Abstract
Background:
Cytomegalovirus (CMV) is a common pathogen affecting the gastrointestinal tract in patients with AIDS. We report a case of CMV-induced pseudotumor of the duodenum in a patient with AIDS and review other reported cases of CMV-induced pseudotumors in the gastrointestinal tract. CMV-induced pseudotumor in patients with AIDS is an exceptionally rare clinical entity, and to our knowledge no reports have previously summarized this clinical entity.
Methods:
All previous cases included in our literature review were found using a PubMed search (1980–November 2008) of the English-language medical literature applying the terms ‘CMV infection’, ‘inflammatory mass’, ‘pseudotumor’, and ‘gastrointestinal tract’. The references cited in these articles were examined to identify additional reports.
Results:
Although CMV-induced duodenitis has been described in patients with HIV infection, to our knowledge CMV-induced pseudotumor of the duodenum has not been previously reported in the literature. We describe the first case of an AIDS patient with CMV pseudotumor responding to oral treatment with valganciclovir with complete resolution of the CMV mass. Among reports of non-duodenal pseudotumor reported in the English literature, we found only 14 cases of CMV-induced gastrointestinal pseudotumors in HIV-positive patients. The clinical manifestations, pathologic findings of the CMV pseudotumors, as well as the treatment and outcome of these HIV patients are reviewed.
Conclusion:
CMV pseudotumor should be included in the differential diagnosis of gastrointestinal mass lesions in AIDS patients and in other immunocompromised patients. The tumor often responds to antiviral therapy, but resolution of a CMV mass as a result of oral antiviral therapy has not been previously described. Since pseudotumors secondary to CMV often respond to medical treatment, it is important that the physicians treating severely immunocompromised patients are aware of this entity.
Keywords: Cytomegalovirus, Inflammatory pseudotumor, AIDS
1. Introduction
Cytomegalovirus (CMV) is one of the most common viral pathogens affecting the gastrointestinal tract in patients with AIDS. The clinical and radiographic findings of CMV esophagitis, gastritis, enteritis, and colitis have been well documented.1,2 CMV-induced duodenitis is rare but has been described in patients with HIV infection.3–5 To our knowledge CMV-induced pseudotumor of the duodenum has not been previously reported in the literature in this patient population. Among reports of non-duodenal pseudotumor, we found only 14 cases of CMV-induced gastrointestinal pseudotumors in HIV-positive patients reported in the English literature (Table 1).6–15 The pseudotumors were found in the esophagus,8,12 stomach,6,7,10,12,14 ileum,9–11,15 and colon.9–11,13,16 Since pseudotumors secondary to CMV often respond to medical treatment, it is important that the physicians treating severely immunocompromised patients are aware of this entity. We report a case of CMV-induced pseudotumor of the duodenum in a patient with AIDS and review other reported cases of CMV-induced pseudotumors in the gastrointestinal tract.
Table 1.
Cases of cytomegalovirus pseudotumor of the gastrointestinal tract in patients with HIV infection
| Author (year) [Ref.] | Location | Age | Sex | HIV status/ART | Clinical picture | Method of diagnosis | Characteristics of pseudotumor | Outcome | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Victoria (1985)6 | Stomach, pylorus | 16 months | M | A child with AIDS | Pyloric obstruction with nausea, vomiting, fever | Open biopsy from stomach and duodenum. Postmortem examination | Occlusion of the pylorus with thickening of the walls. CMV inclusion bodies on the glandular epithelium and muscle layer of pylorus with extensive inflammatory infiltration | The patient died in his hospital course of progressive respiratory failure | Not described |
| Elta (1986)7 | Stomach (gastric antrum) | 29 years | M | Homosexual transmission. AIDS with CD4/CD8 ratio of 0.36 | Abdominal pain, watery diarrhea and fever. Nonobstructive prepyloric mass | Submucosal mass was identified in CT abdomen and upper endoscopy. Open biopsy after exploratory laparoscopy | Irregular mass, mucosal sparing, marked inflammation. Infiltrate of predominantly mononuclear cells, edema, and numerous stromal and epithelium cells with CMV inclusion bodies | Patient died 2 months later after diagnosis of CMV infection | Surgery. No information was provided about medical treatment |
| Dolgin (1990)15 | Small bowel | Infant | M | Infant with AIDS | Enteritis presenting with massive hemorrhage and subsequent fatal small bowel obstruction | Laparotomy. Postmortem examination | Striking, diffuse, large yellowish plaques along the entire length of the small bowel. Each of these had a central ulceration. Each eventually caused a partial narrowing resulting in refractory small bowel obstruction. Biopsy of one of these lesions demonstrated many cells with typical cytomegalic inclusion bodies | Patient died of bleeding and small bowel obstruction | Surgery |
| Wilcox (1990)8 | Esophagus | NR | NR | AIDS for 8 months | Epigastric pain for 6 months | Endoscopy | Exophytic mass. Characteristic inclusion bodies mostly in endothelial cells and in fibroblasts, epithelial cells | Could not be evaluated by authors. Patient died after 4 months | Ganciclovir |
| Duva-Frissora (1991)9 | Cecum, ileum | 36 years | M | AIDS. No other data specified | One month watery diarrhea and fevers, CT finding | Open biopsy after right side ileocolectomy | Pericolonic mass with adenopathy, mucosal sparing, inflammation. Pathologic examination revealed a cytomegalovirus-mediated inflammatory pseudotumor within the muscularis and submucosa with complete mucosal sparing | NR | Surgery. No information was provided about medical treatment |
| Rich (1992)10 | Stomach (gastric fundus) | 41 years | M | Homosexual transmission. HIV seropositivity 5 years prior to diagnosis of AIDS. Other opportunistic infection prior to CMV infection were cerebral toxoplasmosis | Epigastric mass, LUQ palpable mass | Endoscopy. Serology positive for CMV | Polypoid 5-cm wide necrotic mass with ulceration, necrosis, fibrosis. Cytomegalic change was present within glandular epithelial cells, which stained positive for CMV with immunoperoxidase | His gastrointestinal symptoms completely resolved following treatment | Ganciclovir |
| Rich (1992)10 | Cecum | 41 years | M | Homosexual transmission. HIV seropositivity 3 years prior to diagnosis of AIDS | Abdominal pain w/o diarrhea | Colonoscopy. Excision biopsy (right colectomy) | Focal polypoid friable, firm near circumferential mass with ulceration, fibrosis. Microscopic examination revealed granulation tissue in the ulcer bed, overlying acute inflammatory exudate, CMV like inclusions identified in endothelial cells and fibroblasts within the granulation tissue. Presence of CMV was confirmed by immunoperoxidase staining | The patient’s pain was completely resolved following surgery and did not recur | Surgery, ganciclovir |
| Rich (1992)10 | Cecum (primary mass), ileum (recurrence) | 34 years | M | Homosexual transmission. AIDS diagnosed 1 year prior to presentation. Other opportunistic infection prior to CMV infection was cerebral toxoplasmosis | Abdominal pain w/o diarrhea, fever (primary mass). Recurrence of abdominal pain (relapse) | Colonoscopy. Excision biopsy (right colectomy) | 4 cm polypoid mass with ulceration, necrosis, fibrosis (primary mass and relapse). Microscopic examination revealed extensive fibrosis, granulation tissue and chronic inflammation. Numerous CMV like inclusions identified in mesenchymal cells and were confirmed by immunoperoxidase staining. Relapse mass was a 2 cm inflammatory polyp with similar to the previous mass microscopic findings | The abdominal pain resolved after surgery. One year later he developed relapse, which was again successfully treated with ganciclovir and surgery | Surgery for primary mass. Surgery and ganciclovir for recurrent mass |
| Wisser (1992)11 | Cecum/ terminal ileum | 36 years | M | Homosexual transmission. AIDS diagnosed 1 year prior to admission. Zidovudine alone for a year | Generalized abdominal pain, bloody diarrhea, night sweats, 1 month h/o tenesmus, 40 lb weight loss | Excision biopsy after right colectomy | Size: 6 5.3 5.1 cm, irregular exophytic mass. Histology showed necrosis and fibrosis, particularly in the muscularis propia, as well as fibroblastic and vascular proliferation with pleomorphic and mononuclear cell infiltrates. CMV inclusion bodies were present in endothelial cells, fibroblasts, muscle, ganglion cells. In situ hybridization confirmed CMV inclusions | Patient died 12 days postoperatively from bacterial sepsis | Surgery. No medical treatment reported |
| Laguna (1993)12 | Stomach (gastric antrum) | 42 years | M | Homosexual transmission. AIDS diagnosed 1 year prior to admission. On zidovudine. Other opportunistic infection prior to CMV infection was PCP | Epigastric pain | Endoscopy CMV was cultured in urine | Large rigid folds in the stomach without ulcers. Characteristic large intranuclear inclusions were identified on routine hematoxylin and eosin staining of esophageal and gastric biopsies | 3 days after treatment with ganciclovir he developed a psychotic syndrome and treatment was changed to foscarnet. Patient improved after 3 weeks of treatment | Ganciclovir, foscarnet |
| Laguna (1993)12 | Esophagus | 40 years | M | Homosexual transmission. AIDS diagnosed 14 months prior to admission. On zidovudine. Other opportunistic infection prior to CMV infection was PCP | Epigastric pain, fever | Endoscopy | Polypoid, exophytic mass without ulceration in the distal esophagus. Immunohistochemical staining with a CMV monoclonal antibody showed intranuclear inclusions in the esophagus biopsies | After 3 weeks of treatment he remained febrile but abdominal pain had improved. Patient died 12 days after cardiac arrest | Ganciclovir (6 weeks), foscarnet (2 weeks) |
| Swansiger (1996)16 | Colon | 28 years | M | Homosexual transmission. HIV infection diagnosed 7 years before presentation. Patient was on zidovudine | RLQ abdominal pain of 1-year duration. Weight loss, intermittent fever and chills | Biopsies obtained at first colonoscopy showed intranuclear inclusion bodies, but viral culture was negative. Cultures of biopsies obtained at repeat colonoscopy after 4 months grew CMV | Multilobulated intraluminal mass with an overall length of 4.5 cm. Biopsies from repeat colonoscopy showed chronic inflammation with vascular endothelial thickening, and culture of the biopsy grew CMV | NR | 5 mg/kg ganciclovir given IV every 12 h |
| Chow (1997)13 | Colon (ascending colon) | 46 years | F | Heterosexual transmission through unprotected sexual exposure to multiple partners. HIV infection diagnosed on presentation. Unknown duration of infection. CD4 count was 129 | Abdominal discomfort, weight loss and change of bowel habits of 1-month duration | Barium enema. Excision biopsy after right hemicolectomy | Multiple tiny ulcers in the ascending colon 0.2 to 0.3 cm in diameter. Histologic examination of the resected specimen revealed that the ulcers were superficial involving the mucosa and submucosa. Epithelial cells with CMV inclusion were identified and stained positive for CMV with immunoperoxidase | The patient was readmitted after a few weeks for proctitis | Surgery |
| Mohan (2007)14 | Stomach/ antral region | 49 years | M | Heterosexual transmission. HIV infection diagnosed on presentation. Unknown duration of infection. CD4 count could not be obtained | Pseudotumor simulating gastric malignancy with 1-month h/ o epigastric discomfort, loose stools, loss of appetite, weight loss | Excision biopsy after subtotal gastrectomy with Roux-en-Y gastrojejunotomy | Size: 5 3 1.5 cm. Gastric epithelial cells and vascular endothelial cells showed intranuclear inclusions of CMV | Patient died on postoperative day 10 because of overwhelming sepsis | Surgery. No medical treatment reported |
ART, antiretroviral therapy; M, male; F, female; NR, not reported; CT, computed tomography; CMV, cytomegalovirus; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; PCP, Pneumocystis carinii pneumonia; LUQ, left upper quadrant; RLQ, right lower quadrant; w/o, without; h/o, history of.
2. Case report
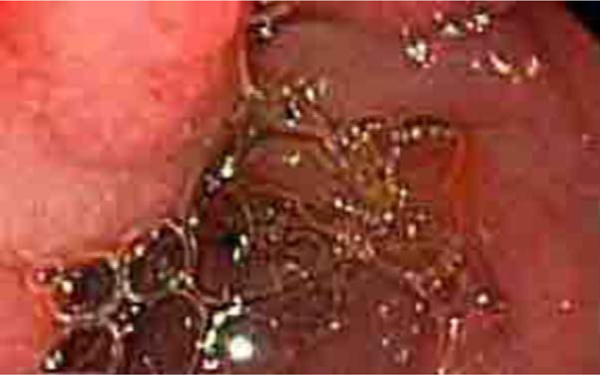
A 52-year-old homosexual male recently diagnosed with AIDS after18 years of HIV infection, on highly active antiretroviral therapy (HAART) for the last seven years, presented with a one-week history of abdominal pain associated with increased abdominal distension, decreased oral intake, nausea, vomiting, and progressive constipation. Past medical history included multiple episodes of small bowel obstruction of unclear etiology. Physical examination revealed an afebrile, hemodynamically stable patient with hypoactive bowel sounds and diffuse abdominal tenderness without any rebound tenderness or guarding. Pertinent laboratory data included a white blood cell count of 8.9 × 109/l with a normal metabolic panel, liver function tests, as well as amylase and lipase. He had a plasma HIV RNA level of <50 copies/ml and a recent CD4 count of 79 cells/ μl. CMV was not detected by PCR in the blood. Serology for CMV revealed positive IgM anti-CMV and IgG anti-CMV. A computed tomography (CT) of the abdomen revealed a distal small bowel obstruction. An endoscopic study revealed esophagitis, duodenitis, and a large mass in the duodenal bulb causing luminal narrowing, which prevented passing the endoscope into the second part of the duodenum (Figure 1). No bleeding or ulcers were found. Histologic examination of multiple biopsies from the mass revealed cytopathic changes compatible with CMV (Figure 2). More specifically, marked acute and chronic inflammation in the mucosa and submucosa was found with endothelial cell atypia suggestive of viral cytopathic effect. Similar findings have been reported in other CMV-induced pseudotumors (Table 1). Immunohistochemical staining using a CMV monoclonal antibody (Dako CCH2/DDG9) against CMV early antigen was positive (Figure 3). Stains for other microorganisms (acid-fastbacillus, Gomori methenamine silver, periodic acid-Schiff) and for other viral pathogens (herpes simplex virus, adenovirus) were negative. The patient’s symptoms improved after hydration and bowel rest and he was discharged after six days. He was treated with oral valganciclovir (900 mg twice daily) for five weeks. Before the diagnosis of the CMV-induced pseudotumor the patient was on an antiretroviral regimen that included abacavir, lamivudine, and zidovudine. However, a genotype analysis of the virus revealed resistance of the virus to all nucleoside reverse transcriptase inhibitors (NRTIs). Thus, the antiretroviral regimen was changed to efavirenz, lopinavir, ritonavir, and tenofovir DF (tenofovir was used for the treatment of co-infection with hepatitis B virus). The HIVRNA levels remained <50 copies/ml during all this period and the CD4 count slowly increased. A repeat endoscopy after six weeks revealed persistent mass in the duodenum with slight decrease in size. Repeat biopsies were negative for CMV. One year later an endoscopy confirmed resolution of the mass and at that time the CD4 count was 179 cells/μl.
Figure 1.
Endoscopic view of the inflammatory mass seen in the duodenum (on the left side).
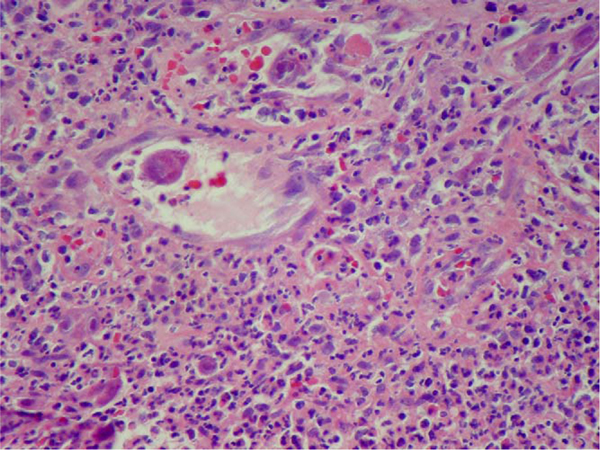
Figure 2.
Vascular proliferation at the periphery of the inflammatory mass with viral cytopathic effect in endothelial cells (H&E × 400).
Figure 3.
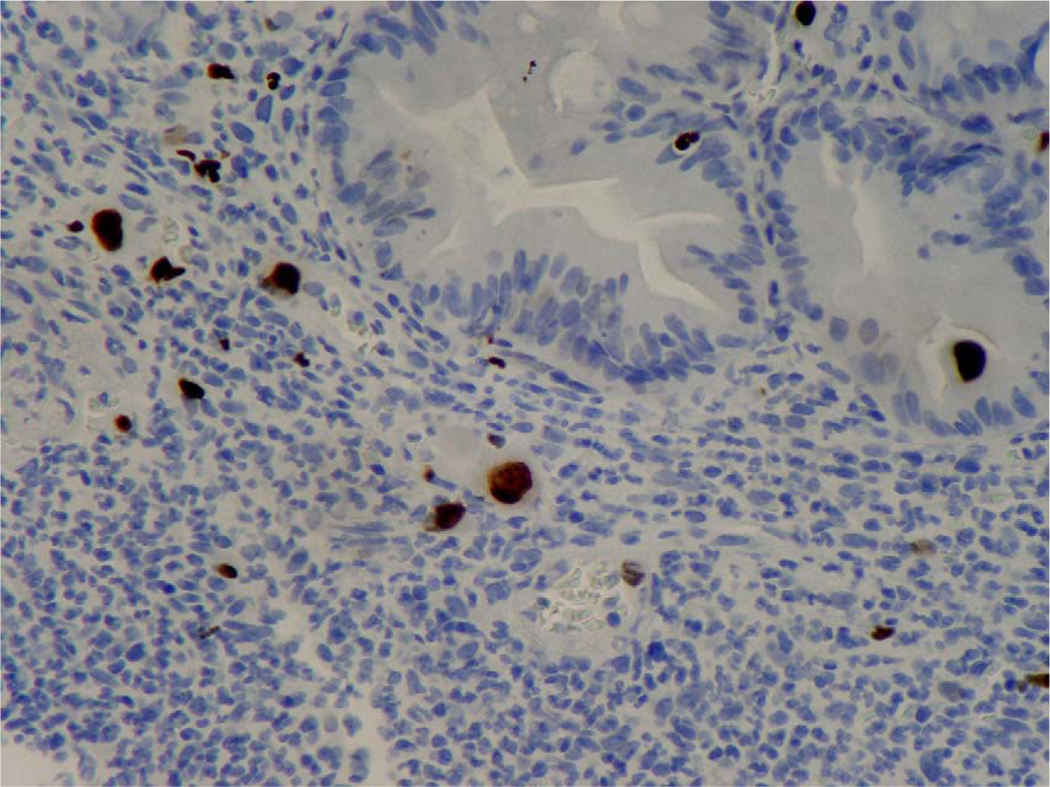
Cytomegalovirus (CMV) intranuclear inclusions in endothelial cells and epithelial cells of duodenal crypts. Immunohistochemical staining using a CMV monoclonal antibody (Dako CCH2/DDG9) against CMV early antigen was positive (×400).
3. Discussion
CMV infection of the alimentary tract is common in immunocompromised patients, including patients with AIDS.2,17,18 The infection may affect any part of the gastrointestinal tract from the esophagus to the rectum, either by diffuse or isolated involvement. It can produce perforation or present subclinically as an incidental pathologic finding with or without any associated opportunistic pathogens. It may also involve the pancreas, gallbladder, and liver.19,20
CMV involvement of the duodenum is a rare entity. Isolated CMV duodenitis has been described in patients with HIV infection.3,4 Inflammation of the ampulla of Vater secondary to CMV infection has been reported,5,21 but usually in association with AIDS and cholangiopathy.22 As in patients with CMV involving other portions of the gastrointestinal tract, patients with CMV duodenitis are at risk for developing complications such as bleeding, perforation, and peritonitis. Early treatment with appropriate antiviral agents may be essential before these complications ensue. However, the clinical entity of CMV-associated gastrointestinal pseudotumors has not been reviewed previously and we summarize the available scientific evidence for the first time, to our knowledge.
4. Literature review
4.1. Search strategy and selection criteria
All previous cases included in our literature review were found using a PubMed search (1980–November 2008) of the English-language medical literature applying the terms ‘CMV infection’, ‘inflammatory mass’, ‘pseudotumor’, and ‘gastrointestinal tract’. The references cited in these articles were examined to identify additional reports.
4.2. Results
Our review of the literature yielded a total of 25 cases with CMV-associated gastrointestinal pseudotumors, 14 of which were AIDS patients (Table 1)6–15 and 11 non-HIV patients (Table 2), including five immunocompetent patients,21,23,24 four patients after organ transplantation,25–28 one patient with common variable immunodeficiency syndrome,29 and one patient with chronic renal failure.30
Table 2.
Cytomegalovirus pseudotumors of the gastrointestinal tract in patients without HIV infection
| Author (year) [Ref.] | Location | Age | Sex | Immunocompromised status | Clinical picture | Method of diagnosis | Characteristics of pseudotumor | Outcome | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Garcia (1987)23 | Stomach antral area | 44 | M | No history of immunosuppression except concurrent heterophil-negative mononucleosis; the HIV status was not reported | Fever, abdominal pain | Endoscopy. Positive serology for CMV | Rigid narrowed gastric antrum and a deformed duodenal bulb suggestive of antral neoplasia. Histologic examination revealed ulcerated gastric mucosa and CMV inclusions in endothelial cells, macrophages, and rare epithelial cells | The pseudotumor remitted spontaneously and showed radiographic improvement without surgery or antiviral therapy | No specific treatment |
| Roberts (1989)25 | Esophagus | NR | NR | Patient with a renal transplant; no other information was given | NR | NR | CMV-positive cells in a biopsy specimen of an esophageal mass | NR | NR |
| Lewis-Jones (1990)26 | Colon | 53 | M | Renal transplantation on maintenance immunosuppression (azathioprine and prednisolone) | Masquerading as colonic neoplasia: epigastric pain and diarrhea for 2 months | Barium enema. Surgery (excision biopsy) | 5 cm long eccentric stricture involving the proximal transverse colon with mucosal irregularity and ulceration. Histology of a 4 × 2 cm ulcer revealed multiple nuclear and cellular inclusions characteristic of CMV with normal mucosa and no signs of malignancy | NR | Surgery |
| Cheung (1993)21 | Duodenum (ampulla of Vater) (2 cases) | NR | NR | Non-immunocompromised patients | Obstructive jaundice | Endoscopy | Obstructive jaundice as a result of exuberant formation of granulation tissue, fibrosis, inflammation at the ampulla of Vater, resembling malignancy and resulting in bile flow obstruction. Histologic identification of CMV inclusion bodies and positive immunohistochemical staining with anti-CMV antibodies | NR | NR |
| Falagas (1996)30 | Colon | 57 | M | Chronic renal failure | Abdominal pain, bloody diarrhea, fever | Colonoscopy. Exploratory laparotomy | Large polypoid mass at the hepatic flexure of colon. Large cell with intranuclear inclusions and submucosal fibrosis | Symptoms resolved after 2 weeks of treatment | Ganciclovir for 2 weeks |
| Shrestha (1996)37 | Colon. Not a true mass just pseudo-obstruction (Ogilvie) | 69 | F | 10 weeks post renal transplantation | Acute colonic pseudo-obstruction with symptoms of abdominal pain, diarrhea, fever, weight loss | Sigmoidoscopy. Positive CMV antigenemia and anti-CMV IgM | Inflamed rectum and sigmoid colon. Biopsy showed intranuclear inclusions | NR | Ganciclovir |
| Diaz-Gonzalez (1997)27 | Colon (midtransverse colon) | 57 | F | 14 months after heart transplantation on cyclosporine, azathioprine | Abdominal pain, diarrhea, fever | CT. Colonoscopy. Right hemicolectomy | Thickening of the colon surrounding the base of ulcer in mid transverse colon, which lead to formation of stricture. The pathologic specimen showed CMV inclusion bodies with acute suppurative ulceration | At 14 months of follow-up the patient had no evidence of rejection or CMV infection | Surgery. Ganciclovir for 2 weeks after surgery |
| Crespo (1998)28 | Colon (descending colon) | 62 | M | Heart transplantation on cyclosporine, azathioprine and prednisone | Abdominal pain, constipation, and hematochezia of 12 h in duration | Colonoscopy. Barium enema. Left colectomy | Colonoscopy revealed a friable, 6 cm stenosing lesion in the left colon and endoscopic biopsies revealed deep ulcers with frequent fibrin thrombi, large fibroblasts, and endothelial cells with the intranuclear inclusions typical of CMV infection. Immunohistochemical staining with anti-CMV antibodies was positive | Patient underwent left colectomy but had persistent symptoms and he underwent a repeat colonoscopy. He was placed on foscarnet and his subsequent course was satisfactory | The patient was treated with a 4-week course of intravenous ganciclovir and hyperimmune anti-CMV globulin for 1 week. After surgery the patient was again treated with intravenous ganciclovir for 3 weeks. Due to persistent symptoms, resistance to ganciclovir was suspected, and the patient was treated for 3 weeks with foscarnet (60 mg/kg every 8 h) |
| Tahan (2000)29 | Stomach, small bowel | 40 | M | Common variable immunodeficiency syndrome | Abdominal pain, fatigue, and weight loss. Bowel obstruction developed later, which lead to surgery | Endoscopy. Surgery (excision biopsy) | Biopsy specimens showed intranuclear CMV inclusions and staining with both hematoxylin eosin and immunofluorescence methods for CMV was strongly positive. The CMV-DNA test on mononuclear cells in blood by nested PCR was also found to be positive | After two months of therapy, there was both a clinical improvement and regression in the size of both esophageal and gastric ulcers. This was regarded as a partial response to therapy | IV ganciclovir for 2 months |
| Maiorana (2003)24 | Colon (ascending colon) | 84 | F | Hypertension, non-immunocompromised | Abdominal pain and diarrhea of 1-week duration, with fever. Peritoneal inflammation secondary to bowel perforation was present | Colonoscopy. Excision biopsy (right hemicolectomy) | Friable, stenosing lesion in ascending colon. Microscopic examination showed chronic inflammatory infiltrates and ulcerations with necrotic material and granulation tissue. Numerous intranuclear inclusions were detected in macrophages, stromal cells, and isolated endothelial cells. Positive immunohistochemical reaction to anti-HCMV antibody. Positive PCR of histological sections for CMV | Patient recovered without any recurrence of pain or diarrhea and was doing well on follow up visit 2 weeks and 3 months after discharge, then she was lost to follow-up | Surgery (Right hemicolectomy) Antiviral treatment (ganciclovir) was administered for 2 weeks and was tolerated |
| Maiorana (2003)24 | Colon (Sigmoid colon) | 57 | M | Hypertension and depression, non-immunocompromised | Painful abdominal cramps associated with diarrhea and fever for 5 days | Rectosigmoidoscopy. Surgery | Intraluminal, polypoid, soft, broad based, vegetant ulcerated mass that measured 5 × 2 cm and occupied one half of the intestinal lumen and mass and was strongly suggestive of carcinoma. Positive immunohistochemical reaction to anti-HCMV antibody. Positive PCR of histological sections for CMV | Patient had no evidence of recurrence of CMV colitis or of neoplastic disease after a follow-up period of 4 years | Surgery. The patient was treated for 2 weeks with intravenous ganciclovir and tolerated the treatment |
M, male; F, female; NR, not reported; CT, computed tomography; CMV, cytomegalovirus; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; PCR, polymerase chain reaction.
The distribution of pseudotumors in the gastrointestinal tract seems to parallel the predilection of CMV for the esophagus and right colon/ileocecal area.10 In our review of 14 cases, the colon was the most commonly involved area (42.9% of cases),9–11,13,16 followed by the antrum of the stomach (21.4% of cases),7,12,14 the esophagus (14.3% of cases),8,12 the gastric fundus (7.1% of cases),10 and the gastric pylorus (7.1% of cases).6 Isolated small-bowel involvement with CMV was noticed in one case.15
Most of the reports of AIDS patients who developed gastrointestinal CMV pseudotumor acquired HIV infection through homosexual transmission, similar to our case (8/12).7,10–12,16 In three cases opportunistic infections had developed prior to CMV infection,10,12 whereas in two cases there was co-infection in the lesion with cryptosporidia14 and Mycobacterium avium complex.12
4.3. Pathogenesis of CMV pseudotumor
The pathogenesis of CMV pseudotumor remains unclear. The thickening of the bowel wall in patients with CMV is thought to be caused histologically by submucosal edema, granulation tissue, and fibrosis.31 More specifically, the histologic characteristics of the pseudotumor include inflammatory infiltration in the mucosa and submucosa, characteristic inclusion bodies mostly in endothelial cells, fibroblasts and epithelial cells, with associated extensive fibrosis, granulation tissue, and chronic inflammation (Table 1). These changes may result in a gastrointestinal inflammatory mass capable of causing bowel obstruction and mimicking exophytic neoplastic luminal mass lesions on barium studies, endoscopy, and CT scans.
Some authors propose that CMV is a secondary superinfection of previously damaged tissues.21 Except for a few cases, information regarding the duration of AIDS, concurrent opportunistic infections and malignancies, response to therapy, and survival were not available from the reports of CMV-induced pseudotumors reviewed herein. Interestingly, other pathogens (cryptosporidia, mycobacteria) were also detected in the inflammatory pseudotumor in two cases.12,14 Thus it remains unclear if these pathogens are superinfection to an inflammatory process that has already been initiated by an unknown mechanism. Other authors believe that CMV is a primary enteric pathogen since CMV has been detected with immunohistochemistry in certain cells.13,21 In all reported cases reviewed herein, documentation of CMV infection was done either by typical inclusion bodies seen histologically or by immunohistological staining. CMV most commonly infects mesenchymal cells and tends to target endothelial cells, while glandular or surface epithelium cells are infrequently affected.25,32 In one case, culture of the biopsy from a pseudotumor grew CMV.16 Although epithelial cell involvement often lacks an inflammatory reaction, mesenchymal cell involvement is often accompanied by patchy nonspecific infiltration of acute and chronic inflammatory cells. Exuberant formation of granulation tissue and inflammation in response to CMV could theoretically be responsible for formation of a CMV-induced inflammatory mass or pseudotumor. Consistent with this theory is our finding that in all the cases of CMV-induced pseudotumors described in the literature in patients with AIDS, the predominant cells where CMV inclusions were identified were stromal and epithelium cells, whereas in CMV-induced ulcerations the predominant cells involved are usually endothelial cells (Table 1).
Autopsy findings on CMV-associated gastrointestinal masses in simian immunodeficiency virus (SIV)-infected macaques33 revealed that histologically the masses were composed of hyperplastic glandular tissue, dense neutrophilic infiltrates within the lamina propia, and frequent cytomegalic cells with intranuclear inclusions in affected regions. Immunohistochemistry demonstrated frequent CMV immunopositive cells within affected areas. Furthermore, immunohistochemistry for the proliferation marker Ki-67 demonstrated increased proliferation in hyperplastic glands and crypts.33 The authors in this study concluded that CMV should be considered a cause of discrete mass lesions in the gastrointestinal tract of SIV-infected macaques.33
In the cases listed above it is not possible to establish whether the CMV infection occurred as a primary event or as a reactivation, partly due to limitations of serological studies in immunocompromised patients and partly since serological studies were not performed in most of the described cases. However, since the frequency of CMV reactivation has been shown to increase linearly with age because of the decline of immunity,34 the occurrence of large intestinal inflammatory pseudotumors might represent an advanced stage of a previously unidentified CMV infection.11 Further studies are needed to elucidate the pathogenesis of CMV-induced pseudotumors.
4.4. Clinical manifestations
CMV infection of the bowel typically presents as enterocolitis, with chronic diarrhea, fever, abdominal pain, weight loss, melena, and hematochezia.1 Perforations, massive hemorrhage, and toxic megacolon have also been reported.35,36
The most common clinical symptom of CMV-induced gastrointestinal pseudotumors in patients with AIDS was abdominal pain, which was present in 71.4% of cases in our series.7,8,10–14,16 Fever,6,7,9,10,12,16 change of bowel habits,7,9,11,13,14 and weight loss11,13,14,16 were present in 42.9% (6/14), 35.7% (5/14), and 28.6% of the cases, respectively, and a palpable mass was present in one case.10 CMV pseudotumor presented as a non-obstructing mass in 78.6% of the cases, as a small bowel obstruction in 14.2% (2/14) of cases,11,15 and in 7.1% (1/14) it presented as obstruction of the gastric pylorus.6 In two cases the CMV pseudotumor was diagnosed in the setting of gastrointestinal bleeding.11,15
4.5. Diagnostic methods
Routine hematoxylin and eosin staining of biopsy specimens supplemented by immunohistochemical stains is the diagnostic method of choice and was done in all the cases reviewed herein, including our case. From a review of 14 reported cases of CMV-induced gastrointestinal pseudotumors in HIV-positive patients, serology was used for diagnosis of CMV infection in only one case.10 We could not find any data on the use of PCR or detection of CMV antigen in these cases. Thus, although serum analysis is a widespread method for the diagnosis of CMV infection, it has not been used widely as a tool for the diagnosis of CMV-induced pseudotumors. The fact that PCR of the blood in our case was negative for CMV could represent a more indolent chronic CMV infection at the tissue level, which may not necessarily be associated with CMV viremia. Positive PCR for CMV does not always translate to end-organ involvement in HIV patients, and histology is the gold standard for the diagnosis of end-organ involvement of CMV infection in this group of patients. Viral culture for CMV is unreliable and may be positive in the absence of invasive CMV disease. Of the cases reviewed it was only available in two cases.12,16 More experience is clearly needed regarding the use of conventional methods of diagnosis of CMV infection such as PCR and serologic studies in cases of CMV-induced pseudotumors in HIV patients.
4.6. Treatment
Treatment of CMV infection of the gastrointestinal tract is well established and usually includes treatment with ganciclovir,2 however data regarding the treatment of CMV-induced pseudotumors are sparse. Of the 14 AIDS cases reviewed, five patients were treated medically with ganciclovir;8,10,12,16 in two of those cases the patients were treated with both surgery and ganciclovir.10 Two additional cases were initially treated with ganciclovir but treatment was changed to foscarnet due to side effects.12 No information with regards to medical treatment was given in the remainder of the reviewed cases. The CMV-induced pseudotumor often responds to antiviral therapy but only intravenous antiviral therapy has been described.8,10,12,16 We describe the first case of an AIDS patient with CMV pseudotumor responding to oral treatment with valganciclovir with complete resolution of the CMV mass. The increasing CD4 count most likely contributed significantly to the resolution of the CMV mass, but it is difficult to determine in vivo the relative contribution of oral valganciclovir versus the immune reconstitution effect in the resolution of the CMV mass. A repeat endoscopy one week after completion of antiviral treatment revealed slight reduction in the size of the mass. Given the lack of data regarding the natural history of CMV pseudotumors it is difficult to assess if the reduced size was a treatment effect or a response to immune reconstitution.
A diagnostic surgical approach was performed in eight cases (57.1%),7,9–11,13–15 while for one case no data on treatment were available.6 Operative intervention was elective in all but two patients, in whom emergency surgery was necessary for bowel and pyloric outlet obstructions.6,11 Both of these patients died as a result of postoperative complications (Table 1).
The duration of treatment in HIV-positive patients varied from 3 weeks in one case12 to 8 weeks in another case (6 weeks of ganciclovir and 2 weeks of foscarnet),12 while the treatment duration was not reported in three cases.8,10,16
Of the 11 non-HIV patients who developed CMV-induced gastrointestinal pseudotumor, ganciclovir was given to three patients,29,30,37 surgery was performed in one case,26 while in four cases the patients were treated with both surgery and ganciclovir.24,24,27,28 Data on treatment were not reported in three cases (Table 2).21,23,25 In patients without HIV infection the duration of medical treatment varied from 2 to 8 weeks.29 Information regarding the duration of AIDS, concurrent opportunistic infections, HAART therapy, and associated malignancies, or response to therapy and survival was not consistently available for comparison.6,8,9
4.7. Outcomes
HIV patients with CMV masses have a grim prognosis, and poor outcome (death or relapse) was documented in 63% of the cases with available data.6–8,11,12,14,15 In one case, similar to our case, symptoms abated and the focal mass appeared to resolve endoscopically following treatment with ganciclovir alone.10 Thus, in our opinion, and based on the sparse available data, surgical intervention should be reserved for patients whose lesions fail to respond to medical therapy alone or those who present with a surgical indication such as outlet obstruction.
Recurrence of the CMV pseudotumors was noted at the anastomic site in one patient who required a second laparotomy for resection of the recurrent mass 14 months after the original resection.10 Only one case of gastric pseudotumor in an immunocompetent patient remitted spontaneously and showed radiographic improvement without surgery or antiviral therapy.23 No deaths were reported in the non-AIDS patients.
5. Conclusions
Although CMV has been described to involve any part of the gastrointestinal tract, localization to the small bowel and especially the duodenum is rare. We have described, to our knowledge, the first case report of isolated CMV-induced mass in the duodenum of a patient with AIDS. As the length of survival of patients with AIDS increases, CMV pseudotumors are not likely to remain unique. CMV pseudotumor should be included in the differential diagnosis of gastrointestinal mass lesions in AIDS patients and in other immunocompromised patients. The tumor often responds to antiviral therapy.8,10,12,16 We have described the first case of an AIDS patient with CMV pseudotumor responding to oral treatment with valganciclovir with complete resolution of the CMV mass. The role of surgery as treatment for a CMV-induced mass has not been clearly defined. It is hard to know what the role of immune reconstitution would be in this group of patients, and if resolution of the pseudotumor may be achievable by HAART therapy alone. Larger studies are needed to further study this entity and to outline treatment guidelines.
Footnotes
Conflict of interest
No conflict of interest to declare.
References
- 1.Drew WL. Cytomegalovirus infection in patients with AIDS. J Infect Dis 1988;158:449–56. [DOI] [PubMed] [Google Scholar]
- 2.Goodgame RW. Gastrointestinal cytomegalovirus disease. Ann Intern Med 1993;119:924–35. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox CM, Schwartz DA. Symptomatic CMV duodenitis. An important clinical problem in AIDS. J Clin Gastroenterol 1992;14:293–7. [PubMed] [Google Scholar]
- 4.Mong A, Levine MS, Furth EE, Laufer I. Cytomegalovirus duodenitis in an AIDS patient. AJR Am J Roentgenol 1999;172:939–40. [DOI] [PubMed] [Google Scholar]
- 5.Kim YS, Cho YD, Lee JS, Jin SY, Shim CS. Cytomegalovirus infection in an HIV patient with duodenal papillitis. Endoscopy 2007;39(Suppl 1):E23. [DOI] [PubMed] [Google Scholar]
- 6.Victoria MS, Nangia BS, Jindrak K. Cytomegalovirus pyloric obstruction in a child with acquired immunodeficiency syndrome. Pediatr Infect Dis 1985;4:550–2. [DOI] [PubMed] [Google Scholar]
- 7.Elta G, Turnage R, Eckhauser FE, Agha F, Ross S. A submucosal antral mass caused by cytomegalovirus infection in a patient with acquired immunodeficiency syndrome. Am J Gastroenterol 1986;81:714–7. [PubMed] [Google Scholar]
- 8.Wilcox CM, Diehl DL, Cello JP, Margaretten W, Jacobson MA. Cytomegalovirus esophagitis in patients with AIDS. A clinical, endoscopic, and pathologic correlation. Ann Intern Med 1990;113:589–93. [DOI] [PubMed] [Google Scholar]
- 9.Duva-Frissora AD. Abdominal case of the day. Cytomegalovirus pseudotumor of the cecum. AJR Am J Roentgenol 1991;156:1302–4. [DOI] [PubMed] [Google Scholar]
- 10.Rich JD, Crawford JM, Kazanjian SN, Kazanjian PH. Discrete gastrointestinal mass lesions caused by cytomegalovirus in patients with AIDS: report of three cases and review. Clin Infect Dis 1992;15:609–14. [DOI] [PubMed] [Google Scholar]
- 11.Wisser J, Zingman B, Wasik M, Duva-Frissora A, Beazley R, McAneny D. Cytomegalovirus pseudotumor presenting as bowel obstruction in a patient with acquired immunodeficiency syndrome. Am J Gastroenterol 1992;87:771–4. [PubMed] [Google Scholar]
- 12.Laguna F, Garcia-Samaniego J, Alonso MJ, Alvarez I, Gonzalez-Lahoz JM. Pseudotumoral appearance of cytomegalovirus esophagitis and gastritis in AIDS patients. Am J Gastroenterol 1993;88:1108–11. [PubMed] [Google Scholar]
- 13.Chow PK, Ho JM, Ling AE, Goh HS. CMV colitis masquerading as colon cancer—an unusual presentation of acquired immunodeficiency syndrome. Singapore Med J 1997;38:32–4. [PubMed] [Google Scholar]
- 14.Mohan H, Bal A, Garg S, Dalal U. Cytomegalovirus-associated pseudotumor simulating gastric malignancy in acquired immunodeficiency syndrome: a case report with review of literature. Jpn J Infect Dis 2007;60:134–6. [PubMed] [Google Scholar]
- 15.Dolgin SE, Larsen JG, Shah KD, David E. CMV enteritis causing hemorrhage and obstruction in an infant with AIDS. J Pediatr Surg 1990;25:696–8. [DOI] [PubMed] [Google Scholar]
- 16.Swansiger B, Orchard JL. A colonic mass lesion due to cytomegalovirus in an immunocompromised patient. J Clin Gastroenterol 1996;22:41–4. [DOI] [PubMed] [Google Scholar]
- 17.Francis ND, Boylston AW, Roberts AH, Parkin JM, Pinching AJ. Cytomegalovirus infection in gastrointestinal tracts of patients infected with HIV-1 or AIDS. J Clin Pathol 1989;42:1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson MA, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS). Clinical findings, diagnosis, and treatment. Ann Intern Med 1988;108:585–94. [DOI] [PubMed] [Google Scholar]
- 19.Sissons JG, Borysiewicz LK. Human cytomegalovirus infection. Thorax 1989;44: 241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galloway PG. Widespread cytomegalovirus infection involving the gastrointestinal tract, biliary tree, and gallbladder in an immunocompromised patient. Gastroenterology 1984;87:1407. [PubMed] [Google Scholar]
- 21.Cheung AN, Ng IO. Cytomegalovirus infection of the gastrointestinal tract in non-AIDS patients. Am J Gastroenterol 1993;88:1882–6. [PubMed] [Google Scholar]
- 22.Cello JP. Acquired immunodeficiency syndrome cholangiopathy: spectrum of disease. Am J Med 1989;86:539–46. [DOI] [PubMed] [Google Scholar]
- 23.Garcia F, Garau J, Sierra M, Marco V. Cytomegalovirus mononucleosis-associated antral gastritis simulating malignancy. Arch Intern Med 1987;147: 787–8. [PubMed] [Google Scholar]
- 24.Maiorana A, Torricelli P, Giusti F, Bellini N. Pseudoneoplastic appearance of cytomegalovirus-associated colitis in nonimmunocompromised patients: report of 2 cases. Clin Infect Dis 2003;37:e68–71. [DOI] [PubMed] [Google Scholar]
- 25.Roberts WH, Sneddon JM, Waldman J, Stephens RE. Cytomegalovirus infection of gastrointestinal endothelium demonstrated by simultaneous nucleic acid hybridization and immunohistochemistry. Arch Pathol Lab Med 1989;113: 461–4. [PubMed] [Google Scholar]
- 26.Lewis-Jones HG, Ward RG, Garvey C. Cytomegalovirus infection masquerading as colonic neoplasia. Br J Radiol 1990;63:573–4. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Gonzalez VM, Altemose GT, Ogorek C, Palazzo I, Pina IL. Cytomegalovirus infection presenting as an apple-core lesion of the colon. J Heart Lung Transplant 1997;16:1171–5. [PubMed] [Google Scholar]
- 28.Crespo MG, Arnal FM, Gomez M, Monserrat L, Suarez F, Rodriguez JA, et al. Cytomegalovirus colitis mimicking a colonic neoplasm or ischemic colitis 4 years after heart transplantation. Transplantation 1998;66:1562–5. [DOI] [PubMed] [Google Scholar]
- 29.Tahan V, Dobrucali A, Canbakan B, Hamzaoglu I, Ozaras R, Biyikli M, et al. Cytomegalovirus infection of gastrointestinal tract with multiple ulcers and strictures, causing obstruction in a patient with common variable immunodeficiency syndrome. Dig Dis Sci 2000;45:1781–5. [DOI] [PubMed] [Google Scholar]
- 30.Falagas ME, Griffiths J, Prekezes J, Worthington M. Cytomegalovirus colitis mimicking colon carcinoma in an HIV-negative patient with chronic renal failure. Am J Gastroenterol 1996;91:168–9. [PubMed] [Google Scholar]
- 31.Teixidor HS, Honig CL, Norsoph E, Albert S, Mouradian JA, Whalen JP. Cytomegalovirus infection of the alimentary canal: radiologic findings with pathologic correlation. Radiology 1987;163:317–23. [DOI] [PubMed] [Google Scholar]
- 32.Hinnant KL, Rotterdam HZ, Bell ET, Tapper ML. Cytomegalovirus infection of the alimentary tract: a clinicopathological correlation. Am J Gastroenterol 1986; 81:944–50. [PubMed] [Google Scholar]
- 33.Hutto EH, Anderson DC, Mansfield KG. Cytomegalovirus-associated discrete gastrointestinal masses in macaques infected with the simian immunodeficiency virus. Vet Pathol 2004;41:691–5. [DOI] [PubMed] [Google Scholar]
- 34.Kano Y, Shiohara T. Current understanding of cytomegalovirus infection in immunocompetent individuals. J Dermatol Sci 2000;22:196–204. [DOI] [PubMed] [Google Scholar]
- 35.Orloff JJ, Saito R, Lasky S, Dave H. Toxic megacolon in cytomegalovirus colitis. Am J Gastroenterol 1989;84:794–7. [PubMed] [Google Scholar]
- 36.DeRiso AJ, Kemeny MM, Torres RA, Oliver JM. Multiple jejunal perforations secondary to cytomegalovirus in a patient with acquired immune deficiency syndrome. Case report and review. Dig Dis Sci 1989;34:623–9. [DOI] [PubMed] [Google Scholar]
- 37.Shrestha BM, Darby C, Fergusson C, Lord R, Salaman JR, Moore RH. Cytomegalovirus causing acute colonic pseudo-obstruction in a renal transplant recipient. Postgrad Med J 1996;72:429–30. [DOI] [PMC free article] [PubMed] [Google Scholar]