Abstract
Motivation
Tumours are composed of distinct cancer cell populations (clones), which continuously adapt to their local micro-environment. Standard methods for clonal deconvolution seek to identify groups of mutations and estimate the prevalence of each group in the tumour, while considering its purity and copy number profile. These methods have been applied on cross-sectional data and on longitudinal data after discarding information on the timing of sample collection. Two key questions are how can we incorporate such information in our analyses and is there any benefit in doing so?
Results
We developed a clonal deconvolution method, which incorporates explicitly the temporal spacing of longitudinally sampled tumours. By merging a Dirichlet Process Mixture Model with Gaussian Process priors and using as input a sequence of several sparsely collected samples, our method can reconstruct the temporal profile of the abundance of any mutation cluster supported by the data as a continuous function of time. We benchmarked our method on whole genome, whole exome and targeted sequencing data from patients with chronic lymphocytic leukaemia, on liquid biopsy data from a patient with melanoma and on synthetic data and we found that incorporating information on the timing of tissue collection improves model performance, as long as data of sufficient volume and complexity are available for estimating free model parameters. Thus, our approach is particularly useful when collecting a relatively long sequence of tumour samples is feasible, as in liquid cancers (e.g. leukaemia) and liquid biopsies.
Availability and implementation
The statistical methodology presented in this paper is freely available at github.com/dvav/clonosGP.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
Cancer cells undergo a process of Darwinian evolution in response to selective pressures in their local micro-environment, for example, as a result of therapeutic intervention (Merlo et al., 2006; Nowell, 1976). This induces cell propagation and diversification during tumour growth, which result in a heterogeneous population of phylogenetically related, but genotypically and phenotypically distinct cancer cell populations, known as clones. Tumour heterogeneity is clinically important because it complicates the molecular profiling of tumours and enables the fittest cancer cells to escape treatment leading to relapse. Monitoring this process of continuous adaptation requires a detailed characterization (through the use of next-generation sequencing, bioinformatics and statistical analysis) of the somatic aberrations harboured by the tumour at various time points over the course of the disease.
A major challenge in solving the problem of clonal deconvolution using bulk sequencing data is the fact that tumour heterogeneity is not directly observed, but rather inferred through the analysis of samples, each of which is a mixture of normal and cancer cells from various clones. Despite (or because of) this, clonal deconvolution has been the subject of much statistical innovation (see Beerenwinkel et al., 2015; Dentro et al., 2017; Ismail et al., 2019; Salcedo et al., 2020 for a review). Current statistical methodologies seek to identify the number of clones in a tumour, their somatic mutation content, prevalence and phylogenetic relations and they can be used for the analysis of cross-sectional data (obtained, for example, through multiple biopsies from the same patient) or longitudinal data after discarding any information on the timing of tissue sample collection (Abécassis et al., 2019; Deshwar et al., 2015; Donmez et al., 2017; El-Kebir et al., 2015; Fischer et al., 2014; Jiang et al., 2016; Marass et al., 2016; Miller et al., 2014; Myers et al., 2019; Roth et al., 2014; Rubanova et al., 2018; Sengupta et al., 2014; Yuan et al., 2015, 2018; Zare et al., 2014; Zucker et al., 2019).
In this article, we pose the following two questions: (i) how can we incorporate temporal spacing information in the analysis of sequentially collected samples (typically over several months or years) and (ii) is there any benefit in doing so? We begin with a standard Bayesian non-parametric model for clustering somatic mutations with similar observed frequencies, while simultaneously correcting for sample purity and local copy number variation. We extend this model by treating the cluster prevalences as functions of time, which follow a Gaussian process prior. The advantage of this approach is that we do not need to impose a particular functional form on the time dependence of cluster abundances, but only some general properties (e.g. smoothness, amplitude and time scale), which are estimated from the data. In return, we obtain a continuous reconstruction of the time course of each cluster during the course of the disease from a small number of sequentially collected samples. We test various model configurations on whole genome sequencing (WGS), whole exome sequencing (WES) and targeted genome sequencing (TGS) data from patients with chronic lymphocytic leukaemia (CLL; González-Rincón et al., 2019; Schuh et al., 2012), on data from the liquid biopsy of a patient with melanoma (Cutts et al., 2017) and on synthetic data, and we demonstrate that incorporating temporal information in our analysis can boost the performance of clonal deconvolution.
2 Materials and methods
We present a series of models of increasing complexity starting with the statistical model for a single tumour sample.
2.1 Model for a single tumour sample
We assume that a tumour has been sequenced at N bi-allelic genomic loci harbouring somatic mutations. For each locus i, we can calculate the observed variant allele fraction (VAF) as the ratio , where ri and are the number of reads harbouring the alternative and reference alleles, respectively. The expected value θi of the VAF for mutation i is a function f of the cancer cell fraction (CCF), i.e. the fraction of cancer cells that harbour the mutation, . The population of cancer cells is partitioned in a finite, but unknown, number of clones, each harbouring a unique set of mutations. This implies that different mutations share the same CCF value, i.e. the mutation-specific fractions are not all distinct. We model this structure with a Dirichlet Process prior on with concentration parameter α and a uniform base distribution (Gelman et al., 2013; Nik-Zainal et al., 2012). Using the stick-breaking representation of the Dirichlet Process, is modelled as an infinite mixture, as shown below:
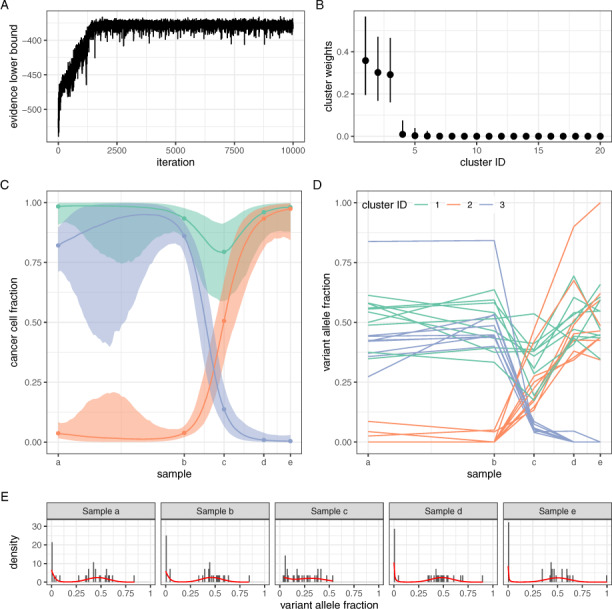
where is the Dirac delta function centred at and indicates a beta distribution. The uniform prior on the mean of the beta function implies that the prior on the concentration parameter is , which is similar to the standard exponential distribution, but with higher kurtosis, resulting in a heavier tail. In practice, we approximate the above infinite sum by truncating at a large value K (Ishwaran and James, 2001). Here, we take K = 20, which is more than twice the largest number of estimated clusters across all examined datasets (see Section 3; Fig. 4).
Fig. 4.
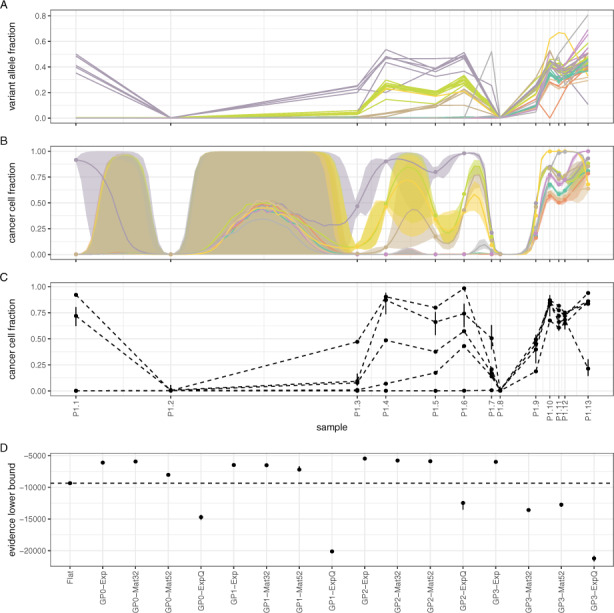
Assessing model performance on CLL data from Patient 1 (González-Rincón et al., 2019). (A) Observed VAF values for each somatic mutation over 6.5 years and their cluster assignments (colours are the same as in (B). B) Mutation clusters identified by model GP0-Mat32. (C) Mutation clusters identified by the Flat model. (D) Comparative performance of various models. Notice that simpler models (GP0) often perform equivalently to or better than more complex ones (GP1, GP2 and GP3)
2.2 Joint model for clonally related tumour samples
The above model can be extended to multiple clonally related samples by allowing the CCF variables to vary between samples (Bolli et al., 2014; Roth et al., 2014). For M samples (and truncation K), we have:
where the rest of the model remains the same as for the one-sample case. Effectively, we incorporate multiple samples in the model by allowing the cluster centres to vary across samples. As a prelude to the next section, we note that the transformed variable follows a standard logistic distribution, . Below, instead of the logistic distribution, we use a parameterized multivariate normal distribution, as explained in more detail in the next section.
2.3 Single-output Gaussian process model for longitudinal tumour samples
The above model does not consider the temporal spacing of the M samples, in case these have been collected longitudinally. If such information is indeed available, it can be included in the model by treating the transformed CCF variables as functions of time, . On these functions, we impose a Gaussian Process prior (Gelman et al., 2013; Rasmussen and Williams, 2006; Roberts et al., 2013):
where the kernel function encodes the covariance of at times t and . This non-parametric approach permits modelling the time-dependency of the transformed CCF variables without any strong prior assumptions on the functional form of this dependency. The above implies that if M samples have been collected at times , then the variables follow a multivariate Normal distribution:
where is the M-dimensional zero vector. The elements of the covariance matrix encode the covariance between the values of at all possible pairs of sampling times tj and .
We consider kernels of the form where h is an amplitude parameter, while the function , which is parameterized by an inverse squared time scale parameter τ, takes any of the following forms: (i) exponential: , (ii) Mat32: , (iii) Mat52: and (iv) exponentiated quadratic: . These four kernels are members of the Matérn family of covariance functions ordered in terms of increasing smoothness (Rasmussen and Williams, 2006). Finally, we impose gamma priors on the amplitude and time scale parameters, and .
2.4 Multi-output Gaussian process model for longitudinal tumour samples
In the above model, the cluster-specific scalar-valued functions share the same Gaussian Process prior, but they are otherwise independent. We can directly model possible correlations between different clusters (i.e. different values of k) by assuming that the vector-valued function of time, , follows a Gaussian Process prior:
where is a matrix-valued kernel encoding the K × K covariance matrix between vectors and . Given M longitudinally observed samples, the above implies that the matrix of CCF values follows a multivariate Normal distribution of dimensionality MK:
where the operator vectorizes its matrix argument by stacking its columns on top of each other, is a matrix of zeros and is a positive semi-definite block matrix encoding the covariance between ψjk and .
Assuming that the above kernel is separable (Álvarez et al., 2012), we can write the factorization , where is the same as in the previous section. is a positive semi-definite matrix factorized as , where and is a correlation matrix following the LKJ prior (Stan Development Team, 2020) with concentration parameter η. A value of η = 1 implies a uniform prior over correlation matrices, while η = 2 (the value we adopt here) concentrates more probability mass around the identity matrix. This structure for implies both cluster-specific amplitudes , as well as correlations between clusters. Alternatively, we can assume that , which implies that different clusters have different values of the amplitude parameters .
Finally, we examine the case where is a block-diagonal matrix, with each of the K matrices along its main diagonal induced by the kernel , where both amplitude and time scale τk parameters are cluster-specific.
2.5 Relation between VAF and CCF
In this section, we give more details about the form of the function , which encodes the relationship between VAF and CCF of mutation i in sample j. With respect to mutation/locus i, each sample is viewed as a mixture of three cell populations (Roth et al., 2014): (i) a normal population of non-cancer cells, (ii) a reference population of cancer cells, which do not harbour mutation i and (iii) a variant population of cancer cells, which harbour mutation i. The total number of cancer cells in the sample is . The reference and variant populations may each be further subdivided into sub-populations, where a different number of chromosomes covers locus i in each sub-population. The total number of chromosomes in the normal, reference and variant populations overlapping locus i in sample j are, respectively, equal to (assuming diploid normal cells), and , where and are the average numbers of chromosomes per cell covering locus i in sample j in each of the two cancer cell populations. Similarly, the total number of chromosomes harbouring mutation i in sample j is equal to , where is the multiplicity, i.e. the average number of chromosomes per cell in the variant cancer cell population harbouring mutation i in sample j. We write:
where is the purity of the tumour and . At this stage, two simplifying assumptions are often made: (i) there are no sub-clonal copy number events, which implies that and are whole numbers, and (ii) the reference and variant cancer cell populations have the same copy number profile at locus i in sample j, i.e. . Under these assumptions, the above expression simplifies to:
where ζij is the value of θij if mutation i in sample j is clonal (i.e. ). The quantities ρj and Dij can be independently estimated using software such as ASCAT (Van Loo et al., 2010), ABSOLUTE (Carter et al., 2012), TITAN (Ha et al., 2014) and others, and they are considered fixed. One way to approximate the multiplicity is as follows: first, we calculate . Then, we estimate using the following rule:
where is the closest integer to uij. For a justification of this estimation procedure, see Dentro et al. (2017).
2.6 Observation models
We complete the above models by introducing expressions for the distribution of the read counts rij harbouring mutation i in sample j. Since high-throughput sequencing data often exhibit over-dispersion, we consider a beta-binomial model:
where Rij is the sum of reads harbouring the alternative and reference alleles at locus i in sample j and vj is a precision parameter. In the absence of over-dispersion (i.e. when ), the above reduces to the binomial model, . Both error models capture the discrete sampling of reads in the NGS data-generating process and they account for read depth variability due to non-uniform coverage across the genome. Parameter vj in the beta-binomial model is sample-specific, which allows the model to adapt to different degrees of over-dispersion across samples. Alternatively, a common precision parameter can be used for all samples in the absence of a sufficiently large volume of data. In Supplementary Methods, we further present a version of the above model, which explicitly accounts for possible sequencing errors.
2.7 Inference
We implemented the above models using the probabilistic programming language PyMC3 v3.8 (Salvatier et al., 2016) and inference was conducted using Automatic Differentiation Variational Inference (ADVI; Kucukelbir et al., 2017), instead of developing bespoke estimation algorithms, which is a rather laborious process particularly when multiple candidate models are considered (Vavoulis, 2020; Vavoulis et al., 2015, 2017). Variational inference (VI; Blei et al., 2017; Zhang et al., 2019) is a computationally efficient approach for Bayesian inference, which aims to approximate the posterior density of latent variables z given data y using a surrogate probability density parameterized by a vector of variational parameters . In our case, the data y are the locus- and sample-specific read counts rij and Rij, the local copy numbers Dij, the sample-specific purities ρj and the sample collection times tj, while the latent variables z are the CCFs , the cluster weights wk, the amplitudes , the time-scales τk and the sample-specific dispersions vj. VI approximates by maximizing the lower bound of the marginal likelihood (or evidence) , which is known as the evidence lower bound (ELBO), with respect to the variational parameters :
Maximizing the ELBO is equivalent to jointly maximizing the entropy term (which leads to a more spread out variational distribution q and prevents over-fitting) and minimizing the average energy term (i.e. the discrepancy between q and p). Furthermore, the maximized ELBO, being a lower bound of the evidence , can be used for model comparison (see below).
2.8 Performance metrics
We fit the above models against actual or simulated tumour samples (see Section 3). In the first case, the ground truth or latent structure of the data (i.e. the true CCFs, the number and composition of mutation clusters) is by definition unobservable and therefore unknown. This situation is the rule, not the exception, in the study of complex systems and it complicates model validation and selection, since model estimates cannot be verified against their true values. In this case, we compare the performance of different models using the model evidence, as approximated by the maximized ELBO (with a higher value indicating a better model). This criterion naturally favours simple models over complex ones, thus protecting against over-fitting (a manifestation of Occam’s razor). It is implied that models with higher ELBO better approximate the data generating process and, by extension, the underlying latent structure of the data. In the case of simulated data, the ground truth is known a priori and different models are compared using the Adjusted Rand Index (ARI), as implemented in the Python package scikit-learn v0.22 (Pedregosa et al., 2011). ARI takes values between -1 and 1, with negative or close to 0 values indicating deviation from the ground truth, while values close to 1 indicate close agreement to it. ARI is symmetric, and for this reason, we also use it for estimating the concordance between any two clustering models when these are fitted on actual data. In Supplementary Methods, we give further details on ARI and on two additional metrics, the Adjusted Mutual Information (AMI) and the Fowlkes-Mallows Index (FMI). All three scores are robust against agreement-by-chance and anisotropic cluster shapes.
2.9 Model nomenclature
In Section 3, the various models described above are referred to as follows. The model that assumes a uniform (i.e. flat) prior over the CCF variables is the Flat model. The model that assumes a single-output Gaussian Process prior over the transformed CCF variables ψjk is the GP0 model. The models assuming a multi-output Gaussian Process prior on ψjk are labelled GP1 (when is diagonal), GP2 (when is full rank) and GP3 (when is block-diagonal with cluster-specific and τk parameters), respectively. Each of the models GP0 to GP3 admits exponential (Exp), Mat32, Mat52 or exponentiated quadratic (ExpQ) kernels and are labelled accordingly, e.g. GP0-Exp, GP0-ExpQ, etc. In total, we examined 17 models. If the number of parameters in the Flat model is (where L is the number of clusters with non-zero weights), the number of parameters in the GP0 to GP3 models is , and , respectively.
3 Results
We conducted a series of computational experiments on WES and WGS data from patients with CLL (González-Rincón et al., 2019; Schuh et al., 2012), on TGS data from the liquid biopsy of a patient with melanoma (Cutts et al., 2017) and on simulated data. The aim of these experiments was to demonstrate the application of the above models on longitudinal data and to assess their relative performance.
3.1 The case of patient CLL003
First, we demonstrate the application of model GP0-Mat32 on WGS data from patient CLL003 reported by Schuh et al. (2012) (Fig. 1; the performance of other models on the same dataset is summarized in Figures 2A and 3, top-left panel; see also Supplementary Figs S1–S3). Details on sequencing and bioinformatics analysis for obtaining this data are given in the original paper. Briefly, peripheral blood was collected at five specific time points during disease progression, treatment and relapse together with a matched buccal swab (for germinal DNA). All samples underwent WGS followed by bioinformatics analysis, which identified 28 somatic mutations. Fitting the model to this data was performed by maximizing the ELBO (see Section 2), which can be used for assessing convergence of the estimation algorithm (typically achieved in less than 3K iterations; Fig. 1A). Following a non-parametric approach for clustering mutations using a Dirichlet Process prior on the CCFs (see Section 2) means that the number of clusters is not selected a priori, but rather estimated along with other model parameters (Fig. 1B). We identified three major mutation clusters: one with median weight 35% (i.e. any mutation has approximately 35% probability of belonging to this cluster) and two slightly smaller clusters with median 30%. In Figure 1C, we illustrate the evolution of each cluster in time. Sample (a) was collected before commencing treatment with chlorambucil; sample (b) before treatment with fludarabine, cyclophosphamide and rituximab (FCR); sample (c) immediately after six cycles of FCR; sample (d) before treatment with ofatumumab; and sample (e) after treatment with ofatumumab, spanning in total a period of 35 months. Initial treatment with chlorambucil did not alter significantly the prevalence of the three mutation clusters, with median CCF > 75% for clusters 1 and 3 and median CCF < 10% for cluster 2. The second treatment regime (FCR) induced a dramatic reduction in the prevalence of cluster 3, but only a minor reduction of cluster 1. Concomitantly, the prevalence of cluster 2 increased substantially. By the end of the 35-months period, cluster 1 had recovered and, along with cluster 2, it reached CCF values higher than 95%, while cluster 3 collapsed. Our algorithm soft-clusters mutations, i.e. for each mutation, it calculates the probability of membership to each cluster. From these, a hard clustering can be obtained by assigning each mutation to the cluster with the highest median membership probability. Figure 1D illustrates the hard cluster assignment for each mutation in the CLL003 dataset. It is interesting to observe that, by considering multiple time-separated samples, our method manages to deconvolve mutation clusters with similar VAF values, which would otherwise be hard to distinguish [e.g. observe the mixing of clusters 1 and 3 at time points (a) and (b) or clusters 1 and 2 at time points (d) and (e)]. Finally, we can visually confirm the goodness of fit of the model to the data by overlaying the posterior predictive distribution (red lines in Fig. 1E) on the histograms of observed VAF values for each sample.
Fig. 1.
Application of model GP0-Mat32 on data from patient CLL003 (Schuh et al., 2012). (A) Parameter estimation was achieved via maximization of the evidence lower bound. Convergence was attained in less than 3K iterations. (B) The number of clusters in the data was automatically estimated through the use of a Dirichlet Process prior. In this example, three major clusters were identified. (C) The temporal profile of the three major clusters during disease treatment and progression. The median and 95% credible intervals are shown. Sample collection took place over the course of 35 months. (D) Observed VAF values for each somatic mutation and their cluster assignment. (E) The fitted model (red lines) against the data in each sample
Fig. 2.
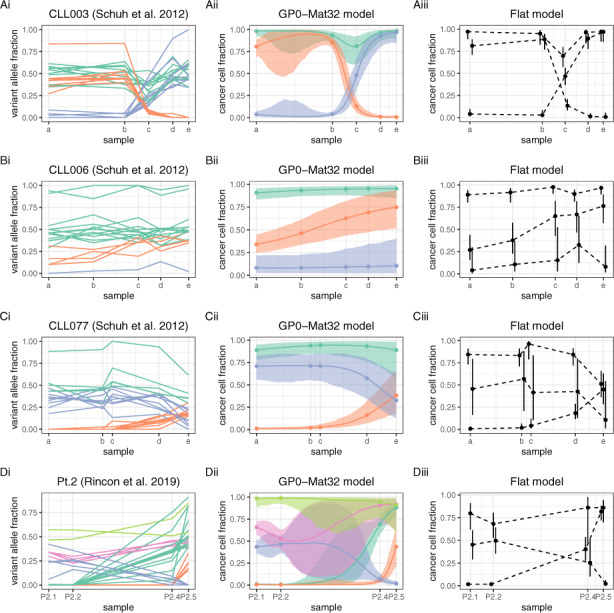
Overview of CLL data and fitted models Flat and GP0-Mat32. Unlike GP0-Mat32, the Flat model estimates the CCF of each cluster only at the points of sample collection (dashed lines). Although both models identified the same number of clusters in datasets CLL003 to CLL077, these were not concordant (see main text for details)
3.2 Benchmarks on CLL data with four or five samples
Next, we applied the remaining models on the data from patient CLL003, as well as all models on data from patients CL006 and CLL077 reported in Schuh et al. (2012) (models Flat and GP0-Mat32 are illustrated in Fig. 2; the performance of all models, except GP2, is summarized in Fig. 3). WGS and bioinformatics analysis were conducted as for patient CLL003 (see original paper for details). For patients CLL006 and CLL077, samples were collected over a period of 50 and 57 months, respectively. In addition, we examined WES data from Patient 2 reported in the study by González-Rincón et al. (2019), where collected samples spanned 79 months in total (for details of sequencing and bioinformatics analysis, see original paper).
Fig. 3.
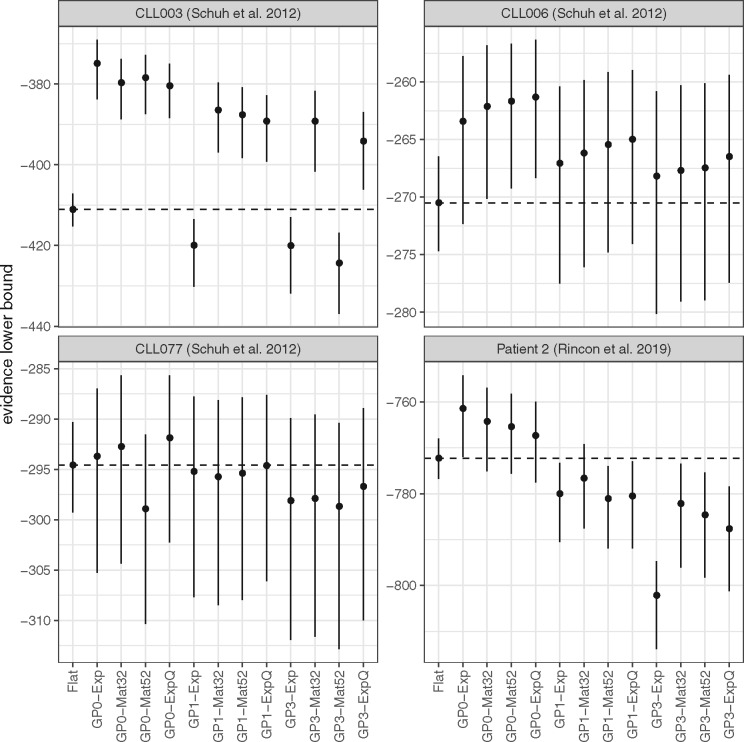
Comparison of all models using the data in the previous figure and the evidence lower bound for assessing performance. GP0 models perform at least as well as the Flat model in all cases. Models GP2 had the worst performance and they were omitted from the figure
There were 18, 21 and 32 somatic mutations in patients CLL006, CLL077 and Patient 2, respectively (as well as 28 somatic mutations in patient CLL003, as previously mentioned; Fig. 2Ai–Di). A preliminary comparison indicates that, for patients CLL003 to CLL077, model GP0-Mat32 (Figs. 2Aii–Cii) identified the same number of mutation clusters as the simpler Flat model (Fig. 2Aiii–Ciii), i.e. three clusters with similar temporal dynamics. To assess the clustering concordance between the two models (i.e. whether they assign the same mutations to the same clusters), we calculated the values of ARI, which were equal to 0.54, 0.79 and 0.58, respectively. This indicates that the two models are not perfectly concordant in any of these three datasets (despite both identifying the same number of clusters) presumably due to the partial overlap between different mutation groups, as illustrated in Figure 2Ai–Ci. One striking difference between the Flat and GP-based models is that while the former estimates the latent state of the tumour only at the time points of sample collection (this is indicated by the dashed connecting lines in Fig. 2Aiii–Ciii), the latter provides an estimate of the complete history of this latent state, i.e. both at and between these fixed time points. This is a major difference in favour of the use of GP-based models. In the case of Patient 2, the Flat and GP0-Mat32 models identify three and five clusters, respectively (ARI = 0.63; Fig. 2Di–iii). For comparison, in the original paper, the authors identified seven clusters using PyClone (Roth et al., 2014).
To further assess the relative performance of different models (and without knowledge of the true clonal state of each tumour), we used the ELBO as performance metric (see Section 2). The ELBO provides a lower bound on the marginal likelihood of the data (i.e. the evidence) and, at the same time, it includes an internal mechanism that prevents over-fitting. Thus, it is often used in practise for model comparison and selection, with higher ELBO values indicating a better model. As illustrated in Figure 3A, all GP0 models, all but one GP1 models and all but two GP3 models outperform the Flat model on the CLL003 data. The GP2 models, which have the largest number of parameters, were by far the worst performers on these datasets and they are omitted from the figure. There is a clear trend of decreasing performance with increasing number of parameters among the GP-based models, which is not surprising given that the lower the number of time points, the lower the capacity of the data to support overly complex models (as, for example, in the case of GP2 models). In the case of CLL006 (Fig. 3B), the same trend is observed, although the difference of the GP-based models from the Flat model is less pronounced. In the case of CLL077 (Fig. 3C), models GP0-Mat32 and GP0-ExpQ perform better than the Flat model (although this difference is not particularly pronounced because of the high variance of the ELBO), but the remaining GP-based models perform either clearly worse or comparably to the Flat model. In the case of Patient 2 (Fig. 3D), the GP0 models are again the best performers, unlike GP1and GP3 models, which are clearly worse than the Flat model. In summary, there is always at least one member of the relatively parsimonious (in terms of the number of model parameters) GP0 family of models that performs better than the Flat model in the above benchmarks.
3.3 Benchmarks on CLL and melanoma data with 10 or 13 samples
Next, we tested our models on longitudinal genomic data involving a higher number of time points. The first dataset comes from Patient 1 in González-Rincón et al. (2019). A total of 13 peripheral blood mononuclear cell samples (P1.1–P1.13) were collected over the course of 6.5 years and underwent TGS. Samples were collected before or after treatment commenced. In particular, sample P1.1 was collected before the patient received a stem cell transplant and the same holds for sample P1.8. Bioinformatics analysis identified 46 somatic mutations over all 13 samples (Fig. 4A; see original paper for details). Model GP0-Mat32 identified nine mutation clusters (Fig. 4B), while the Flat model identified five (Fig. 4C). For comparison, in the original paper, the authors estimated four clusters using PyClone (Roth et al., 2014). Overall, models GP0, GP1 and GP2 perform better than the Flat model, unless an exponentiated quadratic kernel (ExpQ) is used (Fig. 4D). We speculate that this is because ExpQ encodes perfectly smooth dynamics, which presumably cannot model sufficiently well the non-smooth bottleneck points P1.2 and P1.8 which precede stem cell transplantation. Model GP3-Exp is also performing better than the Flat model.
The second multi-sample dataset comes from the liquid biopsy of a patient with metastatic melanoma (Cutts et al., 2017). Peripheral blood samples were collected at 10 different time points during pre-treatment, post-treatment and relapse over the course of 13 months. Targeted sequencing was conducted on extracted cell-free DNA followed by bioinformatics analysis, which revealed 63 somatic mutations. Visual inspection of the data indicates the absence of a definitive cluster structure (Fig. 5A) and, for this reason, this is an interesting dataset to use for model evaluation. Both the Flat and GP0-Exp models identified five mutation clusters with little concordance between them (ARI = 0.27) due to the extended overlap between different mutations bundles (Fig. 5B, C). The median performance of model GP0-Exp is nominally higher than the Flat model, although it is doubtful whether the difference is substantial due to the high variance of the ELBO (Fig. 5D). The remaining GP-based models perform worse than either Flat or GP0-Exp.
Fig. 5.
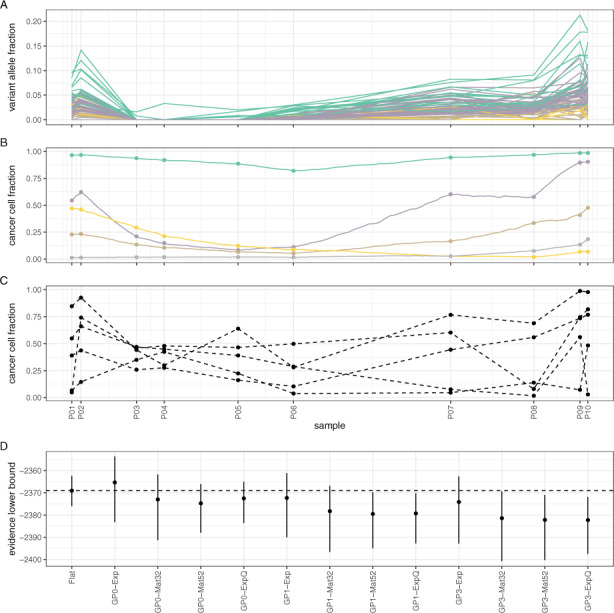
Assessing model performance using data from a liquid biopsy on a subject with melanoma (Cutts et al., 2017). (A) Observed VAF values for each somatic mutation over 13 months of treatment and their cluster assignments (colours are the same as in B). B) Mutation clusters identified by model GP0-Exp (due to extensive overlap, credible intervals are omitted for clarity). (C) Mutation clusters identified by the Flat model. (D) Comparative performance of various models. Model GP0-Exp performs comparably to Flat
3.4 Computational experiments on simulated data
Overall, models GP0 (particularly GP0-Exp and GP0-Mat32) perform at least as well as the Flat model in all the above datasets. More complex models (i.e. models with a larger number of parameters), such as GP1, GP2 and GP3, require a higher number of longitudinally collected samples for improved performance (Fig. 4). However, this is not a sufficient condition, since data of low complexity (i.e. with trivial or non-obvious cluster structure and dynamics) can negatively affect the performance of the GP-based models (Fig. 5).
We wanted to test whether these trends (i.e. the reduction in the performance of the GP-based models in relation to the Flat model as data size and complexity decreases) can be replicated using synthetic genomic data. For a given number of samples M, mutations N and mutation clusters K, data were simulated using actual experimental data as template (see Supplementary Methods for details). In total, we generated 729 datasets, each of which was processed using the Flat and GP0 models (which were top performers on the actual data) and their performance was assessed against the true cluster structure of the dataset.
We may observe that when few samples are available (M = 3), the baseline model (Flat) performs comparably to GP0 at all values of N and K (Fig. 6). For large (M = 12) datasets, the Flat model falls behind the other models, when the number of clusters in the data is relatively high (K = 4 or 8). At medium sample numbers (M = 6), the same effect is observed at small mutation numbers (N = 25). These results indicate that in the presence of non-trivial cluster dynamics, the baseline model is comparable to GP0 models, but only when the number of samples or data complexity (here, the number of clusters) is low. In Supplementary Results, we provide further benchmarks against PyClone (Roth et al., 2014) and Canopy (Jiang et al., 2016), as well as additional performance metrics (Supplementary Figs S4–S6).
Fig. 6.
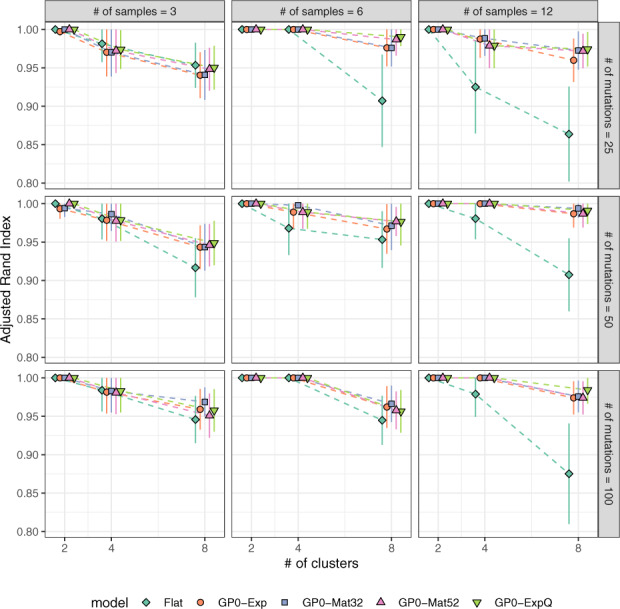
Benchmarks on synthetic data. When the number of samples is small (M = 3) or data complexity (i.e. the number of clusters) is low (K = 2), the Flat model performs comparably to the GP0 models. In all other cases, it is outperformed by them. Parameters used in data simulation were informed by the experimental data (see main text for details)
4 Discussion
Tumour heterogeneity in the form of distinct cancer cell populations or clones is the outcome of a process of continuous adaptation of the component cells to their local micro-environment. The outcome of any therapeutic intervention depends on this latent cellular diversity and, for this reason, statistical methodologies that help deconvolve the clonal structure of tumours are valuable tools at the disposal of clinicians and bioinformaticians.
Building on previous works (Nik-Zainal et al., 2012; Roth et al., 2014), we propose a statistical methodology for clonal deconvolution based on longitudinal data, which explicitly considers the temporal spacing of sample collection. Our approach combines two Bayesian non-parametric statistical frameworks, namely Dirichlet Process Mixture Models (for clustering in the absence of prior knowledge on the number of clusters supported by the data) and Gaussian Process Latent Variable Models (for modelling the time-dependence of clone prevalence without any explicit assumptions on the form of this dependence). The models we present in this article are sufficiently flexible to capture many common scenarios without additional assumptions or constraints, including monotonic increase and/or decrease of CCFs, as well as stability of CCFs near (or at) 0 or 1. More elaborate scenarios (e.g. the introduction of change-points in the function domain) are possible through appropriate design of the kernel function, possibly at the cost of increased model complexity.
Using a combination of experimental data from patients with CLL or melanoma, as well as synthetic data simulated using experimental data as template, we demonstrate that there are advantages in this approach, when compared to several baseline models (Fig. 6 and Supplementary Figs S4–S6). These benefits are particularly evident when longitudinal data of sufficient volume and complexity are available. When this is not the case, our methodology performs comparably to baseline models, but it also manages to reconstruct the time dependence of mutation clusters continuously in time (i.e. not only at the points of sample collection, which is what baseline models do, but also between them) from a small number of sequentially collected samples.
CLL is an ideal experimental model for the study of cancer evolution, because it develops over many years and because the collection of a long sequence of blood samples from the same patient for genomic analysis is easy, at least when compared to solid tumours. Thus, we expect that our methodology will find applications in the study of CLL and other liquid cancers. It can also be used as a general purpose clustering tool for identifying populations of mutations based on sequencing of circulating tumour DNA obtained through a liquid biopsy.
As with other approaches for clustering mutations based on bulk sequencing data, a phylogeny is not derived directly, but it can be calculated retrospectively using the output of our method as input to bespoke software (Dang et al., 2017; Niknafs et al., 2015; Qiao et al., 2014). Furthermore, single-cell sequencing promises to alleviate the confounding of clones inherent in methods based on bulk sequencing by permitting direct observation of the genotypes of the cells that compose each clone. However, it is in turn plagued by its own technical limitations, namely high levels of noise, error rates and missing values (Borgsmueller et al., 2020; Chen et al., 2019; El-Kebir, 2018; Jahn et al., 2016; Malikic et al., 2019; Ramazzotti et al., 2020; Ross and Markowetz, 2016; Roth et al., 2016; Zafar et al., 2017, 2019).
Finally, an important assumption in our approach is the absence of sub-clonal copy number variation [see assumptions (a) and (b) in Section 5]. Although commonly adopted by statistical methods of sub-clonal reconstruction based on single nucleotide variants (SNV), we recognize that these assumptions may not always be exact. It is possible to relax these assumptions, however, this would lead to an intractable estimation problem in the resulting model. A possible mitigation strategy is to complement a SNV-based approach as the one we present in this article (which, as we show, does incorporate a correction for copy number variation, CNV) by subsequently applying a CNV-based approach as well. Several such methods are available aiming to identify the copy number state of each sub-clone in a tumour by typically using the measured B-allele frequency as input to downstream estimation procedures (Carter et al., 2012; Fischer et al., 2014; Ha et al., 2014; Nik-Zainal et al., 2012).
5 Conclusion
In conclusion, we propose that considering information on the temporal spacing of longitudinal tumour samples can improve clonal deconvolution and we show how this can be achieved in the context of non-parametric Bayesian statistics.
Supplementary Material
Acknowledgements
The authors thank two anonymous reviewers for their constructive criticism on an earlier version of this manuscript.
Funding
This research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre Programme and a Wellcome Trust Core Award [203141/Z/16/Z]. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Wellcome Trust.
Conflict of Interest: none declared.
References
- Abécassis J. et al. (2019) CloneSig: joint inference of intra-tumor heterogeneity and signature deconvolution in tumor bulk sequencing data. bioRxiv, doi: 10.1101/825778 [DOI]
- Álvarez M.A. et al. (2012) Kernels for Vector-Valued functions: a review. Found. Trends Mach. Learn., 4, 195–266. [Google Scholar]
- Beerenwinkel N. et al. (2015) Cancer evolution: mathematical models and computational inference. Syst. Biol., 64, e1–25–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blei D.M. et al. (2017) Variational inference: a review for statisticians. J. Am. Stat. Assoc., 112, 859–877. [Google Scholar]
- Bolli N. et al. (2014) Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun., 5, 2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgsmüller,N. et al. (2020) BnpC: Bayesian non-parametric clustering of single-cell mutation profiles. Bioinformatics, 10.1093/bioinformatics/btaa599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter S.L. et al. (2012) Absolute quantification of somatic DNA alterations in human cancer. Nat. Biotechnol., 30, 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen,Z. et al. (2020) RobustClone: a robust PCA method for tumor clone and evolution inference from single-cell sequencing data. Bioinformatics, 36, 3299–3306. 10.1093/bioinformatics/btaa172 [DOI] [PubMed] [Google Scholar]
- Cutts A. et al. (2017) Characterisation of the changing genomic landscape of metastatic melanoma using cell free DNA. NPJ Genomic Med., 2, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang H.X. et al. (2017) ClonEvol: clonal ordering and visualization in cancer sequencing. Ann. Oncol., 28, 3076–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentro S.C. et al. (2017) Principles of reconstructing the subclonal architecture of cancers. Cold Spring Harb. Perspect. Med., 7, a026625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshwar A.G. et al. (2015) PhyloWGS: reconstructing subclonal composition and evolution from whole-genome sequencing of tumors. Genome Biol., 16, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez N. et al. (2017) Clonality inference from single tumor samples using low-coverage sequence data. J. Comput. Biol., 24, 515–523. [DOI] [PubMed] [Google Scholar]
- El-Kebir M. (2018) SPhyR: tumor phylogeny estimation from single-cell sequencing data under loss and error. Bioinformatics, 34, i671–i679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kebir M. et al. (2015) Reconstruction of clonal trees and tumor composition from multi-sample sequencing data. Bioinformatics, 31, i62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A. et al. (2014) High-definition reconstruction of clonal composition in cancer. Cell Rep., 7, 1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A. et al. (2013) Bayesian Data Analysis (Chapman & Hall/CRC Texts in Statistical Science), 3rd edn. Chapman and Hall/CRC. London, England.
- González-Rincón J. et al. (2019) Clonal dynamics monitoring during clinical evolution in chronic lymphocytic leukaemia. Sci. Rep., 9, 975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha G. et al. (2014) TITAN: inference of copy number architectures in clonal cell populations from tumor whole-genome sequence data. Genome Res., 24, 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishwaran H., James L.F. (2001) Gibbs sampling methods for stick-breaking priors. J. Am. Stat. Assoc., 96, 161–173. [Google Scholar]
- Ismail W.M. et al. (2019) Algorithmic approaches to clonal reconstruction in heterogeneous cell populations. Quant. Biol., 7, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K. et al. (2016) Tree inference for single-cell data. Genome Biol., 17, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y. et al. (2016) Assessing intratumor heterogeneity and tracking longitudinal and spatial clonal evolutionary history by next-generation sequencing. Proc. Natl. Acad. Sci. USA, 113, E5528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucukelbir A. et al. (2017) Automatic differentiation variational inference. J. Mach. Learn. Res., 18, 1–45. [Google Scholar]
- Malikic S. et al. (2019) PhISCS: a combinatorial approach for subperfect tumor phylogeny reconstruction via integrative use of single-cell and bulk sequencing data. Genome Res., 29, 1860–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marass F. et al. (2016) A phylogenetic latent feature model for clonal deconvolution. Ann. Appl. Stat., 10, 2377–2404. [Google Scholar]
- Merlo L.M.F. et al. (2006) Cancer as an evolutionary and ecological process. Nat. Rev. Cancer, 6, 924–935. [DOI] [PubMed] [Google Scholar]
- Miller C.A. et al. (2014) SciClone: inferring clonal architecture and tracking the spatial and temporal patterns of tumor evolution. PLoS Comput. Biol., 10, e1003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M.A. et al. (2019) CALDER: inferring phylogenetic trees from longitudinal tumor samples. Cell Syst., 8, 514–522.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nik-Zainal S. et al. ; Breast Cancer Working Group of the International Cancer Genome Consortium. (2012) The life history of 21 breast cancers. Cell, 149, 994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niknafs N. et al. (2015) SubClonal hierarchy inference from somatic mutations: automatic reconstruction of cancer evolutionary trees from multi-region next generation sequencing. PLoS Comput. Biol., 11, e1004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P.C. (1976) The clonal evolution of tumor cell populations. Science, 194, 23–28. [DOI] [PubMed] [Google Scholar]
- Pedregosa F. et al. (2011) Scikit-learn: machine learning in python. J. Mach. Learn. Res., 12, 2825–2830. [Google Scholar]
- Qiao Y. et al. (2014) SubcloneSeeker: a computational framework for reconstructing tumor clone structure for cancer variant interpretation and prioritization. Genome Biol., 15, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramazzotti D. et al. (2020) Longitudinal cancer evolution from single cells. bioRxiv, doi: 10.1101/2020.01.14.906453 [DOI]
- Rasmussen C.E., Williams C.K.I. (2006) Gaussian Processes for Machine Learning. MIT Press. London, England. [Google Scholar]
- Roberts S. et al. (2013) Gaussian processes for time-series modelling. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci., 371, 20110550. [DOI] [PubMed] [Google Scholar]
- Ross E.M., Markowetz F. (2016) OncoNEM: inferring tumor evolution from single-cell sequencing data. Genome Biol., 17, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A. et al. (2014) PyClone: statistical inference of clonal population structure in cancer. Nat. Methods, 11, 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A. et al. (2016) Clonal genotype and population structure inference from single-cell tumor sequencing. Nat. Methods, 13, 573–576. [DOI] [PubMed] [Google Scholar]
- Rubanova Y. et al. ; PCAWG Evolution and Heterogeneity Working Group, and PCAWG Network. (2020) Reconstructing evolutionary trajectories of mutation signature activities in cancer using TrackSig. Nat.Commun, 11, 731. [DOI] [PMC free article] [PubMed]
- Salcedo A. et al. ; DREAM SMC-Het Participants. (2020) A community effort to create standards for evaluating tumor subclonal reconstruction. Nat. Biotechnol., 38, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatier J. et al. (2016) Probabilistic programming in python using PyMC3. PeerJ Comput. Sci., 2, e55. [Google Scholar]
- Schuh A. et al. (2012) Monitoring chronic lymphocytic leukemia progression by whole genome sequencing reveals heterogeneous clonal evolution patterns. Blood, 120, 4191–4196. [DOI] [PubMed] [Google Scholar]
- Sengupta S. et al. (2014) BayClone: Bayesian non-parametric inference of tumour subclones using NGS data. In: Biocomputing 2015. WORLD SCIENTIFIC, Singapore, pp. 467–478. [PubMed]
- Stan Development Team (2020). Stan user’s guide: Multivariate priors for hierarchical models. (17 January 2020, date last accessed).
- Van Loo P. et al. (2010) Allele-specific copy number analysis of tumors. Proc. Natl. Acad. Sci. USA, 107, 16910–16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavoulis D.V. (2020) Exploring Bayesian approaches to eQTL mapping through probabilistic programming. Methods Mol. Biol., 2082, 123–146. [DOI] [PubMed] [Google Scholar]
- Vavoulis D.V. et al. (2015) DGEclust: differential expression analysis of clustered count data. Genome Biol., 16, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavoulis D.V. et al. (2017) Hierarchical probabilistic models for multiple gene/variant associations based on next-generation sequencing data. Bioinformatics, 33, 3058–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K. et al. (2015) BitPhylogeny: a probabilistic framework for reconstructing intra-tumor phylogenies. Genome Biol., 16, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K. et al. (2018) Ccube: a fast and robust method for estimating cancer cell fractions. bioRxiv, doi: 10.1101/484402 [DOI]
- Zafar H. et al. (2017) SiFit: inferring tumor trees from single-cell sequencing data under finite-sites models. Genome Biol., 18, 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar H. et al. (2019) SiCloneFit: Bayesian inference of population structure, genotype, and phylogeny of tumor clones from single-cell genome sequencing data. Genome Res., 29, 1847–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare H. et al. (2014) Inferring clonal composition from multiple sections of a breast cancer. PLoS Comput. Biol., 10, e1003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. et al. (2019) Advances in variational inference. IEEE Trans. Pattern Anal. Mach. Intell., 41, 2008–2026. [DOI] [PubMed] [Google Scholar]
- Zucker M.R. et al. (2019) Inferring clonal heterogeneity in cancer using SNP arrays and whole genome sequencing. Bioinformatics, 35, 2924–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.