Abstract
Purpose
Our purpose was to establish the prevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in asymptomatic patients scheduled to receive radiation therapy and its effect on management decisions.
Methods and Materials
Between April 2020 and July 2020, patients without influenza-like illness symptoms at four radiation oncology departments (two academic university hospitals and two community hospitals) underwent polymerase chain reaction testing for SARS-CoV-2 before the initiation of treatment. Patients were tested either before radiation therapy simulation or after simulation but before treatment initiation. Patients tested for indications of influenza-like illness symptoms were excluded from this analysis. Management of SARS-CoV-2-positive patients was individualized based on disease site and acuity.
Results
Over a 3-month period, a total of 385 tests were performed in 336 asymptomatic patients either before simulation (n = 75), post-simulation, before treatment (n = 230), or on-treatment (n = 49). A total of five patients tested positive for SARS-CoV-2, for a pretreatment prevalence of 1.3% (2.6% in north/central New Jersey and 0.4% in southern New Jersey/southeast Pennsylvania). The median age of positive patients was 58 years (range, 38-78 years). All positive patients were white and were relatively equally distributed with regard to sex (2 male, 3 female) and ethnicity (2 Hispanic and 3 non-Hispanic). The median Charlson comorbidity score among positive patients was five. All five patients were treated for different primary tumor sites, the large majority had advanced disease (80%), and all were treated for curative intent. The majority of positive patients were being treated with either sequential or concurrent immunosuppressive systemic therapy (80%). Initiation of treatment was delayed for 14 days with the addition of retesting for four patients, and one patient was treated without delay but with additional infectious-disease precautions.
Conclusions
Broad-based pretreatment asymptomatic testing of radiation oncology patients for SARS-CoV-2 is of limited value, even in a high-incidence region. Future strategies may include focused risk-stratified asymptomatic testing.
Introduction
In spring 2020, the rapid spread of COVID-19 caused widespread concern throughout the country. At the peak of the pandemic, New Jersey had the second-highest incidence of cases and death per capita due to COVID-19, and a state of emergency was declared in March 2020. Although testing in early April was limited to symptomatic patients only, the positivity rate was over 50%, and by the middle of the month the COVID-19 deaths peaked at 412 deaths on April 13—over 20% of all COVID-19 related deaths in the country.1
Though cases and death rates throughout the United States were rising, there were no clear guidelines in place for testing asymptomatic patients with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Early data during the pandemic revealed that immunocompromised patients were a high-risk cohort and that patients with cancer were particularly vulnerable to COVID-19, making early detection invaluable for their oncologic care.2 However, even among academic societies for cancer care, there was no clear consensus on testing. American Society of Radiation Oncology began issuing clinical guidance in late March, with site-specific guidelines beginning in April.3 The Yale School of Medicine had also put forth their strategy for triaging patients, delaying treatment, using personal protective equipment (PPE), and limiting exposure to patients with the use of telehealth.4 Notably, however, oncologic societies did not comment on testing for asymptomatic patients.
This study aims to better clarify the utility of asymptomatic SARS-COV-2 testing in the radiation oncology setting. The logic underpinning an asymptomatic testing strategy is that it would not only prevent adverse outcomes among potentially immunosuppressed patients with cancer but would also mitigate the risk of asymptomatic transmission to other patients and staff who share the linear accelerator room environment. The limitations of such testing was also understood, in that it provides only a “snapshot” of infection status at a moment in time and is not protective or predictive of future infection. Therefore, our testing also did not take into account exposures after the first day of treatment. This study represents an unprecedented interinstitutional collaboration, deployed in record time, to address this crucial clinical question.
Methods and Materials
Implementation of asymptomatic testing strategy
In mid-April 2020, during the peak of symptomatic daily confirmed case incidence of COVID-19, the New Jersey Statewide Cancer Program Collaboration Group (NJCPCG) was formed to share best practices for the safe treatment of patients with cancer in the pandemic setting. A radiation-oncology subspecialty division of this group met weekly to discuss PPE use, sanitization practices, screening of patients, and preventative measures, as well as the treatment of patients with confirmed COVID-19. During this period (extending to June 1, 2020), state governors’ executive orders in the region (New Jersey, New York, Pennsylvania) restricted so-called “elective” surgeries. At the same time, increased testing capabilities were deployed within the region as a component of the US federal response.
The US Centers for Disease Control (CDC) was discouraging the practice of asymptomatic testing among the general public; however, there was increasing interest in testing asymptomatic patients with cancer before the initiation of treatment. Routine pretreatment testing was first deployed to inpatient (and, soon after, outpatient) chemotherapy patients with the rationale of identifying early infection in a potentially immunosuppressed population. With increased testing capacity, attention turned to outpatient radiation oncology, where the main rationale for pretreatment asymptomatic testing was preventing occult transmission to therapy staff and other patients who all share the same linear accelerator space. Although limitations of one-time testing were understood, by late-April 2020, most institutions in the NJCPCG had implemented an asymptomatic testing strategy to complement other widely adopted preventative measures (symptom and exposure questionnaires, limiting visits, social distancing, and PPE).
Institutional participation
This study represents a retrospective review of prospectively collected databases of asymptomatic patients tested for COVID-19 at 4 institutions participating in the NJCPCG: 2 academic medical centers (MD Anderson at Cooper University Hospital, Camden, NJ and Rutgers Cancer Institute of NJ, New Brunswick, NJ) and 2 community hospitals (Trinitas Health System, Elizabeth, NJ and Holy Redeemer Hospital, Medowbrook, PA). These institutions matched their COVID-19 testing databases to patient information, including demographic, disease-site, medical comorbidity, and treatment details. Databases were then deidentified, and interinstitutional contracting was executed to allow sharing of limited data sets and compilation at the coordinating institution (MD Anderson at Cooper University Hospital ) under an expedited retrospective institutional review board protocol (#20-437).
Patients who commenced outpatient radiation therapy during a 3-month period (April 27, 2020-July 31, 2020) and who had no symptoms of influenza-like illness and who underwent polymerase chain reaction testing for SARS-CoV-2 before the initiation of treatment were eligible for inclusion in this analysis. Patients who were tested for the indication of influenza-like illness symptoms, inpatients, and those who refused testing were excluded. Testing strategy varied among institutions and included testing of all patients or selected patients based on risk (nursing home patients, those undergoing chemotherapy, inability to wear a mask). Patients were tested before simulation, after simulation but before first treatment, and/or at selected intervals on-treatment, according to varying institutional capabilities. Patients who were found to be asymptomatic positive for COVID-19 were managed according to best practices, considering disease factors and evolving CDC guidelines.
Statistical analysis
The coordinating institution carried out statistical analysis of the limited data sets using R Core Team software (Vienna, Austria; http://www.R-project.org/). Two-tailed Fisher's exact test was used to assess whether the dependence of positive pretreatment COVID-19 results on an explanatory variable was statistically significant (α < 0.05). To analyze combination of variables, 2-tailed Fisher's exact test was used, but, for the contingency table, the independent variable was considered positive if both explanatory variables were positive. If either or both were negative, the independent variable was considered negative. This allowed analyzing multiple explanatory variables in combination to assess if having both increased the proportions of positive pretreatment COVID-19 results. The null hypothesis was that the relative proportions of the positive pretreatment COVID-19 results were independent of the explanatory variable.
Results
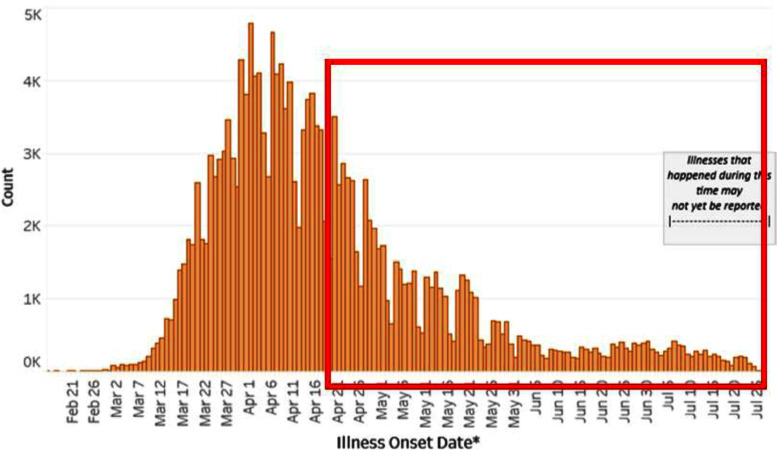
During a 3-month period, a total of 385 polymerase chain reaction nasal swab tests for SARS-CoV-2 were performed in 336 asymptomatic radiation oncology patients. According to the CDC, the 7-day average of daily confirmed cases in our region ranged from 3197 in late April to 295 in late July 2020 (Fig 1). The percent positivity rate ranged from 43% to under 2%.1 The demographics and medical comorbidities of the 336 analyzed patients are shown in Table 1. A majority of patients were ≥45 years (89.9%), approximately 20% were African American, 15% were Hispanic, and a majority (53.3%) had a comorbidity score >5.
Fig. 1.
Laboratory-confirmed cases of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in the state of New Jersey from February to July 2020 (source: www.cdc.gov). The red highlighted box indicates the period of time for the presented study.
Table 1.
Patient demographics (N= 336)
| Age (years) | ||
|---|---|---|
| Median (range) | 62 (4-89) | |
| N | % | |
| Age category | ||
| <45 y | 34 | 10.1 |
| 45-65 y | 178 | 53.0 |
| >65 y | 124 | 36.9 |
| Regional location | ||
| North/central New Jersey | 106 | 31.5 |
| Southern New Jersey | 201 | 59.8 |
| Pennsylvania | 29 | 8.6 |
| Treatment institution | ||
| CUH | 201 | 59.8 |
| HRH | 29 | 8.6 |
| R-CINJ | 67 | 19.9 |
| THS | 49 | 14.6 |
| Race | ||
| African American | 66 | 19.6 |
| Asian | 21 | 6.3 |
| White | 249 | 74.1 |
| Ethnicity | ||
| Hispanic | 49 | 14.6 |
| Non-Hispanic | 287 | 85.4 |
| Sex | ||
| Female | 191 | 56.8 |
| Male | 145 | 43.2 |
| Charlson comorbidity score | ||
| ≤5 | 157 | 46.7 |
| 6-10 | 134 | 39.9 |
| >10 | 45 | 13.4 |
Disease site, stage, and treatment information are presented in Table 2. The top 3 disease-sites for patients undergoing treatment were breast (27.1%), thoracic (18.5%), and genitourinary (12.8%) malignancies. In terms of stage, the overwhelming majority of patients were treated for locally advanced or metastatic disease (74.4%). Radiation therapy was largely external-beam treatment without brachytherapy (93.2%), and a majority of patients were undergoing some form of sequential and/or concurrent chemotherapy (56.8%).
Table 2.
Disease site and treatment specifications (n = 336)
| N | % | |
|---|---|---|
| Primary disease category | ||
| Central nervous system | 20 | 6.0 |
| Head and neck | 26 | 7.7 |
| Thoracic | 62 | 18.5 |
| Breast | 91 | 27.1 |
| Gastrointestinal | 29 | 8.6 |
| Genitourinary | 43 | 12.8 |
| Gynecologic | 27 | 8.0 |
| Other* | 38 | 11.3 |
| AJCC stage category | ||
| 0-1 | 82 | 24.4 |
| 2-3 | 123 | 36.6 |
| 4 | 127 | 37.8 |
| N/A | 4 | 1.2 |
| Treatment intent | ||
| Curative | 245 | 72.9 |
| Palliative | 91 | 27.1 |
| Role of radiation therapy | ||
| Definitive | 103 | 30.7 |
| Adjuvant | 128 | 38.1 |
| Neoadjuvant | 6 | 1.8 |
| Oligometastatic | 12 | 3.6 |
| Palliative | 87 | 25.9 |
| Type of radiation therapy planned | ||
| EBRT | 313 | 93.2 |
| Brachytherapy | 5 | 1.5 |
| EBRT + brachytherapy | 18 | 5.4 |
| Radiopharmaceutical | 0 | 0.0 |
| Role of chemotherapy | ||
| None | 145 | 43.2 |
| Concurrent | 63 | 18.8 |
| Sequential (prior) | 112 | 33.3 |
| Sequential + concurrent | 16 | 4.8 |
Abbreviations: AJCC = American Joint Committee on Cancer; EBRT = external beam radiation therapy.
Includes sarcoma, lymphoma, skin cancers, and benign conditions.
The testing strategy for the 385 tests that were performed as well as the testing results are shown in Table 3. Approximately 70% of patients tested were undergoing radiation therapy alone with no other patient risk factors. The overwhelming majority of patients were tested after simulation but before first treatment (67.8%), and most tests results were available within 24 to 72 hours (78.4%). A total of five positive tests were observed, for a pretreatment asymptomatic prevalence rate of 1.3%. The details and management strategy of the five positive patents are shown in Table 4. Initiation of treatment was delayed for 14 days with the addition of retesting for four patients, and one patient with locally advanced head and neck cancer was treated without delay but with additional infectious-disease precautions (therapists in full PPE, treatment at the end of the day with a terminal room clean).
Table 3.
COVID testing details
| N | % | |
|---|---|---|
| Radiation oncology-ordered tests: N = 385 | ||
| Rationale for testing | ||
| Routine (radiation therapy only) | 270 | 70.1 |
| Concurrent chemo-radiation therapy | 69 | 17.9 |
| Nursing facility/recent hospitalization | 8 | 2.1 |
| Previous positive COVID test | 1 | 0.3 |
| Unable to wear mask | 29 | 7.5 |
| Multiple aforementioned reasons | 8 | 2.1 |
| Timing of COVID testing | ||
| Presimulation | 75 | 19.5 |
| Postsimulation, pretreatment | 261 | 67.8 |
| On-treatment | 49 | 12.7 |
| Type of COVID test | ||
| PCR swab (outside laboratory) | 56 | 14.5 |
| PCR swab (on-site) | 329 | 85.5 |
| In-house antigen | 0.0 | |
| Sputum | 0.0 | |
| Time to COVID results | ||
| <24 hours | 47 | 12.2 |
| 24-72 hours | 302 | 78.4 |
| >72 hours | 36 | 9.4 |
| Radiation oncology patients: N = 336 | ||
| Prior COVID testing | ||
| None | 277 | 82.4 |
| Yes (asymptomatic) | 57 | 17.0 |
| Yes (symptomatic) | 1 | 0.3 |
| Prior COVID results | ||
| N/A | 277 | 82.4 |
| Negative | 56 | 16.7 |
| Positive | 2 | 0.6 |
| Radiation oncology-ordered tests: N = 385 | ||
| COVID result | ||
| Negative | 380 | 99.0 |
| Positive | 5 | 1.3 |
| Plan of care for COVID+ | ||
| N/A | 380 | 99.0 |
| Delay radiation therapy +/– retest | 4 | 1.0 |
| Proceed with radiation therapy + precautions | 1 | 0.3 |
| Radiation oncology patients: N = 336 | ||
| On-treatment retesting | ||
| None | 287 | 85.4 |
| Yes (asymptomatic) | 49 | 14.6 |
| Yes (symptomatic) | 0 | 0.0 |
| Interruption of radiation therapy course* | ||
| None | 277 | 82.4 |
| Yes (non-COVID-related) | 57 | 17.0 |
| Yes (COVID-related) | 1 | 0.3 |
Abbreviations: COVID = coronavirus disease; PCR = polymerase chain reaction.
Unplanned early termination of radiation therapy or interruptions >7 days.
Table 4.
Details of asymptomatic COVID+ patients
| COVID+ subject | Demographics | Region | Comorbidity score | Primary diagnosis | Triage strategy |
|---|---|---|---|---|---|
| 1 | 47 y white, Hispanic female | North/central | 2 | Stage 2, breast | Delay 14 d, retest |
| 2 | 38 y white, Hispanic female | North/central | 2 | Stage 1, GYN | Delay 14 d, retest |
| 3 | 78 y white, non-Hispanic female | North/central | 5 | Stage 2, GU | Delay 14 d, retest |
| 4 | 70 y white, non-Hispanic male | North/central | 8 | Stage 4, H&N | Treatment with additional precautions* |
| 5 | 58 y white, Hispanic male | South | 5 | Stage 3, lung | Delay 14 d, retest |
Abbreviations: COVID = coronavirus disease; GU = genitourinary; GYN = gynecologic; H&N = head and neck; PPE = personal protective equipment.
Treated at the end of the day with terminal cleaning. Staff with additional PPE.
Statistical analysis using univariate and multivariate Fisher's exact test to determine factors predictive of a positive test are presented in Table 5. Regional location (north/central New Jersey, P = .024) as well as Hispanic ethnicity (P = .036) were found to be statistically significant on univariate analysis. On multivariate analysis, patients treated with concurrent and/or sequential chemotherapy who were Hispanic (P = .005) or who had locally advanced disease (P = .019) were at highest likelihood for a positive asymptomatic test.
Table 5.
Univariate and multivariate analyses
| Univariate analysis using Fisher's Exact Test | ||||
|---|---|---|---|---|
| Variable | Odds ratio | P value | 95% confidence interval | |
| Ethnicity: Hispanic | 9.293 | .024 | 1.4-75.8 | |
| Regional location: North/central | 8.980 | .036 | 1.1-218.6 | |
| Primary disease stage: Locally advanced | 7.126 | .062 | 0.9-173.5 | |
| Regional location: South | 0.164 | .162 | 0.8-148.8 | |
| Role of radiation therapy: Definitive | 3.416 | .174 | 0.5-27.8 | |
| Type of radiation planned: EBRT + brachy | 4.618 | .242 | 0.2-37.5 | |
| Type of radiation planned: EBRT | 0.285 | .300 | 0.1-28.2 | |
| Primary disease category: H&N | 3.060 | .333 | 0.1-24.4 | |
| Primary disease category: GYN | 2.933 | .344 | 0.1-23.4 | |
| Chemotherapy: Yes | 3.080 | .395 | 0.3-152.6 | |
| Age: <45 y | 2.258 | .415 | 0.1-17.9 | |
| Multivariate analysis | ||||
|---|---|---|---|---|
| Variable 1 | Variable 2 | Odds ratio | P value | 95% confidence interval |
| Ethnicity: Hispanic | Chemotherapy: Yes | 17.596 | .005 | 2.6-144.3 |
| Primary disease stage: Locally advanced | Chemotherapy: Yes | 11.576 | .019 | 1.1-570.5 |
Abbreviations: EBRT = external beam radiation therapy; GYN = gynecologic; H&N = head and neck.
Discussion
Asymptomatic COVID-19 infections have been reported since the beginning of the pandemic, with the CDC estimating 40% of infections to be asymptomatic and a latency time of six days.5 Several prior studies have found variable asymptomatic rates ranging from 20% to 75% of cases, thus suggesting that isolation of symptomatic patients as the sole strategy of controlling the pandemic is likely to be ineffective.6,7 Specifically, Hellewell et al8 demonstrated that the probability of control decreases with an increase in asymptomatic transmission, a decrease in proper contact tracing, and a longer time from infection to isolation. Although masks have been shown to mitigate transmission among both symptomatic and asymptomatic individuals, prior studies have indicated the need to impose stricter contact tracing and testing regardless of symptoms, especially given that the spread of COVID-19 is largely driven by asymptomatic cases.9
Data on testing in asymptomatic patients with cancer remains scarce, and early studies have found the asymptomatic rate to be uncertain and highly variable. An early study from Germany tested 139 asymptomatic patients with cancer before beginning radiation treatment from April 17 to May 8 and found one positive patient, translating into a COVID prevalence rate of 0.72%.10 In another study by a hospital in Dubai, 85 asymptomatic patients with cancer were tested between March 13 and April 4. Seven patients (8.24%) of those tested were positive for COVID-19. All of them subsequently developed symptomatic disease.11 In a study by Lee et al12 from the United Kingdom, 1226 patients undergoing chemotherapy from April 3 to June 22 were tested, with an asymptomatic rate of 0.6%.
Other than our initial results published in abstract form,13 to our knowledge there have been no published results of asymptomatic testing in radiation oncology patients in the United States. Although asymptomatic cases contribute to a significant amount of transmission, our study indicates that blanket asymptomatic testing may not be the optimal strategy in reducing this transmission in this population. Rather, a more targeted approach based on high-risk factors may provide more value. There are several important factors that are associated with an increased risk for transmission and adverse outcomes. For example, minority populations have been shown to be disproportionately affected by the pandemic, with a higher rate of cases, hospitalizations, and deaths.14, 15, 16 In a study by Goyal et al,15 minority children were found to have higher rates of infection, whereas children with the highest median family income had lower rates of infection. Moreover, Kim et al16 showed that non-English speakers were not only less likely to have completed testing, but also had a 4.6-fold higher proportion of positive cases compared with English speakers. Finally, Muñoz-Price et al17 showed that black race, age greater than 60, and male sex were associated with positive COVID-19 tests in a study of 2595 patients.
In addition to race-based disparities in COVID-19 outcomes, cancer continues to be a negative risk factor for outcomes in patients with COVID-19. Specifically, Al-Shamsi et al18 showed that compared with the general population, patients with cancer not only had a significantly higher rate of coronavirus (29.4% vs 0.5%), but also that these patients were significantly more likely to be hospitalized (28.1% vs 10.4%). Furthermore, Pinato et al19 showed European patients with cancer with COVID-19 had a 33% mortality rate, and that male sex, age greater than 65, and the presence of fewer than two comorbidities before infection were significantly associated with the development of a complication from COVID-19.
Our study found limited utility with regard to generalized COVID-19 testing of radiation oncology patients despite the high incidence of cases in the area. Our study is in agreement with prior studies that found low positivity rates in asymptomatic patients with cancer, including the aforementioned study from Germany, which found a positivity rate of less than 1%.10 Additionally, in a recent study by Jan et al,20 which was performed at one of the institutions included in the current study during the same time-period, multiple surfaces were tested for SARS-COV-2 in a radiation oncology clinic, but zero positive samples were collected, thereby indicating the safety of radiation oncology clinics and the importance of treating without delay.
Although the strength of our study is that it is the only study detailing the results of asymptomatic testing in radiation oncology patients in the United States, it also has limitations. First, given that each institution had varying methods for determining risk and the need for COVID testing, our study may be limited by sampling bias. Nevertheless, the demographics of our patient population as seen in Tables 1 and 2 demonstrate that we have a representative sample of the radiation oncology population. Second, our study is limited by the fact that only a small percentage of patients tested positive, resulting in large 95% confidence intervals and limited statistical comparisons. However, the fraction of asymptomatic patients was hypothesized to be small, and the statistically significant findings in this study do warrant further consideration.
In summary, our study sought to determine the prevalence of asymptomatic COVID-19 rates in patients with cancer. We found a low pretreatment prevalence of 1.3%, thereby suggesting that broad-based pretreatment asymptomatic testing of radiation oncology patients for SARS-COV-2 is of limited value even in the setting of high community prevalence. Should there be another wave of infections, this study may help with implementing testing in asymptomatic patients, ultimately leading to a decrease in asymptomatic transmission and a reduction in disruptions in care. Although current measures to mitigate risk such as the use of masks, social distancing, and hand hygiene are important, future strategies should focus on targeted asymptomatic testing for high-risk patients, including those with advanced stage cancer, those in active chemotherapy, and underserved populations.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: none.
Research data are not available at this time.
References
- 1.COVID-19 NJ. NJ COVID-19 Hub. Available at: https://covid19.nj.gov/. Accessed May 25, 2021.
- 2.Kuderer NM, Choueiri TK, Shah DP. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ASTRO. COVID-19 clinical guidance. Available at:https://www.astro.org/Daily-Practice/COVID-19-Recommendations-and-Information/Clinical-Guidance. Accessed December 7, 2020.
- 4.Zaorsky NG, Yu JB, McBride SM. Prostate cancer radiation therapy recommendations in response to COVID-19. Adv RadiatOncol. 2020;5:659–665. doi: 10.1016/j.adro.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CDC. Pandemic planning scenarios. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. Accessed November 15, 2020.
- 6.Yanes-Lane M, Winters N, Fregonese F. Proportion of asymptomatic infection among COVID-19 positive persons and their transmission potential: A systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, Lau EHY, Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 8.Hellewell J, Abbott S, Gimma A. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng H-Y, Jian S-W, Liu D-P. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Int Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marschner S, Corradini S, Rauch J. SARS-CoV-2 prevalence in an asymptomatic cancer cohort - results and consequences for clinical routine. Radiat Oncol. 2020;15:165. doi: 10.1186/s13014-020-01609-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HO Al-Shamsi, Coomes EA, Alrawi S. Screening for COVID-19 in asymptomatic patients with cancer in a hospital in the United Arab Emirates. JAMA Oncol. 2020;6:1627–1628. doi: 10.1001/jamaoncol.2020.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LYW, Hill T, Topping O. Utility of COVID-19 screening in cancer patients. Cancer Cell. 2020;38:306–307. doi: 10.1016/j.ccell.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dragun AE, Modi C, Henson CF. A statewide multi-institutional study of asymptomatic pre-treatment testing of radiation therapy patients for SARS-CoV-2 in a high-incidence region of the United States. Int J Radiat Oncol Biol Phys. 2020;108:1401–1402. doi: 10.1016/j.ijrobp.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. COVID-19 hospitalization and death by race/ethnicity. Available at:https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Accessed November 15, 2020.
- 15.Goyal MK, Simpson JN, Boyle MD. Racial and/or ethnic and socioeconomic disparities of SARS-CoV-2 infection among children. Pediatrics. 2020;146 doi: 10.1542/peds.2020-009951. [DOI] [PubMed] [Google Scholar]
- 16.Kim HN, Lan KF, Nkyekyer E. Assessment of disparities in COVID-19 testing and infection across language groups in Seattle, Washington. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz-Price LS, Nattinger AB, Rivera F. Racial disparities in incidence and outcomes among patients with COVID-19. JAMA Network Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Shamsi HO, Coomes EA, Aldhaheri K, Alrawi S. Serial screening for COVID-19 in asymptomatic patients receiving anticancer therapy in the United Arab Emirates. JAMA Oncol. 2021;7:129–131. doi: 10.1001/jamaoncol.2020.5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinato DJ, Zambelli A, Aguilar-Company J. Clinical portrait of the SARS-CoV-2 epidemic in European patients with cancer. Cancer Disc. 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jan I, Chen K, Sayan M. Prevalence of surface contamination with SARS-CoV-2 in a radiation oncology clinic. JAMA Oncol. 2020;6:1632–1634. doi: 10.1001/jamaoncol.2020.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]