Abstract
Background
COVID-19 pandemic-related disruptions to EUS-based pancreatic cancer surveillance in high-risk individuals remain uncertain.
Methods
Analysis of enrolled participants in the CAPS5 Study, a prospective multicenter study of pancreatic cancer surveillance in high-risk individuals.
Results
Amongst 693 enrolled high-risk individuals under active surveillance, 108 (16%) had an EUS scheduled during the COVID-19 pandemic-related shutdown (median length of 78 days) in the spring of 2020, with 97% of these procedures being canceled. Of these canceled surveillance EUSs, 83% were rescheduled in a median of 4.1 months, however 17% were not rescheduled after 6 months follow-up. Prior history of cancer was associated with increased likelihood of rescheduling. To date no pancreatic cancer has been diagnosed among those whose surveillance was delayed.
Conclusions
COVID-19 delayed pancreatic cancer surveillance with no adverse outcomes in efficiently rescheduled individuals. However, 1 in 6 high-risk individuals had not rescheduled surveillance, indicating the need for vigilance to ensure timely surveillance rescheduling.
Keywords: COVID-19, EUS, Pancreatic cancer, Surveillance
Introduction
The COVID-19 pandemic led to unprecedented disruptions to medical care worldwide, including cancer screening [1,2]. Significant delays in cancer screening due to the pandemic risks increased advanced cancer diagnoses [[3], [4], [5]], increased cancer-related deaths [6], and widened disparities in cancer screening and prevention [7]. Screening delays may have the largest impact on individuals with elevated cancer risk [8], including those with increased risk of pancreatic ductal adenocarcinoma (PDAC) due to a family history of PDAC and/or a defined genetic predisposition. Recent guidelines recommend consideration of annual PDAC surveillance in these high-risk individuals [9,10], with early studies demonstrating that PDAC surveillance increases resectability and overall survival for screen-detected PDACs [11,12].
How COVID-19 pandemic-related delays will affect patients at high-risk of PDAC remains uncertain. Therefore, herein we examine pandemic-related disruptions to PDAC surveillance in the prospective, multicenter Cancer of the Pancreas Sceening-5 (CAPS5) Study, including rescheduling efficiency, factors associated with surveillance delays, and the impact of delayed surveillance on clinical outcomes.
Methods
The CAPS5 Study (NCT02000089) is a prospective, multicenter study of PDAC surveillance in high-risk individuals, that has primary approval from the Johns Hopkins Institutional Review Board with site-specific approval from each participating institution. Enrollment for CAPS5 began in 2014, and eligibility included individuals at increased risk of PDAC due to genetic and/or familial risk factors (see Supplemental Methods for eligible high-risk groups). Enrolled participants undergo yearly pancreatic cancer surveillance with either EUS, MRI, or CT. We identified all enrolled CAPS5 participants who had an endoscopic ultrasound (EUS) scheduled during the initial COVID-19 pandemic shutdown. This shutdown period was defined on an institution-specific basis given variations in local restrictions as the time from initial implementation of procedural restrictions during the initial height of the COVID-19 pandemic in the spring of 2020 until lifting of stay-at-home orders with resumption of routine EUS surveillance (Table 1 ). All participating CAPS5 sites made attempts to reschedule surveillance for individuals whose surveillance EUSs were canceled due to the pandemic. For the purpose of this study, individuals under active surveillance were defined as enrolled CAPS5 participants who had prior pancreatic surveillance (EUS, MRI, or CT) within 2 years of the start of the pandemic plus those who had their initial surveillance procedure scheduled during the pandemic shutdown. Data were collected through December 1, 2020. Descriptive statistics were presented as medians and interquartile ranges (IQR) or as percentages. We compared characteristics between participants who rescheduled PDAC surveillance after a canceled EUS and those who did not. Continuous and categorical variables were compared using Wilcoxon rank-sum and Fisher’s exact tests, respectively. Likert scale data were visualized using stacked bar graphs, stratified by rescheduling status, and compared using Wilcoxon rank-sum tests.
Table 1.
Institution-specific routine EUS surveillance shutdown intervals due to the COVID-19 pandemic.
| Institution | City, State | Date of initial procedural restrictions | Date of routine EUS surveillance resumption | Pandemic shutdown duration (days) |
|---|---|---|---|---|
| Case Western Reserve University | Cleveland, OH | March 3rd | May 5th | 63 |
| Columbia University | New York, NY | March 19th | June 22nd | 95 |
| Dana Farber Cancer Institute/Brigham and Women’s Hospital | Boston, MA | March 16th | May 25th | 70 |
| Johns Hopkins University | Baltimore, MD | March 16th | June 1st | 77 |
| University of Michigan | Ann Arbor, MI | March 14th | June 1st | 79 |
| University of Pennsylvania | Philadelphia, PA | March 16th | June 4th | 80 |
| University of Pittsburgh | Pittsburgh, PA | March 23rd | May 11th | 49 |
| Yale University | New Haven, CT | March 16th | June 17th | 93 |
All dates are from the year 2020.
Results
Amongst 693 high-risk CAPS5 participants actively undergoing PDAC surveillance, 108 (16%) had an EUS scheduled during the COVID-19 pandemic shutdown, which lasted for a median of 78 days [IQR 68, 83 days] (Table 1). With pandemic-related procedural restrictions in place, 105 (97%) of these procedures were canceled, whereas 3 (3%) were performed as scheduled. Of the 105 participants with a canceled EUS, 87 (83%) were rescheduled as either an EUS (n = 73, 84%) or MRI (n = 14, 16%) during the approximately 6-month follow-up period. The median time between EUS cancelation and rescheduled EUS/MRI was 4.1 months [IQR 2.3, 5.7 months]. No individual with a rescheduled EUS/MRI was diagnosed with PDAC nor found to have a high-risk pancreatic lesion during the follow-up interval. Eighteen (17%) participants did not have rescheduled surveillance during the follow-up period.
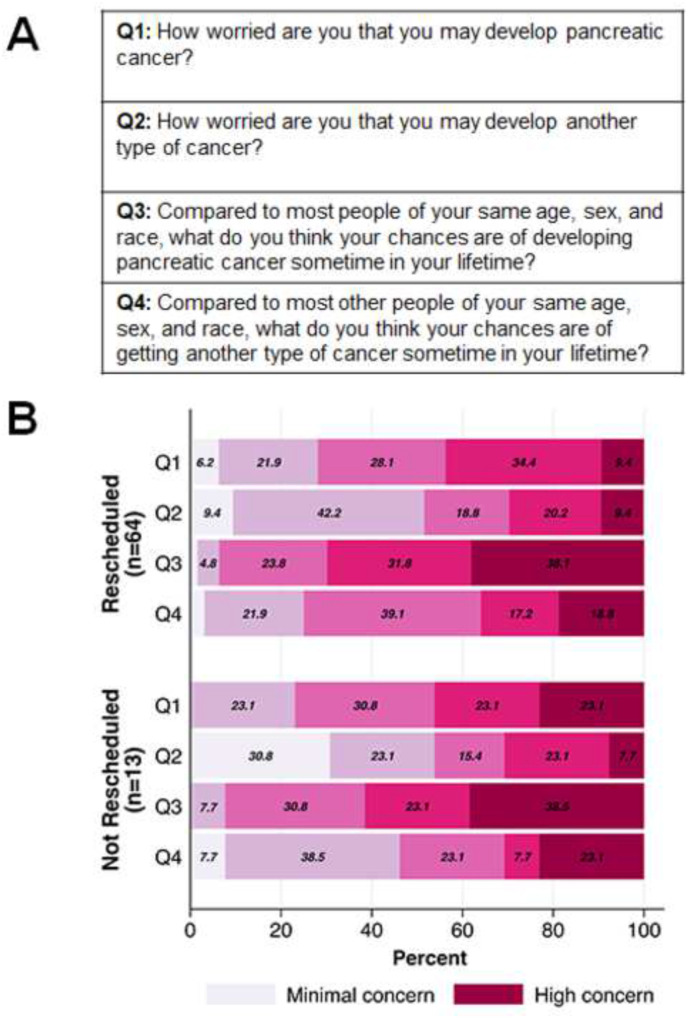
Individuals with a personal history of cancer were more likely to have a rescheduled procedure compared to those without a personal history of cancer (93% [39 of 42] versus 76% [48 of 63], p = 0.034, Table 2 ). Other characteristics between those with and without a rescheduled procedure, including age, race/ancestry, smoking/alcohol status, family history of PDAC, high-risk category, whether the canceled EUS was the participant’s first surveillance EUS, whether a pancreatic abnormality was identified on a prior surveillance exam, number of prior surveillance exams, and time in surveillance, were not significantly different between the groups. Survey questionnaire data about baseline concern for cancer risk were available on 77 (73%) individuals with canceled procedures, including 64 with, and 13 without, a rescheduled EUS/MRI. There were no significant differences in responses between participants who rescheduled and those who did not (each p > 0.05) (Fig. 1 ).
Table 2.
Participant characteristics by rescheduling status.
| Factor | Not Rescheduled (N = 18) | Rescheduled (N = 87) | p-value |
|---|---|---|---|
| Age, median (IQR) | 63.5 (58, 71) | 60 (55, 68) | 0.16 |
| Sex | 0.80 | ||
| Female | 10 (56%) | 52 (60%) | |
| Male | 8 (44%) | 35 (40%) | |
| Race | 0.12 | ||
| White | 17 (94%) | 80 (92%) | |
| Black | 0 (0%) | 7 (8%) | |
| Asian | 1 (6%) | 0 (0%) | |
| Smoking status | 0.28 | ||
| Current | 2 (11%) | 4 (5%) | |
| Former | 3 (17%) | 27 (31%) | |
| Never | 13 (72%) | 56 (64%) | |
| Alcohol use | 0.20 | ||
| Current | 4 (22%) | 37 (43%) | |
| Former | 2 (11%) | 13 (15%) | |
| Never | 12 (67%) | 37 (43%) | |
| Personal cancer history | 0.034 | ||
| No | 15 (83%) | 48 (55%) | |
| Yes | 3 (17%) | 39 (45%) | |
| Jewish ancestry | 0.23 | ||
| No | 11 (61%) | 67 (77%) | |
| Yes | 7 (39%) | 20 (23%) | |
| Relatives with PDAC | 0.19 | ||
| 0–1 | 2 (11%) | 28 (32%) | |
| 2 | 10 (56%) | 35 (40%) | |
| 3+ | 6 (33%) | 24 (28%) | |
| High-risk category | 0.40 | ||
| Familial PDAC without a known disease-causing gene variant | 12 (67%) | 42 (48%) | |
| Disease-causing gene variant (ATM, BRCA1, BRCA2, PALB2, or Lynch syndrome genes) with PDAC family history |
6 (33%) | 34 (39%) | |
| CDKN2A | 0 (0%) | 9 (10%) | |
| Other | 0 (0%) | 2 (2%) | |
| First surveillance EUS | 2 (11%) | 18 (21%) | 0.35 |
| Pancreatic abnormality on prior surveillance exam | 6 (33%) | 21 (24%) | 0.75 |
| Number of prior surveillance exams, median (IQR) | 2.5 (2, 4) | 2 (1, 4) | 0.46 |
| Time in surveillance (months), median (IQR) | 28.9 (12.3, 46.8) | 22.8 (10.0, 45.4) | 0.42 |
Fig. 1.
Baseline questionaireresponses from CAPS5 study participants with a canceled EUS. A) Questions answered by CAPS5 study participants during their initial PDAC surveillance procedure after enrollment. B) Questionnaire responses stratified by those with a rescheduled EUS/MRI (n = 64) compared to those without (n = 13).
Discussion
COVID-19 pandemic-related delays in cancer screening are widespread, and patients at high-risk of PDAC are no exception. Our study demonstrates the remarkable PDAC surveillance disruptions the COVID-19 pandemic produced in our multicenter high-risk cohort, with 97% of surveillance EUSs scheduled during the pandemic-related shutdown being canceled. While most of these participants (83%) had their surveillance rescheduled as an EUS or MRI within 6 months of procedural restrictions being lifted, it is notable that 1 in 6 participants did not yet have a surveillance EUS/MRI rescheduled.
We found individuals with a rescheduled EUS/MRI were more likely to have a prior history of cancer. This is consistent with data showing increased screening adherence amongst cancer survivors [13]. While extra steps should be taken to ensure all high-risk individuals are rescheduled for PDAC surveillance, extra attention may need to be paid to the sub-group without prior cancer. Furthermore, compliance with PDAC surveillance in high-risk individuals remains a significantly understudied area that merits dedicated longitudinal study to identify factors associated with surveillance compliance in high-risk individuals.
Whether screening delays in these high-risk patients are clinically relevant is also important. Amongst our cohort, with a median rescheduling time of 4.1 months, there were no PDACs detected nor high-risk lesions identified on subsequent surveillance. However, further longitudinal follow-up of high-risk individuals who have not yet rescheduled surveillance exams will be important to determine if longer delays in rescheduling are associated with negative clinical outcomes. Additionally, this data also brings up the question of whether yearly surveillance is the ideal interval for individuals at increased PDAC risk, and whether this interval should be increased. While our data may initially appear to support that increasing the surveillance interval may be safe, given the overall small number of patients analyzed and the overall low rate of PDAC conversion in surveillance populations [12], this data should be interpreted with caution. Ultimately, determining the ideal surveillance interval will require continued prospective data collection from high-risk individuals in large surveillance cohorts.
PDAC surveillance can be performed by either EUS or MRI [14]. In our cohort we observed that of patients with a canceled EUS who rescheduled pancreatic cancer surveillance, 16% chose to reschedule this surveillance as an MRI. Unlike EUSs, MRIs are not aerosolizing procedures and typically do not require pre-procedure COVID screening. Therefore, performing pancreatic cancer surveillance in high-risk individuals via MRI has potential benefits during the pandemic, and thus it is not surprising that some patients changed their surveillance modality to MRI.
Limitations of our study include the relative lack of racial diversity of the CAPS5 population, limited geographic diversity with most participating centers located in the northeastern United States, and lack of detail about why some high-risk individuals did not have a rescheduled surveillance procedure. Another limitation is that our analysis only focused on pandemic-related EUS cancelations. The pandemic shutdown had a much greater effect on the scheduling of endoscopic procedures compared to MRI [15], and EUS scheduling is more easily tracked than MRI, which is sometimes performed locally outside of CAPS5 centers.
With the potential for additional PDAC surveillance delays due to COVID-19, it is important to understand factors associated with delayed PDAC surveillance and the outcomes of these surveillance delays. It also remains critical to diligently monitor individuals from high-risk PDAC cohorts to ensure that their surveillance exams are rescheduled efficiently.
Acknowledgements
Smith Family Research Fund (BWK), ACG Junior Faculty Development Award ACG-JR-010-2020 (NM), Bowen-Chapman Family Fund (SS), NIH/NCI grant U01CA210170 (MG).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pan.2021.04.005.
Disclosures
BWK: Consulting (Exact Sciences), Travel (Janssen). SS: Consulting (Myriad Genetics, Inc.).
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Patt D., Gordan L., Diaz M., Okon T., Grady L., Harmison M. Impact of covid-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for american seniors. JCO Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakouny Z., Paciotti M., Schmidt A.L., Lipsitz S.R., Choueiri T.K., Trinh Q.D. Cancer screening tests and cancer diagnoses during the covid-19 pandemic. JAMA Oncol. 2021;7(3):458–460. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gathani T., Clayton G., MacInnes E., Horgan K. The covid-19 pandemic and impact on breast cancer diagnoses: what happened in england in the first half of 2020. Br J Canc. 2020;124(4):710–712. doi: 10.1038/s41416-020-01182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricciardiello L., Ferrari C., Cameletti M., Gaiani F., Buttitta F., Bazzoli F. Impact of sars-cov-2 pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turkington R.C., Lavery A., Donnelly D., Cairnduff V., McManus D.T., Coleman H.G. The impact of the covid-19 pandemic on barrett’s esophagus and esophagogastric cancer. Gastroenterology. 2021 doi: 10.1053/j.gastro.2021.01.208. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R. The impact of the covid-19 pandemic on cancer deaths due to delays in diagnosis in england, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carethers J.M., Sengupta R., Blakey R., Ribas A., D’Souza G. Disparities in cancer prevention in the covid-19 era. Canc Prev Res. 2020;13:893–896. doi: 10.1158/1940-6207.CAPR-20-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mckenna D.B., Dudzik C.M., Kumar S., Mahud N., Katona B.W. 2021. Covid-19 disruptions to endoscopic surveillance in lynch syndrome Cancer Prev Res (Phila) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goggins M., Overbeek K.A., Brand R., Syngal S., Del Chiaro M., Bartsch D.K. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the international cancer of the pancreas screening (caps) consortium. Gut. 2020;69:7–17. doi: 10.1136/gutjnl-2019-319352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daly M.B., Pilarski R., Yurgelun M.B., Berry M.P., Buys S.S., Dickson P. Nccn guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw. 2020;18:380–391. doi: 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]
- 11.Vasen H., Ibrahim I., Ponce C.G., Slater E.P., Matthai E., Carrato A. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long-term prospective follow-up studies from three european expert centers. J Clin Oncol. 2016;34:2010–2019. doi: 10.1200/JCO.2015.64.0730. [DOI] [PubMed] [Google Scholar]
- 12.Canto M.I., Almario J.A., Schulick R.D., Yeo C.J., Klein A., Blackford A. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology. 2018;155:740–751 e742. doi: 10.1053/j.gastro.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke T.C., Soler-Vila H., Fleming L.E., Christ S.L., Lee D.J., Arheart K.L. Trends in adherence to recommended cancer screening: the us population and working cancer survivors. Front Oncol. 2012;2:190. doi: 10.3389/fonc.2012.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harinck F., Konings I.C., Kluijt I., Poley J.W., van Hooft J.E., van Dullemen H.M. A multicentre comparative prospective blinded analysis of eus and mri for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505–1513. doi: 10.1136/gutjnl-2014-308008. [DOI] [PubMed] [Google Scholar]
- 15.Markar S.R., Clarke J., Kinross J., PanSurg Collaborative g. Practice patterns of diagnostic upper gastrointestinal endoscopy during the initial covid-19 outbreak in england. Lancet Gastroenterol Hepatol. 2020;5:804–805. doi: 10.1016/S2468-1253(20)30236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.