Abstract
Objective
To determine the incidence of venous thromboembolism (VTE) in lower-grade gliomas (LGGs, WHO grades II–III) and to stratify the risk of VTE by molecular subtype in gliomas grade II–IV, we performed a retrospective review of a large cohort of patients with glioma.
Methods
We performed a retrospective analysis of a cohort of 635 adult patients with glioma with molecular testing seen at the University of Virginia with a diagnosis of diffuse glioma established from January 2005 to August 2017. Estimates of cumulative incidence of VTE were calculated with death as competing risk; significance was determined using the Fine and Gray model.
Results
Of 256 patients with LGG, 81 were isocitrate dehydrogenase (IDH) wild-type; 113 IDH mutant, 1p/19q codeleted; and 62 IDH mutant, 1p/19q intact. With a median follow-up of 17.9 months, the overall cumulative incidence of VTE was 8.2% for grade II (147 patients), 9.2% for grade III (109 patients), and 30.5% for grade IV (334 patients). In grade II–IV patients, absence of an IDH mutation was associated with a threefold increase in VTE risk when compared to IDH-mutant patients (hazard ratio 3.06, 95% confidence interval 2.03–4.64). In patients with glioblastoma, there was no difference in VTE incidence according to O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status.
Conclusion
Patients with LGG have a higher VTE risk compared to the general population, which is decreased, but not eliminated, in the presence of an IDH mutation. MGMT promoter methylation in glioblastoma does not affect the incidence of VTE.
Venous thromboembolism (VTE) is a known complication of glioblastoma (GBM, grade IV of the WHO classification), with an estimated incidence of 20%–30% over the course of the disease, and an association with a 30% increase in the risk of death within 2 years.1,2 However, the incidence of VTE in lower-grade gliomas (LGGs, WHO grades II and III) is not clearly established. Multiple risk factors have been associated with increased risk of VTE, including some that are applicable to thromboembolic risk in general (such as prior history of VTE or postoperative immobility) and others that are specific for gliomas (e.g., tumor size >5 cm or subtotal resection).3 In recent years, the classification of gliomas has shifted from a purely histologic perspective to a molecular paradigm, as reflected by the 2016 WHO classification of CNS tumors, and there has been interest in correlating the new molecular subtypes with different clinical characteristics. It has been suggested that isocitrate dehydrogenase (IDH) mutation status dramatically affects the incidence of VTE in patients with glioma,4,5 but the influence of other cornerstone molecular alterations such as 1p/19q codeletion has not been well-characterized. In this study, we aimed to determine the incidence of VTE in a large cohort of patients with LGGs and to explore the differences in risk of VTE according to several molecular characteristics, including 1p/19q codeletion in patients with LGG, O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in patients with GBM, and IDH mutation in patients of all grades.
Methods
Patient Selection and Molecular Data
The Neuro-Oncology database, a prospectively collected database at University of Virginia that includes details of VTE events due to our group's longstanding interest in this complication in brain tumors, was screened for patients 18 years or older with a pathologic diagnosis of glioma grade II–IV obtained from January 2008 to June 2017. All patients for whom information regarding status of IDH mutation, 1p/19q codeletion, or MGMT promoter methylation was available were included in our data set. A few patients had their original diagnosis prior to 2008, but were included in our cohort by having a second or third surgery within the established dates. For all patients, the date of initial diagnosis was considered to be the date of the first surgery of any kind that established a pathologic diagnosis of glioma. Molecular information was obtained through review of pathology slides by neuropathologists at University of Virginia in all but 15 patients, for whom only local data were available. As is standard clinical practice at our institution, IDH mutational status was initially determined using immunohistochemistry testing for IDH1 R132H mutant protein, followed by genetic sequencing of IDH1 and IDH2 through PCR, in some cases with negative immunohistochemistry; immunohistochemistry-negative cases in patients older than 55 years were assigned IDHwt without routine further testing, per WHO guidelines. 1p/19q codeletion status was established through fluorescence in situ hybridization.
Outcome Measures
Medical charts were reviewed and information regarding age, sex, duration of follow-up, histologic diagnosis, pathologically confirmed progression, and VTE occurrence was collected. VTE was defined as an event of deep venous thrombosis (DVT), pulmonary embolus (PE), or cerebral venous sinus thrombosis (CVST), identified by radiologic studies and occurring any time after the date of histologic diagnosis of glioma. There were several patients with an initial diagnosis of LGG who over time had progression to GBM, confirmed through a pathologic sample; given that one of our specific objectives was to determine the incidence of VTE in LGG as it compares to GBM, episodes of VTE that occurred after progression to GBM in these patients were not counted. For patients with multiple VTE events throughout the follow-up period, only the first VTE was included.
Statistical Analysis
VTE incidence was calculated at 6 months and 1 and 2 years postdiagnosis overall, by grade, IDH mutation status, 1p/19q codeletion status, and MGMT promoter methylation status. The primary outcome was time to first VTE after pathologic diagnosis of glioma, with death as a competing event. Competing-risks regressions were performed, as these provide a more accurate estimate of the risk of an outcome over time for patients with short life expectancy than traditional survival analyses, while controlling for different lengths of follow-up given the disparity in prognosis in our patient groups (LGG vs GBM). Specifically, Fine and Gray models were used to compare differences in time to first VTE between grade, IDH mutation status, 1p/19q codeletion status, and MGMT promoter methylation status. Pairwise comparisons between categories were adjusted for multiple comparisons using a Sidak-Holm adjustment. Analyses were conducted using StataSE 15.
Standard Protocol Approvals, Registrations, and Patient Consents
Approval from an ethical standards committee to conduct this study was received. Institutional waiver of informed consent was obtained due to minimal patient risk associated with participation in the study.
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
Patient Demographics and Clinical Characteristics
A total of 635 patient charts were reviewed, of which 301 were patients with LGG and 334 were patients with GBM. Among patients with LGG, there were 256 patients whose charts contained enough information to be classified into one of the following molecular groups based on the WHO 2016 classification6: IDH mutant (IDHmt) and 1p/19q codeleted (113 patients, 44%), IDHmt and 1p/19q intact (62 patients, 24%), and IDH wild-type (IDHwt, 81 patients, 32%). IDHmt patients with no information on 1p/19q codeletion but evidence of ATRX loss were included in the 1p/19q-intact group, as these genetic alterations are generally exclusive. A separate analysis was performed dividing the 593 patients of grades II–IV with information on IDH mutation status into 2 groups, IDHwt (385 patients, 65%) and IDHmt (208 patients, 35%). Finally, patients with GBM were divided according to their MGMT status (263 patients with available data, 165 [63%] methylated and 98 [37%] unmethylated) (figure 1).
Figure 1. Patient Groups.
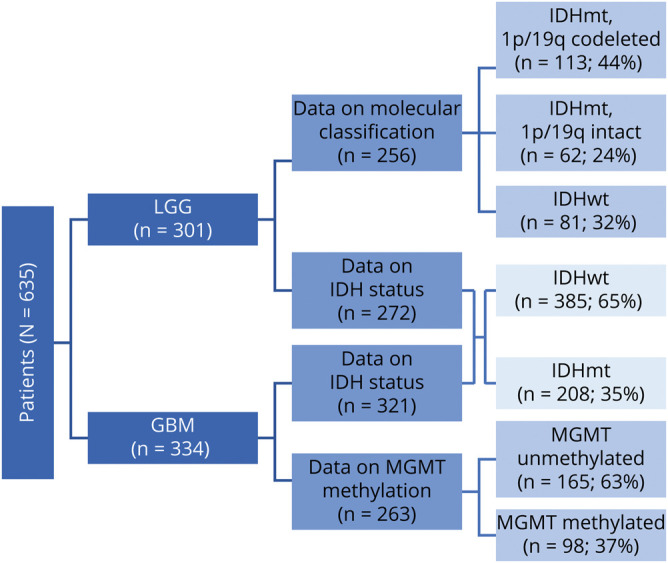
GBM = glioblastoma; IDH = isocitrate dehydrogenase; LGG = lower-grade glioma.
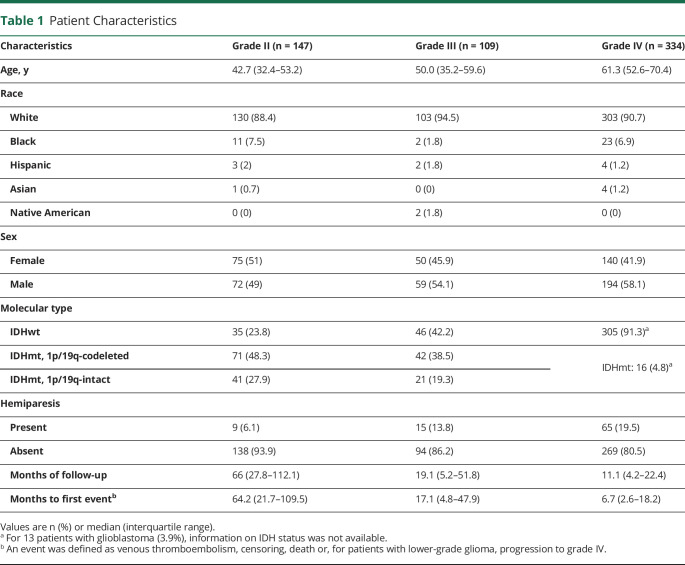
Table 1 summarizes the characteristics of our patients, including the 256 patients with LGG fully classified according to their molecular characteristics and all 334 patients with GBM. As expected, median age increased with tumor grade (42.7 years in grade II vs 50 in grade III and 61.3 in grade IV, p < 0.001), and there was a higher percentage of IDHwt tumors in higher tumor grades (23.8% in grade II vs 42.2% in grade III and 91.3% in grade IV). Overall median follow-up was 17.9 months, but lower tumor grades were associated with a lengthier follow-up period (median 66 months for grade II vs 19.1 for grade III and 11.1 for grade IV). Episodes of VTE and other competing events (including death, censoring, and for patients with LGG, progression to grade IV) also occurred considerably later in the follow-up period in lower tumor grades (median time to first event 64.2 months in grade II vs 17.1 in grade III and 6.7 in grade IV, p < 0.001).
Table 1.
Patient Characteristics
VTE Cumulative Incidence
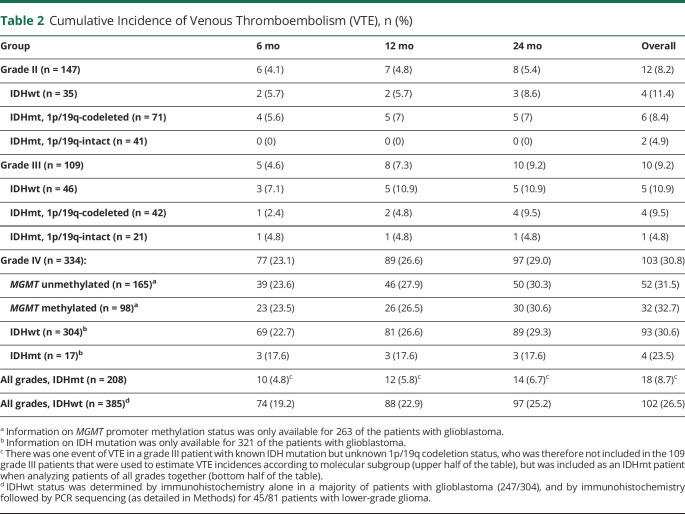
During the length of follow-up, a total of 12/147 patients (8.2%) from the grade II group had a VTE event, compared to 10/109 patients (9.2%) in the grade III group and 103/334 patients (30.8%) in grade IV. Most of these patients did not have hemiparesis (1/12 patients, or 8.3%, in the grade II group, and 2/10 patients, or 20%, in the grade III group). Although the median time to an event was very prolonged in grade II patients, the majority of VTE episodes (8/12) occurred within the first 24 months of follow-up, being particularly frequent in the first 6 months after diagnosis (6/12). Of the total of 125 events of VTE in patients of all grades, 27 (21.6%) occurred in the postoperative period (first 30 days after surgery; mean time from surgery to VTE 12.5 days for this group). This proportion was only slightly higher in the LGG group (6/22 events [27.2%] in the postoperative period) than in the GBM group (21/103 events [20.4%]).
Among patients with GBM, cumulative incidence of VTE was very similar in patients with or without MGMT promoter methylation (overall cumulative incidence 52/165 [30.8%] for unmethylated patients vs 32/98 [32.7%] for methylated patients). Although it was not part of our original objectives, we also had information on increased epidermal growth factor receptor (EGFR) expression by immunohistochemistry available for 92 of our patients, most of them (73) in the GBM group. Increased EGFR expression was present in 62 patients of all grades, of whom 15 had a VTE (24.2%), compared to 8/30 (26.7%) in patients without overexpression.
When comparing based on IDH mutation status for patients of all grades, VTE occurred more frequently in IDHwt patients (overall cumulative incidence 102/385 patients, 26.5%), but there were infrequent cases of VTE among the IDHmt group (18/208 patients, 8.7%), most of which (13/18) were patients with LGG (table 2). The majority of events consisted of DVT, PE, or both, and there were only 2 instances of CVST in the entire dataset, both occurring in IDHwt, MGMT-promoter-methylated patients with GBM.
Table 2.
Cumulative Incidence of Venous Thromboembolism (VTE), n (%)
Competing-Risks Regression
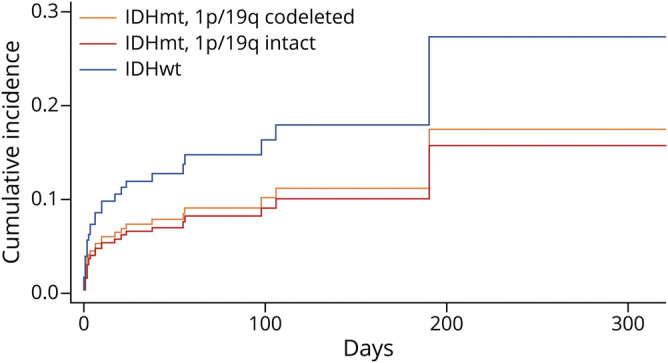
The competing-risks regression for time to VTE in patients of all grades demonstrated that lack of IDH mutation was associated with a threefold increase in VTE risk when compared to IDHmt patients (hazard ratio [HR] 3.06, 95% confidence interval [CI] 2.03–4.64, p < 0.001) (figure 2). After adjusting for age, tumor grade, and presence or absence of hemiparesis, the risk of VTE was still over double for patients without an IDH mutation (HR 2.11, 95% CI 1.17–3.79, p = 0.013). This same type of analysis in the LGG group demonstrated a higher risk of VTE in IDHwt patients compared to both IDHmt, 1p/19-codeleted and IDHmt, 1p/19q-intact patients, but this result did not reach statistical significance (IDHwt vs IDHmt, 1p/19q-codeleted, subdistribution HR [SHR] 1.67, 95% CI 0.59–4.72, p = 0.56; IDHwt vs IDHmt, 1p/19q-intact, SHR 1.87, 95% CI 0.54–6.53, p = 0.55) (figure 3). Adjusting for age and hemiparesis yielded similar results (IDHwt vs IDHmt, 1p/19q-codeleted, SHR 1.42, 95% CI 0.46–4.40, p = 0.84; IDHwt vs IDHmt, 1p/19q-intact, SHR 1.34, 95% CI 0.43–4.19, p = 0.90). There was no difference in the risk of VTE for patients with GBM according to MGMT promoter methylation (for methylated vs unmethylated, HR 0.99, 95% CI 0.64–1.54, p = 0.97).
Figure 2. Competing Risks Regression for Venous Thromboembolism Incidence in Patients of All Grades According to IDH Mutation.
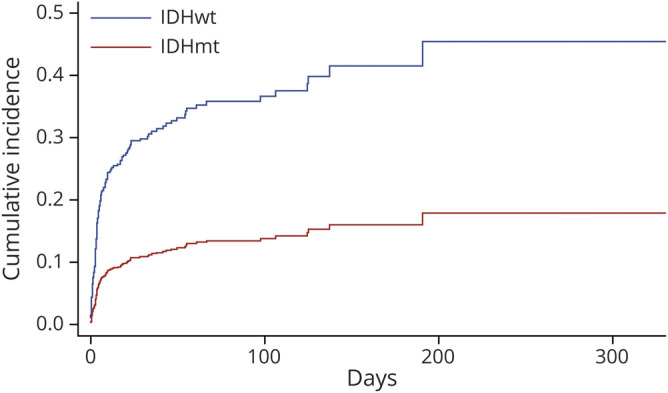
Figure 3. Competing Risks Regression for Venous Thromboembolism Incidence in Patients With Lower-Grade Glioma According to Molecular Type.
Discussion
In recent years, classification of gliomas has shifted from a purely histologic perspective to the current system based on molecular profiling, upon which grade II and III gliomas have emerged as a more similar disease entity than they were previously considered, in turn separate from GBMs on account of their different molecular characteristics.7 However, most studies looking at VTE incidence in gliomas occurred prior to this paradigm change, and while some of them included gliomas of all grades or, more commonly, what has classically been referred to as high-grade gliomas (grades III and IV), the number of grade II and III patients was overall too small to establish solid conclusions,8–13 or they were not analyzed separately from GBMs.14–16 Only one large series analyzing gliomas of all grades included sufficient numbers of grade III patients to determine their risk of VTE, finding a cumulative 2-year incidence of VTE of 6.8% in 843 patients with anaplastic astrocytoma and 7.8% in 192 patients with anaplastic oligodendroglioma.1 However, the data for this study were extracted from a statewide database containing information from dozens of different institutions; since at the time of that analysis classification into different tumor categories was based exclusively on histology rather than molecular characteristics, the heterogeneous backgrounds of the diagnosing pathologists may have affected the accuracy of the results.17 Moreover, a recent review of thousands of patients with glioma from a nationwide cancer database demonstrated that, based on 1p/19q codeletion status alone, up to 8.8% of histologically diagnosed astrocytomas and over 20% of histologically diagnosed oligodendrogliomas were historically misclassified according to the current WHO 2016 criteria, underscoring the importance of relying on molecular data to correctly interpret study results in patients with LGG.18
Our study, which examines large numbers of grade II and III disease, shows that the risk of VTE extends well beyond the postoperative period and is similar for both grades at 6 months (4.1% in grade II and 4.6% in grade III), then decelerates slightly for grade II at the 12- and 24-month mark before becoming again similar at the end of the follow-up period (8.2% for grade II and 9.2% in grade III). Although the incidence of VTE in LGG in our study was lower than for patients with GBM, the numbers still represent a major increase in the risk of VTE when compared to the general population, in which epidemiologic studies estimate VTE incidence at less than 0.2% per year.19 This finding suggests that gliomas in general and not only GBMs are associated with a state of hypercoagulability.
Several studies have recently investigated the relationship between IDH1 mutation status and VTE incidence in gliomas of all grades. The largest of these looked at 2 separate cohorts totaling 317 patients with glioma (of which 79 were grade II and III), and found a striking 0% incidence of VTE in the 76 patients who had an IDH mutation, compared to 27% in IDHwt patients.4 Two subsequent articles reported similar results (1 with only 1 VTE in 42 IDHmt patients, occurring in a patient with a previous history of thrombosis,5 and another one with 1 VTE in 27 IDHmt patients20). IDH1 mutation in gliomas has been associated with hypermethylation and downregulation of F3, which in turn decreases the expression of procoagulant tissue factor (TF); this reduction in TF has been linked not only with an inhibition of coagulation,4 but also with a decline in tumor proliferation and invasion, which may partially explain the lower level of malignancy seen in IDHmt gliomas.21 D-2-hydroxyglutarate, the direct metabolite of mutant IDH, has been shown to inhibit platelet aggregation, although it is unclear if the levels of this substance are high enough in peripheral serum to have a distant effect on coagulation.4 Our data confirm that IDH mutation status substantially affects the risk of VTE, with a threefold increase in VTE in IDHwt compared to IDHmt; this heightened risk persists when adjusting for known risk factors for VTE that tend to be disproportionately more frequent in IDHwt patients, including older age, higher tumor grade, and presence of hemiparesis. However, in contrast to previous studies, we did find a considerable number of VTE events in IDHmt patients, with 10/208 cases (4.8%) in the first 6 months, and 18 (8.7%) overall. Our larger number of patients (208 IDHmt patients) may partially explain this discrepancy. We identified our patients from a research database that specifically aimed to record VTE events, which may have increased our ability to capture those events that occurred outside our institution (5 out of 18 VTE episodes in IDHmt patients were not diagnosed at our center).
On the other hand, our results indicate that other routinely tested molecular alterations such as MGMT promoter methylation and EGFR overexpression have no influence in the risk of VTE. This is concordant with a recent retrospective study showing no difference in VTE risk according to MGMT promoter methylation status in patients with GBM.22 Although EGFR amplification and mutation has been associated with an upregulation in tissue factor expression and an in vitro procoagulant effect,23–25 our sample suggests that this may not translate into a heightened systemic hypercoagulability in clinical practice.
This study is a large single-center study of thromboembolic complications in patients with grade II and III gliomas. Weaknesses include its retrospective nature; data were collected from a retrospective database spanning several years and included patients diagnosed at other institutions, which limits the amount of clinical and molecular information available for some patients; for example, not all of our patients with LGG underwent genetic sequencing for rare non-canonical mutations in IDH1 and IDH2, and subsequently some of those patients may have been misclassified as IDHwt. Strengths include the large number of patients and long follow-up time. Although our results do not fully overlap with other studies looking at the risk of VTE in IDHmt patients, they do add to the evidence that this mutation is linked with a decreased risk of systemic thrombosis.
Given the high risk of VTE in patients with glioma and its associated morbidity, there has been interest in determining whether long-term prophylaxis could be beneficial. Whereas a few recent randomized clinical trials have demonstrated a possible benefit in the use of direct oral anticoagulants as primary prophylaxis in select high-risk ambulatory cancer patients,26–28 the applicability of these data to brain tumor patients in general is unclear. The only randomized trial that analyzed this issue in grade III and IV patients showed a reduction in VTE incidence in patients receiving low-molecular-weight heparin compared to placebo, accompanied by an increased risk of intracranial hemorrhage in these patients, neither of which was statistically significant.29 Based on the results of our group and others demonstrating that IDH mutation is associated with a considerably lower VTE risk, a future trial that includes only IDHwt patients could provide more insight into a subpopulation with a favorable benefit–risk ratio from long-term VTE prophylaxis.
Patients with LGG have an increased risk of VTE compared to the general population that is decreased, but not eliminated, in the presence of an IDH mutation. Prospective trials analyzing anticoagulant use for primary prophylaxis specifically in IDHwt gliomas could help clarify the best management for these patients.
Glossary
- CI
confidence interval
- CVST
cerebral venous sinus thrombosis
- DVT
deep venous thrombosis
- EGFR
epidermal growth factor receptor
- GBM
glioblastoma
- HR
hazard ratio
- IDH
isocitrate dehydrogenase
- LGG
lower-grade glioma
- PE
pulmonary embolus
- SHR
subdistribution hazard ratio
- TF
tissue factor
- VTE
venous thromboembolism
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Semrad TJ, O'Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007;106:601–608. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins EO, Schiff D, Mackman N, Key NS. Venous thromboembolism in malignant gliomas. J Thromb Haemost 2010;8:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo JT, Schiff D, Perry JR. Thrombosis in brain tumors. Semin Thromb Hemost 2014;40:325–331. [DOI] [PubMed] [Google Scholar]
- 4.Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol 2016;132:917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mir Seyed Nazari P, Riedl J, Preusser M, et al. Combination of isocitrate dehydrogenase 1 (IDH1) mutation and podoplanin expression in brain tumors identifies patients at high or low risk of venous thromboembolism. J Thromb Haemost 2018;16:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016;131:803–820. [DOI] [PubMed] [Google Scholar]
- 7.Schiff D, van den Bent M, Vogelbaum MA, et al. Recent developments and future directions in adult lower-grade gliomas: Society for Neuro-oncology (SNO) and European Association of Neuro-oncology (EANO) consensus. Neuro Oncol 2019;21:837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandes AA, Scelzi E, Salmistraro G, et al. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer 1997;33:1592–1596. [DOI] [PubMed] [Google Scholar]
- 9.Streiff MB, Segal J, Grossman SA, Kickler TS, Weir EG. ABO blood group is a potent risk factor for venous thromboembolism in patients with malignant gliomas. Cancer 2004;100:1717–1723. [DOI] [PubMed] [Google Scholar]
- 10.Simanek R, Vormittag R, Hassler M, et al. Venous thromboembolism and survival in patients with high-grade glioma. Neuro Oncol 2007;9:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaler J, Preusser M, Ay C, et al. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res 2013;131:162–165. [DOI] [PubMed] [Google Scholar]
- 12.Streiff MB, Ye X, Kickler TS, et al. A prospective multicenter study of venous thromboembolism in patients with newly-diagnosed high-grade glioma: hazard rate and risk factors. J Neurooncol 2015;124:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senders JT, Snijders TJ, van Essen M, et al. Length of thromboprophylaxis in patients operated on for a high-grade glioma: a retrospective study. World Neurosurg 2018;115:e723–e730. [DOI] [PubMed] [Google Scholar]
- 14.Ay C, Vormittag R, Dunkler D, et al. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 2009;27:4124–4129. [DOI] [PubMed] [Google Scholar]
- 15.Chaichana KL, Pendleton C, Jackson C, et al. Deep venous thrombosis and pulmonary embolisms in adult patients undergoing craniotomy for brain tumors. Neurol Res 2013;35:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith TR, Lall RR, Graham RB, et al. Venous thromboembolism in high grade glioma among surgical patients: results from a single center over a 10 year period. J Neurooncol 2014;120:347–352. [DOI] [PubMed] [Google Scholar]
- 17.Bruner JM, Inouye L, Fuller GN, Langford LA. Diagnostic discrepancies and their clinical impact in a neuropathology referral practice. Cancer 1997;79:796–803. [DOI] [PubMed] [Google Scholar]
- 18.Iorgulescu JB, Torre M, Harary M, et al. The misclassification of diffuse gliomas: rates and outcomes. Clin Cancer Res 2019;25:2656–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe J, Natsumeda M, Okada M, et al. Podoplanin expression and IDH-wildtype status predict venous thromboembolism in patients with high-grade gliomas in the early postoperative period. World Neurosurg 2019;128:e982–e988. [DOI] [PubMed] [Google Scholar]
- 21.Unruh D, Mirkov S, Wray B, et al. Methylation-dependent tissue factor suppression contributes to the reduced malignancy of IDH1-mutant gliomas. Clin Cancer Res 2019;25:747–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim G, Ho C, Roldan Urgoti G, Leugner D, Easaw J. Risk of venous thromboembolism in glioblastoma patients. Cureus 2018;10:e2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milsom CC, Yu JL, Mackman N, et al. Tissue factor regulation by epidermal growth factor receptor and epithelial-to-mesenchymal transitions: effect on tumor initiation and angiogenesis. Cancer Res 2008;68:10068–10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rong Y, Belozerov VE, Tucker-Burden C, et al. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res 2009;69:2540–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood 2010;116:815–818. [DOI] [PubMed] [Google Scholar]
- 26.Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 2019;380:711–719. [DOI] [PubMed] [Google Scholar]
- 27.Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 2019;380:720–728. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost 2019;17:1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry JR, Julian JA, Laperriere NJ, et al. PRODIGE: a randomized placebo-controlled trial of dalteparin low-molecular-weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost 2010;8:1959–1965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.