Abstract
Objective
This tertiary analysis from A Very Early Rehabilitation Trial (AVERT) examined fatal and nonfatal serious adverse events (SAEs) at 14 days.
Method
AVERT was a prospective, parallel group, assessor blinded, randomized international clinical trial comparing mobility training commenced <24 hours poststroke, termed very early mobilization (VEM), to usual care (UC). Primary outcome was assessed at 3 months. Patients with ischemic or hemorrhagic stroke within 24 hours of onset were included. Treatment with thrombolytics was allowed. Patients with severe premorbid disability or comorbidities were excluded. Interventions continued for 14 days or hospital discharge if less. The primary early safety outcome was fatal SAEs within 14 days. Secondary outcomes were nonfatal SAEs classified as neurologic, immobility-related, and other. Mortality influences were assessed using binary logistic regression adjusted for baseline stroke severity (NIH Stroke Scale [NIHSS] score) and age.
Results
A total of 2,104 participants were randomized to VEM (n = 1,054) or UC (n = 1,050) with a median age of 72 years (interquartile range [IQR] 63–80) and NIHSS 7 (IQR 4–12). By 14 days, 48 had died in VEM, 32 in UC, age and stroke severity adjusted odds ratio of 1.76 (95% confidence interval 1.06–2.92, p = 0.029). Stroke progression was more common in VEM. Exploratory subgroup analyses showed higher odds of death in intracerebral hemorrhage and >80 years subgroups, but there was no significant treatment by subgroup interaction. No difference in nonfatal SAEs was found.
Conclusion
While the overall case fatality at 14 days poststroke was only 3.8%, mortality adjusted for age and stroke severity was increased with high dose and intensive training compared to usual care. Stroke progression was more common in VEM.
Registration
Australian New Zealand Clinical Trials Registry, ACTRN12606000185561.
Classification of Evidence
This study provides Class I evidence that very early mobilization increases mortality at 14 days poststroke.
Uncertainty about the benefits and risks of early mobilization (out-of-bed activity and mobility training) started soon after stroke onset remains.1,2 We addressed this question in A Very Early Rehabilitation Trial (AVERT), in which higher dose (amount, frequency, intensity) of out-of-bed, task-specific training of sitting, standing, and walking, termed very early mobilization (VEM), was commenced within 24 hours of stroke onset and continued for 14 days or hospital discharge if sooner. Higher dose training (VEM) was associated with significant reduction in the odds of a favorable outcome (modified Rankin Scale score 0–2) at 3 months, compared to usual care.3 In this primary report of 3-month (primary endpoint) outcomes,3 the 3-month case fatality was 7.6% (95% confidence interval [CI] 6.5–8.8), lower than the case fatality reported in a recent review of stroke incidence studies of 10%–42% over a similar time period, 28–30 days poststroke.4 There were no significant treatment-by-subgroup interactions, and by 3 months, the number of fatal or nonfatal serious stroke or immobility-related adverse events (SAEs) did not differ significantly between groups.
In line with our prespecified analysis plan,5 this tertiary analysis aimed to further elucidate factors associated with the safety of this intervention in the first weeks after stroke. Therefore, the objectives of this article were to report the 14-day (end of intervention period) safety analyses of the number and nature of fatal and nonfatal SAEs during the trial intervention period, and to undertake exploratory analyses in prespecified subgroups.
Methods
Study Design, Participants, and Procedures
Details of the AVERT trial design, participants, procedures, sample size estimation, analysis plan, and primary outcomes have been published.3,5 In brief, AVERT was a parallel-group, international, multicenter, randomized controlled trial incorporating 56 sites in 5 countries: Australia, New Zealand, Singapore, Malaysia, and the United Kingdom. The eligibility criteria were broad, with no upper age limit, including patients with both ischemic and hemorrhagic stroke, and treatment with thrombolysis (recombinant tissue plasminogen activator [rtPA]) was allowed at the discretion of the recruiting physician. Those with a fever, low oxygen saturation, or blood pressure (BP) <110 mm Hg or >220 mm Hg were excluded.3 Participants were recruited within 24 hours of stroke onset and randomized to either the more frequent, higher dose mobility training regimen (VEM) or to usual stroke unit care (UC), which includes mobilization, but was not standardized.3 A remote, web-based, computer-generated randomization procedure was used to balance randomization by site and stratify by stroke severity based upon the participant's baseline NIH Stroke Scale score (NIHSS: mild [1–7], moderate [8–16], or severe [>16]). Participants were blinded to group allocation, but therapists and nurses were not blinded. For those in the VEM group, intervention was delivered by ward-based, intervention-trained physiotherapy and nursing staff, starting ≤24 hours after stroke. VEM comprised out-of-bed activities and task-specific training to promote recovery of mobility in addition to usual care on 5–6 days per week. Prolonged sitting or standing activities were not part of the intervention and were actively discouraged, particularly in the first 3 days after stroke onset. Bed rest between activities was encouraged. For VEM participants, the first out of bed intervention occurred if physiologic measures were within broad safety limits (BP, temperature, O2 saturation) detailed in the protocol, and if the treating therapist believed it was safe and appropriate to do so. Physiologic data were not recorded in the trial. An episode of orthostatic hypotension (>30 mm Hg) meant the session would cease. Daily interventions were guided by the participant's functional status as judged by the physiotherapist, with 4 levels (1–4) specified, and intervention staff used their clinical judgement of a participant's ability and response to treatment to commence or continue a prescribed session. The intervention period continued for 14 days or until acute hospital discharge, whichever was earlier.
Outcomes
The prespecified safety outcome in this analysis was fatal SAEs within the first 14 days after stroke (Class 1 evidence). The secondary outcome was nonfatal SAEs within 14 days (Class 1 evidence). We predefined safety events as important medical events (IMEs) or adverse events (AEs), both of which were termed SAEs if at least one of the following were met: (1) resulted in death, (2) life-threatening, (3) required inpatient hospitalization or prolonged existing hospitalization, or (4) resulted in persistent disability. Stroke-related IMEs included progression and recurrence of stroke. Stroke progression was defined as worsening of symptoms in the same vascular territory as the initial stroke event, commencing within 14 days; recurrent strokes were new stroke events in a different vascular territory in the same period, or any new event beyond 14 days. Immobility-related IMEs comprised pneumonia, urinary tract infection, pulmonary embolism, deep vein thrombosis, and pressure sores. Other IMEs were myocardial infarct, angina, depression, and falls. Falls were categorized as fall with no soft tissue injury, fall with soft tissue injury, or fall with bone fracture or head injury. AEs were defined as any other untoward medical occurrence and included worsening of a preexisting event. AEs were categorized as neurologic, cardiovascular, pulmonary, gastrointestinal, hepato-biliary, metabolic and endocrine, renal and urinary, musculoskeletal, psychiatric, oncologic, dermatologic, infections, hematologic, stroke-related fever, or support services.
Trained site investigators reported SAEs via submission of case report forms and de-identified supporting medical record documentation. At 3-month assessments, blinded assessors interviewed participants or carers and examined medical records to ensure that all SAEs were reported. For each SAE, assessors recorded (1) medical diagnosis and (2) blinded assessment of the relationship to treatment: probably, possibly, probably not, or not related, according to the clinicians' determination of the relationship between the intervention and the SAE. All SAE documentation was reviewed by trial management for accuracy and completeness. At site monitoring visits, trial management staff performed risk-based independent audits using random participant selection and blinded medical record review to verify source data. For participants discharged prior to 14 days and not contactable at 3 months, assessors performed a search of death registers in the United Kingdom, Australia, and New Zealand. No death registers were available in Singapore or Malaysia.
Safety Adjudication and Monitoring
An independent outcomes committee comprising a chairperson (S.M.), consultant neurologist (J.H.F.), and stroke physician (V.S.) reviewed all SAEs throughout the trial. Members were blinded to participants' group allocation. Prior to each meeting, events were independently adjudicated by each medical expert for cause of event and relationship to treatment. At meetings, held approximately every 2 months via teleconference, divergent opinions were resolved by discussion, where necessary requesting additional medical information from assessors prior to adjudication. When insufficient additional information was available, events were adjudicated as “unable to be determined” or “sudden unexplained death.”
The independent Data Monitoring Committee adhered to a charter of responsibilities. The committee (P.B., C.B., S.J.R., C.M.R., C.M.S.) comprised experienced trial monitors with medical, physiotherapy, research, and statistical expertise. Meetings were held at least annually, with meeting frequency dependent on trial progress. Unblinded group allocation data were provided to the Data Monitoring Committee, with adjudicated individual and group summaries of SAEs that occurred in the acute intervention (0–14 days) and early (15 days–3 months) time periods. The committee reviewed current trial data for evidence of relative harm and made recommendations to the steering committee on whether to continue, modify, or stop the trial. Author and coinvestigator contributions are detailed in appendices 1 and 2 at links.lww.com/WNL/B261.
Analysis
Our statistical analysis plan5 outlines the 14-day tertiary safety analyses reported here. These include number of fatal and nonfatal SAEs, with specific reporting of stroke-related, immobility-related, and fall-related SAEs.
Mortality was investigated using the binary logistic regression model with treatment group as the independent variable and death at 14 days as the dependent variable, with baseline NIHSS and age as covariates for adjustment purposes. The treatment effect is presented as an adjusted odds ratio (aOR) with the corresponding 95% CI. Mortality was analyzed on an intention-to-treat basis, with an assumption that the data were missing at random.6 We explored the sensitivity of the results to plausible departures from the missing-at-random assumption as part of our intention-to-treat analysis, with use of both a selection model and a pattern mixture model.
Additional exploratory analyses undertaken were (1) time to death, that is, days from stroke onset to death, censored at 14 days, compared between groups using Cox regression, with the treatment effect presented as an aHR; (2) exploratory subgroup analyses were the same as for the primary analyses and included prespecified age, stroke severity, stroke type, rtPA treatment, time from stroke onset to first mobilization, and geographic region of recruitment subgroups, adjusted for age and stroke severity.
Nonfatal SAEs were analyzed using regression models for count data; Poisson or negative binomial regression were used depending on the validity of distributional assumptions. Incidence rate ratios adjusted for age and baseline stroke severity, with stroke-related and immobility-related SAEs reported in addition to total SAEs by group.
To supplement these analyses, we also provide participant profiles for everyone who died within 14 days of stroke. These pictorially illustrate the interventions delivered per day, day of fatal and nonfatal SAEs, and day of death. Interventions delivered per day are shown as total daily minutes spent in out-of-bed mobilizations with physiotherapists, in addition to the number of nursing and physiotherapy sessions reported.
All analyses were conducted using Stata 13IC statistical software (StataCorp, College Station, TX); the graphical participant summaries were produced using R statistical software (R Core Team 2017, R Foundation for Statistical Computing, Vienna, Austria).
Standard Protocols Approvals, Registrations, and Patient Consents
Human research ethics approval was obtained from all sites. Written informed consent was obtained from participants or guardians.
Data Availability
Data may be shared by writing to the corresponding author to obtain details of procedures and processes.
Results
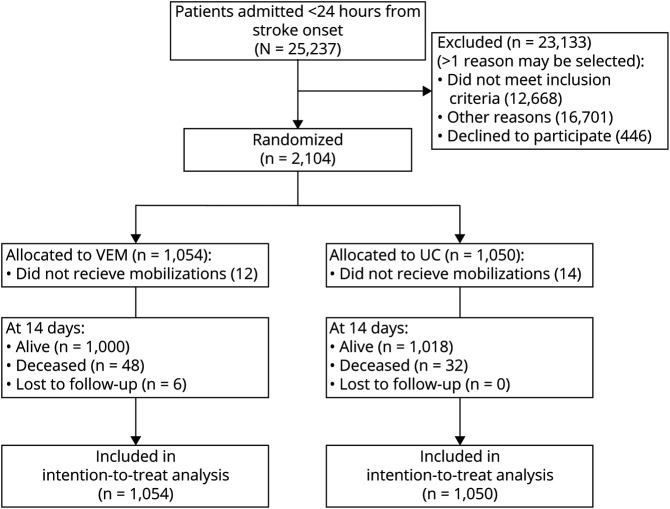
In all, 2,104 participants were recruited and randomly allocated to VEM (n = 1,054) or UC (n = 1,050; figure 1). The baseline characteristics were similar between groups, with a median age of 72 years (interquartile range [IQR] 63–80) and median NIHSS of 7 (IQR 4–12).3 Intracerebral hemorrhage was present in 12% and 22% had a total anterior circulation infarct (table 1). Median acute length of stay was 7 days for both groups. VEM participants commenced 4.8 hours earlier, and had a median of 3 additional sessions/day and a median of 20 additional minutes of training per day compared to UC.3 Twelve VEM participants (1.1%) were never mobilized, in comparison with 14 in UC (1.3%). Mortality status at 14 days was unknown for 6 (<0.5%) participants, all of whom were in VEM. Of these 6 participants, 2 withdrew from the trial before day 14. Four participants were discharged from hospital prior to 14 days (at days 2, 3, 4, and 6 post stroke) and were lost to follow- up at 3 months. Their mortality status at day 14 was unknown as there was no searchable death register in these countries.
Figure 1. Participant Progress Through Trial to 14 Days.
Primary reason for not receiving mobilization within 14 days: very early mobilization (VEM), n = 12; serious adverse event (SAE) and death outcome, n = 9; alive at 14 days: discharged home from hospital ≤24 hours, n = 1; SAE then transfer to intensive care unit ≤24 hours, n = 1; lost to follow-up: withdrawal, n = 1. Usual care (UC), n = 14: SAE and death outcome, n = 10; alive at 14 days: SAE, n = 2; palliated, n = 1; transferred to another ward, n = 1.
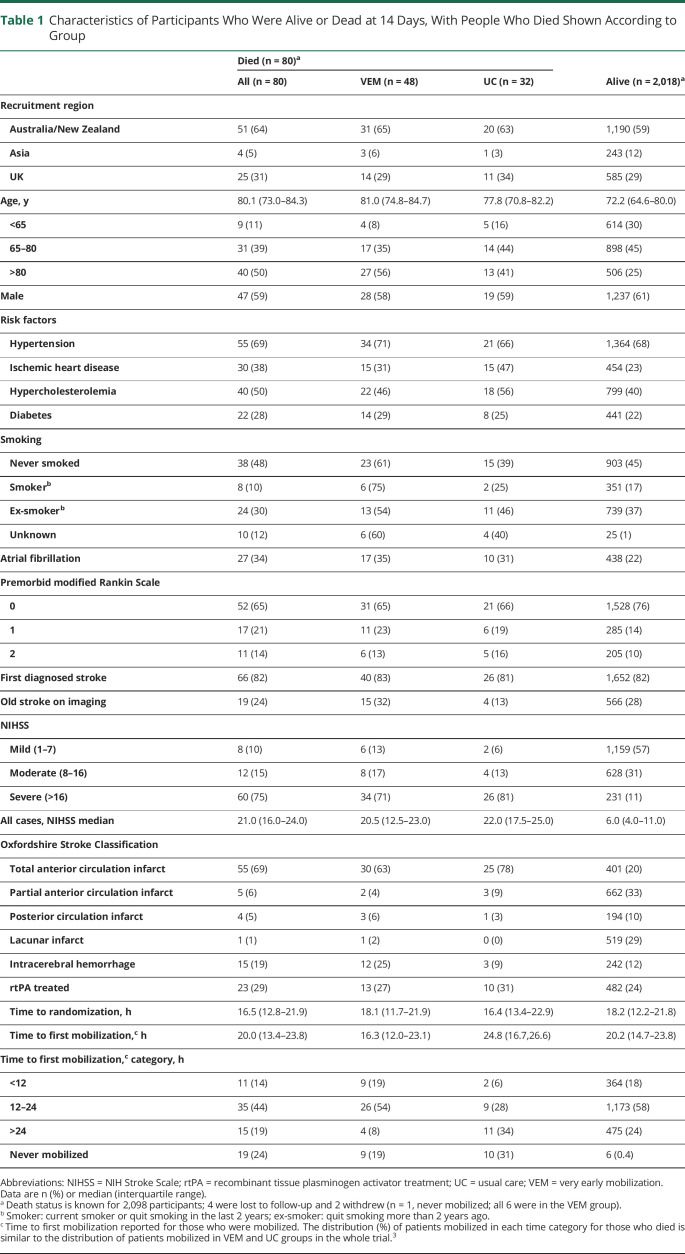
Table 1.
Characteristics of Participants Who Were Alive or Dead at 14 Days, With People Who Died Shown According to Group
Deaths
Eighty participants had died by 14 days, an overall case fatality rate of 3.8%. Of these, 48 (4.6%) were in the VEM group and 32 (3.0%) in UC. Of those who died within 14 days, 9 VEM and 10 UC participants had SAEs prior to any mobilization (table 1). Baseline characteristics of those who died were similar between treatment groups (table 1).
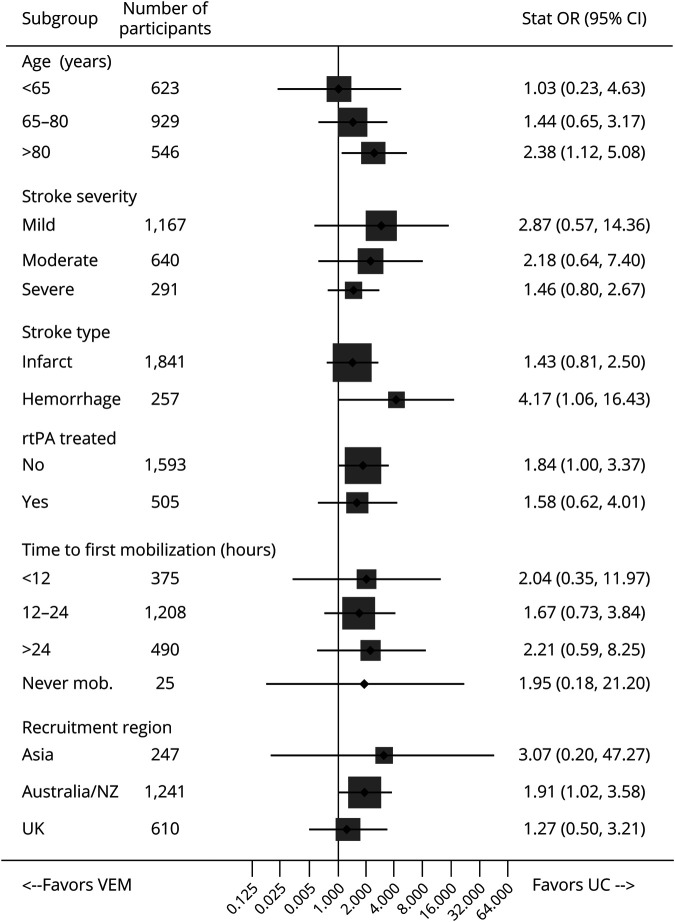
Participants in VEM had significantly greater odds of death by day 14 compared to those in UC (aOR 1.76, 95% CI 1.06–2.92, p = 0.029). In Cox regression analysis, the participants in the VEM group had a greater risk of dying at any point within 14 days than those in the standard care group (aHR 1.59, 95% CI 1.01–2.48, p = 0.044). Sensitivity analyses using selection and pattern mixture models supported these results (aOR 1.76, 95% CI 1.06–2.92, p = 0.029). The age- and severity-adjusted exploratory subgroup analyses (figure 2) indicated that there was a greater odds of death within 14 days in those aged over 80 years (aOR 2.38, 95% CI 1.12–5.08) and with intracerebral haemorrhage stroke (aOR 4.17, 95% CI 1.06–16.43) who were treated with VEM compared to UC. However, treatment by subgroup interactions were not significant (all p > 0.05) and there was poor estimate precision due to the small number of deaths in these subgroups (>80 years: VEM, n = 27; UC, n = 13; intracerebral hemorrhage: VEM, n = 12; UC, n = 3).
Figure 2. Forest Plot: Subgroup Analysis for Death at 14 Days.
Total number of deaths at 14 days, n = 80 (very early mobilization [VEM] 48, usual care [UC] 32). Missing data: unknown status at day 14, n = 6. Subgroup interactions were not significant. CI = confidence interval; OR = odds ratio; rtPA = recombinant tissue plasminogen activator.
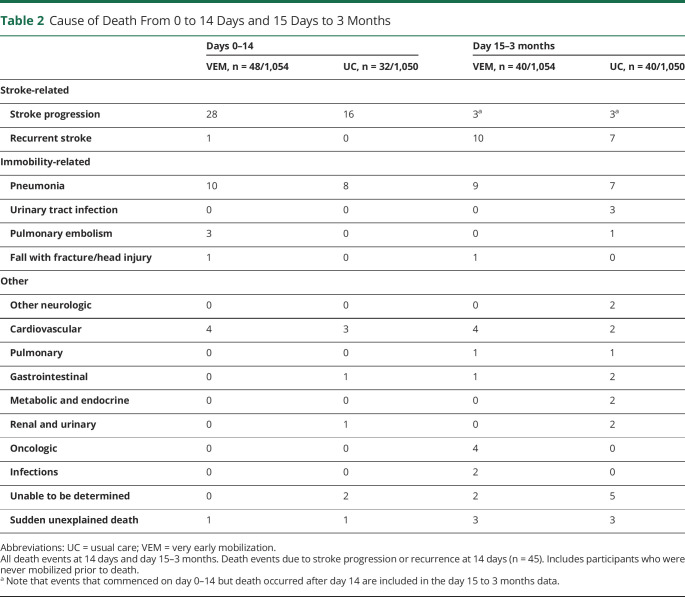
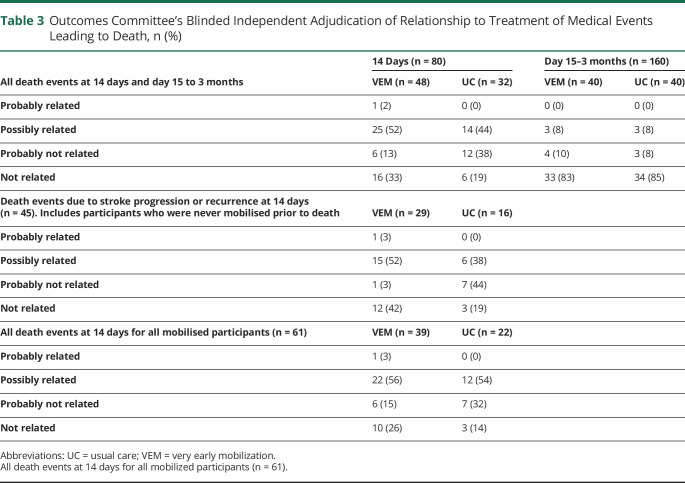
During the intervention period (0–14 days), stroke-related events were the most common cause of death (VEM, n = 29/48; UC, n = 16/32) (table 2). The outcomes committee adjudicated that death events were “probably” or “possibly” related to treatment in 26/48 (54%) of VEM and 14/32 (44%) of UC participants (table 3).
Table 2.
Cause of Death From 0 to 14 Days and 15 Days to 3 Months
Table 3.
Outcomes Committee's Blinded Independent Adjudication of Relationship to Treatment of Medical Events Leading to Death, n (%)
The clinical profiles of those who died within 14 days of stroke, including age, stroke severity (NIHSS), and stroke type (ischemic/hemorrhage), as well as the interventions received, the number and timing of SAEs, and day of death, are displayed in figure e-1 (doi.org/10.5061/dryad.9s4mw6mcq). Median time from stroke onset to death was 4 (VEM) and 5 (UC) days. Of those who died, 73% (35/48) from the VEM group were mobilized within 24 hours, compared to 34% (11/32) of those in UC. Nine VEM participants who died were never mobilized (8 died from stroke events), compared with 10 in UC (7 died from stroke events). The remainder were mobilized later than 24 hours.
Nonfatal SAEs to 14 Days
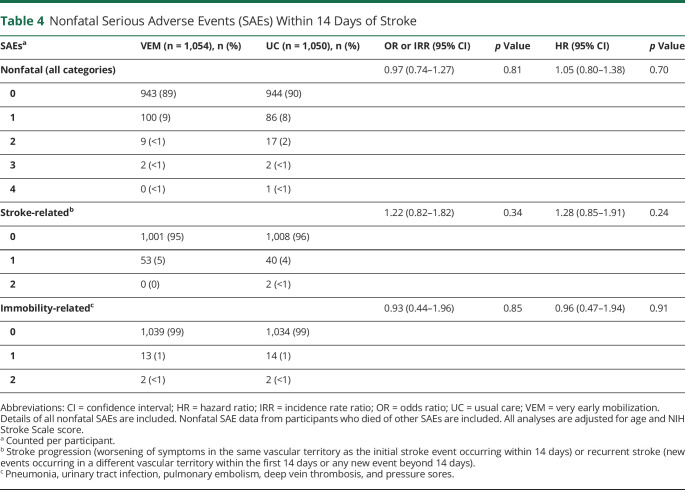
There were 124 nonfatal SAEs recorded for the VEM group and 130 in the UC group (table 4). The proportion of participants with nonfatal, stroke-related, and immobility-related SAEs did not differ significantly between groups (negative binomial regression analyses, table 4). By 14 days, 6 nonfatal serious falls were reported (VEM, 2; UC, 4); the numbers were too few to analyze.
Table 4.
Nonfatal Serious Adverse Events (SAEs) Within 14 Days of Stroke
Discussion
Whereas the overall case fatality at 14 days poststroke was only 3.8%, this prespecified analysis has shown that risk of death was significantly greater in those who undertook higher dose out-of-bed mobilization (VEM) as compared to a lesser dose mobilization in UC. The absolute difference in deaths by day 14 was 16 more in the VEM group, and this difference remained at 3 months (VEM, 88; UC 72).
Prior to AVERT, small trials and meta-analyses showed a higher number of deaths in participants undertaking early mobility-based interventions compared to controls, but these results failed to reach clinical or statistical significance.1 In the primary AVERT report of 3-month outcomes,3 deaths at 3 months were not significantly different between groups. A recent Cochrane review2 of all relevant trials shows higher, but nonsignificant differences in death at 3 months when compared with delayed or lower dose mobilization (8.5% VEM; 6.7% UC; odds ratio [OR] 1.27, 95% CI 0.95 to 1.70; p = 0.11, n = 2,570; moderate-quality evidence). This tertiary safety analysis from the AVERT trial suggests that when VEM-related death occurs, it occurs within the first 14 days after onset.
Our exploratory analysis suggests that people aged over 80 years and those with intracerebral haemorrhage have greater odds of early death with higher dose intervention, and the most common cause of death was stroke-related events (stroke progression or recurrence). However, no significant treatment by subgroup interaction was found, and observation numbers were small. Nevertheless, understanding why subgroups of participants carry higher risk of harm is imperative for development of safe treatment protocols.
Substantial attention has been paid to understanding early neurologic deterioration in acute ischemic stroke, particularly in those treated with rtPA. In their 2015 systematic review, which included 31 studies (26 focused on IV rtPA-treated patients, 5 on nonthrombolysed patients), Seners et al.7 concluded that while symptomatic ICH and malignant edema may account for approximately 20% of cases of early neurologic deterioration, in the majority of cases no clear cause was identified. It should be noted that in AVERT only 23 of the 504 (4.6%) participants treated with rtPA died within 14 days, with no between-group differences observed. The association between a range of clinical factors, such as age, hyperglycemia, admission BP and its early variation, temperature, stroke etiology, and severe neurologic impairment on admission, with early neurologic deterioration were explored in this review. A range of plausible reasons exist why one or more of these factors may be associated with early deterioration and a larger risk of death or dependency, but there appears to be no clear evidence to underpin accurate prediction of who may experience early deterioration after ischemic stroke. Seners et al.7 also raise the issue of considerable variation in definitions of early neurologic deterioration. In AVERT, stroke progression was defined as “worsening of symptoms in the same vascular territory as the initial stroke event within 14 days of initial stroke onset.” Although we are confident that assessors were diligent in identifying stroke progression according to this definition, in future studies, applying a neurologic worsening cutoff (for example, NIHSS increase by ≥ 4) would improve confidence in the accuracy of the diagnosis.8,9 More importantly, the mechanism by which upright activity contributes to greater rates of stroke events and an outcome of early death is unknown. Understanding who is most at risk with early activity is important for development of safe protocols for acute stroke patients. The larger number of deaths in those with intracerebral haemorrhage in intervention compared to control (12 VEM vs 3 UC) is concerning, although individual treatment plots show no clear pattern of intervention timing or amount that could explain these results. One hypothesis that could be advanced to explain early harm with upright activity relates to disturbance of cerebral autoregulation in this hyperacute phase. Our recent systematic review of cerebral hemodynamic changes with head position change10 highlights ongoing uncertainty around the effect of head position on cerebral blood flow in stroke patients. We found 10 studies (n = 323) conducted within 48 hours poststroke, mostly with transcranial Doppler, most examining cerebral blood flow (CBF) changes in patients with ischemic stroke moving between flat and 30° or 45° head elevation.10 No studies were found that examined change from flat to active sitting or standing positions, which engage large postural muscle groups, within 48 hours of stroke. Changes in CBF were not uniform across studies, although we did find a trend toward reduced CBF velocity in the affected hemisphere with increased head elevation. When studied, BP was not a reliable proxy for changes in CBF. In only 2 studies performed within 48 hours, BP changed significantly. In both studies, BP and CBF velocity both decreased when elevating the head of bed angle. Further explanatory research in this domain is needed. A significant limitation of the current study is that we did not collect physiologic measures before, during, and after training, nor did we determine whether patients with ischemic stroke had ongoing occlusion, which may also be important. This limits our ability to further explore or speculate in more detail about patient responses to the intervention, and we would recommend that data such as these are collected in any future explanatory studies or trials.
While it is difficult to find comparable data given the varying inclusion criteria between trials, the 3.8% death rate in this study was similar to the other large nonpharmacologic clinical study addressing positioning in the acute phase of care (HeadPoST), with death rate estimates at 14 days obtained from hazard plots of 5% in lesser impaired acute stroke survivors (HeadPoST, n = 11,093).11 In AVERT, 45 of 2,104 (2.1%) patients died from stroke progression or recurrence at 14 days. Fatalities at 30 days due to the same cause were 2.5% (n = 275) of deaths in HeadPoST.11
The main results of AVERT demonstrated less favorable outcome (significantly fewer patients achieving modified Rankin Scale score 0–2) at 3 months with early, high-dose training.3 This analysis shows higher odds of death (age and severity-adjusted OR 1.76, 95% CI 1.06–2.92) with excess early deaths (n = 16) in the same group. Clinical practice guidelines have been revised to reflect this finding and, while not uniform, recommendations to delay or modify mobilization practices now exist. However, the clinical and research community continue to seek greater guidance for clinical protocols in this early period poststroke.12 Further research is required to advance rehabilitation practice very early after stroke.
Acknowledgment
The authors thank the participants, families, and carers for supporting the trial; and Liudmyla Olenko and Hannah Johns for their assistance with statistical coding and analysis. The Florey Institute of Neuroscience and Mental Health received funds from the Victorian Government via the Operational Infrastructure Support Scheme.
Glossary
- AE
adverse event
- aOR
adjusted odds ratio
- AVERT
A Very Early Rehabilitation Trial
- BP
blood pressure
- CBF
cerebral blood flow
- CI
confidence interval
- IME
important medical event
- IQR
interquartile range
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- rtPA
recombinant tissue plasminogen activator
- SAE
serious adverse event
- UC
usual stroke unit care
- VEM
very early mobilization
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
Patient page e1269
Study Funding
Supported by National Health and Medical Council Australia (386201, 1041401), Chest Heart and Stroke Scotland (Res08/A114), Northern Ireland Chest Heart and Stroke, Singapore Health (SHF/FG401P/2008), UK Stroke Association (TSA2009/09), UK National Institute Health Research (Health Technology Assessment Project 12/01/16), and Australian Research Council (0991086).
Disclosure
J. Bernhardt received fellowship funding from the NHMRC (1058635) and National Heart Foundation (G04M1571). K. Borschmann and J. Collier report no disclosures. A. Thrift received NHMRC fellowship funding (1042600). P. Langhorne, S. Middleton, and R. Lindley report no disclosures. H. Dewey received NHMRC fellowship funding (336102). P. Bath is a Stroke Association Professor of Stroke Medicine and an NIHR Senior Investigator. C. Said, L. Churilov, F. Ellery, and C. Bladin report no disclosures. C. Reid received NHMRC fellowship funding (1136372). J. Frayne reports no disclosures. V. Srikanth received NHMRC fellowship funding (1061453). S. Read and G. Donnan report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Lynch E, Hillier S, Cadilhac D. When should physical rehabilitation commence after stroke: a systematic review. Int J Stroke 2014;9:468–478. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne P, Collier JM, Bate PJ, Thuy MNT, Bernhardt J. Very early versus delayed mobilisation after stroke. Cochrane Database Syst Rev 2018;10:CD006187. [DOI] [PMC free article] [PubMed]
- 3.The AVERT Trial Collaboration Group, Bernhardt J, Langhorne P, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet 2015;386:46–55. [DOI] [PubMed] [Google Scholar]
- 4.Thrift AG, Thayabaranathan T, Howard G, et al. Global stroke statistics. Int J Stroke 2017;12:13–32. [DOI] [PubMed] [Google Scholar]
- 5.Bernhardt J, Churilov L, Dewey H, et al. Statistical Analysis Plan (SAP) for A Very Early Rehabilitation Trial (AVERT): an international trial to determine the efficacy and safety of commencing out of bed standing and walking training (very early mobilisation) within 24 h of stroke onset vs usual stroke unit care. Int J Stroke 2015;10:23–24. [DOI] [PubMed] [Google Scholar]
- 6.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342:d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry 2015;86:87–94. [DOI] [PubMed] [Google Scholar]
- 8.Birschel P, Ellul J, Barer D. Progressing stroke: towards an internationally agreed definition. Cerebrovasc Dis 2004;17:242–252. [DOI] [PubMed] [Google Scholar]
- 9.Siegler JE, Boehme AK, Kumar AD, Gillette MA, Albright KC, Martin-Schild S. What change in the National Institutes of Health Stroke Scale should define neurologic deterioration in acute ischemic stroke? J Stroke Cerebrovasc Dis 2013;22:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho LB, Kramer S, Borschmann K, Chambers B, Thijs V, Bernhardt J. Cerebral hemodynamics with head position changes post-ischaemic stroke: a systematic review and meta-analysis. J Cereb Blood Flow Metab Epub 2020 May 13. [DOI] [PMC free article] [PubMed]
- 11.Anderson CS, Arima H, Lavados P, et al. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med 2017;376:2437–2447. [DOI] [PubMed] [Google Scholar]
- 12.Bayley MT, Bowen A, English C, Teasell R, Eng JJ. Where to now? AVERT answered an important question, but raised many more. Int J Stroke 2017;12:683–686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be shared by writing to the corresponding author to obtain details of procedures and processes.