Abstract
Background
Robust evidence shows that schizophrenia is associated with significant cognitive impairments, including deficits in visuospatial abilities. While other cognitive domains have sparked several functional neuroimaging studies in schizophrenia, only a few brain activation studies have examined the neural correlates of visuospatial abilities in schizophrenia.
Purpose
Here, we propose to perform a functional connectivity study on visuospatial processing in schizophrenia, and to determine the classification accuracy of the observed alterations.
Methods
Thirty-nine schizophrenia patients and 42 healthy controls were scanned using functional magnetic resonance imaging while performing a mental rotation task. Task-based functional connectivity was examined using a region-of-interest (ROI) to ROI approach, as implemented in the CONN Toolbox. ROIs were selected from a previous meta-analysis on mental rotation. Logistic regression with Lasso regularization was performed, using train-test cross-validation.
Results
Schizophrenia was associated with a complex pattern of dysconnectivity between the superior, middle and inferior frontal gyrus, the precentral gyrus, the superior parietal lobule (SPL) and the inferior lateral occipital cortex. The classification accuracy was 86.1%. Mental rotation performance was predicted by the dysconnectivity between the right and left superior frontal gyrus (SFG), as well as between the left SFG and left SPL.
Conclusion
The results of the current study highlight that visuospatial processing is useful for examining the widespread dysconnectivity between executive, motor and visual brain regions in schizophrenia. We also demonstrate that very good classification accuracy can be achieved using visuospatial-related functional connectivity data.
Keywords: schizophrenia, mental rotation, functional connectivity, machine learning
Introduction
Schizophrenia is a complex psychiatric disorder characterized by positive (eg, delusions and hallucinations) and negative (eg, blunted affect, alogia) symptoms. The disorder is further associated with significant deficits in several cognitive domains, including attention, executive functions, speed of processing, episodic memory, working memory, as well as social cognition.1–3 Considering the fact that cognitive deficits are amongst the strongest predictors of social and occupational dysfunction in schizophrenia,4,5 several functional neuroimaging studies have examined the neural correlates of cognitive impairments in schizophrenia in order to elucidate the pathophysiology of the disorder.6 While several functional neuroimaging studies have used tasks assessing working memory, episodic memory and emotion recognition in this population,7–9 relatively little attention has been paid to visuospatial abilities. However, large-scale evidence demonstrates significant impairments in visuospatial abilities of schizophrenia patients throughout their lifespan.10–12
In healthy subjects, several functional magnetic resonance imaging (fMRI) studies have examined the neurophysiologic mechanisms involved in visuospatial abilities using predominantly mental rotation tasks. Such tasks typically require participants to mentally rotate representations of 2- or 3-dimensional objects and determine if the objects are identical or not.13 Due to its complexity, mental rotation engages several cognitive abilities, including attention, object orientation discrimination, visual imagery, spatial transformation, action simulation, and ultimately, decision-making.14,15 Reflecting this complexity, a meta-analysis of 60 functional neuroimaging studies performed in healthy volunteers has shown that mental rotation engages widespread activations in brain regions involved in executive functions (superior frontal gyrus, middle frontal gyrus and inferior frontal gyrus), motor regions (precentral gyrus) and brain regions of the extended visual system (superior parietal lobule, angular gyrus and inferior occipital gyrus).16 In schizophrenia, visuospatial abilities have been investigated in a few fMRI studies, which have shown reduced activity in the lateral prefrontal cortex, the superior parietal lobule, and occipital cortex.17,18 To the best of our knowledge, no task-based functional connectivity study has been performed on visuospatial abilities in schizophrenia, even though mental rotation offers a rich cognitive context for examining the functional connectivity between executive, motor and visual brain regions in this population. In comparison to task-based functional connectivity studies on working memory, the study of visuospatial abilities in schizophrenia holds the promise of revealing a more diffuse pattern of dysconnectivity.19,20
Over the last two decades, a significant shift in the neuroimaging literature has promoted the emergence of research investigating the classification of psychiatric disorders, in lieu of research examining solely their pathophysiological bases. In 2015, Kambeitz and colleagues conducted a meta-analysis of multivariate pattern recognition studies involving the classification of schizophrenia patients.21 On the basis of the 38 (structural and functional) neuroimaging studies involving 1602 patients and 1637 controls, the authors determined that patients were differentiated from controls with a sensitivity and a specificity of approximately 80%. Greater sensitivity was achieved in older patients, and in resting-state functional connectivity studies. Since then, similar analyses have been performed in larger samples of participants, with levels of accuracy varying significantly across studies (generally between 70% and 95%).22–25 To date, however, most neuroimaging studies involving schizophrenia classification have been performed using either structural imaging or resting-state functional connectivity data, with little attention being paid to task-based brain activity and/or functional connectivity.
In light of the robust evidence regarding the significant deficits in visuospatial abilities of schizophrenia patients, and the relative lack of fMRI studies on this cognitive domain, the primary objective of the current study was to examine the potential differences between schizophrenia patients and healthy controls in task-based functional connectivity. We hypothesized that schizophrenia patients would display widespread dysconnectivity between brain regions involved in executive functions, motor regions and brain regions of the extended visual system. As a secondary objective, we sought to determine the classification accuracy of task-based dysconnectivity data in schizophrenia.
Methods
Participants
Thirty-nine schizophrenia patients meeting the DSM-IV criteria for schizophrenia in a stable phase of their illness (defined as no relapse within the last two months and no change in their antipsychotic treatment within the last month) and 42 healthy controls participated in the study. Severity of symptoms was evaluated using the Positive and Negative Syndrome Scale.26 Patients were stabilized on one or more of the following second-generation antipsychotics: clozapine (n=18), olanzapine (n=10), risperidone (n=15), quetiapine (n=8), and ziprasidone (n=2). Antipsychotic dosage was compared between patients using olanzapine equivalents.27 Healthy participants were screened with the non-patient edition of the Structured Clinical Interview for DSM-IV.28 All participants were free of concomitant neurological disorders, substance use disorders (lifetime for healthy controls; last 12 months for patients), and magnetic resonance imaging (MRI) contra-indications. No participant had an IQ lower than 70.29 Urine drug screenings were administered. Patients and controls did not differ in age, sex ratio, and handedness (Table 1).
Table 1.
Characteristics of Participants
| Schizophrenia (n=39) | Controls (n=42) | Statistics | |
|---|---|---|---|
| Age, mean (SD) | 32.5 (6.9) | 30.5 (8.5) | F=1.34; p=0.250 |
| Sex | 20M/19F | 20M/22F | χ2=0.1; p=0.457 |
| Handedness | 36 right | 38 right | χ2=0.09; p=0.542 |
| Duration of illness (years) | 9.8 (7.1) | — | — |
| Education (years) | 11.6 (3.1) | 17.9 (3.7) | F=68.3; p<0.001 |
| PANSS-Positive | 18.9 (6.7) | — | — |
| PANSS-Negative | 20.8 (6.9) | — | — |
| PANSS-General | 41.2 (9.9) | — | — |
| Olanzapine equivalents (mg/d) | 18.9 (10.4) | — | — |
| Mental rotation accuracy (%) | 71.3 (17.8) | 84.8 (14.9) | F=13.1; p=0.001 |
Abbreviations: PANSS, Positive and Negative Syndrome Scale; SD, standard deviation.
In accord with the Declaration of Helsinki, written informed consent was obtained from each participant before any assessment. The study was approved by the local ethics committees from the Centre de recherche de l’Institut Universitaire en Santé Mentale de Montréal.
fMRI Task
The mental rotation task lasted 8 minutes and consisted of alternating 38-second blocks of experimental and control conditions. The blocks were separated from one another with 20-second rest periods. Experimental and control blocks were both repeated four times during the course of the functional run. During the blocks were presented pairs of black-and-white drawings of 3-D shapes, adopted from Shepard and Metzler’s mental rotation task.13 In the experimental condition, one shape was rotated along its vertical axis relative to the other shape. In half of the trials, the figures were identical to each other, whereas they were mirror images of each other in the other half. In the control condition, participants were presented with identical or mirror 3-D drawings that were un-rotated. In both conditions, participants had to determine whether the two shapes were identical or mirror images of each other by pressing a button. Each picture was presented for a duration of 3 seconds, followed by a blank screen with a fixation point for an average of 1.75 seconds.
fMRI Acquisition Parameters
Functional magnetic resonance imaging (fMRI) blood oxygenation level dependent (BOLD) procedure was used during the mental rotation task [1, 23, 24]. BOLD signals were recorded using a T2-weighted gradient echo-planar imaging (EPI) sequence [repetition time (TR)=3000 ms, echo time (TE)=30 ms, flip angle=90°, matrix 64×64; voxel size=3.5mm3; 41 axial slices] on a 3.0 Tesla TRIO-TIM MRI system at the Functional Neuroimaging Unit at the University of Montreal Geriatric Institute. The functional slices were angled to be parallel to the AC-PC line. An inline retrospective motion correction algorithm was employed while the EPI images were acquired. Individual high-resolution co-planar anatomical images were also acquired using a three-dimensional, spoiled gradient-echo sequence (TR=19 ms; TE=4.92 ms; FA=25°; matrix size: 256x256; voxel size; 1mm3; 176 sagittal slices).
fMRI Data Pre-Processing
fMRI data was analyzed with the CONN v.18b functional connectivity toolbox (http://www.nitrc.org/projects/conn).30 We used the default preprocessing pipeline, which is based on SPM12 functions (The Wellcome Department of Cognitive Neurology, London, UK).31 Functional images were realigned, slice-time corrected, corrected for motion artifacts (global signal Z-value threshold=5; subject-motion threshold=0.9 mm) with the Artifact Detection Toolbox,32 and co-registered to the corresponding anatomical image. The anatomical images were segmented (into grey matter, white matter, and cerebrospinal fluid) and normalized to the Montreal Neurological Institute (MNI) stereotaxic space. Functional images were then normalized to MNI stereotaxic space, and spatially smoothed with an 8-mm 3D isotropic Gaussian kernel.
Functional Connectivity Analyses
For functional connectivity analyses, we adopted a region of interest (ROI)-to-ROI approach.30 The anatomical component-based noise correction method (aCompCor) was used to estimate the physiological BOLD signal noise from the white matter and cerebrospinal fluid.33 The aCompCor strategy was found to increase sensitivity of analyses.34 These physiological noise processes, together with the 6 realignment parameters and the scans impacted by movement artifacts (eg, scrubbing) were regressed out as first-level nuisance covariates from the BOLD time-series at each voxel. To avoid spurious effects due to task co-activation, the main activation effects of the conditions were also accounted for.35 The residual BOLD time series were then weighted by the appropriate predictor to derive condition-specific time series for functional connectivity analyses. The current investigation examined the mental rotation relative to the reference condition as the main contrast of interest.
The ROIs were chosen from the default Harvard-Oxford atlas (www.fmrib.ox.ac.uk/fsl/) available in the CONN toolbox. The following 14 regions were included: the bilateral superior frontal gyrus (SFG), the middle frontal gyrus (MFG), the inferior frontal gyrus, opercularis (IFG), the bilateral precentral gyrus (PrCG), the bilateral superior parietal lobule (SPL), the bilateral angular gyrus (AG) and the bilateral inferior lateral occipital gyrus (iLOC). ROIs were chosen based on the meta-analysis of Tomasino et al of fMRI brain activation studies involving mental rotations in healthy volunteers,16 as well as a previous study in schizophrenia patients from our research team.18
In the first-level analyses, Pearson’s correlation coefficients were calculated between the time courses of each pair of ROIs for each subject. These correlation coefficients were then converted to normally distributed z-scores (using Fisher’s transform) in order to improve second-level analyses (eg, General Linear Model).30 In the second-level analysis, a one-sample t-test was performed across groups (schizophrenia patients and controls). Fisher’s z-scores were extracted for each participant for each connection between the 14 brain regions (for a total of 91 connections), and were used to perform machine learning analyses (see below). Prior to the machine learning analyses, we verified that there was no association between the connections of interest and potential confounding factors, namely sex, antipsychotic dosage and clozapine. Across and between groups (schizophrenia and controls), there were no differences in ROI-to-ROI connectivity between sexes (p>0.05, FDR corrected). There were no significant differences in ROI-to-ROI connectivity between patients on clozapine and those taking other antipsychotics (p>0.05, FDR corrected). Finally, there was no significant relationship between antipsychotic dosage (ie, olanzapine equivalents) and the connections of interest (p>0.05, FDR corrected).
Test/Training Samples and Validation
The 81 participants were split into two groups, the training sample (n=60) and the test sample (n=21), regardless of diagnosis. The two samples were randomly drawn from the total sample. We verified that the percentage of controls and patients were similar in the two subsamples, using a χ2 test, and we could not reject the null hypothesis that the two samples have a similar number of controls and patients (p=0.76) (Table 2). Likewise, there was no significant difference in age between participants allocated to the training (30.9 ±7.6) and the test samples (33.0 ±8.5) (t=−1.02; p=0.31).
Table 2.
Number of Patients and Controls in Each Random Sample
| Controls | Schizophrenia | Total | |
|---|---|---|---|
| Training sample | 30 (37.0%) | 30 (37.0%) | 60 (74.1%) |
| Test sample | 12 (14.8%) | 9 (11.1%) | 21 (25.9%) |
| Total | 42 (51.9%) | 39 (48.1%) | 81 (100%) |
Notes: χ2= 0.096; df = 1; p=0.76.
Machine Learning Analyses
In order to identify the pairs of regions predicting membership in the schizophrenia group, we applied five machine-learning algorithms to the data, namely logistic regression with lasso and Ridge regularization, random forest, linear discriminant analysis (LDA) and support vector machine (SVM). Analyses were performed using R version 4.0.2.36 Each algorithm described below was optimized in the training sample using leave-one-out cross-validation. Finally, a prediction was made for each algorithm using the test sample and summarized with the percentage of good classification and the area under the curve (AUC) of a receiver operating characteristic (ROC) curve. 95% confidence intervals were computed using bootstrap resampling. In the method displaying the greatest classification accuracy, we ran two separate analyses, one with and one without mental rotation accuracy being included in the list of predictors. Finally, we identified the connections between brain regions that were the best predictors in the machine learning algorithm with the greatest classification accuracy.
Because the number of pairs of regions was greater than the number of subjects in this project, we completed a pre-screening of pairs of brain regions for which the p-value of a t-test between the two groups were below 0.20. This yielded the following 31 candidate pairs presented in Table 3. All 31 variables were scaled and centered before they were entered in the analyses.
Table 3.
Connections Used in the Machine Learning Algorithms
| Right superior frontal gyrus | Left superior frontal gyrus |
| Right inferior frontal gyrus | |
| Right superior parietal lobule | |
| Left superior parietal lobule | |
| Right angular gyrus | |
| Left inferior lateral occipital cortex | |
| Left superior frontal gyrus | Right middle frontal gyrus |
| Right inferior frontal gyrus | |
| Left inferior frontal gyrus | |
| Left superior parietal lobule | |
| Right angular gyrus | |
| Left inferior lateral occipital cortex | |
| Right middle frontal gyrus | Left middle frontal gyrus |
| Left inferior frontal gyrus | |
| Left precentral gyrus | |
| Right angular gyrus | |
| Left angular gyrus | |
| Left middle frontal gyrus | Left superior parietal lobule |
| Right angular gyrus | |
| Left inferior lateral occipital cortex | |
| Right inferior frontal gyrus | Left precentral gyrus |
| Right angular gyrus | |
| Right inferior lateral occipital cortex | |
| Left inferior frontal gyrus | Right superior parietal lobule |
| Left inferior lateral occipital cortex | |
| Right precentral gyrus | Left inferior lateral occipital cortex |
| Left precentral gyrus | Right inferior lateral occipital cortex |
| Left inferior lateral occipital cortex | |
| Left superior parietal lobule | Left inferior lateral occipital cortex |
| Right angular gyrus | Left angular gyrus |
| Left angular gyrus | Left inferior lateral occipital cortex |
Logistic Regression
Logistic regression was fitted to the data using lasso and ridge regularization using the glmnet package.37,38 The lasso regularization is a penalty function added to the objective function that reduces the absolute value of the sum of coefficients in the model to be under a certain threshold. This threshold dependent on parameter λ was determined using leave-one-out cross-validation. In this kind of regularization, some coefficients may be set at a value of zero. Alternatively, Ridge regularization adopts a similar concept, however, the objective function is penalized using a quadratic function instead of the absolute value. In the latter regularization, some coefficients may be set to small values, yet are never set to zero.
Random Forest
A random forest was also used, whereby three hundred trees were formed, and 5 variables were tried at each split. The model was fitted using the randomForest package.39
Linear Discriminant Analyses (LDA)
LDA was performed using the MASS package.40 A linear discriminant function assumes that each group has a particular multinormal distribution, which is then used to compute the probabilities of being in each group. The LDA method is considered to be linear, as by adding the constraint of homoscedasticity (equal covariance across groups), the solution is linear and has a closed form. As the method implies, LDA works best if the input is normal.
Support Vector Machine (SVM)
The final adopted method, SVM, is an extension of the linear classifier similar to LDA. Instead of a separation defined by a single line, we used a margin defined by two lines, which created a zone of uncertainty of being in the control group or the schizophrenia group. This model was adjusted using the e1071 package.41
Inferential Statistics
For comparative purposes, we performed in the CONN Toolbox a classic 2-sample t-test to examine potential differences in functional connectivity between groups, based on the Mental rotation minus Reference contrast. For this analysis, we used the 91 connections of interest. These second-level analyses were corrected for multiple comparison using a p<0.05 threshold corrected for the false discovery rate (FDR) applied at the analysis level.
Association with Mental Rotation Performance
The relationship between the connections having the most discriminative value and mental rotation performance was examined using step-wise linear regression analyses with motion parameters (eg, mean motion, max motion and number of valid scans) being entered as covariates of no interest. The statistical threshold was set at p<0.05, FDR-corrected.
Results
Cognitive Performance
Mental rotation accuracy was lower in schizophrenia patients relative to healthy controls (see Table 1).
Comparison of Predictions
Tables 4 and 5 show a comparison of the performance of the 5 algorithms, including the AUC of the ROC curve, which measures the discriminative power of the methods. For the training data, random forest revealed to be the best method, as it yielded an AUC of 0.96. As for the test data, it appears that the best method was logistic regression with Lasso regularization (λ=0.065), which yielded an AUC of 0.861, a percentage of good classification of 85.7%, a sensitivity of 83.3%, and a specificity of 88.9%. The Lasso algorithm also had less variance, and its AUC was similar for the training data (0.871).
Table 4.
Comparison of the Performance of the Five Machine Learning Algorithms (Training; n=60*)
| Lasso | Ridge | Random Forest | LDA | SVM | |
|---|---|---|---|---|---|
| % of good classification (95% CI) | 81.7% (68.3, 91.7) | 81.7% (71.7, 91.7) | 88.3% (83.3, 95.0) | 81.7% (69.1, 93) | 81.7% (5.0, 95) |
| Sensibility | 83.6% (58.8, 95.6) | 85.2% (64.5, 97.1) | 89.3% (71.1, 98.7) | 84.8% (64.3, 93.8) | 79.4% (2.9, 95.6) |
| Specificity | 79.7% (55.5, 93.2) | 80.3% (56.0, 96.0) | 89.5% (69.7, 97.3) | 80.7% (64.5, 95.9 | 77.5% (6.1, 97.0) |
| ROC curve AUC | 0.871 (0.70, 0.95) | 0.878 (0.79, 0.95) | 0.962 (0.93, 0.99) | 0.784 (0.62, 0.93) | 0.907 (0.82, 0.98) |
Note: *Leave-one-out cross-validation.
Abbreviations: AUC, area under the curve; LDA, linear discriminant analysis; ROC, receiving.
Table 5.
Comparison of the Performance of the Five Machine Learning Algorithms (Test; n=21)
| Lasso | Ridge | Random Forest | LDA | SVM | |
|---|---|---|---|---|---|
| % of good classification (95% CI) | 85.7% (71.4, 95.2) | 81.0% (61.9, 95.2) | 76.2% (52.4, 90.5) | 81.0% (66.7, 95.2) | 85.7% (68.9, 1.00) |
| Sensitivity | 83.3% (60.7, 100) | 83.3% (57.7, 100) | 83.3% (61.5, 100) | 83.3% (60.7, 100) | 91.7% (72.0, 100) |
| Specificity | 88.9% (65.1, 100) | 77.8% (50.0, 100) | 66.7% (26.7, 92.3) | 77.8% (46.3, 100) | 77.8% (46.3, 100) |
| ROC curve AUC | 0.861 (0.64, 1.00) | 0.806 (0.52, 0.98) | 0.796 (0.50, 0.94) | 0.870 (0.58, 1.00) | 0.806 (0.56, 1.00) |
Abbreviations: AUC, area under the curve; LDA, linear discriminant analysis; ROC, Receiving Operating Characteristic; SVM, support vector machine.
The inclusion of mental rotation accuracy in the list of predictors had only a marginal effect on the discriminative power of the Lasso regression algorithm in both the training ([AUC=0.872 (0.725–0.957); percentage of good classification=81.7% (67.5–93.3); sensitivity=77.7% (56.3–97.1); and specificity=86.7% (50.0–94.4)] and test data [AUC=0.870 (0.708–1.00); percentage of good classification=81.0% (64.2–95.2); sensitivity=75.0% (54.2–96.6); and specificity=88.9% (73.1–1.00)].
Predictive Connections
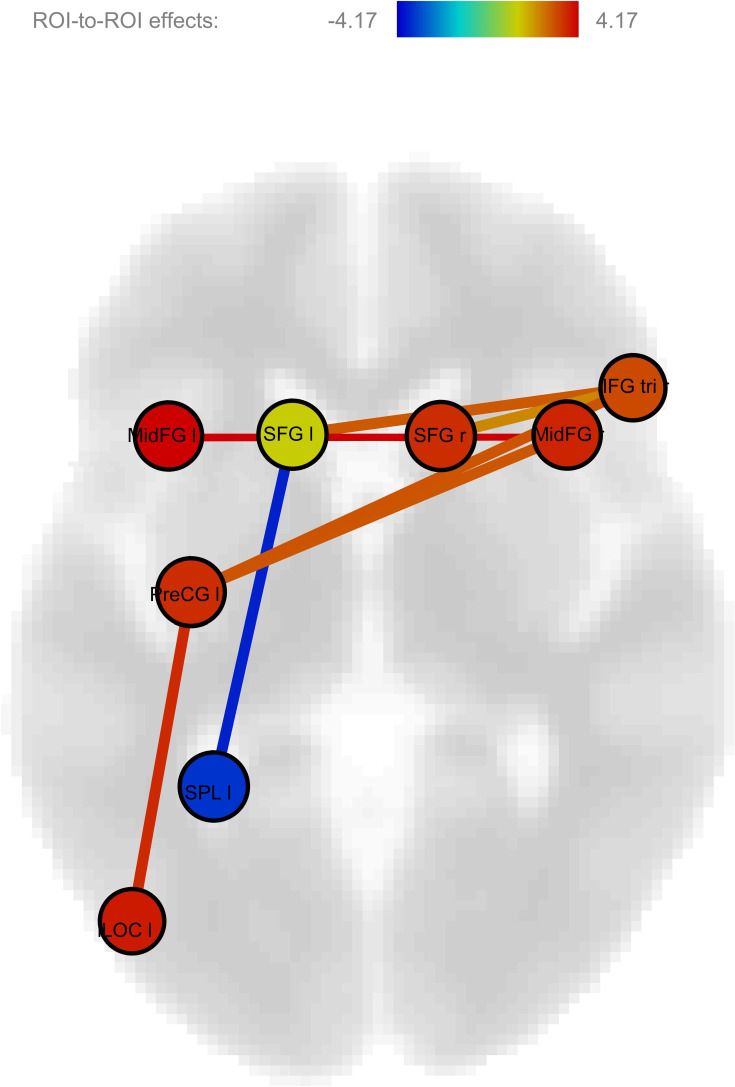
According to the logistic regression with lasso regularization algorithm, the pairs of regions which are the most predictive are the connections between the right and left SFG; between the left SFG and left SPL; between the left SFG and left iLOC; between the right and left MFG; between the right IFG and left PrCG; as well as between the left PrCG and left iLOC. Connectivity was reduced in schizophrenia in all pairs of regions, apart from the connectivity between the left SFG and left SPL, which was actually increased in schizophrenia (Table 6) (Figure 1). Noteworthy, the same connections emerged as being the most predictive in the secondary analysis including mental rotation accuracy in the list of potential predictors, apart from the connection between the right inferior frontal gyrus and the left precentral gyrus (Table 6).
Table 6.
Coefficients from the Logistic Regression with Lasso Regularization
| Connection | Lasso Beta (No MR) | Lasso Beta (with MR) |
|---|---|---|
| Intercept | 0.019 | 0.16 |
| Right and left superior frontal gyrus | −0.055 | −0.036 |
| Left superior frontal gyrus and left superior parietal lobule | 0.254 | 0.183 |
| Left superior frontal gyrus and left inferior lateral occipital cortex | −0.063 | −0.016 |
| Right and left middle frontal gyrus | −0.196 | −0.172 |
| Right inferior frontal gyrus and left precentral gyrus | −0.019 | — |
| Left precentral gyrus and left inferior lateral occipital cortex | −0.199 | −0.183 |
Abbreviation: MR, mental rotation accuracy.
Figure 1.
Impaired connectivity in schizophrenia during visuospatial processing. Figure 1 illustrates the complex pattern of dysconnectivity observed in schizophrenia during visuospatial processing. The figure was generated using a two-sample t-test performed in CONN Toolbox.
Abbreviations: IFG, inferior frontal gyrus; iLOC, inferior lateral occipital gyrus; MFG, middle frontal gyrus; PreCG, precentral gyrus; ROI, region-of-interest; SFG, superior frontal gyrus; SPL, superior parietal lobule.
Inferential Statistics
Using a classic 2-sample t-test, we found that schizophrenia was associated with reduced connectivity between the right and left SFG; between the right and left MFG; between the right IFG and left PrCG; and between the left PrCG and left iLOC; as well as increased connectivity between the left SFG and left SPL. In addition to these alterations that were also detected using the Lasso regression algorithm, the 2 sample t-test showed additional alterations, namely reduced connectivity, in schizophrenia, between the left and right AG, between the left PrCG and right MFG; and between the left SFG and right IFG (Table 7).
Table 7.
ROI-to-ROI Connectivity Between Schizophrenia Patients and Healthy Controls During Mental Rotation (Inferential Statistics)
| Connexion | t-Value | Schizophrenia Fischer’s-z | Controls Fischer’s-z |
|---|---|---|---|
| Controls > schizophrenia | |||
| Left middle frontal gyrus - right middle frontal gyrus | 4.17 * | −0.060 (0.208) | 0.133 (0.208) |
| Left superior frontal gyrus - right superior frontal gyrus | 3.78 | −0.050 (0.263) | 0.143 (0.195) |
| Left precentral gyrus - left inferior lateral occipital cortex | 3.62 | −0.0630 (0.264) | 0.114 (0.170) |
| Left angular gyrus - right angular gyrus | 3.41 | −0.090 (0.225) | 0.073 (0.204) |
| Left precentral gyrus - right interior frontal gyrus (triangularis) | 3.04 | −0.058 (0.246) | 0.109 (0.249) |
| Left precentral gyrus - right middle frontal gyrus | 2.99 | −0.024 (0.201) | 0.125 (0.243) |
| Left superior frontal gyrus - right inferior frontal gyrus (triangularis) | 2.94 | −0.053 (0.213) | 0.094 (0.237) |
| Schizophrenia > controls | |||
| Left superior parietal gyrus - left superior frontal gyrus | 3.68 | 0.084 (0.200) | −0.077 (0.194) |
Note: *All ps<0.05, corrected for false discovery rate.
Linear Regression Analysis
The stepwise linear regression analysis was performed using the connections described in Table 6. Across groups, the connectivity between the left and right SFG, and between the left SFG and the left SPL, accounted for 15% (r2) of variance of MR accuracy (F=7.8; p=0.001). More precisely, a positive association was found between MR accuracy and the connectivity between the left and right SFG (t=2.8; β=0.298; p=0.006), whereas a negative association was found in the connexion between the left SFG and the left SPL (t= −2.7; β= −0.287; p=0.008). For both predictors, results remained significant after FDR correction (p<0.05).
Discussion
The current task-based functional connectivity study sought to better characterize the neurophysiologic mechanisms involved in visuospatial impairments in schizophrenia, and determine the classification accuracy of the functional connections found to be altered in this disorder. Several machine algorithms were implemented (Lasso and Ridge regression, random forest, LDA and SVM). Among them, Lasso regression achieved the greatest accuracy. This classification performance was based on a complex pattern of decreased functional connectivity in schizophrenia patients between the right and left SFG, the left SFG and left iLOC, the right and left MFG, the right IFG and left PrCG, and the left PrCG and left iLOC, as well as increased functional connectivity between the left SFG and left SPL. Mental rotation accuracy was reduced in schizophrenia patients, relative to healthy controls. Finally, mental rotation performance was predicted, across groups, by the reduced functional connectivity between the right and left SFG and the increased connectivity between the left SFG and left SPL.
As previously noted, mental rotation requires several sub-abilities and engages the activity of widespread brain regions.16 Reflecting this complexity, we discovered that during visuospatial processing, patients with schizophrenia displayed reduced connectivity between executive (SFG, MFG and IFG), motor (PrCG) and visual brain regions (iLOC). These results are consistent with the findings of several previous resting-state functional connectivity studies which consistently demonstrated that the connectivity between frontoparietal (executive), sensorimotor and visual networks is altered in schizophrenia.42–44 They are also consistent with the contemporary notion that schizophrenia is a disorder of global dysconnectivity.45 Additional noteworthy findings of our study include a reduced functional connectivity between the right and left SFG, as well as between the right and left MFG, in schizophrenia. These results show that alterations in inter-hemispheric connectivity may contribute to visuospatial impairments in schizophrenia. The positive correlation between bilateral SFG connectivity and mental rotation accuracy is coherent with this interpretation. In the last decade or so, several resting-state functional connectivity studies have examined inter-hemispheric connectivity in schizophrenia. Although the majority of these studies yielded an association between inter-hemispheric dysconnectivity in bilateral auditory cortices and verbal hallucinations in schizophrenia,46 a few studies have shown that inter-hemispheric connectivity between frontal regions (among others) also contribute to cognitive impairment in the disorder.47,48 Finally, we observed that the connectivity between the left SFG and left SPL was actually increased in schizophrenia. In classic, task-based activity fMRI studies, increased brain activations in frontal and parietal regions have been regularly observed, and such aberrant activations have been traditionally interpreted as compensatory mechanisms.49 Consistent with this interpretation, a negative correlation was observed across groups between the left SGF-SPL connectivity and mental rotation performance. Overall, the pattern of dysconnectivity observed here is more complex than the alterations that have been observed in task-based functional connectivity studies using visuospatial working memory paradigms in schizophrenia, which have shown alterations mostly in the frontoparietal executive network.19,50,51
Using task-based functional connectivity data and Lasso regularization, a very good classification accuracy (AUC=0.861) was achieved, with a sensitivity of 83.3% and a specificity of 88.9%. Lasso regularization forces some of the coefficient estimates to be equal to zero, and produces sparse models likely to be advantageous in studies involving small samples.37 When compared to the results of pattern recognition neuroimaging studies published prior to 2015, the classification performance of the Lasso regression in the current study is relatively superior (especially its specificity: 88.9% versus 80%).21
It is in the same range, however, as the accuracies achieved by most studies that have been performed since then.23–25 This performance level suggests that the examination of task-based functional connectivity holds promise for future diagnostic classification investigations in schizophrenia. However, this possibility must be considered cautiously. Indeed, in a similar study to ours, Antonucci and colleagues (2020) examined functional connectivity during an attentional control task in schizophrenia. Using SVM, the study achieved only modest accuracy (eg, 66.9%).52 Another potential explanation for the very satisfactory classification performance of our study is that it involved schizophrenia patients with a mean age of 32.5 years. In their meta-analysis from 2015, Kambeitz and colleagues conducted a meta-regression analysis which demonstrated a positive linear association between the mean age of patients and classification performance, with better accuracy being achieved in studies involving schizophrenia patients older than 30 years of age.21 It must be acknowledged, however, that age seemed to influence sensitivity more than specificity in the meta-analysis. Finally, it is noteworthy that the Lasso algorithm achieved the very same level of performance even when including mental rotation accuracy in the list of predictors. This attests to the robustness of results.
The main limitation of the current study is the relatively small sample size. While a sample of 81 participants may be sufficient to detect significant differences in functional connectivity between schizophrenia patients and healthy controls,53 it may not be optimal for performing train-test cross-validation.54 Here, the test was not performed on a sample from an independent study, but rather on a sub-sample of the same study. A second limitation of our study relates to the fact that all patients were treated with antipsychotics, which may have confounded results. The exact impact of antipsychotics on cognition remains uncertain. The data of several clinical trials suggests that some second-generation antipsychotics may produce beneficial effects on cognition in schizophrenia. However, these benefits seem rather small in general, and in the specific case of visuospatial abilities, we are unaware of any evidence showing significant (beneficial or detrimental) effects of antipsychotics.55 In order to reduce the variability of medication effects in the current study, we only recruited patients taking second-generation antipsychotics. Moreover, prior to the implementation of the machine learning algorithms, we verified that antipsychotic dosage and clozapine treatment had no impact on the connections of interest.
Conclusion
To the best of our knowledge, this is the first study to examine functional connectivity in schizophrenia patients performing a task assessing visuospatial abilities, despite the fact that these abilities are significantly impaired in these patients. Our results revealed a complex pattern of dysconnectivity in executive, motor and visual regions in schizophrenia. Using Lasso regression, we observed that this pattern of task-related dysconnectivity achieved very good classification accuracy. Further neuroimaging studies on the neural alterations associated with visuospatial impairments in schizophrenia are warranted. Future studies in the field will need to involve larger samples of patients, and incorporate other biological measures to increase classification accuracy.
Acknowledgments
SP is a holder of the Eli Lilly Canada Chair on schizophrenia research.
Funding Statement
This study was funded by an operating grant from the Canadian Institutes of Health Research to AM.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Nuechterlein KH. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007 [DOI] [PubMed] [Google Scholar]
- 2.Savla GN, Vella L, Armstrong CC, Penn DL, Twamley EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2013;39:979–992. doi: 10.1093/schbul/sbs080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42–50. doi: 10.1016/j.schres.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fett A-KJ, Viechtbauer W, Penn DL, Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35:573–588. doi: 10.1016/j.neubiorev.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Tolman AW, Kurtz MM. Neurocognitive predictors of objective and subjective quality of life in individuals with schizophrenia: a meta-analytic investigation. Schizophr Bull. 2012;38:304–315. doi: 10.1093/schbul/sbq077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crossley NA, Mechelli A, Ginestet C, Rubinov M, Bullmore ET, McGuire P. Altered hub functioning and compensatory activations in the connectome: a meta-analysis of functional neuroimaging studies in schizophrenia. Schizophr Bull. 2016;42(2):434–442. doi: 10.1093/schbul/sbv146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong D, Wang Y, Jia X, et al. Abnormal brain activation during threatening face processing in schizophrenia: a meta-analysis of functional neuroimaging studies. Schizophr Res. 2018;197:200–208. doi: 10.1016/j.schres.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 8.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174(7):676–685. doi: 10.1176/appi.ajp.2017.16040400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC. Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry. 2009;166(8):863–874. doi: 10.1176/appi.ajp.2009.08091307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieto RG, Castellanos FX. A meta-analysis of neuropsychological functioning in patients with early onset schizophrenia and pediatric bipolar disorder. J Clin Child Adolesc Psychol. 2011;40(2):266–280. doi: 10.1080/15374416.2011.546049 [DOI] [PubMed] [Google Scholar]
- 11.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–336. doi: 10.1037/a0014708 [DOI] [PubMed] [Google Scholar]
- 12.Irani F, Kalkstein S, Moberg EA, Moberg PJ. Neuropsychological performance in older patients with schizophrenia: a meta-analysis of cross-sectional and longitudinal studies. Schizophr Bull. 2011;37(6):1318–1326. doi: 10.1093/schbul/sbq057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171(3972):701–703. doi: 10.1126/science.171.3972.701 [DOI] [PubMed] [Google Scholar]
- 14.Wexler M, Kosslyn SM, Berthoz A. Motor processes in mental rotation. Cognition. 1998;68(1):77–94. doi: 10.1016/S0010-0277(98)00032-8 [DOI] [PubMed] [Google Scholar]
- 15.Michelon P, Zacks JM. Two kinds of visual perspective taking. Percept Psychophys. 2006;68(2):327–337. doi: 10.3758/BF03193680 [DOI] [PubMed] [Google Scholar]
- 16.Tomasino B, Gremese M. Effects of stimulus type and strategy on mental rotation network: an activation likelihood estimation meta-analysis. Front Hum Neurosci. 2016;9:693. doi: 10.3389/fnhum.2015.00693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehatpour P, Dias EC, Butler PD, et al. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67(8):772–782. doi: 10.1001/archgenpsychiatry.2010.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiménez JA, Mancini-Marïe A, Lakis N, Rinaldi M, Mendrek A. Disturbed sexual dimorphism of brain activation during mental rotation in schizophrenia. Schizophr Res. 2010;122:53–62. doi: 10.1016/j.schres.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 19.Jalbrzikowski M, Murty VP, Stan PL, et al. Differentiating between clinical and behavioral phenotypes in first-episode psychosis during maintenance of visuospatial working memory. Schizophr Res. 2018;197:357–364. doi: 10.1016/j.schres.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manivannan A, Foran W, Jalbrzikowski M, et al. Association between duration of untreated psychosis and frontostriatal connectivity during maintenance of visuospatial working memory. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(5):454–461. doi: 10.1016/j.bpsc.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambeitz J, Kambeitz-Ilankovic L, Leucht S, et al. Detecting neuroimaging biomarkers for schizophrenia: a meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology. 2015;40(7):1742–1751. doi: 10.1038/npp.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steardo LJ, Carbone E, de Filippis R, et al. Application of support vector machine on fMRI data as biomarkers in schizophrenia diagnosis: a systematic review. Front Psychiatry. 2020. doi: 10.3389/fpsyt.2020.00588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Sun Y, Huang Y, Bezerianos A, Yu R. Machine learning technique reveals intrinsic characteristics of schizophrenia: an alternative method. Brain Imaging Behav. 2019;13:1386–1396. doi: 10.1007/s11682-018-9947-4 [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Guo W, Zhang Y, Lv L, Hu F, Wu R. Decreased resting-state interhemispheric functional connectivity correlated with neurocognitive deficits in drug-naive first-episode adolescent-onset schizophrenia. Int J Neuropsychopharmacol. 2018;21:33–41. doi: 10.1093/ijnp/pyx095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing R, Li P, Ding Z, Lin X, Zhao R, Shi L Machine learning identifies unaffected first-degree relatives with functional network patterns and cognitive impairment similar to those of schizophrenia patients. 40. Hum Brain Mapp; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- 27.Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40(2):314–326. doi: 10.1093/schbul/sbu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005 [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. WASI-II: Wechsler abbreviated scale of intelligence. PsychCorp. 2011. [Google Scholar]
- 30.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J, Barnes G, Chen C, Daunizeau J, Flandin G, Friston K. SPM12 Manual. Wellcome Trust Centre for Neuroimaging; 2014. [Google Scholar]
- 32.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chai XJ, Castañón AN, Öngür D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheldon S, McAndrews MP, Pruessner J, Moscovitch M. Dissociating patterns of anterior and posterior hippocampal activity and connectivity during distinct forms of category fluency. Neuropsychologia. 2016;90:148–158. doi: 10.1016/j.neuropsychologia.2016.06.028 [DOI] [PubMed] [Google Scholar]
- 36.R Core Team. The R Project for Statistical Computing; 2020. Available from: https://www.r-project.org/.
- 37.Friedman J, Hastie T, Tibshirani R The elements of statistical learning. 1; 2001. [Google Scholar]
- 38.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. doi: 10.18637/jss.v033.i01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liaw A, Wiener M. Classification and Regression by randomForest. R News. 2002;2(3):18–22. [Google Scholar]
- 40.Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. Springer; 2002. Available from: http://www.stats.ox.ac.uk/pub/MASS4. [Google Scholar]
- 41.Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F. E1071: misc functions of the department of statistics, probability theory group.; 2019. Available from: https://CRAN.R-project.org/package=e1071. Accessed March27, 2021.
- 42.Lee WH, Doucet GE, Leibu E, Frangou S. Resting-state network connectivity and metastability predict clinical symptoms in schizophrenia. Schizophr Res. 2018;201:208–216. doi: 10.1016/j.schres.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qureshi MNI, Oh J, Lee B. 3D-CNN based discrimination of schizophrenia using resting-state fMRI. Artif Intell Med. 2019. doi: 10.1016/j.artmed.2019.06.003 [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, Zhuo C, Xu L, Liu F, Qin W, Yu C. Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr Bull. 2017;43(6):1363–1374. doi: 10.1093/schbul/sbx051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168–181. doi: 10.1093/schbul/sbx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinmann S, Leicht G, Mulert C. The interhemispheric miscommunication theory of auditory verbal hallucinations in schizophrenia. Int J Psychophysiol. 2019;145:83–90. doi: 10.1016/j.ijpsycho.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 47.Wheeler AL, Chakravarty MM, Lerch JP, et al. Disrupted prefrontal interhemispheric structural coupling in schizophrenia related to working memory performance. Schizophr Bull. 2014;40(ue4):914–924. doi: 10.1093/schbul/sbt100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016;61:108–120. doi: 10.1016/j.neubiorev.2015.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MA, Tura E, Potkin SG, et al. Working memory circuitry in schizophrenia shows widespread cortical inefficiency and compensation. Schizophr Res. 2010;117(1):42–51. doi: 10.1016/j.schres.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Godwin D, Ji A, Kandala S, Mamah D. Functional connectivity of cognitive brain networks in schizophrenia during a working memory task. Front Psychiatry. 2017;22(8):294. doi: 10.3389/fpsyt.2017.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang SS, Sponheim SR, Chafee MV, MacDonald AW 3rd. Disrupted functional connectivity for controlled visual processing as a basis for impaired spatial working memory in schizophrenia. Neuropsychologia. 2011;49(10):2836–2847. doi: 10.1016/j.neuropsychologia.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonucci LA, Penzel N, Pergola G, et al. Multivariate classification of schizophrenia and its familial risk based on load-dependent attentional control brain functional connectivity. Neuropsychopharmacology. 2020;45(4):613–621. doi: 10.1038/s41386-019-0532-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Potvin S, Lungu O, Tikàsz A, Mendrek A. Abnormal effective fronto-limbic connectivity during emotion processing in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2017;72:1–8. doi: 10.1016/j.pnpbp.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 54.Chen YJ, Liu CM, Hsu YC, et al. Individualized prediction of schizophrenia based on the whole-brain pattern of altered white matter tract integrity. Hum Brain Mapp. 2018;39(1):575–587. doi: 10.1002/hbm.23867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Désaméricq G, Schurhoff F, Meary A, et al. Long-term neurocognitive effects of antipsychotics in schizophrenia: a network meta-analysis. Eur J Clin Pharmacol. 2014;70:127–134. doi: 10.1007/s00228-013-1600-y [DOI] [PubMed] [Google Scholar]