Abstract
Background:
Psilocybin is a predominant agonist of 5HT1A and 5HT2A/C receptors and was first isolated in 1958, shortly before it became a controlled substance. Research on the potential therapeutic effects of this compound has recently re-emerged alongside what is being addressed as a psychedelic renaissance.
Methods:
In this paper we performed a systematic review of the clinical trials conducted so far regarding the therapeutic effects of psilocybin on psychiatric disorders. The eligibility criteria included clinical trials that assessed psilocybin's potential therapeutic effects on patients with psychiatric disorders. Nine hundred seven articles were found and screened in regard to the title, from which 94 were screened through abstract and 9 met the eligibility criteria and were included.
Results:
The papers published focused on 3 disorders: depression, obsessive-compulsive disorder (OCD) and substance use disorder (namely tobacco and alcohol). Psilocybin has shown a relatively safe profile and very promising results, with reductions found on most of the psychiatric rating scales’ scores. Research on depression showed the most solid evidence, supported by 3 randomized controlled trials. Studies on OCD and substance use disorder showed more limitations due to their open-label design.
Conclusions:
Altogether, the results from the studies reviewed in this paper suggest a substantial therapeutic potential. This calls for further research to confirm the results observed so far and further explain the underlying mechanisms.
Keywords: anxiety, depression, obsessive-compulsive disorder, psilocybin, substance-related disorders
Introduction
Psilocybin is a substituted indolealkylamine from the tryptamine group of compounds. Its main active metabolite, psilocin, is obtained after desphosphorylation of psilocybin in the intestinal mucosa.1,2 Psilocybin administration can lead to changes in perception, derealization, depersonalization, impaired attention, thought content disorder, symptoms of anxiety or elation and change of intuition.3 Psilocybin as well as psilocin are substances with predominant agonist activity on serotonin's 5HT1A and 5HT2A/C receptors,2 the latter being considered necessary for the hallucinogenic effects.4
Psilocybin can be found in over 100 species of mushrooms, many of them belonging to the genus Psilocybe,5 and their use dates back more than 3500 years in Mexico.6 However, psilocybin was first isolated only in 1958 by Albert Hofmann.7 Shortly after, it became marketed as Indocybin; however, clinical research on psilocybin was scarce, limited to anecdotal case-reports.8,9 As a result of the increasing widespread use of psychedelics in the 1960s, psilocybin research stopped almost completely after the Controlled Substances Act classified it as a Schedule I substance.10 Nevertheless, psychedelics, such as psilocybin, are one of the safest known classes of central nervous system drugs, showing a very low potential for addiction.2,11,12 A pooled analysis13 of 8 double-blind placebo-controlled studies with 110 healthy subjects receiving psilocybin suggested that the administration of moderate doses of psilocybin to healthy, high-functioning and well-prepared subjects in the context of a carefully monitored environment was associated with an acceptable level of risk. Given the risks associated with its use, Johnson and his colleagues have developed guidelines for research safety that include measures such as careful volunteer preparation and having a safe physical session environment.12
After decades of suspension, human hallucinogen research has resumed in what is being addressed as a Psychedelic Renaissance.14 In fact, by 2005, around 2000 subjects had undergone psychotherapy in clinical studies with psilocybin.15 In this paper, we review all the clinical trials conducted so far on the potential therapeutic effects of psilocybin on patients with psychiatric disorders.
Methods
The search was last performed in the PubMed database on the 5th of April 2019 for the term “psilocybin” on PubMed (search details: “psilocybin”[MeSH Terms] OR “psilocybin”[All Fields]). The eligibility criteria included clinical trials that assessed psilocybin's potential therapeutic effects on patients with psychiatric disorders. Studies were excluded if they were not in English, abstract was not available, they were studies with only healthy volunteers, they did not assess therapeutic effects, or they were secondary analyses. No further restrictions were imposed in regard to the type of intervention, outcomes, population of study or comparators. Nine hundred seven articles were found and screened in regard to the title, from which 94 were screened through abstract and 9 met the eligibility criteria and were included (see Fig. 1). A summary of the studies included is presented in Table 1.
Figure 1.
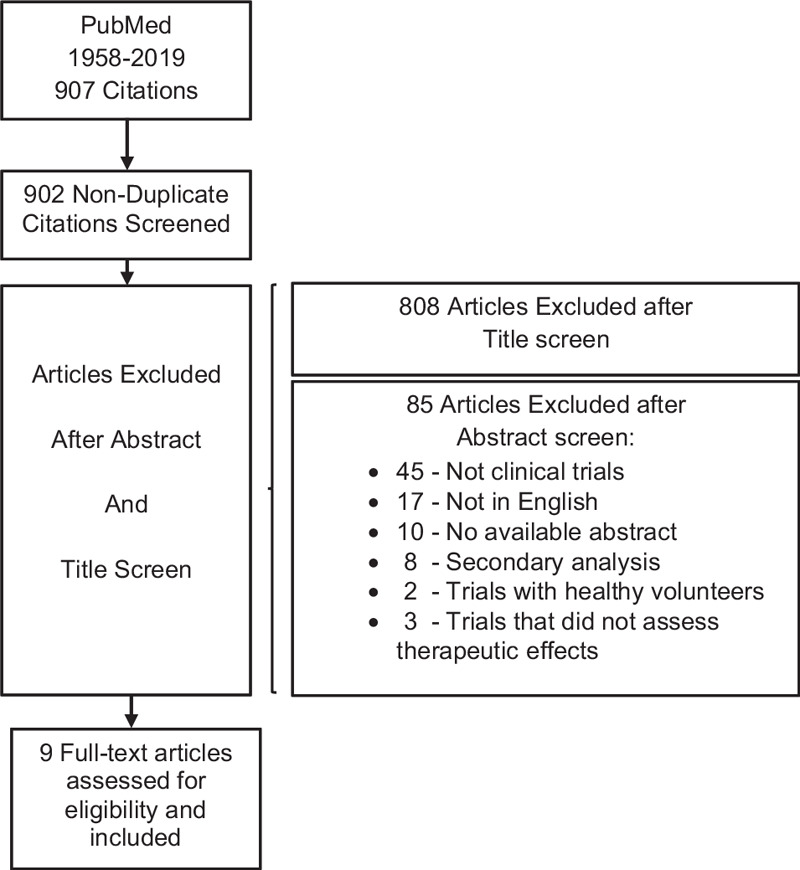
Flow of citations according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta).
Table 1.
Summary of the studies included in this systematic review
| Publication | Study design | Disorder/diagnosis | Sample | Intervention | Primary efficacy measures | Main findings |
|---|---|---|---|---|---|---|
| Grob et al 2011 | Randomized controlled double-blind with crossover | Advanced-stage cancer and anxiety | n = 12 | 2 sessions several weeks apart: | BDI for depression | BDI score reduction at 6 month follow-up |
| psilocybin (0.2 mg/kg) | Profile of Mood States Brief | STAI-T score reduction at 1 and 3 months follow up | ||||
| niacin (250 mg) | STAI-T and STAI-S for anxiety | |||||
| BPRS | ||||||
| 5-DASC | ||||||
| Ross et al 2016 | Randomized controlled double-blind with crossover | Depression and anxiety in life-threatening cancer | n = 29 | 2 dose sessions combined with psychotherapy, 7 weeks apart: | STAI-T and STAI-S for anxiety | Reductions on STAI-T, STAI-S, HADS-A, HADS-D, HAD-T and BDI 1 day, 2, 6 and 7 weeks post 1st psilocybin dose session |
| psilocybin (0.3 mg/kg) | HADS-A for anxiety; HADS-D for depression; HAD-T | Within group reductions on anxiety and depression at every time point until the last one—26 weeks post dose 2 | ||||
| niacin (250 mg) | BDI for depression | BDI 83% anti-depressant response vs 14% in the control group at 7 weeks post dose 1 | ||||
| Remission and Response rates | HAD-A 58% anxiolytic response vs 14% in the control group at 7 weeks post dose 1 | |||||
| BDI ∼85% of anti-depressant remission vs ∼15% in the control group at 7 weeks post dose 1 | ||||||
| Griffiths et al 2016 | Randomized controlled double-blind with crossover | Depression and anxiety in life-threatening cancer | n = 51 | 2 dose sessions + psychological support, ∼5 weeks apart: | GRID-HAMD-17 for depression | GRID-HAMD-17 higher response in high dose first group—92% vs 32% at the 5-week time-point after session 1 |
| psilocybin (1 or 3 mg/70kg) | HAM-A for anxiety | GRID-HAMD-17 higher remission in high dose first group—60% vs 16% at the 5-week time-point after session 1 | ||||
| psilocybin (22 or 30mg/70kg) | Remission and Response rates | HAM-A higher response in high dose first group—76% vs 24% at the 5-week time-point after session 1 | ||||
| HAM-A higher remission in high dose first group—52% vs 12% at the 5-week time-point after session 1 | ||||||
| Carhart Harris et al 2016 | Open-label, no control group | Treatment-resistant depression | n = 12 | 2 sessions combined with psychological support, 1 week apart: | QIDS for depression | QIDS score reduction at 1, 2, 3 and 5 weeks and 3 months post high dose session |
| 1st: psilocybin (10mg) | BDI for depression | BDI score reduction at 1 week and 3 months post high dose session | ||||
| 2nd: psilocybin (25mg) | STAI-T for anxiety | STAI-T score reduction at 1 week and 3 months post high dose session | ||||
| SHAPS for anhedonia | SHAPS score reduction at 1 week and 3 months post high dose session | |||||
| HAM-D for depression | HAM-D mean score (SD) reduction at 1 week post high dose session | |||||
| MADRS for depression | MADRS mean score (SD) reduction at 1 week post high dose session | |||||
| GAF—Global Assession of Function | Increase on MADRS mean score (SD) at 1 week post high dose session | |||||
| Remission and Response Rates | 8 of the 12 (67%) patients achieved complete remission at 1 week and 7 (57%) patients continued to meet criteria for response at 3 months | |||||
| GAF score increase at 1 week post high dose session | ||||||
| Carhart Harris et al 2017 | Follow-up, Open-label, no control group | Treatment-resistant depression | n = 20 | 2 sessions combined with psychological support, 1 week apart: | QIDS-SR16 | QIDS-SR16 score reduction at 1, 2, 3 and 5 weeks, 3 and 6 months post high dose session |
| 1st: psilocybin (10 mg) | BDI for depression | BDI score reduction at 1 week, 3 and 6 months post high dose session | ||||
| 2nd: psilocybin (25 mg) | STAI-T for anxiety | STAI-T score reduction at 1 week, 3 months and 6 months post high dose session | ||||
| SHAPS for anhedonia | SHAPS score reduction at 1 week and 3 months post high dose session | |||||
| HAM-D for depression | HAM-D mean score (SD) reduction at 1week post high dose session | |||||
| GAF—Global Assession of Function | GAF score increase at 1 week post high dose session | |||||
| Moreno et al 2006 | Open-label, dose-escalation | Obsessive-compulsive disorder | n = 9 | 4 escalating dose sessions ≥ 1 week apart: | Yale-Brown Obsessive Compulsive Scale | Combined baseline mean YBOCS scores, when stratified by dose groups, reduced 24 hours after psilocybin administration |
| 1st psilocybin 100 μg/kg | Visual Analog Scale (VAS) | from a range of 18.3 to 24.1 to a range of 10.7 to 11.3 | ||||
| 2nd psilocybin 200 μg/kg | ||||||
| 3rd psilocybin 300 μg/kg | ||||||
| psilocybin 25 μg/kg given randomly in a session after the 1st one | ||||||
| Bogenshutz et al 2015 | Open-label, no control group | Alcohol addiction | n = 10 | 2 sessions: at week 4 and at week 8, combined with psychotherapy | Timeline follow-back (TLFB) change in: | mean %HDD reduction on weeks 5–36 vs baseline |
| psilocybin (0.3 mg/kg) | - Percent of Heavy Drinking Days (%HDD) | mean %HDD reduction on weeks 5–36 vs weeks 1–4 | ||||
| psilocybin (0.4 mg/kg) | - Percent of Drinking Days (%DD) | mean %DD reduction on weeks 5–36 vs baseline | ||||
| mean %DD reduction on weeks 5–36 vs weeks 1–4 | ||||||
| Higher scores on HRS intensity subscale, 5D-ASC, and MEQ correlated with reduction on heavy drinking days | ||||||
| Johnson et al 2014 | Open-label, no control group | Tobacco addiction | n = 15 | 3 sessions at 5, 7 and 13-week time points, combined w/psychotherapy: | Timeline follow-back (TLFB) | 12 (80%) patients showed 7-day point prevalence abstinence at the 6-month follow-up |
| 1st: psilocybin (20 mg/70 kg) | Exhaled carbon monoxide (CO) | Reduction in mean self-reported daily smoking: ∼15 at intake vs ∼3 cigarettes a day at the 6-month follow-up | ||||
| 2nd: psilocybin (30 mg/70kg) | Urinary cotinine | |||||
| 3rd: psilocybin (30 mg/70 kg) | ||||||
| Johnson et al 2016 | Follow-up, open-label, no control group | Tobacco addiction | n = 15 | No intervention | Timeline follow-back (TLFB) | 10 (67%) patients biologically verified as smoking abstinent at the 12-month follow-up |
| Exhaled carbon monoxide (CO) | 9 (60%) patients biologically verified as smoking abstinent at the long-term follow up (∼30 months post-TQD) | |||||
| Urinary cotinine | Reduction in TLFB at 10 weeks, 6 months, 12 months, and a mean of 30 months (long-term follow-up) post-TQD |
Results
Depression
Anxiety and depression on life-threatening illness
On a randomized double-blind placebo-controlled crossover study,16 12 patients with advanced-stage cancer and reactive anxiety were recruited. They underwent 2 sessions in a comfortable environment, spaced several weeks apart, during which they randomly ingested 0.2 mg/kg of psilocybin, on 1 session, and 250 mg of niacin on the other. The main goals were to establish cardiac safety [through blood pressure (BP) and heart rate (HR) control], evaluate subjective experience during the sessions (with the 5-Dimensional Altered states of Consciousness Scale17) and follow-up with the Beck Depression Inventory (BDI),18 the Profile of Mood States (POMS)19 and the State-Trait Anxiety Inventory (STAI)20 efficacy measures.
All 12 participants completed the 3 month-follow up, 11 completed the 4-month follow-up, and 8 completed the 6-month follow-up. The mean score for BDI decreased at the 6-month follow-up (BDI mean ∼7.4) in comparison to baseline (BDI mean = 16.1). The mean STAI Trait anxiety score was lower than baseline (mean STAI ∼43.0) after 1 month (mean STAI ∼34.0) and after 3 months (mean STAI ∼32.6). No significant changes were found in POMS mean scores. Compared to niacin, psilocybin increased the HR and BP (Table 2). Additionally, no adverse psychological effects were observed and all subjects tolerated well the treatment sessions.16
Table 2.
Incidence of adverse effects attributable to psilocybin administration across the studies
| Publication | Sample | Intervention | Blood pressure (BP) increase | Heart rate increase | Headaches | Nausea or vomiting | Transient anxiety | Transient psychotic-like symptoms | Other |
|---|---|---|---|---|---|---|---|---|---|
| Grob et al 2011 | n = 12 | 2 sessions several weeks apart: | Mean peak systolic BP (SEM): 138.9 (6.4) mmHg | Mean (SEM) peak: 81.5 (5.8) bpm | – | – | – | – | No psychological adverse events registered |
| psilocybin (0.2 mg/kg) | Mean peak diastolic BP (SEM): 75.9 (3.4) mmHg | ||||||||
| niacin (250 mg) | |||||||||
| Ross et al 2016 | n = 29 | 2 dose sessions combined with psychotherapy, 7 weeks apart: | “Non-clinically significant elevation” in 76% | 28% | 14% | 17% | Transient thought disorder in 7% | – | |
| psilocybin (0.3 mg/kg) | peak mean systolic BP 142 mmHg | Mean peak: HR 71 bpm | Paranoid ideation in 3% | ||||||
| niacin (250 mg) | peak mean diastolic BP 83 mmHg | ||||||||
| Griffiths et al 2016 | n = 51 | 2 dose sessions + psychological support, ∼5 weeks apart: | Systolic BP (>160 mmHg): 34% in the high-dose session; 17% in the low-dose session | – | 6% in the high-dose session | 15% in the high-dose session | 26% in the high-dose session | 2% in the high-dose session | Physical discomfort: 21% in the high-dose session vs 8% in the low-dose session |
| psilocybin (1 or 3 mg/70 kg) | Diastolic BP (>100 mmHg): 13% in the high-dose session; 2% in the low-dose session | – | 0% in the low-dose session | 0% in the low-dose session | 15% in the low-dose session | 0% in the low-dose session | Psychological discomfort: 32% in the high-dose session vs 12% in the low-dose session | ||
| psilocybin (22 or 30 mg/70 kg) | |||||||||
| Carhart Harris et al 2016 | n = 12 | 2 sessions combined with psychological support, 1 week apart: | – | – | 33% | 33% | 100% | Transient paranoia in 8% | – |
| 1st: psilocybin (10 mg) | Transient thought disorder in 75% | ||||||||
| 2nd: psilocybin (25 mg) | |||||||||
| Carhart Harris et al 2017 | n = 20 | 2 sessions combined with psychological support, 1 week apart: | – | – | 40% | 25% | 75% | Transient paranoia in 15% | 1 patient became transiently uncommunicative |
| 1st: psilocybin (10 mg) | Visual hallucinations in 70% | ||||||||
| 2nd: psilocybin (25 mg) | |||||||||
| Moreno et al 2006 | n = 9 | 4 escalating dose sessions ≥ 1 week apart: | One subject (11%) experienced transient hypertension with peak BP values of 142/105 mmHg | – | – | – | – | – | – |
| 1st psilocybin 100 μg/kg | |||||||||
| 2nd psilocybin 200 μg/kg | |||||||||
| 3rd psilocybin 300 μg/kg | |||||||||
| psilocybin 25 μg/kg given randomly in a session after the 1st one | |||||||||
| Bogenshutz et al 2015 | n = 10 | 2 sessions: at week 4 and at week 8, combined with psychotherapy | Peak systolic BP: ∼150 mmHg | No significant changes | 50% | 10% | – | – | 1 patient reported insomnia on the night following a psilocybin session |
| psilocybin (0.3 mg/kg) | Peak dyastolic BP: ∼90 mmHg | 1 participant experienced diarrhea during 1 psilocybin session | |||||||
| psilocybin (0.4 mg/kg) | |||||||||
| Johnson et al 2014 | n = 15 | 3 sessions at 5, 7 and 13-week time points, combined w/psychotherapy: | Systolic BP mean peak (SD): 153 (11) mmHg | Mean peak (SD): 68 bpm | 80% | – | – | Transient thought disorder in 40% | – |
| 1st: psilocybin (20 mg/70 kg) | Diastolic BP mean peak (SD): 87(11) mmHg | ||||||||
| 2nd: psilocybin (30 mg/70 kg) | |||||||||
| 3rd: psilocybin (30 mg/70 kg) | |||||||||
| Johnson et al 2016 | n = 15 | No intervention | – | – | – | – | – | – | One participant showed decrease in well-being or life-satisfaction at the 12-month follow-up |
| reportedly due to re-experiencing traumatic childhood memories |
Another randomized double-blind placebo-controlled crossover study21 assessed the efficacy of a single dose of 0.3 mg/kg psilocybin vs 250 mg of niacin, both in combination with psychotherapy, to treat anxiety and depression in 29 patients with life-threatening cancer. The crossover occurred 7 weeks after dose 1 and patients were then followed for another 6.5 months. Primary outcome measures were assessed with self-reports on: STAI20 of State (STAI-S) and of Trait (STAI-T) subscales; Hospital Anxiety and Depression Scale (HADS)22 subscales of anxiety (HADS-A), depression (HADS-D) and total (HAD-T); and BDI.18 No serious adverse events were reported.
For the pre-crossover period, the psilocybin group showed statistically significant reductions on all 6 primary outcome measures at every time point, with large effect sizes (d ≥ 0.80). Similarly, at every time point after the second session, on every primary outcome measure, the psilocybin-first group reported significant within-group reductions on anxiety and depression scores. Clinically significant response rates were defined as a 50% or greater reduction in a score relative to baseline. Accordingly, at the 7-week time point post-dose 1, 83% of the subjects in the psilocybin-first group vs 14% in the niacin-first group met criteria for anti-depressant response (with BDI). Regarding anxiolytic response, for the same time point, 58% vs 14% (with HAD-A) and ∼75% vs ∼25% (with HAD-T) met criteria, favouring the psilocybin-first group. In addition, about 85% of psilocybin-first group vs ∼15% (with BDI) of the control group met criteria for anti-depressant remission, defined as 50% or greater reduction plus HADS-D ≤723 or BDI ≤12.24,25 The assessment of the Mystical Experience Questionnaire (MEQ 30)26 at the end of the first session showed a significant positive correlation with the scores, from baseline to 6 weeks after dose 1, on HADS-T (Spearman's r = 0.39), HADS-A (r = 0.36), HADS-D (r = 0.30), BDI (r = 0.49), STAI-S (r = 0.42), STAI-T (r = 0.39).
Thirdly, a randomized double-blind crossover study27 compared the effects of a low dose (1 or 3 mg/70 kg) vs a high dose (22 or 30 mg/70 kg) of psilocybin on 51 patients with life-threatening cancer and symptoms of depression or anxiety. The doses were administered in 2 sessions, 5 weeks apart. Preparatory meetings were conducted to establish rapport. The drug sessions took place in a living-room-like environment where psychological support was available. The primary outcome measures chosen were the GRID-Hamilton Depression Rating Scale (GRID-HAM-D-17)28 and the Structured Interview Guide for the Hamilton Anxiety Rating Scale (HAM-A).29 A clinically significant response was defined as ≥50% score decrease compared to baseline. Symptom remission was defined as ≥50% reduction from baseline scores plus a score of ≤7 on either GRID-HAMD or HAM-A.30,31 Fifteen secondary measures were also applied, including BDI,18 HADS22 and STAI.20
No serious adverse events were reported although some adverse events were registered. 5 weeks after session 1, 92% in the high-dose-first group vs 32% in the low-dose-first group showed clinically significant responses, according to GRID-HAMD-17 scores. For the same time point and outcome measure, 60% vs 16% of the participants showed symptom remission, favouring the high-dose-first group. According to the HAM-A scores, the high-dose-first group also presented larger response rates (76% vs 24%) and remission rates (52% vs 12%) comparing to the low-dose-first group. Furthermore, MEQ3026 scores were substantially correlated with score reductions in HADS Anxiety (−1.50), HADS Depression (−1.11), HADS Total (−2.62), and HAM-A (−3.93).
Treatment-resistant depression
An open-label study has investigated the feasibility and efficacy of psilocybin administration alongside psychological support on treatment-resistant depression.32 The study included 12 subjects with major depression of a moderate to severe degree (17+ on the 21-item Hamilton Depression Rating scale [HAM-D]), and no improvement after treatment with 2 adequate courses of antidepressants from distinct pharmacological classes, lasting at least 6 weeks within the current depressive episode. Every subject took an initial low dose of 10 mg and a high dose of 25 mg of psilocybin, 7 days apart. Previously, a 4-hour preparatory session with psychiatrists was provided. Dosing sessions took place in a pre-decorated room and patients were suggested to relax and listen to music while under supervision by 2 psychiatrists. To evaluate feasibility and safety, BP, HR, observer ratings of psilocybin's acute effects as well as the revised 11-Dimension Altered States of Consciousness questionnaire (11D ASC)33 were assessed. The primary outcome chosen for efficacy was the mean change in the severity of depressive symptoms, assessed with QIDS, from baseline to 1 week after the high-dose session. Additionally, the HAM-D, Montgomery-Åsberg Depression Rating Scale (MADRS),34 Global Assessment of Functioning (GAF),35 BDI,18 State-Trait Anxiety Inventory20 STAI-T (trait version), 16-item Quick Inventory of Depressive Symptoms [QIDS]36 and Snaith-Hamilton Pleasure Scale (SHAPS)37 were assessed.
One patient reported deterioration during the 3-month follow-up. However, mean QIDS scores were significantly lower from baseline to 1, 2, 3, 5 weeks and 3 months (final follow-up) after treatment. Maximum reduction was seen at 2 weeks, with a mean difference of −12.9 points compared to baseline. All patients showed reduced depression severity 1 week [mean BDI (SD) = 8.7 (8.4)] and 3 months [mean BDI (SD) = 15.2(11.0)] after the high dose session, in comparison to baseline [mean BDI (SD) = 33.7 (7.1)]. Taking into account the criteria for remission (BDI score of ≤9), 8 patients achieved complete remission 1 week after treatment. Furthermore, 7 patients continued to meet criteria for response (defined as a 50% BDI score reduction vs baseline) 3 months after treatment, from which 5 were still in remission at this point. STAI-T anxiety scores were significantly reduced 1 week [STAI-T mean score (SD) = 40.6(14.2)] and 3 months [STAI-T mean score (SD) = 54.8(14.5)] post high-dose session vs baseline scores [STAI-T mean score (SD) = 70.1(5.8)]. Similarly, SHAPS scores were significantly lower 1 week [mean SHAPS (SD) = 1.4(2.7)] and 3 months [mean SHAPS (SD) = 2.8(3.7)] post-treatment vs baseline [mean SHAPS (SD) = 7.5(3.7)]. HAM-D mean scores (SD) decreased from baseline [21.4(4.5)] to the 1-week follow-up [7.4(6.9)]. Significant reductions on mean MADRS (SD) [31.0 (5.0) at baseline to 9.7(9.8) 1 week post-treatment] were also reported alongside increases on Global Assessment of Function [50.3 (9.2) at baseline to 77.7(13.0) 1 week post-treatment].
Later, a 6-month follow-up study38 was conducted with a sample increase to 20 patients. Treatment with psilocybin was well tolerated and no serious adverse events were registered. 19 subjects completed all assessments. QIDS-SR16 scores were significantly decreased at all post-treatment time points, with maximum effect size seen at 5 weeks (−9.2, Cohen's d = 2.3), compared to baseline. BDI scores were significantly lower at 1 week (mean reduction = −22.7), 3 months (mean reduction = −15.3) and 6 months post-treatment (mean reduction = −14.9); STAI-T anxiety scores were significantly reduced 1 week (mean reduction = −23.8), 3 months (mean reduction = −12.2) and 6 months after treatment (mean reduction = −14.8); SHAPS anhedonia scores decreased 1 week (mean reduction = −4.6) and 3 months after treatment (mean reduction = −3.3); HAM-D scores dropped week post-treatment (mean reduction = −14.8); and GAF scores increased 1 week post-treatment (mean increase = +25.3). A significant reduction on the suicidality scores of QIDS-SR16 was seen 1 week (mean reduction = −0.9), 2 weeks (mean reduction = −0.85), 3 weeks (mean reduction = −0.8) and 5 weeks post-treatment (mean reduction = −0.7). Similarly, reductions were observed on the suicide item of the HAM-D, 1-week post-treatment (mean reduction = −0.95), at which point 16 of the 19 patients were scoring 0 and none was showing increase from baseline nor reaching the maximum score on this measure. After assessing relapse at 6 months, out of the 9 subjects that met response criteria at 5 weeks post treatment, 3 had relapsed, according to criteria of QIDS score of 6 or more.
Obsessive-compulsive disorder (OCD)
On a modified double-blind study39 that was set to evaluate the safety, tolerability and the potential therapeutic effects of psilocybin in OCD, 9 subjects who met criteria for current OCD were recruited. Inclusion criteria included at least 1 “treatment failure”, defined as a lack of significant improvement after an adequate treatment course with a serotonin reuptake inhibitor (SRI) for at least 12 weeks. Subjects were required to have tolerated well at least 1 prior exposure to indole-based psychedelics and took psilocybin in a dose escalation protocol in up to 4 sessions. The doses used were 25 (very low dose [VLD]), 100 (low dose [LD]), 200 (medium dose [MD]), and 300 (high dose [HD]) μg/kg of body weight. LD, MD, and HD were administered in that order, whereas the VLD was given randomly in a double-blind fashion at any session after the first dose (LD) session. During sessions, subjects wore eyeshades and listened to a standardized set of music in the presence of trained sitters. In order to evaluate obsessive-compulsive symptom severity, Yale-Brown Obsessive Compulsive Scale (YBOCS) and a visual analog scale (VAS) were used. In addition, the Hallucinogen Rating Scale (HRS)40 was assessed and vital signs were monitored.
Two subjects discontinued their participation after the first session (LD) due to discomfort with hospitalization. Regarding efficacy, a significant main effect of time on YBOCS scores was found, but no significant effect of dose or interaction of time and dose. Mean YBOCS scores immediately before (baseline) and 24 hours after psilocybin ingestion (T-24h) for each dose were as follows: 25 μg/kg (VLD), baseline = 18.29, T-24h = 11.14; 100 μg/kg (LD), baseline = 24.11, T-24h = 10.67; 200 μg/kg (MD), baseline = 19.57, T-24h = 11.00; 300 μg/kg (HD), baseline = 18.83, T-24h = 11.33. The combined baseline mean YBOCS scores, after stratification by dose groups, was significantly reduced 24 hours after administration, from a range of 18.3 to 24.1 to a range of 10.7 to 11.3. The comparison of baseline vs post-ingestion VAS scores for all doses combined was also statistically significant; however, no significant effect of time or dose was found on VAS scores for all doses combined. HRS total score and each of its subscales, with the exception of volition, showed a statistically significant linear main effect of dose.
Substance use disorder
Alcohol
An open-label study41 investigated the acute effects of psilocybin and its preliminary efficacy and safety in 10 subjects diagnosed with active alcohol dependence. Volunteers were required to have had at least 2 heavy drinking days (defined as ≥5 drinks/day for males and ≥4 drinks/day for females) in the past 30 days, to not being under treatment and to express concern about their drinking habit. Participants underwent 14 sessions of manualized intervention, including 2 psilocybin sessions. The other sessions consisted of 7 motivational enhancement therapy sessions, 3 preparation sessions and 2 debriefing sessions. Participants had to be abstinent and not in alcohol withdrawal. The drug sessions took place in a living-room-like environment, in the presence of 2 therapists. On week 4, participants took 0.3 mg/kg of psilocybin and, on week 8, 0.4 mg/kg. To measure the psilocybin's acute effects, the HRS Intensity subscale,40 the 5D-ASC17 (which includes MEQ26) and a Monitor Session Rating Form42 were assessed. Moreover, after each psilocybin session, the Addiction Research Center Inventory (ARCI), 49-item version,43 was evaluated. To evaluate substance use, the past 3-month version of Short Inventory of Problems (SIP)44 and Breath Alcohol Concentration (BAC) were assessed. The primary efficacy outcome measure was change in percent heavy drinking days (%HDD) at baseline and at weeks 5 to 12, assessed with the Time-Line Follow-Back procedure. Heavy drinking days were defined as days during which participants consumed ≥5 standard drinks, if the participant was male, or ≥4 standard drinks if the participant was female. A standard drink was defined as containing 14 g of alcohol. An analysis of the percent drinking days (%DD), defined as days during which participants consumed any amount of alcohol, was also performed. In addition, the following measures were assessed: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES 8A),45 the Alcohol Abstinence Self-Efficacy Scale (AASE),46 the Penn Alcohol Craving Scale (PACS)47 and the Profile of Mood States (POMS).19 Safety was controlled through vital signs.
One subject left the study with no justification provided and its data was not used. No serious adverse events were reported. Mean percent heavy drinking days (%HDD) dropped during weeks 5 to 12 (∼9%HDD) compared to baseline (∼35%HDD), and compared to weeks 1 to 4 (∼27%HDD)—during which only psychosocial treatment was provided. These %HDD reductions persisted from 13 to 24 weeks and from 25 to 36 weeks, compared to baseline values. The mean %HDD decrease observed at weeks 1 to 4 was not statistically significant, in comparison to baseline. On the other hand, the mean %DD showed significant reductions in every time point when compared to baseline (∼42%DD). For this measure, the mean (SD) reduction on weeks 5 to 12 was 27.2 (23.7) vs baseline, and 21.9 (21.8) vs weeks 1 to 4. The reductions reported on both mean %HDD and mean %DD, on weeks 1 to 4, were not significant in comparison to baseline. However, compared to weeks 1 to 4, these outcomes showed statistically substantial decreases at all time points. The only exception was %HDD at weeks 9 to 12, at which the reduction did not reach significance. Furthermore, significant correlations were found between measures of acute effects (HRS Intensity subscale, MEQ total and ASC summary score) and favorable changes regarding drinking, craving and self-efficacy (%HDD, %DD, PACS scores and AASE). In addition, higher scores on the HRS intensity subscale (r = −0.76), 5D-ASC (r = −0.89), and MEQ(r = −0.85) were correlated with fewer heavy drinking days.
Tobacco
An open-label pilot study researched the safety and preliminary efficacy of psilocybin on treating tobacco addiction.48 This study included 15 subjects that smoked a minimum of 10 cigarettes a day, that had multiple unsuccessful quit attempts and that still showed desire to stop smoking. In a 15-week treatment course, subjects attended weekly preparatory meetings in the first 4 weeks and took psilocybin on weeks 5, 7 and 13. Nevertheless, the last session was optional. A moderate dose (20 mg/70 kg) was administered in the first session and a high dose (30 mg/70 kg) was provided in the other 2 sessions; however, participants were allowed to repeat the moderate dose instead. A Target to Quit Date (TQD) was set for the same day as the first psilocybin session. Participants underwent cognitive behavior therapy as well as preparation for the psilocybin sessions. Treatment also included 2 components of an effective group based smoking cessation therapy.49 In addition, integration meetings and weekly support meetings were conducted after the TQD for 10 weeks. Short daily phone calls were made to participants for 2 weeks post-TQD to encourage abstinence. The primary efficacy outcome measures were the self-reported Timeline follow-back (TLFB),50 for retrospective number of cigarettes smoked per day, and the biological markers exhaled carbon monoxide (CO) and urinary cotinine levels. Safety was evaluated through BP and HR monitoring during psilocybin sessions. Additionally, post-session States of Consciousness Questionnaire42,51 ratings of acute adverse psychological effects, next-day headache ratings and Visual Effects Questionnaire data were assessed.
Three patients did not undergo the third session and 1 participant chose the moderate dose for the second psilocybin session. The remaining followed the default doses for each session. No clinically significant adverse events occurred. According to the TLFB and confirmed by CO and urinary cotinine measures, out of the 15 participants, 12 (80%) showed 7-day point prevalence abstinence at the 6-month follow-up. One of these 12 participants self-reported quitting on the TQD and was biologically verified as abstinent at all attended meetings, but was unable to attend the third psilocybin session or provide CO and urine samples for weeks 6 to 10 post-TQD. The other 11 participants self-reported quitting smoking on the TQD and showed biologically confirmed smoking abstinence up to 10 weeks post-TQD. Three of these 12 patients reported self-corrected lapses (defined as any discrete instances of smoking post-TQD) and 1 participant reported a relapse (defined as smoking on 7 or more consecutive days post-TQD52), after 13 weeks of continuous abstinence. Further analysis showed substantial reductions in mean self-reported daily smoking: ∼15 at intake vs ∼3 cigarettes a day at the 6-month follow-up. For the 3 participants that tested positive for smoking at the 6-month follow-up, an analysis on their TLFB assessments also revealed a significant reduction, from a reported mean of 20 cigarettes/day, at intake, vs 14 at the 6-month follow-up. Post-hoc testing for linear contrast showed significantly higher confidence to abstain from administration to the 6-month follow-up, and also reported significantly reduced craving and temptation to smoke across all time points.
Later, a long-term follow-up53 was conducted on the same 15 subjects. The primary outcome measures chosen were the CO, urinary cotinine, the TLFB50 and a Persisting effects questionnaire. Out of the 15 subjects, 3 did not complete the long-term (at a mean of 30months post-TQD) follow-up and were confirmed as daily smokers at the 12-month follow-up. For the latter time point, 10 (67%) participants were biologically verified as smoking abstinent from which 8 self-reported continuous abstinence since the TQD. At the long-term follow-up, 9 (60%) participants were biologically confirmed as smoking abstinent, from which 7 reported continuous abstinence since their TQD. Furthermore, statistically significant reductions in the self-reported TLFB were observed, in comparison to intake, at 10 weeks, 6 months, 12 months, and at a mean of 30months (long-term follow-up) post-TQD as follows: from a mean (SD) of 16.5 (4.3) cigarettes per day (CPD) at study intake, to 1.4 (3.8) CPD at 10 weeks; 2.7 (5.5) CPD at 6 months; 3.3 (6.5) CPD at 12 months; and 4.3 (6.6) CPD at the long-term follow-up.
Discussion
All studies included in this review have suggested that psilocybin has a favorable safety profile, being well tolerated in general (see Table 2). The most common adverse events reported were transient hypertension, anxiety and nausea, and headaches that were limited to the experimental sessions in the majority of cases. These events go in accordance with previous reports.13,42,54,55 The occurrence of adverse events was more often reported with higher doses of psilocybin27; however, out of all studies, there were no reports of serious adverse events and all adverse events were readily managed by the staff without the need of pharmacological intervention. The safety of psilocybin use is conditioned mainly by the individual's expectations and the surrounding environment, which explains the wide amplitude of subjective effects4 and the concern about the conditions under which drug sessions were conducted in many of the studies cited.
Regarding results, on depression and anxiety symptomatology, 4 studies16,21,27,32 consistently showed immediate and enduring anti-depressant and anxiolytic effects. Notably, results have shown BDI score reductions lasting as long as 6 months16 and reaching as high as 83% anti-depressant response and 85% remission rates (defined by BDI scores), 7 weeks following a single dose of psilocybin.21 Significant reductions were also reported on other depression measures such as QIDS, GRID-HAMD-17 or MADRS. Furthermore, significant evidence for anxiety symptom reduction was also observed on scales such as STAI and HAM-A, with reports of 76% and 52% of response and remission rates, respectively27 (defined by HAM-A scores). Regarding OCD, 1 open-label study has found symptomatic relief and significant reductions on YBOCS scores.39,56–58 On tobacco addiction, 2 open-label studies regarding the same experiment have showed abstinence on 80% of the subjects after 3 months and on 67% after 9 months from the final dose session. In addition, 60% were biologically verified as abstinent roughly 27 months after the last dose session. Nevertheless, these 2 studies did not differentiate the effects of moderate and high doses. In regard to alcohol abuse, the open-label study reported significant reductions on the percent of heavy drinking days and percent of drinking days to up to 28 weeks. However, it was not able to differentiate the effects of the 2 doses used. Although the level and quality of evidence for psilocybin on substance use disorder are low, the preliminary results published so far are promising and have motivated researchers to conduct larger controlled trials: a 50-participant study on psilocybin-facilitated smoking cessation treatment (NCT01943994), a 180-participant study on psilocybin-assisted treatment of alcohol dependence (NCT02061293), the first study on psilocybin-facilitated treatment for cocaine use (NCT02037126) and multiple studies on depression (NCT03775200, NCT03429075, NCT03715127, NCT03181529, NCT03380442, NCT03554174, NCT03866174) and OCD (NCT03356483, NCT03300947). Overall the results obtained across studies are impressive, given that few administrations show long lasting effects, well beyond the time-course of the acute drug effects. Moreover, the studies on different disorders have coherently shown a significant therapeutic effect.
The mechanisms underlying the effects measured are, however, yet to be confirmed and several explanations have been proposed. Given that the agonism of psilocybin on the 5-HT2A receptor is well-established, there is evidence supporting that it plays a role on the therapeutic effects reported, especially on depression. Some authors59 propose that one of the mechanisms through which psilocybin improves depression symptoms is by blocking the activity of inflammatory cytokines, namely TNF-α, whose levels have been found to be significantly higher in depressed patients.60 Research with fMRI in healthy volunteers after psilocybin administration has shown reduced activity in the medial Pre-Frontal Cortex (PFC) and decreased connectivity within the default mode network (DMN).61,62 This becomes more relevant given that depressive symptoms have been associated with increased activity in the medial PFC63,64 and that activity normalization of medial PFC has been shown with anti-depressant treatment.65–67 In the last paper regarding fMRI studies in patients with treatment-resistant depression under psilocybin treatment,68 increased DMN connectivity was observed 1 day after psilocybin administration. The authors proposed that, after psilocybin administration, DMN connectivity is reduced acutely and normalized afterwards with mood improvements, in a sort of “reset” mechanism. In the same study,68 a significant relationship was observed between reductions in amygdala cerebral blood flow (CBF) and reductions in depression symptomatology after psilocybin administration. Moreover, psycho-spiritual mechanisms have been proposed and explored before,51,69 as well as on several studies21,27,41,53 included in this review. Significant correlations have been found between mystical-type experiences and the outcomes. These mystical-type experiences are defined by feelings of positive mood, sacredness, a noetic quality, transcendence of time and space and ineffability.42 In one of the trials27 the correlations were still significant when the overall psilocybin effects’ intensity was controlled in a partial correlation analysis, suggesting that mystical-type experiences per se play an important role, independent from the overall intensity of psilocybin effects. A mediation analysis also suggested that mystical-type experiences mediated the therapeutic effects of psilocybin.
Regarding limitations, generalizability is limited since it includes 6 open-label studies. When it comes to OCD, the single open-label study's results39 should be analyzed carefully. Because even the lowest dose of psilocybin produced significant symptom reduction, this means that either there is a placebo effect present that cannot be measured for lack of a true placebo, or that psilocybin can be effective in such a low dose. Thus, further research on the effects of psilocybin in this disorder should try to clarify this by comparing it with a true placebo or a non-psychedelic active comparator. Furthermore, authors could not find a clear dose-response relationship to the change in YBOCS score nor correlation between YBOCS score reduction and the perceived psychedelic intensity. Additionally, the dose escalation protocol, which was also conducted on the tobacco addiction study, may have contributed to expectancy bias in both subjects and staff. Moreover, the need for staying at the hospital overnight may have introduced bias by selecting patients that could tolerate hospitalization. On alcohol dependence41 study, the lack of biological verification of alcohol use would decrease the bias risk of self-reported-only measures if conducted. Another limitation refers to the significant number of subjects across studies that reported previous psychedelic use, which contributes to expectancy bias. On the other hand, because psilocybin produces highly discriminable effects, blinding becomes a challenge, thus increasing the bias risk. The best option seems to be the use of an inactive low dose of psilocybin, as it showed some protection against monitor expectancy and it assures the benefit of the instruction that psilocybin is going to be administered on each session.27 Additionally, because psilocybin administration was associated with psychological support on most studies, it is not possible to make strong inferences regarding the extent of the effects. Nevertheless, the possibility of a synergistic interaction between the psilocybin administration and the psychological support is likely and should be explored.
Further trials with larger samples should be sought to confirm the results found so far, including research on the mechanisms underlying the effects reported. Altogether, the results from the studies reviewed in this paper suggest a very promising therapeutic potential from psilocybin. The results obtained so far, alongside the need for more effective psychiatric treatment, justify a call for further research.
Author contributions
Both authors, Henrique Castro Santos and João Gama Marques, have contributed to the study conception and design, including material preparation, data collection and analysis. The first draft of the manuscript was written by the first author and both authors commented on every version of the manuscript. Both authors read and approved the final manuscript.
Financial support and sponsorship
None.
Presentation
None.
Conflicts of interest
None.
Glossary
Abbreviations: %DD = percent of drinking days, %HDD = percent of heavy drinking days, 11D ASC = 11-dimension altered states of consciousness questionnaire, 5-DASC = 5-dimension altered states of consciousness questionnaire, 5HT = 5-hydroxytryptophan, AASE = alcohol abstinence self-efficacy scale, ARCI = addiction research center inventory, BAC = breath alcohol concentration, BDI = beck depression inventory, BP = blood pressure, CBF = cerebral blood flow, CO = carbon monoxide, CPD = cigarettes per day, DMN = default mode network, fMRI = functional magnetic ressonance imaging, GAF = global assessment of function, GRID-HAM-D-17 = 17-item grid-hamilton depression rating scale, HADS = hospital anxiety and depression scale, HADS-A = hospital anxiety and depression scale subscale of anxiety, HADS-D = hospital anxiety and depression scale subscale of depression, HADS-T = hospital anxiety and depression scale total, HAM-A = hamilton anxiety rating scale, HAM-D = hamilton depression rating scale, HD = high dose, HR = heart rate, HRS = hallucinogen rating scale, LD = low dose, MADRS = montgomery-åsberg depression rating scale, MD = medium dose, MEQ 30 = 30-item mystical experience questionnaire, OCD = obsessive compulsive disorder, PACS = penn alcohol craving scale, PFC = pre-prontal cortex, POMS = profile of mood states, QIDS = quick inventory of depressive symptoms, QIDS-SR16 = 16-item quick inventory of depressive symptoms-self report, SD = standard deviation, SEM = standard error of the mean, SHAPS = snaith-hamilton pleasure scale, SIP = short inventory of problems, STAI = state-trait anxiety inventory, STAI-S = state-trait anxiety inventory of state, STAI-T = state-trait anxiety inventory of trait, TLFB = timeline follow-back, TNF-α = tumor necrosis factor alpha, TQD = target to quit day, VAS = visual analog scale, VLD = very low dose, YBOCS = yale-brown compulsive scale.
References
- [1].Passie T, Seifert J, Schneider U, Emrich HM. The pharmacology of psilocybin. Addict Biol. 2002;7:357–364. [DOI] [PubMed] [Google Scholar]
- [2].Tylš F, Páleníček T, Horáček J. Psilocybin—summary of knowledge and new perspectives. Eur Neuropsychopharmacol. 2014;24:342–356. [DOI] [PubMed] [Google Scholar]
- [3].Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological affects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145–156. [DOI] [PubMed] [Google Scholar]
- [4].Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. [DOI] [PubMed] [Google Scholar]
- [5].Stamets P. Psilocybin Mushrooms of the World: An Identification Guide [Internet]. Ten Speed Press; 1996: 258 p. Available from: http://books.google.com/books?id=GAmfNNwHkjIC. Accessed November 2018. [Google Scholar]
- [6].Carod-Artal FJ. Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurol (Barcelona, Spain) [Internet]. 2015;30:42–49. Available from: http://www.sciencedirect.com/science/article/pii/S0213485311002696. Accessed November 2018 [DOI] [PubMed] [Google Scholar]
- [7].Hofmann A, Frey A, Ott H, Petrzilka T, Troxler F. Determination of the structure and synthesis of psilocybin. Experientia. 1958;14:397–399. [DOI] [PubMed] [Google Scholar]
- [8].Fisher G. The psycholytic treatment of a childhood schizophrenic girl. Int J Soc Psychiatry [Internet]. 1970;16:112–130. Available from: https://journals.sagepub.com/doi/abs/10.1177/002076407001600204. Accessed November 2018 [DOI] [PubMed] [Google Scholar]
- [9].Jorgensen EG. Further observations regarding hallucinogenic treatment. Acta Psychiatr Scand. 1968;43 (203S):195–200. [DOI] [PubMed] [Google Scholar]
- [10].Bogenschutz MP, Johnson MW. Classic hallucinogens in the treatment of addictions. Prog Neuro-Psychopharmacol Biol Psychiatry [Internet]. 2016;64:250–258. Available from: 10.1016/j.pnpbp.2015.03.002. Accessed November 2018 [DOI] [PubMed] [Google Scholar]
- [11].Nichols DE. Psychedelics. Barker EL, editor. Pharmacol Rev [Internet]. 2016;68:264–355. Available from: http://pharmrev.aspetjournals.org/lookup/doi/10.1124/pr.115.011478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnson MW. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22:603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. J Psychopharmacol (Oxford, England) [Internet]. 2011;25:1434–1452. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20855349. Accessed November 2018 [DOI] [PubMed] [Google Scholar]
- [14].Sessa B. Shaping the renaissance of psychedelic research. The Lancet [Internet]. 2012;380:200–201. Available from: 10.1016/S0140-6736(12)60600-X. Accessed November 2018 [DOI] [PubMed] [Google Scholar]
- [15].Metzner R. Reflections on the concord prison project and the follow-up study. J Psychoact Drugs. 1998;30:427–428. [DOI] [PubMed] [Google Scholar]
- [16].Grob CS, Danforth AL, Chopra GS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry [Internet]. 2011;68:71–78. Available from: http://archpsyc.ama-assn.org/cgi/content/abstract/68/1/71. Accessed December 2018 [DOI] [PubMed] [Google Scholar]
- [17].Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry [Internet]. 1998;31 (S2):80–84. Available from: http://www.thieme-connect.de/DOI/DOI?10.1055/s-2007-979351. Accessed December 2018 [DOI] [PubMed] [Google Scholar]
- [18].Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen psychiatry [Internet]. 1961;4:561–571. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13688369. Accessed December 2018 [DOI] [PubMed] [Google Scholar]
- [19].McNair DM, Lorr M, Droppleman LF. Profile of Mood States, POMS. San Diego, CA: Educ Ind Test Serv; 1981. [Google Scholar]
- [20].Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory. Menlo Park, CA: Mind Garden; 1970. Available from: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/210962 [Google Scholar]
- [21].Ross S, Bossis A, Guss J, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharmacol [Internet]. 2016;30:1165–1180. Available from: http://jop.sagepub.com/cgi/doi/10.1177/0269881116675512. Accessed December 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- [23].Hung C-I, Liu C-Y, Wang S-J, Yao YC, Yang CH. The cut-off points of the Depression and Somatic Symptoms Scale and the Hospital Anxiety and Depression Scale in detecting non-full remission and a current major depressive episode. Int J Psychiatry Clin Pract [Internet]. 2012;16 (March 2011):33–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22122659. Accessed December 2018 [DOI] [PubMed] [Google Scholar]
- [24].Reeves GM, Rohan KJ, Langenberg P, Snitker S, Postolache TT. Calibration of response and remission cut-points on the Beck Depression Inventory-Second Edition for monitoring seasonal affective disorder treatment outcomes. J Affect Disord. 2012;138:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Riedel M, Möller HJ, Obermeier M, et al. Response and remission criteria in major depression—a validation of current practice. J Psychiatr Res. 2010;44:1063–1068. [DOI] [PubMed] [Google Scholar]
- [26].Barrett FS, Johnson MW, Griffiths RR. Validation of the revised Mystical Experience Questionnaire in experimental sessions with psilocybin. J Psychopharmacol [Internet]. 2015;29:1182–1190. Available from: http://journals.sagepub.com/doi/10.1177/0269881115609019. Accessed December 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Griffiths RR, Johnson MW, Carducci MA, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: a randomized double-blind trial. J Psychopharmacol (Oxford, England) [Internet]. 2016;30:1181–1197. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27909165. Accessed December 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].ISCDD (2003) GRID-HAMD-17 Structured Interview Guide. San Diego, CA: International Society for CNS Drug. [Google Scholar]
- [29].Katherine Shear M, Bilt J, Vander, et al. Reliability and validity of a Structured Interview Guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress Anxiety. 2001;13:166–178. [PubMed] [Google Scholar]
- [30].Gao K, Wu R, Kemp DE, et al. Efficacy and safety of quetiapine-XR as monotherapy or adjunctive therapy to a mood stabilizer in acute bipolar depression with generalized anxiety disorder and other comorbidities: a randomized, placebo-controlled trial. J Clin Psychiatry. 2014;75:1062–1068. [DOI] [PubMed] [Google Scholar]
- [31].Matza LS, Morlock R, Sexton C, Malley K, Feltner D. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psychiatr Res. 2010;19:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carhart-Harris RL, Bolstridge M, Rucker J, et al. Psilocybin with psychological support for treatment-resistant depression: an open-label feasibility study. The Lancet Psychiatry [Internet]. 2016;3:619–627. Available from: 10.1016/S2215-0366(16)30065-7. Accessed December 2018 [DOI] [PubMed] [Google Scholar]
- [33].Studerus E, Gamma A, Vollenweider FX. Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One [Internet]. 2010;5:e12412.Available from: http://www.ncbi.nlm.nih.gov/pubmed/20824211. Accessed December 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stuart A, Montgomery N, Smeyatksky MR, Montgomery DB. Profiles of antidepressant activity with the Montgomery—Asberg Depression Rating Scale. Acta Psychiat Scand. 1985;72.Available from: https://pubmed.ncbi.nlm.nih.gov/2931947/. Accessed December 2018 [DOI] [PubMed] [Google Scholar]
- [35].Sorensen JL, Hargreaves WA, Friedlander S. Choosing a Global Assessment Scale to describe the functioning of children. Arch Gen Psychiatry [Internet]. 1984;41:1186.Available from: http://www.ncbi.nlm.nih.gov/pubmed/6508506. Accessed December 2018 [DOI] [PubMed] [Google Scholar]
- [36].Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. [DOI] [PubMed] [Google Scholar]
- [37].Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell PA. A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167 (JULY):99–103. [DOI] [PubMed] [Google Scholar]
- [38].Carhart-Harris RL, Bolstridge M, Day CMJ, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology [Internet]. 2017;235:399–408. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29119217. Accessed January 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moreno FA, Wiegand CB, Taitano EK, Delgado PL. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry [Internet]. 2006;67:1735–1740. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17196053. Accessed January 2019 [DOI] [PubMed] [Google Scholar]
- [40].Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen psychiatry. 1994;51:98–108. [DOI] [PubMed] [Google Scholar]
- [41].Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PC, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol [Internet]. 2015;29:289–299. Available from: https://ezp.lib.unimelb.edu.au/login?url=https://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=25586396&site=eds-live&scope=site%5Cnhttp://jop.sagepub.com/content/29/3/289.long. Accessed January 2019 [DOI] [PubMed] [Google Scholar]
- [42].Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–283. [DOI] [PubMed] [Google Scholar]
- [43].Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. [DOI] [PubMed] [Google Scholar]
- [44].Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (Drinc): an instrument for assessing adverse con- sequences of alcohol abuse. Test manual (Vol. 4). Rockville, MD: US Government Printing Office; 1995. [Google Scholar]
- [45].Miller WR, Tonigan JS. Assessing drinkers’ motivation for change: The Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES). Psychol Addict Behav. 1996;10:81–89. [Google Scholar]
- [46].DiClemente CC, Carbonari JP, Montgomery RP, Hughes SO. The Alcohol Abstinence Self-Efficacy Scale. J Stud Alcohol [Internet]. 1994;55:141–148. Available from: http://www.jsad.com/doi/10.15288/jsa.1994.55.141. Accessed January 2019 [DOI] [PubMed] [Google Scholar]
- [47].Flannery Ba, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–1295. [PubMed] [Google Scholar]
- [48].Johnson MW, Garcia-Romeu A, Cosimano MP, Griffiths RR. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol (Oxford, England) [Internet]. 2014;28 (September):0269881114548296-.Available from: http://jop.sagepub.com/content/early/2014/09/06/0269881114548296.abstract. Accessed January 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zernig G, Wallner R, Grohs U, Kriechbaum N, Kemmler G, Saria A. A randomized trial of short psychotherapy versus sustained-release bupropion for smoking cessation. Addiction. 2008;103:2024–2031. [DOI] [PubMed] [Google Scholar]
- [50].Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J (editors). Measuring Alcohol Consumption. Rockville, MD: Humana Press; 1992. p. 207–224. [Google Scholar]
- [51].Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology. 2011;218:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hughes J, Keely J, Niaura R, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res [Internet]. 2003;5:13–26. Available from: https://academic.oup.com/ntr/article-lookup/doi/10.1080/1462220031000070552. Accessed January 2019 [PubMed] [Google Scholar]
- [53].Johnson MW, Garcia-Romeu A, Griffiths RR. Long-term follow-up of psilocybin-facilitated smoking cessation. Am J Drug Alcohol Abus. 2017;43:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hollister LE. Effects of hallucinogens in humans. In Jacobs BL (editor). Hallucinogens: Neurochemical, Behavioral, and Clinical Perspectives. New York: Raven Press; 1984: pp. 19–33. [Google Scholar]
- [55].Johnson MW, Andrew Sewell R, Griffiths RR. Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug Alcohol Depend. 2012;123:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Leonard HL, Rapoport JL. Relief of obsessive-compulsive symptoms by LSD and psilocin. Am J Psychiatry. 1987;144:1239–1240. [DOI] [PubMed] [Google Scholar]
- [57].Moreno FA, Delgado PL. Hallucinogen-induced relief of obsessions and compulsions. Am J Psychiatry. 1997;154:1037–1038. [DOI] [PubMed] [Google Scholar]
- [58].Wilcox JA. Psilocybin and obsessive compulsive disorder. J Psychoact Drugs [Internet]. 2014; 46(5):393–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25364991. Accessed January 2019 [DOI] [PubMed] [Google Scholar]
- [59].Idell RD, Florova G, Komissarov AA, Shetty S, Girard RB, Idell S. The fibrinolytic system: a new target for treatment of depression with psychedelics. Med Hypotheses [Internet]. 2017;100:46–53. Available from: 10.1016/j.mehy.2017.01.013. Accessed January 2019 [DOI] [PubMed] [Google Scholar]
- [60].Dowlati Y, Herrmann N, Swardfater W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. [DOI] [PubMed] [Google Scholar]
- [61].Carhart-Harris RL, Erritzoe D, Williams T, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci [Internet]. 2012;109:2138–2143. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.1119598109. Accessed February 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Carhart-Harris RL, Leech R, Hellyer PJ, et al. The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci [Internet]. 2014;8:20.Available from: http://journal.frontiersin.org/Article/10.3389/fnhum.2016.00423/abstract. Accessed February 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct [Internet]. 2008;213:93–118. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18704495. Accessed February 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Farb NAS, Anderson AK, Bloch RT, Segal ZV. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol Psychiatry. 2011;70:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kennedy SH, Konarski JZ, Segal ZV, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164:778–788. [DOI] [PubMed] [Google Scholar]
- [66].Deakin JFW, Lees J, McKie S, Hallak JEC, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine. Arch Gen Psychiatry [Internet]. 2008;65:154.Available from: http://archpsyc.jamanetwork.com/article.aspx?doi=10.1001/archgenpsychiatry.2007.37. Accessed February 2019 [DOI] [PubMed] [Google Scholar]
- [67].Holtzheimer PE, Mayberg HS. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 2011;34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Carhart-Harris RL, Roseman L, Bolstridge M, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Reports [Internet]. 2017;7:13187.Available from: http://www.nature.com/articles/s41598-017-13282-7. Accessed February 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Garcia-Romeu A, Griffiths RR, Johnson MW. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr drug Abus Rev [Internet]. 2014;7:157–164. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1874-4737&volume=7&issue=3&spage=157. Accessed February 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


