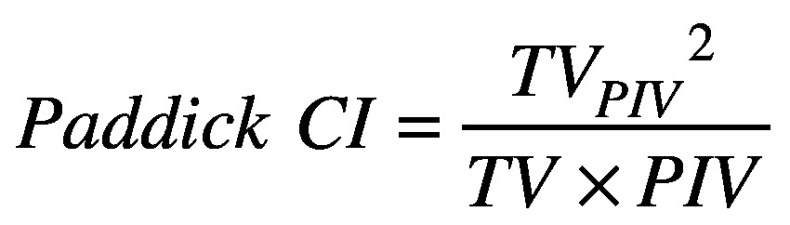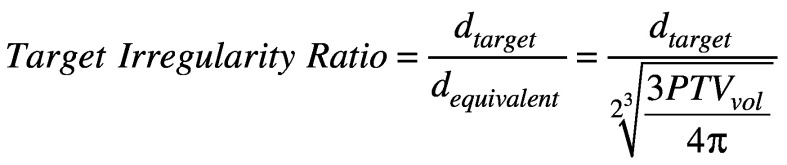Abstract
Our objective is to investigate dosimetric differences between clinically deliverable Gamma Knife® (GK) Icon™ and linac-based FSRT plans on the basis of normal brain dose sparing for large (>14 cm3) recurrent glioblastomas (GBM). Sixteen patients with large, recurrent GBM were treated using re-irradiation via linac-based FSRT, 35 Gy in 10 fractions. For each patient, a new GK FSRT plan was created in Leksell GammaPlan® V11 (LGP). To maintain clinical deliverability, the LGP optimization included a planning goal of treatment time <20 minutes per fraction. Dosimetric comparison of coverage and normal brain dose between the linac and GK treatment plans was performed in MIM. The GK FSRT plans had significantly (p < 0.05) lower mean normal brain dose values (-8.85%), mean values of normal brain V20 (-32.4%) and V12 (-25.9%), and a lower mean V4 (-10.0%). GK FSRT plans have the potential to reduce the risk of radiation-related toxicities.
Keywords: Gamma Knife Icon, fractionated stereotactic radiotherapy, recurrence, glioblastoma
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common and aggressive primary malignant brain tumor in adults.1 Standard primary treatment of GBM includes surgical resection followed by adjuvant conventionally fractionated radiotherapy (~60 Gy/30 fractions) and chemotherapy.2–8 Despite this aggressive treatment regimen, prognosis for GBM remains generally poor as the vast majority of patients experience recurrences.2–8 Although there is not a standard salvage treatment for recurrent GBM, re-irradiation in the form of linear accelerator (linac)-based fractionated stereotactic radiotherapy (FSRT) is a commonly used treatment option for patients with large recurrences (>14 cm3).2,3,9 Re-irradiation of recurrent GBM is a palliative treatment aimed at utilizing the most advanced radiotherapy techniques in order to prolong patient survival while preserving an acceptable quality of life.1
Linac-based FSRT is a multi-fraction stereotactic radiation therapy regimen that is the primary treatment modality for large (>14 cm3) intracranial tumors.2,3,9 It typically utilizes non-coplanar arcs to produce highly conformal plans similar to stereotactic radiosurgery (SRS), while reducing the risk of radiation-related complications by means of fractionation.4 Re-irradiation of recurrent GBM is limited by toxicity due to the majority (80%) of all recurrences being located within 2 cm of the previously irradiated high dose tumor volume; as a result the primary complication of this treatment is radionecrosis.2–9 Review publications that pool together data from past re-irradiation of recurrent GBM survival studies report a radionecrosis rate of 0-60% for linac-based FSRT treatments.1,4–7,10–12 The incidence of radionecrosis is dependent on the total dose of the treatment and the consequential volume of normal brain being re-irradiated.7 The standard method for reporting brain dose for SRS is the volume of the brain, including tumor volume, receiving 12 Gy (V12).13,14 Low V12 is associated with a reduction in normal brain dose, and higher V12 is associated with an increased risk of developing symptomatic radionecrosis.13–17 Currently, there is not a consensus on standard normal brain dose constraints for FSRT.18
Leksell Gamma Knife® (GK) SRS utilizes approximately 200 focused Co-60 beams with millimeter-sized collimators in order to deliver a high and conformal dose to intracranial targets while sparing surrounding normal tissue.19,20 Previous generations of the GK system were typically limited to frame-based single fraction SRS of intracranial tumors with a maximum diameter of ~3 cm (volume ≤14 cm3).15–17,19,21 This tumor size constraint was developed in order to reduce the risk of irreversible central nervous system (CNS) toxicity, primarily radionecrosis, due to the interplay between dose, CNS toxicity, and local control failure with increasing tumor size.15–17,21,22 In order to overcome these tumor size related difficulties, the capability of GK FSRT treatments was introduced with the Gamma Knife PerfexionTM via the ExtendTM bite-block system.19 Dividing the large SRS dose into consecutive daily fractions allows for the treatment of larger intracranial tumors while reducing the risk of treatment limiting complications.23–25 The Gamma Knife IconTM, the successor to the GK PerfexionTM, introduced image-guided repeatable patient setup with non-invasive immobilization via a thermoplastic mask, effectively establishing the capability of efficient and accurate frameless GK FSRT treatments.19 Its main advancements over the GK PerfexionTM include a stereotactic calibrated on-board cone-beam CT (CBCT), patient masking immobilization system, high-definition (HD) infrared patient motion monitoring system, and a dose recalculation workflow based on live 3D patient setup.19 These advancements have led to the emergence of GK FSRT as a potential alternative to standard linac-based FSRT for the treatment of large intracranial tumors.
Recent dosimetric comparisons of GK and linac-based FSRT plans examined patient populations consisting of large metastatic brain tumors.26–28 In these studies, GK plans were able to improve the sparing of normal brain tissue.26–28 However, these studies were limited to spherically shaped metastatic brain tumors and did not address the issue of clinical deliverability among the GK treatment plans, with treatment times up to 122 minutes.26–28 The purpose of our study was to make a single-institution, retrospective dosimetric comparison of GK IconTM and linac-based FSRT treatment plans for the re-irradiation of large (>14 cm3) recurrent GBM on the basis of normal brain dose sparing. Glioblastomas are inherently irregular in shape compared to metastatic brain tumors, which can result in more complex plans with longer delivery times. All GK FSRT plans produced for this study will maintain clinical deliverability with treatment time ideally ≤20 minutes, with a maximum of 40 minutes, due to patient tolerability of a facial mask and our institution’s clinical experience.
MATERIALS AND METHODS
Patient Selection
Forty-two patients receiving re-irradiation had intracranial tumors with target volumes >14 cm3 at our institution from 10/2015-12/2019. Of those, thirty-five patients were treated for recurrent GBM with re-irradiation in the form of linac-based FSRT and were selected for preliminary analysis. We elected to limit the study to targets that could be planned in a single dose matrix in the Leksell GammaPlan® system. The target matrix is limited to ≤7.5 cm in any dimension, which eliminated 43% of the large (>14 cm3) recurrent GBM patients from our initial patient population. Of the twenty eligible patients remaining, sixteen patients with recurrent GBM target volumes >14 cm3 were randomly selected for this study. These linac-based FSRT patients were treated with a dose of 35 Gy in 10 fractions. Patient and plan characteristics for the population are listed in Table 1.
TABLE 1.
Target and plan information for all patients. TIR= Target Irregularity Ratio.
| Patient | Location | Margin (mm) |
PTV (cm3) |
TIR | Beam Configuration | Treatment Time (min) | ||
|---|---|---|---|---|---|---|---|---|
| # Fields (Linac) | # Shots (GK) | Linac | GK | |||||
| 1 | L Frontal | 1 | 64.0 | 1.24 | 3 Arcs | 25 | 4 | 23.7 |
| 2 | L Parietal | 2 | 22.5 | 1.23 | 3 Arcs | 14 | 4 | 15.3 |
| 3 | R Occipital | 2 | 41.3 | 1.09 | 3 Arcs | 19 | 5 | 19.6 |
| 4 | R Frontal | 0 | 16.1 | 1.37 | 4 Arcs + 5 IMRT | 10 | 8 | 13.1 |
| 5 | R Frontal | 2 | 20.1 | 2.35 | 4 Arcs | 15 | 4 | 21.7 |
| 6 | R Insula | 1 | 29.0 | 1.53 | 3 Arcs | 19 | 5 | 27.8 |
| 7 | R Frontal | 0 | 48.3 | 1.27 | 4 Arcs | 29 | 5 | 27.6 |
| 8 | L Temporal | 1 | 74.8 | 1.12 | 4 Arcs | 34 | 6 | 31.3 |
| 9 | L Temporal | 0 | 27.6 | 1.39 | 3 Arcs + 5 IMRT | 13 | 7 | 17.8 |
| 10 | R Frontal | 2 | 34.2 | 1.14 | 4 Arcs + 5 IMRT | 19 | 7 | 17.2 |
| 11 | L Parietal | 2 | 36.2 | 1.11 | 5 Arcs | 17 | 5 | 19.2 |
| 12 | R Parietal | 1 | 47.2 | 1.24 | 5 Arcs + 3 IMRT | 20 | 5 | 23.0 |
| 13 | L Parietal | 1 | 16.8 | 1.06 | 5 Arcs | 12 | 6 | 12.6 |
| 14 | L Temporal | 0 | 36.8 | 1.45 | 8 IMRT | 24 | 7 | 22.6 |
| 15 | R Temporal | 2 | 33.6 | 1.43 | 4 Arcs + 4 IMRT | 23 | 6 | 20.7 |
| 16 | R Temporal | 2 | 15.0 | 1.13 | 3 Arcs + 6 IMRT | 13 | 6 | 17.1 |
Treatment Planning
The linac-based FSRT plans were originally generated using inverse planning in Eclipse V11 (Varian, Anisotropic Analytical Algorithm(AAA)) or iPlan V4.5 (Brainlab, Pencil Beam Algorithm) treatment planning systems for Varian TrueBeam STx delivery, equipped with ExacTrac® and a 6 degree-of-freedom (DOF) couch. At our institution, linac-based patients have a variety of treatment options based on physician preference. These options include VMAT (6 patients), IMRT (1 patient), HybridArcs (dynamic arcs with static IMRT fields at beginning or end of arcs, 6 patients), and StereoDynamic Arcs (3 patients), which are all considered to produce dosimetrically equivalent linac-based plans.29 In our practice, re-irradiation of recurrent GBM linac-based plans use 0-2 mm planning target volume (PTV) margins and the prescription normalization is determined based on the clinical scenario. The following organ-at-risk (OAR) structures were contoured for each patient: brain, normal brain (brain excluding PTV), optic nerves, optic chiasm, brainstem, eyes, and lens. Linac-based FSRT treatment planning goals were PTV V35 ≥95%, but plans were typically normalized to have PTV V35 = 99.5% if possible without exceeding maximum doses (0.03 cm3) to the brainstem, optic nerves, and optic chiasm <15 Gy. PTV maximum dose objectives varied depending on the clinical scenario, with greater heterogeneity allowed for targets near OARs. In this study, all sixteen (6 Eclipse, 10 iPlan) linac-based FSRT patient treatments were calculated with heterogeneity corrections, and delivered with 6 MV photons. Treatment times were obtained from treatment records in ARIA V11 (Varian) and represent a summation of beam-on time and table movement time throughout treatment. No pre- or mid-treatment imaging time was included for this study.
For each patient, the planning CT dataset and contours from Eclipse or iPlan were transferred to the GK treatment planning system (TPS), Leksell GammaPlan® V11 (LGP; Elekta), to generate the GK FSRT plans for comparison. The GK FSRT plans were generated per our clinical practice, using a homogeneous dose calculation, with identical treatment volumes and prescription dose to the linac-based plans. For each patient, the GK FSRT plan was generated with the identical PTV as the corresponding patient specific linac-based plan in order to avoid normal brain dose effects between modalities due to differing PTV margins.30 All GK FSRT plans were prescribed a dose of 35 Gy in 10 fractions to maintain consistency with the linac-based FSRT fractionation schedule, but the dose was prescribed to the 50% isodose line for the GK plans to preserve routine clinical practice. On the GK IconTM, the dose distribution of each shot is determined by 192 Co-60 non-coplanar beams arranged on a hemispherical surface divided into 8 sectors, each with the ability to be independently collimated to 4, 8, or 16 mm shots, or blocked.26,27 In this study, the inverse planning feature of GammaPlan® V11 was utilized to achieve a conformal dose by manipulating the weighting and collimator sizes of multiple shots to cover these large target volumes.
The GK FSRT plans were optimized in stages while attempting to meet all GK planning goals. GK FSRT planning goals include the following values as stated in GammPlan®: target coverage PTV V35 ≥95%, conformity index ≥0.8, gradient index <3, treatment time ≤20 minutes (<40 minutes maximum), and OAR constraints (brainstem, optic nerves, and optic chiasm maximum doses (0.03 cm3)) <15 Gy. The selectivity priority in the optimization was driven as high as possible (e.g. Selectivity ~0.09/Coverage ~0.91) without decreasing target coverage below 95%. Next, the gradient priority in the optimization was increased (e.g. Gradient Index increased to ~0.3) without decreasing target coverage below 95% or decreasing selectivity by more than 0.02. Finally, the beam-on time priority in the optimization was increased (e.g. Beam-on increased to ~0.1) until treatment time was ≤20 minutes without degrading the prior plan parameter optimization. If the hard OAR constraints listed above were not able to be met with target coverage ≥95%, forward planning was used to decrease dose to OARs by manually adjusting shot placement, weight, and collimator sizes. This resulted in decreased coverage for four patients with targets proximal to OARs. All GK FSRT plans were reviewed and deemed clinically acceptable.
GK treatment times were allowed to exceed 20 minutes, with an absolute maximum treatment time of 40 minutes, only in cases where the 20 minute treatment time constraint caused all other GK treatment planning goals to degrade. The maximum time limit of 40 minutes was utilized to ensure deliverability of the GK FSRT treatments per our institution’s clinical experience. GK treatment times, as obtained from GammaPlan®, were scaled from the initial planned value to reflect a nominal 16 mm collimator dose rate of 2.5 Gy/min to generalize the results accounting for decay over the lifespan of GK sources. These treatment times represent beam-on time only, as mid-treatment table movement time is negligible for GK treatments.
Plan Comparison
The FSRT plans from both treatment modalities were calculated with a 1 mm dose grid and imported into MIM Software V6.7 using DICOM-RT, with identical target and OAR volumes verified between FSRT planning software (Eclipse, iPlan, or LGP) and MIM. This import into a third-party software allowed for an unbiased dosimetric comparison for final review and analysis.26,27 In MIM, the GK IconTM and linac-based FSRT plans were compared based on the following parameters: target coverage, Paddick conformity index, gradient index, homogeneity index, the volume of normal brain (excluding PTV) receiving 4 Gy, 12 Gy, and 20 Gy (V4, V12, V20), mean normal brain dose, and treatment time.
Target coverage (TC) is the ratio of the target volume covered by the 35 Gy prescription isodose volume (TVPIV) and the target volume (TV).26
(1).
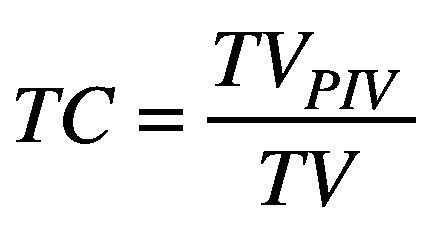
Paddick conformity index (CI) is the ratio of the target volume covered by the prescription isodose volume squared (TVPIV2) and the multiplication result of the target volume and the prescription isodose volume (TVxPIV).26,31
(2).
TC and CI are optimal at unity, and devalue as they decrease from unity. TC and CI values can have an interplay effect for complex target shapes; achieving better conformity and reduced normal brain dose often comes by sacrificing target coverage, and vice versa. Gradient index (GI) is the ratio of the patient volume covered by 50% of the prescription dose (PV50%) and the prescription isodose volume (PIV).26,27,31
(3).
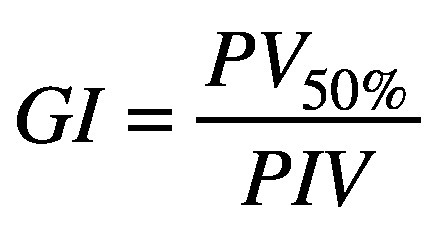
A low gradient index value is associated with a steep dose fall off around the target, resulting in decreased dose to normal tissue.26,27,31 GI, CI, and treatment time values can have an interplay effect as well; plans with lower GI values may have reduced conformity and increased treatment time, and vice versa. Homogeneity index (HI) is the ratio of the maximum target dose and the prescription dose.
(4).
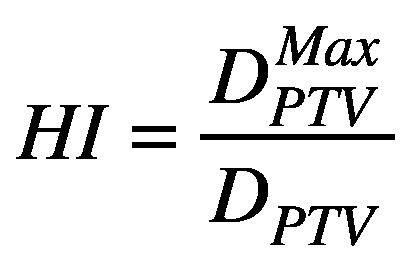
The prescription dose is prescribed to 50% of the maximum dose for GK treatments, resulting in a clinical standard HI value of 2.26 The mean HI for linac-based plans was 1.16 (range 1.05-1.37). In addition, mean normal brain dose values and normal brain V4, V12, and V20 were obtained to represent normal tissue dose level spread.
Two-tailed paired t-tests were conducted to evaluate the statistical significance of the difference between the two treatment modalities for all plan comparison parameters (p < 0.05). Percent and absolute differences were calculated as well to quantify the magnitude of these parameter differences between modalities. The differences in normal brain V12 and mean normal brain dose values between GK IconTM and linac-based FSRT plans were investigated for dependence on PTV size, target location (via GammaPlan® target coordinates), and target irregularity. Target irregularity was defined as the ratio of the largest axial dimension of the target and the equivalent diameter of the PTV. The equivalent diameter is equal to the diameter of a sphere that has a volume identical to that of the PTV.
(5).
As the target irregularity ratio increases from unity, the tumor shape is considered to be more irregular. Figure 1A shows the target with the highest target irregularity ratio of 2.35, and Figure 1B shows the target with the lowest target irregularity ratio of 1.06.
Figure 1.
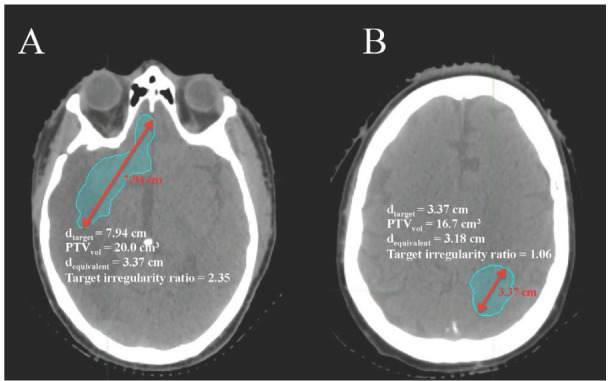
Axial CT slices with targets representing (A) the highest target irregularity ratio 2.35 and (B) the lowest target irregularity ratio 1.06.
RESULTS
A total of 16 patients with a median PTV of 33.9 cm3 (mean 35.2 cm3, range 15.0 – 74.8 cm3) were retrospectively planned for GK. All GK plans were clinically acceptable with LGP target coverage PTV V35 ≥95%, except in cases where OAR constraints would be exceeded to achieve full coverage. The GK FSRT plans resulted in a significant (p < 0.05) mean decrease in normal brain V20, normal brain V12 and mean normal brain dose values by 32.4%, 25.9% and 8.85%, and an average decrease in V4 values by 10.0% compared to the linac-based plans. V12 and V20 values were reduced for all patients within the population, but five patients had GK plans with an increase in mean normal brain dose values compared to the linac-based plans. In four of those five patients, the GK plans produced an increase in V4 values, and a decrease in V12 and V20 values. Figure 2 shows the isodose distributions of linac-based and GK treatment plans in the axial, coronal, and sagittal planes for a representative patient. In this patient, the normal brain V12 decreased from 123.3 cm3 to 67.1 cm3 and the CI was improved from 0.77 to 0.89.
Figure 2.
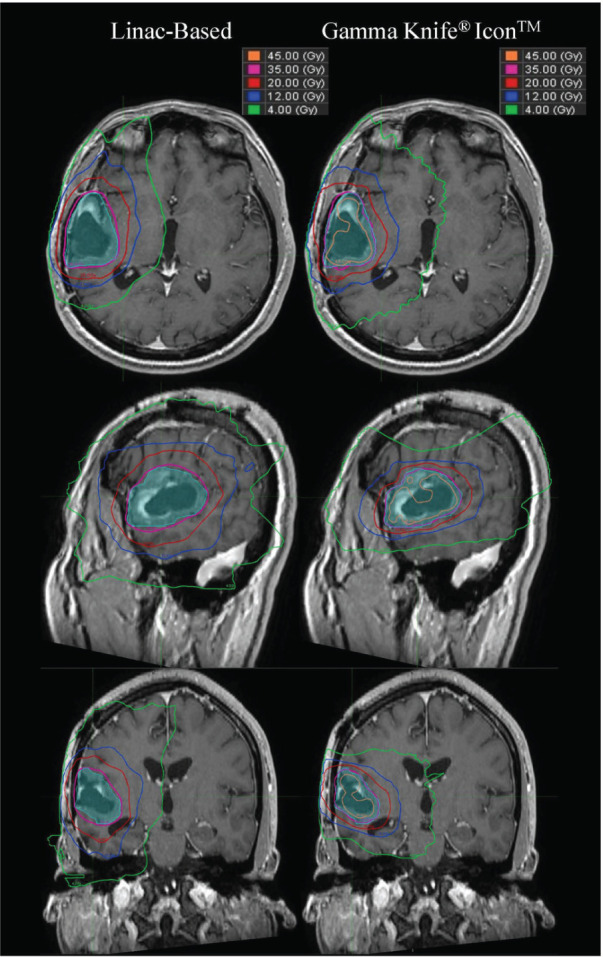
Dose distributions in the axial, sagittal, and coronal views of linac-based (left) and GK plans (right) for patient 15. Maximum dose for the linac plan was 36.8 Gy, and for the GK plan was 69.8 Gy.
Table 2 lists the mean and range TC, CI, GI, V4, V12, V20, and mean normal brain dose of the patient population for both treatment modalities. The linac-based plans showed significantly greater TC values (mean 96.3%, range 76.6% – 100%) compared to the GK plans (mean 92.9%, range 76.0% – 95.2%) due to coverage normalization differences between the two modalities in our clinic. We repeated the dose comparison with linac-based plans normalized to match GK plan target coverage and the normal brain sparing benefits of the GK plans persisted. The GK FSRT plans resulted in a significant (p < 0.05) mean decrease in normal brain V20, normal brain V12 and mean normal brain dose values by 28.3%, 22.3% and 6.04%, and an average decrease in V4 values by 6.20% compared to the re-normalized linac-based plans.
TABLE 2.
Plan comparison parameters for both treatment modalities
| Linac-based | GK | P-value (<0.05) | |
|---|---|---|---|
| Target Coverage (TC)* | 96.3% (76.6% - 100%) | 92.9% (76.0% - 95.2%) | 0.0006* |
| Conformity Index | 0.79 (0.64 - 0.94) | 0.84 (0.69 - 0.89) | 0.0572 |
| Gradient Index | 3.05 (2.32 - 5.85) | 2.70 (2.46 - 3.08) | 0.0827 |
| V4 (cm3) | 470.4 (219.3 - 637.1) | 420.5 (199.3 - 702.2) | 0.0726 |
| V12 (cm3)* | 123.9 (52.8 - 206.9) | 90.8 (45.2 - 162.8) | ≤0.0001* |
| V20 (cm3)* | 51.2 (22.9 - 87.2) | 34.7 (17.8 - 69.9) | ≤0.0001* |
| Mean Brain Dose (Gy)* | 4.4 (2.41 - 6.0) | 4.0 (2.07 - 6.27) | 0.0194* |
Four patients had OARs overlapping or adjacent to the PTV, therefore target coverage was sacrificed in planning with both modalities in order to achieve the OAR constraints for these patients. All other GK plans met the GK target coverage goal of PTV V35 ≥95% as stated in the GammaPlan® optimization workspace, but plan comparison analysis in the independent MIM software resulted in most plans with a target coverage <95% due to a slight difference in target coverage statistics between the optimization workspace and the sum dose which was exported. The GK plans resulted in a mean decrease in TC values by 3.35% compared to the linac-based plans. The GK plans showed improvement in CI values (mean 0.84, range 0.69 – 0.89) compared to the linac-based plans (mean 0.79, range 0.64 – 0.94), with a mean increase in CI values by 7.65%. The mean GI was 3.05 (range 2.32 – 5.85) for linac-based plans and 2.70 (range 2.46 – 3.08) for GK plans. The GK plans resulted in a mean decrease in GI values by 7.49% compared to the linac-based plans. Treatment times, shown in Table 1, differed significantly (p < 0.05) between the two treatment modalities, as expected. The mean treatment time was 5.6 minutes (range 4 – 8 minutes) for linac-based plans and 20.6 minutes (range 12.6 – 31.3 minutes) for GK plans. All GK plans had treatment times less than the maximum allowed time of 40 minutes.
Figure 3 shows mean normal brain dose values and the volume of normal brain receiving doses of at least 4 Gy (V4), 12 Gy (V12), and 20 Gy (V20) for both treatment modalities. The median mean normal brain dose was 4.37 Gy (mean 4.43 Gy, range 3.3 – 6 Gy) for the linac-based plans and 3.63 Gy (mean 4.02 Gy, range 2.07 – 6.27 Gy) for the GK plans. The median V20 was 49.1 cm3 (mean 51.2 cm3, range 22.9 – 87.2 cm3) for the linac-based plans and 28.6 cm3 (mean 34.7 cm3, range 17.8 – 69.9 cm3) for the GK plans. The median V12 was 120.0 cm3 (mean 123.9 cm3, range 52.8 – 206.9 cm3) for the linac-based plans and 77.6 cm3 (mean 90.8 cm3, range 45.2 – 162.8 cm3) for the GK plans. The median V4 was 490.1 cm3 (mean 470.4 cm3, range 219.3 – 637.1 cm3) for the linac-based plans and 372.1 cm3 (mean 420.5 cm3, range 199.3 – 702.2 cm3) for the GK plans.
Figure 3.
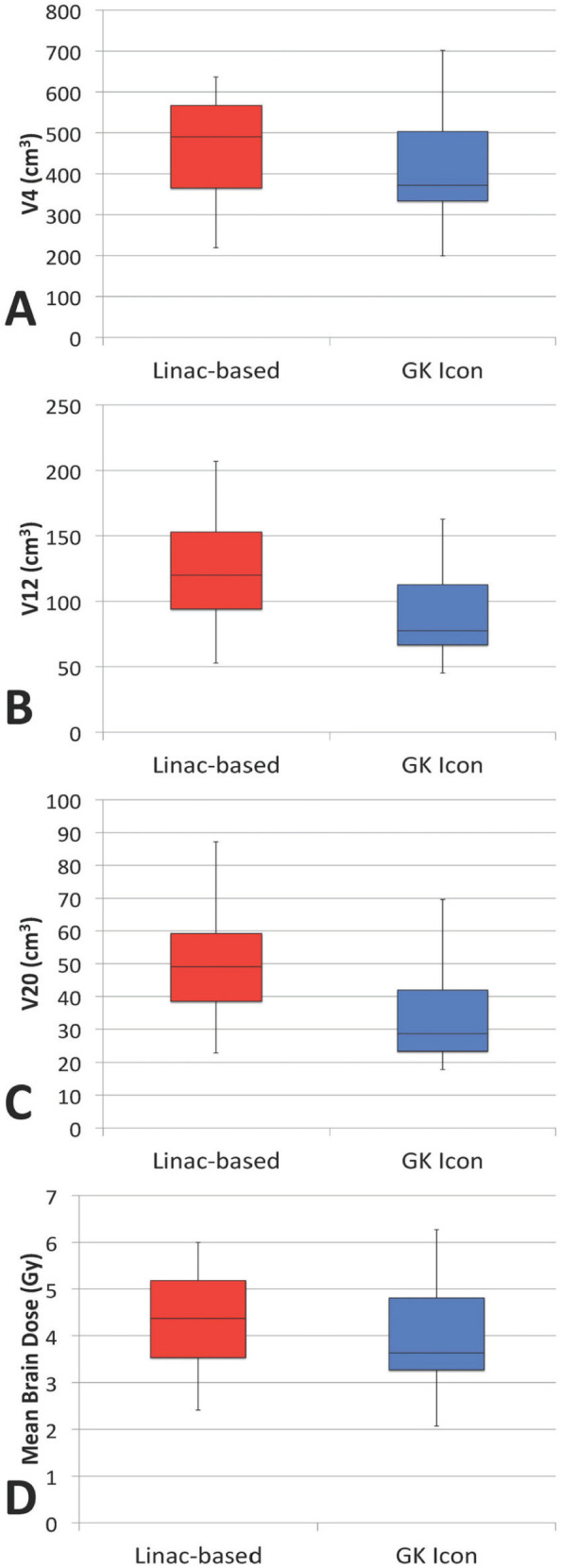
Box-and-whisker plot (25%, 50%, 75% quartiles) for V4 (A), V12 (B), V20 (C), and mean normal brain dose (D) values for linac-based and GK plans.
The normal brain dose sparing benefits (the reduction in V4, V12, V20, and mean normal brain dose values) of GK compared to linac-based plans was found to significantly (p < 0.05) decrease with increasing PTV size, as shown in Figure 4. Regression analysis predicts that PTVs between 14 – 47 cm3 will receive the most benefit from GK planning, resulting in a 36.2 – 20.1% decrease in V12 and a 18.7% - 3.2% decrease in mean normal brain dose with GK FSRT plans compared to linac-based plans. A statistically significant linear relationship was not found between PTV size and target coverage differences between modalities, nor was a statistically significant linear relationship established between target location and target coverage differences.
Figure 4.
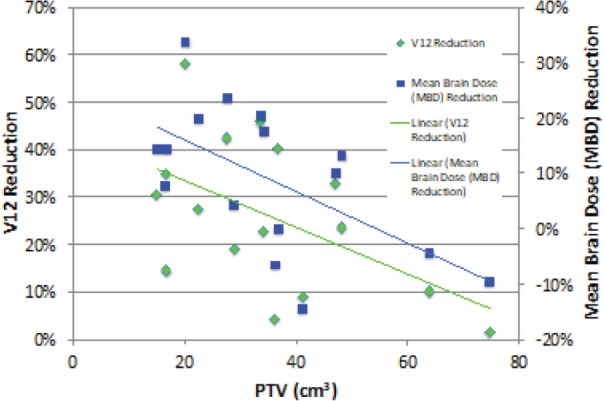
PTV size dependent reduction of normal brain V12 and mean normal brain dose values due to GK plans compared to standard linac-based plans, with linear regression analysis.
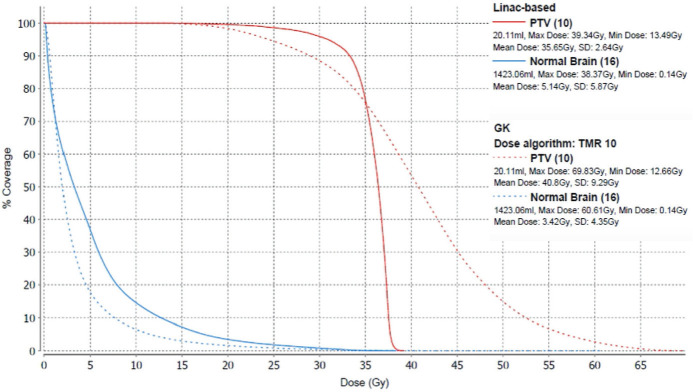
The normal brain dose sparing benefits (the reduction in V12 and mean normal brain dose) of GK compared to linac-based plans were found to be dependent on target location. Regression analysis determined the significance of the relationships between normal brain dose sparing and target volume location in the Right/Left (X), Anterior/Posterior (Y), and Superior/Inferior (Z) directions separately. Normal brain dose sparing differences between modalities was found to have a significant relationship (p < 0.05) with target volume location in the A/P direction, with the largest reduction in normal brain dose predicted to occur in the anterior region of the brain near the optic structures. Significance was not found in the lateral or S/I directions. Multi-regression analysis determined a significant relationship (p < 0.05) between the 3D coordinates of target volumes (R/L, A/P, S/I) and the reduction in V12 values between modalities. The largest reduction in normal brain dose was predicted to occur in the right/center, anterior, center (R/L, A/P, S/I) region of the brain near the optic structures. Figure 5 shows the GK and linac-based dose volume histograms (DVH) for an anterior region target volume that resulted in the largest target irregularity ratio of 2.35 and the greatest reduction in normal brain V4, V12, V20, and mean normal brain dose values in our patient population.
Figure 5.
Dose volume histogram (DVH) of linac-based (solid lines) and GK (dashed lines) plans for patient 5, with an OAR-adjacent target.
The mean target irregularity ratio value for all targets within the patient population was 1.32 (range 1.06 – 2.35). The reduction in normal brain V12 and mean normal brain dose values in GK compared to linac-based plans was found to significantly (p < 0.05) increase as the target irregularity ratio increased from unity. Regression analysis predicts that targets with irregularity ratio values ≥1.165 will receive the most benefit from GK planning, resulting in a ≥20.0% decrease in V12 and a ≥5.2% decrease in mean normal brain dose with GK FSRT plans compared to linac-based plans.
DISCUSSION
Our results show that the GK system is capable of delivering clinically acceptable plans for the re-irradiation of large (>14 cm3) recurrent glioblastomas. Compared to the linac-based deliveries, GK consistently produced plans with sharper dose fall-off, more conformality, and better normal brain dose sparing results, with a slight mean decrease in target coverage. The decrease in target coverage was primarily due to plan normalization differences between GK and linac-based plans in our clinical practice. We chose to present the results with target coverage normalization differences as these plans represent our institutional experience. All GK plans were generated with the prescription isodose line set to 50% of maximum dose, as is our clinical practice. The increase in HI for GK plans compared to linac-based FSRT plans may confer additional clinical benefits such as increased local control, which has been demonstrated in SRS for recurrent tumors.15 It is possible that increasing the prescription isodose line for the GK plans could produce clinically acceptable plans with reduced treatment times and potentially further reduce normal brain dose.32-33
The linac-based FSRT plans were all calculated with heterogeneity corrections, while the GK plans were all calculated with the homogeneous dose calculation in LGP. For the linac-based plans, dose was recalculated with homogeneous density, and differences in the volumetric dose statistics analyzed between homogeneous and heterogeneous dose calculations were negligible for the patients in this study.
Re-irradiation of recurrent GBM was selected for this study due to the expectation that PTV margins would be identical in the linac-based and the GK plans, along with the low daily dose of the fractionation schedule. For other intracranial sites, differences in PTV margins between the two systems may lead to variation within plan comparison parameters. The low daily dose of this treatment allows for clinically deliverable GK FSRT treatments with treatment times ≤20 minutes (<40 minutes maximum), compared to previous GK and linac-based plan comparisons for large brain metastases that had long treatment times and were unlikely to be clinically feasible.26–28 Allowing all GK treatment times to increase above 40 minutes may produce GK plans with even greater normal brain dose sparing than shown here, but we believe the benefits of increased patient comfort are worth maintaining our treatment time limitations in mask-based fractionated cases.34 The tumor dimension size limit for a single dose matrix (one plan per target) implemented in this study seems to be a reasonable clinical cut-off, as it is unlikely that targets with any dimension >7.5 cm would be able to be treated in ≤20 minutes. This limit, as well as evaluation of target irregularity and proximity to OARs, could serve as a method of sorting for patients that would benefit most from GK FSRT and patients that should remain on the linac.
The magnitude of the GK normal brain dose sparing benefits decreased with increasing PTV size, likely due to the GK system’s utilization of more shots or larger collimators to cover these larger target volumes. V12 and V20 values were reduced for all patients within the population, but five patients had GK plans with an increase in mean normal brain dose values compared to the linac-based plans. In four of those five patients, the GK plans produced an increase in V4 values, and a decrease in V12 and V20 values. This suggests that the GK beam configurations produced in those plans spread out lower doses in order to produce plans with more conformal higher value isodose lines, resulting in greater overall normal brain dose sparing at the V12 and V20 level for the GK plans compared to the linac-based plans despite the increase in mean normal brain dose. The mean normal brain dose increase in the patient that did not show an increase in V4 was negligible, therefore likely not clinically significant. It is possible that GK plans with higher prescription isodose lines or longer treatment times than used here may result in decreased lower doses for these patients as well. Based on the inverse relationship between PTV size and normal brain dose sparing, we intend to expand our analysis to include any size recurrence in future work.
V12 and mean normal brain dose value differences between modalities were shown to be significantly (p < 0.05) correlated with target location in the A/P direction and tumor irregularity. The anterior region of the brain showed the greatest differences, with GK outperforming the linac-based plans in the sparing of normal brain tissue from 12 Gy. Furthermore, V12 and mean normal brain dose differences between modalities increased as our measure of tumor shape irregularity (target irregularity ratio) increased. The presence of OARs such as the optic nerves and optic chiasm in the anterior region of the brain increase the likelihood of an irregular target shape, leading to a more complex tumor shape better suited to the increased entrance angles of the GK system.
Four patients had plans which exceeded OAR constraints when initially planned for full target coverage in both the linac-based and GK treatment planning systems. In linac-based treatment planning systems, these constraints can be added to the inverse optimization. The plans in this study were created using LGP V11, therefore forward planning was used to edit these plans in order to decrease OAR doses and meet constraints. The plans used in this study with target volumes adjacent to OARs achieved similar target coverage to the linac-based plans with reduced normal brain dose. Figure 5 shows the GK and linac-based dose volume histograms (DVH) for one of these patients. The target volume of this patient was a right frontal mass in close proximity to the right optic nerve and optic chiasm, resulting in the low TC values produced by both treatment modalities due to hard OAR constraints. The target volume of this patient resulted in the largest target irregularity ratio and the greatest reduction in V4, V12, V20, and mean normal brain dose values in our patient population. The GK system’s ability to produce a highly conformal plan with reduced normal brain dose for this OAR-adjacent irregular target volume indicates the cohort of patients that will most benefit from GK FSRT planning.
Clinically deliverable GK FSRT treatment plans significantly decrease normal brain dose compared to linac-based FSRT for the re-irradiation of large (>14 cm3) recurrent glioblastomas. Patients with targets in close proximity to OARs or an irregular shape will most benefit from GK compared to linac-based treatments. GK FSRT has the potential to improve patient outcomes for this patient population by reducing the risk of CNS toxicities.
ACKNOWLEDGMENTS
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Concept and design: Matthew Schelin, Haisong Liu, Ayehsa Ali, Wenyin Shi, Yan Yu, Karen Mooney
Data collection: Matthew Schelin, Haisong Liu, Karen Mooney
Data analysis and interpretation: Matthew Schelin, Haisong Liu, Wenyin Shi, Yan Yu, Karen Mooney
Manuscript writing: Matthew Schelin, Haisong Liu, Ayesha Ali, Wenyin Shi, Yan Yu, Karen Mooney
Final approval of manuscript: Matthew Schelin, Haisong Liu, Ayesha Ali, Wenyin Shi, Yan Yu, Karen Mooney
REFERENCES
- 1.Romanelli P, Conti A, Pontoriero A, Ricciardi GK, Tomasello F, De Renzis C, Innocenzi G, Esposito V, Cantore G. Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg Focus. 2009;27(6):1-11. doi: 10.3171/2009.9.FOCUS09187 [DOI] [PubMed] [Google Scholar]
- 2.Reynaud T, Bertaut A, Farah W, Thibouw D, Crehange G, Truc G, Vulquin N. Hypofractionated stereotactic radiotherapy as a salvage therapy for recurrent high-grade gliomas: Single-center experience. Technol Cancer Res Treat. 2018;17:1-11. doi: 10.1177/1533033818806498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, Evans JJ, Hyslop T, Pequignot E, Downes B, Comber E, Maltenfort M, Dicker AP, Werner-Wasik M. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28(18):3048-3053. doi: 10.1200/JCO.2009.25.6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y, Fu C, Guan H, Zhang T, Zhang Z, Zhou T, Li B. Re-irradiation alternatives for recurrent high-grade glioma. Oncol Lett. 2016;12(4):2261-2270. doi: 10.3892/ol.2016.4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combs SE, Debus J, Schulz-Ertner D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer. 2007;7:1-11. doi: 10.1186/1471-2407-7-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanker M, Chua B, Bettington C, Foote MC, Pinkham MB. Re-irradiation for recurrent high-grade gliomas: A systematic review and analysis of treatment technique with respect to survival and risk of radionecrosis. Neuro-Oncology Pract. 2019;6(2):144-155. doi: 10.1093/nop/npy019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barney C, Shukla G, Bhamidipati D, Palmer JD. Re-irradiation for recurrent glioblastoma multiforme. Chinese Clin Oncol. 2017;6(4):2-9. doi: 10.21037/cco.2017.06.18 [DOI] [PubMed] [Google Scholar]
- 8.Ene CI, Macomber MW, Barber JK, Ferreira MJ, Ellenbogen RG, Holland EC, Rockhill JK, Silbergeld DL, Halasz LM. Patterns of failure after stereotactic radiosurgery for recurrent high-grade glioma: A single institution experience of 10 years. Neurosurgery. 2019;85(2):E322-E331. doi: 10.1093/neuros/nyy520 [DOI] [PubMed] [Google Scholar]
- 9.Vordermark D, Kölbl O, Ruprecht K, Vince GH, Bratengeier K, Flentje M. Hypofractionated stereotactic re-irradiation: Treatment option in recurrent malignant glioma. BMC Cancer. 2005;5:1-7. doi: 10.1186/1471-2407-5-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amichetti M, Amelio D. A review of the role of re-irradiation in recurrent high-grade Glioma (HGG). Cancers (Basel). 2011;3(4):4061-4089. doi: 10.3390/cancers3044061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel M, Siddiqui F, Jin JY, Mikkelsen T, Rosenblum M, Movsas B, Ryu S. Salvage reirradiation for recurrent glioblastoma with radiosurgery: Radiographic response and improved survival. J Neurooncol. 2009;92(2):185-191. doi: 10.1007/s11060-008-9752-9 [DOI] [PubMed] [Google Scholar]
- 12.Kim H. Appraisal of re-irradiation for the recurrent glioblastoma in the era of MGMT promotor methylation. Radiat Oncol J. 2019;37(1):1-12. doi: 10.3857/roj.2019.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korytko T, Radivoyevitch T, Colussi V, Wessels BW, Pillai K, Maciunas RJ, Einstein DB. 12 Gy Gamma Knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006. doi: 10.1016/j.ijrobp.2005.07.980 [DOI] [PubMed] [Google Scholar]
- 14.Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010. doi: 10.1016/j.ijrobp.2009.02.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291-298. doi: 10.1016/S0360-3016(99)00507-6 [DOI] [PubMed] [Google Scholar]
- 16.Lee CC, Yen CP, Xu Z, Schlesinger D, Sheehan J. Large intracranial metastatic tumors treated by Gamma Knife surgery: Outcomes and prognostic factors. J Neurosurg. 2014;120(1):52-59. doi: 10.3171/2013.9.JNS131163 [DOI] [PubMed] [Google Scholar]
- 17.Yang HC, Kano H, Lunsford LD, Niranjan A, Flickinger JC, Kondziolka D. What factors predict the response of larger brain metastases to radiosurgery? Neurosurgery. 2011;68(3):682-690. doi: 10.1227/NEU.0b013e318207a58b [DOI] [PubMed] [Google Scholar]
- 18.Tsien C, Pugh S, Dicker AP, Raizer JJ, Matuszak MM, Lallana E, Huang J, Algan O, Taylor N, Portelance L, Villano J, Hamm J, Oh KS, Ali Jr AN, Kim MM, Lindhorst S, Mehta MP. Randomized phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): Initial outcomes and RT plan quality report. Int J Radiat Oncol. 2019. doi: 10.1016/j.ijrobp.2019.06.539 [DOI] [Google Scholar]
- 19.Pinnaduwage D, Dong P, Ma L. Image-Guided Hypofractionated Radiosurgery of Large and Complex Brain Lesions. In: From Bench to Bedside - Trauma, Tumors, Spine, Functional Neurosurgery.. InTech; 2016. doi: 10.5772/64481 [DOI] [Google Scholar]
- 20.Young RF. The role of the gamma knife in the treatment of malignant primary and metastatic brain tumors. CA Cancer J Clin. 1998;48(3):177-188. doi: 10.3322/canjclin.48.3.177 [DOI] [PubMed] [Google Scholar]
- 21.Maaks LB, Spencer DP. The Influence of Volume on the Tolerance of the Brain to Radiosurgery. Vol 75.; 1991. [DOI] [PubMed] [Google Scholar]
- 22.Han JH, Kim DG, Chung HT, Paek SH, Park CK, Jung HW. Radiosurgery for large brain metastases. Int J Radiat Oncol Biol Phys. 2012;83(1):113-120. doi: 10.1016/j.ijrobp.2011.06.1965 [DOI] [PubMed] [Google Scholar]
- 23.Park HR, Park KW, Lee JM, Kim JH, Jeong SS, Kim JW, Chung HT, Kim DG, Paek SH. Frameless fractionated Gamma Knife radiosurgery with IconTM for large metastatic brain tumors. J Korean Med Sci. 2019;34(8). doi: 10.3346/jkms.2019.34.e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McTyre E, Helis CA, Farris M, Wilkins L, Sloan D, Hinson WH, Bourland JD, Dezarn WA, Munley MT, Watabe K, Xing F, Laxton AW, Tatter SB, Chan MD. Emerging indications for fractionated Gamma Knife radiosurgery. Neurosurgery. 2017;80(2):210-216. doi: 10.1227/NEU.0000000000001227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JW, Park HR, Lee JM, Kim JW, Chung HT, Kim DG, Jung HW, Paek SH. Fractionated stereotactic Gamma Knife radiosurgery for large brain metastases: A retrospective, single center study. PLoS One. 2016;11(9). doi: 10.1371/journal.pone.0163304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han EY, Wang H, Luo D, Li J, Wang X. Dosimetric comparison of fractionated radiosurgery plans using frameless Gamma Knife ICON and CyberKnife systems with linear accelerator–based radiosurgery plans for multiple large brain metastases. J Neurosurg. April2019:1-7. doi: 10.3171/2019.1.jns182769 [DOI] [PubMed] [Google Scholar]
- 27.Dong P, Pérez-Andújar A, Pinnaduwage D, Braunstein S, Theodosopoulos P, McDermott M, Sneed P, Ma L. Dosimetric characterization of hypofractionated Gamma Knife radiosurgery of large or complex brain tumors versus linear accelerator–based treatments. J Neurosurg. 2016;125(Suppl 1):97-103. doi: 10.3171/2016.7.GKS16881 [DOI] [PubMed] [Google Scholar]
- 28.Phan J, Yang JN, Ghia AJ, Brown PD, Garden AS, Rosenthal DI, Wang C, Tung S, Luo D, Wang H. Dosimetric comparison of Gamma Knife extend to linear accelerator base volumetric modulated arc therapy plans for fractionated stereotactic radiosurgery. Int J Radiat Oncol. 2015. doi: 10.1016/j.ijrobp.2015.07.2118 [DOI] [Google Scholar]
- 29.Li J, To D, Gunn V, Shi W, Yu Y, Liu H. Evaluation of hybrid arc and volumetric-modulated arc therapy treatment plans for fractionated stereotactic intracranial radiotherapy. Technol Cancer Res Treat. 2018;17:1-6. doi: 10.1177/1533033818802804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma L, Sahgal A, Larson DA, Pinnaduwage D, Fogh S, Barani I, Nakamura J, McDermott M, Sneed P. Impact of millimeter-level margins on peripheral normal brain sparing for gamma knife radiosurgery. Int J Radiat Oncol Biol Phys. 2014;89(1):206-213. doi: 10.1016/j.ijrobp.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 31.Huss M, Barsoum P, Dodoo E, Sinclair G, Toma-Dasu I. Fractionated SRT using VMAT and Gamma Knife for brain metastases and gliomas - A planning study. J Appl Clin Med Phys. 2015;16(6):3-16. doi: 10.1120/jacmp.v16i6.5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L. Dependence of normal brain integral dose and normal tissue complication probability on the prescription isodose values for gamma-knife radiosurgery. Phys Med Biol. 200146(11):3031-41. doi: 10.1088/0031-9155/46/11/317 [DOI] [PubMed] [Google Scholar]
- 33.Johnson PB, Monterroso MI, Yang F, Mellon E. Optimization of the prescription isodose line for Gamma Knife radiosurgery using the shot within shot technique. Radiat Oncol 12, 187 (2017). 10.1186/s13014-017-0919-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wegner RE, Xu L, Horne Z, Yu A, Goss M, Liang Y, Sohn J, Karlovits SM. Predictors of Treatment Interruption During Frameless Gamma Knife Icon Stereotactic Radiosurgery. Adv Radiat Oncol. 20205(6):1152-57. doi: 10.1016/j.adro.2020.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]