Abstract
Objective
To determine whether stable polymorphisms that define mitochondrial haplogroups in mitochondrial DNA (mtDNA) are associated with Pick disease risk, we genotyped 52 pathologically confirmed cases of Pick disease and 910 neurologically healthy controls and performed case-control association analysis.
Methods
Fifty-two pathologically confirmed cases of Pick disease from Mayo Clinic Florida (n = 38) and the University of Pennsylvania (n = 14) and 910 neurologically healthy controls collected from Mayo Clinic Florida were genotyped for unique mtDNA haplogroup-defining variants. Mitochondrial haplogroups were determined, and in a case-control analysis, associations of mtDNA haplogroups with risk of Pick disease were evaluated with logistic regression models that were adjusted for age and sex.
Results
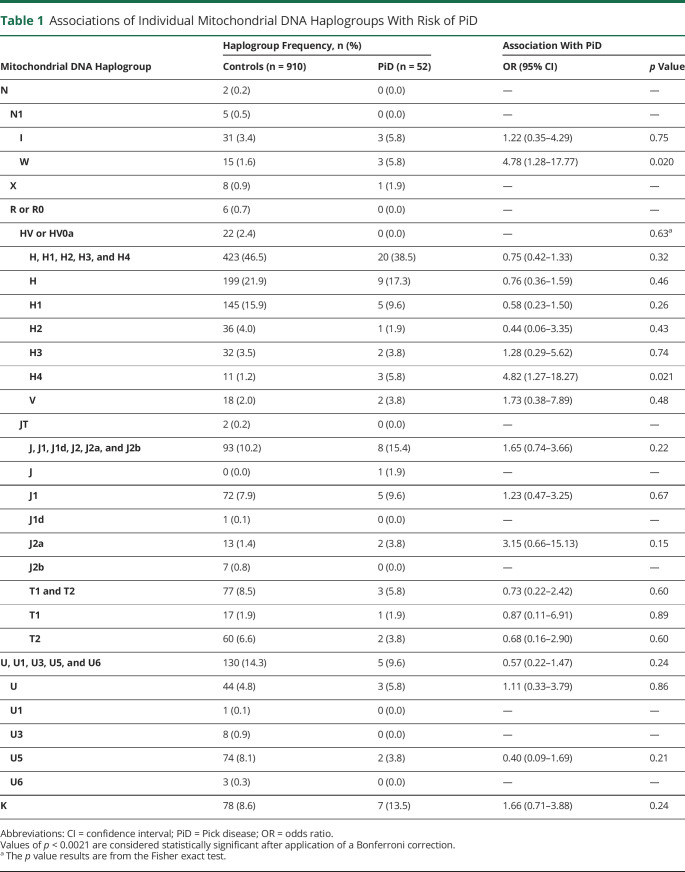
No individual mtDNA haplogroups or superhaplogroups were significantly associated with risk of Pick disease after adjustment for multiple testing (p < 0.0021, considered significant). However, nominally significant (p < 0.05) associations toward an increased risk of Pick disease were observed for mtDNA haplogroup W (5.8% cases vs 1.6% controls, odds ratio [OR] 4.78, p = 0.020) and subhaplogroup H4 (5.8% cases vs 1.2% controls, OR 4.82, p = 0.021).
Conclusion
Our findings indicate that mtDNA variation is not a disease driver but may influence disease susceptibility. Ongoing genetic assessments in larger cohorts of Pick disease are currently underway.
Pick disease (PiD) is a rare and sporadic form of frontotemporal dementia that typically develops in those >65 years of age.1 PiD causes dementia, aphasia, and personality and behavioral changes.2 Although debated, PiD is classified predominantly by biochemical classifications whereby there is frontotemporal lobar degeneration and the presence of ballooned neurons and argyrophilic inclusion Pick bodies, which consist of hyperphosphorylated 3-repeat tau aggregates.2,3 Rare mutations in MAPT, which encodes tau protein, have been identified in a handful of cases1,4; however, the genetic etiology of PiD remains unresolved.
Mitochondrial health is important in healthy aging, and mitochondrial dysfunction has been reported in neurodegeneration.5 Mitochondria contain their own genome (mitochondrial DNA [mtDNA]) that encodes 13 vital oxidative phosphorylation (OXPHOS) subunits that are important in maintaining mitochondrial health. mtDNA contains stable polymorphisms that identify individuals to specific mitochondrial haplogroups.6 These polymorphisms cause subtle changes in OXPHOS subunits, which create unique bioenergetic profiles.7 Considering that PiD is age related and sporadic and that mitochondrial dysfunction has been reported in other tauopathies, we hypothesized that mitochondrial haplogroup background may influence PiD risk. Therefore, the aim of this study was to evaluate associations between mtDNA haplogroups and risk of PiD in a series of pathologically confirmed cases of PiD and controls.
Methods
Study Participants and Data Collection
Fifty-two pathologically confirmed cases with sporadic PiD and 910 neurologically healthy controls were included. Cases were determined postmortem according to published criteria.3 Frozen brain tissue was provided for cases of PiD from the Mayo Clinic Brain Bank for neurodegenerative disorders in Jacksonville, FL (n = 38), and from the University of Pennsylvania (n = 14). Peripheral blood samples were collected from controls, absent of neurologic disease or known family history of parkinsonism or dementia, through the Movement Disorders Center, Mayo Clinic Jacksonville, FL. All participants were unrelated, White, and non-Hispanic.
Standard Protocol Approvals, Registrations, and Patient Consents
The Mayo Clinic Institutional Review Board approved this study, and written informed consent was obtained from all individuals (or next of kin) before their participation in the study (consent for research).
Genetic Analysis
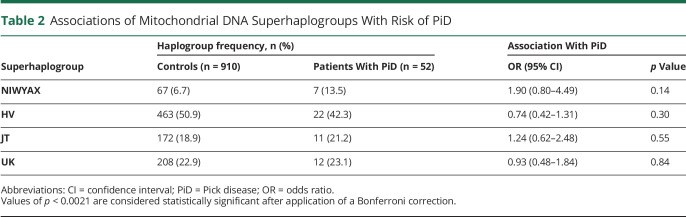
mtDNA was genotyped with Sequenom MassARRAY iPlex technology to detect unique, haplogroup-defining mtDNA sequence variants.6 Individual mtDNA haplogroups were determined for each participant by a single user, as previously described.8 Phylogenetically related haplogroups were combined into 4 superhaplogroups and were also assessed (table 2). Non-European haplogroups (non-IWXRHVJTUK) were excluded. Haplogroups that occurred in <10 participants were summarized descriptively but were not included in association analysis. All samples were negative for MAPT coding mutations after screening using whole-genome or Sanger sequencing.
Table 2.
Associations of Mitochondrial DNA Superhaplogroups With Risk of PiD
Statistical Analysis
Associations of individual mtDNA haplogroups and superhaplogroups with risk of PiD were examined with logistic regression models that were adjusted for age and sex. Odds ratios (ORs) and 95% confidence intervals were estimated. If the given haplogroup did not occur in either controls or cases of PiD, risk of PiD associations was assessed with the Fisher exact test. A Bonferroni correction was applied to adjust for the number of haplogroups and superhaplogroups that were examined in association analysis, after which values of p < 0.0021 were considered statistically significant. All statistical tests were 2 sided. Statistical analyses were performed with R (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
Data Availability
Individual mtDNA genotype or summary statistics data are available on request.
Results
Neuropathologically confirmed cases (n = 52) and 910 controls were assessed; 57.7% of cases of PiD and 42.6% of controls were male. Median age of cases of PiD was 68 years (minimum 55 years, maximum 90 years), and median age of controls was 79 years (minimum 41 years, maximum 102 years). Associations of individual mtDNA haplogroups with risk of PiD are detailed in table 1. In analysis adjusted for age and sex, to remove any potential confounding influence of these 2 variables, no individual mtDNA haplogroups were associated with PiD after correction for multiple testing. However, nominally significant (p < 0.05) associations with increased risk of PiD were observed for haplogroup W (cases 5.8%, controls 1.6%, OR 4.78, p = 0.020) and subhaplogroup H4 (cases 5.8%, controls 1.2%, OR 4.82, p = 0.021). No associations between superhaplogroups and risk of PiD were observed (table 2).
Table 1.
Associations of Individual Mitochondrial DNA Haplogroups With Risk of PiD
Discussion
mtDNA acquired stable polymorphisms as humans migrated and adapted to new environments. These variants define specific mitochondrial haplogroups6 with unique metabolic bioefficiencies.7 Accelerated aging occurs from excessive reactive oxygen species production through mitochondrial stress or dysfunction,9 and certain mitochondrial haplogroups have been associated with neurodegenerative disorders.5 In our study, we assessed associations between individual mitochondrial haplogroups and risk of PiD. Although no associations that withstood correction for multiple testing were identified, our findings did suggest that 2 independent mitochondrial haplogroups, W and H4, may be associated with increased risk of PiD, as evidenced by nominally significant p values and strong ORs (both >4.75).
Both haplogroup W and subhaplogroup H4 are defined by variants located in OXPHOS complex I subunits: missense variant rs28358585 (A3505G) in MT-ND1 and synonymous variant rs41419549 (T5004C) in MT-ND2. Complex I defects have consistently been reported in neurodegeneration, including frontotemporal dementia.10 Mitochondrial subhaplogroup H4 has previously been associated with significantly increased risk of corticobasal degeneration (CBD), and mitochondrial haplogroup W has been linked with increased disease duration in progressive supranuclear palsy (PSP).8 Both CBD and PSP are 4-repeat tauopathies, which suggests that mitochondrial haplogroup background may be influencing tau aggregation independently of MAPT genetic variation. Because the mitochondrial haplogroup W effects in PiD and PSP are opposing, it will be important to further characterize tau aggregation differences between brain regions in both diseases to determine whether the associations reported are region-specific neuropathologic effects.
Clinically, PiD is often misdiagnosed as Alzheimer disease, and the rare and sporadic nature of PiD makes it difficult to study. Although this study included the largest cohort of neuropathologically confirmed cases of PiD to date, the number of samples was still relatively small for a genetic association study, and power to detect associations between haplogroups and PiD was limited. Due to the corresponding possibility of a type II error (i.e., a false-negative finding), especially for rarer haplogroups and after correction for multiple testing, we deemed it reasonable to highlight several nominally significant suggestive associations with the hope that meta-analytic studies will be able to further expand on these initial findings.
Furthermore, it is important for future studies to consider other mitochondrial factors that may be influencing disease pathology and overall risk. mtDNA is a multicopy genome that can be present in hundreds to thousands of copies per cell, depending on its energy demands. Therefore heteroplasmy (proportion of wild-type to mutant mtDNA molecules) is a potential limiting factor in this study. We assessed cerebellum tissue from cases of PiD, which is considered unaffected tissue, however, and we assumed that heteroplasmic levels were low and should not be influencing our haplogroups reported. Moreover, the genotyping methods are considered sensitive enough to accurately determine alleles from pools of recombinants for mtDNA-based population studies.8 Finally, without available genome-wide population control markers, we cannot rule out the possibility that population stratification could have had a small influence on our findings. Future studies should investigate mtDNA variation in affected vs unaffected tissues in PiD to determine whether mtDNA background changes across brain regions and whether heteroplasmic levels influence tau aggregation profiles and overall disease risk. Using whole-genome sequencing data from PiD will be beneficial because it will report rarer variants and regions with lower coverage, which may show whether mtDNA deletions play a role in disease.
We report evidence that haplogroups W and subhaplogroup H4 may be associated with PiD, which has also been recorded in other related tauopathies. These data shows the need to understand better how tau aggregation may be influenced by mitochondrial health determined by mtDNA background.
Acknowledgment
The authors thank the patients, donors, and caregivers who participated in this research, without whom this work would not have been possible. They also acknowledge Audrey Strongosky, who was study coordinator and former Udall grant administrator for Z.K.W. This work was supported in part by; the Mayo Clinic Florida Morris K. Udall Parkinson's Disease Research Center of Excellence (National Institute of Neurological Disorders and Stroke [NINDS] P50 No. NS072187), and American Parkinson Disease Association (APDA), Mayo Clinic Information and Referral Center, and APDA Center for Advanced Research. Samples included in this study were clinical controls or brain donors to the brain bank at Mayo Clinic in Jacksonville, which is supported by CurePSP and the Tau Consortium. R.R.V. designed genotype assays and performed all laboratory analysis, while M.G.H. conducted and is responsible for all statistical data analysis. O.A.R. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Glossary
- CBD
corticobasal degeneration
- mtDNA
mitochondrial DNA
- OR
odds ratio
- OXPHOS
oxidative phosphorylation
- PiD
Pick disease
- PSP
progressive supranuclear palsy
Appendix. Authors

Contributor Information
Rebecca R. Valentino, Email: valentino.rebecca@mayo.edu.
Michael G. Heckman, Email: heckman.michael@mayo.edu.
Patrick W. Johnson, Email: johnson.patrick3@mayo.edu.
Matthew C. Baker, Email: baker.matt@mayo.edu.
Alexandra I. Soto-Beasley, Email: beasley.alexandra@mayo.edu.
Ronald L. Walton, Email: walton.ronald@mayo.edu.
Shunsuke Koga, Email: koga.shunsuke@mayo.edu.
Shanu F. Roemer, Email: roemer.shanu@mayo.edu.
EunRan Suh, Email: suher@pennmedicine.upenn.edu.
Ryan J. Uitti, Email: uitti@mayo.edu.
John Q. Trojanowski, Email: trojanow@upenn.edu.
Murray Grossman, Email: mgrossma@pennmedicine.upenn.edu.
Vivianna M. Van Deerlin, Email: vivianna@upenn.edu.
Rosa Rademakers, Email: rosa.rademakers@uantwerpen.vib.be.
Zbigniew K. Wszolek, Email: wszolek.zbigniew@mayo.edu.
Dennis W. Dickson, Email: dickson.dennis@mayo.edu.
Study Funding
O.A.R. and D.W.D. are both supported by NINDS Tau Center Without Walls Program (U54-NS100693) and NIH (UG3-NS104095). O.A.R. is supported by the NIH (P50-NS072187; R01- NS078086; U54-NS100693; U54- NS110435), Department of Defense (W81XWH-17-1-0249), the Michael J. Fox Foundation, the Little Family Foundation, the Mayo Clinic Foundation, and the Center for Individualized Medicine. D.W.D. receives research support from the NIH (P50-AG016574; U54-NS100693; P01-AG003949), CurePSP, the Tau Consortium, and the Robert E. Jacoby Professorship. Z.K.W. is partially supported by the Mayo Clinic Center for Regenerative Medicine, gifts from The Sol Goldman Charitable Trust, the Donald G. and Jodi P. Heeringa Family, the Haworth Family Professorship in Neurodegenerative Diseases fund, and The Albertson Parkinson's Research Foundation. S.K. is supported by a post-doctoral fellowship from the Karin & Sten Mortstedt CBD Solutions AB. ES, JQT, MG, and VMVD are supported by NIH AG17586 and AG10124.
Disclosure
R.R.V., M.G.H., P.W.J., M.C.B., A.I.S.-B., R.L.W., S.K., S.F.R., E.S., R.J.U., J.Q.T., M.G., V.M.V.D., R.R., D.W.D., and O.A.R. have no financial relationships or sponsorships to disclose. Z.K.W. serves as principal investigator or co–principal investigator on Abbvie, Inc (M15-562 and M15-563), Biogen, Inc (228PD201) grant, and Biohaven Pharmaceuticals, Inc (BHV4157-206 and BHV3241-301). He serves as co–principal investigator of the Mayo Clinic APDA Center for Advanced Research. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011649 for full disclosures.
References
- 1.Tacik P, DeTure M, Hinkle KM, et al. A novel tau mutation in exon 12, p.Q336H, causes hereditary Pick disease. J Neuropathol Exp Neurol 2015;74:1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick's Disease. Arch Neurol 2001;58:1803–1809. [DOI] [PubMed] [Google Scholar]
- 3.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 2011;45:384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bronner IF, ter Meulen BC, Azmani A, et al. Hereditary Pick's disease with the G272V tau mutation shows predominant three-repeat tau pathology. Brain 2005;128:2645–2653. [DOI] [PubMed] [Google Scholar]
- 5.Chinnery PF, Gomez-Duran A. Oldies but goldies mtDNA population variants and neurodegenerative diseases. Front Neurosci 2018;12:682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Oven M, Kayser M. Updated comprehensive phylogenetic tree of global human mitochondrial DNA variation. Hum Mutat 2009;30:E386–E394. [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Durán A, Pacheu-Grau D, López-Gallardo E, et al. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet 2010;19:3343–3353. [DOI] [PubMed] [Google Scholar]
- 8.Valentino RR, Tamvaka N, Heckman MG, et al. Associations of mitochondrial genomic variation with corticobasal degeneration, progressive supranuclear palsy, and neuropathological tau measures. Acta Neuropathol Commun 2020;8:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med 2013;60:1–4. [DOI] [PubMed] [Google Scholar]
- 10.Grazina M, Silva F, Santana I, et al. Frontotemporal dementia and mitochondrial DNA transitions. Neurobiol Dis 2004;15:306–311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual mtDNA genotype or summary statistics data are available on request.