Abstract
Objective
To investigate the association between endovascular therapy (EVT) start time in acute ischemic stroke (AIS) and midterm functional outcome.
Methods
This retrospective cohort study included all patients with AIS treated with EVT from 2 stroke center registries from January 2012 to December 2018. The primary outcome was the score on the modified Rankin Scale (mRS) and the utility-weighted mRS (uw-mRS) at 90 days. A proportional odds model was used to calculate the common odds ratio (OR) as a measure of the likelihood that the intervention at a given EVT start time would lead to lower scores on the mRS (shift analysis).
Results
A total of 1,558 cases were equally allotted into 12 EVT start time periods. The primary outcome favored EVT start times in the morning at 08:00–10:20 and 10:20–11:34 (OR, 0.53; 95% confidence interval [CI], 0.38 to 0.75; p < 0.001; OR, 0.62; 95% CI, 0.44 to 0.87; p = 0.006, respectively), while it disfavored EVT start times at the end of the working day at 15:55–17:15 and 18:55–20:55 (OR, 1.47; 95% CI, 1.03–2.09; p = 0.034; OR, 1.49; 95% CI, 1.03–2.15; p = 0.033). Symptom onset to EVT start time was significantly higher and use of IV tissue plasminogen activator significantly lower between 10:20 and 11:34 (p < 0.004 and p = 0.012, respectively).
Conclusion
EVT for AIS in the morning leads to better midterm functional outcome, while EVT at the end of the work day leads to poorer midterm functional outcome. Difference in baseline factors, standard workflow, and technical efficacy metrics could not be identified as potential mediators of this effect.
Research on occupational accidents indicates that the risk of accidents while working increases as a function of number of hours at work.1–3 Hänecke and colleagues reported an exponential increase in occupational accidents after the 9th hour of work.4 Although most countries officially define the work day as 8 hours, many doctors experience working hours well beyond 9 hours due to the need for 24-hour patient care coverage.5,6 Neurointerventionists and the rest of the stroke management team are currently feeling the effects of changes in acute ischemic stroke (AIS) management,7 due in part to the mounting level of evidence from several large randomized controlled studies since 2015 that demonstrated the value of endovascular therapy (EVT) for first-line and delayed treatment.8–17 The significant increase in the number of EVT-eligible patients has proportionally increased the emergency case load for the neurointerventionists as well as the support staff in the stroke unit, which extends the number of hours at work and could be a potential source of fatigue.18 The need to examine the effects of doctor fatigue on the midterm outcome of treated patients was recognized and reported in the literature.19 Current data on midterm functional outcome in patients treated during work hours vs after hours is limited, requiring more robust studies to elucidate the effects on patients treated at different times of the day.20 We sought to investigate the association between EVT performed by presumed fatigued neurointerventionists and the stroke unit staff after standard working hours and the midterm neurologic outcome in treated patients.
Methods
Patient Population
We used 2 institutional databases—the acute stroke registry and analysis of Lausanne (ASTRAL) from Lausanne University Hospital, Switzerland, and the stroke registry from University Hospital of Bern, Switzerland—to retrospectively include all adult patients with AIS who received EVT, either as thromboaspiration or mechanical thrombectomy according to institutional guidelines, from January 2012 to December 2018. For patient selection, we used the following inclusion criteria: AIS secondary to a large vessel intracranial occlusion, CT-based multimodal imaging performed within 24 hours of last proof of good health showing occlusion of an intracranial vessel amendable to EVT, and brain angiography of good quality showing results following recanalization. Extracranial internal carotid artery occlusion or symptomatic stenosis without intracranial occlusion were excluded.
Both hospitals feature a comprehensive stroke center accredited by the Swiss Federation of Clinical Neurosocieties Swiss Stroke Committee mainly based on recommendations of the Swiss Stroke Society and European Stroke Organisation. Stroke team composition was similar between the centers and included an onsite neurologist trainee, on-call stroke attending neurologist, onsite attending anesthesiologist, and on-call attending interventional neurologist at all times including standard working hours, nighttime, and weekends. Imaging protocol and EVT criteria were based on local institutional guidelines and were applied in the same way regardless of admission day or time. For the University Hospital of Bern, MRI is performed during nighttime and daytime and there are no differences in its availability. Indications for EVT are standardized according to an institutional operating procedure scheme. For Lausanne University Hospital, a dedicated emergency MRI is available 24 hours a day, 7 days a week. It prioritizes stroke cases and there are no differences in its availability during nighttime and weekends relative to daytime. Indications for EVT are identical to those mentioned in the operating procedures of the University Hospital of Bern. Transfer patients were generally not reimaged unless transfer times were greater than 1.5 hours or the patient severely deteriorated after thrombolysis. In these 2 situations, perfusion CT/MRI was repeated prior to EVT.
Both centers have between 4 and 6 interventional neuroradiologists in order to provide 1 on-call physician per center working in 24-hour shifts covering 5 to 7 days per month. Approximately 5 EVTs are performed at Lausanne University and 7 at University Hospital of Bern per week. During EVT, operators work almost exclusively independently, with 1 angiography suite technician working in 8-hour shifts providing technical and material support. Endovascular trainees are often present during EVT, but do not participate as primary or secondary operators. Finally, a wide array of stent retriever devices are used in these centers to perform EVT, including, but not limited to, Trevo (Stryker, Kalamazoo, MI), Solitaire (Medtronic, Irvine, CA), Embotrap (Cerenovus, Galway, Ireland), Catch (Balt Extrusion, Montmorency, France), and Tiger (Rapid Medical, Yokneam, Israel).
Data Collection
Stroke time onset or last time seen without symptoms, groin puncture time, and recanalization time were recorded for each patient. The EVT start time was considered the operator's groin puncture time. A disproportionate number of EVTs were performed during normal working hours, making analysis at fixed time intervals with unequal cohort sizes a potential source of bias. As a result, patients were divided into 12 cohorts containing the same number of patients according to EVT start time, beginning at 08:00, the start of the work day, until 07:59 the following day. Time periods varied between cohorts, with those during the day shorter and those at night longer. To perform a multivariate analysis and eliminate confounding factors, the following patient characteristics were recorded: age, sex, atrial fibrillation, smoking, diabetes, hypertension, and hyperlipidemia, as well as stroke characteristics including NIH Stroke Scale (NIHSS) score at admission, prestroke modified Rankin Scale score (mRS), Alberta Stroke Program Early CT Score (ASPECTS), occlusion site, prestroke utility-weighted mRS score (uw-mRS), concomitant IV tissue plasminogen activator (tPA), Trial of ORG 10172 in Acute Stroke Treatment (TOAST) mechanism type, symptom onset to EVT start time, admission to EVT start time, and occlusion site; and defined as large artery atherosclerosis, cardioembolism, small vessel occlusion, stroke of other determined etiology, or stroke of undetermined etiology.21 The EVT characteristics reported included the following: modified Treatment in Cerebral Ischemia (mTICI) score, number of EVT passes, symptomatic intracranial hemorrhage (sICH), and uw-mRS at 90 days post-EVT.22,23
Primary and Secondary Outcomes
The primary outcome of the study was the score on the mRS at 90 days after EVT, which was assessed by trained personnel who were unaware of the EVT start time. The mRS is a graded interval scale (range, 0 [no symptoms] to 6 [death]) for the assessment of neurologic functional disability. Secondary outcomes included EVT start to recanalization interval time, mTICI score, number of EVT passes, and rate of sICH.
Statistical Analyses
Our analyses were performed to detect a shift in the distribution of scores on the mRS at 90 days between each EVT start time, with scores of 5 (bedbound with severe disability) and 6 (death) combined due to comparable patient-centered outcome and with the assumption that the differential effect would lead to a common odds ratio (OR) (indicating the odds of improvement of 1 point on the mRS) of 0.6 for positive effects and 1.4 for negative effects.24
Comparisons of baseline characteristics between cohorts were conducted using a one-way analysis of variance for continuous variables of interest (age, NIHSS at admission, onset to EVT start time, number of EVT passes, baseline EVT start to recanalization time, and uw-mRS at 90 days). Chi-square analysis was conducted for categorical variables of interest (atrial fibrillation, hypertension, smoking, diabetes, hyperlipidemia, IV tPA, TOAST, baseline mTICI, and sICH). Continuous variables are presented using mean and interquartile range. Categorical variables are presented as frequencies with percentages. Significance was set at p < 0.05.
Detection of statistically significant variables was performed by a univariate analysis using an unpaired t test comparing 1 cohort to all other reference cohorts, then subsequently repeated for other cohorts. Significance was set at p < 0.05.
Adjusted estimates of effect were selected according to clinical relevance and adjusted for age, symptom onset to EVT start time, age, NIHSS score, mTICI score, hypertension, and prestroke mRS score. The assessment of heterogeneity of EVT start time effect was performed with the inclusion of multiplicative interaction terms. Significance was set at p < 0.05.
Statistical analyses were performed using Anaconda version 5.3.1 (Anaconda, Austin, TX) for Python 2.7 linked via module Rpy2 with R version 3.5.1ci (R Foundation, Vienna, Austria). For the primary end point, an ordinal multivariate logistic regression was performed with proportional OR. To perform it, a Vector Generalized Linear and Additive Models (VGLM/VGAM) library was used in R. The confounding factors added into the model were selected by clinical relevance. Noncollinearity was verified using tolerance metric in the R package “olsrr.” All variables had a tolerance greater than 0.2 with the lowest tolerance calculated at 0.23. This confirmed our clinical assumption of no collinearity between variables.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the local institutional review board of each hospital under the auspices of the Swiss ethics committees for research involving humans (Swissethics). No informed consent was required according to the legislation.
Data Availability
Individual deidentified participant data, related documents such as study protocol, and statistical analysis will be shared on request from any qualified investigator for 3 years after the date of publication.
Results
A total of 1,574 consecutive EVT procedures meeting selection criteria were selected for review, with 991 cases derived from University Hospital of Bern, of which 660 were direct presentations (66%), and 581 from Lausanne University Hospital, of which 490 were direct presentations (83%). Sixteen cases were lost to follow-up as no information on midterm functional neurologic status was available. The 1,558 cases available for analysis were distributed according to EVT start time into equal size patient cohort time periods, each containing 127 to 131 patients. The cohorts were as follows: 08:00–10:20, 10:20–11:34, 11:34–12:40, 12:40–13:37, 13:37–14:41, 14:41–15:55, 15:55–17:15, 17:15–18:55, 18:55–20:55, 20:55–22:57, 22:57–02:07, and 02:07–07:58.
Demographics, Cardiovascular Risk Factors, and Stroke Characteristics
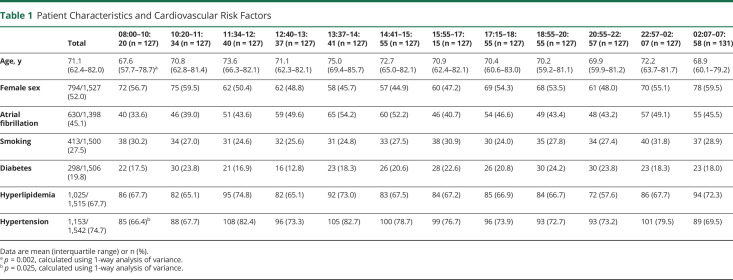
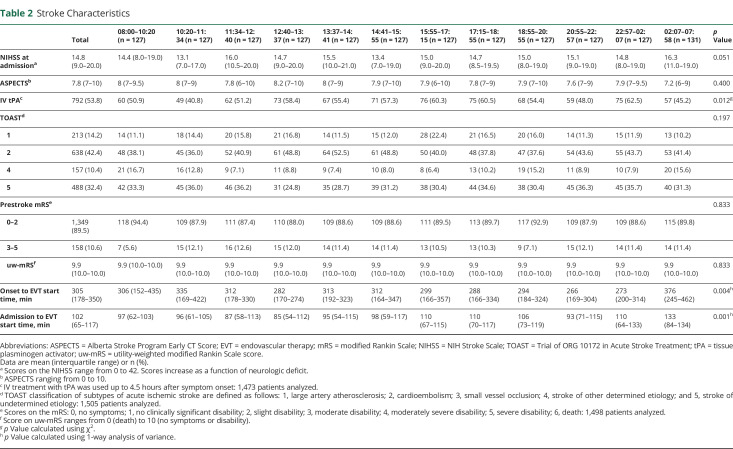
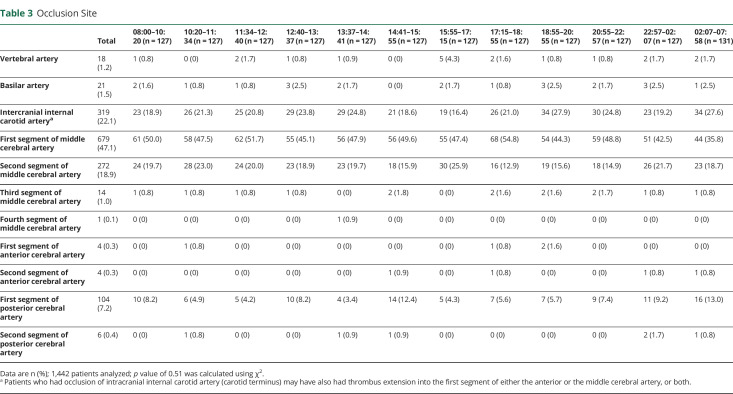
A description of the demographics and cardiovascular risk factors for each patient cohort is shown in table 1. No inhomogeneity was detected in patient cohorts relating to demographics and cardiovascular risk factors with the exception of age; a small, yet statistically significant younger population was observed in the morning 08:00–10:20 cohort (p = 0.002). The absolute difference of mean age between patients in this cohort relative to the mean of all patients included in the study was 3.5 years. Fewer patients in this cohort were hypertensive (p = 0.025). A description of stroke characteristics for each patient cohort is shown in table 2. Stroke characteristics were similarly homogenous with the exception of symptom onset to EVT start time, IV tPA use, and admission to EVT start time. Onset to EVT start time was significantly longer between 10:20–11:34 and 02:07–07:58 and significantly shorter between 20:55–22:57 and 22:57–02:07 (p = 0.004). IV tPA was used less frequently between 10:20–11:34 and 02:07–07:58 (p = 0.012). Admission to EVT start time was significantly longer between 02:07 and 07:58. A description of stroke occlusion site is shown in table 3. Distribution of stroke occlusion site was homogenous among all cohorts.
Table 1.
Patient Characteristics and Cardiovascular Risk Factors
Table 2.
Stroke Characteristics
Table 3.
Occlusion Site
Primary End Point, EVT Start Time, and Midterm Functional Outcome
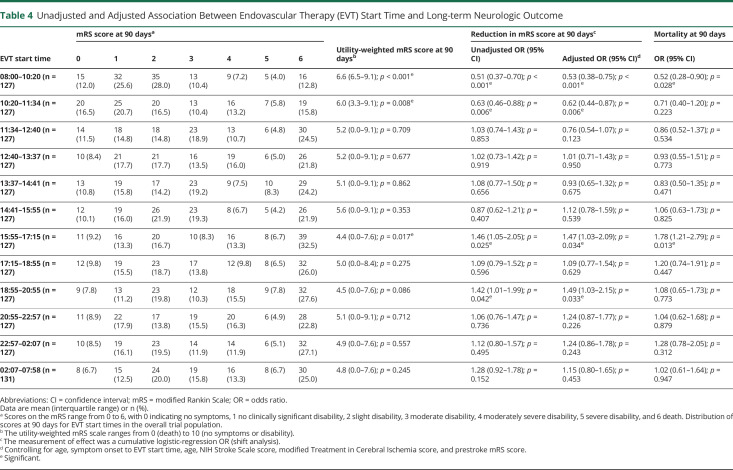
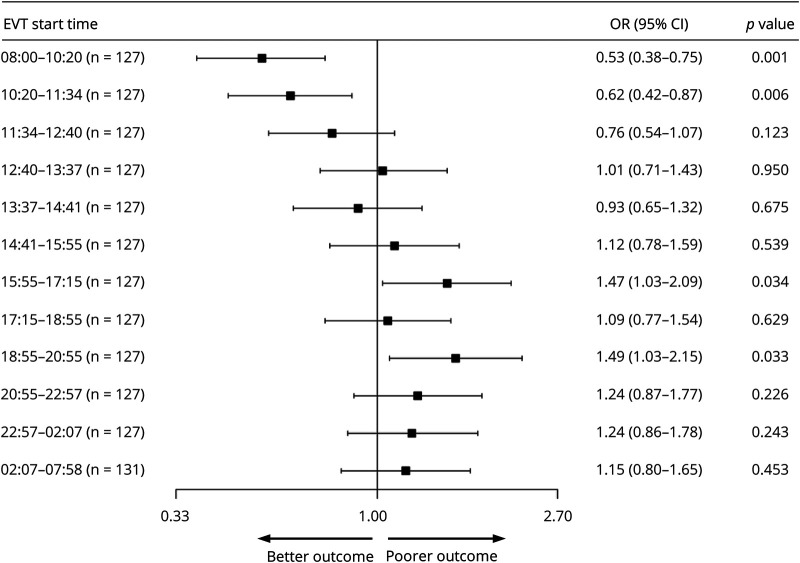
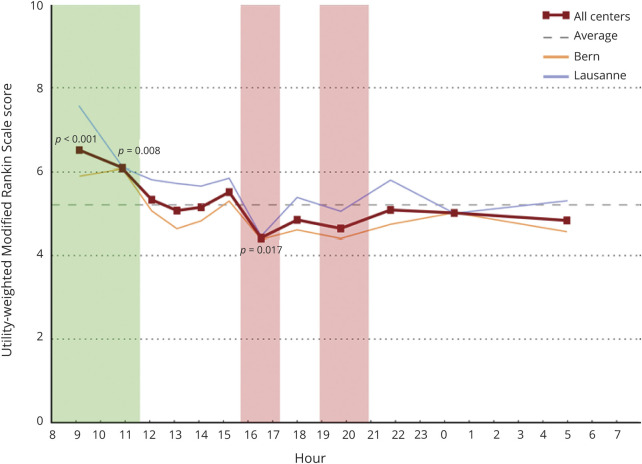
Table 4 describes the univariate and multivariate analyses investigating the relationship between the EVT start time and mRS, uw-mRS, and mortality at 90 days. Analysis of the primary end point showed a common OR (indicating the odds of improvement of 1 point on the mRS) of 0.53 (95% confidence interval [CI], 0.38 to 0.75, p < 0.001) and of 0.62 (95% CI, 0.44 to 0.87, p = 0.006) favoring EVT with start times in the morning at 08:00–10:20 and 10:20–11:34 as well as 1.47 (95% CI, 1.03 to 2.09, p = 0.034) and 1.49 (95% CI, 1.03 to 2.15, p = 0.033) at 15:55–17:15 and 18:55–20:55, respectively, disfavoring EVT at start times at the end of the work day. Figure 1 shows the effect size in the primary end point across all EVT start time cohorts. There was a higher uw-mRS at 90 days, signifying better functional outcome, in patients with EVT performed between 08:00 and 10:20 (5.9; 95% CI, 3.3 to 9.1, p < 0.001) as well as EVT performed between 10:20 and 11:34 (5.2, 95% CI, 0.0 to 9.1, p = 0.008) and significantly lower uw-mRS, signifying poorer functional outcome, in patients treated at 15:55–17:15 (5.0; 95% CI, 0.0 to 8.4, p = 0.017). Figure 2 depicts the uw-mRS at 90 days as a function of time period cohort for both stroke centers. Similar trends in functional outcome are observed in each stroke center.
Table 4.
Unadjusted and Adjusted Association Between Endovascular Therapy (EVT) Start Time and Long-term Neurologic Outcome
Figure 1. Analysis of the Primary End Point.
The forest plot shows the effect size in the primary end point (common odds ratio for improvement on the modified Rankin Scale at 90 days, analyzed according to ordinal logistic regression and adjusted for age, symptom onset to endovascular therapy (EVT) start time, age, NIH Stroke Scale score, modified Treatment in Cerebral Ischemia score, and prestroke modified Rankin Scale score) across all EVT start time cohorts. There are significant differences in the morning cohorts at 08:00–10:20 and 10:20–11:34, suggesting better functional outcome at 3 months, as well as in the end of work day cohorts at 15:55–17:15 and 18:55–20:55, suggesting poorer functional outcome at 3 months. CI = confidence interval; OR = odds ratio.
Figure 2. Scores on the Utility-Weighted Modified Rankin Scale at 90 Days for Each Stroke Center as a Function of Endovascular Therapy Start Time.
Data are organized into 12 equally sized cohorts over 24 hours starting at 8:00. Higher scores indicate better functional outcome. Green shading corresponds to cohorts with favorable functional outcome at 3 months; red shading corresponds to cohorts with poorer functional outcome at 3 months.
The proportion of patients with mRS of 0–2 at 90 days was 37.6% and 37.2% when EVT start times were between 08:00–10:20 and 10:20–11:34, respectively, vs only 22.5% and 19.0% for EVT start times at 15:55 and 18:55–20:55. Mortality at 90 days was lower in the morning in 12.8% patients with EVT start time at 08:00–10:20 (OR, 0.52; 95% CI, 0.28–0.90; p = 0.028) and higher at the end of the work day in 32.5% of patients when EVT start times were 15:55–17:15 (OR, 1.78; 95% CI, 1.21–2.79; p = 0.013).
Secondary End Points, EVT Procedural Characteristics
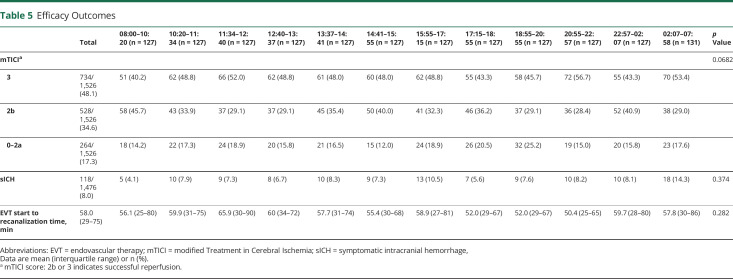
Table 5 describes the univariate analysis of EVT characteristics in all time period cohorts. mTICI score, number of EVT passes, presence of sICH, and EVT start to recanalization time were not significantly different.
Table 5.
Efficacy Outcomes
Discussion
The main finding of the present study is that midterm functional outcome and mortality after EVT in AIS varied according to the start time of thrombectomy. EVT performed in the morning, 08:00–10:15, yielded good functional outcomes, whereas EVT performed after the ninth hour of work, 15:55–17:15, led to poorer functional outcomes. The outcome from EVT performed at nighttime is not significantly worse than that of EVT performed during the day. The difference observed does not seem to be caused by differences in baseline characteristics or due to difference in standard measures of interventional efficacy and safety at different time points.
EVT is a procedure requiring a high degree of vigilance and reflex developed after several years of specialized training, which may become impaired due to extended working hours.25 Patients admitted out of normal work hours are less likely to be managed according to current operational guidelines, leading to poorer short-term outcomes than those of patients admitted during normal work hours.26 We hypothesized that EVT performed outside of the work day or at night would lead to poorer midterm functional outcome because of doctor fatigue. The link between fatigue and performance remains controversial, with some studies failing to demonstrate any correlation between extended working hours and doctor performance or safety outcomes.27 A systematic literature review by Gates and colleagues19 suggests that evidence for possible association of doctor fatigue with performance is varied in part because of the use of heterogenous outcome measurements. In stroke management, the outcomes of interest are midterm functionality graded on the mRS and uw-mRS, a patient-centered scale that measures benefit of a given intervention to the patient.23 We studied effect on mRS as a function of EVT start time. Assuming that the on-call neurointerventionist begins the work day between 07:00 and 08:00, our study suggests that at the start of the work day, between 08:00 and10:31, technical performance is optimal in a rested and vigilant doctor, leading to the significantly higher uw-mRS at 90 days or better midterm functional outcome observed. However, the analyses of the secondary end points measuring technical performance did not reveal higher rates of complete recanalization, fewer numbers of EVT passes, faster procedure times, or lower rates of sICH. Notably, in this cohort, symptom onset to EVT start times were significantly longer and on average beyond 4.5 hours. This may suggest that many patients in this cohort met the stricter EVT requirements and thus had relatively better collateral circulation and smaller core infarct sizes, which could be a potential source of selection bias. Any possible inhomogeneity between cohorts cannot be corroborated because these 2 stroke characteristics were not recorded in the majority of patients in the 2 institutional databases used in this study. We hypothesize that in the morning cohorts, an increased number of patients with stroke of unknown onset with limited infarct core size due to good collateralization were selected for EVT and thus had a higher probability of favorable midterm functional outcome. These patients were statistically younger than other cohorts, thus had better leptomeningeal collateral circulation and better hemodynamic variables with hypothetically small core size volumes. This cannot be substantiated in this study because infarct volume size was not available for analysis in this dataset.28,29 Also, only 66.4% patients at 08:00–10:15 were hypertensive, compared to a frequency of 74.7% for all groups. Whereas concomitant hypertension in AIS is associated with poorer outcome and therefore could in part explain better outcome in the morning cohorts, we do not observe a statistically poorer outcome in cohorts with higher frequency hypertension. Any inhomogeneity in rates of hypertension among the cohorts cannot solely explain, according to the applied statistical model, the difference in midterm functional outcome.30 Moreover, this could also indicate that a large number of patients were deemed unsuitable, but possibly could have benefitted from EVT.
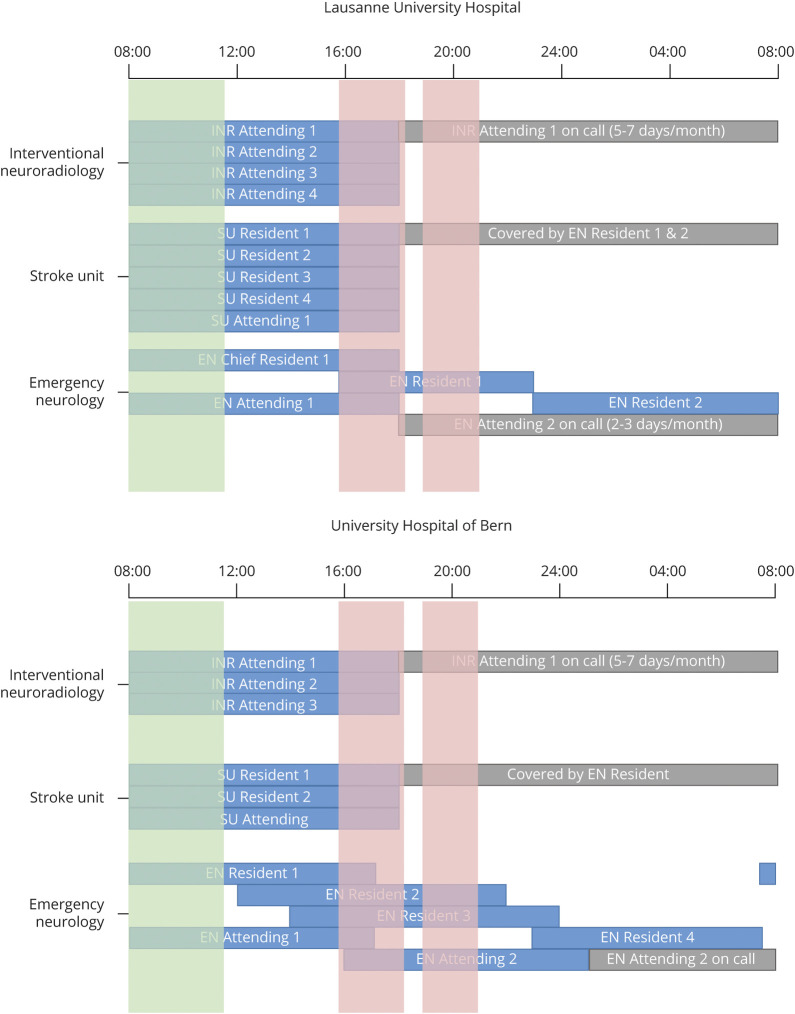
Conversely, EVTs performed between 15:55 and 17:15, after a 9-hour work day, were associated with higher mRS at 90 days or poorer midterm functional outcome. Although our multivariate analysis showed significantly higher mRS at 90 days, intermediate outcome measurements that have predictive effects on midterm functional outcome, like mTICI, number of EVT passes, and rate of sICH, were not significantly altered in this time period.31–33 Moreover, EVT start to recanalization time, a measure of technical skill and rapidity, was not significantly different between time period cohorts. We therefore hypothesize that other factors independent of doctor fatigue, such as stroke team shift changes or hampered performance from other collaborators such as emergency neurologist or support staff in the stroke unit, may contribute to poor functional outcome. Figure 3 shows the work shifts for interventional neuroradiology, stroke unit, and emergency neurology physicians at both centers.
Figure 3. Shift Schedules for Physicians in Interventional Neuroradiology (INR), Stroke Unit (SU), and Emergency Neurology (EN).
Data are organized into 12 equally sized cohorts over 24 hours starting at 8:00. Green shading corresponds to cohorts with favorable functional outcome at 3 months; red shading corresponds to cohorts with poorer functional outcome at 3 months.
The observed outcome decline between 15:55 and 17:15 was only transient, with values of uw-mRS at 90 days actually increasing after 17:15, at the end of the standard work day in our 2 hospitals. Admission to groin puncture delays were significantly longer at night, which has been previously reported.34,35 Corroborating the findings of the MR CLEAN (Multicenter Randomized Clinical Trialof Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) Registry, this delay was not appreciable in our analysis regarding clinical outcome. We conjecture that working conditions for EVT paradoxically improves during the evening or night and may maintain good technical performance and offset fatigue partially because of less work burden or distractions. Studies on work-related injuries as a function of the number of hours worked showed a transient, yet significant increase after the 9th hour of work, but a nonsignificant risk thereafter or at nighttime.4,36 The results of this study show similar findings regarding functional outcome and highlights similar trends in both stroke centers included in this study.
The repercussions of the burgeoning demand for EVT due to recent paradigm shifts of AIS management on neurointerventionists and the stroke unit staff remains controversial.25 Previously, AIS stroke management was limited to IV thrombolysis, requiring knowledge but not depending on technical skill. Nowadays, the complexities involved in EVT during AIS management pose challenges to neurointerventionists, anesthetists, and stroke unit staff faced with increasing caseload, extended working hours, and more on-call duties. Our data show differences in outcome warranting further investigation of current workplace conditions for EVT in AIS on patient outcome.
One of the weaknesses of this study is that many of the confounding variables cannot be controlled in the analyses, such as variability in a doctor's skill, training level, or overall stroke unit performance. For example, interruptions of activity related to shift changes, patient transferring, and diagnostic examinations during the day may decrease the amount of time for direct patient care in certain cohorts, leading to adverse effects prior to or following EVT. Any potential difference may also be observed between the hospitals included in this study, as Lausanne University Hospital performs fewer EVTs than University Hospital of Bern. Nevertheless, the subgroup analysis of uw-mRS at 90 days for each hospital reveals similar trends as a function of EVT start time, most notably at 15:54 to 17:15. Moreover, the addition of center variables, such as hospital site or weekday vs weekend, to the logistic regression did not affect results. Perfusion imaging data and infarct core size score were not available for analysis and inhomogeneity in these characteristics among the cohorts may explain differences in clinical outcome. Other limitations include the fact that this study is retrospective and includes patients with AIS treated by EVT from 2012, an era when EVT was less standardized.
EVT for patients with AIS performed in the morning between 08:00 and 10:20 leads to better midterm functional outcome at 90 days, despite having a statistically longer symptom onset to EVT start time delay, whereas EVT performed at the end of the working day, between 15:54 and 17:15, leads to poorer neurologic outcome. Additional prospective study data are required to support our findings and further analysis regarding causal relation is warranted.
Glossary
- AIS
acute ischemic stroke
- CI
confidence interval
- EVT
endovascular therapy
- mRS
modified Rankin Scale
- mTICI
modified Treatment in Cerebral Ischemia
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- sICH
symptomatic intracranial hemorrhage
- TOAST
Trial of ORG 10172 in Acute Stroke Treatment
- tPA
tissue plasminogen activator
- uw-mRS
utility-weighted modified Rankin Scale score
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011449 for full disclosures.
References
- 1.Akerstedt T. Increased risk of accidents during night shift: an underestimated problem are fatigue-induced accidents. Article in Swedish. Lakartidningen 1995;92:2103–2104. [PubMed] [Google Scholar]
- 2.Akerstedt T. Shift work and disturbed sleep/wakefulness. Sleep Med Rev 1998;2:117–128. [DOI] [PubMed] [Google Scholar]
- 3.Smith L, Folkard S, Poole CJ. Increased injuries on night shift. Lancet 1994;344:1137–1139. [DOI] [PubMed] [Google Scholar]
- 4.Hänecke K, Tiedemann S, Nachreiner F, Grzech-Sukalo H. Accident risk as a function of hour at work and time of day as determined from accident data and exposure models for the German working population. Scand J Work Environ Health 1998;3(24 suppl):43–48. [PubMed] [Google Scholar]
- 5.Colquhoun WP. Shiftwork: Problems and Solutions. Frankfurt am Main: P. Lang; 1996. [Google Scholar]
- 6.Mansukhani MP, Kolla BP, Surani S, Varon J, Ramar K. Sleep deprivation in resident physicians, work hour limitations, and related outcomes: a systematic review of the literature. Postgrad Med 2012;124:241–249. [DOI] [PubMed] [Google Scholar]
- 7.Maingard J, Kok HK, Ranatunga D, et al. The future of interventional and neurointerventional radiology: learning lessons from the past. Br J Radiol 2017;90:20170473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 11.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 12.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 13.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 14.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganesh A, Goyal M. Thrombectomy for acute ischemic stroke: recent insights and future directions. Curr Neurol Neurosci Rep 2018;18:59. [DOI] [PubMed] [Google Scholar]
- 17.Hussain S, Fiorella D, Mocco J, et al. In defense of our patients. J Neurointerv Surg 2017;9:525–526. [DOI] [PubMed] [Google Scholar]
- 18.Hopkins LN, Holmes DR Jr. Public health urgency created by the success of mechanical thrombectomy studies in stroke. Circulation 2017;135:1188–1190. [DOI] [PubMed] [Google Scholar]
- 19.Gates M, Wingert A, Featherstone R, Samuels C, Simon C, Dyson MP. Impact of fatigue and insufficient sleep on physician and patient outcomes: a systematic review. BMJ Open 2018;8:e021967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almallouhi E, Al Kasab S, Harvey JB, et al. Impact of treatment time on the long-term outcome of stroke patients treated with mechanical thrombectomy. J Stroke Cerebrovasc Dis 2019;28:185–190. [DOI] [PubMed] [Google Scholar]
- 21.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 22.Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaisinanunkul N, Adeoye O, Lewis RJ, et al. Adopting a patient-centered approach to primary outcome analysis of acute stroke trials using a utility-weighted modified Rankin scale. Stroke 2015;46:2238–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dijkland SA, Voormolen DC, Venema E, et al. Utility-weighted modified Rankin scale as primary outcome in stroke trials: a simulation study. Stroke 2018;49:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valavanis A, Remonda L, Gralla J. La neuroradiologie: une discipline sous-estimée. Schweiz Arzteztg 2017;98:1639–1640. [Google Scholar]
- 26.Turner M, Barber M, Dodds H, et al. Stroke patients admitted within normal working hours are more likely to achieve process standards and to have better outcomes. J Neurol Neurosurg Psychiatry 2016;87:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eskesen TG, Peponis T, Saillant N, et al. Operating at night does not increase the risk of intraoperative adverse events. Am J Surg 2018;216:19–24. [DOI] [PubMed] [Google Scholar]
- 28.Arsava EM, Vural A, Akpinar E, et al. The detrimental effect of aging on leptomeningeal collaterals in ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:421–426. [DOI] [PubMed] [Google Scholar]
- 29.Nannoni S, Sirimarco G, Cereda CW, et al. Determining factors of better leptomeningeal collaterals: a study of 857 consecutive acute ischemic stroke patients. J Neurol 2019;266:582–588. [DOI] [PubMed] [Google Scholar]
- 30.Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension 2004;43:18–24. [DOI] [PubMed] [Google Scholar]
- 31.Dargazanli C, Consoli A, Barral M, et al. Impact of modified TICI 3 versus modified TICI 2b reperfusion score to predict good outcome following endovascular therapy. AJNR Am J Neuroradiol 2017;38:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baek JH, Kim BM, Heo JH, et al. Number of stent retriever passes associated with futile recanalization in acute stroke. Stroke 2018;49:2088–2095. [DOI] [PubMed] [Google Scholar]
- 33.Ntaios G, Papavasileiou V, Michel P, Tatlisumak T, Strbian D. Predicting functional outcome and symptomatic intracranial hemorrhage in patients with acute ischemic stroke: a glimpse into the crystal ball? Stroke 2015;46:899–908. [DOI] [PubMed] [Google Scholar]
- 34.Mpotsaris A, Kowoll A, Weber W, Kabbasch C, Weber A, Behme D. Endovascular stroke therapy at nighttime and on weekends—as fast and effective as during normal business hours? J Vasc Interv Neurol 2015;8:39–45. [PMC free article] [PubMed] [Google Scholar]
- 35.Hinsenveld WH, de Ridder IR, van Oostenbrugge RJ, et al. Workflow intervals of endovascular acute stroke therapy during on- versus off-hours: the MR CLEAN registry. Stroke 2019;50:2842–2850. [DOI] [PubMed] [Google Scholar]
- 36.Reinberg A, Vieux N, Andlauer P, Permanent Commission and International Association on Occupational Health, Scientific Committee on Shift Work. Night and Shift Work: Biological and Social Aspects: Proceedings of the Fifth International Symposium on Night and Shift Work: Scientific Committee on Shift Work of the Permanent Commission and International Association on Occupational Health (PCIAOH) Rouen, 12–16 1980, 1st ed. Oxford: Pergamon Press; 1981.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual deidentified participant data, related documents such as study protocol, and statistical analysis will be shared on request from any qualified investigator for 3 years after the date of publication.