Supplemental Digital Content is available in the text.
Keywords: blood pressure, diet, hypertension, meta-analysis, public health, sodium, systematic review
Abstract
Background:
The relationship between dietary sodium intake and blood pressure (BP) has been tested in clinical trials and nonexperimental human studies, indicating a direct association. The exact shape of the dose–response relationship has been difficult to assess in clinical trials because of the lack of random-effects dose–response statistical models that can include 2-arm comparisons.
Methods:
After performing a comprehensive literature search for experimental studies that investigated the BP effects of changes in dietary sodium intake, we conducted a dose–response meta-analysis using the new 1-stage cubic spline mixed-effects model. We included trials with at least 4 weeks of follow-up; 24-hour urinary sodium excretion measurements; sodium manipulation through dietary change or supplementation, or both; and measurements of systolic and diastolic BP at the beginning and end of treatment.
Results:
We identified 85 eligible trials with sodium intake ranging from 0.4 to 7.6 g/d and follow-up from 4 weeks to 36 months. The trials were conducted in participants with hypertension (n=65), without hypertension (n=11), or a combination (n=9). Overall, the pooled data were compatible with an approximately linear relationship between achieved sodium intake and mean systolic as well as diastolic BP, with no indication of a flattening of the curve at either the lowest or highest levels of sodium exposure. Results were similar for participants with or without hypertension, but the former group showed a steeper decrease in BP after sodium reduction. Intervention duration (≥12 weeks versus 4 to 11 weeks), type of study design (parallel or crossover), use of antihypertensive medication, and participants’ sex had little influence on the BP effects of sodium reduction. Additional analyses based on the BP effect of difference in sodium exposure between study arms at the end of the trial confirmed the results on the basis of achieved sodium intake.
Conclusions:
In this dose–response analysis of sodium reduction in clinical trials, we identified an approximately linear relationship between sodium intake and reduction in both systolic and diastolic BP across the entire range of dietary sodium exposure. Although this occurred independently of baseline BP, the effect of sodium reduction on level of BP was more pronounced in participants with a higher BP level.
Clinical Perspective.
What Is New?
A comprehensive dose–response meta-analysis of trials detailing the effects of changes in dietary sodium on blood pressure (BP), using the most up-to-date statistical dose–response modeling, shows that the relationship is positive, and almost but not entirely linear.
The sodium change–BP relationship was present in analyses of long-term trials, although slightly attenuated compared with the corresponding finding in short-term studies, and was noted in both analyses based on differences in sodium intake between study arms and achieved sodium intake.
Higher background sodium consumption and BP increase strength and steepness of the effects on BP by changes in sodium intake.
What Are the Clinical Implications?
The clinical implications of a substantially linear positive relationship between sodium intake and BP even in the long-term trials are that a progressively large reduction in BP can be expected with decreases in sodium consumption down to levels as low as 1 to 1.5 g/d, with no evidence for a threshold in benefit.
Advice to reduce dietary sodium intake applies not only to adults with hypertension, who can be expected to derive a substantial reduction in BP, but also to those without hypertension, in whom the expected reduction in BP is smaller but still important.
Editorial, see p 1568
The association between dietary sodium intake and blood pressure (BP) is one of the most widely investigated and relevant issues for nutritionists and cardiovascular disease (CVD) epidemiologists.1–4 Early clinical trial reports of systolic BP (SBP) and diastolic BP (DBP) reduction after experimental exposure to lower levels of dietary sodium intake in humans through a randomized controlled trial (RCT) design were first published in the 1980s and 1990s, and a large number of such studies has been published to date.2–10 Comprehensive reviews of the observational evidence both in children and in adults have consistently identified a positive association across a wide range of intake,11,12 although this type of evidence is limited by 2 major methodologic issues: potential for exposure misclassification and unmeasured confounding. The World Health Organization, professional societies, government agencies, and guidelines recommend sodium intake reduction for prevention and management of high BP.13–20 However, the strength of the sodium–BP relationship has been challenged by some investigators, particularly in individuals without hypertension3,21 and for DBP.2 To our knowledge, no previous meta-analysis has been able to fully characterize the shape of the dose–response relationship throughout the entire range of dietary sodium exposure while adequately taking into account heterogeneity across the studies. This deficit can be explained by lack of a flexible modeling framework capable of incorporating studies encompassing fewer than 3 levels of exposure, as in 2-arm RCTs,2 to assess the dose–response influence of changes in sodium intake on BP levels. Previous reports have ignored either heterogeneity across the studies or the shape of the dose–response relationship. In general, linear dose–response meta-regressions of RCT results2,3 or forest plots on the basis of RCT subgroup pooling4,8 have been computed and presented to describe the sodium–BP relationship in humans. Two reports have presented results of a nonlinear dose–response analysis but they are limited because they only studied SBP effects, were based on differences in sodium exposure between the study arms but not on overall (achieved) sodium intake, and assumed a single common dose–response relationship underlying multiple studies by using a fixed-effects model.10,19
A 1-stage or mixed-effects framework suitable for synthesis of tables of empirical contrasts has recently become available,22,23 with the key advantage that even studies with a single comparison can be included in the estimation of heterogeneous and possibly curvilinear dose–response relationships. Given the importance of this issue for its public health implications, that is, the central role of high BP as a risk factor for CVD, particularly stroke, coronary heart disease, and heart failure,15 the recent availability of a novel statistical approach prompted us to design a dose–response meta-analysis of trials to explore the effect of sodium intake on BP over a wide range of exposure, also stratifying for factors of interest.
Methods
All supporting data are available within the article and its Data Supplement.
Literature Search
We conducted a systematic search of online databases (PubMed/MEDLINE, EMBASE, and CENTRAL [Cochrane Central Register of Controlled Trials]) through October 12, 2020, for reports of RCTs that had tested the effect of dietary sodium reduction on BP levels. No language restriction was applied. We used as key terms “sodium” and “blood pressure.” Detailed search strategies are reported in Table I in the Data Supplement. We checked the reference lists of articles generated by the search and performed backward and forward citation chasing to identify other eligible publications. Title and abstract screening and subsequent full-text evaluation were performed in duplicate by 2 authors (Drs Filippini and Malavolti). A third author (Dr Vinceti) helped resolve differences.
Using PECO (population, exposure, comparator, and outcomes) recommendations,24 the eligibility inclusion criteria were (1) participants with and without hypertension but excluding secondary hypertension; (2) intervention performed comparing low sodium exposure with high sodium exposure within an experimental dietary intervention encompassing either sodium reduction compared with normal diet or sodium reduction followed by supplementation with sodium or placebo tablets; (3) comparator being normal/high sodium diet or placebo administration, without mixed intervention components in which contribution of sodium could not be determined; (4) SBP or DBP, or both, measured as an outcome of interest; (5) 24-hour urinary sodium excretion measured before and after the intervention; and (6) trial duration of at least 4 weeks. Use of a salt substitute that partially replaced sodium with potassium was considered an exclusion criterion if the intervention had been administrated to 1 group only. When relevant data were missing, we sought to contact study authors for retrieval of the necessary information so it could be included in the review.
Risk of Bias Assessment
We conducted an independent assessment of study quality using the revised Risk of Bias assessment tool version 2.0.25 The following 5 risk of bias domains were considered: (1) randomization process errors; (2) deviations from the intended interventions; (3) missing outcome data; (4) measurement of the outcome; and (5) selection of the reported result. Each domain could be characterized as having a low risk of bias, some concerns, or a high risk of bias. A study was assigned an overall higher risk of bias if it was judged to be at higher risk for at least 1 domain and an intermediate risk of bias when some concern existed in at least 1 domain.
Data Extraction
We extracted the following data from included studies: first author name, publication year, country, duration of sodium intervention phase, number of participants and characteristics (including among other factors hypertension status and use of antihypertensive medication), study design (crossover or parallel), modality of BP measurement (type of device: manual or automatic, and position: supine, sitting, standing, 24-hour, others), type of sodium intervention, baseline and achieved sodium excretion level, and SBP and DBP mean difference between intervention and control groups, along with the SE at the end of the intervention periods. When SE was not directly reported, it was calculated from SD, CI, or exact P value following the Cochrane Handbook for Systematic Reviews of Interventions.26
Data Analysis
We performed a random-effects dose–response meta-analysis assessing the relationship between changes in sodium excretion or overall sodium excretion at the end of the trial and changes in SBP and DBP levels using the 1-stage mixed effect meta-analytic model for aggregated data recently described in detail.22,23,27,28 We used restricted cubic splines of sodium with 3 knots at fixed percentiles (10%, 50%, and 90%) having no a priori assumptions regarding the shape of the association. No constraints were imposed on the variances or covariance of the random effects placed on the 2 regression coefficients of the splines. For comparison, we also modeled sodium using a simpler linear function, which is nested within the restricted cubic spline function. Estimates of the measures were obtained with the restricted maximum likelihood method.22,29 Statistical inference was primarily based on the summary dose–response relationship.
We used level of sodium excretion at the end of the trial in each arm as achieved sodium excretion and net difference between urinary sodium excretion at the end minus the beginning of the trial in each randomized arm as difference in sodium excretion. We defined the mean difference in BP after the intervention as the difference for either SBP or DBP at the end minus the corresponding baseline value in the active and control arms of the trial. We used a reference value of 87 mmol/d, which corresponds to 2 g of sodium (or 5 g of salt), the value recently defined as safe and adequate intake for the European adult population by the European Food Safety Authority20 and not to be exceeded by World Health Organization13 and European professional societies,18 being also close to the slightly lower14,17 and higher19 values defined by US bodies. We assumed sodium excretion to be identical to sodium intake (or exposure) in this article, on the basis of the very small difference between the 2 values in individuals and populations.20
We carried out stratified analyses based on study design (parallel versus crossover), hypertension status, use of antihypertensive medication, and length of follow-up. In a sensitivity analysis, we excluded trials at high risk for bias.
We examined small-study bias by using funnel plots and the Egger test. The Egger test aims to measure bias direction and magnitude using the intercept from a linear regression analysis between the effect estimate against its SE.30 Intercept values <0.6, from 0.4 to 1.0, from 0.8 to 2.0, and >1.8 have been suggested as indicators of unimportant, moderate, substantial, and considerable small-study effects, respectively.31 We also used the trim-and-fill method by including the observed studies to estimate the suppressed studies in order to correct for small-study effects based on funnel plot asymmetry.32 Both analyses were based on the restricted maximum likelihood random effects method. We used Stata statistical software (v16.1, StataCorp, College Station, TX) for our data analysis, including the meta routine and the 1-stage approach based on the drmeta command.23,27,33
Results
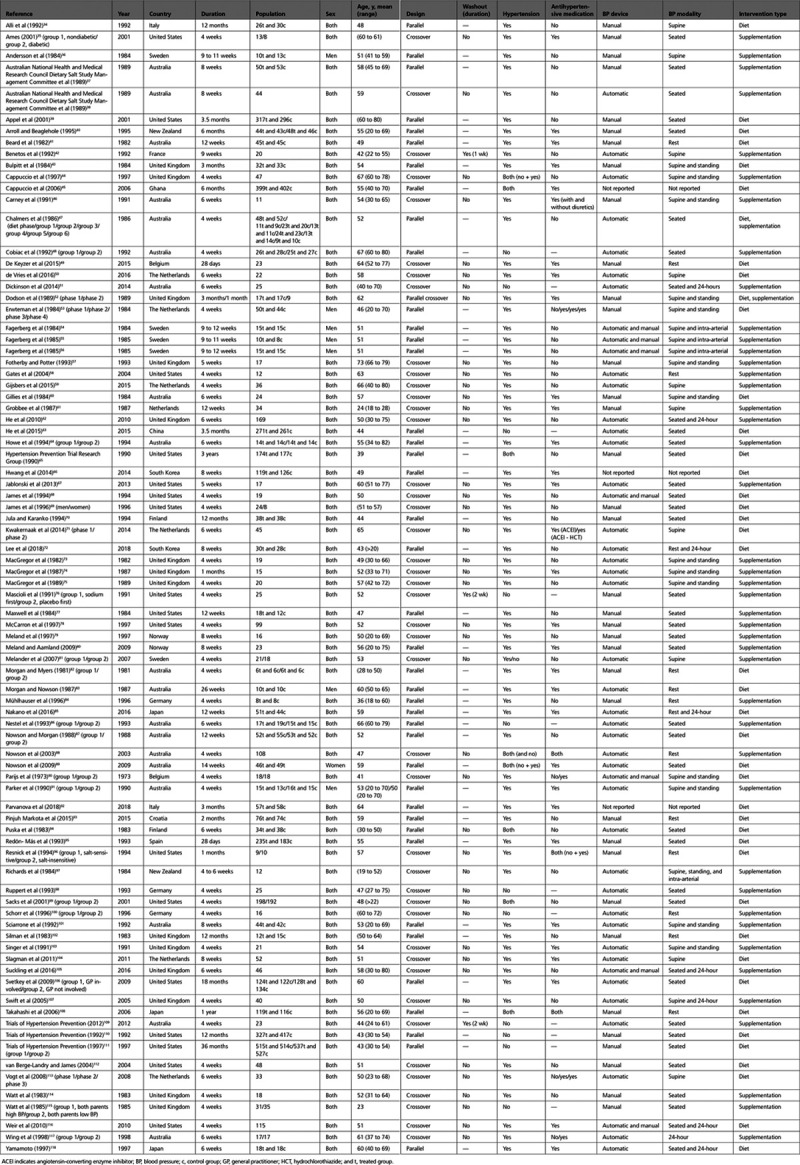
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) literature search flowchart is presented in Figure 1. We retrieved 6744 publication titles, and after title and abstract screening, evaluation of 146 full-text reports resulted in identification of 85 trial articles that could be included in the analysis. Reasons for exclusion included absence of either 24-hour urinary sodium measurements or BP levels (n=25), duplicate reports on the same population (n=18), duration <4 weeks (n=7), determination that sodium reduction was not the trial intervention (n=10), and use of a salt substitute containing potassium in the intervention but not in the control group (n=1). Table 1 presents selected characteristics of the 85 trials included in the analysis.34–118 The reports, which were published between 1973 and 2018, were based on an overall sample size >10 000 participants and included trials conducted in Europe (n=40), Oceania (n=21), North America (n=17), Asia (n=6), and Africa (n=1). Most of the trials (n=76) included men and women, but sex stratification of the results was only available in 4 reports. One study included only women and 7 studies only men. The mean age ranged from 23 to 73 years (overall range, 18 to 82 years). Parallel and crossover designs were equally represented, with only 3 of the latter including a washout period of 1 or 2 weeks. Most trials were limited to participants with hypertension (n=65), but 11 were conducted in participants without hypertension and 9 included adults with and without hypertension, in 2 cases presenting a stratified analysis by hypertension status and in 2 others an analysis restricted to participants without hypertension. In trials including participants with hypertension, treatment with antihypertensive medication was either continued during the trial (n=35) or discontinued (n=28); in 6 trials, results both with and without antihypertensive treatment were presented. Sodium intervention included sodium reduction and then administration of a sodium-containing supplement to 1 arm (n=43), or diet modification through a broad spectrum of interventions aimed at achieving sodium reduction (n=38); in 2 trials, both dietary modification and sodium supplementations were used. Dietary advice for sodium reduction ranged from instructions not to add salt during cooking and at the table, suggestion of dietary regimens as well as tailored diets prepared by a dietitian, to more complex and sophisticated interventions. Examples of the latter include small group counseling for several weeks, followed by large group counseling, and individualized monitoring and feedback to assist participants in achieving and maintaining the desired interventions. Types and modalities of trial interventions are detailed in Table II in the Data Supplement. In almost all of the trials that included sodium supplementation, dietary intake of sodium had been restricted before the trial, and sodium supplements ranged from 50 to 190 mmol/d (1.1 to 4.4 g/d), but most of the trials implemented an amount of 80 to 100 mmol/d (1.8 to 2.3 g/d). All the studies estimated 24-hour sodium excretion in each study arm, both at baseline and at the end of the trial. The difference in sodium excretion between intervention and control groups ranged from to 5 to 309 mmol/d (0.1 to 7.1 g/d) with a median value of ≈80 mmol/d (1.8 g/d). The level of achieved sodium intake at the end of the trials ranged from 17 to 330 mmol, ie, 0.4 to 7.6 g/d.
Figure 1.

Flow chart of systematic literature search for trials, published through October 12, 2020, that met the study inclusion and exclusion criteria.
Table 1.
Study Characteristics of the 85 Trials Included in the Analysis
Risk of bias assessment is presented in Table III in the Data Supplement. Almost all studies resulted in intermediate risk of bias because of lack of evidence of randomization process or missing information about concealment methods. Two trials, both carried out in hypertensive participants and using dietary modification, resulted in a high risk of bias caused by deviations from the intended interventions34 or use of an unblinded study design.49
In the dose–response assessment of the effect of achieved sodium intake on BP levels, we used 87 mmol/d, corresponding to 2 g/d of sodium intake, as the reference value. In Tables 2 and 3, we report the SBP and DBP differences estimated by means of random-effects dose–response spline regression models from this cut point of exposure at different levels of sodium excretion, ie, for 0.5 g increases starting at 1.5 g/d, also taking into account some study characteristics that were effect modifiers. We observed generally stronger dose–response relationships for both SBP and DBP in trials based on overall dietary modification compared with those based on sodium supplementation, in both instances with a steeper dose-response for SBP compared with DBP across the entire range of sodium excretion. We also found a stronger BP effect of achieved sodium intake in participants with hypertension compared with those with normal BP and in women compared with men. In an analysis based on the overall range of exposure (Figure 2), achieved sodium excretion was positively and almost linearly associated with changes in SBP and DBP over a wide range of intake (0 to 300 mmol/d of sodium excretion), although the curve for SBP was steeper than for DBP. The overall BP difference over the entire range of sodium exposure was >15 mm Hg for SBP and nearly 10 mm Hg for DBP. In linear regression analysis, every 100 mmol/d reduction in urinary sodium excretion was associated with a lower mean SBP of 5.56 mm Hg (95% CI, −4.52 to −6.59) and a lower mean DBP of 2.33 mm Hg (95% CI, −1.66 to −3.00). In the trials that used sodium supplementation, the mean (95% CI) decrease in BP for a 100 mmol/d reduction in sodium excretion was 4.47 mm Hg (95% CI, −3.08 to −5.86) for SBP and 1.90 mm Hg (95% CI, −0.99 to −2.81) for DBP and the corresponding reduction in the trials that used a behavior change intervention was 6.63 mm Hg (95% CI, −5.12 to −8.15) for SBP and 2.79 mm Hg (95% CI, −1.80 to −3.78) for DBP. Similarly, every 1 g/d decrease of sodium excretion was associated with a lower mean (95% CI) SBP and DBP of 2.42 mm Hg (95% CI, −1.97 to −2.87) and 1.01 mm Hg (95% CI, −0.72 to −1.31), respectively. Corresponding values of mean (95% CI) SBP and DBP reduction were 1.94 mm Hg (95% CI, −1.34 to −2.55) and 0.83 mm Hg (95% CI, −0.43 to −1.22) in the studies where initial dietary sodium reduction was followed by sodium supplementation in the control arm and 2.88 mm Hg (95% CI, −2.23 to −3.54) and 1.21 mm Hg (95% CI, −0.78 to −1.64) in studies where sodium reduction was achieved by dietary modification.
Table 2.
Dose–Response Relationship Between Achieved Sodium Excretion and Systolic Blood Pressure

Table 3.
Dose–Response Relationship Between Achieved Sodium Excretion and Diastolic Blood Pressure

Figure 2.

Dose–response meta-analysis of changes in SBP and DBP levels (mm Hg) according to achieved sodium excretion in the treatment and control groups at the end of the trials (all studies) and by type of intervention (supplementation or diet). The average curve (solid line) with 95% confidence limits (dashed lines) was estimated with a 1-stage random-effects restricted cubic spline model, using 2 g/d as referent. DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
Figure 3 shows the dose–response range according to presence or absence of hypertension at baseline. Both subgroups showed a tendency for BP lowering after a reduction in dietary sodium and a roughly linear association between achieved sodium intake and BP change at the end of the trials, with the exception that there was little evidence of BP effect in the participants without hypertension whose sodium intake was <2 g/d. The participants with hypertension had a much steeper dose-response for mean SBP and mean DBP over the entire range of achieved sodium excretion, and therefore at the highest and the lowest exposure levels the BP differences were considerably larger compared with those seen in the participants without hypertension. The BP changes at the extremes of sodium intake were more statistically imprecise for those without hypertension (based on 15 studies) than for those with hypertension (based on 67 studies), with wider CIs for the point estimates particularly at the lowest exposure levels for DBP. At a sodium intake as high as 6 g/d compared with 2 g/d, in participants without hypertension, mean (95% CI) SBP and DBP increases were 3.99 mm Hg (95% CI, +0.80 to +7.18) and 1.66 mm Hg (95% CI, −0.58 to +3.91), respectively. In participants with hypertension, the corresponding differences were 10.31 mm Hg (95% CI, +7.86 to +12.75) and 5.13 mm Hg (95% CI, +3.52 to +6.74). Based on use of a linear function in participants without hypertension, a 100 mmol/d decrease in sodium intake was associated with a reduction in mean (95% CI) SBP and DBP of 2.30 mm Hg (95% CI, −1.33 to −3.27) and 0.80 mm Hg (95% CI, +0.29 to −1.89), respectively. The corresponding SBP and DBP reductions in participants with hypertension were 6.50 mm Hg (95% CI, −5.22 to −7.79) and 3.00 mm Hg (95% CI, −2.27 to −3.74), respectively. Limited differences emerged when we further stratified the trials according to the method used to achieve the intervention effect (sodium restriction followed by supplementation versus dietary modification through behavior change; Figure I in the Data Supplement) and the hypertensive status. Similar results were obtained in participants with hypertension whether or not they were being treated with antihypertensive drug therapy, except for a higher SBP level for those with a very high sodium intake who were receiving antihypertensive drug therapy (Figure II in the Data Supplement), particularly in those subject to a dietary intervention (Figure III in the Data Supplement). When we stratified the analysis according to a baseline SBP <140 mm Hg versus ≥140 mm Hg in participants with hypertension, we found substantially similar BP effects for both categories of sodium intake (Figure IV in the Data Supplement). A 100 mmol/d decrease in sodium intake was associated with a reduction in mean (95% CI) SBP and DBP of 7.79 mm Hg (95% CI, −4.90 to −10.67) and of 3.10 mm Hg (95% CI, −1.37 to −4.83), respectively, in the trial participants with a baseline SBP <140 mm Hg, and of 6.06 mm Hg (95% CI, −4.64 to −7.48) and 2.99 mm Hg (95% CI, −2.17 to −3.81) in the trial participants with a baseline SBP ≥140 mm Hg. However, a stronger effect on BP (particularly SBP) was noted at higher sodium intake (>4 g/d) in participants with hypertension whose SBP was <140 or ≥140 mm Hg, after exclusion of individuals taking antihypertensive medication (Figure V in the Data Supplement).
Figure 3.

Dose–response meta-analysis of changes in SBP and DBP levels (mm Hg) according to achieved sodium excretion in the treatment and control groups at the end of the trials divided by hypertension status (no hypertension and hypertension). The average curve (solid line) with 95% confidence limits (dashed lines) was estimated with a 1-stage random-effects restricted cubic spline model, using 2 g/d as referent. DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
When we stratified according to baseline sodium excretion (<109 mmol/d versus ≥109 mmol/d, ie, <2.5 g/d versus ≥2.5 g/d), we found stronger BP effects of increased sodium intake in participants with higher background usual sodium dietary intake for both SBP and DBP (Figure VI in the Data Supplement).
When we considered trial duration (4 to 11 weeks versus ≥12 weeks), the dose–response relationship demonstrated some differences across time and BP end point (Figure 4). The gradient for SBP was steeper in short-term studies. The gradient for DBP was considerably steeper at medium to high levels of urinary sodium excretion in the studies with a duration <12 weeks compared with ≥12 weeks, but the reverse was true at lower levels of urinary sodium during the conduct of the trial. Further exploration by baseline presence or absence of hypertension yielded similar results with a steeper dose–response slope for the shorter studies in participants with hypertension; the corresponding dose–response slope in the shorter studies conducted in participants without hypertension showed similar results compared with the longer studies, apart from a lack of effect on DBP at very low exposure levels, ie, <2 g/d (Figure VII in the Data Supplement).
Figure 4.

Dose–response meta-analysis of changes in SBP and DBP levels (mm Hg) according to achieved sodium excretion in the treatment and control groups at the end of the trials stratified by trial duration (4–11 weeks or ≥12 weeks). The average curve (solid line) with 95% confidence limits (dashed lines) was estimated with a 1-stage random-effects restricted cubic spline model, using 2 g/d as referent. DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
A stratified analysis based on study design (parallel versus crossover) showed a steeper dose–response curve in crossover studies for SBP and DBP at high levels of urinary sodium excretion, with a similar pattern in crossover studies overall considered compared with those without a washout period (the large majority of them; Figure VIII in the Data Supplement). Conversely, <2 g/d of sodium intake crossover trials, in contrast with parallel designs, failed to document a reduction in DBP at low levels of urinary sodium excretion. Subgroup analysis in men and women did not show evidence of sex-related differences except for a slightly steeper association in women, although these estimates were statistically imprecise, being based on only 16 studies (11 in men and 5 in women; Figure IX in the Data Supplement). No age-related difference emerged after restriction of the analysis to participants ≤55 years of age, the only age subgroup for which we could compute a pattern because in most studies participants had a mixed age and no age-specific estimates were reported.
We also assessed the effect of the difference in urinary sodium excretion attributable to the intervention and control treatments on BP, independent of baseline sodium consumption. We found that a larger difference in sodium intake was associated with a larger effect on both SBP and DBP, with an approximately linear positive association (Figure 5). Similarly to the analysis on achieved sodium intake reported in Figure 2, when we stratified the analysis according to the methodology used to achieve the changes in sodium intake across trial arms, we found that the dietary changes were much more effective in producing BP changes compared with sodium supplementation alone. The nearly linear association between sodium difference between the treatment arms and BP changes found in the overall analysis also emerged in an analysis that stratified for baseline presence or absence of hypertension, albeit with a much steeper dose–response curve in the participants with hypertension (Figure 6). Likewise, the pattern was seen in trials of shorter and longer duration (Figure X in the Data Supplement).
Figure 5.

Dose –response meta-analysis of changes in SBP and DBP levels (mm Hg) according to the difference in sodium excretion between the treatment and the control groups at the end of the trials (all studies) and by type of intervention (supplementation or diet). The average curve (solid line) with 95% confidence limits (dashed lines) was estimated with a 1-stage random-effects restricted cubic spline model. DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
Figure 6.

Dose –response meta-analysis of changes in SBP and DBP levels (mm Hg) according to the difference in sodium excretion between the treatment and the control groups at the end of the trials divided by hypertension status (no hypertension and hypertension ). The average curve (solid line) with 95% confidence limits (dashed lines) was estimated with a 1-stage random-effects restricted cubic spline model. DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
Taking into account the risk of bias of the included studies, we reran the main analyses after removing the 2 studies at high risk, with little effect on the results, even after limiting the analysis to trials based on dietary modifications, which was the intervention modality in the 2 excluded studies (Figure XI in the Data Supplement). Funnel plot analyses suggested moderate small-study effects for SBP and moderate to substantial effects for DBP considering all studies (Figure XII in the Data Supplement), as confirmed by the trim-and-fill analysis (Figure XIII in the Data Supplement). Stratified analysis by type of intervention and hypertension status provided some evidence that small-study bias may have occurred in the dietary studies (Figures XIV and XV in the Data Supplement) and in those carried out in participants with hypertension (Figures XVI and XVII in the Data Supplement).
Discussion
We conducted a dose–response meta-analysis of clinical trials that had investigated the effects of sodium reduction on level of BP. Our review was not conducted because of uncertainty regarding whether a reduction in sodium intake lowers BP, for which consistent evidence has been accumulated over decades.1,4,7 Instead, our endeavor was prompted by a desire to determine whether the dose–response relationship between changes in sodium intake and BP is linear or curvilinear, to identify any thresholds for the relationship, and to specifically assess the relationship in the group for whom the sodium–BP association has been most challenged, ie, in adults without hypertension.3,21 We were also influenced by our recent indication of a U-shaped dose–response relationship between potassium intake and BP, previously undetected using traditional meta-analyses that use forest plots or linear models.119 Many systematic reviews and meta-analyses have attempted to assess the sodium–BP relationship in experimental studies, including 2 recent reports,3,4 but they have been unable to fully characterize it because they have used linear models or stratified the exposure by category of sodium reduction between intervention and control arms in each study, or at baseline, thus being unable to smoothly shape the relationship between sodium intake and BP over the entire range of exposure. These recent meta-analyses included a less comprehensive literature database compared with the one we used, and this also resulted in greater statistical precision for the effect estimates we computed, including those for participants without hypertension, the subgroup characterized by the lowest number of available studies.
Our statistical approach differs from previous meta-analyses.10,19 Mozaffarian et al10 reported 2 spline regression analyses of trials of any duration or of at least 1-week duration, based on the literature considered in 2011 and 2013 Cochrane meta-analyses.7,120 The 2019 National Academies of Sciences, Engineering, and Medicine Dietary Reference Intakes Committee reported a meta-analysis based on trials lasting at least 4 weeks.19 These 2 meta-analyses, however, only assessed change in SBP for differences in sodium intake between the study arms, also assuming a single common dose–response relationship underlying multiple studies, as implied by the use of a fixed-effects model. A recent meta-analysis by Huang et al4 also attempted to characterize the dose–response relationship between sodium exposure and BP. In that review, contrasts across studies in each quantile of the dose were combined by computing multiple models. Our 1-stage modeling allowed us to preserve the study-specific structure of contrasts in the estimation of the dose–response relationship within a single model. Huang et al4 also had to categorize sodium exposure dose according to data-dependent quantiles, whereas we could flexibly model the dose as a quantitative variable, fitting a single dose–response random-effects model that takes into account heterogeneity across the studies. We considered the effect of achieved level of sodium intake at the end of the trial on BP in addition to the effect of the difference in sodium excretion between the treatment arms, thus making the results more applicable to risk assessment in clinical practice and public health.
Overall, we found only small departures from linearity for the association between sodium intake and BP lowering across the entire range of exposure tested and achieved in the trials. This was confirmed by the analyses based on difference in sodium intake between intervention and control arms. Dietary sodium reduction was accompanied by an approximately linear decrease in SBP and by a somewhat less steep but still nearly linear decrease in DBP. The SBP reduction of 5.4 mm Hg associated with a 100 mmol/d decrease in achieved sodium intake that we report is similar to a previous finding of 5.8 mm Hg by He et al,7 although our results were much more statistically stable (95% CI, 4.4–6.5 versus 2.5–9.2, respectively). A decrease in achieved sodium intake <2 g/d was accompanied by a considerably larger decrease in SBP compared with DBP. This difference was slightly more apparent when we limited the analysis to trials based on initial sodium restriction followed by behavior change interventions in 1 of the 2 arms, which may modify the effects of changes in sodium intake caused by other dietary changes, compared with studies based on dietary sodium reduction on the basis of sodium supplement administration. In the analysis that is based on sodium supplement administration, the reduction in DBP <2 g/d of sodium intake was almost null, thus providing some support to use of this value as a threshold with reference to the effect of sodium reduction on DBP. However, there was no suggestion of a threshold for the effect of sodium reduction on SBP. Overall, our findings suggest that the effect of sodium reduction on BP is beneficial across a wide range of intake, supporting recommendations to reduce sodium intake as much as possible but in particular to achieve a reduction that would meet the current dietary recommendations of 2 to 2.3 g/d,19,20 mainly based on the aim to reduce BP and the related increased risk of stroke and other CVD complications.8,15,121
The aforementioned assessment is based on the major strength of the present study: use of a novel statistical methodology that allows use of 2-arm comparisons to obtain a continuous modeling of the dose–response relationship between sodium reduction and BP while retaining the matched design of trials. This method avoids the rigidity of linear functions and the pitfalls of forest plot analyses that can result from nonhomogeneous and extreme categories of exposure and is particularly helpful for analysis of nonlinear relationships, as recently reported for potassium.119 Our confirmation of a linear relationship between sodium intake and BP is consistent with the strong positive association noted in recent dose–response meta-analyses between sodium intake and the risk of stroke, a disease for which high BP is the leading risk factor.122–124 This positive dose–response relationship yielded by the experimental evidence in humans overall available also mirrors what has been already noted in the few single RCTs encompassing more than 2 categories of sodium intake, though within a narrower range of sodium exposure compared with the whole set of RCTs. A well-known example is the trial carried out to assess the effects of 3 decreasing amounts of sodium restriction in participants consuming either a typical US diet or a DASH (Dietary Approaches to Stop Hypertension) diet, where a clear dose–response relationship between sodium exposure and both SBP and DBP was noted.99 Another study, of much smaller size, encompassed 3 categories of sodium exposure in normotensive participants and could not find a dose–response relationship with either SBP or DBP.109
A key issue in the controversy regarding the efficacy of sodium reduction for BP lowering is the background level of BP in those being treated. There is general acceptance of a beneficial effect in adults with high BP but not everyone has accepted the premise that reduction in dietary sodium is effective for BP reduction in adults with lower levels of BP.1,3,125,126 Our meta-analysis results are consistent with many previous meta-analysis reports in showing a larger reduction in BP in those with a higher starting level of BP but we noted a similar pattern of effect, albeit with smaller reductions in BP, in those with a lower starting level of BP,4,7,8 contrary to the null effect in some previous reports.2,3,21 This was particularly true for SBP, a major risk predictor for CVD and chronic kidney disease.19,127,128 We found little evidence that decreasing sodium intake <2 g/d lowered DBP in participants without hypertension, but the relevant estimate was imprecise. In participants with hypertension, a higher baseline BP level (as categorized by use of an SBP cut point of 140 mm Hg) was associated with a greater capacity of increased sodium intake to raise BP, suggesting more adverse effects of excessive sodium intake and more evident beneficial effects reducing sodium intake for those with more severe hypertension.
In general, similar reductions in BP levels were achieved by use of interventions that sought to reduce sodium intake by diet modification and by initial dietary sodium reduction followed by selective targeting of sodium intake using different doses of sodium supplement pills, with some interesting exceptions. Specifically, difference in sodium intake induced by sodium supplementations was less effective in modifying both SBP and DBP compared with overall dietary change, particularly at low levels of intake (<2 g/d of sodium). In contrast, dietary changes proved to be more effective in reducing BP at such low levels of sodium intake. Dietary change results in modification of the overall dietary pattern, which may encompass interactions among multiple modifications of nutrients, potentially yielding a greater reduction in BP at very low levels of sodium intake, and highlighting the relevance of targeting overall dietary pattern and not only sodium intake alone when dealing with dietary interventions to reduce BP levels.129–132 On the other hand, the slightly stronger BP decrease induced by selective difference in sodium intake at very high levels of intake, as compared with broader dietary changes, suggests that the simple reduction of sodium intake at such high levels of exposure is a highly effective tool to effectively achieve large reduction in BP levels, also being a more feasible approach at the population level.
In stratified analyses, we noted an apparent modification of the capacity of sodium exposure to increase BP according to study design, with a stronger association in crossover studies compared with parallel trials only at high levels of sodium intake. At low levels of sodium intake, failure of crossover trials, in contrast with parallel designed trials, to document a reduction in DBP after the sodium restriction could have been attributable to the lack of adequate (or any) washout period, although such a hypothesis remains speculative. Failure to detect sex- and age-specific differences in our dose–response meta-analysis is also of interest, suggesting the absence of specific susceptibility for these factors to modify the effect of sodium reduction on BP, although the number of trials for each subgroup was relatively small.
The effect of study duration on the relationship between sodium reduction and BP is of interest. We excluded trials with a duration <4 weeks, as done in other recent reviews and meta-analyses,2,7,8,19,22 because many of them have involved extreme and acute nonphysiologic changes in sodium intake, and because sodium reduction interventions, especially those based on behavioral change, are unlikely to demonstrate their full effect in a period <1 month.133 In our analysis, trials with a duration ≥12 weeks showed weaker effects on BP compared with trials with a duration of 4 to <12 weeks, but the dose–response relationship was still substantially linear and meaningful. Our finding that a positive, almost linear dose–response relationship between sodium intake and BP was still present, without any evidence of a threshold for effect, in trials of longer duration conducted in those with lower levels of BP has special importance for population intake recommendations and public health, given the importance of BP as a risk factor for stroke, heart failure, and many other CVD and renal complications.15,121,128,134 Our analysis also has an important advantage over previous meta-analyses such as that by Huang et al4 in that we could independently assess the effect of duration versus the different intensities of sodium reduction in short-term versus long-term trials. Because the trials with longer duration had a much smaller contrast in sodium intake compared with the trials of shorter duration, the previous analyses did not generally allow for separation of the effects of duration versus dose on change in BP.
We also found some evidence that background habitual sodium intake could influence the relationship between changes in sodium intake and level of BP, with a greater capacity for high-dose sodium supplementation to increase BP in those with a higher usual sodium intake. This suggests a greater susceptibility to the sodium-driven BP increases after consumption of diets with higher sodium content, or conversely that the capacity of reductions in dietary sodium to lower BP is enhanced in those generally consuming more sodium in their diet.
This meta-analysis has strengths in addition to its key feature, the capacity to carry out a comprehensive, flexible dose–response assessment based on RCTs where only 2 levels of contrast were studied. We were able to include a relatively large number of trials that allowed for broad representation of adults, including those with and without high BP, and substantial precision for most of our estimates. We studied a much wider range of dietary sodium exposure than is possible in any individual trial. Moreover, we excluded trials with a duration <4 weeks, allowing for more reliable inferences about the relationship between long-term sodium intake and BP. Our findings, particularly those based on the RCTs with the longest duration, should closely mirror the effects of habitual sodium intake on BP. Our findings were strengthened by the limited evidence of small-study bias in the overall analysis. We acknowledge that such bias might have partially affected the analysis in studies based on dietary modifications and in those with participants who had hypertension.
We are also aware of an important limitation of our meta-analysis, ie, the statistical instability of some point estimates for the highest and lowest dietary sodium exposure, particularly for participants without hypertension and, for those with hypertension, in trials with the longest duration.
Our analysis does not represent a direct assessment of the sodium–CVD risk relationship, a major issue of cardiovascular health and more generally human health.135–137 However, the effect of sodium intake on BP is inherently of major interest, given the importance of BP to CVD morbidity and mortality, and the use of BP as a surrogate end point for CVD.19 Therefore, the findings in this review and dose–response meta-analysis, confirming and strengthening previous reports and providing additional complementary information by summarizing the entire body of the evidence generated by human experimental studies, may provide sound evidence to strengthen recommendations to reduce dietary sodium intake in most populations and individuals.138 Even a small increase in BP is associated with an increase in CVD risk, including for stroke, coronary heart disease, and heart failure.135 Our results are consistent with the recommendations by the US National Academies of Sciences, Engineering, and Medicine,19 the European Food Safety Authority,20 the World Health Organization,13 the American Heart Association,14,17 and the European Societies of Cardiology and of Hypertension18 to limit sodium intake to values between 1.5 and 2.3 g/d, supporting the adequacy of the lowest among these standards.
Our results confirm a positive relationship between sodium intake and average BP in experimental studies and indicate that it is largely but not always compatible with a linear association over the entire large range of exposure experienced by adult trial participants, with no suggestion of thresholds at low or high levels of intake. The results also suggest this relationship is generally true for both SBP and DBP, for adults with and without hypertension, and during shorter and longer periods of sodium reduction.
Acknowledgment
The authors thank Professsor Olle Melander for providing data used in the stratified analysis.
Sources of Funding
Drs Filippini, Malavolti, and Vinceti were supported by grant Dipartimenti di Eccellenza 2018 to 2022 to the UNIMORE Department of Biomedical, Metabolic and Neural Sciences from the Italian Ministry of Education, University and Research, and Dr Filippini by grant UNIMORE FAR IMPULSO 2020 (494/2020) from the University of Modena and Reggio Emilia. Dr Whelton was supported by a Centers of Research Excellence grant from the National Institute of General Medical Sciences (grant no. P20GM109036).
Disclosures
None.
Supplemental Materials
Data Supplement Figures I–XVII
Data Supplement Tables I–III
Supplementary Material
Footnotes
Sources of Funding, see page 1563
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The Data Supplement, podcast, and transcript are available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.120.050371.
Contributor Information
Tommaso Filippini, Email: tommaso.filippini@unimore.it.
Marcella Malavolti, Email: marcella.malavolti@unimore.it.
Paul K. Whelton, Email: pwhelton@tulane.edu.
Androniki Naska, Email: anaska@med.uoa.gr.
Nicola Orsini, Email: Nicola.Orsini@ki.se.
References
- 1.Whelton PK. Body weight, sodium, potassium, and blood pressure. J Clin Hypertens (Greenwich). 2015;17:926–928. doi: 10.1111/jch.12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newberry SJ, Chung M, Anderson CAM, Chen C, Fu Z, Tang A, Zhao N, Booth M, Marks J, Hollands S, et al. Sodium and potassium intake: effects on chronic disease outcomes and risks: AHRQ comparative effectiveness reviews. 2018. Rockville, MD: Agency for Healthcare Research and Quality. doi: 10.23970/AHRQEPCCER206L [PubMed] [Google Scholar]
- 3.Graudal N, Hubeck-Graudal T, Jürgens G, Taylor RS. Dose–response relation between dietary sodium and blood pressure: a meta-regression analysis of 133 randomized controlled trials. Am J Clin Nutr. 2019;109:1273–1278. doi: 10.1093/ajcn/nqy384 [DOI] [PubMed] [Google Scholar]
- 4.Huang L, Trieu K, Yoshimura S, Neal B, Woodward M, Campbell NRC, Li Q, Lackland DT, Leung AA, Anderson CAM, et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials. BMJ. 2020;368:m315. doi: 10.1136/bmj.m315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129:981–989. doi: 10.1161/CIRCULATIONAHA.113.006032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelton PK, Kumanyika SK, Cook NR, Cutler JA, Borhani NO, Hennekens CH, Kuller LH, Langford H, Jones DW, Satterfield S, et al. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention: Trials of Hypertension Prevention Collaborative Research Group. Am J Clin Nutr. 1997;652 Suppl652S–660S. doi: 10.1093/ajcn/65.2.652S [DOI] [PubMed] [Google Scholar]
- 7.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;4:CD004937. doi: 10.1002/14651858.CD004937.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure: DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127 [DOI] [PubMed] [Google Scholar]
- 11.Leyvraz M, Chatelan A, da Costa BR, Taffé P, Paradis G, Bovet P, Bochud M, Chiolero A. Sodium intake and blood pressure in children and adolescents: a systematic review and meta-analysis of experimental and observational studies. Int J Epidemiol. 2018;47:1796–1810. doi: 10.1093/ije/dyy121 [DOI] [PubMed] [Google Scholar]
- 12.Malta D, Petersen KS, Johnson C, Trieu K, Rae S, Jefferson K, Santos JA, Wong MMY, Raj TS, Webster J, et al. High sodium intake increases blood pressure and risk of kidney disease: from the Science of Salt: a regularly updated systematic review of salt and health outcomes (August 2016 to March 2017). J Clin Hypertens (Greenwich). 2018;20:1654–1665. doi: 10.1111/jch.13408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Guideline: sodium intake for adults and children. 2012. Geneva: World Health Organization. https://apps.who.int/iris/handle/10665/77985 [PubMed] [Google Scholar]
- 14.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;12925suppl 2S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1 [DOI] [PubMed] [Google Scholar]
- 15.Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–292. doi: 10.1161/HYPERTENSIONAHA.119.14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell NRC, Webster J, Blanco-Metzler A, He FJ, Tan M, MacGregor GA, Cappuccio FP, Arcand J, Trieu K, Farrand C, et al. Packages of sodium (salt) sold for consumption and salt dispensers should be required to have a front of package health warning label: a position statement of the World Hypertension League, national and international health and scientific organizations. J Clin Hypertens (Greenwich). 2019;21:1623–1625. doi: 10.1111/jch.13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138:e426–e483. doi: 10.1161/cir.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 18.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940 [DOI] [PubMed] [Google Scholar]
- 19.Oria M, Harrison M, Stallings VA, eds. National Academy of Sciences. The National Academies Collection: reports funded by National Institutes of Health. In: Dietary reference intakes for sodium and potassium. 2019. Washington, DC: National Academies Press. doi: 10.17226/25353 [PubMed] [Google Scholar]
- 20.Turck D, Castenmiller J, de Henauw S, Hirsch-Ernst K-I, Kearney J, Knutsen HK, Maciuk A, Mangelsdorf I, McArdle HJ, Pelaez C, et al. EFSA Panel on Nutrition, Novel Foods, and Food Allergens. Dietary reference values for sodium. EFSA Journal. 2019;17:e05778. doi: 10.2903/j.efsa.2019.5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly J, Khalesi S, Dickinson K, Hines S, Coombes JS, Todd AS. The effect of dietary sodium modification on blood pressure in adults with systolic blood pressure less than 140 mm Hg: a systematic review. JBI Database System Rev Implement Rep. 2016;14:196–237. doi: 10.11124/JBISRIR-2016-002410 [DOI] [PubMed] [Google Scholar]
- 22.Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose–response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28:1579–1596. doi: 10.1177/0962280218773122 [DOI] [PubMed] [Google Scholar]
- 23.Vinceti M, Filippini T, Malavolti M, Naska A, Kasdagli MI, Torres D, Lopes C, Carvalho C, Moreira P, Orsini N. Dose-response relationships in health risk assessment of nutritional and toxicological factors in foods: development and application of novel biostatistical methods. EFSA support publ. 2020;17:EN-1899. doi: 10.2903/sp.efsa.2020.EN-1899 [Google Scholar]
- 24.Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. 2018;121:1027–1031. doi: 10.1016/j.envint.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. The Cochrane Collaboration. [updated March 2011].https://handbook-5-1.cochrane.org [Google Scholar]
- 27.Orsini N, Spiegelman D. Schmid CH, Stijnen T, White I, eds. Meta-analysis of dose–response relationships. In: Handbook of Meta-Analysis. 20211st edNew York: Chapman and Hall/CRC. doi: 10.1201/9781315119403 [Google Scholar]
- 28.Sera F, Armstrong B, Blangiardo M, Gasparrini A. An extended mixed-effects framework for meta-analysis. Stat Med. 2019;38:5429–5444. doi: 10.1002/sim.8362 [DOI] [PubMed] [Google Scholar]
- 29.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose–response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73. doi: 10.1093/aje/kwr265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin L, Shi L, Chu H, Murad MH. The magnitude of small-study effects in the Cochrane Database of Systematic Reviews: an empirical study of nearly 30 000 meta-analyses. BMJ Evid Based Med. 2020;25:27–32. doi: 10.1136/bmjebm-2019-111191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. doi: 10.1080/01621459.2000.10473905 [Google Scholar]
- 33.Orsini N. DRMETA: Stata module for dose–response meta-analysis. Statistical Software Components S458546 [revised 25 May 2019]. 2018. Boston: Boston College Department of Economics [Google Scholar]
- 34.Alli C, Avanzini F, Bettelli G, Bonati M, Colombo F, Corso R, Di Tullio M, Gentile MG, Sangalli L, Taioli E. Feasibility of a long-term low-sodium diet in mild hypertension. J Hum Hypertens. 1992;6:281–286. [PubMed] [Google Scholar]
- 35.Ames RP. The effect of sodium supplementation on glucose tolerance and insulin concentrations in patients with hypertension and diabetes mellitus. Am J Hypertens. 2001;14:653–659. doi: 10.1016/s0895-7061(01)01310-3 [DOI] [PubMed] [Google Scholar]
- 36.Andersson OK, Fagerberg B, Hedner T. Importance of dietary salt in the hemodynamic adjustment to weight reduction in obese hypertensive men. Hypertension. 1984;6:814–819. doi: 10.1161/01.hyp.6.6.814 [DOI] [PubMed] [Google Scholar]
- 37.Australian National Health and Medical Research Council Dietary Salt Study Management Committee. Fall in blood pressure with modest reduction in dietary salt intake in mild hypertension. Lancet. 1989;1:399–402. doi: 10.1016/S0140-6736(89)90001-9 [PubMed] [Google Scholar]
- 38.Australian National Health and Medical Research Council Dietary Salt Study Management Committee. Effects of replacing sodium intake in subjects on a low sodium diet: a crossover study. Clin Exp Hypertens A. 1989;11:1011–1024. doi: 10.3109/10641968909035388 [DOI] [PubMed] [Google Scholar]
- 39.Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med. 2001;161:685–693. doi: 10.1001/archinte.161.5.685 [DOI] [PubMed] [Google Scholar]
- 40.Arroll B, Beaglehole R. Salt restriction and physical activity in treated hypertensives. N Z Med J. 1995;108:266–268. [PubMed] [Google Scholar]
- 41.Beard TC, Cooke HM, Gray WR, Barge R. Randomised controlled trial of a no-added-sodium diet for mild hypertension. Lancet. 1982;2:455–458. doi: 10.1016/s0140-6736(82)90491-3 [DOI] [PubMed] [Google Scholar]
- 42.Benetos A, Xiao YY, Cuche JL, Hannaert P, Safar M. Arterial effects of salt restriction in hypertensive patients: a 9-week, randomized, double-blind, crossover study. J Hypertens. 1992;10:355–360. doi: 10.1097/00004872-199204000-00006 [DOI] [PubMed] [Google Scholar]
- 43.Bulpitt CJ, Daymond M, Bulpitt PF, Ferrier G, Harrison R, Lewis PJ, Dollery CT. Is low salt dietary advice a useful therapy in hypertensive patients with poorly controlled blood pressure? Ann Clin Res. 1984;16 Suppl 43:143–149. [PubMed] [Google Scholar]
- 44.Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double-blind randomised trial of modest salt restriction in older people. Lancet. 1997;350:850–854. doi: 10.1016/S0140-6736(97)02264-2 [DOI] [PubMed] [Google Scholar]
- 45.Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643]. BMC Public Health. 2006;6:13. doi: 10.1186/1471-2458-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carney SL, Gillies AH, Smith AJ, Smitham S. Increased dietary sodium chloride in patients treated with antihypertensive drugs. Clin Exp Hypertens A. 1991;13:401–407. doi: 10.3109/10641969109045059 [DOI] [PubMed] [Google Scholar]
- 47.Chalmers J, Morgan T, Doyle A, Dickson B, Hopper J, Mathews J, Matthews G, Moulds R, Myers J, Nowson C. Australian National Health and Medical Research Council dietary salt study in mild hypertension. J Hypertens Suppl. 1986;4:S629–S637. [PubMed] [Google Scholar]
- 48.Cobiac L, Nestel PJ, Wing LM, Howe PR. A low-sodium diet supplemented with fish oil lowers blood pressure in the elderly. J Hypertens. 1992;10:87–92. doi: 10.1097/00004872-199201000-00014 [DOI] [PubMed] [Google Scholar]
- 49.De Keyzer W, Tilleman K, Ampe J, De Henauw S, Huybrechts I. Effect of sodium restriction on blood pressure of unstable or uncontrolled hypertensive patients in primary care. Nutr Res Pract. 2015;9:180–185. doi: 10.4162/nrp.2015.9.2.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Vries LV, Dobrowolski LC, van den Bosch JJ, Riphagen IJ, Krediet CT, Bemelman FJ, Bakker SJ, Navis G. Effects of dietary sodium restriction in kidney transplant recipients treated with renin-angiotensin-aldosterone system blockade: a randomized clinical trial. Am J Kidney Dis. 2016;67:936–944. doi: 10.1053/j.ajkd.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 51.Dickinson KM, Clifton PM, Keogh JB. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin-1 in a randomised crossover study in normotensive overweight and obese subjects. Atherosclerosis. 2014;233:32–38. doi: 10.1016/j.atherosclerosis.2013.11.078 [DOI] [PubMed] [Google Scholar]
- 52.Dodson PM, Beevers M, Hallworth R, Webberley MJ, Fletcher RF, Taylor KG. Sodium restriction and blood pressure in hypertensive type II diabetics: randomised blind controlled and crossover studies of moderate sodium restriction and sodium supplementation. BMJ. 1989;298:227–230. doi: 10.1136/bmj.298.6668.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erwteman TM, Nagelkerke N, Lubsen J, Koster M, Dunning AJ. Beta blockade, diuretics, and salt restriction for the management of mild hypertension: a randomised double blind trial. BMJ. (Clin Res Ed). 1984;289:406–409. doi: 10.1136/bmj.289.6442.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fagerberg B, Andersson OK, Isaksson B, Björntorp P. Blood pressure control during weight reduction in obese hypertensive men: separate effects of sodium and energy restriction. BMJ. (Clin Res Ed). 1984;288:11–14. doi: 10.1136/bmj.288.6410.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fagerberg B, Andersson OK, Persson B, Hedner T. Reactivity to norepinephrine and effect of sodium on blood pressure during weight loss. Hypertension. 1985;7:586–592. doi: 10.1161/01.hyp.7.4.586 [DOI] [PubMed] [Google Scholar]
- 56.Fagerberg B, Andersson OK, Lindstedt G, Waldenström J, Aurell M. The sodium intake modifies the renin-aldosterone and blood pressure changes associated with moderately low energy diets. Acta Med Scand. 1985;218:157–164. doi: 10.1111/j.0954-6820.1985.tb08842.x [DOI] [PubMed] [Google Scholar]
- 57.Fotherby MD, Potter JF. Effects of moderate sodium restriction on clinic and twenty-four-hour ambulatory blood pressure in elderly hypertensive subjects. J Hypertens. 1993;11:657–663. doi: 10.1097/00004872-199306000-00010 [DOI] [PubMed] [Google Scholar]
- 58.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64 [DOI] [PubMed] [Google Scholar]
- 59.Gijsbers L, Dower JI, Schalkwijk CG, Kusters YH, Bakker SJ, Hollman PC, Geleijnse JM. Effects of sodium and potassium supplementation on endothelial function: a fully controlled dietary intervention study. Br J Nutr. 2015;114:1419–1426. doi: 10.1017/S0007114515002986 [DOI] [PubMed] [Google Scholar]
- 60.Gillies AH, Carney SL, Smith AJ, Waga SM. Adjunctive effect of salt restriction on antihypertensive efficacy. Clin Exp Pharmacol Physiol. 1984;11:395–398. doi: 10.1111/j.1440-1681.1984.tb00286.x [DOI] [PubMed] [Google Scholar]
- 61.Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA, Valkenburg HA. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. J Hypertens. 1987;5:115–119. doi: 10.1097/00004872-198702000-00016 [DOI] [PubMed] [Google Scholar]
- 62.He FJ, Marciniak M, Markandu ND, Antonios TF, MacGregor GA. Effect of modest salt reduction on skin capillary rarefaction in white, black, and Asian individuals with mild hypertension. Hypertension. 2010;56:253–259. doi: 10.1161/HYPERTENSIONAHA.110.155747 [DOI] [PubMed] [Google Scholar]
- 63.He FJ, Wu Y, Feng XX, Ma J, Ma Y, Wang H, Zhang J, Yuan J, Lin CP, Nowson C, et al. School based education programme to reduce salt intake in children and their families (School-EduSalt): cluster randomised controlled trial. BMJ. 2015;350:h770. doi: 10.1136/bmj.h770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howe PR, Lungershausen YK, Cobiac L, Dandy G, Nestel PJ. Effect of sodium restriction and fish oil supplementation on BP and thrombotic risk factors in patients treated with ACE inhibitors. J Hum Hypertens. 1994;8:43–49. [PubMed] [Google Scholar]
- 65.Hypertension Prevention Trial Research Group. The Hypertension Prevention Trial: three-year effects of dietary changes on blood pressure. Hypertension Prevention Trial Research Group. Arch Intern Med. 1990;150:153–162. doi: 10.1001/archinte.1990.00390130131021 [PubMed] [Google Scholar]
- 66.Hwang JH, Chin HJ, Kim S, Kim DK, Kim S, Park JH, Shin SJ, Lee SH, Choi BS, Lim CS. Effects of intensive low-salt diet education on albuminuria among nondiabetic patients with hypertension treated with olmesartan: a single-blinded randomized, controlled trial. Clin J Am Soc Nephrol. 2014;9:2059–2069. doi: 10.2215/CJN.01310214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jablonski KL, Racine ML, Geolfos CJ, Gates PE, Chonchol M, McQueen MB, Seals DR. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61:335–343. doi: 10.1016/j.jacc.2012.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.James GD, Pecker MS, Pickering TG, Jackson S, Difabio B, Carroll L, Laragh JH. Extreme changes in dietary sodium effect daily variability and level of blood pressure in borderline hypertensive patients. Am J Hum Biol. 1994;6:283–291. doi: 10.1002/ajhb.1310060303 [DOI] [PubMed] [Google Scholar]
- 69.James GD, Pecker MS, Pickering TG. Sex differences in casual and ambulatory blood pressure responses to extreme changes in dietary sodium. Blood Press Monit. 1996;1:397–401. [PubMed] [Google Scholar]
- 70.Jula AM, Karanko HM. Effects on left ventricular hypertrophy of long-term nonpharmacological treatment with sodium restriction in mild-to-moderate essential hypertension. Circulation. 1994;89:1023–1031. doi: 10.1161/01.cir.89.3.1023 [DOI] [PubMed] [Google Scholar]
- 71.Kwakernaak AJ, Krikken JA, Binnenmars SH, Visser FW, Hemmelder MH, Woittiez AJ, Groen H, Laverman GD, Navis G; Holland Nephrology Study (HONEST) Group. Effects of sodium restriction and hydrochlorothiazide on RAAS blockade efficacy in diabetic nephropathy: a randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2:385–395. doi: 10.1016/S2213-8587(14)70030-0 [DOI] [PubMed] [Google Scholar]
- 72.Lee CJ, Kim JY, Shim E, Hong SH, Lee M, Jeon JY, Park S. The effects of diet alone or in combination with exercise in patients with prehypertension and hypertension: a randomized controlled trial. Korean Circ J. 2018;48:637–651. doi: 10.4070/kcj.2017.0349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacGregor GA, Markandu ND, Best FE, Elder DM, Cam JM, Sagnella GA, Squires M. Double-blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet. 1982;1:351–355. doi: 10.1016/s0140-6736(82)91389-7 [DOI] [PubMed] [Google Scholar]
- 74.MacGregor GA, Markandu ND, Singer DR, Cappuccio FP, Shore AC, Sagnella GA. Moderate sodium restriction with angiotensin converting enzyme inhibitor in essential hypertension: a double blind study. BMJ. (Clin Res Ed). 1987;294:531–534. doi: 10.1136/bmj.294.6571.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet. 1989;2:1244–1247. doi: 10.1016/s0140-6736(89)91852-7 [DOI] [PubMed] [Google Scholar]
- 76.Mascioli S, Grimm R, Jr, Launer C, Svendsen K, Flack J, Gonzalez N, Elmer P, Neaton J. Sodium chloride raises blood pressure in normotensive subjects. The study of sodium and blood pressure. Hypertension. 1991;171 SupplI21–I26. doi: 10.1161/01.hyp.17.1_suppl.i21 [DOI] [PubMed] [Google Scholar]
- 77.Maxwell MH, Kushiro T, Dornfeld LP, Tuck ML, Waks AU. BP changes in obese hypertensive subjects during rapid weight loss: comparison of restricted v unchanged salt intake. Arch Intern Med. 1984;144:1581–1584. doi: 10.1001/archinte.1984.00350200073012 [PubMed] [Google Scholar]
- 78.McCarron DA, Weder AB, Egan BM, Krishna GG, Morris CD, Cohen M, Oparil S. Blood pressure and metabolic responses to moderate sodium restriction in isradipine-treated hypertensive patients. Am J Hypertens. 1997;10:68–76. doi: 10.1016/s0895-7061(96)00295-6 [DOI] [PubMed] [Google Scholar]
- 79.Meland E, Laerum E, Aakvaag A, Ulvik RJ, Høstmark AT. Salt restriction: effects on lipids and insulin production in hypertensive patients. Scand J Clin Lab Invest. 1997;57:501–505. doi: 10.3109/00365519709084600 [DOI] [PubMed] [Google Scholar]
- 80.Meland E, Aamland A. Salt restriction among hypertensive patients: modest blood pressure effect and no adverse effects. Scand J Prim Health Care. 2009;27:97–103. doi: 10.1080/02813430802661795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melander O, von Wowern F, Frandsen E, Burri P, Willsteen G, Aurell M, Hulthén UL. Moderate salt restriction effectively lowers blood pressure and degree of salt sensitivity is related to baseline concentration of renin and N-terminal atrial natriuretic peptide in plasma. J Hypertens. 2007;25:619–627. doi: 10.1097/HJH.0b013e328013cd50 [DOI] [PubMed] [Google Scholar]
- 82.Morgan TO, Myers JB. Hypertension treated by sodium restriction. Med J Aust. 1981;2:396–397. doi: 10.5694/j.1326-5377.1981.tb101026.x [DOI] [PubMed] [Google Scholar]
- 83.Morgan T, Nowson C. Comparative studies of reduced sodium and high potassium diet in hypertension. Nephron. 1987;47(Suppl 1):21–26. doi: 10.1159/000184547 [DOI] [PubMed] [Google Scholar]
- 84.Mühlhauser I, Prange K, Sawicki PT, Bender R, Dworschak A, Schaden W, Berger M. Effects of dietary sodium on blood pressure in IDDM patients with nephropathy. Diabetologia. 1996;39:212–219. doi: 10.1007/BF00403965 [DOI] [PubMed] [Google Scholar]
- 85.Nakano M, Eguchi K, Sato T, Onoguchi A, Hoshide S, Kario K. Effect of intensive salt-restriction education on clinic, home, and ambulatory blood pressure levels in treated hypertensive patients during a 3-month education period. J Clin Hypertens (Greenwich). 2016;18:385–392. doi: 10.1111/jch.12770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nestel PJ, Clifton PM, Noakes M, McArthur R, Howe PR. Enhanced blood pressure response to dietary salt in elderly women, especially those with small waist: hip ratio. J Hypertens. 1993;11:1387–1394. doi: 10.1097/00004872-199312000-00011 [DOI] [PubMed] [Google Scholar]
- 87.Nowson CA, Morgan TO. Change in blood pressure in relation to change in nutrients effected by manipulation of dietary sodium and potassium. Clin Exp Pharmacol Physiol. 1988;15:225–242. doi: 10.1111/j.1440-1681.1988.tb01065.x [DOI] [PubMed] [Google Scholar]
- 88.Nowson CA, Morgan TO, Gibbons C. Decreasing dietary sodium while following a self-selected potassium-rich diet reduces blood pressure. J Nutr. 2003;133:4118–4123. doi: 10.1093/jn/133.12.4118 [DOI] [PubMed] [Google Scholar]
- 89.Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium dietary approaches to stop hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res. 2009;29:8–18. doi: 10.1016/j.nutres.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 90.Parijs J, Joossens JV, Van der Linden L, Verstreken G, Amery AK. Moderate sodium restriction and diuretics in the treatment of hypertension. Am Heart J. 1973;85:22–34. doi: 10.1016/0002-8703(73)90522-x [DOI] [PubMed] [Google Scholar]
- 91.Parker M, Puddey IB, Beilin LJ, Vandongen R. Two-way factorial study of alcohol and salt restriction in treated hypertensive men. Hypertension. 1990;16:398–406. doi: 10.1161/01.hyp.16.4.398 [DOI] [PubMed] [Google Scholar]
- 92.Parvanova A, Trillini M, Podestà MA, Iliev IP, Ruggiero B, Abbate M, Perna A, Peraro F, Diadei O, Rubis N, et al. ; PROCEED Study Organization and the Scientific Writing Academy (SWA) 2016. Moderate salt restriction with or without paricalcitol in type 2 diabetes and losartan-resistant macroalbuminuria (PROCEED): a randomised, double-blind, placebo-controlled, crossover trial. Lancet Diabetes Endocrinol. 2018;6:27–40. doi: 10.1016/S2213-8587(17)30359-5 [DOI] [PubMed] [Google Scholar]
- 93.Pinjuh Markota N, Rumboldt M, Rumboldt Z. Emphasized warning reduces salt intake: a randomized controlled trial. J Am Soc Hypertens. 2015;9:214–220. doi: 10.1016/j.jash.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 94.Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartianinen E, Pietinen P, Dougherty R, Leino U, Mutanen M, Moisio S, et al. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet. 1983;1:1–5. doi: 10.1016/s0140-6736(83)91556-8 [DOI] [PubMed] [Google Scholar]
- 95.Redón-Más J, Abellán-Alemán J, Aranda-Lara P, de la Figuera-von Wichmann M, Luque-Otero M, Rodicio-Díaz JL, Ruilope-Urioste LM, Velasco-Quintana J. Antihypertensive activity of verapamil: impact of dietary sodium: the VERSAL Study Group. J Hypertens. 1993;11:665–671. doi: 10.1097/00004872-199306000-00011 [DOI] [PubMed] [Google Scholar]
- 96.Resnick LM, Gupta RK, DiFabio B, Barbagallo M, Mann S, Marion R, Laragh JH. Intracellular ionic consequences of dietary salt loading in essential hypertension: relation to blood pressure and effects of calcium channel blockade. J Clin Invest. 1994;94:1269–1276. doi: 10.1172/JCI117445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richards AM, Nicholls MG, Espiner EA, Ikram H, Maslowski AH, Hamilton EJ, Wells JE. Blood-pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet. 1984;1:757–761. doi: 10.1016/s0140-6736(84)91276-5 [DOI] [PubMed] [Google Scholar]
- 98.Ruppert M, Overlack A, Kolloch R, Kraft K, Göbel B, Stumpe KO. Neurohormonal and metabolic effects of severe and moderate salt restriction in non-obese normotensive adults. J Hypertens. 1993;11:743–749. doi: 10.1097/00004872-199307000-00010 [DOI] [PubMed] [Google Scholar]
- 99.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, III, Simons-Morton DG, et al. ; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet: DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101 [DOI] [PubMed] [Google Scholar]
- 100.Schorr U, Distler A, Sharma AM. Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: a randomized double-blind crossover trial. J Hypertens. 1996;14:131–135. [PubMed] [Google Scholar]
- 101.Sciarrone SE, Beilin LJ, Rouse IL, Rogers PB. A factorial study of salt restriction and a low-fat/high-fibre diet in hypertensive subjects. J Hypertens. 1992;10:287–298. doi: 10.1097/00004872-199203000-00013 [DOI] [PubMed] [Google Scholar]
- 102.Silman AJ, Locke C, Mitchell P, Humpherson P. Evaluation of the effectiveness of a low sodium diet in the treatment of mild to moderate hypertension. Lancet. 1983;1:1179–1182. doi: 10.1016/s0140-6736(83)92463-7 [DOI] [PubMed] [Google Scholar]
- 103.Singer DR, Markandu ND, Sugden AL, Miller MA, MacGregor GA. Sodium restriction in hypertensive patients treated with a converting enzyme inhibitor and a thiazide. Hypertension. 1991;17:798–803. doi: 10.1161/01.hyp.17.6.798 [DOI] [PubMed] [Google Scholar]
- 104.Slagman MC, Waanders F, Hemmelder MH, Woittiez AJ, Janssen WM, Lambers Heerspink HJ, Navis G, Laverman GD; Holland Nephrology Study Group. Moderate dietary sodium restriction added to angiotensin converting enzyme inhibition compared with dual blockade in lowering proteinuria and blood pressure: randomised controlled trial. BMJ. 2011;343:d4366. doi: 10.1136/bmj.d4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suckling RJ, He FJ, Markandu ND, MacGregor GA. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: a randomized double-blind trial. Hypertension. 2016;67:1189–1195. doi: 10.1161/HYPERTENSIONAHA.115.06637 [DOI] [PubMed] [Google Scholar]
- 106.Svetkey LP, Pollak KI, Yancy WS, Jr, Dolor RJ, Batch BC, Samsa G, Matchar DB, Lin PH. Hypertension improvement project: randomized trial of quality improvement for physicians and lifestyle modification for patients. Hypertension. 2009;54:1226–1233. doi: 10.1161/HYPERTENSIONAHA.109.134874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension. 2005;46:308–312. doi: 10.1161/01.HYP.0000172662.12480.7f [DOI] [PubMed] [Google Scholar]
- 108.Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Blood pressure change in a free-living population-based dietary modification study in Japan. J Hypertens. 2006;24:451–458. doi: 10.1097/01.hjh.0000209980.36359.16 [DOI] [PubMed] [Google Scholar]
- 109.Todd AS, Macginley RJ, Schollum JB, Williams SM, Sutherland WH, Mann JI, Walker RJ. Dietary sodium loading in normotensive healthy volunteers does not increase arterial vascular reactivity or blood pressure. Nephrology (Carlton). 2012;17:249–256. doi: 10.1111/j.1440-1797.2011.01550.x [DOI] [PubMed] [Google Scholar]
- 110.Trials of Hypertension Prevention. Collaborative Research Group. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the Trials of Hypertension Prevention, phase I. JAMA. 1992;267:1213–1220. doi: 10.1001/jama.1992.03480090061028 [DOI] [PubMed] [Google Scholar]
- 111.Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure: the Trials of Hypertension Prevention, phase II. Arch Intern Med. 1997;157:657–667. doi: 10.1001/archinte.1997.00440270105009 [PubMed] [Google Scholar]
- 112.van Berge-Landry H, James GD. Serum electrolyte, serum protein, serum fat and renal responses to a dietary sodium challenge: allostasis and allostatic load. Ann Hum Biol. 2004;31:477–487. doi: 10.1080/03014460412331281746 [DOI] [PubMed] [Google Scholar]
- 113.Vogt L, Waanders F, Boomsma F, de Zeeuw D, Navis G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J Am Soc Nephrol. 2008;19:999–1007. doi: 10.1681/ASN.2007060693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Watt GC, Edwards C, Hart JT, Hart M, Walton P, Foy CJ. Dietary sodium restriction for mild hypertension in general practice. BMJ. (Clin Res Ed). 1983;286:432–436. doi: 10.1136/bmj.286.6363.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watt GC, Foy CJ, Hart JT, Bingham G, Edwards C, Hart M, Thomas E, Walton P. Dietary sodium and arterial blood pressure: evidence against genetic susceptibility. BMJ (Clin Res Ed). 1985;291:1525–1528. doi: 10.1136/bmj.291.6508.1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weir MR, Yadao AM, Purkayastha D, Charney AN. Effects of high- and low-sodium diets on ambulatory blood pressure in patients with hypertension receiving aliskiren. J Cardiovasc Pharmacol Ther. 2010;15:356–363. doi: 10.1177/1074248410377173 [DOI] [PubMed] [Google Scholar]
- 117.Wing LM, Arnolda LF, Harvey PJ, Upton J, Molloy D, Gabb GM, Bune AJ, Chalmers JP. Low-dose diuretic and/or dietary sodium restriction when blood pressure is resistant to ACE inhibitor. Blood Press. 1998;7:299–307. doi: 10.1080/080370598437169 [DOI] [PubMed] [Google Scholar]
- 118.Yamamoto H. Randomized controlled trial of salt-restriction program for primary prevention of hypertension in the community. J Osaka City Med Center. 1997;46:255–267. [Google Scholar]
- 119.Filippini T, Naska A, Kasdagli MI, Torres D, Lopes C, Carvalho C, Moreira P, Malavolti M, Orsini N, Whelton PK, et al. Potassium intake and blood pressure: a dose–response meta-analysis of randomized controlled trials. J Am Heart Assoc. 2020;9:e015719. doi: 10.1161/JAHA.119.015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2011:CD004022. doi: 10.1002/14651858.CD004022.pub3 [DOI] [PubMed] [Google Scholar]
- 121.Jayedi A, Ghomashi F, Zargar MS, Shab-Bidar S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: a systematic review and nonlinear dose–response meta-analysis. Clin Nutr. 2019;38:1092–1100. doi: 10.1016/j.clnu.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 122.Béjot Y. Targeting blood pressure for stroke prevention: current evidence and unanswered questions [published online June 26, 2019]. J Neurol. doi: 10.1007/s00415-019-09443-5https://link.springer.com/article/10.1007%2Fs00415-019-09443-5 [DOI] [PubMed] [Google Scholar]
- 123.Khaku AS, Tadi P. Cerebrovascular disease (stroke). StatPearls. 2020. Treasure Island, FL. https://www.ncbi.nlm.nih.gov/books/NBK430927/ [Google Scholar]
- 124.Wajngarten M, Silva GS. Hypertension and stroke: update on treatment. Eur Cardiol. 2019;14:111–115. doi: 10.15420/ecr.2019.11.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Campbell NRC, He FJ, Tan M, Cappuccio FP, Neal B, Woodward M, Cogswell ME, McLean R, Arcand J, MacGregor G, et al. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J Clin Hypertens (Greenwich). 2019;21:700–709. doi: 10.1111/jch.13551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Campbell NR, Lackland DT, Niebylski ML, Nilsson PM. Is reducing dietary sodium controversial? Is it the conduct of studies with flawed research methods that is controversial? A perspective from the World Hypertension League Executive Committee. J Clin Hypertens (Greenwich). 2015;17:85–86. doi: 10.1111/jch.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 128.Whelton SP, McEvoy JW, Shaw L, Psaty BM, Lima JAC, Budoff M, Nasir K, Szklo M, Blumenthal RS, Blaha MJ. Association of normal systolic blood pressure level with cardiovascular disease in the absence of risk factors. JAMA Cardiol. 2020;5:1011–1018. doi: 10.1001/jamacardio.2020.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Appel LJ. Nonpharmacologic therapies that reduce blood pressure: a fresh perspective. Clin Cardiol. 1999;227 SupplIII1–III5. doi: 10.1002/clc.4960221502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, et al. ; Writing Group of the PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083 [DOI] [PubMed] [Google Scholar]
- 131.Fu J, Liu Y, Zhang L, Zhou L, Li D, Quan H, Zhu L, Hu F, Li X, Meng S, et al. Nonpharmacologic interventions for reducing blood pressure in adults with prehypertension to established hypertension. J Am Heart Assoc. 2020;9:e016804. doi: 10.1161/JAHA.120.016804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Juraschek SP, Woodward M, Sacks FM, Carey VJ, Miller ER, III, Appel LJ. Time course of change in blood pressure from sodium reduction and the DASH diet. Hypertension. 2017;70:923–929. doi: 10.1161/HYPERTENSIONAHA.117.10017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Law MR, Frost CD, Wald NJ. By how much does dietary salt reduction lower blood pressure? III: Analysis of data from trials of salt reduction. BMJ. 1991;302:819–824. doi: 10.1136/bmj.302.6780.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhu Y, Zhang J, Li Z, Liu Y, Fan X, Zhang Y, Zhang Y. Association of sodium intake and major cardiovascular outcomes: a dose–response meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. 2018;18:192. doi: 10.1186/s12872-018-0927-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cook NR, He FJ, MacGregor GA, Graudal N. Sodium and health-concordance and controversy. BMJ. 2020;369:m2440. doi: 10.1136/bmj.m2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Whelton PK, Appel LJ, Sacco RL, Anderson CA, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126:2880–2889. doi: 10.1161/CIR.0b013e318279acbf [DOI] [PubMed] [Google Scholar]
- 137.Whelton PK, Campbell NRC, Lackland DT, Parati G, Ram CVS, Weber MA, Zhang XH. Strategies for prevention of cardiovascular disease in adults with hypertension. J Clin Hypertens (Greenwich). 2020;22:132–134. doi: 10.1111/jch.13797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. doi: 10.1136/bmjopen-2013-003733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


