Abstract
Introduction
The aim of this study was to investigate the role of local radiotherapy in the management of epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancers (NSCLCs) treated with EGFR tyrosine kinase inhibitors (TKIs).
Materials and Methods
Patients with stage IV EGFR-mutant NSCLC treated with radiotherapy concomitant to EGFR TKIs from May 2010 to December 2017 were retrospectively identified. Overall survival (OS) was the primary endpoints of the study.
Results
A total of 205 patients were enrolled in the study. One hundred eleven patients received one-time single-site radiotherapy (SSR), and 94 patients received multiple-site radiotherapy (MSR). Patients who received MSR had longer OS (median OS, 40.0 months; 95% confidence interval [CI], 29.6 to 50.4) than those who received SSR (median OS, 28.9 months; 95% CI, 24.3 to 33.5; P=0.031). Thoracic radiotherapy was associated with prolonged median OS (41.7 months, 95% CI, 29.0 to 54.4 vs 27.1 months, 95% CI 22.7 to 31.5; log-rank P<0.001). Multivariate analysis confirmed that thoracic radiotherapy was independently associated with improved OS (adjusted hazard ratio [HR], 0.514; 95% CI 32.3% to 81.8%; P=0.005).
Conclusion
MSR improves survival outcomes in patients with advanced-stage, EGFR-mutant, lung adenocarcinoma, with thoracic radiotherapy having the most significant effect on prognosis.
Keywords: advanced-stage lung cancer, adenocarcinomas, radiotherapy, EGFR
Introduction
Lung cancer is one of the most common malignancies and the leading cause of cancer death worldwide.1 Non-small cell lung cancer (NSCLC) accounts for nearly 80% of all primary lung cancers. Most patients with NSCLC are diagnosed at an advanced stage and have the a poor prognosis.2
With the technological advances, tumors have been subdivided based on their pathological features, and treatment modalities have gradually shifted from the initial one-size-fits-all model to personalized treatments. Oncogenic mutations in epidermal growth factor receptor (EGFR) are used as a predictive biomarker in NSCLC, and therapies targeting EGFR have greatly improved progression-free survival (PFS) and overall survival (OS) in patients with NSCLC,3,4 especially female non-smoking lung adenocarcinoma patients, who show an 80% EGFR mutation rate.5 First-generation EGFR tyrosine kinase inhibitors (TKIs) provided a median PFS of 8 to 14.7 months,6–8 significantly higher than that of those treated with the previous therapeutic model; first-generation EGFR TKIs also significantly improved the objective response rate (ORR).9–13 Although targeted drugs administered orally are convenient, they offer limited therapeutic benefits. Drug resistance is frequent among patients receiving first, second, or third-generation targeted therapies, limiting the therapeutic potential of EGFR TKIs. KRAS mutations, T790M mutations, c-MET amplification, and PIK3CA are mechanisms contributing to TKI resistance.14,15
Combination therapies have emerged as a promising treatment strategy to overcome resistance to EGFR TKIs.16,17 In the Phase III study NEJ009, the median PFS of patients treated with gefitinib combined with chemotherapy was 20.9 months, twice as long as that of patients treated with gefitinib alone (11.9 months). Similarly, the median OS of patients treated with gefitinib alone was 38.8 months, whereas that of patients treated with gefitinib combined with chemotherapy was 50.9 months.16
However, the therapeutic value of radiotherapy in combination with EGFR TKIs remains unknown. In addition to directly killing tumor cells, radiotherapy also activates antitumor T cells and enhances tumor antigen recognition by affecting the environment of the tumor blood vessels and chemokines.18–21 EGFR TKIs can enhance sensitivity to radiotherapy.22 Therefore, the combination of EGFR TKIs with radiotherapy may be a promising strategy for the treatment of advanced NSCLC. The optimal timing of radiotherapy, the role of the location of radiotherapy in immune responses, and the influence of multiple-site radiotherapy on prognosis remain to be determined. In this retrospective study, we analyzed the relationship between radiotherapy patterns and OS in patients with NSCLC.
Materials and Methods
Patients
Data were collected from more than 400 patients with NSCLC treated at Shandong Cancer Hospital from May 2010 to December 2017. The following inclusion criteria were used:1 pathological diagnosis of primary lung adenocarcinoma;2 presence of EGFR mutations, in exons 18, 19, 20, or 21;3 presence of distant metastasis diagnosed by computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT, or invasive examination (aspiration cytology);4 availability of detailed clinical information, including treatment regimen and clinicopathological characteristics;5 treatment with EGFR TKIs (eg, gefitinib, erlotinib, icotinib, osimertinib) and radiotherapy for at least one site throughout the course of the disease;6 availability of follow-up information. Clinicopathological characteristics included age, sex, tumor stage, smoking history, and EGFR mutation status. Data from 205 patients were included in this retrospective study. All participants provided written informed consent. Tumor staging was evaluated according to the 8th edition of the staging manual of the American Joint Commission on Cancer (AJCC). The study was approved by the Ethics Committee of Shandong Cancer Hospital. This was conducted in accordance with the Declaration of Helsinki.
Treatment and Follow-Up
All patients received EGFR TKIs (gefitinib 250 mg per day, erlotinib 150 mg per day, osimertinib 80 mg per day, and icotinib 125 mg three times per day) as first-line or second-line treatment. Patients also received other systemic treatments, including platinum, pemetrexed, gemcitabine, vinorelbine, docetaxel, albumin paclitaxel, and bevacizumab. Additionally, patients received radiotherapy to at least one site (brain, thorax, bone, adrenal gland, liver, or spleen). The dose for brain radiotherapy ranged from 2 Gy per fraction to 5 Gy per fraction, and the total dosage ranged from 30 Gy to 60 Gy. Radiotherapy for lung tumors and thoracic metastatic lymph nodes was classified as thoracic radiotherapy. The dose of thoracic radiotherapy ranged from 2 Gy per fraction to 5.5 Gy per fraction, and the total dosage ranged from 27 Gy to 75 Gy. The dose of bone (vertebrae, sternum, pelvis, and limb bones) radiotherapy ranged from 1.8 Gy per fraction to 4 Gy per fraction, and the total dosage ranged from 15 Gy to 60 Gy. Radiation doses for the adrenal glands, liver, and spleen ranged from 2 Gy per fraction to 5 Gy per fraction, and the total dosage ranged from 27 Gy to 60 Gy. If radiotherapy was administered to multiple sites from the time of diagnosis to the cut-off date, it was classified as multiple-site radiotherapy (MSR); otherwise, it was classified as single-site radiotherapy (SSR). Patients were followed up by telephone. OS time was calculated as the time from pathological diagnosis of lung cancer to the cut-off date or death.
Statistics
Patient characteristics in the SSR and MSR groups were compared using the chi-squared test (categorical variables) or analysis of variance (continuous variables). The Kaplan-Meier method was used for survival analyses, and the logarithmic rank test was used to compare the effects of individual variables on survival (P < 0.05 was considered statistically significant). Statistically significant variables in univariate analysis were included in multivariate Cox regression analyses to confirm their independent effects on survival. All statistical analyses were carried out using SPSS v.26.0 software (IBM, Armonk, NY, USA). All datasets are available from the corresponding author on reasonable request.
Results
Clinicopathologic and Treatment Characteristics
Of 205 patients who met the inclusion criteria, the majority were female (140, 68.3%) and non-smokers (161, 78.5%). Patient characteristics are listed in Table 1. The vast majority of patients had EGFR mutations in exons 19 and 21, accounting for 44.9% (92/205) and 52.2% (107/205), respectively. All patients received systemic treatment, of which platinum-based treatment was provided to 77.1% (158/205) and 63.4% (130/205) of the patients receiving EGFR TKIs as first-line treatment. During the period from the diagnosis of lung cancer to the last date of follow-up, 111 patients received one-time SSR and 94 patients received MSR, as shown in Table 2. The organs or sites of the patients that were targeted with radiotherapy mainly included the thorax (101/205, 49.3%), brain (113/205, 55.1%), bone (96/205, 46.8%), abdomen (13/205, 6.3%), and soft tissue (1/205, 0.5%).
Table 1.
Characteristics of 205 NSCLC Patients
| Characteristics | Patients |
|---|---|
| N = 205 (%) | |
| Age, years | |
| > 60 | 68 (33.2) |
| ≤ 60 | 137 (66.8) |
| Sex | |
| Female | 140 (68.3) |
| Male | 65 (31.7) |
| Smoking Status | |
| Never | 161 (78.5) |
| Former/current | 44 (21.5) |
| EGFR Mutation | |
| Exon 18 | 4 (2.0) |
| Exon 19 | 92 (44.9) |
| Exon 20 | 1 (0.5) |
| Exon 21 | 107 (52.2) |
| Exon 18 and 20 | 1 (0.5) |
| Systemic Therapy | |
| Platinum treatment | 158 (77.1) |
| First-line EGFR TKIs therapy | 130 (63.4) |
| Second-line EGFR TKIs therapy | 75 (36.6) |
| Location of Metastasis | |
| Brain | 138 (67.3) |
| Bone | 161 (78.5) |
| Abdomen | 13 (6.3) |
| Soft tissue | 1 (0.5) |
| The Site of Radiotherapy | |
| Thoracic | 101 (49.3) |
| Brain | 113 (55.1) |
| Bone | 96 (46.8) |
| Adrenal | 8 (3.9) |
| Liver | 4 (2.0) |
| Spleen | 1 (0.5) |
| Soft tissue | 1 (0.5) |
| Number of Radiotherapy | |
| 1 | 111 (54.1) |
| 2 | 71 (34.6) |
| 3 | 20 (9.8) |
| 4 | 3 (1.5) |
Note: Data are presented as n (%) or median (interquartile range).
Abbreviations: EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors.
Table 2.
Characteristics of Patients in the Two Groups and with the X2 Test for Categorical Variables
| Characteristics | Single-Site Radiotherapy | Multiple-Site Radiotherapy | P |
|---|---|---|---|
| Age(y) | 57 (50–64) | 52 (47.75–59.25) | 0.002 |
| >60 | 47 (42.3) | 21 (22.3) | |
| ≤60 | 64 (57.7) | 73 (77.7) | |
| Sex | 0.808 | ||
| Female | 75 (67.6) | 65 (69.1) | |
| Male | 36 (32.4) | 29 (30.9) | |
| Smoking Status | 0.458 | ||
| Never | 85 (76.6) | 76 (80.9) | |
| Former/current | 26 (23.4) | 18 (19.1) | |
| EGFR Mutation | 0.264 | ||
| Exon 18 | 1 (0.9) | 3 (3.2) | |
| Exon 19 | 55 (49.5) | 37 (39.4) | |
| Exon 20 | 0 (0.0) | 1 (1.1) | |
| Exon 21 | 55 (49.5) | 52 (55.3) | |
| Exon 18 and 20 | 0 (0.0) | 1 (1.1) | |
| Systemic Therapy | |||
| Platinum treatment | 80 (72.1) | 78 (83.0) | 0.064 |
| First-line EGFR TKIs therapy | 74 (66.7) | 56 (59.6) | 0.294 |
| Second-line EGFR TKIs therapy | 37 (33.3) | 38 (40.4) | 0.294 |
| Location of Metastasis | |||
| Brain | 68 (61.3) | 70 (74.5) | 0.045 |
| Bone | 84 (75.7) | 77 (81.9) | 0.278 |
| Abdomen | 2 (1.8) | 11 (11.7) | |
| Soft tissue | 1 (0.9) | 0 (0.0) | |
| The Site of Radiotherapy | |||
| Thoracic | 20 (18.0) | 81 (86.2) | <0.001 |
| Brain | 48 (43.2) | 65 (69.1) | <0.001 |
| Bone | 42 (37.8) | 54 (57.4) | 0.005 |
| Adrenal | 2 (1.8) | 6 (6.4) | |
| Liver | 0 (0.0) | 4 (4.3) | |
| Spleen | 0 (0.0) | 1 (1.0) | |
| Soft tissue | 1 (0.9) | 0 (0.0) | |
| Number of Radiotherapy | |||
| 1 | 111 (100.0) | 0 (0.0) | |
| 2 | 0 (0.0) | 71 (75.5) | |
| 3 | 0 (0.0) | 20 (21.3) | |
| 4 | 0 (0.0) | 3 (3.2) |
Note: Data are presented as n (%) or median (interquartile range).
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Survival Outcomes Among the Entire Study Cohort
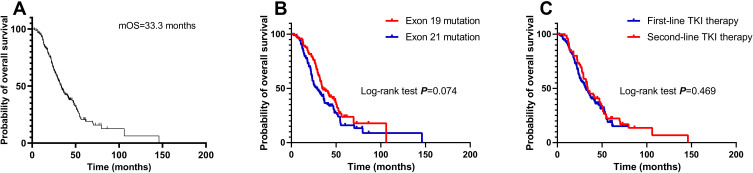
After a median follow-up duration of 50.4 months (interquartile range, 47.5 to 53.2), there were 148 deaths (72.2%) in the entire cohort. The median OS for all the patients was 33.3 months (95% confidence interval [CI], 29.3 to 37.3). Rates of 1-, 3-, and 5-year OS among the entire cohort were 92.2%, 45.1%, and 22.9%, respectively (Figure 1A). The median OS for patients with exon 19 mutation and exon 21 mutation was 34.4 months (95% CI, 27.1 to 41.7) and 28.9 months (95% CI, 22.0 to 35.8), respectively (log-rank P=0.074, Figure 1B). With regard to systemic therapy, the median OS of patients treated with first-line EGFR TKI (95% CI, 25.3 to 37.7) was similar to that of patients treated with second-line EGFR TKI (95% CI, 26.5 to 41.3; P=0.469; Figure 1C).
Figure 1.
Overall survival (OS) of the entire cohort (A) and of patients stratified according to EGFR mutation status (B) and the lines of EGFR TKIs (C).
Multiple-Site Radiotherapy Provided Better Overall Survival Than Single-Site Radiotherapy
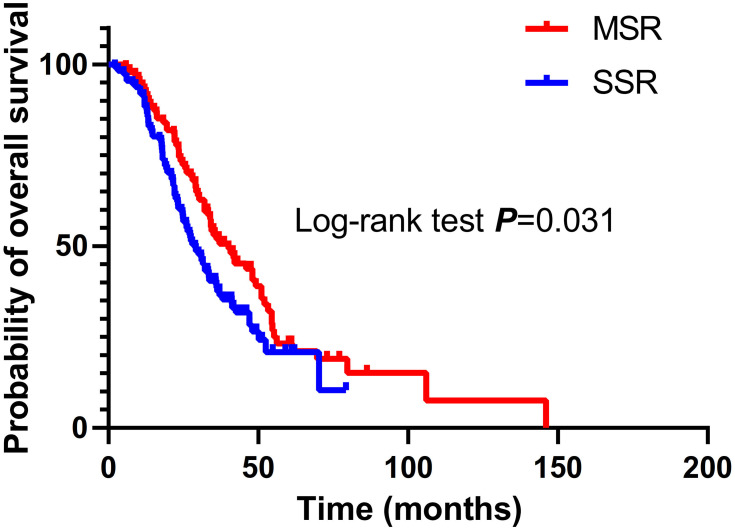
Thoracic radiotherapy (86.2% MSR vs 18.0% SSR; P < 0.001), brain radiotherapy (69.1% MSR vs 43.2% SSR; P < 0.001), and bone radiotherapy (57.4% MSR vs 37.8% SSR; P = 0.005) were more common among patients who received MSR. Fewer patients aged over 60 years underwent MSR (22.3% MMR vs 42.3% SSR; P = 0.002). There were no differences between the two groups with respect to sex, smoking status, EGFR mutations, systemic therapy (platinum-based treatment and first-line EGFR TKIs), or metastatic site. Patients who underwent MSR had longer OS (median OS, 40.0 months; 95% CI, 29.6 to 50.4; 1-year OS, 92.6%; 3-year OS, 51.9%; 5-year OS, 21.1%) than those who received SSR (median OS, 28.9 months; 95% CI, 24.3 to 33.5; 1-year OS, 89.2%; 3-year OS, 38.5%; 5-year OS, 10.4%; P = 0.031; Figure 2).
Figure 2.
Overall survival (OS) of patients treated with multiple-site radiotherapy (MSR) or single-site radiotherapy (SSR).
Effect of Radiotherapy on Survival of Different Organs or Parts
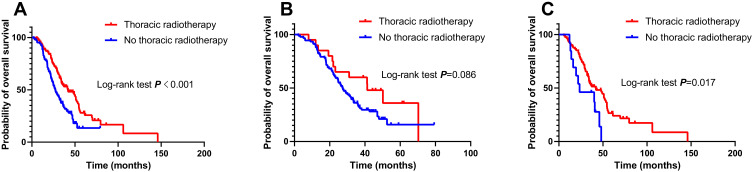
We analyzed the impact of radiotherapy on different organs or sites on patients’ OS. The median OS of patients who received and did not receive thoracic radiotherapy was 41.7 months (95% CI, 29.0 to 54.4) and 27.1 months (95% CI, 22.7 to 31.5), respectively (log-rank P < 0.001; Figure 3A). Among patients receiving MSR, 81/94 (86.2%) underwent thoracic radiotherapy. Subgroup analysis revealed that patients who received thoracic radiotherapy had a longer median OS than those who did not receive thoracic radiotherapy (41.7 months vs 23.5 months; log-rank P = 0.017; Figure 3C). In the SSR subgroup, the median OS of patients who received thoracic radiotherapy was longer than that of patients who did not receive thoracic radiotherapy, although this difference was not statistically significant (41.3 months vs 27.3 months, P = 0.086; Figure 3B).
Figure 3.
Effect of thoracic radiotherapy on overall survival (OS) in the entire cohort (A), in the single-site radiotherapy group (B), and in the multiple-site radiotherapy group (C).
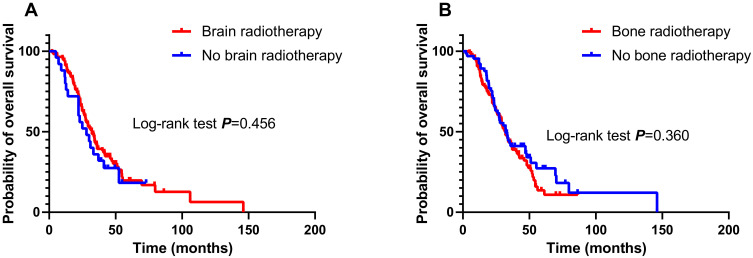
Among patients with brain metastases (138/205), there was no significant difference in OS between patients who received brain radiotherapy (113/138) and those who did not receive brain radiotherapy (25/138; median OS, 32.2 months vs 28.0 months; log-rank P = 0.456; Figure 4A). Among patients with bone metastases (161/205), there was no significant difference in OS between patients who received bone radiotherapy (96/161) and those who did not receive bone radiotherapy (65/161; median OS, 31.8 months vs 32.2 months; log-rank P = 0.360; Figure 4B).
Figure 4.
Effect of brain radiotherapy on overall survival (OS) in patients with brain metastasis (A). Effect of bone radiotherapy on overall survival (OS) in patients with bone metastasis (B).
Multivariate analysis showed that, after adjusting for significant covariates, including thoracic radiotherapy and number of radiotherapy sites, thoracic radiotherapy was independently associated with improved OS (adjusted hazard ratio [HR], 0.514; 95% CI, 32.3% to 81.8%; P = 0.005; Table 3).
Table 3.
Univariable and Multivariable Analyses of Covariable Associated with OS
| Variable | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age(y) | ||||||
| <60 v ≤60 | 0.926 | 0.656 to 1.307 | 0.662 | |||
| Sex | ||||||
| Female v male | 0.945 | 0.670 to 1.333 | 0.746 | |||
| Smoking Status | ||||||
| Never v current/former | 0.866 | 0.589 to 1.275 | 0.466 | |||
| EGFR Mutation | ||||||
| Exon 19 v Exon 21 | 0.740 | 0.531 to 1.031 | 0.075 | |||
| Systemic Therapy | ||||||
| Platinum treatment | ||||||
| Yes v no | 0.749 | 0.500 to 1.120 | 0.159 | |||
| First-line EGFR TKIs therapy | ||||||
| Yes v no | 1.132 | 0.808 to 1.587 | 0.468 | |||
| Location of Metastasis | ||||||
| Brain Metastasis | ||||||
| Yes v no | 1.266 | 0.890 to 1.801 | 0.189 | |||
| Bone Metastasis | ||||||
| Yes v no | 1.300 | 0.855 to 1.975 | 0.220 | |||
| The Site of Radiotherapy | ||||||
| Thoracic Radiotherapy | ||||||
| Yes v no | 0.544 | 0.388 to 0.763 | <0.001 | 0.514 | 0.323 to 0.818 | 0.005 |
| Brain Radiotherapy | ||||||
| Yes v no | 1.105 | 0.796 to 1.534 | 0.550 | |||
| Bone Radiotherapy | ||||||
| Yes v no | 1.273 | 0.919 to 1.762 | 0.146 | |||
| Number of Radiotherapy sites | ||||||
| 2–4 v 1 | 0.696 | 0.499 to 0.970 | 0.032 | 1.087 | 0.688 to 1.719 | 0.721 |
Abbreviations: EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors.
Discussion
The main treatment mode for patients with advanced EGFR-mutant lung adenocarcinoma is systemic therapy, in which EGFR TKIs play an important role. However, PFS in patients treated with first-generation EGFR TKIs rarely exceeds 8 to 14.7 months, and OS is only 26.8 to 34.9 months.23,24 Local treatment with radiotherapy can improve the prognosis of advanced EGFR-mutant lung adenocarcinoma. The feasibility and low toxicity of radiotherapy combined with targeted therapy make it a therapeutic option worth further exploration. A single-arm clinical study demonstrated that EGFR TKIs combined with thoracic radiotherapy as first-line treatment for advanced-stage, EGFR-mutant NSCLC provided long-term control of the primary lung tumor.25 However, the effect of the radiotherapy mode on prognosis remains unclear. Hence, in this retrospective study, we analyzed the impact of radiotherapy mode on the OS of patients with lung adenocarcinoma carrying EGFR mutations. To the best of our knowledge, this is the first study evaluating the effect of MSR on OS.
Notably, MSR provided a longer OS than SSR. Radiotherapy exerts direct cytotoxic effects on tumor cells, providing effective local control and improving patient survival. In a multicenter Phase II study of patients with metastatic NSCLC (≤ 3 metastases) that did not progress after systemic therapy, local consolidative therapy provided superior PFS than maintenance therapy alone.26 In another study of patients with stage IV NSCLC (≤ 3 metastases), local treatment of metastatic lesions prolonged OS.27 Drug resistance after first-line treatment with EGFR TKIs may arise early from drug-resistant clones. In addition to early dominant driver mutations and low background mutation rates, EGFR-mutant lung adenocarcinomas are heterogeneous, especially in Asian patients who have higher intra-tumor heterogeneity due to genomic instability.28 Therefore, some lesions may be controlled with just EGFR TKIs, while others continue to progress. Local treatment with radiotherapy can overcome tumor heterogeneity. MSR can lead to tumor shrinkage and apoptosis of the intractable tumor cells, thereby augmenting the antitumor effects of EGFR TKIs and prolonging patient survival.
Additionally, radiotherapy can activate antitumor immune responses. Preclinical studies have shown that radiation can suppress or stimulate the immune system via numerous pathways. Radiation enhances the ability of the immune system to recognize tumor cells by promoting the release of previously hidden tumor-associated antigens (TAAs) and immunostimulatory molecules, which can activate antitumor immune responses.29,30 Moreover, radiotherapy facilitates the recruitment of activated immune cells to the tumor by modulating the vascular endothelium of the tumor bed and promoting the release of chemokines.31–33 Therefore, we recommend that radiation therapy be administered safely to the tumor burden, with as little exposure as possible to other sites as is allowed under the conditions, rather than limiting radiotherapy to a single focus.
We next analyze the effect of radiotherapy to different sites or organs on OS. Through multivariate analysis, thoracic radiotherapy was independently found to be related to the improvement of OS in patients. Compared with bone and brain radiotherapy, thoracic radiotherapy has the greatest impact on the prognosis of patients. In a retrospective study reported by Tang et al in 2020, 105 patients with advanced lung cancer harboring EGFR mutations who were treated with EGFR TKIs were included for analysis of the failure mode. They found that more than one-third of patients progressed at the primary site.34 This means that increasing the local treatment to pulmonary lesions can prolong the PFS, thus improving the prognosis of patients. As just mentioned, related studies have shown that thoracic radiotherapy can strengthen the control of primary pulmonary lesions in patients with stage IV NSCLC treated with systemic therapy.25 But it should be noted that interstitial lung disease is a serious adverse effect of EGFR TKIs treatment, and pulmonary fibrosis has also been reported in previous studies.35 Therefore, simultaneous application of thoracic radiotherapy and EGFR TKIs may increase the incidence of pulmonary toxicity in patients.
The results of the current study showed that no matter when in the course of the disease radiotherapy is administered, the survival of patients is still improved compared with no chest radiotherapy being administered. This suggests that thoracic radiotherapy plays an important role in the treatment of these patients. According to the results of our study, it is recommended that clinicians add thoracic radiotherapy at the appropriate time based on the actual clinical picture of the patients, but not necessarily concurrently combined with EGFR TKI therapy.
Due to the strong effect of thoracic radiotherapy on OS and the presence of some bias, brain and bone radiotherapy were not found to improve OS in this cohort. A retrospective study have shown that EGFR TKI therapy plus brain radiotherapy could not improve survival in patients with NSCLC and brain metastases.36 The optimal timing of radiotherapy and EGFR TKI treatment has also been explored. Miyawaki et al reported that, in patients with EGFR mutations and 1–4 brain metastases, upfront local therapy combined with EGFR TKIs therapy was more effective than upfront EGFR TKIs alone.37 Because the patients included in this study were also administered radiotherapy to other organs and parts, especially the thoracic area, the impact of thoracic radiotherapy on OS may mask the effects of brain radiotherapy on OS. Regarding bone metastasis radiotherapy, palliative radiotherapy has been shown to improve the quality of life but not OS.38
There are some limitations to this study. First, this retrospective study was conducted in a single center. Specifically, excluding patients with missing covariates could lead to selection bias. Second, we could not evaluate the effect of the timing of MSR (simultaneous or sequential MSR) on patient survival because of the small number of patients in the MSR subgroup. Whether adding thoracic radiotherapy to the therapy plan as soon as possible is more beneficial to improving patient survival has not been studied. Third, the potential toxicity associated with local treatment has not been taken into account in this study.
Taken together, we demonstrated that the use of a comprehensive radiotherapy pattern targeted to multiple sites during the disease course improved survival outcomes in patients with advanced-stage, EGFR-mutant lung adenocarcinoma. Thoracic radiotherapy played the most important role in improving patient prognosis.
Funding Statement
This study was supported jointly by the national natural science foundation of China (Grant No. 81602031), the National Key Research Program of China (No. 2016YFC0904700), Shandong natural science foundation (Grant No. ZR2016HB12) and the national natural science foundation of China (Grant No. 81703038).
Abbreviations
AJCC, American Joint Commission on Cancer; CI, confidence interval; CT, computed tomography; EGFR, epidermal growth factor receptor; HR, hazard ratio; iPFS, intracranial progression-free survival; MRI, magnetic resonance imaging; MSR, multiple-site radiotherapy; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; SSR, single-site radiotherapy; TAA, tumor-associated antigen; TKI, tyrosine kinase inhibitor.
Author Contributions
Jianbin Li and Zhenxiang Li organized the framework and supervised the work. Xuedong Xu and Hui Zhang collected the clinical information. Yankang Li used to have a follow-up. Wei Wang performed the statistical analysis. Yingyun Zhang wrote the paper draft. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.D’Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v116–119. doi: 10.1093/annonc/mdq189 [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314 [DOI] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101(36):13306–13311. doi: 10.1073/pnas.0405220101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124–136. doi: 10.1200/JCO.19.01154 [DOI] [PubMed] [Google Scholar]
- 7.Kelly RJ, Shepherd FA, Krivoshik A, et al. A Phase III, randomized, open-label study of ASP8273 versus erlotinib or gefitinib in patients with advanced stage IIIB/IV non-small-cell lung cancer. Ann Oncol. 2019;30(7):1127–1133. doi: 10.1093/annonc/mdz128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichihara E, Hotta K, Nogami N, et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol. 2015;10(3):486–491. doi: 10.1097/JTO.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi: 10.1016/S1470-2045(11)70184-X [DOI] [PubMed] [Google Scholar]
- 10.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi: 10.1200/JCO.2011.36.8456 [DOI] [PubMed] [Google Scholar]
- 11.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi: 10.1056/NEJMoa0810699 [DOI] [PubMed] [Google Scholar]
- 12.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi: 10.1016/S1470-2045(09)70364-X [DOI] [PubMed] [Google Scholar]
- 14.Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol. 2013;31(31):3987–3996. doi: 10.1200/JCO.2012.45.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosomi Y, Morita S, Sugawara S, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 Study. J Clin Oncol. 2020;38(2):115–123. doi: 10.1200/JCO.19.01488 [DOI] [PubMed] [Google Scholar]
- 17.Sugawara S, Oizumi S, Minato K, et al. Randomized phase II study of concurrent versus sequential alternating gefitinib and chemotherapy in previously untreated non-small cell lung cancer with sensitive EGFR mutations: NEJ005/TCOG0902. Ann Oncol. 2015;26(5):888–894. doi: 10.1093/annonc/mdv063 [DOI] [PubMed] [Google Scholar]
- 18.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58(3):862–870. doi: 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 19.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2 Pt 1):728–734. [PubMed] [Google Scholar]
- 20.Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388. doi: 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verbrugge I, Hagekyriakou J, Sharp LL, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72(13):3163–3174. doi: 10.1158/0008-5472.CAN-12-0210 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Wang Y, Zhao L, et al. EGFR tyrosine kinase inhibitor HS-10182 increases radiation sensitivity in non-small cell lung cancers with EGFR T790M mutation. Cancer Biol Med. 2018;15(1):39–51. doi: 10.20892/j.issn.2095-3941.2017.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36(22):2244–2250. doi: 10.1200/JCO.2018.78.7994 [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka H, Shimokawa M, Seto T, et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann Oncol. 2019;30(12):1978–1984. doi: 10.1093/annonc/mdz399 [DOI] [PubMed] [Google Scholar]
- 25.Zheng L, Wang Y, Xu Z, et al. Concurrent EGFR-TKI and thoracic radiotherapy as first-line treatment for stage IV non-small cell lung cancer harboring EGFR active mutations. Oncologist. 2019;24(8):1031–1031e612. doi: 10.1634/theoncologist.2019-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, Phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. doi: 10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell KG, Farooqi A, Ludmir EB, et al. Improved overall survival with comprehensive local consolidative therapy in synchronous oligometastatic non-small-cell lung cancer. Clin Lung Cancer. 2020;21(1):37–46.e7. doi: 10.1016/j.cllc.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 28.Nahar R, Zhai W, Zhang T, et al. Elucidating the genomic architecture of Asian EGFR-mutant lung adenocarcinoma through multi-region exome sequencing. Nat Commun. 2018;9(1):216. doi: 10.1038/s41467-017-02584-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325–1332. doi: 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 30.Grass GD, Krishna N, Kim S. The immune mechanisms of abscopal effect in radiation therapy. Curr Probl Cancer. 2016;40(1):10–24. doi: 10.1016/j.currproblcancer.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 31.Hellevik T, Martinez-Zubiaurre I. Radiotherapy and the tumor stroma: the importance of dose and fractionation. Front Oncol. 2014;4:1. doi: 10.3389/fonc.2014.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62(5):1462–1470. [PubMed] [Google Scholar]
- 33.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181(5):3099–3107. doi: 10.4049/jimmunol.181.5.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang Y, Xia B, Xie R, et al. Timing in combination with radiotherapy and patterns of disease progression in non-small cell lung cancer treated with EGFR-TKI. Lung Cancer. 2020;140:65–70. doi: 10.1016/j.lungcan.2019.12.009 [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Li A, Lai X, et al. Identification of differentially expressed genes and signaling pathways using bioinformatics in interstitial lung disease due to tyrosine kinase inhibitors targeting the epidermal growth factor receptor. Invest New Drugs. 2019;37(2):384–400. doi: 10.1007/s10637-018-0664-z [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Chen YF, Chen CY, et al. Clinical factors associated with treatment outcomes in EGFR mutant non-small cell lung cancer patients with brain metastases: a case-control observational study. BMC Cancer. 2019;19(1):1006. doi: 10.1186/s12885-019-6140-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyawaki E, Kenmotsu H, Mori K, et al. Optimal sequence of local and EGFR-TKI therapy for EGFR-mutant non-small cell lung cancer with brain metastases stratified by number of brain metastases. Int J Radiat Oncol Biol Phys. 2019;104(3):604–613. doi: 10.1016/j.ijrobp.2019.02.051 [DOI] [PubMed] [Google Scholar]
- 38.Jazieh AM, AlSumai TS, Ali YZ, et al. The pattern of bone involvement, management, and outcomes in patients with nonsmall cell lung cancer: a retrospective study. Ann Thorac Med. 2018;13(3):150–155. doi: 10.4103/atm.ATM_385_17 [DOI] [PMC free article] [PubMed] [Google Scholar]