Abstract
Background
Given the scale of the ongoing COVID-19 pandemic, the development of vaccines based on different platforms is essential, particularly in light of emerging viral variants, the absence of information on vaccine-induced immune durability, and potential paediatric use. We aimed to assess the safety and immunogenicity of an MF59-adjuvanted subunit vaccine for COVID-19 based on recombinant SARS-CoV-2 spike glycoprotein stabilised in a pre-fusion conformation by a novel molecular clamp (spike glycoprotein-clamp [sclamp]).
Methods
We did a phase 1, double-blind, placebo-controlled, block-randomised trial of the sclamp subunit vaccine in a single clinical trial site in Brisbane, QLD, Australia. Healthy adults (aged ≥18 to ≤55 years) who had tested negative for SARS-CoV-2, reported no close contact with anyone with active or previous SARS-CoV-2 infection, and tested negative for pre-existing SARS-CoV-2 immunity were included. Participants were randomly assigned to one of five treatment groups and received two doses via intramuscular injection 28 days apart of either placebo, sclamp vaccine at 5 μg, 15 μg, or 45 μg, or one dose of sclamp vaccine at 45 μg followed by placebo. Participants and study personnel, except the dose administration personnel, were masked to treatment. The primary safety endpoints included solicited local and systemic adverse events in the 7 days after each dose and unsolicited adverse events up to 12 months after dosing. Here, data are reported up until day 57. Primary immunogenicity endpoints were antigen-specific IgG ELISA and SARS-CoV-2 microneutralisation assays assessed at 28 days after each dose. The study is ongoing and registered with ClinicalTrials.gov, NCT04495933.
Findings
Between June 23, 2020, and Aug 17, 2020, of 314 healthy volunteers screened, 120 were randomly assigned (n=24 per group), and 114 (95%) completed the study up to day 57 (mean age 32·5 years [SD 10·4], 65 [54%] male, 55 [46%] female). Severe solicited reactions were infrequent and occurred at similar rates in participants receiving placebo (two [8%] of 24) and the SARS-CoV-2 sclamp vaccine at any dose (three [3%] of 96). Both solicited reactions and unsolicited adverse events occurred at a similar frequency in participants receiving placebo and the SARS-CoV-2 sclamp vaccine. Solicited reactions occurred in 19 (79%) of 24 participants receiving placebo and 86 (90%) of 96 receiving the SARS-CoV-2 sclamp vaccine at any dose. Unsolicited adverse events occurred in seven (29%) of 24 participants receiving placebo and 35 (36%) of 96 participants receiving the SARS-CoV-2 sclamp vaccine at any dose. Vaccination with SARS-CoV-2 sclamp elicited a similar antigen-specific response irrespective of dose: 4 weeks after the initial dose (day 29) with 5 μg dose (geometric mean titre [GMT] 6400, 95% CI 3683–11 122), with 15 μg dose (7492, 4959–11 319), and the two 45 μg dose cohorts (8770, 5526–13 920 in the two-dose 45 μg cohort; 8793, 5570–13 881 in the single-dose 45 μg cohort); 4 weeks after the second dose (day 57) with two 5 μg doses (102 400, 64 857–161 676), with two 15 μg doses (74 725, 51 300–108 847), with two 45 μg doses (79 586, 55 430–114 268), only a single 45 μg dose (4795, 2858–8043). At day 57, 67 (99%) of 68 participants who received two doses of sclamp vaccine at any concentration produced a neutralising immune response, compared with six (25%) of 24 who received a single 45 μg dose and none of 22 who received placebo. Participants receiving two doses of sclamp vaccine elicited similar neutralisation titres, irrespective of dose: two 5 μg doses (GMT 228, 95% CI 146–356), two 15 μg doses (230, 170–312), and two 45 μg doses (239, 187–307).
Interpretation
This first-in-human trial shows that a subunit vaccine comprising mammalian cell culture-derived, MF59-adjuvanted, molecular clamp-stabilised recombinant spike protein elicits strong immune responses with a promising safety profile. However, the glycoprotein 41 peptide present in the clamp created HIV diagnostic assay interference, a possible barrier to widespread use highlighting the criticality of potential non-spike directed immunogenicity during vaccine development. Studies are ongoing with alternative molecular clamp trimerisation domains to ameliorate this response.
Funding
Coalition for Epidemic Preparedness Innovations, National Health and Medical Research Council, Queensland Government, and further philanthropic sources listed in the acknowledgments.
Introduction
The COVID-19 pandemic is placing unprecedented pressure on patients, communities, health-care systems, and economies worldwide. As a result, accelerated development of safe and effective COVID-19 vaccines has become the focus of globally coordinated research activities. The desired attributes of a successful pandemic vaccine include the ability to confer long-term protection, demonstrated efficacy with an acceptable safety profile at a maximum of two doses, the ability for rapid and large-scale manufacturing, and widespread distribution using existing cold-chain infrastructure.1 The candidate COVID-19 vaccines under development can be broadly categorised into inactivated virus vaccines, protein subunit vaccines, nucleic acid vaccines, and viral vectors, each with specific advantages and disadvantages.2
The SARS-CoV-2 spike glycoprotein binds the human angiotensin-converting enzyme 2 (ACE2) receptor, which mediates cell entry and is the main target of the neutralising antibody response during infection.3 The candidate SARS-CoV-2 spike glycoprotein-clamp (sclamp) vaccine is an adjuvanted protein subunit vaccine that comprises a recombinant SARS-CoV-2 spike glycoprotein and squalene-oil-in-water adjuvant (MF59).4 A molecular clamp is used to stabilise the spike glycoprotein in the authentic pre-fusion conformation that preserves neutralising epitopes present on the virion surface. Immunisation with the stabilised antigen therefore stimulates neutralising antibodies against SARS-CoV-2, which mimics the response induced by natural infection and minimises the induction of potentially confounding non-neutralising antibodies.4 Similar to other vaccine platforms that stabilise the pre-fusion conformation of spike glycoprotein,5, 6, 7 this approach is anticipated to generate vaccines with enhanced stability, immunogenicity, and safety profiles.8 In addition, MF59 is a commercially approved adjuvant with a well established safety record in children, adults, and older people9, 10, 11 and that elicits strong antibody responses, along with a balanced T-helper 1 (Th1) and T-helper 2 (Th2) cell response.12 Although Alhydrogel (aluminium adjuvant) is widely available and recognised as safe in humans, it is noted for driving a predominantly Th2 immune response. A strong Th2 response has previously been associated with immunopathology in humans immunised with a candidate respiratory syncytial virus vaccine, and in preclinical animal studies for MERS-CoV and SARS-CoV.13, 14, 15 Early in the pandemic it was the recommendation of regulators that Alhydrogel be avoided in human trials of SARS-CoV-2 vaccines. Due to the limited amount of information available at this early stage in the pandemic and the combination of the preferred immune stimulation, the established safety profile, and availability, the decision was made to focus on MF59 as the adjuvant of choice for this study. Preclinical data have shown that the SARS-CoV-2 sclamp vaccine elicits a robust neutralising antibody response to wild-type SARS-CoV-2 virus, including three of the currently dominant strains, and spike glycoprotein-specific T-cell immunity in animals that protects against SARS-CoV-2 infection.4
Here, we report the primary findings of our trial of the SARS-CoV-2 sclamp vaccine in healthy adults. We aimed to assess the safety, tolerability, and immunogenicity of 5 μg, 15 μg, and 45 μg doses of vaccine compared with placebo. In addition, humoral and cellular responses to the vaccine are reported as exploratory outcomes.
Research in context.
Evidence before this study
We searched PubMed on Dec 22, 2020, for clinical trials of COVID-19 vaccines, using the search terms “SARS-CoV-2”, “vaccine”, and “clinical trial” with no language restrictions. Only peer-reviewed publications were included. Although many vaccines are in development, we found only one published trial of a vaccine based on the recombinant SARS-CoV-2 spike protein, packaged as a nanoparticle. In preclinical studies, an adjuvanted vaccine based on the recombinant spike protein stabilised in the pre-fusion conformation by a molecular clamp (spike glycoprotein-clamp [sclamp]) elicited strong, neutralising immune responses in mice, and reduced viral load and conferred protection against pulmonary disease in SARS-CoV-2-challenged hamsters.
Added value of this study
In this phase 1, double-blind, randomised, placebo-controlled trial, two doses of the SARS-CoV-2 sclamp vaccine elicited robust, highly correlated humoral and cellular immune responses, with very low rates of systemic reactions. However, sequences of the HIV-1 glycoprotein 41 used in the molecular clamp elicited antibodies that cross-reacted with rapid HIV diagnostic tests that included recombinant glycoprotein 41.
Implications of all the available evidence
Although this specific vaccine design will not be progressed further because of cross-reactivity with some HIV diagnostics, these first-in-human results validate the molecular clamp technology as a viable platform for vaccine development with potential for large-scale manufacturability and distribution.
Methods
Study design and participants
This phase 1, randomised, double-blind, placebo-controlled, single and multiple-ascending dose trial was done at a single centre (Nucleus Network, Brisbane, QLD, Australia). This study comprised two populations: healthy adults (aged ≥18 to ≤55 years on Oct 21, 2020) and healthy adults aged 56 years and older (to be subsequently reported). All participants tested negative for SARS-CoV-2 infection at screening or day 1, reported no close contact with anybody with active or previous SARS-CoV-2 infection, and tested negative for pre-existing SARS-CoV-2 immunity. Detailed information on inclusion and exclusion criteria is provided in the appendix (p 2).
The protocol was approved by the Alfred Health Human Research Ethics Committee (2020001376/334/20). The study was done in accordance with the protocol, Good Clinical Practice, the Declaration of Helsinki, and all local regulations. All participants provided written informed consent before any study procedures were undertaken.
Randomisation and masking
Participants were randomly assigned to either a placebo group or one of four treatment groups (1:1:1:1:1): two 5 μg doses of vaccine; two 15 μg doses of vaccine; two 45 μg doses of vaccine; and one 45 μg dose of vaccine followed by one dose of placebo. Participants were administered vaccine or placebo according to a block randomisation scheme. Participants and study personnel were masked to treatment, except the dose administration personnel who were not otherwise involved in the study. After day 57, specific study personnel were unmasked to allow data analysis; participants will remain masked until the end of the study.
Procedures
The study comprised three cohorts in a step-wise dose-escalation design. In cohort 1, participants received two 5 μg doses of vaccine on days 1 and 29. In cohort 2, participants received two 15 μg doses of vaccine on days 1 and 29. In cohort 3, participants received one 45 μg dose of vaccine on day 1 followed by placebo on day 29, or participants received two 45 μg doses on days 1 and 29. In each cohort, a third of participants received placebo on days 1 and 29.
SARS-CoV-2 sclamp antigen (CSIRO Manufacturing, Clayton, VIC, Australia) was produced in Chinese hamster ovary cells and comprises a trimeric glycosylated SARS-CoV-2 spike glycoprotein ectodomain (GenBank accession number MN908947) fused to a molecular clamp. The recombinant protein was based on the sequence of the prototypic SARS-CoV-2 strain first detected in Wuhan, China. This protein includes the spike glycoprotein native signal peptide, replacement of the furin cleavage site of spike glycoprotein amino acids 680–690 with a glycine-serine-glycine linker, and truncation at amino acid 1204 to remove the C-terminal domain and replace this with the molecular clamp trimerisation sequence (HIV-1 glycoprotein 41 heptad repeat [HR] elements, HR1 [amino acids 540–576], and HR2 [amino acids 619–656] separated by a glycine2-serine-glycine2 linker).4 The SARS-CoV-2 sclamp antigen has been shown to adopt the native pre-fusion conformation and to bind to the human ACE2 receptor and SARS-CoV-2-specific monoclonal antibodies.4 The SARS-CoV-2 sclamp and MF59 adjuvant were stored at 2–8°C.
SARS-CoV-2 sclamp antigen and squalene adjuvant MF59C (Seqirus, Parkville, VIC, Australia) were mixed at the clinical site and 0·5 mL of suspension drawn into a syringe. Sterile 0·9% saline (0·5 mL) was used as placebo. Because physical masking of prepared syringes was not possible, doses were transported in a boxed blinding bag, and unmasked study personnel administered each dose. All doses of vaccine and placebo were administered as a single intramuscular injection into the deltoid region. Within each cohort, immediate post-vaccination (≥24 h after first dose); safety data from the first two participants (so-called sentinels; one vaccine, one placebo) were reviewed by the safety review committee before proceeding with dosing of the remaining participants in the cohort. Cumulative safety data from the first 7 days after the first vaccination of each cohort were reviewed by the safety review committee before proceeding to the next higher-dose cohort.
Outcomes
The primary safety endpoints included the frequency, duration, and intensity of solicited local and systemic adverse events for 7 days after each dose; the frequency, duration, intensity, and relatedness of unsolicited adverse events up to 12 months; and the frequency of serious adverse events and adverse events leading to study discontinuation throughout the study. The toxicity grading system for solicited local and systemic adverse events are detailed in the appendix (pp 5–6).
The primary immunogenicity endpoints included the geometric mean of the serum antibody response to SARS-CoV-2 sclamp vaccine compared with placebo by antigen-specific ELISA at day 29 and day 57, and the geometric mean of the serum neutralising antibody titres to SARS-CoV-2 virus compared with placebo at day 29 and day 57 by microneutralisation assay.
Secondary immunogenicity endpoints included the geometric mean of antigen-specific serum antibody response on day 43, and seroconversion rate (the proportion of participants with a four times or more increase above baseline) for antigen-specific serum antibody response at days 29, 43, and 57. Additional exploratory humoral and cellular immunogenicity endpoints were examined. Detailed information on all methods is provided in the appendix (pp 2–5).
Serum samples for immunological assessments were taken at screening and on days 1 (pre-dose on day of first dose), 8, 15, 29 (pre-dose on day of second dose), 36, 43, and 57. Serum samples collected with written informed consent from 67 Australian adults between 14 and 90 days after recovery from mild-to-moderate SARS-CoV-2 infection were used as a reference. The demographics of this patient cohort have been previously described.16, 17
Antigen-specific serum antibody responses against the SARS-CoV-2 sclamp or clamp-specific Nipah virus Fclamp vaccines were measured by ELISA. Detection of neutralising antibodies against infectious SARS-CoV-2 (CoV/Australia/VIC01/2020)18 was assessed with a traditional microneutralisation assay19 and the neutralising antibody titre, expressed as 50% microneutralisation titre (MN50), was calculated using the Reed–Muench method as previously described.19 The limit of detection was the reciprocal of the highest concentration of serum tested and any values falling below the limit of detection were reported as half that of the limit of detection. National Institute for Biological Standards and Control reference serum 130/20 was included as an internal control for assay variation. Each patient serum sample was assayed twice on different days and in the event that the repeat MN50 differed by more than two times, the sample was analysed a third time. MN50 titres are reported as the geometric mean of the two or three assay repeats.
Neutralising antibodies were also assessed against pseudotype viruses encoding the spike glycoprotein from the reference Wuhan sequence (GenBank accession number NC_045512), as well as circulating SARS-CoV-2 virus spike glycoprotein variants (mutations Ser477Asn and Asp614Gly, Gly485Arg and Asp614Gly, and Asn501Tyr and Asp614Gly; GenBank accession numbers 519263, 456508, and 480701).20, 21, 22 HIV reporter virus pseudotyped with SARS-CoV-2 spike glycoprotein was produced by lipofectamine co-transfection, and pseudovirus neutralisation was measured and expressed as the reciprocal titre of the participant serum sample required for 50% reduction of relative luciferase units compared with controls.
A blocking ELISA was used to measure neutralising antibodies against SARS-CoV-2, which block the interaction between the spike glycoprotein and ACE2 receptors (SARS-CoV-2 surrogate virus neutralisation test, GenScript, Piscataway, NJ, USA) as previously described.23 The absorbance at 450 nm is inversely proportional to the titre of anti-SARS-CoV-2 neutralising antibodies.
The potential for the immune response directed to the HIV glycoprotein 41 sequences, which comprised the clamp, to cause diagnostic interference was assessed (Queensland Health Pathology Services, Brisbane QLD, Australia) using a three-tiered routine procedure (Abbot Architect Ag/Ab Combo Quantitative Assay, Abbot, Chicago, IL, USA; MP Diagnostics HIV Blot 2, 2 Western Blot assay, MP Biomedicals, Santa Ana, CA, USA; and nucleic acid testing). Cross-reactivity was assessed with nine additional HIV diagnostic platforms (laboratory-based, point-of-care, and self-test diagnostics).
A custom multiplex bead array was done to examine the elicited antibody response, including analysis of antibody isotypes and subclasses; specificity to SARS-CoV-2 sclamp, S1 (amino subunit), S2 (carboxyl subunit), spike receptor binding domain, and clamp; as well as cross-reactivity to human coronaviruses (229E, NL63, HKU1, OC43).
Fresh whole blood was used to measure spike-specific CD4+ and CD8+ T cells, T follicular helper (Tfh) cells, and antibody secreting cells using flow cytometry to detect cytokine expression after stimulation with a SARS-CoV-2 spike peptide library as described previously.24, 25, 26, 27
Statistical analysis
No sample size calculations for statistical power were done due to the absence at the time of any pre-existing data on effect size or SD for SARS-CoV-2 neutralisation in humans, and an enrolment of 120 participants (n=32 in cohorts 1 and 2, n=56 in cohort 3) was planned. Baseline demographic and safety data are summarised using descriptive statistics. Safety data are presented for all randomly assigned participants (intention-to-treat population); immunogenicity data are presented for the per-protocol population. Detailed information on all the statistical analyses is provided in the appendix (p 5). This study is registered with ClinicalTrials.gov, NCT04495933.
Role of the funding source
Coalition for Epidemic Preparedness Innovations provided advice on the study design but had no role in data collection, data analysis, data interpretation, or writing of the report.
Results
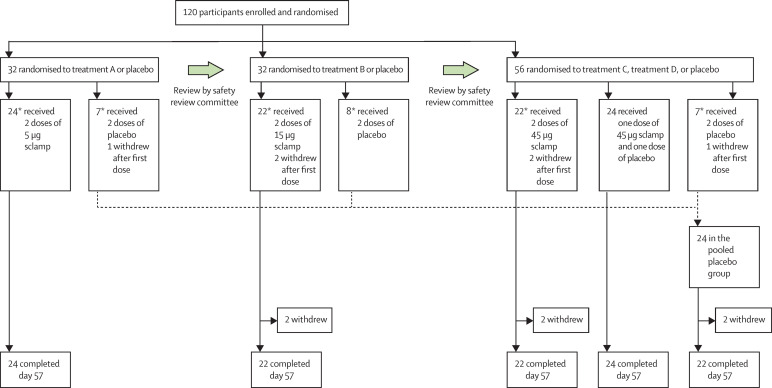
Between June 23, 2020, and Aug 17, 2020, of 314 healthy volunteers screened, 120 met eligibility criteria (mean age 32·5 years [SD 10·4], 65 [54%] male, 55 [46%] female) and were randomly assigned to treatment (figure 1 ). Of the randomly assigned participants, 114 (95%) completed the study (day 57). The six participants who discontinued (two each in the placebo, two dose 15 μg, and two dose 45 μg vaccine groups) decided to withdraw. One participant in the placebo group who withdrew was excluded from the per-protocol population due to early withdrawal (day 9) without at least one post-dose immunogenicity assessment. Participant demographics at baseline were similar across each of the treatment groups (table ). The trial is ongoing at the time of writing, with 12-month follow-up planned for participants in treatments reported here concluding September, 2021.
Figure 1.
Trial profile
*Includes one sentinel participant whose immediate post-dose safety data were reviewed by the safety review committee before proceeding with dosing of the rest of the cohort. † Cumulative safety data from the first 7 days after the first dose of each cohort were reviewed by the safety review committee before proceeding to the next higher-dose cohort.
Table.
Baseline characteristics
| Placebo (n=24) | Two doses of 5 μg vaccine (n=24) | Two doses of 15 μg vaccine (n=24) | Two doses of 45 μg vaccine (n=24) | One dose of 45 μg vaccine (n=24) | ||
|---|---|---|---|---|---|---|
| Age, years | 32·6 (11·0) | 34·1 (11·9) | 31·0 (10·6) | 32·6 (10·2) | 32·0 (8·7) | |
| Sex | ||||||
| Female | 13 (54%) | 13 (54%) | 13 (54%) | 11 (46%) | 5 (21%) | |
| Male | 11 (46%) | 11 (46%) | 11 (46%) | 13 (54%) | 19 (79%) | |
| Body-mass index, kg/m2 | 24·3 (3·8) | 25·0 (4·1) | 25·6 (4·6) | 25·0 (3·7) | 25·8 (4·0) | |
| Ethnicity | ||||||
| White | 21 (88%) | 21 (88%) | 19 (79%) | 16 (67%) | 17 (71%) | |
| Asian | 3 (13%) | 3 (13%) | 4 (17%) | 8 (33%) | 6 (25%) | |
| Other* | 0 | 0 | 1 (4%) | 0 | 1 (4%) | |
Data are mean (SD) or n (%).
Other refers to people of mixed heritage and Indigenous Australians.
Overall, the SARS-CoV-2 sclamp vaccine was well tolerated. Solicited adverse events occurred in 19 (79%) participants in the placebo group, 22 (92%) participants in the 5 μg group, 23 (96%) participants in the 15 μg group, 20 (83%) participants in the single-dose 45 μg group, and 21 (88%) participants in the two-dose 45 μg vaccine group. Unsolicited adverse events occurred in seven (29%) participants in the placebo group, 13 (54%) participants in the 5 μg group, seven (29%) participants in the 15 μg group, eight (33%) participants in the single-dose 45 μg group, and seven (29%) participants in the two-dose 45 μg vaccine group. Vaccine-related unsolicited adverse events occurred in one (4%) participant in the placebo group, four (17%) participants in the 5 μg group, three (13%) participants in the 15 μg group, four (17%) participants in the single-dose 45 μg group, and one (4%) participant in the two-dose 45 μg vaccine group (appendix pp 8–9). No severe treatment-emergent adverse events or serious unsolicited adverse events, discontinuations due to an adverse event, or deaths occurred in any group.
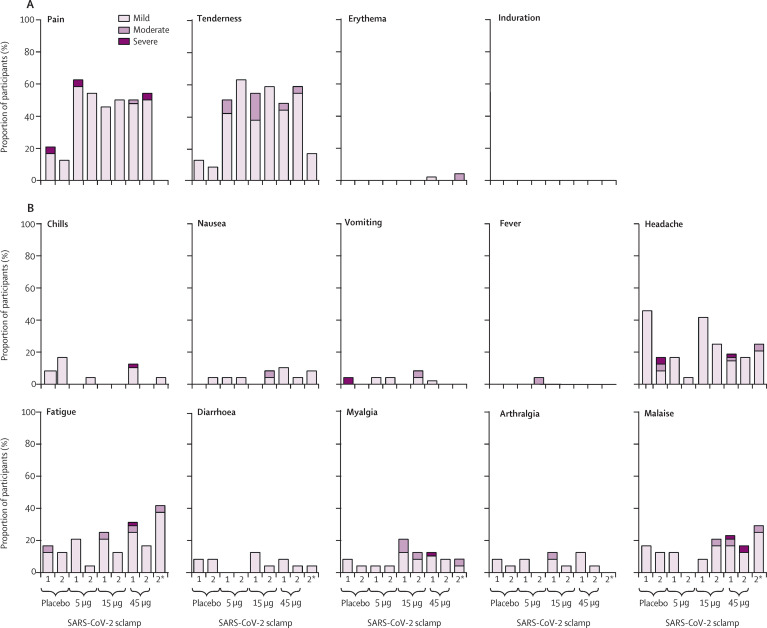
The most common solicited local adverse events occurring within 7 days of vaccination were injection site pain and tenderness. Pain was reported by 88 (54%) of 163 participants who received any dose of SARS-CoV-2 sclamp vaccine compared with eight (17%) of 46 participants who received placebo. Tenderness was reported in 91 (56%) participants who received any dose of SARS-CoV-2 sclamp vaccine compared with five (11%) of 45 participants who received placebo (figure 2A ; appendix p 10). Most local adverse events were mild or moderate. After the first dose, grade 3 (severe) pain was reported by one participant each in the placebo and the 5 μg groups; after the second dose, grade 3 pain was reported by one participant in the two-dose 45 μg group.
Figure 2.
Adverse events
Percentage of (A) solicited local adverse events and (B) solicited systemic adverse events within 7 days of first and second dose. Data for the first dose of treatment C (two doses of 45 μg SARS-CoV-2 sclamp) and D (one dose of 45 μg SARS-CoV-2 sclamp, followed by one dose of placebo) were pooled (n=48), whereas data for the second dose are shown separately. Sclamp=spike glycoprotein-clamp vaccine. *Solicited adverse events after saline dosing in the single 45 μg dose cohort.
The rates of solicited systemic adverse events were similar in placebo and experimental groups (figure 2B; appendix pp 11–12). The most common solicited systemic adverse event was headache, followed by fatigue and malaise. Grade 3 systemic adverse events after the first dose included vomiting reported by one (4%) participant in the placebo group, severe headache reported by one participant who received a 45 μg dose; and severe chills, myalgia, headache, fatigue, and malaise, which were each reported by one participant who received a 45 μg dose. One (4%) participant reported grade 3 headache after the second dose of placebo, and one (4%) participant reported malaise after the second 45 μg dose. There were no reports of fever (temperature >38°C) in any group after the first dose; one participant reported moderate fever (38·5–38·9°C) after the second dose of the 5 μg vaccine. Severe solicited reactions were infrequent and occurred in a similar proportion of patients receiving placebo (two [8%] of 24) and the SARS-CoV-2 sclamp vaccine at any dose (three [3%] of 96).
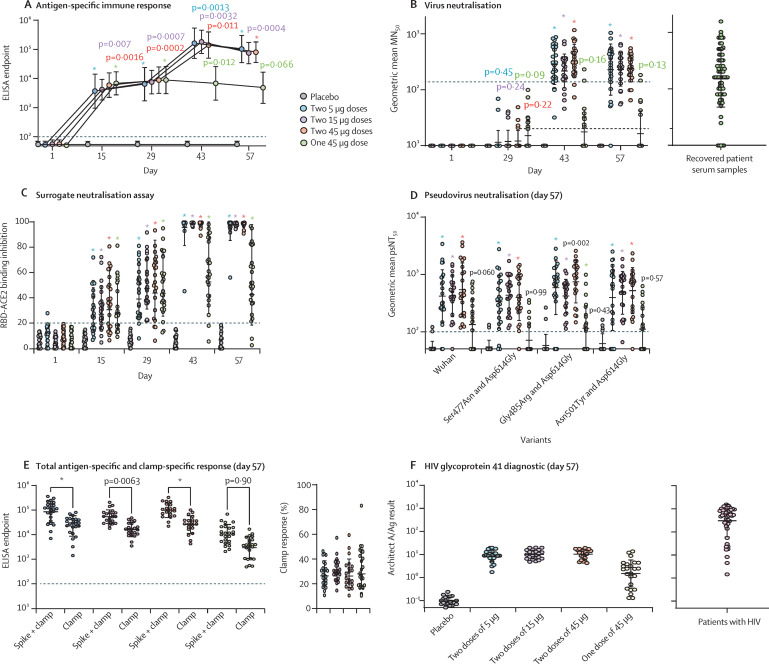
At day 57, 67 (99%) of 68 participants who received two doses of sclamp at any concentration produced a neutralising immune response, compared with six (25%) of 24 who received a single 45 μg dose and none of 22 who received placebo. At day 57, 66 (100%) of 66 samples tested from participants who received two doses of sclamp vaccine were reactive in the Abbott Architect HIV Ag/Ab Combo assay, compared with seven (29%) of 24 participants who received a single 45 μg dose, and none of 21 participants who received placebo.
Serum antibody responses to SARS-CoV-2 sclamp vaccine increased in a similar manner in all two-dose groups, irrespective of dose concentration (figure 3A , appendix p 13). No significant differences were seen between the dosing groups for serum antibody responses. Geometric mean antigen-specific responses were significantly greater than placebo for all dose groups at days 15, 29, and 43, and for the two-dose groups at day 57 (figure 3A). At day 29, geometric mean reciprocal titres were 6400 (95% CI 3683–11 122) for two doses of 5 μg, 7492 (4959–11 319) for two doses of 15 μg, 8770 (5526–13 920) for two doses of 45 μg vaccine, 8793 (5570–13 881) for the single 45 μg dose, and 55 (49–61) for placebo. At day 57, geometric mean reciprocal titres were 102 400 (95% CI 64 857–161 676) for two doses of 5 μg, 74 725 (51 300–108 847) for two doses of 15 μg, 79 586 (55 430–114 268) for two doses of 45 μg vaccine, 4795 (2858–8043) for the single 45 μg dose, and 59 (50–69) for placebo. The seroconversion rate for two doses of 5 μg vaccine was 96% (23 of 24), and it was 100% (44 of 44) for two doses of either the 15 μg and 45 μg vaccine at days 43 and 57. Seroconversion for the single 45 μg dose was 13% (three of 24) on both days 43 and 57.
Figure 3.
Serum antibody response to vaccine
Circles represent geometric mean and error bars represent the geometric mean SD at each timepoint. (A) Anti-SARS-CoV-2 sclamp IgG ELISA responses in trial participants. The dotted line represents the limit of detection. Adjusted p values compare treatment versus placebo (two-way ANOVA Dunnett's multiple comparison test). (B) Live SARS-CoV-2 microneutralisation in trial participants and from serum of convalescent patients with COVID-19 infected with SARS-CoV-2 during the first outbreak of the pandemic in Victoria, Australia. Dashed line represents the geometric mean MN50 of convalescent serum of patients with COVID-19 and the dotted line represents the limit of detection. Adjusted p value versus placebo (two-way ANOVA Dunnett's multiple comparison test). (C) Surrogate virus neutralisation assay results in trial participants at each timepoint. The dotted line represents 20% inhibition below which readings are considered negative. Adjusted p value versus placebo (two-way ANOVA Dunnett's multiple comparison test). (D) SARS-CoV-2 pseudovirus neutralisation in trial participants at day 57. The dotted line represents the limit of detection. Adjusted p value versus placebo (one-way ANOVA Dunn's multiple comparison test). (E) Anti-SARS-CoV-2 sclamp IgG ELISA responses in trial participants; titres of antibodies specific for the SARS-CoV-2 sclamp and Nipah Fclamp antigens at day 57 and the percentage of the anti-SARS-CoV-2 sclamp IgG ELISA response that was specific to the clamp trimerisation domain are shown. The dotted line represents the limit of detection. Adjusted p value of sclamp versus clamp (two-way ANOVA Sidak's multiple comparison test). (F) Reactivity of trial participants' serum at day 57. For comparison of reactivity, samples from 50 patients with HIV are shown (pink). ACE2=angiotensin-converting enzyme 2. MN50=50% microneutralisation. Sclamp=spike glycoprotein-clamp vaccine. RBD=receptor binding domain. psNT50=50% pseudovirus neutralisation titre. *p<0·0001.
At baseline, serum neutralising antibodies to live SARS-CoV-2 virus were lower than the limit of detection for all participants (figure 3B, appendix p 13). At day 29, geometric mean neutralisation titres were not significantly different from placebo in any dose group. At days 43 and 57, geometric mean neutralisation titres were significantly greater (p<0·0001 for each) than placebo for the two-dose groups, but not for the single 45 μg dose group. At day 57, participants receiving two doses of sclamp vaccine elicited similar geometric mean neutralisation titres (normalised against the internal assay control), irrespective of dose; two 5 μg doses (geometric mean titre [GMT] 228, 95% CI 146–356), two 15 μg doses (GMT 230, 170–312), and two 45 μg doses (GMT 239, 187–307). Of the participants receiving a single 45 μg dose, six (25%) of 24 had a detectable neutralising titre and no participants receiving placebo had a neutralising immune response.
For comparison, a panel of serum samples collected from a cohort of adults between 14 and 90 days after recovery from mild-to-moderate SARS-CoV-2 infection had a GMT of 156 (95% CI 117–207, figure 3B). At day 57, of the participants in the two-dose groups, 75% (51 of 68) had a neutralising response greater than the geometric mean for convalescent patient serum samples, and 38% (26 of 68) had a response that was more than twice the geometric mean.
In the surrogate virus neutralisation test, partial inhibition of attachment of the spike glycoprotein receptor binding domain to the ACE2 receptor was achieved after the first dose in all dose groups; inhibition increased to more than 80% for 23 (96%) of 24 participants receiving two doses of the 5 μg vaccine and 100% of participants receiving two doses of the 15 μg and 45 μg vaccines (figure 3C).
Neutralising antibody responses against pseudovirus spike glycoprotein variants, including the index Wuhan and variant Asn501Tyr strains, were similar to responses seen against live SARS-CoV-2 (figure 3D). At day 57, geometric mean neutralisation was significantly greater than placebo for the two-dose groups (p<0·0001). Pseudovirus neutralisation responses appeared to be consistent across each of the spike glycoprotein variants in all dose groups.
Serum antibody responses were elicited to the S1, S2, and receptor binding domain spike glycoprotein subdomains and the clamp domain (appendix p 14). SARS-CoV-2 sclamp induced primarily IgG1 and IgG3, indicative of a strong Th1 response (appendix p 14). Antigen-specific IgG responses were boosted by the second vaccine dose, which also contributed to enhanced engagement of Fc gamma receptor ectodomain dimers. IgA responses were elevated at the first dose, then rapidly waned, despite a second dose (appendix p 14). IgG responses to the S1 subunit of the spike protein of human betacoronaviruses increased after vaccination; however, it was not possible to ascertain whether this increase was through boosting of pre-existing responses or the induction of new cross-reactive antibodies (appendix p 15).
The clamp-specific serum antibody response to the SARS-CoV-2 sclamp vaccine was assessed with an unrelated, similarly clamped antigen comparator, the Nipah virus Fclamp. The geometric mean antibody response at day 57 was significantly greater for SARS-CoV-2 sclamp than Nipah virus Fclamp for all two-dose groups (p<0·0001 for 5 μg dose, p=0·0063 for 15 μg dose, and p<0·0001 for 45 μg dose; figure 3E). For most participants (72 [80%] of 90), the percentage of the geometric mean antibody response that was specific for the clamp trimerisation domain ranged from 10–40% (figure 3E).
The potential for an immune response elicited by the HIV-1 glycoprotein 41 sequences that comprise the clamp to cause HIV screening diagnostic assay interference was assessed (figure 3F). At day 57, all participants in the two-dose SARS-CoV-2 sclamp groups produced a result that was considered indeterminate (figure 3F). The amount of reactivity was more variable for the single 45 μg dose, with some samples showing similar cross-reactivity to participants in the two-dose groups. P24 antigen and nucleic acid testing confirmed that all participants were negative for HIV infection (appendix pp 16–17). Cross-reactivity for participant samples at day 57 was also evident in eight of the 12 HIV screening immunoassay diagnostic platforms assessed (appendix p 18). In summary, samples assessed with diagnostic platforms that used recombinant glycoprotein 41 as a detection reagent were reactive, whereas those using glycoprotein 41 peptides were non-reactive, a finding consistent with the immunodominant sequences used in the peptide-based assays not being present in the molecular clamp.
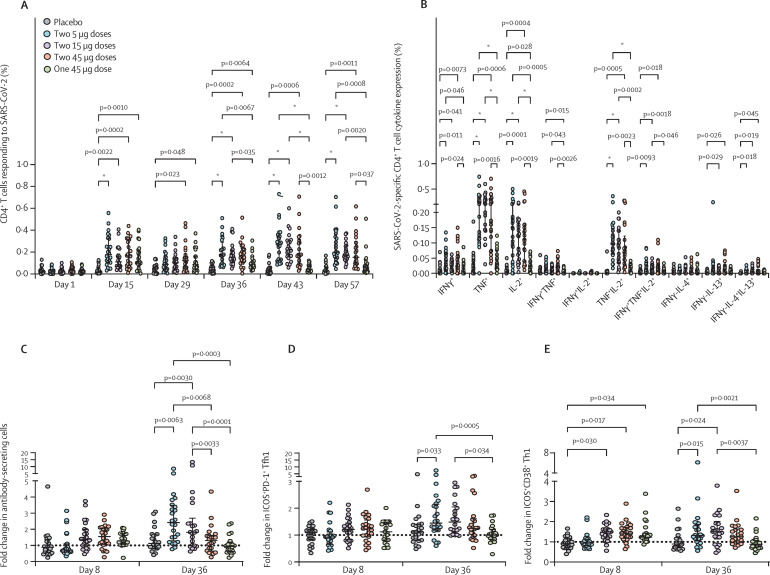
SARS-CoV-2 sclamp elicited robust SARS-CoV-2 spike-specific CD4+ T-cell responses including polyfunctional Th1 and Th2 responses. Analysis of interferon γ, tumour necrosis factor α (TNFα), and IL-2 production of CD4+ T cells after peptide stimulation showed that spike-specific responses were significantly elevated in all four treatment groups at days 15, 36, 43, and 57 compared with placebo and baseline (day 1; figure 4A , appendix pp 19–27). A single dose in all SARS-CoV-2 sclamp vaccination regimens reproducibly elicited CD4+ T-cell responses, but the two-dose regimens were the most effective compared with placebo (figure 4A). Analysis of the polyfunctionality of responding spike-specific CD4+ T cells showed that the two-dose regimens elicited polyfunctional Th1 and Th2 (IL-4 and IL-13) responses, although the increase in the IL-4 or IL-13 expressing Th2 cells was subtle (figure 4B). Notably, the TNFα+IL-2+ Th1 subset was highly elevated compared with other polyfunctional subsets (figure 4B). SARS-CoV-2 sclamp vaccine had no effect on regulating CD8+ T-cell subsets. Detailed analysis of CD4+ and CD8+ T-cell responses is included in the appendix (pp 19–27).
Figure 4.
SARS-CoV-2 spike-specific cytokine expression by CD4+T cells, antibody-secreting cells, and Tfh1 responses in peripheral blood
Summation analysis of SARS-CoV-2 spike-specific Th1 cytokine (IFNγ, TNF, and IL-2) expression by CD4+ T cells at each timepoint (A). SARS-CoV-2 spike-specific Th1 and Th2 cytokine expression by CD4+ T cells at day 43 (B). Fold-change in the frequency of CD3−D19+CD27hiCD38hi antibody-secreting cells (C), activated ICOS+PD-1+CXCR5+CXCR3+CCR6−CD4+ circulating Tfh1 cells (D), and activated ICOS+CD38+CCR5−CD4+ Th1 cells (E), at day 8 and 36. Bars and lines and error bars indicate the median (IQR). Statistical significance between different groups was determined using a fitted mixed-model two-way ANOVA. Tfh1=type 1 T follicular helper cells. Sclamp=spike glycoprotein-clamp vaccine. Th=T-helper. TNF=tumour necrosis factor. ICOS=inducible T cell costimulator. *p<0·0001.
Two doses of SARS-CoV-2 sclamp induced elevated antibody-secreting cells (ASCs) and type-1 Tfh cells (Tfh1; figure 4). Activity appeared to be dose-dependent. After the first dose, a very modest and non-significant increase in ASCs and activated circulating Tfh1 cells, along with a significant increase in activated inducible co-stimulator (ICOS)+CD38+ Th1 was observed in the 15 μg and 45 μg groups but not the 5 μg group. After the second dose, the frequency of ASCs and activated ICOS+CD38+ Th1 cells increased significantly in the 5 μg and 15 μg groups compared with placebo, but not the 45 μg group.
CD4+ T-cell activation strongly correlated with robust humoral responses, as assessed using Spearman's correlation. For most timepoints in the two-dose groups, strong correlation (r s=0·8–1·0) was detected across all antibody assays, including ELISAs, surrogate virus neutralisation tests, neutralisation assays, IgG multiplex serology assays, and reactivity against divergent SARS-CoV-2 strains via the pseudovirus assay (appendix pp 28–29). Importantly, these antibody responses positively correlated with broad CD4+ T-cell activation (intracellular flow cytometry assay) in the two-dose groups, although there were fewer correlations in the single 45 μg group, indicating that robust cellular vaccine responses benefit from a two-dose vaccine regimen (appendix pp 28–29). The highest correlations between antibody and cellular responses occurred in the two-dose 15 μg group, with antibodies strongly correlating with activated CD38+ human leucocyte antigen (HLA)-DR+CD4+ T cells and CD38+ICOS+ Th1 cells on day 7, followed by additional correlations with other CD4+ Th and Tfh cell subsets on day 36. Increase of CD38+ICOS+ Th1 cells on day 7 correlated strongly for all groups, except the two-dose 5 μg group, with antibody responses up to day 57 and could be a potential early predictor of both robust humoral and cellular vaccine responses (appendix pp 28–29). This finding is important, given that Th1 activation has been associated previously with durable immune responses induced by vaccination in mice.28 No correlations were seen between the humoral response and cellular ASC, Th, or Tfh responses at day 7 in the two-dose 5 μg group; activated CD38+HLA-DR+CD4+ T cells and CD38+ICOS+ Th2 cells were negatively correlated, but positive correlations with ASCs were found on day 36 (7 days after the second dose). The two groups receiving one or two doses of 45 μg showed good antibody correlation with ASC, Th, and Tfh responses on day 7, but weaker correlation on day 36, even after the second dose. Notably, the highest correlation with cellular and antibody responses was observed for the neutralisation assay on day 57 in the two-dose 15 μg group (appendix pp 28–29). Multiple comparisons factoring in all antibody and cellular data compared with the placebo group showed that the later antibody titres (days 43 and 57) were enriched in the two-dose groups (>1024 fold change) compared with the single 45 μg dose (appendix pp 28–29).
Discussion
These phase 1 findings in healthy adults show that two doses of the SARS-CoV-2 sclamp vaccine were well tolerated with a similar frequency of adverse events to placebo, irrespective of dose concentration. In addition, two doses of SARS-CoV-2 sclamp vaccine, at all dose concentrations studied (5, 15, and 45 μg), induced a robust neutralising immune response and a spike glycoprotein-specific T-cell response presumed to be indicative of protection against SARS-CoV-2 infection. Analyses of more than 100 immune features showed strong correlations between antibody responses detected across all antibody assays, with the highest correlations observed with two doses of the 15 μg SARS-CoV-2 sclamp, which was also strongly correlated with activated ASCs, CD4+ Th, and Tfh cell subsets. These findings are the first to show the viability of the molecular clamp trimerisation motif for human vaccine development.
The molecular clamp platform of SARS-CoV-2 sclamp was designed based on the well characterised and highly stable six-helix bundle of HIV-1 glycoprotein 41, which comprises two heptad repeat regions, HR1 (amino acids 540–576) and HR2 (amino acids 619–656).4 Inclusion of these sequences enables the manufacture and purification of a highly stable pre-fusion conformation of the SARS-CoV-2 spike glycoprotein.4 Because immunodominant epitopes of glycoprotein 41 are not present in the HR1 and HR2 regions, it was considered possible but unlikely that these regions would cause HIV diagnostic interference.29 All participants who received two doses of SARS-CoV-2 sclamp elicited a humoral response that was detected by several commonly used HIV diagnostic tests. Although the geometric mean for vaccine cross-reactivity was 30 times lower than the geometric mean for samples from a randomly selected set of 50 patients with HIV, it was within the range of nine of these patients with HIV. Therefore, widespread use of this vaccine would have the potential to impact existing HIV screening programmes and processes and could ultimately delay timely diagnosis for a subset of patients with HIV. Additionally, where a climate of vaccine hesitancy exists, the potential negative effects of miscommunication and misunderstanding of this issue, and the potential for societal misconception around the inclusion of HIV-1 glycoprotein 41 regions within a SARS-CoV-2 vaccine, could not be ignored and further development of this vaccine is currently paused.
The candidate SARS-CoV-2 sclamp vaccine was shown to be safe and well tolerated. Most adverse events were injection-site related, mild, and transient. In contrast to other SARS-CoV-2 vaccines,6, 7, 30, 31 only a single participant had a fever (>38°C) and the frequency of solicited systemic adverse events was low and similar to placebo. These safety findings are notable as a first-in-human test of the molecular clamp platform and highlight the benefits of using a subunit vaccine with a well understood and commercially licensed adjuvant.
Studies of several nucleic acid and vectored vaccines in non-human primates have shown that neutralising antibodies against the full-length spike glycoprotein confer protection against SARS-CoV-2 infection.32, 33, 34 In this study, we showed that neutralising antibody titres after the second dose of SARS-CoV-2 sclamp were similar to those of patients with SARS-CoV-2, with 75% of participants having a neutralising response greater than that of convalescent patient serum samples. Importantly, the immunogenicity of the lower doses was similar to that of the highest dose, which is important for antigen sparing and scalability of manufacture. Moreover, the neutralising antibody responses across several spike glycoprotein variants were similar to the responses seen against live SARS-CoV-2, suggesting that SARS-CoV-2 sclamp vaccine is likely to confer protection against divergent strains. This finding is promising given that novel (and potentially more infectious or dangerous) variants will continue to emerge, which might affect the immunogenicity and efficacy of existing and candidate vaccines.
Our findings showed that two doses of SARS-CoV-2 sclamp were required to elicit a strong SARS-CoV-2-specific polyfunctional CD4+ T-cell response. The response had a predominantly Th1 phenotype that was consistent with the ASC, Tfh1, and neutralising antibody responses. Poor CD8+ T-cell and non-cytotoxic T-cell responses were not unexpected as the MF59 adjuvant is well understood to preferentially drive a CD4+ T-cell antibody response in humans,35 and spike-specific CD8+ T-cells have not been observed in humans with similar adjuvanted subunit SARS-CoV-2 vaccines.7
Findings from studies in acute and convalescent patients with COVID-19 suggest that ASCs, and particularly activated circulating Tfh1 cells, are important for recovery from COVID-19.17, 27, 36 In this study, ASCs and activated Tfh1 cells were significantly elevated after two doses of either 5 μg or 15 μg vaccine. The requirement for two doses is not surprising given that ASCs are predominantly derived from memory B cells,37, 38 and circulating Tfh1 cells are known to assist memory B-cells, but not B-cell differentiation into ASCs.39, 40 Unexpectedly, the 45 μg dose led to less activation in the cellular compartments analysed here, possibly indicating that the interplay between antibodies and cellular immunity vary with vaccine dose.41 A range of vaccine platforms that are commercially licenced and available for COVID-19 are needed. The SARS-CoV-2 sclamp vaccine was derived from Chinese hamster ovary cells, a mainstay of the biotechnology industry for which there is extensive global manufacturing capacity. The results of this study provide confidence that mammalian cell-derived spike protein-based vaccines have a safety and immunogenicity profile consistent with other vaccine platforms that have shown very promising efficacy data. Furthermore, adjuvanted protein-based vaccines are known to confer a durable immune response, and the combination with MF59 in this study is of particular importance given its extensive safety and large-scale manufacturing track record.
In conclusion, this study has shown the safety and potential efficacy that can be achieved in humans with molecular clamp-stabilised vaccines and MF59 adjuvant. Coupled with the high thermostability and manufacturability previously shown,4 these vaccines would make a valuable addition to the global COVID-19 response. Further development is underway to identify an alternate molecular clamp stabilisation domain that does not include HIV-1 glycoprotein 41 sequences.
Data sharing
De-identified individual patient data underlying the results will be made available immediately after publication with no end date. Access to data, supported by the clinical study report (or any other specified documents), will be granted on a case-by-case basis at the discretion of the study sponsor and can only be used to achieve the specific aims in the approved proposal in a signed data access agreement. Requests for access can be made via correspondence to the corresponding author Keith Chappell (k.chappell@uq.edu.au).
Acknowledgments
Acknowledgments
We thank every participant who enrolled in the clinical trial. Medical writing assistance was provided by Serina Stretton and Rebecca Lew, of ProScribe (Envision Pharma Group), who were funded by Envision Pharma Group. ProScribe helped verify all the data and their services complied with international guidelines for Good Publication Practice. The comparator convalescent patient population data were made available thanks to Stephen Kent, Jennifer Juno, Helen Kent, Adam Wheatley, Deborah Williamson, Katherine Bond, Allen Cheng, and Denise Doolan. We also thank the patients for providing their samples. The authors thank Marios Koutsakos for his assistance with the antibody-secreting cell and T follicular helper antibodies. The following reagent was produced under HHSN272201400008C and obtained through BEI Resources, NIAID, NIH: SARS-CoV-2 Spike RBD (NR-52366). The study was funded by the Coalition for Epidemic Preparedness Innovations, the National Health and Medical Research Council of Australia, and the Queensland Government. KK was supported by the National Health and Medical Research Council Leadership Investigator Grant (1173871). LH and WZ were supported by the Melbourne International Research Scholarship and the Melbourne International Fee Remission Scholarship from The University of Melbourne. Further philanthropic funding sources included Royal Automobile Club of Queensland, The Golden Casket Foundation, The Bryan Foundation, The a2 Milk Company (Australia), Liming International, The Bowness Family Foundation, Glencore Australia Holdings, BHP Foundation, NewCrest Mining, Dr Jian Zhou Foundation, Paul Ramsay Foundation, Aurizon Operations, and The University of Queensland in America.
Contributors
KJC, TPM, DW, PRY, JB, and PG designed the study. KJC, ZL, DKW, PE, DW, THON, BDW, PCR, TPM, PRY, KK, KS, DP, PMH, and PT designed the assays used. FLM, ZL, DKW, PE, JAL, STMC, NM, MSA, KH, THON, MT, KJS, PMH, AWC, PG, KK, KS, CR, TH, SMH, PT, JB, SC, and LH contributed to data collection. KJC, ZL, DKW, MSA, WZ, DW, THON, MT, KJS, AWC, PCR, SN, PRY, PG, LH, KK, KS, JB, DP, PT, JB, and SC contributed to data analysis and interpretation. KJC and TPM verified the data. KJC, DW, THON, PRY, TPM, and PG wrote the manuscript. TPM was the project director, KJC and PRY were project leaders, and CLH was the project manager. All authors provided intellectual input into the manuscript and approved the final version for submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
KJC, DW, and PRY report grants from the Coalition for Epidemic Preparedness Innovations, the National Health and Medical Research Council of Australia, and the Queensland Government, during the conduct of the study; contract research funding from ViceBio, outside the submitted work; and have patents pending (AU 2018241252; BR112019019813.0; CA 3057171; CH 201880022016.9; EP 18775234.0; IN 201917038666; ID P00201909145; IL 269534; JP 2019-553883; MX/a/2019/011599; NZ 757178; KR 0-2019-7031415; SG 11201908280S; US 16/498865; US 2020/0040042). JB reports personal fees from CSL, during the conduct of the study, and consultancy fees from CSL, Seqirus, and UQ, outside the submitted work. WZ reports grants from the National Health and Medical Research Council of Australia, the Research Grants Council of the Hong Kong Special Administrative Region, China, and the Jack Ma Foundation, during the conduct of the study. SM-H reports grants from Canarian Foundation Doctor Manuel Morales, during the conduct of the study. KJS reports grants from the Australian Medical Research Future Fund, during the conduct of the study. AWC reports grants from the Australian Medical Research Future Fund and a National Health and Medical Research Council of Australia Career Development Fellowship, during the conduct of the study. BDW reports grants from the National Health and Medical Research Council of Australia, the Australian Medical Research Future Fund, and the Victorian State Government, during the conduct of the study. PMH reports grants from the Australian Medical Research Future Fund, during the conduct of the study. DP reports grants from the National Health and Medical Research Council of Australia, the a2 Milk Foundation, and the Jack Ma Foundation, during the conduct of the study. CR reports grants from the Coalition for Epidemic Preparedness Innovations, during the conduct of the study. PRY reports grants from the Coalition for Epidemic Preparedness Innovations, the National Health and Medical Research Council of Australia, and the Queensland Government, during the conduct of the study; grants from ViceBio, outside the submitted work; and a patent issued (US 2020/0040042). All other authors declare no competing interests.
Supplementary Material
References
- 1.WHO . World Health Organization; Geneva: 2020. Accelerating a safe and effective COVID-19 vaccine.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/accelerating-a-safe-and-effective-covid-19-vaccine [Google Scholar]
- 2.WHO . World Health Organization; Geneva: 2021. Draft landscape and tracker of COVID-19 candidate vaccines.https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Google Scholar]
- 3.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watterson D, Wijesundara D, Modhiran N, et al. Preclinical development of a molecular clamp stabilised subunit vaccine for severe acute respiratory syndrome coronavirus 2. Clin Transl Immunol. 2021;10 doi: 10.1002/cti2.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2034201. https://www.nejm.org/doi/10.1056/NEJMoa2034201 published online Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart-Jones GBE, Chuang GY, Xu K, et al. Structure-based design of a quadrivalent fusion glycoprotein vaccine for human parainfluenza virus types 1–4. Proc Natl Acad Sci USA. 2018;115:12265–12270. doi: 10.1073/pnas.1811980115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black S, Della Cioppa G, Malfroot A, et al. Safety of MF59-adjuvanted versus non-adjuvanted influenza vaccines in children and adolescents: an integrated analysis. Vaccine. 2010;28:7331–7336. doi: 10.1016/j.vaccine.2010.08.075. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine. 2009;27:6959–6965. doi: 10.1016/j.vaccine.2009.08.101. [DOI] [PubMed] [Google Scholar]
- 11.Tsai T, Kyaw MH, Novicki D, Nacci P, Rai S, Clemens R. Exposure to MF59-adjuvanted influenza vaccines during pregnancy—a retrospective analysis. Vaccine. 2010;28:1877–1880. doi: 10.1016/j.vaccine.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 12.Knudsen NP, Olsen A, Buonsanti C, et al. Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens. Sci Rep. 2016;6 doi: 10.1038/srep19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acosta PL, Caballero MT, Polack FP. Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol. 2015;23:189–195. doi: 10.1128/CVI.00609-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng CT, Sbrana E, Iwata-Yoshikawa N, et al. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrawal AS, Tao X, Algaissi A, et al. Immunization with inactivated Middle East Respiratory Syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016;12:2351–2356. doi: 10.1080/21645515.2016.1177688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bond K, Nicholson S, Lim SM, et al. Evaluation of serological tests for SARS-CoV-2: implications for serology testing in a low-prevalence setting. J Infect Dis. 2020;222:1280–1288. doi: 10.1093/infdis/jiaa467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juno JA, Tan HX, Lee WS, et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat Med. 2020;26:1428–1434. doi: 10.1038/s41591-020-0995-0. [DOI] [PubMed] [Google Scholar]
- 18.Caly L, Druce J, Roberts J, et al. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med J Aust. 2020;212:459–462. doi: 10.5694/mja2.50569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subbarao K, McAuliffe J, Vogel L, et al. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78:3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Wang R, Wang M, Wei GW. Mutations strengthened SARS-CoV-2 infectivity. J Mol Biol. 2020;432:5212–5226. doi: 10.1016/j.jmb.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu H, Chen Q, Yang G, et al. Adaptation of SARS-CoV-2 in BALB/c mice for testing vaccine efficacy. Science. 2020;369:1603–1607. doi: 10.1126/science.abc4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y, He J, Gong L, et al. Molecular epidemiology of SARS-CoV-2 clusters caused by asymptomatic cases in Anhui Province, China. BMC Infect Dis. 2020;20:930. doi: 10.1186/s12879-020-05612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan CW, Chia WN, Qin X, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein–protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 24.De Rose R, Fernandez CS, Smith MZ, et al. Control of viremia and prevention of AIDS following immunotherapy of SIV-infected macaques with peptide-pulsed blood. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna M, Jackson RJ, Alcantara S, et al. Mucosal and systemic SIV-specific cytotoxic CD4+ T cell hierarchy in protection following intranasal/intramuscular recombinant pox-viral vaccination of pigtail macaques. Sci Rep. 2019;9 doi: 10.1038/s41598-019-41506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Khanna M, Grimley SL, et al. Mucosal IL-4R antagonist HIV vaccination with SOSIP-gp140 booster can induce high-quality cytotoxic CD4+/CD8+ T cells and humoral responses in macaques. Sci Rep. 2020;10 doi: 10.1038/s41598-020-79172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thevarajan I, Nguyen THO, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martins KAO, Cooper CL, Stronsky SM, et al. Adjuvant-enhanced CD4 T cell responses are critical to durable vaccine immunity. EBioMedicine. 2015;3:67–78. doi: 10.1016/j.ebiom.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorn J, Masciotra S, Yang C, et al. Analysis of genetic variability within the immunodominant epitopes of envelope gp41 from human immunodeficiency virus type 1 (HIV-1) group M and its impact on HIV-1 antibody detection. J Clin Microbiol. 2000;38:773–780. doi: 10.1128/jcm.38.2.773-780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 31.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKee AS, Marrack P. Old and new adjuvants. Curr Opin Immunol. 2017;47:44–51. doi: 10.1016/j.coi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koutsakos M, Rowntree L, Hensen L, et al. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Rep Med. 2021;2 doi: 10.1016/j.xcrm.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bentebibel SE, Khurana S, Schmitt N, et al. ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep. 2016;6 doi: 10.1038/srep26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koutsakos M, Wheatley AK, Loh L, et al. Circulating TFH cells, serological memory, and tissue compartmentalization shape human influenza-specific B cell immunity. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan8405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual patient data underlying the results will be made available immediately after publication with no end date. Access to data, supported by the clinical study report (or any other specified documents), will be granted on a case-by-case basis at the discretion of the study sponsor and can only be used to achieve the specific aims in the approved proposal in a signed data access agreement. Requests for access can be made via correspondence to the corresponding author Keith Chappell (k.chappell@uq.edu.au).