google Scholar]


