Abstract
Objective
To determine whether chronic motor deficits secondary to traumatic brain injury (TBI) can be improved by implantation of allogeneic modified bone marrow–derived mesenchymal stromal/stem cells (SB623).
Methods
This 6-month interim analysis of the 1-year double-blind, randomized, surgical sham–controlled, phase 2 Stem Cell Therapy for Traumatic Brain Injury (STEMTRA) trial (NCT02416492) evaluated safety and efficacy of the stereotactic intracranial implantation of SB623 in patients with stable chronic motor deficits secondary to TBI. Patients in this multicenter trial (n = 63) underwent randomization in a 1:1:1:1 ratio to 2.5 × 106, 5.0 × 106, or 10 × 106 SB623 cells or control. Safety was assessed in patients who underwent surgery (n = 61), and efficacy was assessed in the modified intent-to-treat population of randomized patients who underwent surgery (n = 61; SB623 = 46, control = 15).
Results
The primary efficacy endpoint of significant improvement from baseline of Fugl-Meyer Motor Scale score at 6 months for SB623-treated patients was achieved. SB623-treated patients improved by (least square [LS] mean) 8.3 (standard error 1.4) vs 2.3 (standard error 2.5) for control at 6 months, the LS mean difference was 6.0 (95% confidence interval 0.3–11.8, p = 0.040). Secondary efficacy endpoints improved from baseline but were not statistically significant vs control at 6 months. There were no dose-limiting toxicities or deaths, and 100% of SB623-treated patients experienced treatment-emergent adverse events vs 93.3% of control patients (p = 0.25).
Conclusions
SB623 cell implantation appeared to be safe and well tolerated, and patients implanted with SB623 experienced significant improvement from baseline motor status at 6 months compared to controls.
ClinicalTrials.gov Identifier:
Classification of Evidence
This study provides Class I evidence that implantation of SB623 was well tolerated and associated with improvement in motor status.
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide. The estimated global incidence of acute TBI during 2016 was 27 million cases, and the estimated global prevalence of chronic impairment secondary to TBI was 55.5 million cases.1 Overall, TBI and long-term motor deficits secondary to TBI significantly impair patients' self-care, employability, and quality of life and are major burdens on health care systems worldwide.
In the United States, ≈43% of surviving hospitalized patients with TBI experience long-term motor deficits, with 5.3 million people estimated to live with long-term motor deficits secondary to TBI.2,3 In an observational study, >30% of patients with severe TBI had at least 1 neuromotor impairment 2 years after inpatient rehabilitation.4 Overall, the treatment of long-term motor deficits secondary to TBI remains a major unmet medical need.
Mesenchymal stromal/stem cell (MSC) implantation is a promising strategy for the treatment of TBI. Early-stage clinical studies of several cell types implanted during the acute to chronic phases of TBI have shown favorable results.5–7 Allogeneic modified bone marrow–derived MSCs (SB623 cells, SanBio, Inc, Mountain View, CA) are in clinical development for chronic TBI and stroke without concomitant immunosuppressants, which were determined to be unnecessary on the basis of the prior phase 1/2a clinical study and preclinical studies submitted to the Food and Drug Administration (unpublished data, 2010, SanBio, Inc). In a recently completed 2-year open-label phase 1/2a study (NCT01287936) in patients with chronic ischemic stroke, implantation of SB623 cells appeared to be generally safe, with no evidence of immune sensitization, and was associated with statistically significant improvement of measures of motor function.8
This report presents 6-month prespecified interim data from the 1-year, double-blind, randomized, surgical sham–controlled, phase 2 Stem Cell Therapy for Traumatic Brain Injury (STEMTRA) trial (NCT02416492), in which the intracerebral stereotactic implantation of SB623 cells in patients with chronic motor deficits secondary to TBI appeared to be safe and was associated with a statistically significant improvement of the Fugl-Meyer Motor Scale (FMMS) score over surgical sham–controlled patients.
Methods
Primary Research Question
We aimed to determine the safety and efficacy of SB623 cells (allogeneic modified bone marrow–derived MSCs) delivered by stereotactic intracranial implantation to patients with stable chronic motor deficits secondary to TBI in a double-blind, randomized, surgical sham–controlled, phase 2 trial.
Classification of Evidence
This phase 2 trial provides Class I evidence that SB623 implantation appears to be safe and is associated with significant improvement from baseline motor status at 6 months vs controls.
Standard Protocol Approvals, Registrations, and Patient Consents
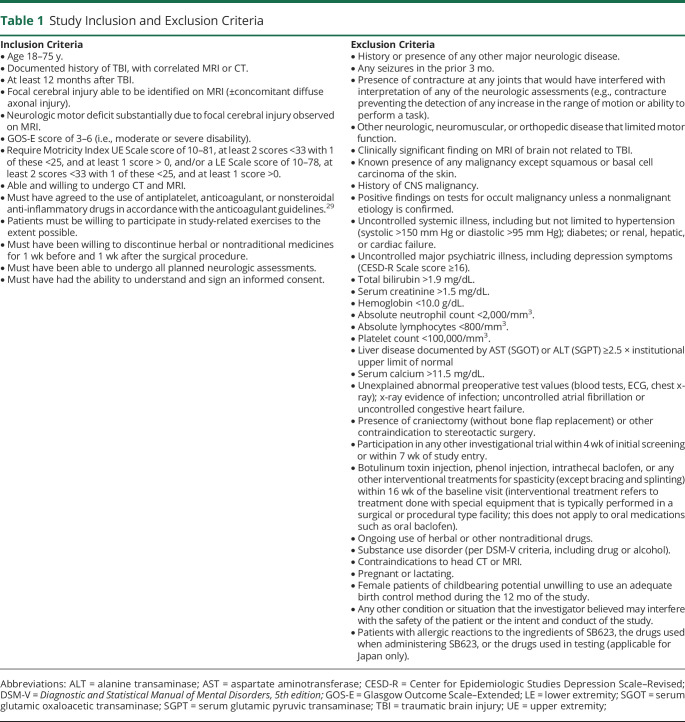
This double-blind, sham surgery–controlled, phase 2 clinical trial (STEMTRA; ClinicalTrials.gov NCT02416492) enrolled patients with moderate or severe TBI (at least 12 months after TBI) who had Glasgow Outcome Scale–Extended (GOS-E) scores of 3 to 6 and chronic motor deficits that correlated with a focal cerebral injury observed on MRI. Patients received physical therapy instruction during the trial, which was conducted between June 2016 and March 2019 at 27 sites in the United States (21), Japan (5), and Ukraine (1) (available from Dryad; site locations, supplementary table 1: doi.org/10.5061/dryad.vdncjsxrb). Clinical study protocols were reviewed and approved by individual institutional review boards, and patients provided written informed consent. Study inclusion and exclusion criteria are listed in table 1.
Table 1.
Study Inclusion and Exclusion Criteria
Patient Selection
The study enrolled only outpatients. In the United States, some patients were prescreened for eligibility by the University of California, San Francisco, which referred patients to study sites. However, other study sites in the United States screened patients directly. In Japan, patients were prescreened for eligibility via a call center or website, which referred patients to study sites. In Ukraine, patients were prescreened for eligibility at peripheral centers and referred to the single assessment/surgery site. In all cases, investigators made a final judgment on eligibility and enrolled patients in the study.
SB623 Cells
Allogeneic modified bone marrow–derived MSCs (SB623 cells) are produced by the transient transfection of MSCs with a plasmid containing the human Notch-1 intracellular domain, which lowers the potential for cells to differentiate into bone, cartilage, or adipose cells and increases their ability to secrete trophic factors and chemotactic factors and to deposit extracellular matrix proteins that may support damaged neural cells.9,10 The transfection is regarded to be transient because the plasmid is lost rapidly by the expansion and passaging of SB623 cells. Notably, 0.6% of implanted SB623 cells are reported to survive at 1 month after implantation in rodent models of stroke and TBI.11,12 Moreover, SB623 is not a cell line, and preclinical studies have shown that SB623 cells have no potential tumorigenicity (unpublished data, SanBio, Inc). Cell preparation details have been described previously.8
Randomization, Blinding, and Surgical Procedure
Enrolled patients with TBI were randomized to SB623 cell treatment or sham surgery in a 3:1 ratio, with the SB623 treatment group being further randomized in a 1:1:1 ratio to receive 2.5 × 106, 5.0 × 106, or 10 × 106 SB623 cells with an interactive web response system.
SB623 cells were implanted stereotactically with methods described previously.8 Briefly, implantation location sites were different for each patient and were determined by MRI to be in cortical or motor sites adjacent to the TBI lesion. SB623 cells were implanted according to the surgeon's judgment of safe implantation trajectory using frame or frameless stereotactic procedures through a single-burr-hole craniostomy (1–1.5 cm), made under local anesthesia and sedation to minimize patient discomfort and to preserve patient blinding. Implantation was carried out with 3 cannula tracks, with 5 × 20 µL cell deposits made at varying depths such that cell deposits were 5 to 6 mm apart on each track. Cells were injected at a rate of ≤10 µL/min with a total volume of 300 µL per patient. Sham surgery control patients received similar treatment including sedation, stereotactic procedure, partial-thickness outer table burr-hole without penetration of the inner table or dura mater, and scalp surgical closure to minimize patient discomfort and to preserve patient blinding. In addition, efficacy assessments were conducted by blinded neurologists, physiatrists, and physical therapists, while treatment-emergent adverse events (TEAEs) were evaluated by blinded rehabilitation physicians throughout the clinical trial.
Study Visit Schedule
Patients attended the following visit schedule: screen (study day −84 to −15), baseline (study day −14 to −1), cell implantation or sham surgical procedure (day 1), visits (days 2 and 8; months 1, 3, 6, and 9), and final visit (month 12). Clinical TBI evaluations were performed at baseline and months 1, 3, and 6 and will be performed at months 9 and 12.
Efficacy Assessments
Efficacy was assessed by measuring mean change from baseline of SB623-treated vs control patients at 24 weeks using clinical TBI evaluations. The primary efficacy endpoint was FMMS (scores range from 0–100, with higher values reflecting better motor status), for which assessors underwent training, certification, and regular recertification.13–15 FMMS was selected because it is widely recognized as a clinically relevant measure of loss of body structure/function (impairment), particularly for motor recovery in an affected limb. Thus, it was an appropriate scale for measuring chronic motor deficits secondary to TBI.13–15 Secondary efficacy endpoints included (1) Disability Rating Scale (DRS), a measure of general functional change selected because it was a sensitive, functional, reliable, and quantitative means of monitoring recovery of patients with TBI16; (2) Action Research Arm Test (ARAT), an assessment of upper extremity function and dexterity in patients with CNS damage resulting in hemiplegia17,18; (3) gait velocity (GV), an outcome measure of lower extremity function assessed by walking speed, a predictor of disability19; (4) T scores of NeuroQOL upper and lower extremity domains, which measure activities of daily living and mobility, respectively20; and (5) Global Rating of Perceived Change assessed by patient and clinician, which assessed perceived changes in patient's motor function.21
Safety
TEAEs were defined as any event not present before the initiation of cell treatment or surgical procedure or any event already present that worsened in either intensity or frequency after exposure to cell treatment or surgical procedure. TEAEs were graded as mild, moderate, severe, or life-threatening. The relationship between TEAEs and cell treatment or surgical procedure was evaluated with the list in supplementary table 2 (available from Dryad: doi.org/10.5061/dryad.vdncjsxrb).
Clinical Laboratory Tests
During the study, hematology and biochemical parameter (including alanine and aspartate aminotransferase) testing was conducted on blood samples collected at baseline and follow-up visits using routine laboratory/clinical procedures. Vital signs were collected at baseline and follow-up visits, and antibodies to donor HLA antigens were detected using panel reactive antibodies and Luminex methods to monitor a possible humoral-mediated immune response.
Genotyping
Genotyping was performed as previously described on blood samples collected at baseline to determine whether patients were homozygous (ApoE2, E3, E4) or heterozygous (ApoE2/E3, E3/E4, or E2/E4) at the ApoE locus and whether the Val66Met polymorphism of brain-derived neurotrophic factor (BDNF) gene was present (yes/no).22,23 The presence of ApoE4 and Val66Met BDNF polymorphisms has previously been reported to be associated with poorer recovery in human patients at 1 month after stroke.24
Statistics
For categorical variables, descriptive statistics, including patient number and patient percentage in each category, were calculated. For continuous variables, descriptive statistics, including patient number, mean, SD, standard error (SE), median, minimum, maximum, and 95% confidence intervals (CIs), were calculated.
For safety comparisons, the Fisher exact test was used to analyze the percentage of patients experiencing at least 1 TEAE.
For primary and (continuous) secondary efficacy endpoints, comparisons for pooled SB623 vs control mixed-model repeated measures (MMRM) analyses were performed, using an unstructured covariance matrix for the restricted maximum likelihood estimation procedure. The MMRM model included the following terms: treatment, visit, treatment-by-visit interaction, baseline score, baseline score–by–visit interaction, GOS-E score at screening, and GOS-E score at screening–by–visit interaction. Least square (LS) means and SEs were calculated for both treatments, together with 95% CIs for the LS means. An MMRM analysis was used in an additional analysis of the SB623 5.0 × 106 treatment arm vs control. The clinically meaningful improvement threshold (FMMS score change of ≥10 points) was analyzed using a generalized linear mixed model with the following terms: treatment (pooled SB623 or control), baseline FMMS score, study visit, GOS-E score at screening, treatment-by-visit interaction, baseline FMMS score–by–visit interaction, and GOS-E score–by–visit interaction.
Within the SB623 treatment groups, the null hypothesis that the coefficient of the interaction between SB623 dose and the indicator variable for the month 6 visit equals 0 was tested using a MMRM model with the following terms: visit; interaction between SB623 dose and indicator variable at months 1, 3, and 6; baseline FMMS score; baseline FMMS score–by–visit interaction; GOS-E score at screening; and GOS-E score at screening–by–visit interaction. Dose was treated as a continuous variable.
To assess potential associations between FMMS score change from baseline and genotype variables for pooled SB623 vs control, an analysis of covariance including treatment, genotypic subgroup, treatment-by-genotypic subgroup interaction, and baseline FMMS score was used. The difference in mean change from baseline (pooled SB623 vs control) for daily activity count was assessed using a 2-sample t test. Values of p < 0.05 were considered statistically significant. Data analyses were performed with SAS version 9.4 (Cary, NC).
Data Availability
Individual deidentified patient data, study protocol, statistical analysis plan, clinical study report, and informed consent forms will be available to qualified external medical and scientific researchers. Data-sharing requests may be submitted 24 months after study completion, with no end date for eligibility. Qualified medical and scientific researchers may submit a data-sharing request containing research objectives, data requirements, statistical analysis plan, endpoints/outcomes of interest, scientific value and impact, and a publication plan to the Chief Medical Officer of SanBio, Inc. The scientific appropriateness of the request will be reviewed by SanBio, Inc.
Results
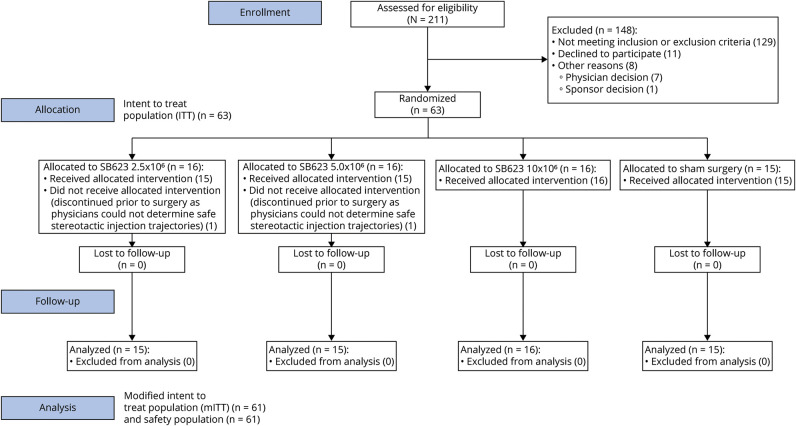
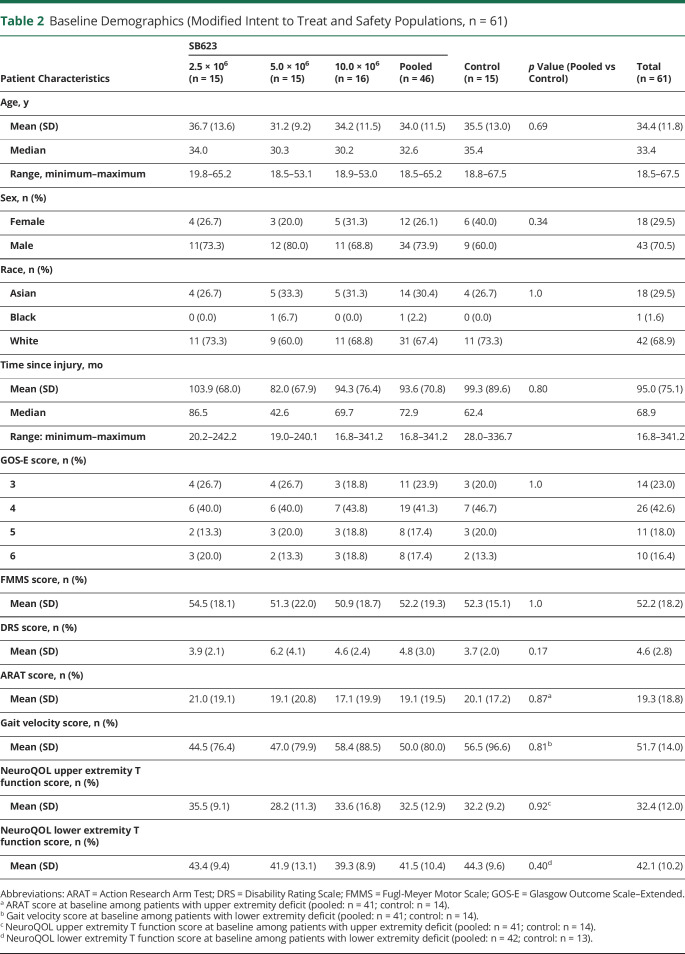
Two hundred eleven patients were screened for this clinical trial, with 63 patients randomized to the intent-to-treat (ITT) population: 16 patients in each of 3 SB623-treated groups (2.5 × 106, 5.0 × 106, and 10 × 106) and 15 patients in the control group (figure 1). The trial screening process was selective, enrolling only 31.8% of screened patients, with the most common causes of screen failure being failure to meet Motricity Index requirements (30.1%), lack of focal cerebral injury identified on MRI (13.1%), and neurologic motor deficit substantially due to focal cerebral injury observed on MRI (13.1%). Therefore, the enrolled population may not be representative of the general population with chronic TBI with motor deficit. Two patients, 1 each in the SB623 2.5 × 106 and 5.0 × 106 treated groups, discontinued before cell treatment as physicians could not determine safe stereotactic injection trajectories. Therefore, both the modified ITT (mITT) and safety populations contained 61 patients (table 2). Sixty-one patients in the mITT and safety populations had completed 6 months of treatment at the time of this interim analysis. The mean age of the study population was 34.4 years, and patients were 1.4 to 28.4 years after injury.
Figure 1. Consort Diagram.
Intent-to-treat (ITT) population (n = 63): patients randomized to SB623 cell treatment or sham surgery. Modified ITT (mITT) population (n = 61): patients randomized to SB623 treatment or sham surgery, minus 2 patients, 1 each from the SB623 2.5 × 106 and 5.0 × 106 treatment groups who discontinued before treatment because physicians could not determine safe cell injection trajectories. Safety population (n = 61): patients who enrolled and underwent SB623 treatment or sham surgery.
Table 2.
Baseline Demographics (Modified Intent to Treat and Safety Populations, n = 61)
Safety Evaluations
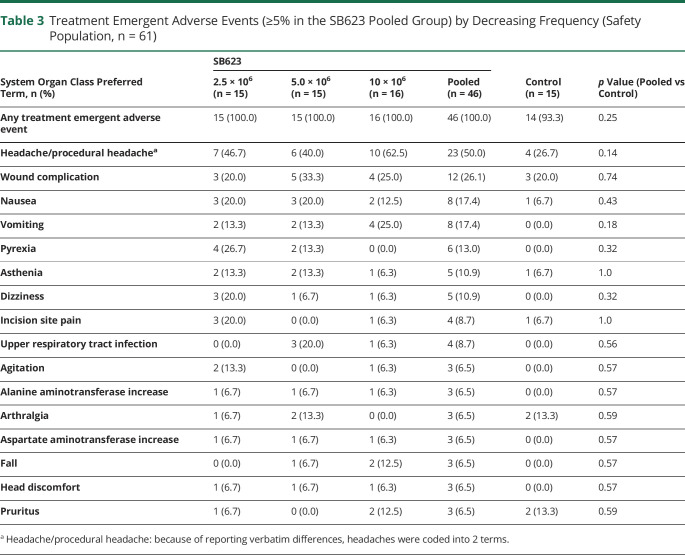
In this 6-month interim analysis, 100% of SB623-treated patients experienced TEAEs vs 93.3% of patients in the control group (p = 0.25) (table 3). There were no dose-limiting toxicities or deaths, and no patients withdrew from the study due to adverse events. Headache/procedural headache was the most frequent TEAE in both the SB623 pooled group (50.0%) and the control group (26.7%, p = 0.14) (table 3), which was mild or moderate in severity. In addition, there were no significant differences in the frequency of TEAEs occurring in the SB623 pooled vs the control group (table 3). The majority of headache/procedural headache TEAEs started between days 1 and 3 after surgery and lasted until day 28 for the SB623 pooled and control groups (available from Dryad: supplementary table 3: doi.org/10.5061/dryad.vdncjsxrb). Four of 6 cases of pyrexia TEAEs started after postsurgery day 3 in the SB623 pooled group, while there were no cases in the control group (available from Dryad: supplementary table 3). Of 17 patients with parenchymal or subdural hematoma in the SB623 pooled group, 8 patients (47%) experienced headache. In comparison, the frequency of headache in the SB623 pooled population was 50%. In the SB623 pooled group (n = 46), patients experienced a total of 223 TEAEs, 98.2% of which were of mild or moderate intensity, vs 65 TEAEs in the control group (n = 15), 93.8% of which were of mild or moderate intensity.
Table 3.
Treatment Emergent Adverse Events (≥5% in the SB623 Pooled Group) by Decreasing Frequency (Safety Population, n = 61)
A total of 92% of TEAEs were classified by investigators as unrelated or unlikely to be related to cell treatment in both the SB623 pooled and control groups (available from Dryad: supplementary table 4: doi.org/10.5061/dryad.vdncjsxrb). In comparison, no TEAEs were definitely related to cell treatment in both treatment groups, and 2 (0.9%) TEAEs (headache and hemiparesis) were classified as probably related to cell treatment in the SB623 pooled group (available from Dryad: supplementary table 4).
A total of 39.5% of TEAEs in the SB623 pooled group and 36.9% of TEAEs in the control group were possibly, probably, or definitely related to surgical procedure (available from Dryad: supplementary table 5: doi.org/10.5061/dryad.vdncjsxrb). In the SB623 pooled group, the most frequent TEAE assessed as probably or definitely related to surgical procedure was headache/procedural headache (63%) (available from Dryad: supplementary table 5). In both groups, more patients experienced TEAEs assessed as possibly, probably, or definitely related to surgical procedure than cell treatment.
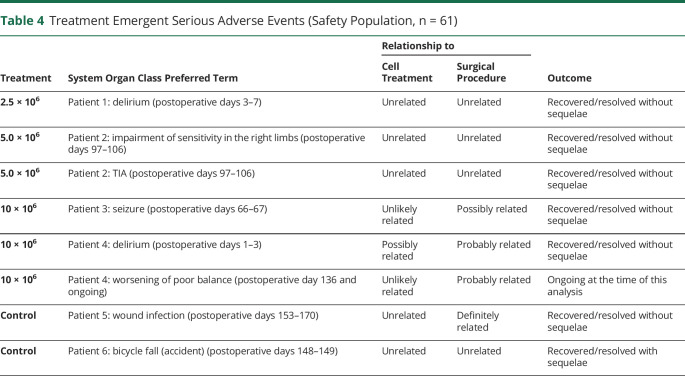
In this 6-month interim analysis, 6 treatment-emergent serious adverse events (TESAEs) occurred in 4 (8.7%) SB623-treated patients vs 2 TESAEs in 2 (13.3%) control patients (table 4). In SB623-treated patients, 5 TESAEs were unrelated or unlikely to be related, and one possibly related (delirium) to cell treatment (table 4). In comparison, 3 TESAEs were unrelated, one possibly related (seizure), and 2 probably related (delirium and worsening of poor balance in 1 patient) to the surgical procedure (table 4). In sham control patients, both TESAEs were unrelated to cell treatment, while single TESAEs were unrelated and definitely related (wound infection) to surgical procedure (table 4). All TESAEs recovered or resolved without sequelae except for in patient 6 in the control group, who recovered from a bicycle fall with sequelae (pain in right hand at discharge from hospital), and patient 4 in the SB623 10 × 106 treated group, whose worsening of poor balance, which was assessed by the investigator as being probably related to surgical procedure, was ongoing at the time of this analysis.
Table 4.
Treatment Emergent Serious Adverse Events (Safety Population, n = 61)
There were no clinically meaningful trends in hematologic or biochemical parameters or vital signs. However, a single (2.2%) patient in the SB623 2.5 × 106 treated group developed new antibodies to a Class I HLA donor cell antigen at month 1 after surgery. Three SB623-treated patients and 1 control patient had preexisting anti-SB623 HLA antibodies. There was no obvious relationship between anti-SB623 HLA antibodies and SB623 cell dose and between anti-SB623 HLA antibodies and TESAEs or efficacy parameters.
MRI examination at day 8 revealed that parenchymal and subdural hematomas were present in 9 (19.6%) and 10 (21.7%) patients in the SB623 pooled group, respectively. Seven subdural hematomas resolved by 6 months without further treatment, while the remaining 3 were stable and improving without treatment at 6 months. A single patient in the SB623 5.0 × 106 group experienced a hematoma of mild severity between days 1 and 7, which was classified by the investigator as not related to cell treatment but probably related to the surgical procedure.
Efficacy Evaluations
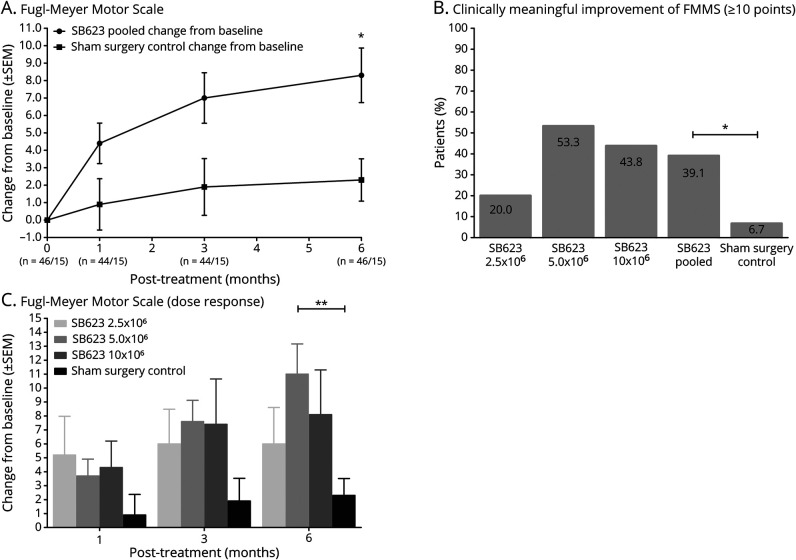
The baseline mean (SD) FMMS scores for SB623 pooled and control groups were 52.2 (19.3) and 52.3 (15.1), respectively. The primary efficacy endpoint of greater FMMS score change from baseline for SB623 pooled was achieved. The SB623 pooled group improved by (LS mean) 8.3 (SE 1.4) vs 2.3 (SE 2.5) for the control group at 6 months; the LS mean difference was 6.0 (95% CI 0.3–11.8, p = 0.040) (figure 2A).
Figure 2. Primary Efficacy Endpoint Measures.
(A) Fugl-Meyer Motor Scale (FMMS) score change from baseline for SB623 pooled and sham control groups at time points up to 6 months (modified intent-to-treat [mITT] population, n = 61). *p < 0.05. (B) Percent of patients in each treatment group who achieved potentially clinically meaningful improvement of FMMS score (≥10 points) at 6 months (mITT population, n = 61). *p < 0.05. (C) FMMS dose response: change from baseline for each treatment group at time points up to 6 months (mITT population, n = 61). Difference found between the 5.0 × 106 treatment and sham surgery groups was calculated separately and did not include comparisons between other SB623 treatment groups and the sham surgery group. **p < 0.01.
More patients in the SB623 pooled vs the control group achieved the FMMS score potentially clinically meaningful improvement of ≥10 points (representing a 19% improvement from baseline for the SB623 pooled group) at 6 months (39.1% vs 6.7%, p = 0.039), with the greatest percentage of patients achieving ≥10 points in the SB623 5.0 × 106 treated group (53.3%) (figure 2B).
When analyzed by treatment group for the SB623 treatment arms, improvement of FMMS score was numerically greatest in the SB623 5.0 × 106 treated group starting at 3 months for the mITT population, while there was no relationship between cell dose and change in FMMS score at 6 months in the mITT population (p = 0.82). SB623 5.0 × 106 treated group improvement was (LS mean) 10.9 (SE 1.8) vs 2.4 (SE 1.8) for the control at 6 months. The LS mean difference was 8.5 (95% CI 3.4–13.7, p = 0.002) (figure 2C).
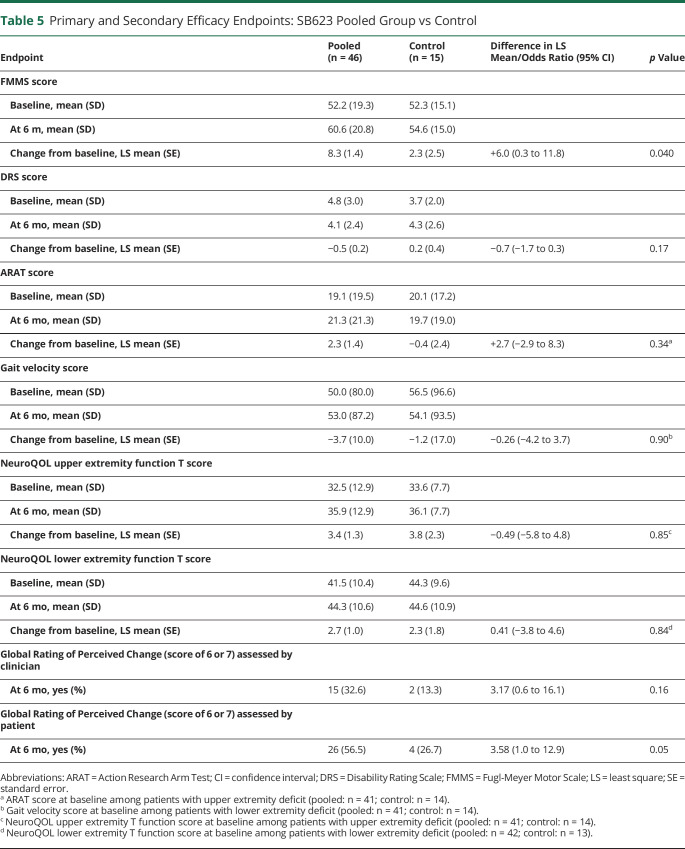
Changes from baseline for DRS score (difference in LS mean −0.7 [95% CI −1.7 to 0.3], p = 0.17), ARAT score (2.7 [95% CI −2.9 to 8.3], p = 0.34), GV (−0.26 m/s [95% CI −4.2 to 3.7], p = 0.90), NeuroQOL lower extremity function T score (0.41 [95% CI −3.8 to 4.6], p = 0.84), and Global Rating of Perceived Change (clinically meaningful score of 6 or 7) assessed by clinician (32.6% vs 13.3%, odds ratio 3.17 [95% CI 0.6–16.1], p = 0.16) and patient (56.5% vs 26.7%, odds ratio 3.58 [95% CI 1.0–12.9], p = 0.05) trended toward improvement but were not statistically significant for the SB623 pooled vs control group at 6 months. In contrast, change from baseline for the NeuroQOL upper extremity function T score (difference in LS mean −0.49 [95% CI −5.8 to 4.8], p = 0.85) was not statistically significantly different for the control vs SB623 pooled group at 6 months. Baseline, 6-month, and change from baseline at 6 months values for primary and secondary efficacy endpoints are shown in table 5.
Table 5.
Primary and Secondary Efficacy Endpoints: SB623 Pooled Group vs Control
At 6 months, across all patients, there were no relationships between FMMS score change from baseline and at least 1 Met allele of BDNF (p = 0.85) and at least 1 ApoE4 allele (p = 0.90). Furthermore, there were no differences in daily activity count change from baseline between the SB623 pooled and control groups for the affected (p = 0.25) and nonaffected (p = 0.96) sides of the body at 6 months.
Discussion
There were no significant differences in the percentage of pooled SB623-treated and control group patients who experienced TEAEs, most of which were of mild or moderate intensity. The vast majority of TEAEs were unrelated or unlikely to be related to cell treatment, while in common with previous studies, many TEAEs were possibly, probably, or definitely related to surgical procedure.8,25
Although a single patient in the SB623-treated group had serologic evidence of immune sensitization and 4 patients had preexisting anti-SB623 HLA antibodies, there were no obvious relationships between the presence of anti-SB623 HLA antibodies and cell dose and between the presence of anti-SB623 HLA antibodies and the incidence of TESAEs or changes in efficacy parameters (data not shown). The presence of preexisting antibodies to donor HLA suggests that patients may have received a blood transfusion with those antigens, may have been pregnant, may have received cells in another study/treatment, or some combination of the above. These findings are important because immunosuppressive agents were not used in this study, and allogeneic SB623 cells have the potential for immunoreactivity. These results are consistent with data from the phase 1/2a chronic stroke study (NCT01287936)8 and provide confidence in the safety of SB623 cell implantation without the use of immunosuppressive agents.
The primary efficacy endpoint of significantly greater improvement of FMMS from baseline for pooled SB623-treated vs control group patients at 6 months was achieved (LS mean) 8.3 (SE 1.4) vs 2.3 (SE 2.5) for control; the LS mean difference was 6.0 (95% CI 0.3–11.8, p = 0.040). The FMMS is well established as a measure of motor impairment14,15 and is widely accepted as an assessment of recovery in chronic stroke.15,26,27 Because there is little consensus on the use of function/impairment scales in TBI trials,5–7 FMMS was adopted as the primary efficacy scale in this study because of its reliability and validity in measuring changes in patients with persistent motor deficits. In addition, a ≥10-point (10%) increase of FMMS score from baseline is recognized as a clinically meaningful threshold of improvement in chronic stroke,8 although this has not been validated in chronic TBI. In this study, more SB623-treated than control group patients achieved improvement of ≥10 points on the FMMS scale at 6 months (39.1% vs 6.7%, p = 0.039).
In this trial, a classic dose response was not seen, with patients in the SB623 5.0 × 106 treated group achieving the most favorable FMMS outcomes at 6 months, specifically the greatest improvement in FMMS score (10.9 vs 2.4, p = 0.002) and the highest percentage of patients with an FMMS score improvement of ≥10 points (53.3%). The SB623 10.0 × 106 cell dose did not confer additional benefit and in general was associated with increased variability (i.e., a greater range in efficacy responses). We speculate that beyond a certain threshold the beneficial effects of additional cell implantation may be counterbalanced by locally increased inflammation secondary to increased cell death or that a biological asymptote had been reached.
The secondary endpoints used in this study included DRS score,16 a measure of global function, and several domain-specific outcome measures that assessed deficits of motor function such as ARAT score and GV for disability in the upper and lower extremity, respectively,17–19 and NeuroQOL upper and lower extremity domain scores for quality of life, satisfaction, and participation in response to changes in upper and lower extremity function.20 Although improvements from baseline for DRS, ARAT, GV, and NeuroQOL lower extremity function T scores and Global Rating of Perceived Change (score of 6 or 7) assessed by clinician and patient were greater for the pooled SB623-treated than the control group at 6 months, these differences were not statistically significant. The reasons for significant treatment-related reduction in motor impairment (FMMS) but not functional scales score are unclear but may include patient need for concomitant occupation therapy or physical therapy, cognitive deficits that may prevent motor gains from being translated into functional improvement, and the study design, which was powered on the primary endpoint, which focused on assessing improvement of motor impairment. Therefore, patients were selected who were likely to respond to motor impairment scales in a time frame needed to see improvement after treatment. In addition, secondary endpoint functional scales, which may have lacked sensitivity due to ceiling/floor effects, were not powered to detect significant change.
Currently, there are no approved pharmacologic or biological treatments for the chronic effects of TBI.2–4 Three early-stage controlled clinical studies for TBI, which implanted different types of cells, show promising results.5–7 However, 2 of these studies enrolled patients in the acute phase of severe TBI who were unconscious (Glasgow Coma Scale score 3–8) and undergoing acute care.5,6 Although these studies reported improved outcomes, they used scales (Glasgow Coma Scale, neurophysiologic measures) that were most appropriate for the acute phase of TBI and did not address changes in long-term motor deficits. Only 1 single-blind controlled study that enrolled patients with chronic deficits secondary to TBI has been published to date.7 This study reported improvement of function (Functional Independence Measure) and impairment (Fugl-Meyer) scores in patients with chronic TBI at 6 months, which support the FMMS findings of our study and the potential utility of MSCs in this indication.7
Although the mode of action of SB623 cells is not completely understood, the biology underpinning neuroplasticity that occurs naturally after injury maybe restimulated by cell implantation through the release of trophic factors or deposition of extracellular matrix, resulting in the recovery of motor function over a similar time frame, although this remains speculative. In a rat contusion model of TBI, implantation of SB623 around the area of injury resulted in significant improvement of motor function. This was associated with a profound increase in numbers of host-originated proliferating nestin-positive cells, which are present in the brain area between the injury site and the subventricular zone in animals receiving the SB623 implant vs those receiving vehicle.12 The observation of increased neural cell proliferation in the presence of SB623 cells correlates with our data from SB623/rat embryonic (E18) cortex cell cocultures.28
In this trial, surgeons determined cell implantation locations in cortical or motor cerebral sites adjacent to the TBI injury, providing a potential source of variability in patient response. Insufficient data currently exist to precisely determine optimal SB623 cell placement in relation to the area of injury. This variability is likely to reflect real-world practice. Because few controlled clinical trials of cell therapy for the treatment of TBI have been published to date, little consensus exists concerning which neurologic outcome measures to use and what degree of change from baseline is clinically meaningful in this population. The wide age range of patients in the study may also be a factor affecting the response to implanted SB623 cells. Furthermore, the sham surgical procedure did not rule out the possibility that motor improvements were caused by surgical manipulation of the peri-injured tissue rather than the effects of SB623 cells. In addition, provision of physical therapy after implantation may need to be greater and provided more consistently.
In this interim analysis of a double-blind, randomized, controlled clinical trial of cell therapy for chronic motor deficits secondary to TBI, treatment with SB623 cells appeared to be safe and was associated with statistically significant improvement of the FMMS score at 6 months. The favorable safety and efficacy outcomes reported here demonstrate the need for functional imaging studies and confirmatory phase 3 clinical trials of SB623 cells for the treatment of chronic motor deficits secondary to TBI.
Acknowledgment
The authors thank the patients and their families for their participation, trust, and partnership.
Glossary
- ARAT
Action Research Arm Test
- BDNF
brain-derived neurotrophic factor
- CI
confidence interval
- DRS
Disability Rating Scale
- FMMS
Fugl-Meyer Motor Scale
- GOS-E
Glasgow Outcome Scale–Extended
- GV
gait velocity
- ITT
intent-to-treat
- LS
least square
- mITT
modified ITT
- MMRM
mixed-model repeated measures
- MSC
mesenchymal stromal/stem cell
- SE
standard error
- STEMTRA
Stem Cell Therapy for Traumatic Brain Injury
- TBI
traumatic brain injury
- TEAE
treatment-emergent adverse event
- TESAE
treatment-emergent serious adverse event
Appendix. Authors

Footnotes
Study Funding
This study was sponsored by SanBio, Inc.
Disclosure
M. Kawabori is a consultant for SanBio, Inc. A.H. Weintraub, H. Imai, and I. Zinkevych report no disclosures relevant to the manuscript. P. McAllister is a consultant for Alder, Allergan, Amgen, Electrocore, Lilly, SanBio, Inc, and Teva. P. McAllister receives research support from Alder, Allergan, Amgen, Electrocore, Genetech, Lilly, Novartis, and Revance. G.K. Steinberg is a consultant for NeuroSave, Qool Therapeutics, SanBio, Inc, and Zeiss. G.K. Steinberg receives royalties from Peter Lazic US. B.M. Frishberg is a consultant for Alexion, Allergan, Argenx, Biogen, Celgene, Genentech, and Sanofi-Genzyme. B.M. Frishberg is a speaker for Alexion, Biogen, EMD-Serono Genentech, Lilly, Novartis, and Sanofi-Genzyme. B.M. Frishberg is a principal investigator for Alexion and Johnson & Johnson. T. Yasuhara and J.W. Chen report no disclosures relevant to the manuscript. S.C. Cramer is a consultant for Abbvie, Biogen, Constant Therapeutics, Fujifilm Toyama Chemical Co, MicroTransponder, Neurolutions, Regenera, SanBio, Inc, Stemedica, and TRCare. A.S. Achrol, N.E. Schwartz, J. Suenaga, D. Lu, I. Semeniv, and H. Nakamura report no disclosures relevant to the manuscript. D. Kondziolka has intellectual property filed on cell therapy for stroke at the University of South Florida. D. Chida is an employee of SanBio, Inc. T. Kaneko was an employee and is currently a paid consultant of SanBio, Inc. Y. Karasawa reports no disclosures relevant to the manuscript. S. Paadre is an employee of Biostatistical Consulting Inc. B. Nejadnik is an employee of SanBio, Inc. D. Bates was an employee and is currently a paid consultant of SanBio, Inc. A.H. Stonehouse and R.M. Richardson are consultants for SanBio, Inc. D.O. Okonkwo reports no disclosures relevant to the manuscript. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011450 for full disclosures.
References
- 1.James SL, Theadom A, Ellenbogen RG; GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:56–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil 2008;23:123–131. [DOI] [PubMed] [Google Scholar]
- 3.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil 1999;14:602–615. [DOI] [PubMed] [Google Scholar]
- 4.Walker WC, Pickett TC. Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J Rehabil Res Dev 2007;44:975–982. [DOI] [PubMed] [Google Scholar]
- 5.Seledtsov VI, Rabinovich SS, Parlyuk OV, et al. Cell transplantation therapy in re-animating severely head-injured patients. Biomed Pharmacother 2005;59:415–420. [DOI] [PubMed] [Google Scholar]
- 6.Cox CS Jr, Hetz RA, Liao GP, et al. Treatment of severe adult traumatic brain injury using bone marrow mononuclear cells. Stem Cells 2017;35:1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang S, Cheng H, Dai G, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res 2013;1532:76–84. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg GK, Kondziolka D, Wechsler LR, et al. Two-year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow-derived mesenchymal stem cells (SB623): a phase 1/2a study. J Neurosurg 2018;1:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Aizman I, Tate CC, McGrogan M, Case CC. Extracellular matrix produced by bone marrow stromal cells and by their derivative, SB623 cells, supports neural cell growth. J Neurosci Res 2009;87:3198–3206. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler LR, Bates D, Stroemer P, Andrews-Zwilling YS, Aizman I. Cell therapy for chronic stroke in focused updates in cerebrovascular disease. Stroke 2018;49:1066–1074. [DOI] [PubMed] [Google Scholar]
- 11.Yasuhara T, Matsukawa N, Hara K, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cell Dev 2009;18:1501–1514. [DOI] [PubMed] [Google Scholar]
- 12.Tajiri N, Kaneko Y, Shinozuka K, et al. Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One 2013;8:e74857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient, 1: a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 14.See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair 2013;27:732–741. [DOI] [PubMed] [Google Scholar]
- 15.Gladstone DJ, Danells CJ, Black SE. The Fugl-Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 2002;16:232–240. [DOI] [PubMed] [Google Scholar]
- 16.Rehabilitation Measures Database. Disability Rating Scale (for TBI). 2012. Available at: sralab.org/rehabilitation-measures/disability-rating-scale-tbi. Accessed June 2, 2019. [Google Scholar]
- 17.Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res 1981;4:483–492. [DOI] [PubMed] [Google Scholar]
- 18.Yozbatiran N, Der-Yeghiaian L, Cramer SC. A standardized approach to performing the action research arm test. Neurorehabil Neural Repair 2008;22:78–90. [DOI] [PubMed] [Google Scholar]
- 19.Rehabilitation Measures Database. 10 Meter walk test. 2014. Available at: sralab.org/rehabilitation-measures/10-meter-walk-test. Accessed June 2, 2019. [Google Scholar]
- 20.National Institute of Neurological Disorders and Stroke. NINDS user manual for the Quality of Life in Neurological Disorders (Neuro-QoL) measures, version 2.0, March 2015. Available at: healthmeasures.net/images/neuro_qol/Neuro-QOL_User_Manual_v2_24Mar2015.pdf. Accessed June 2, 2019.
- 21.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther 2009;17:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990;31:545–548. [PubMed] [Google Scholar]
- 23.Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci 2006;9:735–737. [DOI] [PubMed] [Google Scholar]
- 24.Cramer SC, Procaccio V. Correlation between genetic polymorphisms and stroke recovery: analysis of the GAIN Americas and GAIN International studies. Eur J Neurol 2012;19:718–724. [DOI] [PubMed] [Google Scholar]
- 25.Kondziolka D, Steinberg GK, Wechsler L, Meltzer CC, Elder E, Gebel J. Neurotransplantation for patients with subcortical motor stroke: a phase 2 randomized trial. J Neurosurg 2005;103:38–45. [DOI] [PubMed] [Google Scholar]
- 26.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther 1983;63:1606–1610. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan KJ, Tilson JK, Cen SY, Rose DK, Hershberg J, Correa A. Fugl-Meyer assessment of sensorimotor function after stroke: standardized training procedure for clinical practice and clinical trials. Stroke 2011;42:427–432. [DOI] [PubMed] [Google Scholar]
- 28.Aizman I, Tirumalashetty BJ, McGrogan M, Case CC. Comparison of the neuropoietic activity of gene-modified versus parental mesenchymal stromal cells and the identification of soluble and extracellular matrix-related neuropoietic mediators. Stem Cell Res Ther 2014;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis. 9th ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(suppl):e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual deidentified patient data, study protocol, statistical analysis plan, clinical study report, and informed consent forms will be available to qualified external medical and scientific researchers. Data-sharing requests may be submitted 24 months after study completion, with no end date for eligibility. Qualified medical and scientific researchers may submit a data-sharing request containing research objectives, data requirements, statistical analysis plan, endpoints/outcomes of interest, scientific value and impact, and a publication plan to the Chief Medical Officer of SanBio, Inc. The scientific appropriateness of the request will be reviewed by SanBio, Inc.