Abstract
With the global spread of coronavirus disease 2019 (COVID-19), the important role of natural killer (NK) cells in the control of various viral infections attracted more interest, via non-specific activation, such as antibody-dependent cell-mediated cytotoxicity (ADCC) and activating receptors, as well as specific activation, such as memory-like NK generation. In response to different viral infections, NK cells fight viruses in different ways, and different NK subsets proliferate. For instance, cytomegalovirus (CMV) induces NKG2C + CD57 + KIR+ NK cells to expand 3–6 months after hematopoietic stem cell transplantation (HSCT), but human immunodeficiency virus (HIV) induces KIR3DS1+/KIR3DL1 NK cells to expand in the acute phase of infection. However, the similarities and differences among these processes and their molecular mechanisms have not been fully discussed. In this article, we provide a summary and comparison of antiviral mechanisms, unique subset expansion and time periods in peripheral blood and tissues under different conditions of CMV, HIV, Epstein-Barr virus (EBV), COVID-19 and hepatitis B virus (HBV) infections. Accordingly, we also discuss current clinical NK-associated antiviral applications, including cell therapy and NK-related biological agents, and we state the progress and future prospects of NK cell antiviral treatment.
Keywords: Natural killer cells, CMV, HIV, COVID-19, HBV
1. Introduction
Natural killer (NK) cells are important cytotoxic innate lymphocytes, which contribute to infection control, malignancy and autoimmunity, with a variety of inhibitory and activating receptors expressed on the cell surface. Human NK cells are usually divided into two major populations: the CD56bright/CD16dim subset with NKG2A expression and the absence of killer cell immunoglobulin-like receptors (KIRs) and the CD56dim/CD16bright subset with different proportions of KIRs, NKG2A and NKG2C [1,2]. Usually, CD56dim/CD16bright cells settle in the lung, but the CD56bright/CD16dim subset stays in the lymph gland [3,4]. During different viral infections, NK cells have multiple specific and nonspecific mechanisms to control virus infection.
Traditionally, NK cells have been considered innate immune cells that mediate antigen-independent nonspecific immune responses. Their activation is first regulated by a balance between engagement of its activating and inhibitory receptors in combination with the presence of certain cytokines. Inhibitory killer cell KIRs recognize the “self” and provide inhibitory signals by binding to cognate human leucocyte antigen class I (HLA—I). Viruses escape recognition by T cells by decreasing the expression level of HLA-I, and low HLA-I expression leads to prevailing activating signals, activating NK cells and promoting the recognition and clearance of virus-infected target cells [[5], [6], [7]]. Notably, NK cells can also be activated through Fc receptors (i.e., CD16 or FcγRIII) that identify infected cells by binding antibodies on them, which leads to the release of cytotoxic factors, such as granzyme that lyses cells, called antibody-dependent cell-mediated cytotoxicity (ADCC) [8]. Moreover, NK cells can kill virus-infected cells by various extracellular ligands, including Fas ligand (FasL) and tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), binding to death receptors induced by viral infection [9]. In addition to cytotoxicity, NK cells contribute to the antiviral response by releasing a wide range of proinflammatory cytokines with antiviral activity, such as IFN-γ. In addition, cytokines, such as interferons (IFNs), IL-12, or IL-18, released by accessory cells can also activate bystander NK cells during viral infection, which drives NK cells to proliferate and produce cytokines [10].
However, in recent years, it has been well established that viral infection can generate persistent and antigen-dependent NK cell memory responses called memory-like or adaptive NK cells [11]. Memory-like NK cells have more potent cytotoxicity and effector activity than conventional NK cells [12]. Memory-like NK cells express the activating receptors NKG2C, CD57 (mature marker) and KIR but lack the inhibitory receptor NKG2A, both of which bind to non-classical HLA-I molecules and HLA-E. Notably, human cytomegalovirus (HCMV) usually induces the expansion of NKG2C+ NK cells, resulting from the interaction between NKG2C and HCMV-derived UL40 [13]. As a pioneering strategy, accumulating and maintaining memory NK cells in the long run could be a hopeful viral treatment in the future.
With the global spread of coronavirus disease 2019 (COVID-19) as an international health crisis, the antiviral function of NK cells has been brought into the public eye. At present, NK cell antiviral activity has been extensively studied in cytomegalovirus (CMV), human immunodeficiency virus (HIV), Epstein-Barr virus (EBV) and hepatitis B virus (HBV), especially during the COVID-19 pandemic. In this article, we systematically summarize distinct antiviral mechanisms, amplification subsets, window periods and clinical applications of NK cells during CMV, HIV, EBV, HBV, and COVID-19 virus infections.
2. CMV induces the selective formation and expansion of memory NK cells
NK cells are the earliest reconstituting immune cells, reaching normal numbers within weeks after hematopoietic stem cell transplantation (HSCT) and contributing to the graft-versus-tumour effect along with T cells [14]. However, cytokine-producing and cytotoxic functions of NK cells was decreased until 3–6 months after HSCT [15], and reached normal reactivity levels by first year and maintained at later times [16]. HCMV reactivation is a common post-transplant complication, often accompanied by the maturation of reconstituting NK cells from BM and/or donor grafts, resulting in adaptive NK subset expansion, which is associated with improved disease-free survival [[17], [18], [19]].
The NK cell response to mouse cytomegalovirus (MCMV) has provided important insights into how NK cells specifically recognize and control viral infection and how infection affects NK cells [20]. MCMV infection can induce the selective amplification of Ly49H+ NK cells and skew the NK cell repertoire, dependent on the interaction between m157 and Ly49H [21]. The activating receptor DNAM-1 on Ly49H+ NK cells together with Ly49H cooperatively trigger initial proliferation, allowing differentiation into memory NK cells [22].
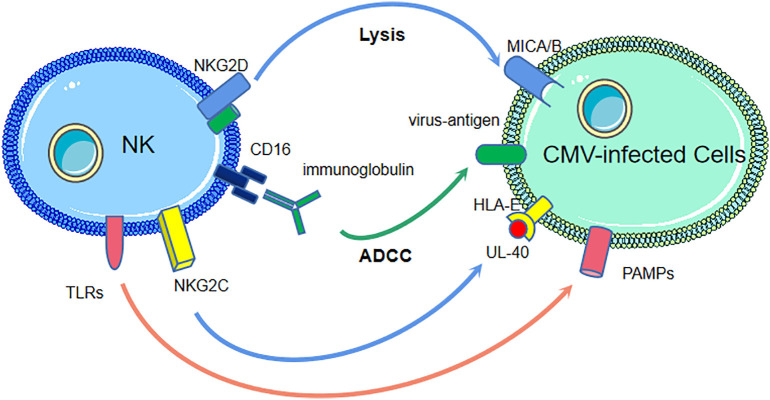
NK cells have several mechanisms to recognize and control HCMV infection. Non-HLA-I-specific activating receptors and co-receptors, including natural cytotoxicity receptors (NCRs), NKG2D, DNAM-1, and 2B4, recognize infected cells through cellular ligands. ADCC via CD16 engaged by the Fc fragment of antiviral immunoglobulins is another way NK cells kill infected cells. In addition, NKG2C interacts with the HLA-E-presenting peptide UL-40 derived from CMV. Moreover, NK cells express different toll-like receptors (TLRs) to recognize various pathogen-associated molecular patterns (PAMPs) derived from CMV [19].
Pioneering studies indicated that, compared to seronegative individuals, HCMV-seropositive individuals had increased NK cells expressing the activating receptor NKG2C, showing a skewed co-expression pattern of receptors that included high expression of CD2 combined with low expression of NKG2A, Siglec-7, NKp30 and FcεR1γ [11,23,24], known as memory-like NK cells [25]. As NKG2A has a higher affinity for ligand participation in competition with NKG2C, the absence of NKG2A is a basic element for the amplification of NK cells with greater cytolytic activity [12].
Interestingly, NKG2C+ NK cells from HCMV-seropositive individuals display high expression of CD57 and KIRs specific for self-MHC class I molecules (sKIRs), as evidence of clonal expansion [23]. The KLRC2/NKG2C gene plays a remarkably similar role to Ly49H in mice, promoting the clonal expansion of NK cells and causing a bias toward self-specific inhibitory KIRs [23,26]. A study demonstrated that HCMV-seropositive individuals with NKG2C gene deficiency can produce memory-like NK cells in the absence of NKG2C, which are similar in phenotype and function to NKG2C-expressing NK cells, suggesting the existence of alternative pathways of NK memory [26]. In CMV-reactivated patients, NK cells expressing NKG2C increased significantly at 3 months and 6 months post-transplant. Beyond 6 months, the percentages of NKG2C+ NK cells did not increase further [27]. Under HCMV infection, memory-like KIR+/NKG2C+/CD57+/CD49a+/CD56dim/CD16- NK cells have been identified in the lungs [28], indicating that this subset is involved in CMV control. A prospective clinical trial has also studied the number of adaptive NK cells (CD56dimCD57 + NKG2C+) of patients undergoing autologous HSCT after CMV-modified vaccinia Ankara (MVA) Triplex vaccination (NCT03383055). Another phase 1 clinical trial (NCT01904136) demonstrated the feasibility and safety of infusion of ex vivo-expanded donor NK cells before and after haploidentical HSCT, which improved NK-cell number and function, decrease viral infections and the relapse rate [29].
Therefore, NKG2C+ NK cells have memory characteristics that are transplantable and require active or latent (subclinical) expression of CMV antigen in the recipient for clonal expansion of NK cells previously exposed to CMV in the donor [27]. However, the causal relationship between the expansion of adaptive NK cells and CMV control is not clear. Meanwhile, it remains to be determined how cytokine-induced memory-like NK cells [30] can be successfully expanded and which genes are essential for adaptive NK cell reconstitution. (Fig. 1 ).
Fig. 1.
The anti-HCMV mechanisms of NK cells.
Non-HLA-I-specific activating receptors and co-receptors on natural killer (NK) cells, such as NKG2D, recognize human cytomegalovirus(HCMV)-infected cells by ligands, MICA/B. The antibody dependent cell-mediated cytotoxicity(ADCC) mediated by CD16 and the Fc fragment of antiviral immunoglobulins is another mechanism. NKG2C on NK cells is engaged by peptide UL-40, derived from HCMV, presented by human leucocyte antigen (HLA)-E. Toll-like receptors(TLRs) on NK cells recognize various pathogen associated molecular patterns (PAMPs) derived from HCMV.
3. IV causes KIR3DS1 + KIR3DL1+ NK cells to expand and activate NK cells by TLRs
NK cells are the first population to expand following HIV infection, preventing HIV replication and controlling disease [31]. During HIV infection in vivo, the KIR3DS1 + KIR3DL1+ NK cell subset expands [32]. A study showed that KIR3DS1+ NK cells can effectively suppress HIV-1 replication in HLA-B Bw480I+ target cells in vitro [33]. Following 1 year of infection, the level of KIR3DS1 + KIR3DL1+ NK cells remained higher and persisted at higher levels in subjects expressing HLA-B Bw480I. KIR3DL1 and KIR3DS1, whose corresponding receptor, the HLA-Bw4 motif, is associated with a slower decrease in the number of CD4+ T cells, were among the first receptors considered to participate in the recognition of HIV by NK cells [34]. However, the mechanisms underlying their protective role are still unclear.
There are three major methods by which NK cells control HIV infection, including DC editing, recognition of TLRs and cytokine secretion. Mature dendritic cells (DCs) are the main antigen-presenting cells (APCs) that initiate the adaptive immune response. The NK/DC interaction results in lysis of immature DCs via NKp30 killing and secretion of IFN-γ and preservation of mature DCs, which upregulate HLA molecules, called “DC editing”, enhancing the antigen presentation function of DCs in infected subjects [35].
TLRs, a type of PRR, on NK cells recognize PAMPs on infected cells, activating NK cells. Notably, TLR agonists efficiently activate NK cells during HIV infection, among which TLR3, TLR7, TRL8 and TRL9 agonists display remarkable effects [36,37]. TLR3 engages viral RNA, inducing the activation of nuclear factor kappa-B (NF-κB) and powerful inflammatory responses mediated by type I IFN release. TLR7 and TLR8 agonists directly activate NK cells or activate NK cells via DC signalling [36,38]. TLR9 activates NK cells mainly through activating pDCs following the secretion of IFN-α, recruiting more cytotoxic NK cells and effector CD8+ T cells [39].
Additionally, it has been reported that NK cells produce increased IFN-γ; tumour necrosis factor (TNF)-α; and the β chemokines CCL3, CCL4, and CCL5, which are ligands for CCR5, in HIV-1-exposed but uninfected individuals, which addresses the role of NK cells in resistance to HIV infection [40,41]. HIV-envelope/CD4 complex binds to the coreceptors CCR5 [42,43], which compete with and mimic cc-chemokines, resulting in activation of T cells [44,45]. It states that HIV-infected environment is both immunosuppressive and inflammatory and cc-chemokines can suppress infection through competing with HIV [46,47].
Nevertheless, the activated signalling pathways and molecular mechanism underlying resistance in repeatedly HIV-1-exposed, uninfected individuals are unclear. Uninfected individuals produce high levels of IL-22, which is involved in the production of acute-phase proteins and results in chemokine receptor 5 (CCR5) phosphorylation, downregulation of CCR5 expression, and strongly decreased susceptibility of these cells to in vitro infection with a primary HIV-1 isolate [48]. In addition, IL-22 can also induce epithelial cells to produce antimicrobial molecules and IL-10 and act on DCs, T cells and B cells, promoting viral antigen presentation and reducing HIV reservoirs [18,49,50].
Several studies have demonstrated that infection and vaccination can induce robust, durable, and antigen-specific NK cell memory responses in primates after SIV infection, but it is unclear whether or how either receptor is directly involved [51,52]. Evaluating the memory-like NK cell phenotype will enable the development of a new HIV vaccine, allowing patients to respond with strong cytotoxic activity and IFN-γ production in a “specific” way at the early stage of infection [35].
Anti-HIV-1 broadly neutralizing Ab (bNAbs) can enhance the cytotoxic effect on target cells through ADCC and inhibit viral replication [28,29]. Recently, bNAbs were expanded and purified from HIV-1-infected patients, overcoming the problem of slow natural production and low levels in plasma. In addition, a type of molecule called bispecific killer engager (BiKE) containing the Fab portion of bNAb linked to a nanobody that binds CD16 improves NK cell-mediated ADCC against HIV-infected targets [53].
As an expected therapy, the IL-15 super-agonist can enhance NK cell responses by production of cc-chemokines [[54], [55], [56]], in the immunosuppressive and inflammatory environment of chronic HIV infection [57]. HIV-infected cells are more infectious than free virus both in vitro and in vivo [58]. IL-15 can boost NK cell activities to kill latently infected cells in the presence of antiretroviral therapy, helping avoid new cell infection and diminish viral reservoir size and viral eradication [59]. In a clinical trial, on day 0, subjects will be infused with IL-15 Super Agonist ALT-803 (N-803)-activated NK cells on days 21 and 42, receiving additional doses of N-803 to evaluate its effect and safety (NCT03899480). As a kind of cell therapy, haploidentical NK cells were infused into HIV-infected subjects with IL-2 in a clinical trial to monitor safety and tolerance on days 2, 4, 6, 8 and 10 after infusion (NCT03346499). (Fig. 2 ).
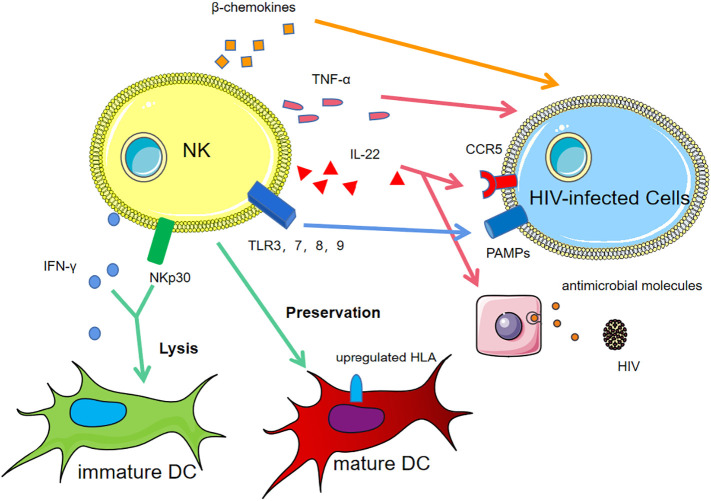
Fig. 2.
The anti-HIV mechanisms of NK cells.
In human immunodeficiency virus (HIV)-infected individuals, the natural killer(NK) cells /dendritic cells (DCs) interaction lyses immature DCs via NKp30 and IFN-γ and preserve mature DCs via upregulating HLA molecules, called “DC editing”. Toll-like receptor (TLR)3, TLR7, TLR8 and TLR9 on NK cells recognize pathogen-associated molecular patterns (PAMPs) on infected cells, activating NK cells. NK cells produce increased tumour necrosis factor (TNF)-α and the β chemokines.
Uninfected individuals produce high levels of IL-22, downregulating chemokine receptor 5 (CCR5) expression and inducing epithelial cells to produce antimicrobial molecules.
4. EBV exhibits distinct expansion of CD56bright cells in tonsils and alters the repertoire of NK cells in the blood
EBV, a double-stranded linear DNA virus, is a member of the gamma-herpes virus family. EBV infection, known to be the first cause of infectious mononucleosis (IM), is one of the risk factors for the development of multiple sclerosis (MS). EBV first infects epithelial submucosal cells where the virus replicates and then results in its malignant transformation. EBV infection can also cause a broad spectrum of B cell or NK/T cell lymphomas.
Tonsils, as the portal of EBV entry, have different NK cell subgroups in naive and germinal centres to limit EBV infection of tonsillar B cells [31]. Anti-EBV tonsillar NK cells are characterized by CD56bright/NKG2A+/CD94+/CD54+/ CD62L– expression, which prevent EBV-induced B cell malignant transformation in vitro by IFN-γ release and NKp44 engagement [60].
Primary infection with human oncogenic EBV can result in infectious mononucleosis (IM). Several studies have demonstrated that NKG2A+/CD56bright NK cells can also restrict early EBV infection in B cells [61,62]. In acute IM, NK cells are significantly elevated in peripheral blood four weeks post-infection at diagnosis compared with healthy controls, in which the proportion of CD56bright cells increased [63,64], identified as CD3 − NKp46+ with increased CD16 expression. This enhanced recruitment to the blood is related to a contraction of NK cell frequencies in both the periphery and spleen [63,64].
However, under CMV infection and chronic graft-versus-host diseases (cGVHD), EBV infection induces expansion of the NKG2A+/CD56dim NK cell subset [65]. In EBV reactivation and EBV-cGVHD patients after HSCT, the subset of NKG2A+/CD56dim NK cells substantially increased in blood and persisted up to 150 days post-transplantation, with the NKG2C+/CD56dim subset scaling down compared with no EBV reactivation cGVHD patients [66]. In addition, CMV and EBV coinfection induces an increased frequency of the CD56dim/NKG2A+/CD57+ NK cell population, which remains elevated in number up to 6 months after infection and results in a decrease in the absolute number of immature CD56bright/CD16- NK cells in the blood [62,65,67].
NK cells play a critical role in controlling EBV infection, mainly promoting cytotoxicity against EBV-transformed cells during the acute phase and limiting EBV viral load [63,68,69]. The anti-EBV mechanism of NK cells is not profoundly studied. The cytotoxicity of NK cells is potently activated by EBV-induced ligands on infected B cells [70]. Additionally, NK cells can be activated by pDCs and cDCs to produce IFN-γ and increase cytotoxicity upon encountering IFN-α/β. NK cells can prevent EBV from entering B cells and prevent B cell transformation via IFN-γ [71]. Peripheral blood human NK cells preferentially lytically recognize EBV-replicating B cells via downregulation of surface MHC class I molecules on infected cells [64,72]. (Fig. 3 ).
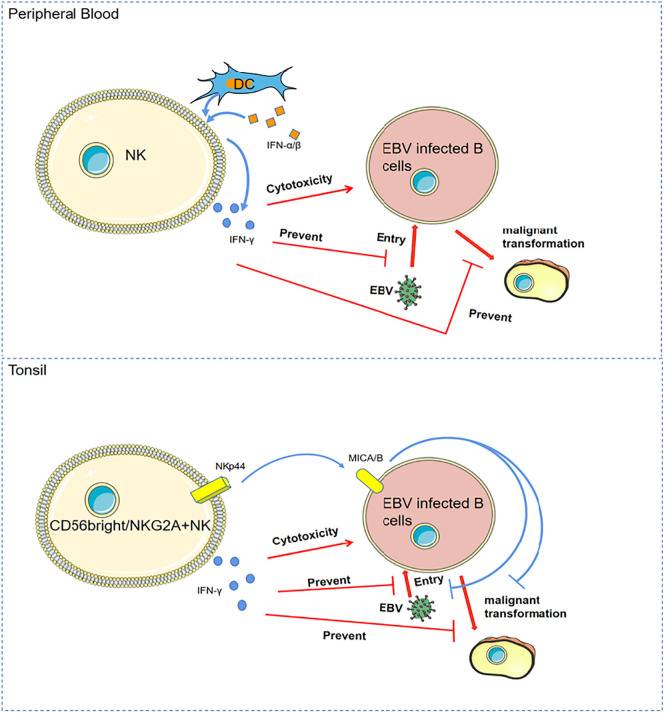
Fig. 3.
The anti-EBV mechanisms of NK cells.
In peripheral blood, natural killer(NK) cells are activated by dendritic cells (DCs) to produce IFN-γ and increase cytotoxicity upon encountering IFN-α/β. IFN-γ prevents epstein-Barr virus (EBV) from entering B cells and prevent EBV-induced B cell malignant transformation.
In tonsils, CD56bright / NKG2A + NK cells prevent EBV-induced B cell malignant transformation in vitro by IFN-γ release and NKp44 engagement.
Nasopharyngeal carcinoma (NPC), linked to EBV infection, is characterized by a lymphomononuclear infiltrate, which is a suitable candidate for cellular-based immunotherapy due to its expression of potentially targetable tumour-associated viral antigens. The number of NK cells in NPC has prognostic significance. NK cells have also attempted to be an alternative approach for adoptive cell therapy in NPC [73]. Due to the shorter lifespan of NK cells compared to cytotoxic T cells (CTLs), it is necessary for a suicide vector to control the over-expansion of transferred cells [73]. Presently, in a phase I/II clinical trials, high-activity NK cells and expanded NK cells combined with cetuximab are currently used to treat refractory NPC (NCT 02507154).
5. COVID-19 causes NK cell decreases in peripheral blood and NK cell increases in adaptive-like tissue residue in the lung
The activation of NK cells plays an important role in controlling the initial stage of severe acute respiratory syndrome coronavirus (SARS-CoV) infection. During COVID-19, NK cells have several mechanisms to control the virus. At the early stage of infection, NK cells, macrophages and plasmacytoid DCs (pDCs) can migrate into the lungs, which occurs after the first wave of enhanced cytokine expression, including TNF-α, IL-6, CXCL10, CCL2, CCL3 and CCL5, produced by airway epithelial cells and alveolar macrophages, with an increased titer of the virus [74]. NK cells control COVID-19 through antiviral cytokine production (e.g., IFN-γ) and lysis of virus-infected cells [75,76]. In addition, abundant production of IgG subclasses IgG1 and IgG3 secreted by B cells may trigger NK cell killing of infected cells via ADCC through the IgG-Fc receptor [77]. A recent study observed efficient SARS-CoV-2 S-glycoproteins (S309- and S306), triggering ADCC in SARS-CoV-2 S-glycoprotein-transfected cells [78]. An analysis revealed the expression levels of perforin and granzyme B in CD56bright NK cells are associated with disease severity, which shows positive correlation with sequential organ failure assessment (SOFA) and activation of effector molecules within cells, and negative correlation with PaO2/FiO2 ratio and inhibitory checkpoint molecule T cell immunoreceptor with Ig and ITIM domains (TIGIT), potentially involving NF-κB, PI3K–Akt, and FoxO signalling identifiedby protein-protein interaction network [79,80]. In addition, the IFN response begins within 12 h post-inoculation and peaks within 18–24 h along with maximal viral replication, secreted by leucocytes in the lung, gut and mesenteric lymph nodes [81]. Moreover, in COVID-19 cases, elevated IL-6, IL-10 and TGF-β are responsible for suppressing NK cell antiviral activity through downregulation of NKG2D [82,83]. However, how NK cells specifically control COVID-19 has not been clearly studied.
Emerging studies have demonstrated that adaptive-like lung tissue resident [tr]NK cells, characterized by KIR and NKG2C expression, can specifically expand during COVID-19. Previous studies have shown that NK cell activation contributes to the control of infection as well as to cytokine storms via the secretion of IL-1β, IL-6, IL-8, and IL-10 [84] [85,86]. Elevated IL-6 and IL-10 levels are observed in severe COVID-19-infected patients, which inhibit NK cell activity [85].
It has been reported that, in a cohort study of 221 patients with SARS, 72 cases of severe SARS showed a significantly lower total number of NK cells and CD158b + (KIR2DL3) NK cells than those in mild cases (National Research Project for SARS, Beijing Group, 2004) for the entire disease course and began to recover after the 40th day of the disease [79]. Recent evidence suggests that respiratory viruses, such as SARS-CoV and Middle East Respiratory Syndrome coronavirus (MERS-CoV), initially inhibit the innate immune system, ensuring stable replication during the early stage of infection [12]. Multiple studies have reported reduced numbers of NK cells in the peripheral blood of COVID-19 patients, whereas the number of CD4+ T cells increases [87,88], likely because of the CXCR3 pathway that recruits NK cells from the peripheral blood to the lungs [85].
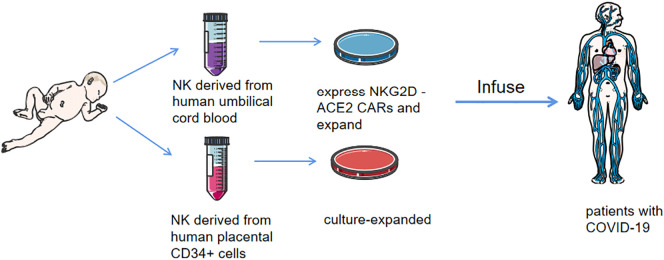
NK cell therapy is highly expected to treat COVID-19 infection in the future. It generally uses either primary NK cells isolated from peripheral blood mononuclear cells (PBMCs), generated from stem cell precursors, or genetically engineered immortalized human NK cell lines, which are often pre-treated and expanded in vitro with cytokines or co-cultured with target cells before infusion into patients [[89], [90], [91]]. The first cell-based drug to be approved by the FDA for clinical testing in COVID-19 patients is an allogeneic, off-the-shelf, cryopreserved NK cell therapy made by Celularity, an immunotherapy containing NK cells derived from human placental CD34+ cells and culture-expanded (CYNK-001), originally developed for cancer immunotherapy. A phase I/II trial is being performed to determine the safety and efficacy of CYNK-001 in patients with moderate COVID-19 disease (NCT04365101). Another phase I/II study in early-stage COVID-19 patients (within 14 days of illness) employing chimaeric antigen receptor (CAR)-NK cell therapy is currently being tested by using off-the-shelf NK cells derived from human umbilical cord blood expressing NKG2D-ACE2 CARs (NCT04324996). NKG2D-ACE2 CAR NK cells secrete a super IL-15 superagonist and GM-CSF neutralizing scFv, which has been shown to be correlated with COVID-19 disease severity in association with pathogenic CD4+ Th1 cells [92]. Additionally, it has been reported that blocking NKG2A in vitro with NKG2A monoclonal antibodies leads to improved NK cytotoxicity [93]. Given the association between NKG2A expression in patients with severe COVID-19, a promising avenue of COVID-19 treatment would be anti-NKG2A therapy, even despite results showing that NKG2A+ NK cells are tuned to present a higher level of responsiveness to stimulation [94].(Fig. 4, Fig. 5 ).
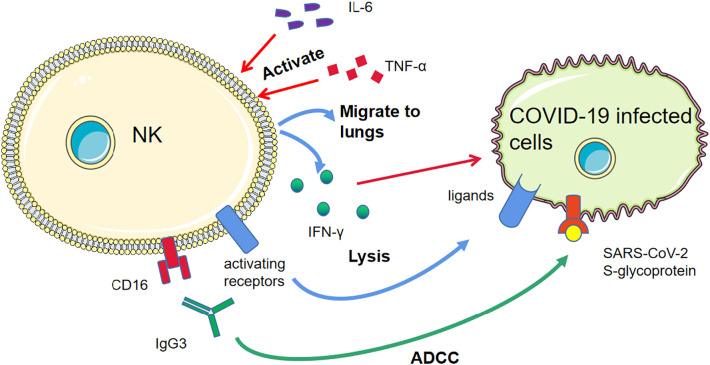
Fig. 4.
The anti-COVID-19 mechanisms of NK cells.
In coronavirus disease 2019 (COVID-19), natural killer (NK) cells activated by tumour necrosis factor (TNF)-α and IL-6, et al., migrate to lungs and release cytokines, such as IFN-γ. The antibody dependent cell-mediated cytotoxicity (ADCC) via severe acute respiratory syndrome coronavirus (SARS-CoV)-2 S-glycoproteins triggers NK cell killing of infected cells .The activating receptors on NK cells recognize the ligands on infected cells, activating NK cells.
Fig. 5.
The anti-COVID-19 clinical therapies of NK cells.
The natural killer (NK) cells derived from human placental CD34+ cells and culture-expanded (CYNK-001) are in clinical test to determine the safety and efficacy in patients with moderate coronavirus disease 2019(COVID-19) disease (NCT04365101). NK cells derived from human umbilical cord blood expressing NKG2D-ACE2 chimeric antigen receptors(CARs) and culture-expanded are in clinical test in early-stage COVID-19 patients (within 14 days of illness) (NCT04324996).
6. HBV increases the number of CD56dim NK cells in acute infection and reduces NK cells in chronic infection
NK cells, accounting for 25%–50% of the total number of liver lymphocytes, play an important role in liver immunity and early viral clearance in acute hepatitis B virus (HBV)-infected patients. Human liver NK cells include the CD56bright and CD56dim subsets. The two subsets exhibit significant differences in their responses to IL-2, intrinsic cytotoxic capacity, cytokine production and adhesion molecule expression. For example, CD56bright NK cells expand in response to low doses of IL-2, whereas CD56dim NK cells respond poorly to IL-2 stimulation and produce lower amounts of cytokines but are more cytotoxic against NK-sensitive targets than CD56bright NK cells. CD56bright NK cells express high levels of the CD94/NKG2 C-type lectin receptors, and few of them express KIRs, while CD56dim NK cells have adverse effects [95].
Interestingly, the cytolytic CD56dim NK-cell subset in the peripheral blood is selectively activated and expands in acute HBV infection, displaying distinct phenotypic and functional profiles associated with efficient and early control of HBV. This implicates NK cell cytolytic responses in the early containment and resolution of HBV infection [60,96]. Furthermore, early clearance of hepatitis B surface antigen (HBsAg) can be predicted by a distinct CD56dim NK cells phenotypic profile [60,96]. In HBV-infected patients, memory-like FcεRIγ-/CD56dim NK cells with higher NKG2C and lower NKG2A expression undergo increased cytokine responses and CD16-mediated ADCC compared to conventional CD56dim NK cells following exposure to HCMV [97].
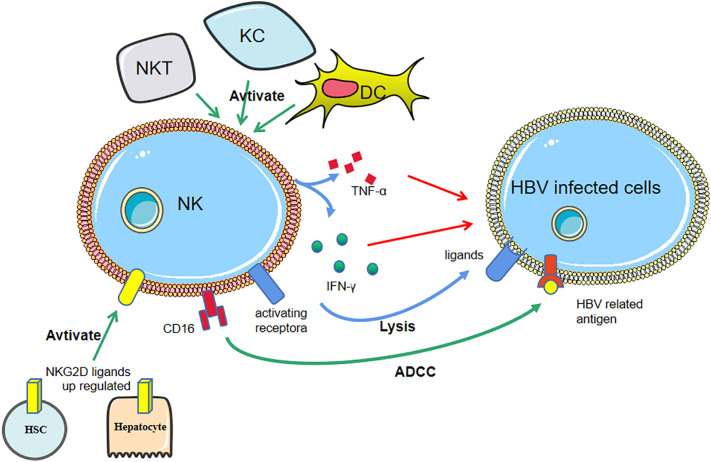
NK cells accumulate within the liver and peripheral blood and can directly kill infected cells or indirectly kill them through the production of cytokines. Activated Kupffer cells (KCs), (DCs), and natural killer T (NKT) cells can induce NK cell activation with higher levels of cytotoxicity and cytokine production and accumulate within the liver via a variety of cytokines [95]. It has been reported that the expression levels of NKG2D ligands are upregulated in hepatocytes, cholangiocytes, activated hepatic stellate cells (HSCs) and KCs in human liver diseases and animal models, binding to NKG2D on NK cells and subsequently activating NK cells [95,98]. NK cells produce a variety of cytotoxic mediators that control HBV infection and inhibit liver fibrosis by killing activated HSCs and alleviating liver fibrogenesis [95,99]. They also induce liver injury by killing hepatocytes and cholangiocytes [100]. Furthermore, in HBV-infected patients, the expression of TRAIL on hepatic NK cells is characteristically upregulated, by which NK cells lyse infected cells [101].
Chronic liver diseases are associated with a decreased number of NK cells and impairments in NK cell cytotoxicity and cytokine production [95]. The number of NK cells is lower in the liver in chronically infected HBV patients than in healthy individuals [102]. Interestingly, intrahepatic NKG2A+ NK cells are more clearly decreased in HBV patients. Most reports have shown reduced expression of activating receptors (NKG2D/DAP10 and 2B4/SAP) and increased expression of inhibitory receptors (NKG2A) on hepatic or peripheral NK cells [103]. In the peripheral blood of hepatocellular carcinoma (HCC) patients, CD56dimCD16pos NK subsets displayed a dramatic reduction with reduced IFN production and cytotoxicity, whereas CD56bright NK cells were dramatically increased [104]. Peripheral activated CD56bright NK cells from HBV-related decompensated liver cirrhosis (HBV-DLC) patients might be in a high immune activation status, expressing lower levels of inhibitory receptor CD158b1/2, higher levels of activating receptor NKG2D, and significantly higher expression of perforin and granzyme A/B, which is positively correlated with cytolytic capacity [105]. Moreover, a significant increase in the frequencies of CD56bright NK cells was observed in patients in the inactive carrier (IC) phase [106]. Notably, the number of NK cells in the blood and tumour tissues of HCC patients was positively correlated with their survival and prognosis [107,108]. Reduced NK cell activity was associated with increased CD4 + CD25+ T regulatory cells in the tumour environment of HCC patients, whose circulating Treg frequency was increased significantly, and Treg-treated NK cells produced less IFN-γ [104].
Immune modulatory therapies via NK cells are widely believed to represent potential therapeutic strategies for chronic HBV infection. However, in patients with chronic HBV infection, NK cells have been described to be more pathogenic than protective, with preserved cytolytic activity but a poor capacity to produce antiviral cytokines. Therefore, modulation of their balance can have potential therapeutic implications [109].(Fig. 6 ).
Fig. 6.
The anti-HBV mechanisms of NK cells.
Activated Kupffer cells (KCs), dendritic cells (DCs), and natural killer T (NKT) cells can activate NK cells with higher cytokines production. NKG2D ligands are upregulated in hepatocytes, hepatic stellate cells (HSC), et al., binding to NKG2D on NK cells and subsequently activating NK cells. Upregulated factor-related apoptosis-inducing ligands (TRAIL) on NK cells, binding to TRAL receptors (TRALR), are involved in lysis of infected cells.
7. Future
Although NK cells are expected to be one of the main effective antiviral treatments for different viruses in the future, virus immune escape, especially NK evasion, remains an unresolved problem. For example, by suppressing NK cell-activating signals or retaining and increasing NK cell-inhibitory signals, herpesviruses evade the killing effect of NK cells [109]. In addition, the expression of UL18, a viral HLA-I decoy on infected cells, whose affinity for leukocyte Ig-like receptor (LIR-1) is higher than that of HLA-I, suppresses the cytotoxicity of LIR-1+ NK cells to avoid NK cell cytotoxicity. Additionally, virus-infected cells express UL40, containing HLA-E binding epitopes, which upregulate the cell surface expression of HLA-E and also increase the cell surface expression of MHC-1 bait protein UL18, thus escaping from anti-viral NK cells [110]. During infection with HIV, viral peptides, which do not interact with inhibitory KIRs, can be presented on HLA-C and thereby abolish the inhibition, as exemplified by a Gag-derived peptide and KIR2DL2+ NK cells [111].
Memory-like NK cell- and CAR NK cell-based approaches have emerged as novel strategies for antiviral therapies.
A memory-like NK cell subset showing increased effector functions when re-encountering viral antigens or following proper activation with proinflammatory cytokines has been identified [75]. Whether viruses are capable of evading NK cell adaptive immune responses is also unclear [112]. A recent finding revealed that enhanced autophagy is essential for the generation of NK cell memory during MCMV infection, since an increasing number of viral products are capable of modulating autophagy through diverse mechanisms [113,114]. However, whether NK cell adaptive memory is suppressed by viral infections and cytokines via autophagy is not well understood.
In addition, induced pluripotent stem cell (iPSC)-derived NK cells, with enhanced activity and efficiency, also provide a promising perspective for CAR NK cell therapy, which helps establish an off-the-shelf clinical-scale cell bank [115].
Funding
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81670166, 81530046), Innovative Research Groups of the National Natural Science Foundation of China (81621001), the National Key Research and Development Program of China (2017YFA0104500), Beijing Municipal Science & Technology Commission (No. Z171100001017098), the Project of Health Collaborative Innovation of Guangzhou City (no. 201704020214), Scientific Research Foundation for Capital Medicine Development (No. 2018-2-4084), and Peking University Clinical Scientist Program (No. BMU2019 LCKXJ003).
Declaration of Competing Interest
None.
References
- 1.Castriconi R., et al. Molecular mechanisms directing migration and retention of natural killer cells in human tissues. Front. Immunol. 2018;9:2324. doi: 10.3389/fimmu.2018.02324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freud A.G., et al. The broad Spectrum of human natural killer cell diversity. Immunity. 2017;47(5):820–833. doi: 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrega P., Ferlazzo G. Natural killer cell distribution and trafficking in human tissues. Front. Immunol. 2012;3:347. doi: 10.3389/fimmu.2012.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquardt N., et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69(−)CD56(dim) cells. J. Allergy Clin. Immunol. 2017;139(4):1321–1330. doi: 10.1016/j.jaci.2016.07.043. e4. [DOI] [PubMed] [Google Scholar]
- 5.Martinet L., Smyth M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015;15(4):243–254. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 6.Mavers M., Bertaina A. High-risk leukemia: past, present, and future role of NK cells. J Immunol Res. 2018;2018:1586905. doi: 10.1155/2018/1586905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Erp E.A., et al. Viral infection of human natural killer cells. Viruses. 2019;11(3) doi: 10.3390/v11030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyth M.J., et al. Activation of NK cell cytotoxicity. Mol. Immunol. 2005;42(4):501–510. doi: 10.1016/j.molimm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 9.Miller J.S. Biology of natural killer cells in cancer and infection. Cancer Investig. 2002;20(3):405–419. doi: 10.1081/cnv-120001185. [DOI] [PubMed] [Google Scholar]
- 10.Lanier L.L. NK cell recognition. Annu. Rev. Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 11.Lee J., et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity. 2015;42(3):431–442. doi: 10.1016/j.immuni.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soleimanian S., Yaghobi R. Harnessing memory NK cell to protect against COVID-19. Front. Pharmacol. 2020;11:1309. doi: 10.3389/fphar.2020.01309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammer Q., et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 2018;19(5):453–463. doi: 10.1038/s41590-018-0082-6. [DOI] [PubMed] [Google Scholar]
- 14.Farag S.S., et al. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100(6):1935–1947. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 15.Foley B., et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118(10):2784–2792. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas P., et al. NK-cell education is shaped by donor HLA genotype after unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2011;117(3):1021–1029. doi: 10.1182/blood-2010-02-269381. [DOI] [PubMed] [Google Scholar]
- 17.Apiwattanakul N., et al. CMV-reactive NK cells in pediatric post-hematopoietic stem cell transplant. Transplant. Proc. 2020;52(1):353–359. doi: 10.1016/j.transproceed.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Cichocki F., et al. Adaptive NK cell reconstitution is associated with better clinical outcomes. JCI Insight. 2019;4(2) doi: 10.1172/jci.insight.125553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashidi A., et al. The association of CMV with NK-cell reconstitution depends on graft source: results from BMT CTN-0201 samples. Blood Adv. 2019;3(16):2465–2469. doi: 10.1182/bloodadvances.2019000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scalzo A.A., Yokoyama W.M. Cmv1 and natural killer cell responses to murine cytomegalovirus infection. Curr. Top. Microbiol. Immunol. 2008;321:101–122. doi: 10.1007/978-3-540-75203-5_5. [DOI] [PubMed] [Google Scholar]
- 21.Dokun A.O., et al. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2001;2(10):951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 22.Nabekura T., et al. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity. 2014;40(2):225–234. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Béziat V., et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood. 2013;121(14):2678–2688. doi: 10.1182/blood-2012-10-459545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlums H., et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–456. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumá M., et al. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood. 2004;104(12):3664–3671. doi: 10.1182/blood-2004-05-2058. [DOI] [PubMed] [Google Scholar]
- 26.Liu L.L., et al. Critical role of CD2 co-stimulation in adaptive natural killer cell responses revealed in NKG2C-deficient humans. Cell Rep. 2016;15(5):1088–1099. doi: 10.1016/j.celrep.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foley B., et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J. Immunol. 2012;189(10):5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dogra P., et al. Tissue determinants of human NK cell development, function, and residence. Cell. 2020;180(4):749–763. doi: 10.1016/j.cell.2020.01.022. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ciurea S.O., et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood. 2017;130(16):1857–1868. doi: 10.1182/blood-2017-05-785659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong J.W., et al. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014;20(4):463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scully E., Alter G. NK cells in HIV disease. Curr. HIV/AIDS Rep. 2016;13(2):85–94. doi: 10.1007/s11904-016-0310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alter G., et al. HLA class I subtype-dependent expansion of KIR3DS1+ and KIR3DL1+ NK cells during acute human immunodeficiency virus type 1 infection. J. Virol. 2009;83(13):6798–6805. doi: 10.1128/JVI.00256-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alter G., et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 2007;204(12):3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores-Villanueva P.O., et al. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc. Natl. Acad. Sci. U. S. A. 2001;98(9):5140–5145. doi: 10.1073/pnas.071548198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flórez-Álvarez L., Hernandez J.C., Zapata W. NK cells in HIV-1 infection: from basic science to vaccine strategies. Front. Immunol. 2018;9:2290. doi: 10.3389/fimmu.2018.02290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu C.L., et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352(6288):1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlaepfer E., Speck R.F. Anti-HIV activity mediated by natural killer and CD8+ cells after toll-like receptor 7/8 triggering. PLoS One. 2008;3(4) doi: 10.1371/journal.pone.0001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alexopoulou L., et al. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 39.Vibholm L., et al. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma Viremia in individuals with human immunodeficiency virus infection. Clin. Infect. Dis. 2017;64(12):1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott-Algara D., et al. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 2003;171(11):5663–5667. doi: 10.4049/jimmunol.171.11.5663. [DOI] [PubMed] [Google Scholar]
- 41.Montoya C.J., et al. Increased IFN-gamma production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin. Immunol. 2006;120(2):138–146. doi: 10.1016/j.clim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Littman D.R. Chemokine receptors: keys to AIDS pathogenesis? Cell. 1998;93(5):677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 43.Dragic T., et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 44.Davis C.B., et al. Signal transduction due to HIV-1 envelope interactions with chernokine receptors CXCR4 or CCR5. J. Exp. Med. 1997;186(10):1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weissman D., et al. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature. 1997;389(6654):981–985. doi: 10.1038/40173. [DOI] [PubMed] [Google Scholar]
- 46.Zagury D., et al. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc. Natl. Acad. Sci. U. S. A. 1998;95(7):3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cocchi F., et al. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270(5243):1811. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 48.Missé D., et al. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J. Immunol. 2007;178(1):407–415. doi: 10.4049/jimmunol.178.1.407. [DOI] [PubMed] [Google Scholar]
- 49.Cogswell A., Ferguson N., Barker E. Presence of inflammatory group i and iii innate lymphoid cells in the colon of simian immunodeficiency virus-infected Rhesus Macaques. J. Virol. 2020;(9):94. doi: 10.1128/JVI.01914-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W., et al. The secretion of IL-22 from mucosal NKp44+ NK cells is associated with microbial translocation and virus infection in SIV/SHIV-infected Chinese macaques. J Immunol Res. 2014;2014:387950. doi: 10.1155/2014/387950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reeves R.K., et al. Antigen-specific NK cell memory in rhesus macaques. Nat. Immunol. 2015;16(9):927–932. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holder K.A., Comeau E.M., Grant M.D. Origins of natural killer cell memory: special creation or adaptive evolution. Immunology. 2018;154(1):38–49. doi: 10.1111/imm.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis Z.B., et al. Natural killer cells unleashed: checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin. Immunol. 2017;31:64–75. doi: 10.1016/j.smim.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perera L.P., Goldman C.K., Waldmann T.A. IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J. Immunol. 1999;162(5):2606–2612. [PubMed] [Google Scholar]
- 55.Bluman E.M., et al. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J. Clin. Invest. 1996;97(12):2722–2727. doi: 10.1172/JCI118726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Becknell B., Caligiuri M.A. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv. Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1. [DOI] [PubMed] [Google Scholar]
- 57.Ram D.R., et al. Adaptive NK cell responses in HIV/SIV infections: a roadmap to cell-based therapeutics? J. Leukoc. Biol. 2019;105(6):1253–1259. doi: 10.1002/JLB.MR0718-303R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anderson D.J., et al. Targeting Trojan horse leukocytes for HIV prevention. Aids. 2010;24(2):163–187. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrido C., et al. Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. J. Virol. 2018;92(12) doi: 10.1128/JVI.00235-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cooper M.A., et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- 61.Strowig T., et al. Tonsilar NK cells restrict B cell transformation by the Epstein-Barr virus via IFN-gamma. PLoS Pathog. 2008;4(2) doi: 10.1371/journal.ppat.0040027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lünemann A., et al. A distinct subpopulation of human NK cells restricts B cell transformation by EBV. J. Immunol. 2013;191(10):4989–4995. doi: 10.4049/jimmunol.1301046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams H., et al. The immune response to primary EBV infection: a role for natural killer cells. Br. J. Haematol. 2005;129(2):266–274. doi: 10.1111/j.1365-2141.2005.05452.x. [DOI] [PubMed] [Google Scholar]
- 64.Chijioke O., et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5(6):1489–1498. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hendricks D.W., et al. Cutting edge: NKG2C(hi)CD57+ NK cells respond specifically to acute infection with cytomegalovirus and not Epstein-Barr virus. J. Immunol. 2014;192(10):4492–4496. doi: 10.4049/jimmunol.1303211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaiswal S.R., et al. Alterations in NKG2A and NKG2C subsets of natural killer cells following Epstein-Barr virus reactivation in CTLA4Ig-based Haploidentical transplantation is associated with increased chronic graft-versus-host disease. Transplantation. 2020;104(1):e23–e30. doi: 10.1097/TP.0000000000002941. [DOI] [PubMed] [Google Scholar]
- 67.Azzi T., et al. Role for early-differentiated natural killer cells in infectious mononucleosis. Blood. 2014;124(16):2533–2543. doi: 10.1182/blood-2014-01-553024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chijioke O., Landtwing V., Münz C. NK cell influence on the outcome of primary Epstein-Barr virus infection. Front. Immunol. 2016;7:323. doi: 10.3389/fimmu.2016.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunmire S.K., et al. The incubation period of primary Epstein-Barr virus infection: viral dynamics and immunologic events. PLoS Pathog. 2015;11(12) doi: 10.1371/journal.ppat.1005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Sullivan T.E., Sun J.C., Lanier L.L. Natural killer cell memory. Immunity. 2015;43(4):634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jud A., et al. Tonsillar CD56brightNKG2A+ NK cells restrict primary Epstein-Barr virus infection in B cells via IFN-γ. Oncotarget. 2017;8(4):6130–6141. doi: 10.18632/oncotarget.14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pappworth I.Y., Wang E.C., Rowe M. The switch from latent to productive infection in epstein-barr virus-infected B cells is associated with sensitization to NK cell killing. J. Virol. 2007;81(2):474–482. doi: 10.1128/JVI.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee A.Z.E., Tan L.S.Y., Lim C.M. Cellular-based immunotherapy in Epstein-Barr virus induced nasopharyngeal cancer. Oral Oncol. 2018;84:61–70. doi: 10.1016/j.oraloncology.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Chen J., et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010;84(3):1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brunetta E., et al. Chronic HIV-1 viremia reverses NKG2A/NKG2C ratio on natural killer cells in patients with human cytomegalovirus co-infection. Aids. 2010;24(1):27–34. doi: 10.1097/QAD.0b013e3283328d1f. [DOI] [PubMed] [Google Scholar]
- 76.Waggoner S.N., et al. Roles of natural killer cells in antiviral immunity. Curr. Opin. Virol. 2016;16:15–23. doi: 10.1016/j.coviro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonam S.R., et al. Adjunct immunotherapies for the Management of Severely ill COVID-19 patients. Cell Rep. Med. 2020;1(2):100016. doi: 10.1016/j.xcrm.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pinto D., et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583(7815):290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 79.The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. Am. J. Clin. Pathol. 2004;121(4):507–511. doi: 10.1309/WPK7Y2XKNF4CBF3R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varchetta S., et al. Unique immunological profile in patients with COVID-19. Cell. Mol. Immunol. 2021;18(3):604–612. doi: 10.1038/s41423-020-00557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Charley B., Riffault S., Van Reeth K. Porcine innate and adaptative immune responses to influenza and coronavirus infections. Ann. N. Y. Acad. Sci. 2006;1081(1):130–136. doi: 10.1196/annals.1373.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osman M.S., van Eeden C., Cohen Tervaert J.W. Fatal COVID-19 infections: is NK cell dysfunction a link with autoimmune HLH? Autoimmun. Rev. 2020;19(7):102561. doi: 10.1016/j.autrev.2020.102561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang K.J., et al. An interferon-gamma-related cytokine storm in SARS patients. J. Med. Virol. 2005;75(2):185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z., Peng C., Zhu T., Liang Q. Virus-Host Interactome and Proteomic Survey Reveal Potential Virulence Factors Influencing SARS-CoV-2 Pathogenesis. Med. (N. Y.) 2021;2(1):99–112.e7. doi: 10.1016/j.medj.2020.07.002. Jan 15, Epub 2020 Jul 21. PMID: 32838362; PMCID: PMC7373048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vabret N., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Von Holle T.A., Moody M.A. Influenza and antibody-dependent cellular cytotoxicity. Front. Immunol. 2019;10:1457. doi: 10.3389/fimmu.2019.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng M., et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17(5):533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang F., et al. The laboratory tests and host immunity of COVID-19 patients with different severity of illness JCI Insight. 2020;5(10) doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fujisaki H., et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimasaki N., Jain A., Campana D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020;19(3):200–218. doi: 10.1038/s41573-019-0052-1. [DOI] [PubMed] [Google Scholar]
- 91.Hu W., et al. Cancer immunotherapy based on natural killer cells: current Progress and new opportunities. Front. Immunol. 2019;10:1205. doi: 10.3389/fimmu.2019.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu X., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li F., et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144(2):392–401. doi: 10.1053/j.gastro.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 94.Shifrin N., Raulet D.H., Ardolino M. NK cell self tolerance, responsiveness and missing self recognition. Semin. Immunol. 2014;26(2):138–144. doi: 10.1016/j.smim.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tian Z., Chen Y., Gao B. Natural killer cells in liver disease. Hepatology. 2013;57(4):1654–1662. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu W.H., et al. ADCC-mediated CD56(DIM) NK cell responses are associated with early HBsAg clearance in acute HBV infection. Pathog. Immun. 2018;3(1):2–18. doi: 10.20411/pai.v3i1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gill U.S., Golden-Mason L. HCMV jogs the ‘memory’ of NK cells in HBV. J. Hepatol. 2019;70(3):343–345. doi: 10.1016/j.jhep.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Y., et al. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46(3):706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 99.Chen Y., et al. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46(3):706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 100.Kahraman A., et al. Major histocompatibility complex class I-related chains A and B (MIC A/B): a novel role in nonalcoholic steatohepatitis. Hepatology. 2010;51(1):92–102. doi: 10.1002/hep.23253. [DOI] [PubMed] [Google Scholar]
- 101.Dunn C., et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J. Exp. Med. 2007;204(3):667–680. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonorino P., et al. Fine characterization of intrahepatic NK cells expressing natural killer receptors in chronic hepatitis B and C. J. Hepatol. 2009;51(3):458–467. doi: 10.1016/j.jhep.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 103.Sun C., et al. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012;8(3) doi: 10.1371/journal.ppat.1002594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cai L., et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin. Immunol. 2008;129(3):428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 105.Jiang Y., et al. Impaired circulating CD56(dim) NK cells are associated with decompensation of HBV-related cirrhosis. Hum. Immunol. 2020;81(1):32–40. doi: 10.1016/j.humimm.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 106.de Groen R.A., et al. NK cell phenotypic and functional shifts coincide with specific clinical phases in the natural history of chronic HBV infection. Antivir. Res. 2017;140:18–24. doi: 10.1016/j.antiviral.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 107.Oliviero B., et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137(3):1151–1160. doi: 10.1053/j.gastro.2009.05.047. 1160.e1–7. [DOI] [PubMed] [Google Scholar]
- 108.Morishima C., et al. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43(3):573–580. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 109.Fisicaro P., et al. The good and the bad of natural killer cells in virus control: perspective for Anti-HBV therapy. Int. J. Mol. Sci. 2019;20(20) doi: 10.3390/ijms20205080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Pelsmaeker S., et al. Herpesvirus evasion of natural killer cells. J. Virol. 2018;92(11) doi: 10.1128/JVI.02105-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hammer Q., Rückert T., Romagnani C. Natural killer cell specificity for viral infections. Nat. Immunol. 2018;19(8):800–808. doi: 10.1038/s41590-018-0163-6. [DOI] [PubMed] [Google Scholar]
- 112.Ma Y., Li X., Kuang E. Viral evasion of natural killer cell activation. Viruses. 2016;8(4):95. doi: 10.3390/v8040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Sullivan T.E., et al. BNIP3- and BNIP3L-mediated Mitophagy promotes the generation of natural killer cell memory. Immunity. 2015;43(2):331–342. doi: 10.1016/j.immuni.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jackson W.T. Viruses and the autophagy pathway. Virology. 2015;479-480:450–456. doi: 10.1016/j.virol.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., et al. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23(2):181–192. doi: 10.1016/j.stem.2018.06.002. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]