Abstract
Probiotics, in particular Lactobacillus (lactic acid bacteria, LAB) strains, are widely used in clinical practice. Despite that these probiotics have GRAS (generally regarded as safe) and qualified presumption of safety (QPS) statuses, the safety of particular strains still needs to be thoroughly studied. The aim of the study was to evaluate the safety of Lact. casei IMV B-7280 strain by investigating toxicity and the effects on gut microbiota in experimental animal model. Male BALB/c mice (7–8 weeks, weight 20–24 g) were treated with amounts of Lact. casei IMV B-7280 strain: 5 × 106, 5 × 108, or 5 × 109 CFU/animal once per day during 7 days, or in the amount of 1 × 1010 CFU/animal once per day during 3 days (most of the proposed probiotic doses for humans—from 108 to 109 CFU) and monitored during 14 days. Blood tests and serum biochemistry were conducted; the cecal content from mice of the experimental and control groups were freshly collected and analyzed. At the end of the experiments (15th day), the presence of LAB in the heart, liver, kidney, and mesenteric lymph nodes and peripheral blood was determined; histology of the brain, liver, heart, fragments of the small and large intestine, and mesenteric lymph nodes was conducted. Survival rate of BALB/c mice treated with Lact. casei IMV B-7280 strain in different concentrations in toxicity experiments during 14 days was 100%. We observed no signs of toxicity as changes in gait, lethargy, sleep, somatomotor activity as well as changes in fur, eyes, skin and mucous membranes, tremors, behavior pattern, convulsions, salivation, diarrhea, and local injuries in mice from all experimental groups. After administration of probiotic strain, the number of opportunistic bacteria in cecal contents, such as Staphylococcus spp., Candida spp., Pseudomonas spp., and total aerobic and optionally anaerobic bacteria decreased compared to controls; the population of beneficial bacteria such as lactobacilli increased in cecal contents of these mice. LAB were not detected in the peripheral blood, heart, liver, kidneys, and mesenteric lymph nodes after administration of this strain to intact mice. Lact. casei IMV B-7280 strain is safe at dose up to 1010 CFU/animal during 3- and 7-day oral administration to mice and has a positive effect on the gut microbiota composition; it could be potentially considered as safe probiotic for humans.
Keywords: Probiotic strain, Safety, Lactobacillus, Lactobacillus casei IMV B-7280, In vivo, Mouse model
Introduction
Probiotics, in particular Lactobacillus (lactic acid bacteria, LAB) strains, are widely used today in clinical practice for the treatment of patients with acute intestinal infections; diseases of the gastrointestinal tract (gastric and duodenal ulcer, pancreatitis, cholecystitis, liver and biliary tract diseases, enteritis, viral hepatitis, gastritis, colitis), respiratory tract (pneumonia, bronchitis, etc.), urogenital system, as well as joint and connective tissue lesions, some cancers, allergies, dermatological and dental diseases, urolithiasis, etc. [1–3]. The results of the meta-analysis have shown the effectiveness of probiotic therapy in other human diseases (type 2 diabetes, irritable bowel syndrome, depressive states, atopic dermatitis in children) [4–9]. The effectiveness of probiotic therapy has also been proven in various experimental models in animals: experimental acute pancreatitis [10], necrotic enterocolitis [11], obesity [12], and staphylococcal infection [13].
At the same time, various clinical and experimental studies have shown limited efficacy or even ineffectiveness of probiotic therapy [14–21] due primarily to the fact that only a small number of living probiotic microorganisms can survive in the intestine; further, they do not integrate into biofilms and do not perform their physiological functions or have side effects. It is suggested that the optimal positive effect of probiotics will depend on the individual features of the quantitative and qualitative composition of the gut microbiota and the clinical manifestations of each specific disease. Therefore, in order to increase the effectiveness of probiotic therapy, it is recommended to create probiotics that are specific to each individual disease [22], as well as to introduce into the clinical practice rational schemes of their personalized use [23], taking into account their safety, optimal dosage regimens, and duration of probiotic therapy and the severity of the pathological process, the level of dysbiotic and immune disorders as well as a rational way of their administration (oral, rectal, vaginal). The main criteria for the development of probiotics are their quality (qualitative and quantitative composition of probiotic cultures, confirmed by microbiological analysis and genotyping) as well as efficiency and safety, confirmed in experimental studies, and positive results of their use in clinical practice.
According to the regulations of the World Health Organization (WHO) and the Food and Drug Administration (FDA), despite that probiotics are primarily representatives of the genera Lactococcus and Lactobacillus and have GRAS (generally regarded as safe) status, their safety must still be confirmed [24, 25]. The several theoretical concepts regarding the safety of probiotic therapy can be formulated: (1) the development of local or systemic infections, including bacteremia, fungemia, meningitis, endocarditis, etc.; (2) the occurrence of metabolic and toxic disorders (e.g., invasion of epithelial cells, degradation of the intestinal layer of mucin, production of toxins, etc.); (3) transfer of plasmids containing antibiotic resistance genes and virulence factors into the gut microbiota resulting in the formation of new clones of bacterial strains; (4) excessive stimulation of the immune system in susceptible individuals [3, 6, 24, 25, 25–30], which threatens human health.
The Guidelines for the Evaluation of Probiotics in Foods, adopted jointly by the FAO and WHO Working Groups in 2002 set out key principles for the assessment of probiotics in food [31], which provide the identification of bacteria by phenotypic and genotypic methods (determination of bacteria genus, species, strain) and their deposition in the international collection of microorganisms; determination of functional properties in vitro and in vivo using animal models; assessment of in vitro and in vivo safety using animal models as well as in clinical studies; conducting double-blind, randomized placebo-controlled clinical trials; conducting prolonged observations after probiotic therapy; determination of their side effects.
The evaluation of the safety of probiotic cultures is carried out in vitro and in vivo. Animal models are widely used in the study of safety of probiotic cultures in vivo and most often—different lines of immunodeficient animals (including gnotobiotic animals) [32].
The European Food Safety Authority (EFSA) in Europe have granted a number of Lactobacillus species (36 species up to date, including Lact. casei) for qualified presumption of safety (QPS) status based on their safety evaluation criteria [33].
Sequenced original Lactobacillus casei IMV B-7280 (Lact. casei IMV B-7280) strain with the probiotic attributes, deposited in the Depository of Microorganisms of Zabolotny Institute of Microbiology and Virology of NAS of Ukraine, is non-pathogenic, genetically homogeneous. It was not susceptible to mutagenic effects and genetic transformations. Its tinctorial, cultural-morphological, and biochemical properties, sensitivity to antibiotics, and adhesion to epitheliocytes have already been determined [34]. Antibacterial efficacy against a wide range of pathogenic and opportunistic microorganisms, immunomodulatory, anti-inflammatory, and hypocholesterolemic properties has been demonstrated in vitro and in vivo in animal experimental models [34, 35]. Lact. casei IMV B-7280 is promising probiotic strain for the clinical use, yet has no history of safe use as a component of probiotics or functional foods. Therefore, the safety of this LAB strain should be thoroughly investigated.
In this context, the aim of the current study was to estimate the safety of Lact. casei IMV B-7280 strain by investigating its acute and subchronic toxicity and its effect on cecal microbiota in experimental animal model.
Materials and Methods
Probiotic Bacteria Culture
The lyophilized (Cuddon Freeze Dryer FD1500, New Zealand) probiotic Lact. casei IMV B-7280 was used in our studies as a stock culture. Briefly, the lyophilized culture of Lact. casei IMV B-7280 strain was cultured in De Man, Rogosa, and Sharpe (MRS) medium (Merck, Darmstadt, Germany) and incubated for 24 h at 37 °C. After incubation, the suspension of this probiotic bacteria was plated to Petri dishes with De Man, Rogosa, and Sharpe Agar (MRSA) medium (Merck) and its colonies were investigated and colony forming units (CFU) were counted. Cell suspensions in normal saline were freshly prepared for use from the lyophilized stocks every day before gavages to the animals.
Animals
Male BALB/c mice age 7–8 weeks and weighing over 20 g were used for studies of oral toxicity. Animals were kept under standard conditions in plastic cages with adequate space and had free access to tap water and standard rodent diet in a separate room at constant air temperature (22–25 °C). These animals were quarantined for at least 7 days before the experiments began. The mice were fed at a fixed time, as ingestion may change animals’ sensitivity to the effects of the drugs. All our studies have been carried out in accordance with the Law of Ukraine No 3447-IV “On protection of animals from cruelty”, “European Convention for the Protection of vertebrate animals used for experimental and other scientific purposes” from 18 March 1986 (Strasburg, 1986), and “General ethics for animal experimentation” (First National Congress on bioethics, Ukraine, 2001).
Scheme of Oral Toxicity Study
The scheme of study of oral toxicity of Lact. casei IMV B-7280 strain in different doses and animal groups presented in Fig. 1. The joint control group included 12 intact animals (control 1). In toxicity experiments, animals of the experimental group (12 animals) were given an oral suspension of this LAB strain in 0.15 M NaCl daily using a probe at the maximum tolerable dose (e.g., 5 × 1010 CFU/animal) once a day (experimental group 1). The term of administration was 3 days. The maximum tolerable dose was determined by the results of previous studies and the analysis of literature data. The standard rodent diet was given to mice 3 h after treatment with these probiotic bacteria. Animals of the control group (12 animals) were similarly administered with 0.15 M NaCl (control 2). Animals were monitored daily for 14 days. After that, 6 animals from each group were used for histological analysis of organs and tissues, the remaining 6 animals were monitored for the next 12 months.
Fig. 1.
The scheme of oral toxicity study of Lact. casei IMV B-7280 strain
Before carrying out animal studies, the doses were determined as equivalent to the dose received by humans. The suspension of Lact. casei IMV B-7280 strain was administered to the animals orally daily in different amounts—5 × 106 (experimental group 2, 12 animals), 5 × 108 (experimental group 3, 12 animals), and 5 x 109 (experimental group 4, 12 animals) CFU/animal. The standard rodent diet was given to mice 3 h after treatment with these probiotic bacteria. The term of administration was 7 days. Twelve intact mice were orally administered with 0.15 M NaCl (control 3) during 7 days. The other control group included 12 intact animals (control 1). Animals were monitored daily for 14 days. After that, 6 animals from each group were used for histological analysis of organs and tissues, the remaining 6 animals were monitored for the next 12 months. Toxicity experiments were performed simultaneously; therefore, common control group 1 was used to reduce the number of animals.
All animals were weighed daily for 14 days. Other physical parameters such as feed intake, and local injuries, and mortality (if any) were also observed. The signs of toxicity as changes in gait, lethargy, sleep, somatomotor activity, as well as changes in fur, eyes, skin and mucous membranes, tremors, behavior pattern, convulsions, salivation, and diarrhea were investigated.
The minimum daily dose of Lact. casei IMV B-7280 strain, which was used in the study, was a dose equivalent to the human daily dose per 1 kg of animal body weight (DD).
We calculated it according to the formula: DD = ABM × HD / HBM, where ABM is the animal body mass; HD—dose for human; HBM—human body mass. For mice, this dose averages 5 × 106 CFU/animal.
Blood Tests and Serum Biochemistry
On the 15th day of the experiment, the animals of experimental and control groups were anesthetized and killed by cervical dislocation, and hematological and biochemical studies were conducted. For biochemical analyses, blood was collected for non-heparinized blood collection tubes. Blood was centrifuged at 3000×g for 15 min after clot at room temperature, and serum was collected in Eppendorf tubes. Serum biochemical parameters were investigated by colorimetric analysis using specific test systems (Felicit-Diagnostic, Ukraine) to determine the level of total cholesterol, alanine aminotransferase (ALT), phosphorus, calcium, uric acid, iron, total iron-binding ability of serum, and transferrin saturation. Serum glucose level was examined using a glucometer (FreeStyle, Abbot Diabetes Care Ltd., England).
For hematological analyses, blood was collected in heparinized blood collection tubes and analyzed using an automated hematological analyzer (Particle counter PCE-210, Elmer ERMA INC). Such parameters of blood were investigated as the leukocytes (WBC), lymphocytes (LY), monocytes (MO), granulocytes (GR), erythrocytes (RBC) cell count, hemoglobin (Hgb), hematocrit (HCT), average size of red blood cells (MCV), hemoglobin content of red blood cells (MCH), and mean hemoglobin content of red blood cells, not whole blood (MCHC), red cell distribution width (RDW), absolute platelet content (PLT), plasma thromboplastin component (PTC), mean platelet volume (MPV), and platelet distribution width (PDW).
Histological Analyses
On the 15th day of the experiment, the brain, liver, heart, fragments of the small and large intestine, mesenteric lymph nodes, and spleen were received from mice of the experimental and control groups (6 mice from each group) that were anesthetized and killed by cervical dislocation. The tissues were fixed in 10% formalin, washed in water for 20–24 h, and dehydrated in ethyl alcohol of increasing concentration—50 and 60% for 4–6 h, 70, 80, and 90% for 8–12 h, as well as in two portions of absolute ethyl alcohol for 12–24 h. After that, the samples of tissue were passed through a mixture of absolute ethyl alcohol and chloroform for 2–3 h, immersed in pure chloroform (twice-thrice for 1.5 h), impregnated initially with chloroform-paraffin (for 3–6 h at 37 °C), and then in paraffin (in three portions, for 1–1.5 h). These samples of tissue were poured into paraffin blocks after complete dehydration, and histologic sections 5–6 μm thick were prepared on the microtome. The paraffin sections were freed from paraffin using a solvent prior to staining, and the obtained preparations were stained with Ehrlich hematoxylin (Merck, Buchs, Switzerland). A 1% aqueous eosin solution was used for cytoplasmic staining. Microscopic examination of the specimens was performed using the Olympus BX 51 microscope, an Olympus C 5050 Z digital camera, and Olympus DP-Soft software (Olympus Corporation, Tokyo, Japan).
Microbiological Analyses of Cecal Content
On the 15th day of the experiment, the presence of the LAB in the internal organs and peripheral blood was determined. Heart, liver, kidney, and mesenteric lymph nodes were obtained from the animals of the experimental and control groups, which were killed by cervical dislocation after anesthesia; they were thoroughly ground in sterile sand and homogenized in 1 ml of normal saline in sterile Eppendorf tubes to obtain the initial dilution of the material (10−1). Next, a series of further dilutions of the material in normal saline to 10−3 and 10−5 were prepared from the initial dilution. Samples were plated on the nutrient selective MRSA (Merck) medium. A series of dilutions in normal saline to 10−3 and 10−5 were prepared from heparinized peripheral blood and were plated on the nutrient selective MRSA medium.
After 24 h of incubation, preliminary results were recorded, and after 48 h, final results were recorded. The number of colonies of microorganisms in each of the single crop dilutions was counted. The result was expressed in lg CFU in 1 g of tested tissue or 1 ml of blood.
On the 1st, 3rd, 6th, 9th, and 15th day of the experiments, the cecal content from mice of the experimental and control groups was freshly collected, and the lactobacilli, bifidobacteria, staphylococci, streptococci, coliform bacteria, microscopic fungi, and total amount of aerobic and optionally anaerobic bacteria were enumerated. A total of 100 μm of cecal content was homogenized in 1 ml of normal saline in sterile Eppendorf tubes and vortexed for 1 min, obtaining the initial dilution of the material (10−1), and left at room temperature for 40–60 min. Consequently, from the initial dilution, we prepared a series of dilutions of cecal content in normal saline to 10−5 and 10−7 and 50 μl of dilutions was plated on appropriate selective media for the cultivation and enumeration of different groups of microorganisms.
Meat-peptone agar (MPA) was used for the cultivation and enumeration of total amount of aerobic and optionally anaerobic bacteria; MRS (Merck) and Bifidobacterium Agar (HiMedia, Mumbai, India)—for the cultivation and enumeration of lactobacilli and bifidobacteria, respectively; BAIRD-PARKER-Agar (Merck)—for the cultivation and enumeration of staphylococci; KF-Streptococcus agar (Merck)—for the cultivation and enumeration of streptococci; ENDO (HiMedia)—for the cultivation and enumeration of polymorphic bacteria; Sabouraud agar (HiMedia)—for the cultivation and enumeration of microscopic fungi; Candida Medium (HiMedia)—for the cultivation and enumeration of Candida spp.; and Pseudomonas agar (HiMedia)—for the cultivation and enumeration of Pseudomonas spp.
After 24 h of incubation in appropriate condition for each group of microorganisms, preliminary results were recorded, and after 48 h, final results were recorded. CFU in each of the single crop dilutions were counted. According to the results, the arithmetic mean of the number of colonies in the crops of a single dilution or initial sample was determined. The multiplicity of sample dilution was taken into account during the calculation. The result was expressed as lg of CFU in 1 g of cecal content.
The presence of lactobacilli in the internal organs and peripheral blood was determined on the 15th day of the experiment. Heart, liver, kidney, and mesenteric lymph nodes were taken from the animals of the experimental and control groups, which were killed by cervical dislocation after being anesthetized; they were thoroughly ground in sterile sand and homogenized in 1 ml of 0.15 M NaCl in sterile Eppendorf tubes to obtain the initial dilution of the material (10−1). Next, a series of further dilutions of the material in 0.15 M NaCl to 10−3 and 10−5 were prepared from the initial dilution. Samples were plated on the nutrient selective MRSA (Merck) medium. A series of dilutions in 0.15 M NaCl to 10−3 and 10−5 were prepared from heparinized peripheral blood and were plated on the nutrient selective MRSA (Merck) medium.
After 24 h of incubation, preliminary results were recorded, and after 48 h, final results were recorded. The number of colonies of microorganisms in each of the single crop dilutions was counted. The result was expressed in lg CFU in 1 g of tested tissue or 1 ml of blood.
Statistical Analysis
Experimental data were analyzed using the STATISTICA computer program using the method of variational statistics, as well as the Excel program from Microsoft Office-2007 and 2010. The numerical data are presented as arithmetic mean and standard error. The null hypothesis for the comparison groups was tested using the nonparametric Kolmogorov-Smirnov and Wilcoxon-Mann-Whitney (U) criteria. The differences were considered significant if p < 0.05.
Results
General Signs
Survival rate of BALB/c mice treated with Lact. casei IMV B-7280 strain in different concentrations in toxicity experiments during 14 days and for the next 12 months was 100%. The weight of mice in all groups is presented in Table 1. Mean body weight did not differ in the experimental and control mice during 14 days of observation. After administration of Lact. casei IMV B-7280 strain, the physiological condition of the mice (appearance or behavior and feed intake) did not deteriorate. Other physical parameter such as local injuries was also not identified.
Table 1.
Body weights of mice
| Groups of mice | Body weight (g) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 14 | |
| Control 1 | 22.2 ± 1.8 | 23.0 ± 1.0 | 23.1 ± 1.6 | 23.0 ± 3.8 | 23.2 ± 1.6 | 23.7 ± 1.0 | 23.4 ± 1.7 | 23.3 ± 3.0 | 23.2 ± 1.6 | 23.1 ± 1.3 | 23.8 ± 1.0 | 23.3 ± 3.2 | 23.3 ± 1.0 | 23.3 ± 3.2 | 23.1 ± 3.3 |
| Control 2 | 23.2 ± 1.0 | 23.6 ± 1.0 | 23.1 ± 1.6 | 23.2 ± 1.1 | 23.3 ± 3.6 | 23.7 ± 1.0 | 23.0 ± 1.1 | 23.5 ± 1.0 | 23.8 ± 1.2 | 23.9 ± 1.5 | 23.7 ± 1.1 | 23.9 ± 1.2 | 23.2 ± 1.0 | 23.0 ± 1.7 | 23.5 ± 1.3 |
| Control 3 | 23.1 ± 1.9 | 23.6 ± 1.2 | 23.5 ± 1.0 | 23.9 ± 1.9 | 23.1 ± 2.3 | 23.9 ± 1.0 | 23.8 ± 1.9 | 23.4 ± 1.2 | 23.5 ± 1.8 | 23.4 ± 2.0 | 23.8 ± 1.7 | 23.7 ± 1.8 | 23.9 ± 2.0 | 23.8 ± 1.9 | 23.9 ± 1.7 |
| Experimental group 1 | 23.3 ± 2.6 | 23.2 ± 2.4 | 22.9 ± 2.5 | 23.2 ± 2.6 | 23.8 ± 2.2 | 24.4 ± 2.0 | 24.4 ± 2.0 | 23.8 ± 4.2 | 23.7 ± 1.9 | 23.9 ± 1.4 | 23.7 ± 1.4 | 23.8 ± 1.4 | 23.8 ± 1.5 | 24.1 ± 1.1 | 24.9 ± 1.2 |
| Experimental group 2 | 23.9 ± 2.9 | 23.2 ± 3.3 | 23.0 ± 2.9 | 22.7 ± 3.0 | 22.6 ± 2.9 | 23.3 ± 3.0 | 23.3 ± 3.1 | 23.3 ± 3.0 | 23.2 ± 3.1 | 22.7 ± 3.0 | 22.8 ± 3.1 | 22.8 ± 3.1 | 23.5 ± 2.4 | 23.5 ± 2.4 | 23.5 ± 2.7 |
| Experimental group 3 | 23.2 ± 1.3 | 23.6 ± 1.0 | 23.6 ± 1.0 | 23.7 ± 1.0 | 23.8 ± 0.9 | 24.0 ± 1.0 | 24.1 ± 0.5 | 24.0 ± 0.7 | 23.8 ± 1.5 | 23.9 ± 3.0 | 23.8 ± 3.1 | 23.9 ± 3.0 | 23.9 ± 3.1 | 23.4 ± 2.9 | 23.9 ± 2.8 |
| Experimental group 4 | 23.0 ± 4.1 | 23.1 ± 4.3 | 23.2 ± 4.4 | 23.1 ± 4.3 | 23.7 ± 4.0 | 24.2 ± 4.0 | 23.9 ± 3.7 | 23.4 ± 4.3 | 23.6 ± 4.3 | 23.5 ± 4.3 | 23.9 ± 4.1 | 23.5 ± 2.3 | 24.0 ± 2.9 | 23.7 ± 4.5 | 23.9 ± 2.1 |
No signs of toxicity as changes in gait, lethargy, sleep, somatomotor activity as well as changes in fur, eyes, skin and mucous membranes, tremors, behavior pattern, convulsions, salivation, diarrhea in mice from all experimental groups were observed during 14 days and for the next 12 months.
Blood Tests and Serum Biochemistry
It was found that blood tests in mice administrated with Lact. casei IMV B-7280 strain were unchanged compared to control group (Table 2 and Table 3). Thus, the absolute numbers of leukocytes, lymphocytes, monocytes, granulocytes, red blood cells and hemoglobin, erythrocyte volume index to the volume of total blood sample, mean corpuscular volume of erythrocyte, mean hemoglobin content in particular erythrocyte, and average concentration of hemoglobin in the red cell mass vs in whole blood showed no significant difference between the control and experimental groups. As shown in the Table 2, after LAB administration, relative width of distribution of platelets by volume (indicator of heterogeneity of platelets), absolute platelet content, platelets amount (percentage, %) in whole blood volume occupied by platelets, mean platelet volume, and relative platelet volume distribution (platelet heterogeneity indicator) were also at the control level. We found tendency to decrease the absolute platelet count in mice administrated with Lact. casei IMV B-7280 strain in the dose of 5 × 109 CFU/animal during 7 days, but the difference from control was not significant.
Table 2.
Blood tests I
| Groups of mice | WBC × 103/µl | LY × 103/µl | MO × 103/µl | GR × 103/µl | LY % | MO % | GR % | Hgb g/dl | RBC × 106/µl | HCT % | MCV fl | MCN pg | MCHC g/dl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | 6.2 ± 2.2 | 4.8 ± 1.9 | 0.4 ± 0.2 | 1.3 ± 0.4 | 75.3 ± 5.8 | 6.0 ± 1.3 | 18.6 ± 4.7 | 9.8 ± 4.1 | 6.8 ± 2.8 | 18.2 ± 3.1 | 46.9 ± 1.8 | 13.9 ± 2.1 | 29.7 ± 4.8 |
| Control 2 | 6.9 ± 1.4 | 5.1 ± 1.1 | 0.5 ± 0.1 | 1.4 ± 0.9 | 74.0 ± 4.6 | 5.9 ± 1.8 | 18.9 ± 3.1 | 9.1 ± 1.8 | 6.7 ± 1.9 | 19.6 ± 4.9 | 47.0 ± 1.2 | 12.0 ± 1.8 | 30.4 ± 3.5 |
| Control 3 | 6.1 ± 1.2 | 5.3 ± 1.0 | 0.5 ± 0.2 | 1.4 ± 0.3 | 74.9 ± 3.1 | 6.1 ± 1.2 | 18.1 ± 4.9 | 9.0 ± 2.0 | 6.1 ± 2.1 | 19.0 ± 2.5 | 47.2 ± 2.3 | 12.2 ± 1.6 | 30.3 ± 4.1 |
| Experimental group 1 | 7.1 ± 3.3 | 5.7 ± 2.5 | 0.5 ± 0.2 | 1.4 ± 0.6 | 77.8 ± 3.9 | 5.7 ± 1.5 | 16.5 ± 2.9 | 9.7 ± 4.1 | 6.9 ± 3.2 | 19.6 ± 5.0 | 46.2 ± 2.7 | 14.3 ± 1.4 | 33.7 ± 6.9 |
| Experimental group 2 | 8.2 ± 3.8 | 5.6 ± 2.9 | 0.6 ± 0.3 | 1.9 ± 0.6 | 69.9 ± 3.1 | 7.7 ± 1.3 | 23,6 ± 2.8 | 8.4 ± 1.4 | 5.8 ± 1.8 | 20.1 ± 3.1 | 48.4 ± 1.6 | 14.2 ± 0.5 | 29.4 ± 1.9 |
| Experimental group 3 | 8.1 ± 1.3 | 5.6 ± 1.0 | 0.7 ± 0.2 | 1.9 ± 0.3 | 68.7 ± 8.2 | 7.9 ± 1.6 | 23.1 ± 2.0 | 8.3 ± 0.8 | 5.9 ± 1.3 | 21.1 ± 6.2 | 48.9 ± 0.8 | 14.6 ± 2.4 | 29.8 ± 4.6 |
| Experimental group 4 | 5.2 ± 0.9 | 4.4 ± 0.5 | 0.4 ± 0.4 | 1.4 ± 0.4 | 70.0 ± 5.0 | 7.5 ± 1.6 | 23.9 ± 4.4 | 8.8 ± 0.5 | 5.0 ± 2.9 | 20.3 ± 1.2 | 48.9 ± 0.8 | 14.3 ± 1.7 | 29.2 ± 3.1 |
Table 3.
Blood tests II
| Groups of mice | RDW % | PLT × 103/µl | PCT % | MPV fl | PDW fl |
|---|---|---|---|---|---|
| Control 1 | 17.65 ± 0.55 | 72.33 ± 18.58 | 0.21 ± 0.02 | 14.99 ± 1.93 | 27.16 ± 0.99 |
| Control 2 | 17.13 ± 1.65 | 73.12 ± 10.12 | 0.20 ± 0.02 | 14.00 ± 1.09 | 27.99 ± 1.99 |
| Control 3 | 16.87 ± 0.98 | 71.45 ± 12.45 | 0.19 ± 0.43 | 14.23 ± 2.34 | 26.45 ± 1.23 |
| Experimental group 1 | 16.10 ± 1.20 | 66.71 ± 20.23 | 0.19 ± 0.05 | 14.38 ± 3.20 | 26.40 ± 1.72 |
| Experimental group 2 | 14.34 ± 0.68 | 75.80 ± 21.45 | 0.10 ± 0.09 | 15.04 ± 0.65 | 22.16 ± 7.11 |
| Experimental group 3 | 15.50 ± 0.75 | 65.67 ± 10.29 | 0.10 ± 0.08 | 16.33 ± 2.21 | 26.87 ± 1.79 |
| Experimental group 4 | 14.96 ± 0.29 | 66.40 ± 17.56 | 0.39 ± 0.14 | 17.20 ± 0.65 | 26.56 ± 1.60 |
After administration of this LAB strain into mice, serum biochemical parameters such as glucose, total cholesterol, ALT, phosphorus, calcium, uric acid, serum iron levels, as well as indicators of total serum iron-binding capacity, unsaturated serum iron-binding ability, and transferrin saturation remained at the control level (Table 4). Uric acid level was slightly increased in the serum of mice administrated with Lact. casei IMV B-7280 strain in the doses of 5 × 106, 5 × 108, or 5 × 109 CFU/animal for 7 days, but the difference vs the controls was not significant.
Table 4.
Serum biochemistry
| Groups of mice | Glucose (g/l) | Total cholesterol (mmol/l) | ALT (mmol/l) | Phosphorus (mmol/l) | Calcium (mmol/l) | Uric acid (μm/l) | Iron (μm/l) | Total serum iron-binding capacity (μmol/l) | Unsaturated serum-binding serum (μmol/l) | Transferrin saturation (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control 1 | 8.30 ± 1.20 | 3.46 ± 0.39 | 0.41 ± 0.02 | 1.38 ± 0.26 | 2.60 ± 0.05 | 379.59 ± 56.12 | 23.03 ± 9.10 | 71.08 ± 24.72 | 48.05 ± 19.64 | 33.06 ± 12.20 |
| Control 2 | 8.45 ± 1.10 | 3.43 ± 0.27 | 0.40 ± 0.01 | 1.36 ± 0.13 | 2.62 ± 0.03 | 375.23 ± 34.23 | 23.58 ± 7.34 | 69.12 ± 15.67 | 45.05 ± 11.12 | 34.15 ± 9.32 |
| Control 3 | 8.47 ± 0.94 | 3.52 ± 0.12 | 0.44 ± 0.06 | 1.26 ± 0.11 | 2.54 ± 0.09 | 368.12 ± 12.66 | 25.12 ± 3.73 | 65.58 ± 12.52 | 43.19 ± 5.24 | 36.52 ± 8.22 |
| Experimental group 1 | 8.50 ± 1.20 | 3.40 ± 0.69 | 0.42 ± 0.03 | 1.34 ± 0.07 | 2.65 ± 0.08 | 371.43 ± 29.91 | 23.92 ± 12.70 | 59.08 ± 13.37 | 38.88 ± 13.81 | 40.74 ± 22.91 |
| Experimental group 2 | 7.90 ± 0.90 | 3.59 ± 0.69 | 0.44 ± 0.05 | 1.15 ± 0.20 | 2.47 ± 0.12 | 453.06 ± 90.96 | 27.13 ± 8.11 | 72.09 ± 20.95 | 44.97 ± 10.05 | 39.31 ± 12.51 |
| Experimental group 3 | 8.20 ± 0.60 | 3.84 ± 0.35 | 0.39 ± 0.03 | 1.30 ± 0.18 | 2.52 ± 0.13 | 464.29 ± 72.17 | 35.29 ± 4.20 | 72.09 ± 20.95 | 36.50 ± 13.94 | 40.55 ± 10.94 |
| Experimental group 4 | 8.50 ± 0.60 | 3.19 ± 0.48 | 0.41 ± 0.01 | 1.11 ± 0.13 | 2.50 ± 0.05 | 407.14 ± 34.80 | 21.29 ± 6.77 | 71.02 ± 15.70 | 49.73 ± 10.73 | 29.73 ± 6.21 |
Histological Study
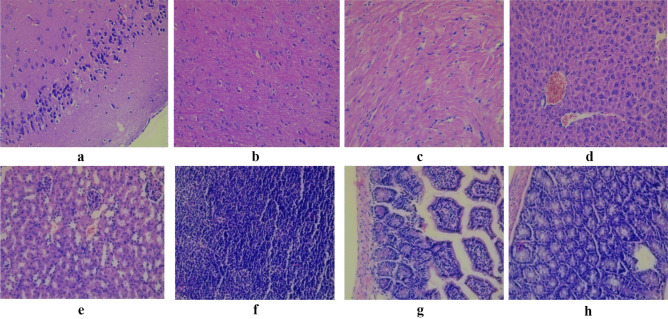
The results of histological examination of the cortex and white matter of the brain (cerebrum), cardiac muscle fibers, liver, cortical substance of the kidney, mesenteric lymph node, fragments of the small intestine, fragments of the colon of intact mice that did not receive probiotic bacteria, and mice of all experimental groups are presented in the Figs. 2, 3, 4, 5, and 6.
Fig. 2.
Light micrograph of the cortex a and white matter b of the brain cerebrum; cardiac muscle fibers c; lever d; cortical substance of the kidney e; mesenteric lymph node f; fragments of the small intestine g; fragments of the colon h intact mice that did not receive probiotic bacteria. Staining with hematoxylin and eosin. × 200. No cellular and inflammatory changes as well as hemorrhage were observed in cortex and white matter of the brain (cerebrum); cardiac muscle fibers; lever; cortical substance of the kidney; mesenteric lymph node; fragments of the small intestine, and colon
Fig. 3.
Light micrograph of the cortex a and white matter b of the brain (cerebrum); cardiac muscle fibers c; liver d; cortical substance of the kidney e; mesenteric lymph node f; fragments of the small intestine g; fragments of the colon h of mice that administrated with Lact. casei IMV B-7280 strain in the dose of 5 × 1010 CFU/animal (experimental group 1). Staining with hematoxylin and eosin. × 200. No cellular and inflammatory changes as well as hemorrhage were observed in cortex and white matter of the brain (cerebrum); cardiac muscle fibers; liver; cortical substance of the kidney; mesenteric lymph node; fragments of the small intestine, and colon. There was a slight perivascular edema in the cerebral cortex and white matter of the brain. In the cerebral cortex, there were no signs of pericellular edema. There were no perivascular or parenchymal hemorrhages in the brain. Inflammatory infiltration of the cerebral cortex and white matter is absent. There was mild interstitial edema in the myocardium of the heart, but inflammatory stroma infiltration was absent. Myocardial vessels are moderately full-blooded; no evidence of vascular wall edema, or perivascular hemorrhage in the myocardium of the heart was detected. There was no hyperplasia of lymphoid tissue in mesenteric lymph nodes, small and large intestine
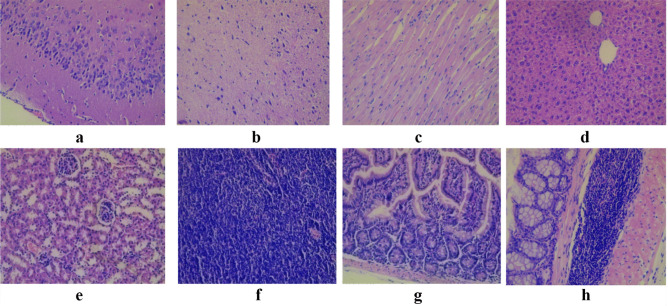
Fig. 4.
Light micrograph of the cortex a and white matter b of the brain (cerebrum); cardiac muscle fibers c; liver d; cortical substance of the kidney e; mesenteric lymph node f; fragments of the small intestine g; fragments of the colon h of mice that administrated with Lact. casei IMV B-7280 strain in the dose of 5 × 106 CFU/animal (experimental group 2). Staining with hematoxylin and eosin. × 200. No cellular and inflammatory changes as well as hemorrhage were observed in cortex and white matter of the brain (cerebrum); cardiac muscle fibers; liver; cortical substance of the kidney; mesenteric lymph node; fragments of the small intestine, and colon. A slight perivascular edema was observed focally in the brain. Inflammatory infiltration of the cerebral cortex, meninges, and white matter was not detected. Perivascular or parenchymal hemorrhage in the brain was absent. In the liver, a small fraction of hepatocytes had more hyperchromic nucleus with multiple nucleoli d. The cell cytoplasm had fine-grained evenly eosinophilic staining; however, histological signs of hepatocyte damage, inflammatory infiltration or fibrotic changes, signs of bile stasis were not detected. Mild interstitial edema was observed in the myocardium of the heart, kidneys, and muscle layer, the walls of the small and large intestine. There was no hyperplasia of lymphoid tissue in mesenteric lymph nodes, small and large intestine
Fig. 5.
Light micrograph of the cortex a and white matter b of the brain (cerebrum); cardiac muscle fibers c; liver d; cortical substance of the kidney e; mesenteric lymph node f; fragments of the small intestine g; fragments of the colon h of mice that administrated with Lact. casei IMV B-7280 strain in the dose of 5 × 108 CFU/animal (experimental group 3). Staining with hematoxylin and eosin. × 200. No cellular and inflammatory changes as well as hemorrhage were observed in cortex and white matter of the brain (cerebrum); cardiac muscle fibers; liver; cortical substance of the kidney; mesenteric lymph node; fragments of the small intestine, and colon. Slightly expressed perivascular edema was observed in the cortex and white matter of the brain. Inflammatory infiltration of the cerebral cortex, meninges, and white matter was not detected. Perivascular or parenchymal hemorrhage in the brain was absent. There was mild interstitial edema in the heart myocardium, but inflammatory infiltration, vascular wall edema, or perivascular hemorrhages were absent c. Liver hepatocytes were polymorphic, of different size, had rounded, clearly delimited, moderately basophilic nucleus with nucleolus. No histological signs of hepatocyte damage, bile stasis, inflammatory infiltration, or fibrotic changes were detected d. A slight interstitial edema was found in the kidneys and the muscular layer of the wall of the small and large intestine. There was no hyperplasia of lymphoid tissue in mesenteric lymph nodes, fragments of the small and large intestine
Fig. 6.
Light micrograph of the cortex a and white matter b of the brain (cerebrum); cardiac muscle fibers c; liver d; cortical substance of the kidney e; mesenteric lymph node f; fragments of the small intestine g; fragments of the colon h of mice that administrated with Lact. casei IMV B-7280 strain in the dose of 5 × 108 CFU/animal (experimental group 4). Staining with hematoxylin and eosin. × 200. No cellular and inflammatory changes were observed in cortex and white matter of the brain (cerebrum); cardiac muscle fibers; liver; cortical substance of the kidney; mesenteric lymph node; fragments of the small intestine, and colon. There were no signs of pericellular edema in the cerebral cortex and white matter of the brain. Perivascular edema and perivascular hemorrhage in the major cortex were also absent. Mild interstitial edema was detected in the myocardium of the heart, but there was no inflammatory stroma infiltration. Myocardial vessels were moderately full-blooded, no signs of vascular wall swelling or perivascular hemorrhage were observed in the mice of this group. Hepatocytes had different sizes and rounded, clearly delineated, moderately basophilic nucleus with nucleolus. The cytoplasm of cells had fine-grained eosinophilic staining. Histological signs of hepatocyte damage and signs of bile stasis were not detected. Mild interstitial edema was detected in the kidneys and the muscular layer, the walls of the small and large intestine. There was no hyperplasia of lymphoid tissue in mesenteric lymph nodes, small and large intestine
Administration of Lact. casei IMV B-7280 strain into mice did not lead to the appearance of any signs of damage in tissue and cellular structures of the internal organs: histological structure of the cortex and white matter of the brain, heart myocardium, liver, kidney, mesenteric lymph nodes, the walls of the small and large intestines have been preserved, indicating the preservation of their function. Inflammatory infiltration of these organs and tissues, sclerotic changes, and hyperplasia of lymphoid tissue in mesenteric lymph nodes, tissue of the small and large intestine in mice of all experimental groups were absent.
In mice treated with this LAB strain in the doses of 5 × 106, 5 × 108, or 5 × 109 CFU/animal for 7 days, mild perivascular edema was detected in the cortex and white matter of mice brain, and mild interstitial edema was observed in the myocardium, kidneys, and muscle layer of the small and large intestine wall. Also, in these mice, some polymorphism of hepatocytes, which was characterized by different size of cells and their nuclei, as well as different intensity of nuclei staining, were detected in the liver.
Microbiological Study of Blood, Organs, and Cecal Contents
The results of the evaluation of the presence of LAB in the internal organs and peripheral blood of mice administrated with Lact. casei IMV B-7280 strain are presented in Table 5. Lactobacilli were not detected in peripheral blood, heart, liver, kidneys, and mesenteric lymph nodes after LAB administration. Cecal contents of mice administrated with Lact. casei IMV B-7280 strain were changed. The content of beneficial bacteria such as LAB in cecal contents of these mice was increased. There was a decrease in the number of bifidobacteria in the cecal contents of mice administrated with Lact. casei IMV B-7280 strain (experimental groups 1, 4), which later restored to normal levels. After administration of LAB strain, the number of opportunistic bacteria, such as Staphylococcus spp., Candida spp., Pseudomonas spp., and total aerobic and optionally anaerobic bacteria levels decreased compared to controls.
Table 5.
Microbiological study of cecal contents
| Groups of mice | The number of bacteria (lg CFU/g)/elective nutrient medium | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MPA | BAIRD-PARKER-Agar | KF-Streptococcus agar | MRSA | Bifidobacterium Agar | Sabouraud agar | Candida medium | ENDO | Pseudomonas agar | ||
| Control 1 | 7.33 ± 0.19 | 4.92 ± 0.08 | 3.77 ± 0.14 | 3.45 ± 0.08 | 2.16 ± 0.11 | 2.35 ± 0.09 | 1.92 ± 0.06 | 6.98 ± 0.22 | 2.57 ± 0.12 | |
| Control 2 | 6.93 ± 0.13 | 4.57 ± 0.06 | 3.92 ± 0.11 | 3.05 ± 0.12 | 2.01 ± 0.14 | 2.67 ± 0.12 | 2.19 ± 0.08 | 7.34 ± 0.29 | 3.01 ± 0.20 | |
| Control 3 | 6.74 ± 0.13 | 4.73 ± 0.11 | 4.02 ± 0.10 | 3.52 ± 0.14 | 2.21 ± 0.09 | 2.41 ± 0.16 | 2.01 ± 0.05 | 6.77 ± 0.17 | 3.25 ± 0.09 | |
| Experimental group 1 | 4.43 ± 0.10* | 1.92 ± 0.04* | 2.77 ± 0.05* | 6.22 ± 0.11* | 1.05 ± 0.02* | ≤ 1.00* | ≤ 1.00* | 4.62 ± 0.08* | ≤ 1.00* | |
| Experimental group 2 | 6.01 ± 0.06* | 2.41 ± 0.05* | 4.92 ± 0.07* | 6.73 ± 0.06* | 3.14 ± 0.06* | 1.22 ± 0.04* | 1.12 ± 0.02* | 6.94 ± 0.07 | ≤ 1.00* | |
| Experimental group 3 | 6.22 ± 0.12* | 2.25 ± 0.03* | 4.13 ± 0.13 | 6.88 ± 0.13* | 2.76 ± 0.07* | ≤ 1.00* | ≤ 1.00* | 7.15 ± 0.06 | ≤ 1.00* | |
| Experimental group 4 | 5.18 ± 0.07* | 1.14 ± 0.01* | 3.78 ± 0.09 | 7.14 ± 0.08* | 1.14 ± 0.04* | ≤ 1.00* | ≤ 1.00* | 6.73 ± 0.12 | ≤ 1.00* | |
*The difference is significant compared with control groups (p < 0.05)
Discussion
The safety of each individual strain of LAB probiotic cultures should be determined via the properly documented results of studies on their safe use for humans, for example, in food, as well as the results of experimental studies and clinical observations [31–33]. The current study was conducted to assess the safety of Lact. casei IMV B-7280 strain in mouse model. Male BALB/c mice (7–8 weeks, weight more 20–24 g) were treated with this LAB strain in different doses: 5 × 106, 5 × 108, or 5 × 109 CFU/animal once per day during 7 days, or in the amount of 1 × 1010 CFU/animal once per day during 3 days (most of the proposed probiotic doses for humans—from 108 to 109 CFU) and monitored for 14 days and for the next 12 months.
The oral toxicity of Lact. casei IMV B-7280 strain was investigated according to the methodological recommendations of Preclinical Drug Research (in Ukraine) and analysis of data from literary sources [36–38]. Mice that were treated with Lact. casei IMV B-7280 strain had a 100% survival rate. LAB in the peripheral blood, heart, liver, kidney, and mesenteric lymph nodes of these mice were not detected. Weight and physiological parameters such as appearance or behavior and feed intake in mice administrated with Lact. casei IMV B-7280 strain did not change. The local injuries were not identified. There were also no signs of toxicity in any of the mice, as well as changes in blood and serum biochemistry parameters that were critical in determining toxicological effects. Cholesterol and glucose levels in the serum of the experimental mice were normal. Thus, Lact. casei IMV B-7280 strain did not affect lipid metabolism and did not induce the development of diabetes.
There was also no difference between the levels of calcium and phosphorus in the blood serum of mice of the control and experimental groups. Abnormalities of calcium, phosphorus, and magnesium homeostasis are associated with disorders of mineral metabolism [39]. High levels of serum phosphorus may indicate impairment of endothelial function by increasing oxidative stress and decreasing nitric oxide production, etc. [40]. Serum iron level, total serum iron-binding ability, unsaturated serum-binding capacity, and transferrin level did not change in mice of the experimental groups, i.e., Lact. casei IMV B-7280 strain did not induce iron-deficiency anemia.
The histological studies of the organs of mice administered with this LAB strain revealed no abnormalities or histopathological changes in the organs. Inflammatory infiltration of these organs and tissues, sclerotic changes, as well as hyperplasia of lymphoid tissue in mesenteric lymph nodes, tissue of the small, and large intestine in mice of all experimental groups were not revealed. Mild perivascular edema was determined in the cortex and white matter of the brain after administration of Lact. casei IMV B-7280 strain to mice in the doses of 5 × 106, 5 × 108, or 5 × 109 CFU/animal. But there were no changes in the behavior of these mice or neurological disorders. This LAB strain administration to mice did not lead to any damage of the tissue and cellular structures of the myocardium of the heart, kidneys, walls of the small and large intestine, although they evoked a mild interstitial edema, which can be reversible. There was no kidney injury detected and no tendency to increase the level of urea in mice treated with Lact. casei IMV B-7280 strain in the doses of 5 × 106, 5 × 108, or 5 × 109 CFU/animal, and also in the dose of 5 × 1010 CFU/animal. It should be noted that studies of the strain effect on blood biochemistry are extremely important to develop optimal schemes for their individualized use and predictive diagnosis and preventing urolithiasis, kidney disease, or calculous prostatitis during administration probiotic therapy.
The histological structure of the liver of mice after administration Lact. casei IMV B-7280 strain also was not altered. The preservation of liver function was evidenced by the normal levels of ALT in the blood serum. Mild polymorphism of hepatocytes, characterized by different size of cells and their nuclei, as well as different intensity of staining of nuclei that were observed after administration of this LAB strain into mice in the doses of 5 × 106, 5 × 108, or 5 × 109 CFU/animal, can be explained by activation of metabolic processes in the liver of these mice. Therefore, Lact. casei IMV B-7280 strain had no adverse effect on circulating blood cells and their production, blood serum biochemical parameters, histological structure, and function of the organs that were studied. Previously, we showed [41] that this LAB strain had no immunotoxic effect after administration to intact mice in a dose of 5 × 106 CFU/animal for 7 days.
Safety has been proven for other Lact. casei strains in various animal experimental models. In particular, the safety of oral and anal administration of Lact. casei GG strain has been demonstrated in gnotobiotic bg/bg-nu/nu and bg/bg-nu/+ mice strains (males and females), although LAB translocation to the internal organs was detected in 27 and 26% of cases, respectively, and abscesses of the stomach and small intestine in 29% of cases. The survival rate of all adult mice of both strains was 100%, their weight did not change. Mice born from females of the bg/bg-nu/+ line administered with Lact. casei GG strain also had a 100% survival rate. However, deaths of bg/bg-nu/nu (4 weeks old, but not 8–12 weeks old) mice born by mothers receiving this LAB strain were observed. Lact. casei GG strain is safe for immunocompetent and immunodeficient adult animals, but additional studies are needed to confirm its safety in immunodeficient infants [42]. Lact. casei Shirota strain demonstrated safety in a non-bacterial endocarditis model in rabbits. Instead, Lact. casei PHLS A357/84 strain caused bacteremia in rabbits with endocarditis; LAB have been found in vegetation, peripheral blood, liver, and spleen. Histopathological analysis of the heart of these rabbits revealed that vegetations were formed on the heart valve with a fibrin clot and bacterial colonies; their infiltration by cells involved in the development of inflammation was observed [43].
The safety of Lact. casei 5s strain isolated from Serbian homemade cheese has been demonstrated in the Wistar rat model of hyperlipidemia. Under the influence of this LAB strain, lipid metabolism and the gut microbiota improved. The Lact. casei 5s strain had no acute toxicity and was not translocated to the internal organs of animals, but was identified on the surface of the intestinal mucosa and in fecal samples of treated animals, indicating its adhesiveness and ability to colonize [44]. Safety of Lact. casei var. rhamnosus strain (Lcr35, Antibiophilus™, France) was proven on diarrhea model in immunodeficient SCID/NOD mice. This LAB strain after oral daily administration to mice for 5 days in the amount of 1 × 107 CFU did not lead to bacteremia, did not translocate into peripheral blood and improve the course of diarrhea, increased the weight of mice, restored the depth of crypts, and suppressed production of tumor necrosis factor-α, interferon-γ, interleukin (IL)-1β, IL-6, IL-4, IL-10, and IL-17 [38]. Lact. paracasei subsp. paracasei LC-01 strain was also safe in case of oral administration to BALB/c mice (mice weight did not change; translocation of LAB to internal organs was not detected). In contrast, intraperitoneal administration of this strain to mice resulted in a significant translocation of LAB to the liver, spleen, and kidneys, but did not cause pathologies or death throughout the experiment [45].
In clinical practice, the safety has also been proven for Lact. casei Shirota strain in the probiotic composition in the treatment of critically ill children receiving intensive care [46], Lact. casei subsp. casei 327 strain in female volunteers who received it to improve skin [47], Lact. casei when consumed by intensive care units (ICU) patients [48]. Lact. casei, Lact. fermentum [49] and Lact. casei HDS-01 [50] strains have proven to be safe for the development of functional fermented products. It should be noted that in clinical practice, cases of local and systemic infectious processes induced by LAB are extremely rare. For example, infectious endocarditis and bacteremia after probiotic therapy were found only in 0.05–0.40% of cases [51, 52].
Complications after probiotic therapy were most commonly reported in critically ill patients in ICU and in patients with severe immunodeficiencies [1, 6, 53]. Given this, before probiotic therapy, a careful analysis is necessary for the presence of certain microecological disorders, which can lead to the immunodeficiency conditions, when even weakly virulent bacteria can induce the development of infectious processes. It can be considered to include in the protocols of the safety study of each particular LAB strain the determination of their effect on the microbiota of gut or other biotopes. Such studies have particular importance, since probiotic bacteria may influence the levels of potentially harmful intestinal bacterial enzymes (β-glucosidase and β-glucuronidase) [54].
The population of beneficial bacteria such as LAB and bifidobacteria was significantly increased in mice and the number of opportunistic bacteria, such as staphylococci, microscopic fungi, and Pseudomonas spp. decreased after administration of Lact. casei IMV B-7280 strain in comparison with mice of control groups. Oral administration of this LAB strain normalized microbiota of different biotopes in the case of experimental infections and inflammatory processes, and metabolic syndrome [36, 55] and demonstrated efficacy in simultaneous administration with cerium nanoparticles as a novel prebiotic, and significantly enhanced positive individual effects [56].
Although this study has limitations (e.g., one sex of animals used), the Lactobacillus casei IMV B-7280 strain was thoroughly assessed via methods which adhere recent approaches for assessing safety of probiotics needed before using in human subjects [33]; and the results can justify to conduct further clinical trials according to recent knowledge in the safety of probiotics [57, 58], current regulations, and future vision of food safety [59].
The potential of using probiotics in periocular Lactobacillus and Bifidobacterium spp. as a preventive measure against viral epidemics like COVID-19 [60] motivates new studies to determine whether the prescription of routine longer treatment oral probiotics is completely safe. In this respect, further studies can be considered to include a robust preclinical assessment longer effects (also, e.g., 30- to 90-day outcomes) and can be considered to model chronic toxicity [61], and other animal models (e.g., rats) should be involved in the studies.
Conclusion
The results of this study showed that Lact. casei IMV B-7280 strain is safe at a dose up to 1010 CFU/animal during 3- and 7-day oral administration to mice and has a positive effect on the gut microbiota and can be considered a potentially safe probiotic for humans.
Acknowledgements
The research work was partially done as a part of the project titled «Development of probiotics for the prevention and treatment of infectious-inflammatory diseases» (DZ/48-2018) that was funded by the Ministry of Education and Science of Ukraine. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- LAB
Lactobacillus (lactic acid bacteria)
- QPS
Qualified presumption of safety status
- GRAS
(Generally regarded as safe) status
- CFU
Colony forming units
- Lg
Logarithm
- WBC
Leukocytes
- LY
Lymphocytes
- MO
Monocytes
- GR
Granulocytes
- RBC
Erythrocytes
- Hgb
Hemoglobin
- HCT
Hematocrit
- MCV
Average size of red blood cells
- MCH
Hemoglobin content of red blood cells
- MCHC
Mean hemoglobin content of red blood cells, not whole blood
- RDW
Red cell distribution width
- PLT
Absolute platelet content
- PTC
Plasma thromboplastin component
- MPV
Mean platelet volume
- PDW
Platelet distribution width
Availability of Data and Materials
Data are available on reasonable request to the authors.
Declarations
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams NT (2010) Probiotics. Am J Health Syst Pharm 67:449-458. 10.2146/ajhp090168 [DOI] [PubMed]
- 2.Falagas ME, Betsi GI, Tokas T, Athanasiou S (2006) Probiotics for prevention of recurrent urinary tract infections in women: a review of the evidence from microbiological and clinical studies. Drugs 66:1253-1261. 10.2165/00003495-200666090-00007 [DOI] [PubMed]
- 3.Di Cerbo A, Palmieri B, Aponte M, Morales-Medina JC, Iannitti T (2016) Mechanisms and therapeutic effectiveness of lactobacilli. J Clin Pathol 69:187-203. 10.1136/jclinpath-2015-202976 [DOI] [PMC free article] [PubMed]
- 4.Kasińska MA, Drzewoski J (2015) Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn 125:803-813. 10.20452/pamw.3156 [DOI] [PubMed]
- 5.Hendijani F, Akbari V (2018) Probiotic supplementation for management of cardiovascular risk factors in adults with type II diabetes: A systematic review and meta-analysis. Clin Nutr 37:532-541. 10.1016/j.clnu.2017.02.015 [DOI] [PubMed]
- 6.Didari T, Mozaffari S, Nikfar S, Abdollahi M (2015) Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol 21:3072-30784. 10.3748/wjg.v21.i10.3072 [DOI] [PMC free article] [PubMed]
- 7.Wang F, Feng J, Chen P, Liu X, Ma M, Zhou R, Chang Y, Liu J, Li J, Zhao Q (2017) Probiotics in Helicobacter pylori eradication therapy: Systematic review and network meta-analysis. Clin Res Hepatol Gastroenterol 41:466-475. 10.1016/j.clinre.2017.04.004 [DOI] [PubMed]
- 8.Huang R, Wang K, Hu J (2016) Effect of probiotics on depression: a systematic review and meta-Analysis of randomized controlled trials. Nutrients 8:483. 10.3390/nu8080483 [DOI] [PMC free article] [PubMed]
- 9.Chang YS, Trivedi MK, Jha A, Lin YF, Dimaano L, García-Romero MT (2016) Synbiotics for prevention and treatment of atopic dermatitis: a meta-analysis of randomized clinical trials. JAMA Pediatr 170:236-242. 10.1001/jamapediatrics.2015.3943 [DOI] [PubMed]
- 10.Hooijmans CR, de Vries RB, Rovers MM, Gooszen HG, Ritskes-Hoitinga M (2012) The effects of probiotic supplementation on experimental acute pancreatitis: a systematic review and meta-analysis. PLoS One 7:e48811. 10.1371/journal.pone.0048811 [DOI] [PMC free article] [PubMed]
- 11.Athalye-Jape G, Rao S, Patole S (2018) Effects of probiotics on experimental necrotizing enterocolitis: a systematic review and meta-analysis. Pediatr Res 83:16-22. 10.1038/pr.2017.218 [DOI] [PubMed]
- 12.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, Raoult D (2012) Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animalis. Microb Pathog 53:100-108. 10.1016/j.micpath.2012.05.007 [DOI] [PubMed]
- 13.Mokrozub VV, Lazarenko LM, Babenko LP, Shinkarenko LM, Demchenko OM, Spivak MY, Bila VV. Antistaphylococcal action of lacto- and bifidobacteria and interleukin-2. Mikrobiol Z. 2013;75(6):17–21. [PubMed] [Google Scholar]
- 14.Bo L, Li J, Tao T, Bai Y, Ye X, Hotchkiss RS, Kollef MH, Crooks NH, Deng X (2014) Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 10:CD009066. 10.1002/14651858.CD009066.pub2 [DOI] [PMC free article] [PubMed]
- 15.Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, Soffer EE, Spiegel BM, Moayyedi P. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1547–1561. doi: 10.1038/ajg.2014.202. [DOI] [PubMed] [Google Scholar]
- 16.Korterink JJ, Ockeloen L, Benninga MA, Tabbers MM, Hilbink M, Deckers-Kocken JM. Probiotics for childhood functional gastrointestinal disorders: a systematic review and meta-analysis. Acta Paediatr. 2014;103:365–372. doi: 10.1111/apa.12513. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira LVN, Bastos RW, Ribeiro NQ, Costa MC, Acurcio LB, Rocha KM, Santos JRA, de Carvalho Cruz R, Soares BM, Santos DA (2017) In vivo probiotic and antimicrobial photodynamic therapy as alternative therapies against cryptococcosis are ineffective. Vet Microbiol 211:169-173. 10.1016/j.vetmic.2017.08.015 [DOI] [PubMed]
- 18.Wojtyniak K, Szajewska H (2017) Systematic review: probiotics for functional constipation in children. Eur J Pediatr 176:1155-1162. 10.1007/s00431-017-2972-2 [DOI] [PMC free article] [PubMed]
- 19.Zhong C, Qu C, Wang B, Liang S, Zeng B (2017) Probiotics for preventing and treating small intestinal bacterial overgrowth: a meta-analysis and systematic review of current evidence. J Clin Gastroenterol 51:300-311. 10.1097/MCG.0000000000000814 [DOI] [PubMed]
- 20.Suez J, Zmora N, Zilberman-Schapira G et al (2018) Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous. Cell 174:1406-1423.e16. 10.1016/j.cell.2018.08.047 [DOI] [PubMed]
- 21.Ng QX, Soh AYS, Venkatanarayanan N, Ho CYX, Lim DY, Yeo WS (2019) A Systematic Review of the Effect of Probiotic Supplementation on Schizophrenia Symptoms. Neuropsychobiology 78:1-6. 10.1159/000498862 [DOI] [PubMed]
- 22.Timmerman HM, Niers LE, Ridwan BU, Koning CJ, Mulder L, Akkermans LM, Rombouts FM, Rijkers GT (2007) Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin Nutr 26:450-459. 10.1016/j.clnu.2007.04.008 [DOI] [PubMed]
- 23.Zmora N, Zilberman-Schapira G, Suez J et al (2018) Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features. Cell 174:1388-1405.e21. 10.1016/j.cell.2018.08.041 [DOI] [PubMed]
- 24.Salminen S, von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, Fondén R, Saxelin M, Collins K, Mogensen G, Birkeland SE, Mattila-Sandholm T (1998) Demonstration of safety of probiotics – a review. Int J Food Microbiol 44:93-106. 10.1016/s0168-1605(98)00128-7 [DOI] [PubMed]
- 25.Marteau P, Seksik P, Jian R (2002) Probiotics and health: new facts and ideas. Curr Opin Biotechnol 13:486-489. 10.1016/s0958-1669(02)00368-3 [DOI] [PubMed]
- 26.Snydman DR (2008) The safety of probiotics. Clin Infect Dis 46 Suppl 2:S104-11; discussion S144-151. 10.1086/523331 [DOI] [PubMed]
- 27.Mayes T, Gottschlich MM, James LE, Allgeier C, Weitz J, Kagan RJ (2015) Clinical safety and efficacy of probiotic administration following burn injury. J Burn Care Res 36:92-99. 10.1097/BCR.0000000000000139 [DOI] [PubMed]
- 28.Marteau PR (2002) Probiotics in clinical conditions. Clin Rev Allergy Immunol 22:255-273. 10.1007/s12016-002-0011-0 [DOI] [PubMed]
- 29.Shanahan F (2012) A commentary on the safety of probiotics. Gastroenterol Clin North Am 41:869-868. 10.1016/j.gtc.2012.08.006 [DOI] [PubMed]
- 30.Doron S, Snydman DR (2015) Risk and safety of probiotics. Clin Infect Dis 60 Suppl 2(Suppl 2):S129-34. 10.1093/cid/civ085 [DOI] [PMC free article] [PubMed]
- 31.Guidelines for the Evaluation of Probiotics (2002) Food Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food London Ontario, Canada April 30 and May 1
- 32.Ishibashi N, Yamazaki S (2001) Probiotics and safety. Am J Clin Nutr 73(2 Suppl):465S-470S. 10.1093/ajcn/73.2.465s [DOI] [PubMed]
- 33.Ricci A, et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 8: suitability of taxonomic units notified to EFSA until March 2018. EFSA Journal. 2018;16:e05315. doi: 10.2903/j.efsa.2018.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Spivak MY (2018) Specific properties of probiotic strains: relevance and benefits for the host. EPMA J 9:205-223. 10.1007/s13167-018-0132-z [DOI] [PMC free article] [PubMed]
- 35.Lazarenko LM, Babenko LP, Bubnov RV, Demchenko OM, Zotsenko VM, BoykoSpivak MYa, NV. Immunobiotics are the novel biotech drugs with antibacterial and immunomodulatory properties. Microbiol J. 2017;79:66–75. [Google Scholar]
- 36.Zhou JS, Shu Q, Rutherfurd KJ, Prasad J, Birtles MJ, Gopal PK, Gill HS (2000) Safety assessment of potential probiotic lactic acid aacterial strains Lactobacillus rhamnosus HN001, Lb. acidophilus HN017, and Bifidobacterium lactis HN019 in BALB/c mice. Int J Food Microbiol 56:87-96. 10.1016/s0168-1605(00)00219-1 [DOI] [PubMed]
- 37.Gill HS, Shu Q, Lin H, Rutherfurd KJ, Cross ML (2001) Protection agains translocating Salmonella typhimurium infection in mice by feeding the inununo-enhancing probiotic Lactohucillus rhumnosus strain HN001. Med Microbiol Immunol 190:97-104. 10.1007/s004300100095 [DOI] [PubMed]
- 38.Huang L, Chiang Chiau JS, Cheng ML, Chan WT, Jiang CB, Chang SW, Yeung CY, Lee HC (2019) SCID/NOD mice model for 5-FU induced intestinal mucositis: Safety and effects of probiotics as therapy. Pediatr Neonatol 60:252-260. 10.1016/j.pedneo.2018.07.007 [DOI] [PubMed]
- 39.Moe SM. Disorders involving calcium, phosphorus, and magnesium. Prim Care. 2008;35(215–237):v–vi. doi: 10.1016/j.pop.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watari E, Taketani Y, Kitamura T, Tanaka T, Ohminami H, Abuduli M, Harada N, Yamanaka-Okumura H, Yamamoto H, Takeda E (2015) Fluctuating plasma phosphorus level by changes in dietary phosphorus intake induces endothelial dysfunction. J Clin Biochem Nutr 56:35-42. 10.3164/jcbn.14-96 [DOI] [PMC free article] [PubMed]
- 41.Lazarenko LM, Babenko LP, Spivak MY (2019) Immunomodulatory effect of probiotic strain Lactobacillus casei IMV B-7280 on physiological norm in experimental animals. Mikrobiol Z 51:69-82. Ukrainian. 10.15407/microbiolj81.06.069
- 42.Wagner RD, Warner T, Roberts L, Farmer J, Balish E. Colonization of congenitally immunodeficient mice with probiotic bacteria. Infect Immun. 1997;65:3345–3351. doi: 10.1128/iai.65.8.3345-3351.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asahara T, Takahashi M, Nomoto K, Takayama H, Onoue M, Morotomi M, Tanaka R, Yokokura T, Yamashita N (2003) Assessment of safety of lactobacillus strains based on resistance to host innate defense mechanisms. Clin Diagn Lab Immunol 10:169-173. 10.1128/cdli.10.1.169-173.2003 [DOI] [PMC free article] [PubMed]
- 44.Zavišić G, Ristić S, Petrièević S, Novaković Jovanović J, Janać Petković B, Strahinić I, Piperski V (2015) Characterisation and preliminary lipid-lowering evaluation of Lactobacillus isolated from a traditional Serbian dairy product. Benef Microbes 6:119-128. 10.3920/BM2014.0018 [DOI] [PubMed]
- 45.Zhang H, Wang Y, Sun J, Guo Z, Guo H, Ren F (2013) Safety evaluation of Lactobacillus paracasei subsp. paracasei LC-01, a probiotic bacterium. J Microbiol 51:633-638. 10.1007/s12275-013-3336-x [DOI] [PubMed]
- 46.Srinivasan R, Meyer R, Padmanabhan R, Britto J (2006) Clinical safety of Lactobacillus casei shirota as a probiotic in critically ill children. J Pediatr Gastroenterol 42:171-173. 10.1097/01.mpg.0000189335.62397.cf. [DOI] [PubMed]
- 47.Saito Y, Mihara T, Maruyama K, Saito J, Ikeda M, Tomonaga A, Kumagai T (2017) Effects of intake of Lactobacillus casei subsp. casei 327 on skin conditions: a randomized, double-blind, placebo-controlled, parallel-group study in women. Biosci Microbiota Food Health 36:111-120. 10.12938/bmfh.16-031 [DOI] [PMC free article] [PubMed]
- 48.Alberda C, Marcushamer S, Hewer T, Journault N, Kutsogiannis D (2018) Feasibility of a Lactobacillus casei Drink in the Intensive Care Unit for Prevention of Antibiotic Associated Diarrhea and Clostridium difficile. Nutrients 10:539. 10.3390/nu10050539 [DOI] [PMC free article] [PubMed]
- 49.de Souza BMS, Borgonovi TF, Casarotti SN, Todorov SD, Penna ALB (2019) Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics Antimicrob Proteins 11:382-396. 10.1007/s12602-018-9406-y [DOI] [PubMed]
- 50.Zhao D, Wang Y, Na J, Ping W, Ge J (2019) The response surface optimization of β-mannanase produced by Lactobacillus casei HDS-01 and its potential in juice clarification. Prep Biochem Biotechnol 49:202-207. 10.1080/10826068.2019.1566151 [DOI] [PubMed]
- 51.Gasser F. Safety of lactic-acid bacteria and their occurrence in human clinical infections. Bulletin de L'Institut Pasteur. 1994;92:45–67. [Google Scholar]
- 52.Saxelin M, Chuang NH, Chassy B, Rautelin H, Mäkelä PH, Salminen S, Gorbach SL (1996) Lactobacilli and bacteremia in southern Finland, 1989–1992. Clin Infect Dis 22:564-566. 10.1093/clinids/22.3.564 [DOI] [PubMed]
- 53.Singhi SC, Kumar S. (2016) Probiotics in critically ill children. F1000Res 5:F1000 Faculty Rev-407. 10.12688/f1000research.7630.1
- 54.Huang Y, Kotula L, Adams MC (2003) The in vivo assessment of safety and gastrointestinal survival of an orally administered novel probiotic, Propionibacterium jensenii 702, in a male Wistar rat model. Food Chem Toxicol 41:1781-1787. 10.1016/s0278-6915(03)00215-1 [DOI] [PubMed]
- 55.Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Demchenko OA, Nechypurenko OV, Spivak MY (2017) Comparative study of probiotic effects of Lactobacillus and Bifidobacteria strains on cholesterol levels, liver morphology and the gut microbiota in obese mice. EPMA J 8:357-376. 10.1007/s13167-017-0117-3 [DOI] [PMC free article] [PubMed]
- 56.Bubnov R, Babenko L, Lazarenko L et al (2019) Can tailored nanoceria act as a prebiotic? Report on improved lipid profile and gut microbiota in obese mice. EPMA J 10, 317–335. 10.1007/s13167-019-00190-1 [DOI] [PMC free article] [PubMed]
- 57.Pradhan D, et al. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control. 2020;108:106872. doi: 10.1016/J.FOODCONT.2019.106872. [DOI] [Google Scholar]
- 58.Pradhan D, Singh R, Tyagi A, et al. Assessing the safety and efficacy of Lactobacillus plantarum MTCC 5690 and Lactobacillus fermentum MTCC 5689 in colitis mouse model. Probiotics & Antimicro. Prot. 2019;11:910–920. doi: 10.1007/s12602-018-9489-5. [DOI] [PubMed] [Google Scholar]
- 59.Bronzwaer S et al (2019) Food Safety Regulatory Research Needs 2030 EFSA J. 17(7):e170622. 10.2903/j.efsa.2019.e170622 [DOI] [PMC free article] [PubMed]
- 60.Tiwari SK, Dicks LMT, Popov IV, Karaseva A, Ermakov AM, Suvorov A, Tagg JR, Weeks R, Chikindas ML. Probiotics at war against viruses: what is missing from the picture? Front. Microbiol. 2020;11:1877. doi: 10.3389/fmicb.2020.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Druart C, Plovier H, Van Hul M, et al. Toxicological safety evaluation of pasteurized Akkermansia muciniphila. J Appl Toxicol. 2020 doi: 10.1002/jat.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request to the authors.