Abstract
Background
Propofol (PPF) ameliorates ischemia/reperfusion (I/R) injury in multiple organs by reducing apoptosis and release of pro-inflammatory cytokines. This study aims to explore the mechanism of PPF in attenuating myocardial ischemia-reperfusion injury (MIRI).
Materials and Methods
Rat MIRI model was established, and PPF pre-treatment was performed 10 min before I/R. H9c2 cardiomyocytes treated with hypoxia/reoxygenation (H/R) were used to establish an in vitro model. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to evaluate HOTAIR and miR-17-5p expression levels. Flow cytometry was employed to detect the apoptosis of H9c2 cells. The interaction between HOTAIR and miR-17-5p was determined by bioinformatics analysis, luciferase reporter gene analysis, and RNA immunoprecipitation experiments. STAT3 and p-STAT3 expressions were detected by Western blot.
Results
PPF pre-treatment significantly reduced creatine kinase isoenzyme (CK-MB) and serum lactate dehydrogenase (LDH) levels in the serum of the rats with MIRI. PPF pre-treatment remarkably up-regulated HOTAIR expression and down-regulated miR-17-5p expression in both in vivo and in vitro models. HOTAIR adsorbed miR-17-5p to repress the expression of miR-17-5p. PPF pre-treatment markedly inhibited cardiomyocyte apoptosis induced by I/R or H/R. HOTAIR knockdown could partially reverse the protective effects of PPF on MIRI. HOTAIR could activate STAT3 signaling via repressing miR-17-5p expression.
Conclusion
PPF protects the MIRI by modulating the HOTAIR/miR-17-5p/STAT3 axis.
Keywords: PPF, HOTAIR, miR-17-5p
Introduction
Myocardial ischemia-reperfusion injury (MIRI) is a common problem in clinical practice.1,2 Propofol (2,6–Diisopropylphenol, PPF) is a kind of intravenous anesthetic widely used in clinical anesthesia and sedation.3 Importantly, PPF has a variety of biological effects on organ protection, including reducing the generation of reactive oxygen species (ROS), reducing chemotaxis of inflammatory cells, and improving microcirculation.4–7 For example, by activating the PI3K/AKT/mTOR signaling pathway, PPF can reduce the apoptosis and release of inflammatory cytokines, thereby inhibiting renal ischemia-reperfusion (I/R) injury;4 PPF pre-treatment can decrease the injury of neurons after cerebral I/R and reduce blood-brain barrier damage and cerebral edema.5 Importantly, it is reported that PPF has certain protective effects on MIRI.6 However, the specific mechanism by which PPF ameliorates MIRI has not been fully clarified.
A lot of evidence indicates that long non-coding RNAs (lncRNAs) have important biological functions, including the regulation of I/R-induced injury.7 For example, in cerebral I/R injury, lncRNA MALAT1 protects cerebral vascular endothelial cells from apoptosis by activating PI3K/Akt signaling and reducing the activity of caspase-3.8 Hox transcript antisense intergenic RNA (HOTAIR) is a lncRNA, which has a regulatory effect on I/R–induced injury. For example, during liver I/R injury, HOTAIR overexpression promotes the autophagy of hepatocytes, thereby exacerbating liver I/R injury.9 Nevertheless, the role of HOTAIR in MIRI and its regulatory mechanism have not been fully elucidated.
In recent years, microRNAs (miRNAs) are linked to the pathogenesis of myocardial injury.10–12 Additionally, miRNAs also have regulatory effects on I/R-induced injury of multiple organs. For instance, miR-146a improves intestinal epithelial cell survival during I/R by regulating TLR4/TRAF6/NF-κB signaling;13 inhibiting miR-125b expression protects the brain from I/R injury by modulating the CK2α/NADPH signaling pathway.14 It is reported that miR-17-5p inhibits death receptor 6 (DR6) expression and attenuates renal I/R injury.15 Nonetheless, the role of miR-17-5p in MIRI is still undefined.
In this study, we aimed to investigate the mechanism of PPF in ameliorating MIRI. With in vivo and in vitro models, we demonstrated that during I/R, PPF protected cardiomyocytes by modulating HOTAIR/miR-17-5p/STAT3 axis.
Materials and Methods
The Establishment of Animal Model with MIRI
In this study, the animal experiments were approved by the Animal Care and Use Committee of China-Japan Union Hospital of Jilin University (Approval number: 20190107C007). All procedures were performed in accordance with the Guidelines of Care and Use of Laboratory Animals issued by the China Laboratory Animal Care Association. Sprague-Dawley rats were divided into sham group, MIRI group, and PPF treatment group (n=3 in each group). The rats were anesthetized with 10% chloral hydrate (330 mg kg−1) by intraperitoneal injection and fixed in supine position, and intravenous access was established. After shearing and disinfecting, the skins and muscles of the neck were cut and opened. The trachea was exposed, tracheotomy was performed, and animal ventilator was connected. Subsequently, the skins and muscles at the left margin of the sternum were opened. At the 4th and 5th intercostal space, the chest and pericardium were opened, and then the left anterior descending coronary artery was ligated to induce myocardial ischemia for 30 min. Next, the thread was loosened and the myocardium was reperfused for 2 h. Below were the signs of successful MIRI modeling: local myocardial tissue was pale or cyanotic during ischemia, and myocardial tissue in the ischemic area turned red during reperfusion. In PPF treatment group, PPF (60 mg/kg) was injected via intravenous access into each rat 10 min before the ligation; in MIRI group, equal amount of normal saline was injected; in sham group, left anterior descending coronary artery was not ligated, and normal saline was injected. To establish rat model with HOTAIR knockdown, adeno-associated virus type 9 (AAV9) carrying HOTAIR shRNA (and control shRNA) was designed and packaged by Hanbio (Shanghai, China). AAV9 (1×1011 vg/rat) was injected via caudal vein 1 week before MIRI modeling.
After 3 h of reperfusion, 5 mL of blood sample was obtained from each rat through abdominal aortic puncture, and the levels of creatine kinase isoenzyme (CK-MB) and lactate dehydrogenase (LDH) in the serum were detected by enzyme-linked immunosorbent assay (ELISA) according to the manufacture’s instructions (Solarbio, Beijing, China). Then the rats were euthanized, and the myocardium was collected for subsequent experiments.
Cell Culture and H/R Treatment
Rat H9c2 cardiomyocytes were purchased from the China Center for Type Culture Collection (CCTCC, Wuhan, China). The cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Biyuntian, Shanghai, China) in 5% CO2 at 37°C. H9c2 cells were grouped as follows: (1) Control group: cells were cultured under normoxic conditions (21% O2, 5% CO2, and 74% N2), added with equal volume of dimethyl sulfoxide (DMSO), without PPF treatment; (2) H/R group: cells, added with equal volume of DMSO were exposed to hypoxia (2 h, 1% O2, 5% CO2, 94% N2) and then reoxygenated (2 h, 21% O2, 5% CO2, and 74% N2). (3) H/R+PPF group: cells with PPF pre-treatment were treated with H/R. PPF (Aladdin, Shanghai, China) was dissolved in DMSO and subsequently applied to treat the cells (working concentration: 50 µM) 1 h before the H/R treatment.
Cell Transfection
HOTAIR overexpression plasmid (pcDNA3.1-HOTAIR), siRNAs against HOTAIR (si-HOTAIR), STAT3 over-expression plasmid (pcDNA3.1-STAT3), and their negative controls were obtained from the GeneChem (Shanghai, China). H9c2 cells were transfected using Lipofectamine® 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacture’s instruction.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The concentration and purity of RNA were measured by NanoDrop spectrophotometer, and then the RNA was reversely transcribed into cDNA using reverse transcription kit (Takara, Dalian, China). Then, 1 μg of cDNA in each group was then used as the template to amplify the target genes. qRT-PCR was conducted on ABI 7900 Fast Real-Time PCR System (Applied Biosystem, Foster City, CA, USA) with SYBR Green Master Mix kit (Takara, Otsu, Japan). The relative expressions of HOTAIR and miR-17-5p were calculated using the 2 −ΔΔCT method. The primer sequences are shown in Table 1.
Table 1.
Primer Sequences Used for qPCR
| Name | Primer Sequences |
|---|---|
| HOTAIR | Forward: 5′- GGGTGGCTCACTCTTCTGGC −3′ |
| Reverse: 5′- TGGCCTTGCCCGGGCTTGTC −3′ | |
| miR-17-5p | Forward:5ʹ- GGGGCAAAGTGCTTACAGTG −3’ |
| Reverse:5′- GTGCGTGTCGTGGAGTCG −3′ | |
| U6 | Forward:5ʹ- GCTTCGGCAGCACATATACTAAAAT −3’ |
| Reverse:5ʹ- CGCTTCACGAATTTGCGTGTCAT −3’ | |
| STAT3 | Forward: 5ʹ- AGAGGCGGCAGCAGATAGC −3’ |
| Reverse: 5ʹ- TTGTTGGCGGGTCTGAAGTT −3’ | |
| GAPDH | Forward: 5ʹ- ACCACAGTCCATGCCATCAC −3’ |
| Reverse: 5ʹ- TCCACCACCCTGTTGCTGTA −3’ |
Flow Cytometry
H9c2 cells (about 1×105 cells) in each group were collected, rinsed once with PBS, and then resuspended with 500 µL of binding buffer (Beyotime, Shanghai, China). Subsequently, 5 µL of Annexin V-FITC staining solution (Beyotime, Shanghai, China) and 10 µL of PI staining solution (Beyotime, Shanghai, China) were added into the cell suspension, with which the cells were incubated in the dark for 30 min. Next, the apoptosis of H9c2 cells was detected by a flow cytometer (FACScan; BD Biosciences, San Jose, CA, USA), and at least 1×104 cells in each group were used for calculating the apoptosis rate.
Western Blot
With density gradient centrifugation, cytosolic and nuclear fractions were separated after H9c2 cells from each group were lysed in RIPA buffer (Beyotime, Shanghai, China). Subsequently, the mixture was centrifuged for 15 min (12,000 r/min, 4°C) and the supernatant was collected, which was considered as the protein sample, before protein concentration was measured using Bicinchoninic Acid (BCA) Protein Assay Kit (Boster, Wuhan, China). After the protein samples were denatured, 20 μg of protein sample in each group was dissolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and then the proteins were transferred to polyvinylidene fluoride (PVDF) membrane (Beyotime, Shanghai, China). Following that, the PVDF membrane was blocked with 5% skim milk for 2 h and then incubated with anti-histone H3 (Abcam, ab215728, Cambridge, UK, 1:1000), anti-GAPDH (Abcam, ab8245, Cambridge, UK, 1:1000), anti-STAT3 (Proteintech, 10253-2-AP, Wuhan, China, 1:1000) and anti-p-STAT3 (Abcam, ab76315, Cambridge, UK, 1:500) primary antibodies at 4°C overnight. Next, the membrane was incubated with the secondary antibody (Proteintech, 10253-2-AP, Wuhan, China) at room temperature for 2 h. Finally, the protein bands were detected with the enhanced chemiluminescence method (Beyotime, Shanghai, China).
Dual-Luciferase Reporter Assay
The sequence of HOTAIR containing miR-17-5p binding site was amplified by PCR. The amplified product was then inserted into basic pGL3 vector (Promega, Madison, WI, USA) to construct a HOTAIR wild-type (HOTAIR WT) reporter vector. Thereafter, the amplified product of the HOTAIR mutant sequence was inserted into pGL3 vector to construct a HOTAIR mutant (HOTAIR MUT) reporter vector. Next, H9c2 cells were transfected with the reporter vectors, together with miR-17-5p mimics or negative control, respectively. After 48 h, the luciferase activity was measured using dual–luciferase reporter assay system (Promega, Madison, WI, USA). Firefly luciferase activity was normalized to renilla luciferase activity.
RNA Immunoprecipitation (RIP)
RIP analysis was performed using the EZMagna RIP kit (Millipore, Billerica, MA, USA) according to the protocol provided by the manufacturer. H9c2 cells transfected with Ago2 plasmids or control plasmids with 80–90% confluency were collected and lysed in RIP lysis buffer. The lysate was incubated at 4°C for 6 h with anti-Ago2 or anti-IgG antibodies conjugated with magnetic beads. Following that, proteins were removed with proteinase K, and then the immunoprecipitated RNA was isolated with TRIzol method. Finally, qRT-PCR was performed to detect HOTAIR and miR-17-5p expression levels.
Statistical Analysis
All experiments were performed in triplicate. The data were analyzed using SPSS 19.0 statistical software (SPSS, Chicago, IL, USA). All experimental data were expressed as mean ± standard deviation (x ± s). Student’s t-test was used for making the comparison between the two groups, and P<0.05 signified statistical significance.
Results
PPF Ameliorated MIRI and Regulated the Expressions of HOTAIR and miR-17-5p in Myocardium
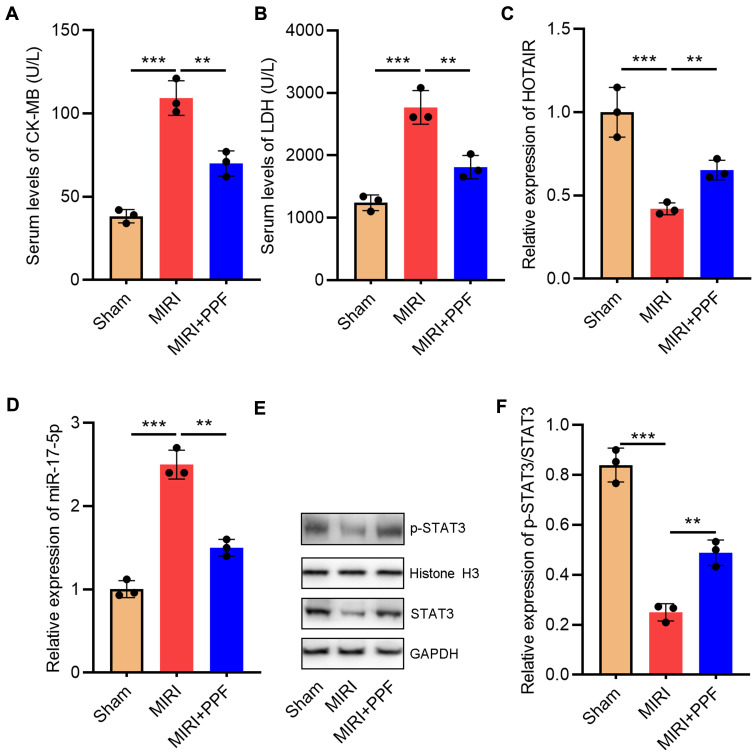
To validate the protective effects of PPF on MIRI, we divided the rats into 3 groups: the control group, the MIRI group, and the PPF pre-treatment group. The results suggested that the levels of CK-MB and LDH in the serum of the rats in MIRI group were remarkably higher than those in the control group, suggesting that the establishment of the MIRI model was successful (Figure 1A and B). Notably, PPF treatment decreased CK-MB and LDH levels compared with MIRI group, indicating that PPF ameliorated the injury of heart (Figure 1A and B). Additionally, both HOTAIR and miR-17-5p play important regulatory roles in MIRI.16,17 In this research, qRT-PCR was employed to detect the changes of HOTAIR and miR-17-5p expression levels in myocardial tissue of rats which received PPF pre-treatment. The results implied that HOTAIR and p-STAT3 expression in the rats of the MIRI group, compared with that in control group, was significantly reduced while miR-17-5p expression was notably increased (Figure 1C–F). Furthermore, PPF intervention could partially reverse the above-mentioned changes (Figure 1C–F). These results implied the potential regulatory functions of PPF on HOTAIR and miR-17-5p expression during MIRI.
Figure 1.
The effect of PPF treatment on rats with MIRI and its effect on HOTAIR and miR-17-5p expression. (A) CK-MB in the serum of the rats in each group was detected with ELISA (N=3). (B) LDH in the serum of the rats in each group was detected with ELISA (N=3). (C) qRT-PCR was used to detect the expression level of HOTAIR in the heart tissue of the rats (N=3). (D) MiR-17-5p expression in heart tissue of the rats was detected by qRT-PCR. (E and F) Western blot was used to detect by the expression of p-STAT3 and STAT3 in heart tissue of the rats. ** P<0.01 and *** P<0.001.
Abbreviations: PPF, propofol; MIRI, myocardial ischemia-reperfusion injury; CK-MB, creatine kinase isoenzyme; LDH, lactate dehydrogenase; qRT-PCR, quantitative real-time polymerase chain reaction.
PPF Regulated HOTAIR and miR-17-5p Expression Levels in H9c2 Cells Exposed to H/R
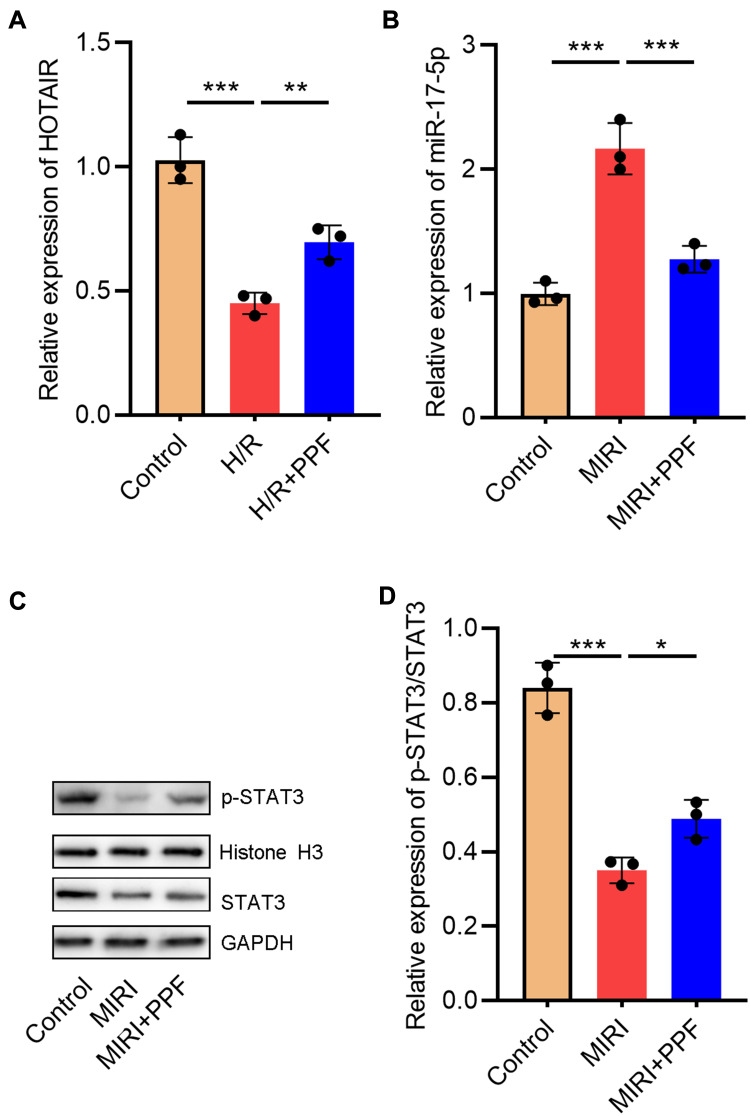
Furthermore, in order to further delve into the effects of PPF on HOTAIR and miR-17-5p expression in cardiomyocytes, H9c2 cells were exposed to H/R. We then performed qRT-PCR, the results of which showed that H/R significantly inhibited HOTAIR expression in H9c2 cells, and this inhibitory effect could be partially reversed after PPF pre-treatment (Figure 2A). PPF also reversed the up-regulation of miR-17-5p expression induced by H/R (Figure 2B). Moreover, STAT3, as a downstream target of miR-17-5p, plays a prominent regulatory role in the I/R-induced injury in multiple organs.18,19 Western blot results demonstrated that H/R inhibited p-STAT3 expressions in H9c2 cells, and this inhibitory effect could be partially reversed by PPF treatment (Figure 2C and D). Based on the above results, we supposed that PPF played a protective role in MIRI by regulating HOTAIR, miR-17-5p and STAT3 signaling.
Figure 2.
The effects of PPF on HOTAIR, miR-17-5p, and STAT3 expressions in H9c2 cells. (A and B) The expression levels of HOTAIR and miR-17-5p in H9c2 cells were detected by qRT-PCR (N=3). (C and D) Western blot was used to detect the expressions of STAT3 and p-STAT3 in H9c2 cells (N=3). * P<0.05, ** P<0.01 and *** P<0.001.
Abbreviations: PPF, propofol; qRT-PCR, quantitative real-time polymerase chain reaction.
HOTAIR Targeted miR-17-5p in H9c2 Cells
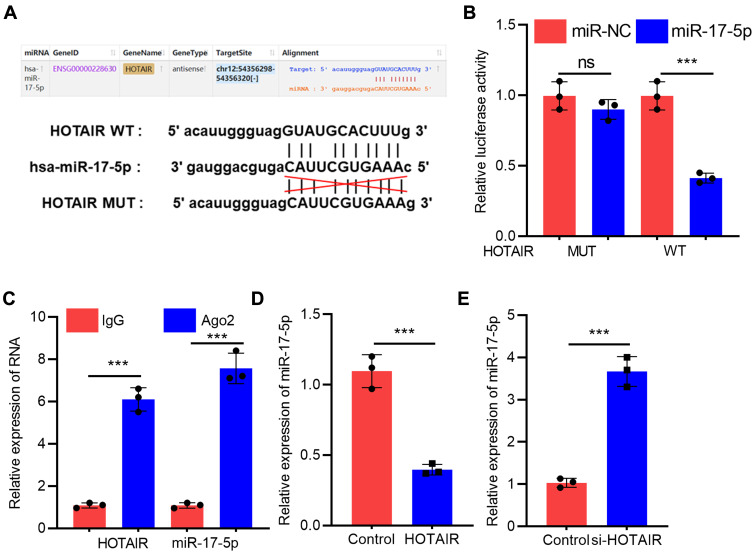
To further elaborate on the interaction between HOTAIR and miR-17-5p, bioinformatics analysis was performed with StarBase (http://starbase.sysu.edu.cn/panCancer.php) database, and it was demonstrated that HOTAIR contained a binding sequence for miR-17-5p (Figure 3A). Dual-luciferase reporter assay confirmed that miR-17-5p mimics markedly suppressed the luciferase activity of wild-type HOTAIR reporter, but the luciferase activity of mutant HOTAIR reporter was not significantly changed (Figure 3B). Furthermore, RIP experiments manifested that HOTAIR and miR-17-5p were enriched in Ago2 immunoprecipitation compared to the IgG immunoprecipitation, suggesting a direct interaction between them (Figure 3C). We subsequently discovered that HOTAIR overexpression in H9c2 cells significantly inhibited miR-17-5p expression while knockdown of HOTAIR worked oppositely (Figure 3D). Taken together, these studies suggested that miR-17-5p was a downstream target of HOTAIR in H9c2 cells.
Figure 3.
HOTAIR adsorbed miR-17-5p and repressed its expression in H9c2 cells. (A) StarBase database analysis revealed that there was a potential binding site between HOTAIR and miR-17-5p. (B) Dual-luciferase reporter analysis showed that miR-17-5p could inhibit the luciferase activity of HOTAIR-WT, but could not inhibit that of HOTAIR-MUT reporter (N=3). (C) RIP experiments indicated the direct interaction between HOTAIR and miR-17-5p (N=3). (D and E) qRT-PCR results showed that HOTAIR negatively regulated miR-17-5p expression in H9c2 cells (N=3). *** P<0.001.
Abbreviations: qRT-PCR, quantitative real-time polymerase chain reaction; WT, wild-type; MUT, mutant type.
The Regulatory Function of PPF on miR-17-5p and STAT3 Was Dependent on HOTAIR in H9c2 Cells Treated with H/R
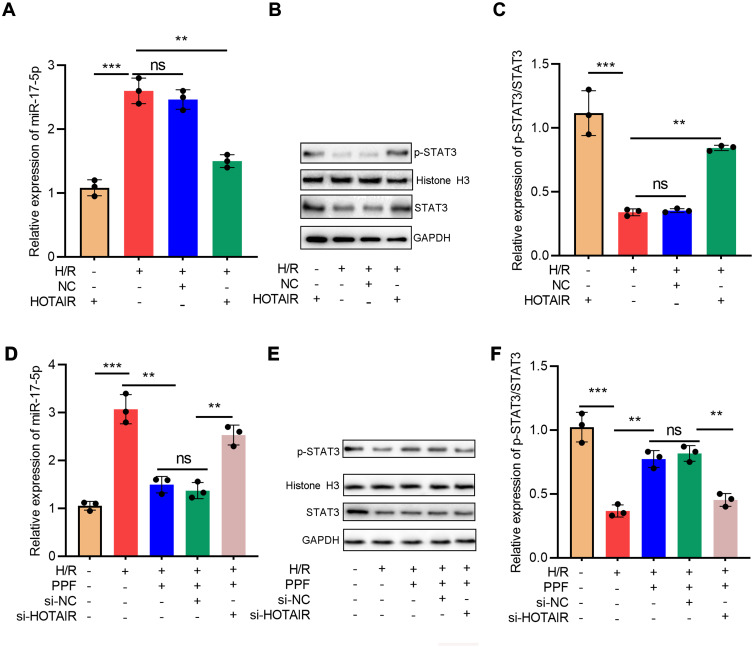
Next, HOTAIR overexpression was transfected into H9c2 cells and exposed to H/R. qRT-PCR results showed that HOTAIR overexpression attenuated the up-regulation of miR-17-5p expression induced by H/R (Figure 4A). Western blot verified that HOTAIR overexpression partially reversed H/R-induced down-regulation of p-STAT3 expressions (Figure 4B and C). Next, after the transfection of HOTAIR siRNA, H9c2 cells were pre-treated with PPF and exposed to H/R. The results implied that PPF decreased H/R-induced up-regulation of miR-17-5p expression, and this effect was partially attenuated by si-HOTAIR (Figure 4D). In addition, si-HOTAIR weakened the effects of PPF on promoting p-STAT3 expression (Figure 4E and F). These results indicated that HOTAIR was a mediator for PPF to regulate miR-17-5p expression and STAT3 signaling.
Figure 4.
H/R treatment regulated miR-17-5p and STAT3 expressions via HOTAIR. (A) Empty plasmids and HOTAIR overexpression plasmids were transfected into H9c2 cells treated with H/R, respectively, and the expression of miR-17-5p was detected by qRT-PCR (N=3). (B and C) The expressions of STAT3 and p-STAT3 in H9c2 cells were detected by Western blot (N=3). (D) Control siRNA and HOTAIR siRNA were transfected into H9c2 cells treated with H/R and PPF, and the expression of miR-17-5p was detected by qRT-PCR (N=3). (E and F) The expressions of STAT3 and p-STAT3 in H9c2 cells were detected by Western blot (N=3). ** P<0.01 and *** P<0.001. ns, P>0.05.
Abbreviations: H/R, hypoxia/reoxygenation; siRNA, small interfering RNA.
HOTAIR and STAT3 Were Involved in the Effects of PPF on H/R-Induced Apoptosis
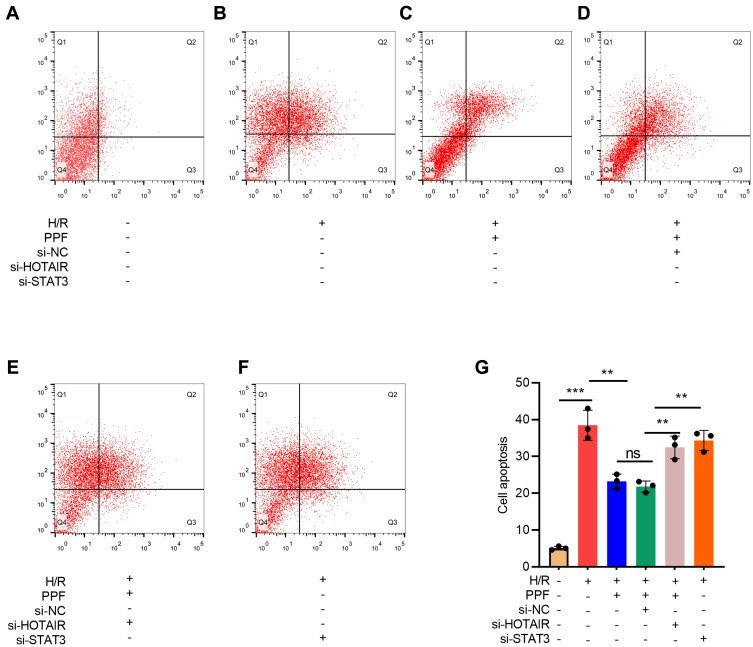
To investigate the role of HOTAIR/miR-17-5p/STAT3 axis in H/R-induced apoptosis of cardiomyocytes, flow cytometry was performed. The results suggested that PPF reduced H/R-induced apoptosis, and this effect could be partially attenuated by HOTAIR knockdown or STAT3 knockdown (Figure 5A–E).
Figure 5.
The effects of HOTAIR, STAT3, and PPF on H/R-induced apoptosis of H9c2 cells. (A–G) Apoptosis of H9c2 cells was detected by flow cytometry after HOTAIR or STAT3 was selectively regulated (N=3). ** P<0.01, and *** P<0.001. ns, P>0.05.
HOTAIR Knockdown Partially Reversed the Protective Effects of PPF on MIRI Models in vivo
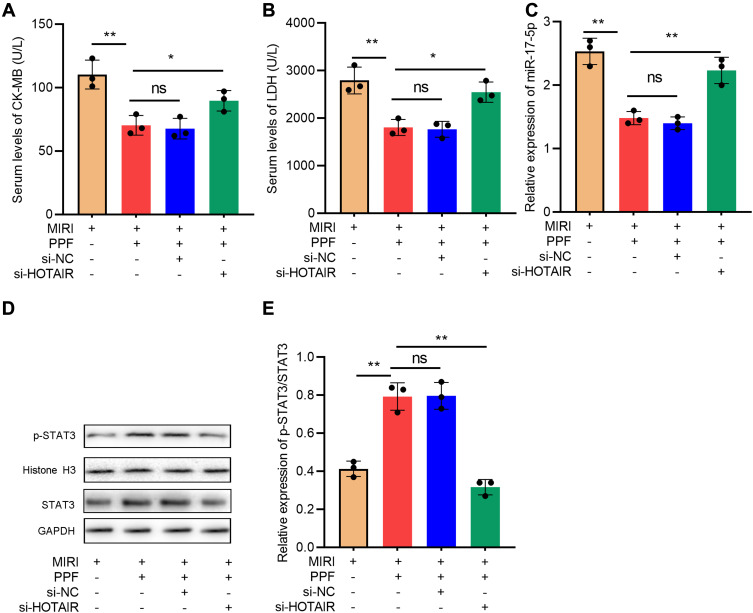
To further decipher the regulatory effects of HOTAIR in the cardioprotective effect of PPF, rats were divided into 4 groups: MIRI group, MIRI+PPF group, MIRI+PPF+NC group, MIRI+PPF+HOTAIR knockdown group (3 rats in each group). As shown, compared with the MIRI+PPF group, HOTAIR knockdown mediated by AAV9 increased the CK-MB and LDH levels (Figure 6A and B). In addition, HOTAIR knockdown attenuated the inhibition of miR-17-5p expression and the activation of STAT3 signaling, which were induced by PPF pre-treatment (Figure 6C–E). These data further suggested that HOTAIR was a crucial effector in PPF’s cardioprotective function.
Figure 6.
The effects of HOTAIR on the protective function of PPF in MIRI rats. (A) CK-MB in the serum of the rats in each group was detected with ELISA (N=3). (B) LDH in the serum of the rats in each group was detected with ELISA (N=3). (C) The expression level of miR-17-5p in the myocardial tissue of rats was detected by qRT-PCR (N=3). (D and E) Western blot was used to detect the expressions of STAT3 and p-STAT3 protein in cardiac tissue of the rats (N=3). * P<0.05, ** P<0.01. ns, P>0.05.
Abbreviations: PPF, propofol; MIRI, myocardial ischemia-reperfusion injury.
Discussion
MIRI is a common pathological factor, which limits the therapeutic effect of thrombolytic therapy, coronary artery bypass grafting, and heart transplantation.20 PPF has many advantages, such as rapid induction of anesthesia, rapid recovery, good function recovery, and low incidence of postoperative nausea and vomiting, so it becomes one of the most commonly used intravenous anesthetic in clinical practice.21 In addition, PPF has a variety of non-narcotic effects. For example, PPF reduces sympathetic nerve activity and angiotensin II-induced calcium influx, and in turn affects the release of calcium in smooth muscle cells, reduces circulation disorders, and improves blood supply to the organs.22 PPF can also ameliorate dysregulation of ATP, Ca2+, Na+, K+, and inhibit ROS-mediated lipid peroxidation catalyzed by cyclooxygenase, reducing inflammation and organ damage.23–25 Clinical data indicate that PPF can reduce myocardial lipid peroxidation of the patients induced by coronary artery bypass grafting.26 In this research, with in vitro and in vivo data, our results further confirmed that PPF could reduce the injury of myocardium/cardiomyocytes, which was consistent with previous reports.
As mentioned above, the cardioprotective effects of PPF mainly depend on its regulatory function on circulatory disturbance, generation of ROS, lipid peroxidation, and inflammatory responses.5–7,23–27 However, the detailed molecular mechanism is still obscure. In recent years, the role of non-coding RNA in the pathogenesis of cardiovascular diseases has attracted a lot of attention.27,28 During MIRI, a large amount of released ROS exceeds the ability of cardiomyocytes to scavenge, and ROS can directly react with DNA and proteins and damage cell membrane and ion channels, leading to cardiomyocytes injury and apoptosis.29 Interestingly, it is reported that HOTAIR overexpression can protect H9c2 cells from oxidative stress-induced damage; in this process, HOTAIR attenuates I/R-induced cardiomyocyte apoptosis by acting as an inhibitor of reactive oxygen species.16 Additionally, miR-17-5p also figures prominently in regulating I/R-induced injury. For example, miR-17-5p expression is abnormally increased during the maintenance and recovery stages of renal I/R injury.30,31 In MIRI, miR-17-5p aggravates cardiomyocyte apoptosis through repressing STAT3 expression.17 In this work, we found that HOTAIR expression in H9c2 cells in MIRI models was markedly reduced while miR-17-5p expression was significantly increased, which was consistent with previous reports.17,18 Bioinformatics analysis implied a potential binding site between HOTAIR and miR-17-5p, and dual-luciferase reporter gene experiments, RIP experiments, and qRT-PCR confirmed that HOTAIR was able to adsorb miR-17-5p and repress miR-17-5p expression in cardiomyocytes. Additionally, the target gene of miR-17-5p, STAT3 was positively regulated by HOTAIR. STAT3 signaling is a crucial pro-survival pathway during I/R injury of different organs, and in animal models, the cytokines modulated by STAT3, such as erythropoietin and granulocyte-colony stimulating factor (G-CSF), ameliorate MIRI.32,33 Collectively, these data indicated that, in the pathogenesis of MIRI, HOTAIR exerted its protective role partly via modulating miR-17-5p/STAT3 axis, and the dysfunction of miR-17-5p was partially due to the dysregulation of HOTAIR.
Some previous studies report that PPF can regulate the expression levels of multiple non-coding RNAs, thereby playing a protective role in I/R-induced injury.32,33 For example, PPF protects the liver from I/R injury by modulating MAPK/miR-133a-5p axis;34 PPF regulates HMGB1miR-451/HMGB1 axis to attenuate MIRI expression.22 In this study, we found that the down-regulation of HOTAIR expression and up-regulation of miR-17-5p expression in MIRI/HR could be partially attenuated by PPF pre-treatment. In addition, knocking down HOTAIR could significantly reverse the reduction in apoptosis of H9c2 cells induced by PPF and attenuate the effects of PPF on inhibiting the release of CK-MB and LDH. These results suggested that PPF functioned by modulating the HOTAIR/miR-17-5p axis. Furthermore, it was found that PPF could promote STAT3 activation; compared with the PPF group, knocking down STAT3 could significantly eliminate the effects of PPF on ameliorating MIRI. Therefore, it was concluded that PPF attenuated MIRI by regulating HOTAIR/miR-17-5p/STAT3 axis.
In summary, we provide conclusive evidence that PPF exerts cardioprotective effects following I/R injury and protects MIRI by regulating HOTAIR/miR-17-5p/STAT3 axis. Our findings help clarify the molecular mechanism of MIRI pathogenesis and offer theoretical basis for the clinical application of PPF. Our data support that PPF is an ideal anesthetic for patients with cardiovascular diseases who receive surgery.
Acknowledgments
We thank Hubei Yican Health Industry Co., Ltd. for its linguistic assistance during the preparation of this manuscript.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
Our study was approved by the China-Japan Union Hospital of Jilin University (Approval number: 20190107C007).
Disclosure
The authors declare that they have no competing interests.
References
- 1.Boag SE, Andreano E, Spyridopoulos I. Lymphocyte communication in myocardial ischemia/reperfusion injury. Antioxid Redox Signal. 2017;26(12):660–675. doi: 10.1089/ars.2016.6940 [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Guo Z, Ding Z, Mehta JL. Inflammation, autophagy, and apoptosis after myocardial infarction. J Am Heart Assoc. 2018;7(9):e008024. doi: 10.1161/JAHA.117.008024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinis-Oliveira RJ. Metabolic profiles of propofol and fospropofol: clinical and forensic interpretative aspects. Biomed Res Int. 2018;2018:6852857. doi: 10.1155/2018/6852857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei Q, Zhao J, Zhou X, Yu L, Liu Z, Chang Y. Propofol can suppress renal ischemia-reperfusion injury through the activation of PI3K/AKT/mTOR signal pathway. Gene. 2019;708:14–20. doi: 10.1016/j.gene.2019.05.023 [DOI] [PubMed] [Google Scholar]
- 5.Ji F-T, Liang -J-J, Miao L-P, Wu Q, Cao M-H. Propofol post-conditioning protects the blood brain barrier by decreasing matrix metalloproteinase-9 and aquaporin-4 expression and improves the neurobehavioral outcome in a rat model of focal cerebral ischemia-reperfusion injury. Mol Med Rep. 2015;12(2):2049–2055. doi: 10.3892/mmr.2015.3585 [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Wu Q, Liao J, et al. Propofol induces cardioprotection against ischemia-reperfusion injury via suppression of transient receptor potential vanilloid 4 channel. Front Pharmacol. 2019;10:1150. doi: 10.3389/fphar.2019.01150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Zhu H, Zhu S, Hao M, Li Q. lncRNA expression character associated with ischemic reperfusion injury. Mol Med Rep. 2017;16(4):3745–3752. doi: 10.3892/mmr.2017.7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xin J-W, Jiang Y-G. Long noncoding RNA MALAT1 inhibits apoptosis induced by oxygen-glucose deprivation and reoxygenation in human brain microvascular endothelial cells. Exp Ther Med. 2017;13(4):1225–1234. doi: 10.3892/etm.2017.4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang B, Bao N, He G, Wang J. Long noncoding RNA HOTAIR regulates autophagy via the miR-20b-5p/ATG7 axis in hepatic ischemia/reperfusion injury. Gene. 2019;686:56–62. doi: 10.1016/j.gene.2018.10.059 [DOI] [PubMed] [Google Scholar]
- 10.Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;141(4):1202–1207. doi: 10.1016/j.jaci.2017.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16(3):203–222. doi: 10.1038/nrd.2016.246 [DOI] [PubMed] [Google Scholar]
- 12.Qiao L, Hu S, Liu S, et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J Clin Invest. 2019;129(6):2237–2250. doi: 10.1172/JCI123135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He X, Zheng Y, Liu S, et al. MiR-146a protects small intestine against ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB pathway. J Cell Physiol. 2018;233(3):2476–2488. doi: 10.1002/jcp.26124 [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Xu J, Wang Y, et al. Inhibition of MiRNA-125b decreases cerebral ischemia/reperfusion injury by targeting CK2α/NADPH oxidase signaling. Cell Physiol Biochem. 2018;45(5):1818–1826. doi: 10.1159/000487873 [DOI] [PubMed] [Google Scholar]
- 15.Hao J, Wei Q, Mei S, et al. Induction of microRNA-17-5p by p53 protects against renal ischemia-reperfusion injury by targeting death receptor 6. Kidney Int. 2017;91(1):106–118. doi: 10.1016/j.kint.2016.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Zhang M, Chen W, et al. LncRNA-HOTAIR inhibition aggravates oxidative stress-induced H9c2 cells injury through suppression of MMP2 by miR-125. Acta Biochim Biophys Sin (Shanghai). 2018;50(10):996–1006. doi: 10.1093/abbs/gmy102 [DOI] [PubMed] [Google Scholar]
- 17.Du W, Pan Z, Chen X, et al. By targeting Stat3 microRNA-17-5p promotes cardiomyocyte apoptosis in response to ischemia followed by reperfusion. Cell Physiol Biochem. 2014;34(3):955–965. doi: 10.1159/000366312 [DOI] [PubMed] [Google Scholar]
- 18.Luo N, Liu J, Chen Y, Li H, Hu Z, Abbott GW. Remote ischemic preconditioning STAT3-dependently ameliorates pulmonary ischemia/reperfusion injury. PLoS One. 2018;13(5):e0196186. doi: 10.1371/journal.pone.0196186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Tan J-L, Chen Z-Y, Huang G. Cardioprotection of post-ischemic moderate ROS against ischemia/reperfusion via STAT3-induced the inhibition of MCU opening. Basic Res Cardiol. 2019;114(5):39. doi: 10.1007/s00395-019-0747-9 [DOI] [PubMed] [Google Scholar]
- 20.Shah M, Yellon DM, Davidson SM. The role of extracellular DNA and histones in ischaemia-reperfusion injury of the myocardium. Cardiovasc Drugs Ther. 2020;34(1):123–131. doi: 10.1007/s10557-10020-06946-10556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gersner R, Paredes C, Hameed MQ, Dhamne SC, Pascual-Leone A, Rotenberg A. Transcranial magnetic stimulation tracks subminute changes in cortical excitability during propofol anesthesia. Ann Clin Transl Neurol. 2020;7(3):384–389. doi: 10.1002/acn1003.50981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y-M, Sun J-G, Hu L-H, Ma X-C, Zhou G, Huang X-Z. Propofol-mediated cardioprotection dependent of microRNA-451/HMGB1 against myocardial ischemia-reperfusion injury. J Cell Physiol. 2019;234(12):23289–23301. doi: 10.1002/jcp.28897 [DOI] [PubMed] [Google Scholar]
- 23.Liu NH, Zhu L, Zhang XB, Chen Y. Metformin with propofol enhances the scavenging ability of free radicals and inhibits lipid peroxidation in mice. Eur Rev Med Pharmacol Sci. 2019;23(11):4980–4987. doi: 10.26355/eurrev_201906_18089 [DOI] [PubMed] [Google Scholar]
- 24.Wu G-J, Lin Y-W, Tsai H-C, Lee Y-W, Chen J-T, Chen R-M. Sepsis-induced liver dysfunction was ameliorated by propofol via suppressing hepatic lipid peroxidation, inflammation, and drug interactions. Life Sci. 2018;213:279–286. doi: 10.1016/j.lfs.2018.10.038 [DOI] [PubMed] [Google Scholar]
- 25.Ge YW, Gao HM, Wang ZM. [Advances in study of genus Curcuma]. Zhongguo Zhong Yao Za Zhi. 2007;32(23):2461–2467. Chinese. [PubMed] [Google Scholar]
- 26.Sayin MM, Ozatamer O, Taşöz R, Kilinç K, Unal N. Propofol attenuates myocardial lipid peroxidation during coronary artery bypass grafting surgery. Br J Anaesth. 2002;89(2):242–246. doi: 10.1093/bja/aef173 [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Li Y, Wang P. Long non-coding RNA-ROR aggravates myocardial ischemia/reperfusion injury. Braz J Med Biol Res. 2018;51(6):e6555–e6555. doi: 10.1590/1414-431x20186555 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Wang JB, Cui HR, Bai ZF, Xiao XH. [Precision medicine-oriented safety assessment strategy for traditional Chinese medicines: disease-syndrome-based toxicology]. Yao Xue Xue Bao. 2016;51(11):1681–1688. Chinese. [PubMed] [Google Scholar]
- 29.Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. [DOI] [PubMed] [Google Scholar]
- 30.Kaucsár T, Révész C, Godó M, et al. Activation of the miR-17 family and miR-21 during murine kidney ischemia-reperfusion injury. Nucleic Acid Ther. 2013;23(5):344–354. doi: 10.1089/nat.2013.0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma L, Wu K, Liu K, et al. Changes of miRNA-17-5p, miRNA-21 and miRNA-106a level during rat kidney ischemia-reperfusion injury. Zhonghua Yi Xue Za Zhi. 2015;95(19):1488–1492. [PubMed] [Google Scholar]
- 32.Chen X, Wang Y, Xiao ZY, Hou DN, Li DB, Zhang XP. Effect of propofol on myocardial ischemia/reperfusion injury in rats through JAK/STAT signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(14):6330–6338. doi: 10.26355/eurrev_201907_18456 [DOI] [PubMed] [Google Scholar]
- 33.Ren Y, Yao MC, Huo XQ, et al. [Study on treatment of “cytokine storm” by anti-2019-nCoV prescriptions based on arachidonic acid metabolic pathway]. Zhongguo Zhong Yao Za Zhi. 2020;45(6):1225–1231. Chinese. doi: 10.19540/j.cnki.cjcmm.20200224.405 [DOI] [PubMed] [Google Scholar]
- 34.Hao W, Zhao Z-H, Meng Q-T, Tie M-E, Lei S-Q, Xia Z-Y. Propofol protects against hepatic ischemia/reperfusion injury via miR-133a-5p regulating the expression of MAPK6. Cell Biol Int. 2017;41(5):495–504. doi: 10.1002/cbin.10745 [DOI] [PubMed] [Google Scholar]