Abstract
Background:
People who inject drugs (PWID) are at high risk for skin and soft tissue infections (SSTIs), but few interventions have targeted their reduction. The goal of the current study was to test the effects of a brief skin and needle hygiene behavioral intervention (SKIN) in a two-group randomized controlled trial with 12-month follow-up.
Method:
PWID (N = 252) were recruited from inpatient hospital units at a single urban medical center site and randomly assigned to an assessment-only (AO) condition or SKIN, which was a two-session intervention that included psychoeducation, behavioral skills demonstrations, and motivational interviewing. Mixed effects generalized linear models assessed the impact of the intervention on frequency of: 1) self-reported SSTIs, 2) uncleaned skin injections, and 3) injection.
Results:
Participants were 58.3% male, 59.5% White, and averaged 38 years of age. SKIN participants had 35% fewer SSTIs compared to AO (p=.179), a difference of nearly one infection per year. The mean rate of uncleaned skin injections was about 66% lower (IRR=0.34, 95% CI 0.20; 0.59, p<.001) among SKIN participants compared to AO. Almost one-third of participants reported no injection over follow-up and the mean rate of injection during follow-up was about 39% lower (IRR=0.61; 95% CI 0.36; 1.02, p=.058) among persons randomized to SKIN than AO.
Conclusions:
The SKIN intervention reduced uncleaned skin injections but did not reduce SSTIs significantly more than a control condition. Brief interventions can improve high-risk practices among PWID and lead to clinically meaningful outcomes.
Keywords: skin infections, abscesses, bacterial infections, people who inject drugs, injection drug use, heroin
1. Introduction
People who inject drugs (PWID) are at risk for a number of injection-related health conditions, such as overdose, viral disease (HIV, HCV), and bacterial infections (e.g., skin and soft tissue infection [SSTI], infective endocarditis) (Gordon and Lowy 2005). Of these, SSTIs (e.g., abscesses) are the most common and can lead to significant morbidity and mortality (Saldana, Vyas, and Wurcel 2020). If untreated, SSTIs can produce more widespread (e.g., cellulitis) or debilitating skin conditions (e.g., necrotizing fasciitis) and life-threatening infections (e.g., sepsis, endocarditis) that may require costly emergency department (ED) and inpatient hospitalization (Marks et al. 2013; Palepu et al. 2001). Though rates vary, past studies have reported high rates of SSTIs in PWID, with up to three-fourths reporting lifetime infections and one-third to one-half experiencing a recent infection (Binswanger et al. 2000; Dahlman et al. 2015; Phillips and Stein 2010; Larney et al. 2017).
Demographic and high-risk injection and drug use practices contribute to SSTIs. Women appear more susceptible to SSTIs compared to men, likely due to problems with venous access and relying on others for injection assistance (Lloyd-Smith et al. 2008; Hope et al. 2008; Smith et al. 2015). Individuals with unstable housing, possibly due to an inability to maintain hygiene practices while living on the street, are also at greater risk (Lloyd-Smith et al. 2008; Rhodes et al. 2006). Racial and ethnic disparities influence injection risk and treatment utilization for SSTIs, though few studies have examined racial/ethnic differences in SSTI risk (Cooper, Linton, et al. 2016; Cooper, West, et al. 2016; Fink et al. 2013). Variations in drug supply and use of certain types of drugs, such as black tar heroin, crushed/liquified prescription drugs, desomorphine, and stimulants, have been shown to portend risk (Lloyd-Smith et al. 2008; Hearne et al. 2016; Hope et al. 2008; Dwyer et al. 2009; Summers et al. 2017). Injection frequency and practices are also associated with SSTIs, including booting or jacking (repeatedly drawing blood/syringe contents in/out of vein), reusing dull needles repeatedly or sharing syringes with others, and injecting subcutaneously (under the skin), intramuscularly, or missing the vein (Binswanger et al. 2000; McElrath 2006; Murphy et al. 2001; Gordon and Lowy 2005; Doran et al. 2020; Hope et al. 2016; Moradi-Joo et al. 2019). These latter methods are often a last resort for PWID who have injected for long periods and begin to lose venous access (Saldana, Vyas, and Wurcel 2020). Hygiene practices, such as failing to wash hands and clean skin with alcohol or other anti-bacterials prior to injecting, also contribute significantly to SSTI risk (Dwyer et al. 2009; Larney et al. 2017). PWID may introduce bacteria from uncleaned skin into the vein or subcutaneous space when a needle pierces the skin (Raff and Kroshinsky 2016; Tuazon, Hill, and Sheagren 1974). Lack of skin cleaning at the injection site is associated with SSTIs (Fink et al. 2013; Murphy et al. 2001; Smith et al. 2015; Vlahov et al. 1992) and many PWID report not skin cleaning (Varga, Chitwood, and Fernandez 2006).
Evidence-based interventions for PWID, such as medication-based treatment for opioid use disorder (MOUD; e.g., methadone, buprenorphine, extended-release naltrexone), naloxone provision/training, supervised injection facilities, and needle exchange, have been shown to reduce illicit drug use, overdose, and HIV/HCV transmission respectively (Reddon, Marshall, and Milloy 2019; Fernandes et al. 2017; National Academies of Sciences and Medicine 2019); however, less work has focused on potentially modifiable behavioral factors (e.g., safer needle use) to reduce bacterial infections. To our knowledge, only two studies (Phillips et al. 2012; Stein et al. 2020; Roux et al. 2016; Mezaache et al. 2018) have targeted SSTIs and other bacterial infections as outcomes. Two recent papers reported on findings from a non-randomized cluster trial that tested a community-focused, educational face-to-face intervention for PWID in France (Mezaache et al. 2018; Roux et al. 2016). The intervention included one or more sessions focused on staff observation and training of injection practice, as well as information on safer injection and injection-related complications (HIV, HCV, bacterial infections). Roux et al. (2016) found that intervention participants decreased unsafe HIV–HCV practices (from 44% to 25%) at a 6-month follow-up and had fewer injection-related complications (from 66% to 39%) at a 12-month follow-up, while control participant behaviors remained stable. Mezaache and colleagues (2018) found that participants who attended at least one intervention session significantly improved from pre- to post-intervention on 8 of 17 measured injection-related outcomes, including hand washing (AOR=7.16) and skin cleaning (AOR=5.56).
Using a stage-based behavior therapy development approach recommended by NIDA, we designed and piloted a skin and needle hygiene intervention (SKIN) as part of a Stage 1 trial among a sample of PWID recruited through street outreach in Denver (Phillips et al. 2013; Phillips et al. 2012). This small randomized controlled trial (RCT) showed effects in the small to large range for improved skin and needle cleaning (ds=.53–1.00), reduced bacterial infection risk (d=.32), fewer days of injection (d=.12), and a lower incidence of bacterial infections (HR=.80) up to 6 months post-intervention (Phillips et al. 2012). In our Boston-based expansion of the SKIN intervention to a population of 252 hospitalized PWID, we demonstrated that SKIN reduced likelihood of ED visits over the next year for injection-related infections (as assessed with electronic medical record [EMR] data) following hospitalization compared to an assessment-only (AO) control condition (Stein et al. 2020).
The current analysis examined the impact of the SKIN intervention on our primary outcome – SSTIs – over the year following hospitalization. In addition, we examined the effect of SKIN on secondary outcomes, including skin cleaning practices and injection frequency. Because many SSTIs are self-treated (Monteiro et al. 2020) and are not associated with medical system encounters, this paper focuses on self-report data of our outcomes. We hypothesized that participants assigned to SKIN would report fewer SSTIs compared to those assigned to AO. This hypothesis was centered on the belief that the SKIN intervention would reduce two of the most pertinent risk practices associated with SSTIs – number of injections and uncleaned skin injections.
2. Methods
2.1. Study Design
This study was an RCT of the SKIN intervention (n=128) compared to an AO control group (n=124) at a single site with follow-ups to 12 months.
2.2. Participants and Recruitment
PWID were recruited from inpatient medical units at Boston Medical Center (BMC), an academic safety-net hospital, between January 2014 – August 2018. Research staff reviewed medical records daily for any indication of injection drug use (IDU) or SSTI diagnosis and screened for eligibility. Though not part of eligibility, 61% of participants had an injection- related diagnosis (including events coded as SSTI, endocarditis/sepsis, or deep tissue bacterial infection) at baseline.
Patients were eligible to participate if they: 1) were age 18 or older, 2) injected drugs at least three days in the week prior to hospitalization, 3) could speak English, 4) were not actively psychotic or homicidal/suicidal, 5) could provide informed consent, 6) were able to provide names and contact information for at least two locator persons, and 7) did not plan to move out of the Boston area in the next year. The Boston University Medical Center Institutional Review Board approved all study procedures.
2.3. Procedures
After informed consent, eligible persons completed a 90-minute baseline assessment in their hospital room and then were randomly assigned using permuted-block randomization with randomly ordered blocks of 8, 12 and 16 to either the SKIN intervention or AO (standard care), which were created by the study statistician (BJA). Sealed envelopes designating group assignment were prepared in advance. Following the baseline assessment, a research assistant (RA) selected an envelope noting the assigned group. Participants assigned to the SKIN intervention then participated in the first 60-minute intervention session.
Follow-up appointments took place at 1-week and 1-, 3-, 6-, 9-, and 12-months post-baseline. Compensation at baseline included either a $25 gift card, with increasing amounts at each additional assessment, or an equivalent pre-paid cell phone with service to use for the entire study (both compensation options valued at $415). As part of protocol and in addition to their medical care, all patients actively using illicit substances were provided with a brochure listing substance use treatment and needle exchange facilities in the city of Boston. To improve retention, RAs conducted regular appointment reminders (via phone, email, letter, or contact with participant’s locator contacts) and check-ins with participants. If unsuccessful in reaching participants, RAs contacted correctional institutions, shelters, and treatment facilities in the area (all with participant permission) and checked online inmate databases and obituaries. A jail retention plan allowed staff to complete follow-ups during participant incarceration.
2.4. Skin and Needle Hygiene (SKIN) Intervention
2.4.1. Overview
The SKIN intervention, developed initially following focus group work with PWID (Phillips et al. 2013; Phillips et al. 2012), included two individually delivered in-person sessions. Session 1 took approximately 60 minutes and Session 2 (conducted after 1-month follow-up assessment) about 30 minutes. SKIN is a manualized intervention and includes a workbook for participant use. The intervention is based on the Information-Motivation-Behavioral Skills (IMB) Model (Fisher and Fisher 1992), a well-established theoretical framework that has been utilized successfully in HIV prevention work (Li et al. 2020; Avants et al. 2004). The model postulates that risk behavior results from a lack of information about a particular disease state or ways to prevent it, low motivation to change risk behavior, and a lack of behavioral skills to engage in risk reduction. These three domains make up key portions of the SKIN intervention. To address low motivation, MI was utilized throughout both sessions. As an approach, MI is a non-confrontational client-centered style that guides participants to explore their ambivalence about changing a problematic behavior (Miller and Rollnick 2012).
Session 1 began with a review of goals and building rapport. Initial psychoeducation about SSTIs, other bacterial infections, and HIV/HCV, including a description of each infection and select images, was provided. Training included identification of SSTIs and the importance of seeking treatment if an infection worsens (e.g., signs of sepsis). Participants were provided with information about high-risk practices that can lead to infection, with emphasis on reducing the frequency with which one injects and methods for safer injection (e.g., using new equipment, not sharing, reducing subcutaneous/intramuscular injection) and hygiene (e.g., disinfecting skin before every injection, using clean water to inject). Participants were then guided through behavioral skills demonstrations on hand washing, skin cleaning at the injection site, and needle cleaning. Hand washing included discussion of best practices both when a sink (soap/water) is and is not available. In the latter context, participants were instructed on and shown how to use alcohol-based hand sanitizer. The skin cleaning protocol included use of alcohol wipes at the injection site in a circular manner (King County, 2002). Needle cleaning (Royer et al. 2004) focused on a three-step process of rinsing a used syringe with water, bleach, and water. Following each demonstration, participants were asked to complete the steps associated with each task.
A personalized risk assessment was performed to identify key high-risk practices that put the participant at risk for bacterial or viral infections. Working with interventionists, the majority of participants selected several harm reduction or drug use/abstinence goals consistent with their readiness to change. A change plan worksheet was completed and listed steps to reach each goal. At the end of the session, participants were provided with a copy of the change plan and client workbook, a starter risk reduction kit (e.g., including alcohol pads, bleach, etc.), and brochures with step-by-step instructions for bleach-cleaning needles, washing hands, and cleaning skin.
Session 2 was a booster session to assess progress toward goals set in Session 1. Interventionists asked participants about any risk-related events (e.g., sharing equipment episodes) that occurred since the last session. The participant’s initial change plan was reviewed to assess progress towards goals and any barriers that interfered with goal attainment. When needed, the interventionist assisted the participant in setting new goals and plans for achieving these goals and provided refresher information about risk reduction or hygiene skills.
2.4.2. Interventionist Training, Supervision, and Fidelity
Five BA/MA-level study interventionists were trained and administered the SKIN intervention. All SKIN sessions were audio recorded with participant permission and adherence and fidelity were monitored by the first author (KP). Of persons assigned to the intervention condition (n=128), 66 completed two sessions, 55 completed one session, and seven completed zero sessions.
2.5. Measures
2.5.1. Outcomes
The primary outcome of the current study was frequency (number) of SSTIs. At baseline, participants were asked to report how many times in the past year they had SSTIs, which were defined as “abscesses (red, hardish infected lumps that contain pockets of pus), ulcers (open infected sores), and cellulitis (more widespread skin infection) that occur at the injection site.” At follow-ups, and using the same definition, participants were asked about any SSTIs since their last assessment.
Secondary outcomes included the frequency of injections and uncleaned skin injections, assessed over the last three months at baseline and since the last assessment at follow-ups. Frequency of injection included the total number of injections (days*number of times injected/day), while injection without skin cleaning was calculated based on the total number of times injected minus the number of times the participant reported cleaning their skin prior to injection. We also assessed the relative frequency of injection without skin cleaning, which was a proportion based on the number of times participants cleaned their skin divided by the total number of injections.
2.5.2. Demographics, background characteristics, and covariates
To better describe the sample and control for select variables, we assessed the following variables at baseline: age, sex, race/ethnicity, years of education, and nights spent homeless (i.e., in a shelter or on the street) over the last 3 months. Participants also reported the primary drug they injected (opiates, cocaine, methamphetamine).
2.6. Sample Size
The current study was powered for two outcomes – SSTI incidence and ED visits, assessed via self-report and through EMR data. The current analyses were powered to detect differences in 12-month SSTI infection rates of approximately 15%, with a required sample size of 280. With α/2 and 1 - β = .8, the current study did not achieve this goal.
2.7. Analytical Methods
Descriptive statistics were used to summarize sample characteristics. Outcomes were analyzed using mixed effects generalized linear models (GLMs). Planned covariates included age, sex (1 if male), race (1 if White), ethnicity (1 if Latinx), number of homeless nights in the 3-months prior to baseline, and indicator variables for month of follow-up intent-to-treat assessment. Models also included the time invariant baseline instance of the specific outcome being analyzed. We used the robust Huber-White variance estimator for all tests of significance and to estimate 95% confidence intervals. All tests of significance were based on 2-tailed alpha < .05. We report exponentiated coefficients and 95% confidence estimates.
The primary outcome, number of self-reported SSTIs, was analyzed with a log link function and negative-binomial family error distribution. The number of days between assessments was entered as an offset to control for time at risk. Methods to impute missing observations generally assume data are missing completely at random or at random. Here, loss to follow-up may be systematically associated with the outcomes being assessed. To determine if results were sensitive to subject attrition, we estimated models in which observations lost to follow-up were imputed under a range of scenarios; for the primary outcome sensitivity testing, we also analyzed models in which missing observations were assumed to have 0, 1, 2, 3, 4, or 5 SSTIs.
Number of uncleaned skin injections and total number of injections were also analyzed with a log link and negative binomial error distribution. The relative frequency of skin cleaning (proportion of total number of injections on which participants reported skin cleaning) was analyzed with a mixed-effects GLM with logit link and binomial family error distribution. Relative frequency of skin cleaning is not defined at assessments in which participants reported no drug injection. Exponentiated coefficients are reported.
3. Results
3.1. Sample Enrollment and Characteristics
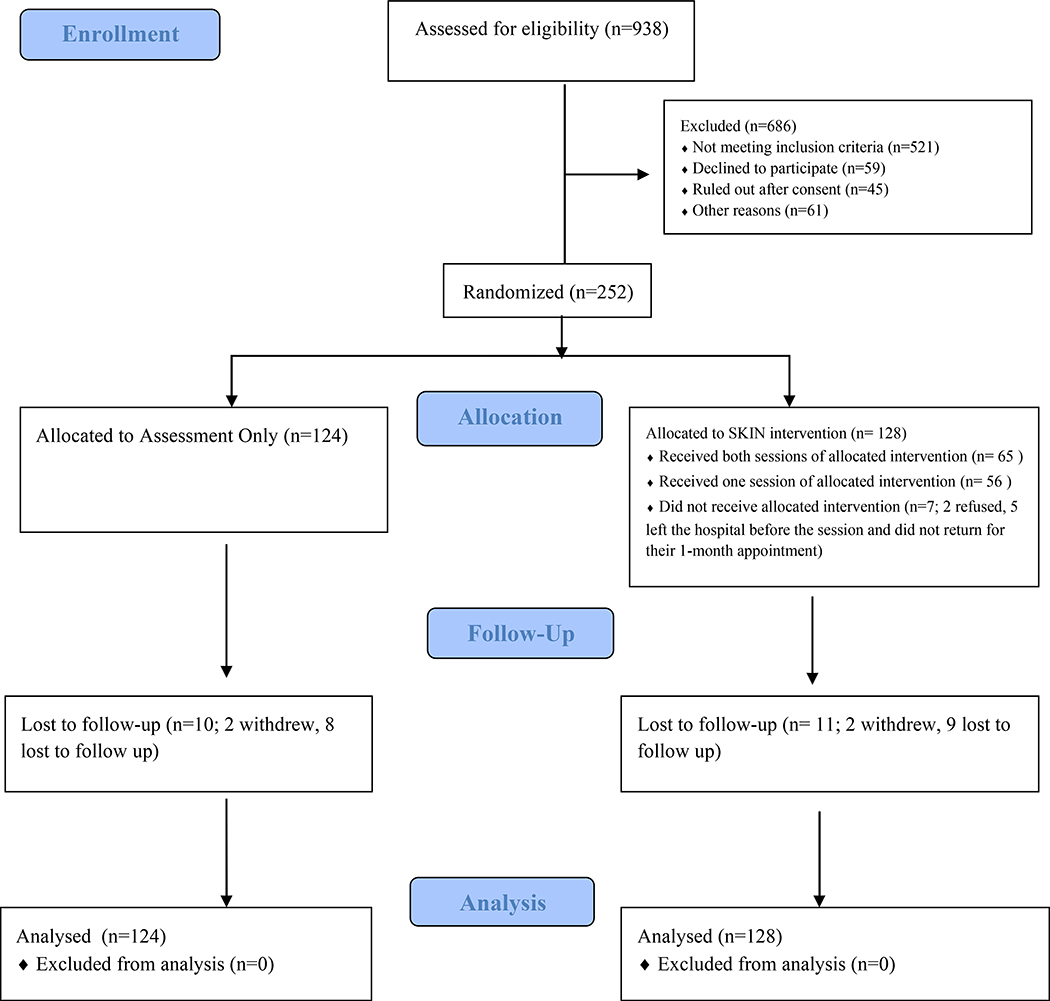
Figure 1 outlines study recruitment, enrollment, and follow-ups using the CONSORT diagram. Out of 938 screened patients, 686 were excluded, most due to not meeting eligibility criteria (e.g., did not inject drugs). The remaining 252 participants (n=128 SKIN; n=124 AO) agreed to participate, provided informed consent, and completed the baseline assessment.
Figure 1. CONSORT Flow Diagram.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram
Participants averaged 37.9 (± 10.7) years of age and were 58.3% male, 59.5% White, 20.6% Black, 15.9% Latinx, with 11.6 (± 2.4) mean years of education. Most (90.1%) reported opiates as their primary injection drug. On average at baseline, participants injected on 60.4 (± 25.8) of the past 90 days, with an average of 5.6 (± 5.2) times per day. Participants reported a mean of 5.35 (± 20.62) lifetime SSTIs and 1.58 (± 2.35) SSTIs during the year prior to baseline. At baseline participants reported cleaning their skin on about 23.8% (± 34.1) of the total times they injected. Additional demographic and follow-up rates for both treatment arms can be found in Table 1.
Table 1.
Baseline Characteristics by Intervention Arm. Cell entries are mean (± SD) or n (%).
| INTERVENTION ARM |
|||
|---|---|---|---|
| Sample (n = 252) | Control (n = 124) | SKIN (n = 128) | |
| Age | 37.9 (± 10.7) | 37.2 (± 10.6) | 38.6 (± 10.8) |
| Sex (Male) | 147 (58.3%) | 67 (54.0%) | 80 (62.5%) |
| Race (White) | 150 (59.5%) | 71 (57.3%) | 79 (61.7%) |
| Latinx (Yes) | 40 (15.9%) | 20 (16.1%) | 20 (15.6%) |
| Yrs. Educationa | 11.6 (± 2.4) | 11.9 (± 2.3) | 11.2 (± 2.5) |
| # Nights Homeless (90-Days) | 18.6 (± 36.9) | 20.8 (± 37.2) | 16.5 (± 36.7) |
| Primary IDU Drug (Opiates) | 227 (90.1) | 110 (88.7%) | 17 (91.4%) |
| Days IDU (90-Days) | 60.4 (± 25.8) | 60.4 (± 27.0) | 60.5 (± 24.6) |
| # IDU / Day | 5.6 (± 5.2) | 5.3 (± 4.4) | 5.9 (± 5.9) |
| Total # IDU (90-Days) | 357.6 (± 414.0) | 325.2 (± 309.2) | 388.9 (± 494.1) |
| # SSTIs (Lifetime) | 5.35 (± 20.62) | 4.56 (± 10.32) | 6.12 (± 27.13) |
| # SSTIs (Past Yr) | 1.58 (± 2.35) | 1.55 (± 2.60) | 1.58 (± 2.09) |
| % Total IDU Cleaned Skin | 23.8 (± 34.1) | 23.8 (± 35.2) | 23.7 (± 33.1) |
| Follow-Up Rates | |||
| @ Month 1 (Yes) | 142 (56.3%) | 65 (52.4%) | 77 (60.2%) |
| @ Month 3 (Yes) | 133 (52.8%) | 63 (50.8%) | 70 (54.7%) |
| @ Month 6 (Yes) | 117 (46.4%) | 55 (44.4%) | 62 (48.4%) |
| @ Month 9 (Yes) | 128 (50.8%) | 62 (50.0%) | 66 (51.6%) |
| @ Month 12 (Yes) | 150 (59.5%) | 77 (62.1%) | 73 (57.0%) |
| Any Assessment (Yes) | 213 (84.5%) | 105 (84.7%) | 108 (84.4%) |
| # Times Assessed | 2.7 (± 1.8) | 2.60 (± 1.8) | 2.7 (± 1.9) |
3.2. Outcomes
3.2.1. Number of SSTIs
We estimated the effect of intervention on number of self-reported SSTIs (Table 2). Participants randomized to the intervention arm reported about 35% (IRR = 0.65) fewer infections than those randomized to control, although this was not statistically significant (p = .179). The estimated average marginal mean 12-month infection rates, evaluated at the median (1) value of reported baseline SSTI, were 2.79 in the control arm and 1.82 in the active intervention arm; a difference of nearly one infection per year.
Table 2.
Mixed Effects Generalized Linear Models Estimating the Adjusted Effect of Intervention on Number of Self-Reported SSTIs, Total Number of Injections, Number of Uncleaned Skin Injections, and The Relative Frequency of Injections without Skin Cleaning.a
| Model Outcome | IRR/exp(b)b | 95% CIc | z (p =)c |
|---|---|---|---|
| # Self Reported SSTIsd | 0.65 | (0.35; 1.22) | −1.34 (.179) |
| Total # of Injectionsd | 0.61 | (0.36; 1.02) | −1.90 (.058) |
| # of Uncleaned Skin Injectionsd | 0.34 | (0.20; 0.60) | −3.78 (<.001) |
| Relative Frequency of Skin Cleaning when IDUe | 1.82 | (0.77; 4.39) | 1.36 (.173) |
Covariates included in all models were indicator variables for month of assessment, number of infections reported in the year prior to baseline, years age, gender, race (White), ethnicity Latinx) and # of nights spent on the street or in a shelter during 3-months prior to baseline.
IRR reported for # self-reported SSTIs, total # of injections, # of uncleaned skin injections; exp(b) reported for relative frequency of skin cleaning when IDU
Confidence interval estimates and tests of significance were based on the robust Huber-White variance estimator.
Model specified with log link function and negative binomial error distribution. To adjust for time at risk the models also included log(number of days at risk during each assessment) as an offset with coefficient fixed to 1. The n was 213 persons observed on 669 occasions.
Operationally defined as ((times cleaned skin / total injections) * 100). Model specified a logit link function with binomial family error distribution. The n was 182 persons observed on 460 occasions.).
To determine if our results were sensitive to subject attrition, we estimated models in which all missing follow-up observations were assumed to have 0 to 5 SSTIs in that period. Under the assumption that all participants lost to attrition had 0 infections the estimated rate of skin infection was about 39% (IRR = 0.61, 95% CI 0.32; 1.17, p = .139) lower in the intervention arm. Estimated incidence rate ratios were between 0.79 – .82 (all nonsignificant) when observations lost to follow-up were assumed to have 1, 2, 3, 4, and 5 SSTIs during the reporting period.
3.2.2. Number of injections
Overall, we found that large numbers of participants reported no injection during follow-up. Specifically, 28.9%, 20.3%, 33.3%, 32.8%, and 39.6% of the participants reported no injection at the 1-, 3-, 6-, 9-, and 12-month assessments, respectively. Using mixed-effects negative binomial regression (Table 2) the adjusted mean rate of injection during follow-up was about 39% lower (IRR = 0.61; 95% CI 0.36; 1.02, p = .058) among persons randomized to SKIN than for persons randomized to AO. The estimated average marginal 12-month total injection rates, evaluated at the median value of total injection reported at baseline were 743.2 and 483.8 in the control and active intervention arms, respectively.
3.2.3. Number of uncleaned skin injections
The mean rate of uncleaned skin injections (i.e., number of times injected minus the number of times the participant reported cleaning their skin prior to injection) was about 66% lower (IRR = 0.34, 95% CI 0.20; 0.59, p < .001) among persons randomized to active intervention than for persons randomized to AO (Table 2). The estimated average marginal 12-month rates of uncleaned skin injections were about 625 in the control arm and 212 in the active intervention arm when evaluated at the median value of uncleaned skin injections observed at baseline.
3.2.4. Relative frequency of injections without cleaning skin
We also estimated a mixed effect GLM with logit link and binomial error distribution to estimate the adjusted effect of intervention on the relative frequency of injection without skin cleaning (i.e., number of times participants cleaned their skin divided by the total number of injections). This analysis necessarily excludes observations in which no injection was reported during the assessment period; this limited the analysis to 182 persons and 460 observation periods (Table 2). Covariates in the model were indicator variables for assessment month, age, sex, race, ethnicity, days homeless, and the proportion of total baseline injections without skin cleaning. For each injection during follow-up, participants randomized to active intervention were about 1.82 times (95% CI 0.77; 4.39, p = .173) times more likely to report they had cleaned their skin.
4. Discussion
We found that SKIN participants experienced 35% fewer SSTIs over the year following a medical hospitalization compared to a control condition -- approximately one less infection in 12 months -- but this group difference was not statistically significant. Still, we believe this finding is clinically meaningful. SSTIs can result in significant pain and life-threatening infections and any reduction in incidence is important. Analyses of secondary outcomes found that SKIN participants reduced the number of uncleaned skin injections and total injections over follow-up (the latter of which approached statistical significance). Our findings hint at a possible mechanism by which the SKIN intervention may be operating – SSTIs are reduced as a function of unclean skin injections, which is the product of decreases in overall injection and increases in skin cleaning. However, a fully-powered mediation model would need to test this explanation in a future study.
Participants assigned to the SKIN intervention reported significantly fewer uncleaned skin injections compared to those assigned to the assessment-only condition. A 66% reduction in uncleaned injections is notable and demonstrates that PWID successfully utilized this harm reduction strategy, which was emphasized extensively during the intervention. Encouraging PWID to use skin cleaning and teaching proper techniques is warranted. However, we understand that it isn’t practical to expect PWID to engage in skin cleaning 100% of the time. Those who inject frequently and use certain drugs (e.g., methamphetamine) are less likely to skin clean (Varga, Chitwood, and Fernandez 2006; Gibbs et al. 2020). A subset of participants in our study had unstable housing, which may affect access to supplies and overall adoption. Potential barriers should be considered when working with individual PWID on setting harm reduction goals.
Though not an a priori study outcome, we found that the adjusted mean rate of IDU during follow-up was 39% lower among SKIN participants compared to controls. It is possible that SKIN reduced overall injection as a result of discussion of potential harm reduction strategies during intervention sessions. However, over one-third of participants reported no injection at all at their 12-month follow-up, suggesting the possibility that injection may have ceased due to other interventions not assessed, such as use of MOUD following the index hospitalization. Similarly, participants may have stopped using drugs due to incarceration or through participating in residential treatment. Hospitalization itself may have contributed to increased motivation to change.
PWID are a vulnerable population with significant needs (e.g., unstable housing, co-morbid mental health issues). Past literature indicates that a complex combination of factors contributes to SSTI risk. In addition, larger sociopolitical factors can influence access to harm reduction services and drug treatment, which are known to be protective. Stigma towards PWID, both within the community and by medical professionals (Monteiro et al. 2020; Motavalli et al. 2020), influence decisions to seek regular medical care and treatment for SSTIs, which can worsen into more serious infections if untreated. Our study took place in Boston, where white powder heroin dominates, but access to harm reduction (e.g., needle exchange) and other treatment services, as well as state-funded healthcare access, are high. Risk of SSTIs and other injection-related conditions may differ depending on the risk environment or larger context in which IDU occurs, which likely impacts the success of any intervention (Rhodes 2009).
The current study had several limitations. First, this is a single-site trial and this difficult-to-track urban cohort had low follow-up rates despite repeated contact attempts and receipt of cell phones as compensation. In addition, we used a needle cleaning protocol with three rounds of multiple bleach and water rinses that may not be realistic for PWID living on the street. SSTIs were self-reported, though we have previously shown that SSTIs requiring ED visits, documented using EMR data, were also reduced by the SKIN intervention (Stein et al. 2020). Because annual SSTI incidence is low, power to detect changes over time may have been impacted. Compensation related to frequent follow-up assessments may also have contributed to behavior change among control participants, thus possibly diluting intervention effects.
Conclusions
Overall, findings from the current study show that a brief intervention can promote longer term change in skin hygiene among PWID, with successful implementation in a hospital setting when many PWID may be considering health changes. The intervention also showed promise in its ability to reduce SSTIs and injection, consistent with our recent work (Stein et al. 2020) demonstrating that the SKIN intervention reduced the likelihood of ED visits for bacterial infections over the 12-month follow-up. Future studies may consider testing the effectiveness of SKIN in less controlled settings (e.g., needle exchange or drop-in clinics). It will be important to examine the cost-effectiveness of the intervention over time, though we believe that avoiding SSTIs are important to the health of PWID regardless of potential cost savings.
Supplementary Material
Highlights.
We tested a brief intervention (SKIN) for hospitalized people who inject drugs.
A two-group RCT (SKIN vs assessment-only) was conducted with 12-months of follow-up.
SKIN participants reduced the number of uncleaned skin injections.
SKIN participants had fewer skin infections, but differences were not significant.
Acknowledgements
Role of Funding Source
National Institutes of Health (R01DA034957)
This study was funded by the National Institutes of Health (R01DA034957). Trial registered at clinicaltrials.gov; Clinical Trial #01892358.
Conflict of Interest
Dr. Stein received $7,000 from Alkermes, Inc to review grants for the Young Investigator Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avants SK, Margolin A, Usubiaga MH, and Doebrick C 2004. ‘Targeting HIV-related outcomes with intravenous drug users maintained on methadone: a randomized clinical trial of a harm reduction group therapy’, J Subst Abuse Treat, 26: 67–78. [DOI] [PubMed] [Google Scholar]
- Binswanger IA, Kral AH, Bluthenthal RN, Rybold DJ, and Edlin BR 2000. ‘High prevalence of abscesses and cellulitis among community-recruited injection drug users in San Francisco’, Clin Infect Dis, 30: 579–81. [DOI] [PubMed] [Google Scholar]
- Cooper HL, Linton S, Kelley ME, Ross Z, Wolfe ME, Chen YT, Zlotorzynska M, Hunter-Jones J, Friedman SR, Des Jarlais D, Semaan S, Tempalski B, DiNenno E, Broz D, Wejnert C, and Paz-Bailey G 2016. ‘Racialized risk environments in a large sample of people who inject drugs in the United States’, Int J Drug Policy, 27: 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HL, West B, Linton S, Hunter-Jones J, Zlotorzynska M, Stall R, Wolfe ME, Williams L, Hall HI, Cleland C, Tempalski B, and Friedman SR 2016. ‘Contextual Predictors of Injection Drug Use Among Black Adolescents and Adults in US Metropolitan Areas, 1993–2007’, Am J Public Health, 106: 517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- County Seattle & King. 2002. “All about abscesses.” In, edited by Public Health Department. [Google Scholar]
- Dahlman D, Håkansson A, Björkman P, Blomé MA, and Kral AH 2015. ‘Correlates of Skin and Soft Tissue Infections in Injection Drug Users in a Syringe-Exchange Program in Malmö, Sweden’, Subst Use Misuse, 50: 1529–35. [DOI] [PubMed] [Google Scholar]
- Doran J, Harris M, Hope VD, Wright T, Edmundson C, Sinka K, and Heinsbroek E 2020. ‘Factors associated with skin and soft tissue infections among people who inject drugs in the United Kingdom: A comparative examination of data from two surveys’, Drug Alcohol Depend, 213: 108080. [DOI] [PubMed] [Google Scholar]
- Dwyer R, Topp L, Maher L, Power R, Hellard M, Walsh N, Jauncey M, Conroy A, Lewis J, and Aitken C 2009. ‘Prevalences and correlates of non-viral injecting-related injuries and diseases in a convenience sample of Australian injecting drug users’, Drug Alcohol Depend, 100: 9–16. [DOI] [PubMed] [Google Scholar]
- Fernandes RM, Cary M, Duarte G, Jesus G, Alarcão J, Torre C, Costa S, Costa J, and Carneiro AV 2017. ‘Effectiveness of needle and syringe Programmes in people who inject drugs - An overview of systematic reviews’, BMC Public Health, 17: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink DS, Lindsay SP, Slymen DJ, Kral AH, and Bluthenthal RN 2013. ‘Abscess and self-treatment among injection drug users at four California syringe exchanges and their surrounding communities’, Subst Use Misuse, 48: 523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JD, and Fisher WA 1992. ‘Changing AIDS-risk behavior’, Psychol Bull, 111: 455–74. [DOI] [PubMed] [Google Scholar]
- Gibbs D, Peacock A, O’Keefe D, Butler K, Bruno R, Lenton S, Burns L, and Larney S 2020. ‘Use of alcohol swabs to clean injecting sites among people who regularly inject drugs in Australia’, Drug Alcohol Rev, 39: 83–92. [DOI] [PubMed] [Google Scholar]
- Gordon RJ, and Lowy FD 2005. ‘Bacterial infections in drug users’, N Engl J Med, 353: 1945–54. [DOI] [PubMed] [Google Scholar]
- Hearne E, Grund JP, Van Hout MC, and McVeigh J 2016. ‘A scoping review of home-produced heroin and amphetamine-type stimulant substitutes: implications for prevention, treatment, and policy’, Harm Reduct J, 13: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope VD, Parry JV, Ncube F, and Hickman M 2016. ‘Not in the vein: ‘missed hits’, subcutaneous and intramuscular injections and associated harms among people who inject psychoactive drugs in Bristol, United Kingdom’, Int J Drug Policy, 28: 83–90. [DOI] [PubMed] [Google Scholar]
- Hope V, Kimber J, Vickerman P, Hickman M, and Ncube F 2008. ‘Frequency, factors and costs associated with injection site infections: findings from a national multi-site survey of injecting drug users in England’, BMC Infect Dis, 8: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larney S, Peacock A, Mathers BM, Hickman M, and Degenhardt L 2017. ‘A systematic review of injecting-related injury and disease among people who inject drugs’, Drug Alcohol Depend, 171: 39–49. [DOI] [PubMed] [Google Scholar]
- Li DH, Moskowitz DA, Macapagal K, Saber R, and Mustanski B 2020. ‘Using Intervention Mapping to Developmentally Adapt an Online HIV Risk Reduction Program for Adolescent Men Who Have Sex with Men’, Prev Sci, 21: 885–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith E, Wood E, Zhang R, Tyndall MW, Montaner JS, and Kerr T 2008. ‘Risk factors for developing a cutaneous injection-related infection among injection drug users: a cohort study’, BMC Public Health, 8: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M, Pollock E, Armstrong M, Morris-Jones S, Kidd M, Gothard P, Noursadeghi M, and Doherty JF 2013. ‘Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London Teaching Hospital’, J Infect, 66: 95–102. [DOI] [PubMed] [Google Scholar]
- McElrath Karen %J Journal of Substance Use. 2006. ‘Booting and flushing: needle rituals and risk for bloodborne viruses’, 11: 177–89. [Google Scholar]
- Mezaache S, Protopopescu C, Debrus M, Morel S, Mora M, Suzan-Monti M, Rojas Castro D, Carrieri P, and Roux P 2018. ‘Changes in supervised drug-injecting practices following a community-based educational intervention: A longitudinal analysis’, Drug Alcohol Depend, 192: 1–7. [DOI] [PubMed] [Google Scholar]
- Miller William R, and Stephen Rollnick. 2012. Motivational interviewing: Helping people change (Guilford press; ). [Google Scholar]
- Monteiro J, Phillips KT, Herman DS, Stewart C, Keosaian J, Anderson BJ, and Stein MD 2020. ‘Self-treatment of skin infections by people who inject drugs’, Drug Alcohol Depend, 206: 107695. [DOI] [PubMed] [Google Scholar]
- Moradi-Joo Mohammad, Ghiasvand Hesam, Noroozi Mehdi, Armoon Bahram, Noroozi Alireza, Karimy Mahmood, Rostami Ali, Mirzaee Mohammad Saeed, and Morteza %J Journal of Substance Use Hemmat. 2019. ‘Prevalence of skin and soft tissue infections and its related high-risk behaviors among people who inject drugs: a systematic review and meta-analysis’, 24: 350–60. [Google Scholar]
- Motavalli D, Taylor JL, Childs E, Valente PK, Salhaney P, Olson J, Biancarelli DL, Edeza A, Earlywine JJ, Marshall BDL, Drainoni ML, Mimiaga MJ, Biello KB, and Bazzi AR 2020. ‘“Health Is on the Back Burner:” Multilevel Barriers and Facilitators to Primary Care Among People Who Inject Drugs’, J Gen Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EL, DeVita D, Liu H, Vittinghoff E, Leung P, Ciccarone DH, and Edlin BR 2001. ‘Risk factors for skin and soft-tissue abscesses among injection drug users: a case-control study’, Clin Infect Dis, 33: 35–40. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2019. Medications for Opioid Use Disorder Save Lives (The National Academies Press: Washington, DC: ). [PubMed] [Google Scholar]
- Palepu A, Tyndall MW, Leon H, Muller J, O’Shaughnessy MV, Schechter MT, and Anis AH 2001. ‘Hospital utilization and costs in a cohort of injection drug users’, Cmaj, 165: 415–20. [PMC free article] [PubMed] [Google Scholar]
- Phillips KT, Altman JK, Corsi KF, and Stein MD 2013. ‘Development of a risk reduction intervention to reduce bacterial and viral infections for injection drug users’, Subst Use Misuse, 48: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KT, and Stein MD 2010. ‘Risk practices associated with bacterial infections among injection drug users in Denver, Colorado’, Am J Drug Alcohol Abuse, 36: 92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KT, Stein MD, Anderson BJ, and Corsi KF 2012. ‘Skin and needle hygiene intervention for injection drug users: results from a randomized, controlled Stage I pilot trial’, J Subst Abuse Treat, 43: 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff AB, and Kroshinsky D 2016. ‘Cellulitis: A Review’, Jama, 316: 325–37. [DOI] [PubMed] [Google Scholar]
- Reddon H, Marshall BDL, and Milloy MJ 2019. ‘Elimination of HIV transmission through novel and established prevention strategies among people who inject drugs’, Lancet HIV, 6: e128–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes T 2009. ‘Risk environments and drug harms: a social science for harm reduction approach’, Int J Drug Policy, 20: 193–201. [DOI] [PubMed] [Google Scholar]
- Rhodes T, Kimber J, Small W, Fitzgerald J, Kerr T, Hickman M, and Holloway G 2006. ‘Public injecting and the need for ‘safer environment interventions’ in the reduction of drug-related harm’, Addiction, 101: 1384–93. [DOI] [PubMed] [Google Scholar]
- Roux P, Le Gall JM, Debrus M, Protopopescu C, Ndiaye K, Demoulin B, Lions C, Haas A, Mora M, Spire B, Suzan-Monti M, and Carrieri MP 2016. ‘Innovative community-based educational face-to-face intervention to reduce HIV, hepatitis C virus and other blood-borne infectious risks in difficult-to-reach people who inject drugs: results from the ANRS-AERLI intervention study’, Addiction, 111: 94–106. [DOI] [PubMed] [Google Scholar]
- Royer M, Fuller BE, Ober A, and Booth RE. 2004. ‘HIV and HCV counseling and education (C&E) intervention training manual, Version 3.0 (NIH Publication No. 93–3580)’. [Google Scholar]
- Saldana CS, Vyas DA, and Wurcel AG 2020. ‘Soft Tissue, Bone, and Joint Infections in People Who Inject Drugs’, Infect Dis Clin North Am, 34: 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Robinowitz N, Chaulk P, and Johnson KE 2015. ‘High rates of abscesses and chronic wounds in community-recruited injection drug users and associated risk factors’, J Addict Med, 9: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Phillips KT, Herman DS, Keosaian J, Stewart C, Anderson BJ, Weinstein Z, and Liebschutz J 2020. ‘Skin Cleaning among Hospitalized Persons Who Inject Drugs: A randomized controlled trial’, Addiction. [DOI] [PubMed] [Google Scholar]
- Summers PJ, Struve IA, Wilkes MS, and Rees VW 2017. ‘Injection-site vein loss and soft tissue abscesses associated with black tar heroin injection: A cross-sectional study of two distinct populations in USA’, Int J Drug Policy, 39: 21–27. [DOI] [PubMed] [Google Scholar]
- Tuazon Carmelita U, Ruth Hill, and John N %J Journal of Infectious Diseases Sheagren. 1974. ‘Microbiologic study of street heroin and injection paraphernalia’, 129: 327–29. [DOI] [PubMed] [Google Scholar]
- Varga Leah M., Chitwood Dale D., and Fernandez M. Isabel. 2006. ‘Research Note: Factors Associated with Skin Cleaning Prior to Injection among Drug Users’, 36: 1015–29. [Google Scholar]
- Vlahov David, Sullivan Marian, Astemborski Jacqueline, and Edwin Kenrad %J Public health reports Nelson. 1992. ‘Bacterial infections and skin cleaning prior to injection among intravenous drug users’, 107: 595. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.