Abstract
Background
Coronavirus disease 2019 (COVID-19) is an emerged pandemic disease with no specific treatment. One of the potential treatments in newly found infectious disease is plasma exchange (PE) with convalescent plasma transfusion (CPT). This case series aimed to evaluate the primary PE and CPT in five Iranian COVID-19 patients.
Methods
Five patients with confirmed COVID-19 who had acute respiratory distress syndrome and were supported by mechanical ventilation were treated with two consecutive PE containing fresh frozen plasma (FFP) of healthy donors and 0.9 % saline solution containing 5 % human albumin. Thereafter, CPT was performed just like PE, except that the FFP in this step was substituted with convalescent ABO-matched plasma. Clinical and laboratory factors were evaluated before and after treatments.
Results
Three to Four patients showed lower body temperature and improved oxygen saturation as well as reduced laboratory factors such as c-reactive protein, lactate dehydrogenase, creatine phosphokinase (total and myocardial isoform), aspartate aminotransferase, blood urea nitrogen, bilirubin (total and direct), D-dimer, interleukin-6, and CD4+/CD8 + T cells ratio initially after PE and continued to improve so that they were discharged. One patient due to secondary hemophagocytic lymphohistiocytosis and extensive lung fungal infection was expired.
Discussion
Overall, the PE followed by CPT was beneficial in reducing acute inflammation led to a considerable improvement in patients’ clinical features. It seems that PE along with CPT could provide clearance of pro-inflammatory mediators as well as the positive effects of CPT. Controlled studies are required to confirm the effect of PE/CPT compared with other therapeutic approaches.
Keywords: COVID-19, SARS-CoV-2, Plasma exchange, Convalescent plasma
1. Introduction
The outbreak of coronaviruses has caused three epidemic diseases during the recent two decades, namely, severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019 (COVID-19). These diseases could cause acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) which leads to pulmonary failure and eventually death [[1], [2], [3]]. In December 2019, COVID-19 emerged in Wuhan, China, and on 11 March 2020 the World Health Organization (WHO) declared it as a pandemic disease [4].
Patients with COVID-19 present clinical symptoms including high fever, dry cough, dyspnea, malaise, fatigue, headache, and gastrointestinal problems. In progressed conditions, patients may suffer from breath shortness, ARDS, and even septic shock, metabolic acidosis, irreversible bleeding, and coagulation dysfunction might develop [5,6]. Massive cytokines overproduction by the immune system in SARS, MERS, and COVID-19 patients result in a cytokine storm. Cytokine storm is characterized by the release of a series of cytokines like tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-6, interferon (IFN)-α, IFN-β, and IFN-γ, that could potentially cause ARDS [7].
Different treatment procedures have been proposed to treat SARS, MERS, and other viral diseases. For COVID-19 patients, however, there is not much experience and mainly supportive care and other treatment procedures for viral diseases could be provided. Suggested therapies include antiviral drugs like lopinavir/ritonavir, remdesivir, ribavirin, and IFN-α to reduce the virus load. Oxygen therapy for respiratory supports, steroids to reduce the severity of inflammatory damages, convalescent plasma (CP) therapy and plasma exchange (PE) are also supportive treatments. PE and CP are two old approaches which was used to treat infectious disease as passive therapy before drug or vaccine development [[7], [8], [9]].
The application of convalescent plasma collected from patients who had recovered from the Ebola virus, SARS-CoV, H5N1 avian influenza, and H1N1 influenza was recommended by WHO as an empirical treatment during outbreaks. Recently, studies have reported that the administration of CP containing neutralizing antibody to patients with COVID-19 and ARDS, could improve their clinical status [10,11].
Moreover, PE is a potential therapeutic procedure in which some amount of plasma is removed to decrease large-molecular weight substances include anti-immune complexes, endotoxins, pathogenic autoantibodies, cryoglobulins, and protein-binding agents [12]. Noteworthy, TPE also remove the anti-inflammatory mediators and anti-SARS-CoV-2 antibodies that are potentially beneficial for patients [13]. However, due to the inflammatory condition of severe COVID-19 patients, the amount of removed pro-inflammatory mediators are much more than anti-inflammatory molecules. Hence, the overall result of TPE is ameliorating the inflammation through the removal of pro-inflammatory mediators and harmful molecules. It is necessary to replace the discarded plasma with a replacement fluid to avoid hypotension and hypovolemia [14,15]. The 4–5 % human albumin in physiologic saline (0.9 %) is the most common replacement fluid using for PE [16]. Huang C et al. published the first case series of patients infected with COVID-19 and declared that severe conditions in these patients had caused increased plasma cytokine concentrations [17]. Sungim Choi et al. showed that treating a patient with severe fever and thrombocytopenia syndrome (SFTS) complicated by SFTS-associated encephalopathy with 4-day PE followed by two-time CP therapy could successfully decrease the plasma cytokines IFN-α, inducible protein-10 and plasma viral load [18].
In this case series, we used CP from patients recovered from COVID-19 after two cycles of PE to evaluate the effectiveness of PE followed by CP therapy in ameliorating the symptoms and management of the disease in five COVID-19 patients.
2. Material and Methods
2.1. Patients
This study was conducted in Taleghani Hospital, Tehran, Iran between March and April 2020. The study was approved by the ethics committee from Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.REC.1398.148) and each patient gave written informed consent. Five patients (2 males and 3 females) with COVID-19 were recruited for this study. The patients’ age was 48–76 years. The SARS-CoV-2 detection was assessed on nasopharyngeal specimens using a quantitative reverse-transcriptase polymerase chain reaction (qRT- PCR) kit specific for E and N genes of SARS-CoV-2 (OSANG Healthcare, South Korea) based on the manufacturer and WHO guideline. The inclusion criteria in our study include patients with respiratory distress; respiratory rate (RR) ≥ 30 beats/min, required mechanical ventilation, oxygen saturation level ≤ 90 % in resting-state wearing an oxygen mask, and positive SARS-CoV-2 after antiviral treatment. Allergic patients to plasma or injectable albumin, patients with severe organ failure, and those who were positive for HBV, HCV, and HIV were excluded from the study. The reason for excluding patients with severe organ failure is that these patients usually receive multimodal treatment (such as dialysis and severe corticosteroids) that could affect the results of PE/CP therapy. Patients continued receiving the conventional treatment (Lopinavir/ritonavir, hydroxychloroquine, azithromycin, steroids, and special treatments for their comorbidities) along with the PE/CP therapy.
2.2. Convalescent plasma Donors
The donors had recovered from COVID-19 infection and donated their convalescent plasma after giving written informed consent. All donors showed negative results of the qRT-PCR assay for SARS-CoV-2 (double-checked in different days) and other respiratory viruses. They were also negative for hepatitis B virus, hepatitis C virus, HIV (1 and 2), varicella-zoster virus, cytomegalovirus, herpes simplex virus (I and II), Epstein-Barr virus, toxoplasma and syphilis antigens/IgM antibody at the time of plasma donation. The donors were men between the ages of 30 and 67 years and had been afebrile with alleviated respiratory symptoms for at least 14 days after the second negative SARS-CoV-2 result. The donors were checked for SARS-CoV-2 specific immunoglobulin G (IgG) and IgM in serum. The titration of IgG and IgM on donor serum was performed using commercial ELISA kits (PishtazTeb, Iran). Briefly, 100 μL of titrated serum or positive/negative control was added to the pre-coated wells and incubated for 30 min. at a 37 °C incubator. The wells were washed five times with the working wash solution and 100 μL of HRP-conjugated anti-human IgG/IgM was added to the wells for an extra 30 min. at 37 °C incubation. After a five-time wash, 100 μL chromogen-substrate solution was dispensed to the wells and incubated for 15 min. at room temperature in dark. The reaction was stopped by addition of 100 μL stop solution, and the optical density was read at 450 nm. The sensitivity and specificity of the IgG and IgM kits are 94.1 %, 98.3 %, 79.4 %, and 97.3 %, respectively. Donors with IgM titer lower than 1:10 and IgG titer higher than 1:1000 were selected for plasma donation. Approximately, 600−900 mL of CP were collected from each donor and infused (exchanged with their plasma) on the same day to the patients (Table 1 ). All mentioned donor tests were performed before the day of CP collection.
Table 1.
Characteristics and specific antibody titer of convalescent plasma of donors.
| Characteristic |
Donors |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sex | Male | Male | Male | Male | Male |
| Age (year) | 30 | 66 | 30 | 67 | 32 |
| Blood group | A+ | O+ | O+ | A+ | A+ |
| Interval between symptom onset and discharge (day) | 10 | 17 | 19 | 20 | 12 |
| Interval between discharge and plasma donation (day) | 16 | 20 | 17 | 16 | 22 |
| Total donated plasma volume (mL)/cycle | 900/3 | 600/1 | 700/1 | 750/1 | 650/1 |
| SARS-CoV-2 specific IgM titer | 1:1400 | 1:2200 | 1:1800 | 1:1600 | 1:1600 |
| SARS-CoV-2 specific IgG titer | 1:6 | 1:8 | 1:6 | 1:4 | 1:8 |
2.3. Therapeutic plasma exchange
A total of 1500−2000 mL of patients’ plasma was replaced with an equal amount of solution containing the same proportions of 0.9 % saline with 5 % human albumin (Biotest, Germany) and ABO-compatible fresh frozen plasma (FFP) from healthy donors for two consecutive days. The plasma volume (PV) for each patients was calculated using the following formula [19,20] : PV = 0.065 × weight (kg) × (1-hematocrit). Due to the critical status of patients such as cardiovascular disorders and coagulopathy, the plasma volume replaced for all patients was less than one PV (0.75 PV). The day after second PE, CP therapy was performed just like PE, except that the FFP in this step was substituted with convalescent ABO-matched plasma. All the PE and CP therapy was performed using the apheresis system (MCS 3 P, Haemonetics, Braintree, MA). In one case (patient 1), two doses of 200 mL convalescent plasma were infused to the patient at two consecutive days after CP therapy.
2.4. Evaluation of clinical and laboratory features
Demographic and primary clinical data of patients, such as principal symptoms, comorbidities, intervals between symptom onset and admission as well as therapy onset, and given treatments were collected. Clinical and laboratory findings, including body temperature, oxygen saturation, C-reactive protein (CRP), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), creatine kinase myocardial band (CK-MB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (Cr), total and direct bilirubin, fibrinogen, D-dimer, white blood cell (WBC), and platelet (PLT) count were measured before and several days after therapy (PE and CP). The number of CD4 + T and CD8 + T cells, as well as serum IL-6, were measured before and after the treatment procedure.
Patient fresh blood samples were collected from COVID-19 patients. Plasma, serum, and whole blood were used based on the purpose of the study. Serum concentrations of IL-6 were measured by electrochemiluminescent immunoassay (ECLIA) method using the Elecsys IL-6 kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer protocol. The analytical sensitivity of the kit was 1.5 pg/mL. The number of CD3+ (APC-conjugated), CD4+ (PE-conjugated), and CD8+ (FITC-conjugated, all from BD Bioscience, USA) cells were measured on lymphocyte population using flow cytometry (BD FACSLyric, BD Bioscience, USA).
The computed tomography (CT) scan of patients’ lung were performed before the first PE and after the CP therapy.
3. Results
In the present case series, five patients (age 48–76 years) in the critical stage were chosen for PE and CP therapy. All patients’ comorbidity beside COVID-19 were treated accordingly. One male patient was diagnosed with CLL. All patients were supported by an oxygen mask and are currently alive except for one (patient 4) who developed secondary hemophagocytic lymphohistiocytosis (sHLH) before the PE/CP therapy as well as an extensive lung fungal infection, despite anti-fungal treatment and intubation. PE was performed within 10 days post-admission (1–9 days), except patient 4 for which the onset of PE was one month after her admission. Antiviral and antibacterial treatments were prescribed for all patients, while steroid and anti-fungal treatments were administered based on the patients’ conditions (Table 2 ). Patient 1, experienced a myocardial infarction (MI) before PE/CP therapy and underwent cardiopulmonary resuscitation as well as medical treatment (furosemide). He also received two extra infusions of CP due to his heart failure.
Table 2.
Clinical characteristics of COVID-19 patients.
| Clinical Characteristics |
Patients |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Sex | Male | Female | Male | Female | Female |
| Age | 55 | 48 | 76 | 71 | 62 |
| Weight | 80 | 67 | 72 | 52 | 60 |
| Blood Group | A+ | O+ | O+ | A+ | A+ |
| Smoking history | No | No | No | No | No |
| Principal symptoms | Shortness of breath | Cough, Shortness of breath | Cough, Shortness of breath | Cough, Fever, Shortness of breath, Sputum | Cough, Shortness of breath, Chest pain |
| Comorbidity | Hypothyroidism MI | Diabetes | Anemia, CLL, Hypertension | sHLH | Diabetes, Asthma, Hypertension, Hypercholesterolemia CABG |
| Disease severity1 | Critical | Critical | Critical | Critical | Critical |
| Mechanical Ventilation | Mask | Mask | Mask | Mask/Intubation | Mask |
| Duration (day) | 10 | 13 | 18 | 34/3 | 8 |
| Interval between symptom onset and admission (day) | 20 | NA | 30 | 16 | NA |
| Interval between admission and plasma exchange (day) | 1 | 3 | 9 | 30 | 3 |
| Interval between admission and convalescent plasma transfusion (day) | 4 | 6 | 12 | 33 | 6 |
| Bacterial/viral/fungal infection | Yes/Yes/No | Yes/Yes/No | Yes/Yes/No | Yes/Yes/Yes | Yes/Yes/No |
| Length of admission (day) | 12 | 13 | 20 | 38 | 10 |
| Current status | Alive /discharged | Alive /discharged | Alive/ discharged | Dead | Alive/ discharged |
| Treatments | |||||
| Steroids | Yes | Yes | Yes | Yes | No |
| Antiviral | Yes | Yes | Yes | Yes | Yes |
| Antibiotic/antifungal | Yes/No | Yes/No | Yes/No | Yes/Yes | Yes/No |
| Other treatments2 | Yes | No | Yes | Yes | Yes |
MI. Myocardial infarction; CLL. Chronic lymphocytic leukemia; sHLH. Secondary Hemophagocytic lymphohistiocytosis; CABG. Coronary artery bypass grafting; NA. Not available.
Patients with respiratory problem requiring ventilation support, have comorbidity and requiring intensive care.
Treatments based on the comorbidity of patients.
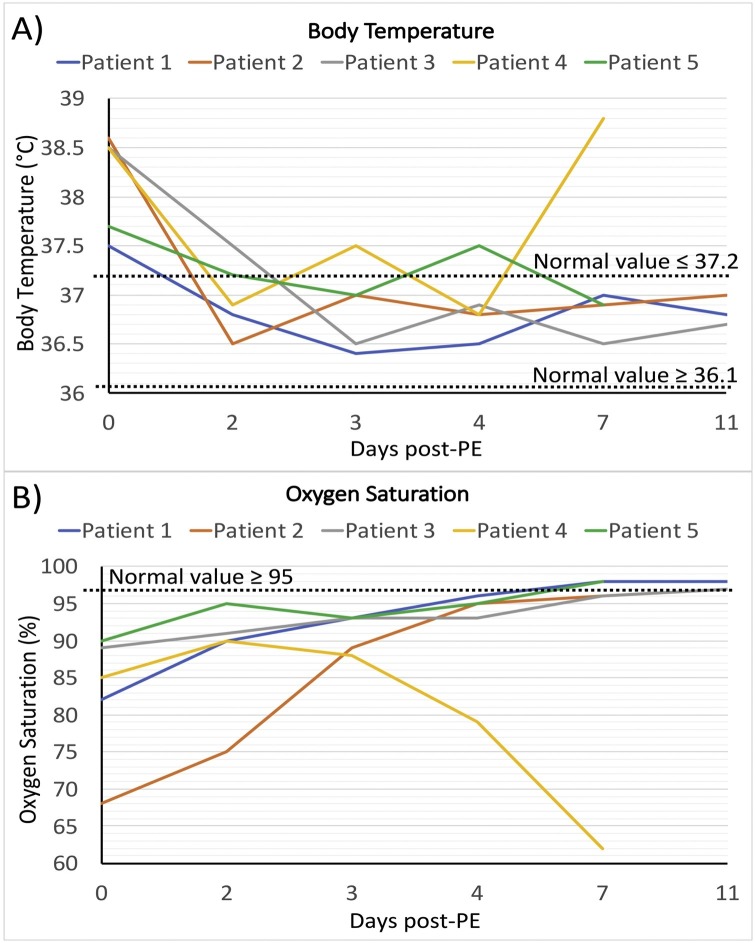
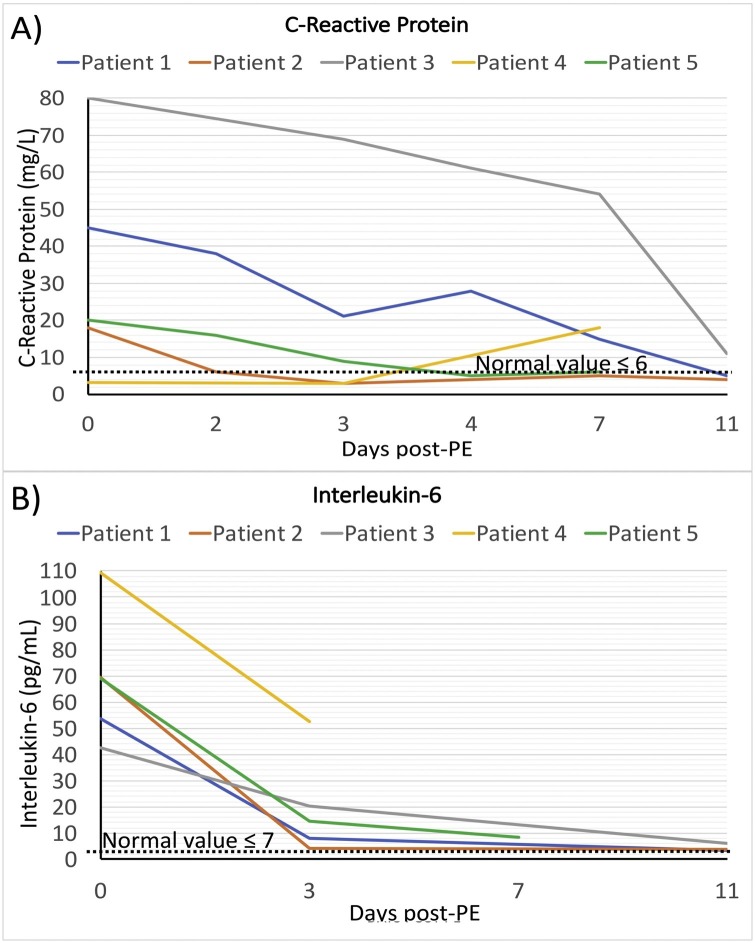
Six time-points were considered to assess the treatment responses, comprising the day before the first PE (day 0, before treatment), day after first PE (day 2), day after second PE and before CP therapy (day3) to assess the effect of PE, one day after CP therapy (day 4), four days after CP therapy (day 7), and one week after CP therapy (day 11). The body temperature of patients was between 37.5 and 38.6 °C prior to treatment and decreased to normal range in 4 alive patients (36.7−37 °C). Oxygen saturation with oxygen mask was between 68 and 90 % before PE and raised time-dependently to ≥97 % in 4 alive patients (Table 3 and Fig. 1 A and B). Fever of patient 4 was eliminated after PE/CP therapy and also oxygen saturation increased in initial days, but then started to get worst in days later. The laboratory findings such as CRP, LDH, CPK, CK-MB, AST, BUN, total and direct bilirubin, and D-dimer were high in patients before treatments and started to decrease toward normal range in 3–4 of patients after treatment (Table 3). The majority of these inflammatory factors were decreased after the first and second PEs and continue to decrease after CP therapy (Table 3). CPK and CK-MB were considerably high in patient 1 who had MI, however, they fell to the normal range following PE and remained normal in the next days. ALT levels in patients 1 and 5 were high and came into the normal range after first PE. Creatine and fibrinogen levels were in the normal range in patients (Table 3). As the treatment proceeds, the CRP level showed a decreasing trend for all patients, except for patient 4 which was in the normal range and increased 4 days after CP transfusion (Fig. 2 A). The serum level of IL-6 as a key inflammatory cytokine in COVID-19 was measured in patients before and after PE/CP therapy. The level of IL-6 in patients was 6–15 times higher than the normal level prior to treatment. PE eliminated more than half of IL-6 and CP therapy further decreased IL-6 to normal range in 3 patients (Fig. 2B).
Table 3.
Clinical and laboratory findings before and after PE/CP therapy.
| Clinical findings |
Patients |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Body temperature (°C) | |||||
| Before first PE | 37.5 | 38.6 | 38.5 | 38.5 | 37.7 |
| Before second PE | 36.8 | 36.5 | 37.5 | 36.9 | 37.2 |
| Before CPT | 36.4 | 37 | 36.5 | 37.5 | 37 |
| Day 1 After CPT | 36.5 | 36.8 | 36.9 | 36.8 | 37.5 |
| Day 4 after CPT | 37 | 36.9 | 36.5 | 38.8 | 36.9 |
| Day 7 after CPT | 36.8 | 37 | 36.7 | NA | NA |
| Oxygen saturation (%) | |||||
| Before first PE | 82 | 68 | 89 | 85 | 90 |
| Before second PE | 90 | 75 | 91 | 90 | 95 |
| Before CPT | 93 | 89 | 93 | 88 | 93 |
| Day 1 After CPT | 96 | 95 | 93 | 79 | 95 |
| Day 4 after CPT | 98 | 96 | 96 | 62 | 98 |
| Day 7 after CPT | 98 | 97 | 97 | NA | NA |
| Laboratory findings | |||||
| C-reactive protein (mg/L) | |||||
| Before first PE | 45 | 18 | 80 | 3.2 | 20 |
| Before second PE | 38 | 6 | NA | NA | 16 |
| Before CPT | 21 | 3 | 69 | 3 | 9 |
| Day 1 After CPT | 28 | NA | 61 | NA | 5 |
| Day 4 after CPT | 15 | 5 | 54 | 18 | 6 |
| Day 7 after CPT | 5 | 4 | 11 | NA | NA |
| Lactate dehydrogenase (IU/L) | |||||
| Before first PE | 1338 | 1430 | 1124 | 897 | 816 |
| Before second PE | 642 | 1253 | 836 | NA | 462 |
| Before CPT | 649 | 1122 | 690 | 612 | 414 |
| Day 1 After CPT | 602 | 905 | 553 | 715 | 512 |
| Day 4 after CPT | 674 | 851 | 479 | 950 | 603 |
| Day 7 after CPT | 557 | 704 | 576 | NA | NA |
| Creatine phosphokinase (IU/L) | |||||
| Before first PE | 369 | 143 | 254 | 74 | 74 |
| Before second PE | NA | 161 | NA | NA | 91 |
| Before CPT | 120 | 132 | 228 | NA | 68 |
| Day 1 After CPT | 118 | NA | NA | 71 | 39 |
| Day 4 after CPT | 95 | 167 | 78 | 91 | 45 |
| Day 7 after CPT | 46 | NA | NA | NA | NA |
| Creatine kinase-myocardial band (IU/L) | |||||
| Before first PE | 71 | 32 | 41 | 11 | 17 |
| Before second PE | NA | 28 | NA | NA | 25 |
| Before CPT | 27 | 25 | 39 | NA | 19 |
| Day 1 After CPT | 26 | NA | NA | 18 | 12 |
| Day 4 after CPT | 24 | 24 | 21 | 21 | 18 |
| Day 7 after CPT | 18 | NA | NA | NA | NA |
| Alanine aminotransferase (IU/L) | |||||
| Before first PE | 66 | 28 | 32 | 28 | 73 |
| Before second PE | 35 | 20 | NA | NA | 40 |
| Before CPT | 30 | 22 | 50 | 29 | 35 |
| Day 1 After CPT | 35 | 21 | 54 | 23 | 22 |
| Day 4 after CPT | 30 | 23 | 58 | 10 | 69 |
| Day 7 after CPT | 12 | 31 | 24 | NA | NA |
| Aspartate aminotransferase (IU/L) | |||||
| Before first PE | 111 | 42 | 50 | 11 | 43 |
| Before second PE | 38 | 36 | NA | NA | 26 |
| Before CPT | 27 | 32 | 45 | 12 | 14 |
| Day 1 After CPT | 26 | 42 | 42 | 58 | 29 |
| Day 4 after CPT | 21 | 31 | 22 | 64 | 54 |
| Day 7 after CPT | 20 | 26 | 19 | NA | NA |
| Blood urea nitrogen (mg/dL) | |||||
| Before first PE | 29 | 24.7 | 21 | 29.8 | 52 |
| Before second PE | 29 | 24 | 21 | NA | 64 |
| Before CPT | 37 | 23 | 19 | 19 | 63 |
| Day 1 After CPT | 23 | 22 | 20 | 64 | 68 |
| Day 4 after CPT | 28 | 15 | 22 | 58 | 63 |
| Day 7 after CPT | 24 | 16 | 17 | NA | NA |
| Creatinine (mg/dL) | |||||
| Before first PE | 0.9 | 1.03 | 0.9 | 0.71 | 1.7 |
| Before second PE | 0.9 | 0.9 | 0.8 | NA | 1.7 |
| Before CPT | 0.8 | 0.8 | 0.8 | 0.8 | 1.7 |
| Day 1 After CPT | 0.9 | NA | 0.9 | 1.5 | 1.7 |
| Day 4 after CPT | 1.1 | 0.80 | 1 | 0.9 | 1.8 |
| Day 7 after CPT | 0.9 | 0.76 | 0.8 | NA | NA |
| Total bilirubin (mg/dL) | |||||
| Before first PE | 3.4 | 0.8 | 1.2 | 0.6 | 2 |
| Before second PE | 2 | 0.8 | 1 | NA | 0.9 |
| Before CPT | 1.1 | 0.7 | NA | 0.4 | 0.6 |
| Day 1 After CPT | 1.5 | 0.8 | 0.9 | 1 | NA |
| Day 4 after CPT | 1 | 0.8 | 0.6 | NA | 0.5 |
| Day 7 after CPT | 0.8 | 0.5 | 0.9 | NA | NA |
| Direct bilirubin (mg/dL) | |||||
| Before first PE | 2.9 | 0.2 | 0.5 | 0.2 | 1.2 |
| Before second PE | 1.6 | 0.4 | 0.5 | NA | 0.4 |
| Before CPT | 0.7 | 0.4 | NA | 0.2 | 0.3 |
| Day 1 After CPT | 0.9 | 0.3 | 0.4 | 0.6 | NA |
| Day 4 after CPT | 0.8 | 0.3 | 0.3 | NA | 0.2 |
| Day 7 after CPT | 0.4 | 0.2 | 0.3 | NA | NA |
| Fibrinogen (mg/dL) | |||||
| Before first PE | 186 | 332 | 346 | NA | 269 |
| Before second PE | 161 | 269 | 255 | 173 | 340 |
| Before CPT | 151 | NA | 210 | NA | NA |
| Day 1 After CPT | 194 | 186 | 303 | NA | 303 |
| Day 4 after CPT | NA | 210 | 180 | NA | 220 |
| Day 7 after CPT | 167 | 346 | 173 | NA | NA |
| D-dimer (mg/dL) | |||||
| Before first PE | 0.40 | 12 | 3.8 | 2.9 | 14 |
| Before second PE | 0.30 | 9.1 | NA | 1.3 | 6 |
| Before CPT | 1 | 7.2 | 1 | 0.4 | 5 |
| Day 1 After CPT | 0.5 | 6.4 | 2.9 | NA | 9 |
| Day 4 after CPT | 8 | 2 | 3.3 | NA | 7 |
| Day 7 after CPT | 2.3 | 1.1 | 1.6 | NA | NA |
| IL-6 (pg/mL) | |||||
| Before PE | 53.68 | 69.29 | 42.5 | 109.4 | 69.07 |
| Before CPT | 7.86 | 4.27 | 20.43 | 52.4 | 14.55 |
| Day 7 After CPT | 3.52 | 3.66 | 5.98 | NA | NA |
NA. Not available; PE. Plasma exchange; CPT. Convalescent plasma transfusion.
Fig. 1.
Trends of body temperature and oxygen saturation. Trends of changes in body temperature (A) and oxygen saturation (B), in different time-points. The day before the first PE was considered as day 0. Values in day 0, 2, 3, 4, 7, and 11 are attributed to the baseline value (the day before the first PE), before second PE, before CP, one day post-CP, 4 days post-CP, and one week post-CP, respectively. Each line represents a patient. Normal range is illustrated as dashed lines in each figure.
Fig. 2.
Trends of CRP and IL-6 levels. Trends of changes c-reactive protein (CRP) level (C), and IL-6 level (D) in different time-points. The day before the first PE was considered as day 0. Values in day 0, 2, 3, 4, 7, and 11 are attributed to the baseline value (the day before the first PE), before second PE, before CP, one day post-CP, 4 days post-CP, and one week post-CP, respectively. Each line represents a patient. Normal range is illustrated as dashed lines in each figure.
WBC of two patients increased post-CP transfusion and the others showed subtle changes during the treatment. Lymphocyte and neutrophil percent increased and decreased, respectively in 4 patients in a time-dependent manner. PLT count remained high in patient 1 and reached high in the patient 2 post-CP therapy, while it fell in the patient 4 after treatment. Patient 3 showed a fall in PLT count after PE but got back to normal range 4 days after CP. The absolute number of total T cells, CD4 + T cells, and CD8 + T cells increased in patients 1, 2, and 5 after PE and CP therapy. CD4 + T cells/ CD8 + T cells ratio was increased in patients 1 and 5 after treatment, while it slightly decreased in patients 2 and 3 (Table 4 ).
Table 4.
Blood cell findings before and after PE/CP therapy.
| Blood cell findings |
Patients |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| White blood cell count (×1000/μL) | |||||
| Before first PE | 12.20 | 9.3 | 15.9 | 20.1 | 10.8 |
| Before second PE | 13.34 | 10.4 | NA | 19.9 | 10.8 |
| Before CPT | 13.1 | 12.5 | 16.9 | 12.9 | 10.6 |
| Day 1 After CPT | 11.9 | 15.8 | 19.6 | 19 | 11 |
| Lymphocyte percent (%) | |||||
| Before first PE | 8 | 9 | 71 | 10 | 5 |
| Before second PE | 6 | 10 | NA | 2 | 6 |
| Before CPT | 10 | 10 | 87 | 2 | 9 |
| Day 1 After CPT | 13 | 11 | 88 | 3 | 8 |
| Day 4 after CPT | 14 | 12 | 89 | 5 | 14 |
| Day 7 after CPT | 33 | 15 | 82 | NA | NA |
| Neutrophil percent (%) | |||||
| Before first PE | 90 | 85 | 25 | 95 | 91 |
| Before second PE | 83 | 88 | NA | 95 | 84 |
| Before CPT | 85 | 85 | 6 | 96 | 80 |
| Day 1 After CPT | 85 | 85 | 7 | 95 | 82 |
| Day 4 after CPT | 88 | 78 | 10 | 92 | 76 |
| Day 7 after CPT | 62 | 80 | 13 | NA | NA |
| Platelet count (×1000/μL) | |||||
| Before first PE | 529 | 302 | 121 | 186 | 155 |
| Before second PE | 466 | 252 | 73 | 74 | 163 |
| Before CPT | 453 | 216 | 83 | 52 | 180 |
| Day 1 After CPT | 563 | 282 | 84 | 55 | 203 |
| Day 4 after CPT | 685 | 481 | 125 | 27 | 235 |
| Day 7 after CPT | 491 | 669 | 177 | NA | NA |
| T cell count/μL, (%) | |||||
| Before PE | 429, (39) | 646, (64) | 466, (5) | 273, (74) | 474, (60) |
| After CPT | 723, (54) | 753, (54) | 361, (2) | NA | 1500, (67) |
| CD4+ T cell count/μL, (%) | |||||
| Before PE | 297, (27) | 524, (52) | 280, (3) | 213, (58) | 308, (39) |
| After CPT | 535, (40) | 595, (41) | 180, (1) | NA | 1074, (48) |
| CD8+ T cell count/μL, (%) | |||||
| Before PE | 121, (11) | 111, (11) | 186, (2) | 44, (12) | 189, (24) |
| After CPT | 174, (13) | 145, (10) | 180, (1) | NA | 447, (20) |
| CD4+ T cell/CD8+ T cell ratio (%) | |||||
| Before PE | 2.45 | 4.7 | 1.5 | 4.8 | 1.63 |
| After CPT | 3.07 | 4.1 | 1 | NA | 2.40 |
NA. Not available; PE. Plasma exchange; CPT. Convalescent plasma transfusion.
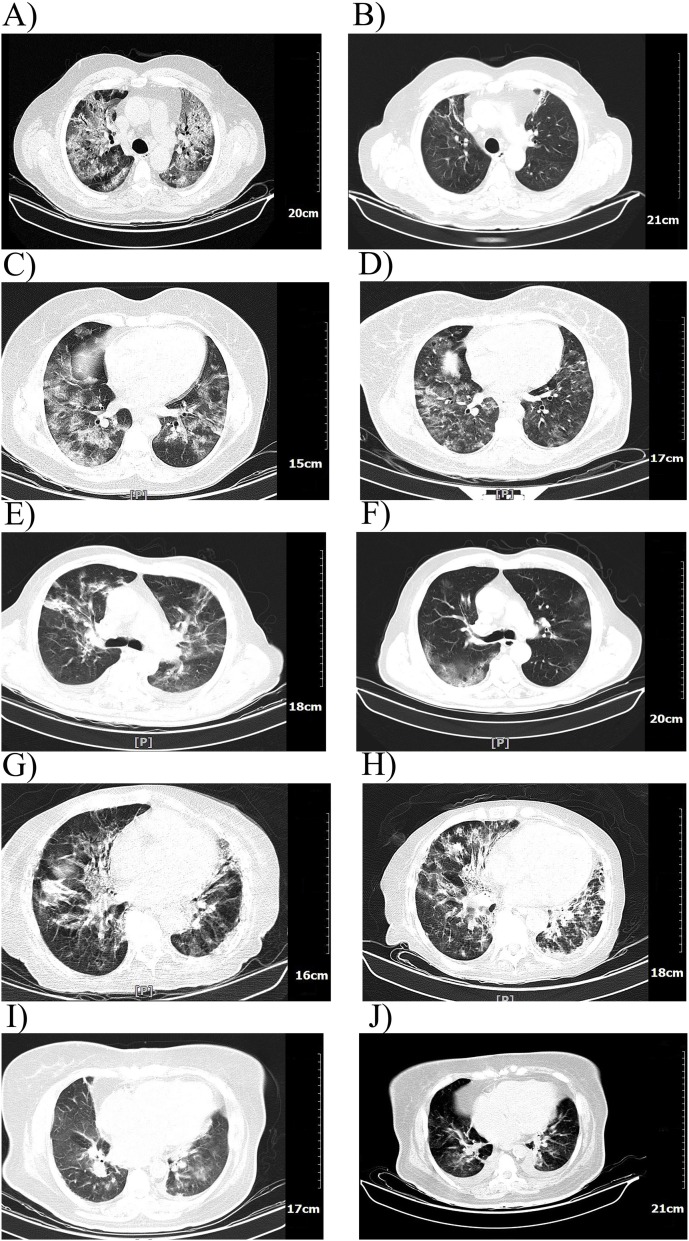
The CT scan of patients, generally, showed sever pneumonia and ground-glass opacity before the treatment (Fig. 3 ). An overall resolution of the pulmonary lesions is seen in the patients’ lung after the treatment. Patient 1 illustrated a significant improvement in CT scan and the others showed an acceptable lesion healing after the PE/CP therapy (Fig. 3).
Fig. 3.
Computed tomography (CT) scan of the lung of patients. The CT scan of the lung of patients before (A, C, E, G, I) and 4-9 days after the first plasma exchange (B, D, F, H, J) is illustrated. The images are in the order of the patients.
4. Discussion
Most studies identified ARDS as the leading cause of death in COVID-19 patients [16]. The immune dysregulation in SARS-CoV-2 infection causes uncontrolled systemic inflammatory responses and releases an extensive amount of pro-inflammatory cytokines and chemokines called cytokine storm [17,21,22]. The cytokine storm can provoke the immune response against organs that cause organ failure and ARDS which could be fatal [23]. Hence, one of the potential treatment in such an acute inflammation is removing the inflammatory mediators through PE [24]. Accordingly, researchers are performing PE along with the other treatments such as steroids and showed beneficial effects in decreasing inflammatory mediators in COVID-19 [23,24]. Similar studies in ARDS caused by other etiologies reported respiratory function improvement by decreasing the inflammation following PE [[26], [27], [28]]. Basically, PE is the substitution of the patients’ plasma with FFP from healthy donors [29]. This technique is widespread to reduce the concentration of cytokines and inflammatory mediators. It could be used even in non-infectious inflammation [30]. FFP used in the PE could decrease the inflammatory cytokines and modulate the hypercoagulation by providing the ADAMTS13 enzyme [29]. In the case of COVID-19, it has been shown that PE alone was able to modulate the hyperinflammatory state and reduce mortality [31].
On the other hand, CP therapy is commonly used in infectious diseases [10,27]. CP is enriched with neutralizing antibodies against certain infectious agents and could be beneficial in controlling the infectious agent [32]. So, the purposes of PE therapy and CP therapy are different. In the case of COVID-19, which is a hyper-inflammation following an infectious disease, we can reduce the inflammation using PE and then provide neutralizing antibodies using CP therapy [32]. CP as a replacement for patients’ plasma could afford the blood proteins and enriched the patient’s plasma with anti-SARS-CoV-2 antibodies [33,34]. Several case studies have shown the effectiveness of CP therapy in COVID-19, two most prominent of which are Duan et al. [27] and Shen et al. [10] studies. In the first study, 10 patients were given CP and 3 patients showed improvement of respiratory status and laboratory parameters [35]. The latter study, showed fever alleviation within three days post-CP transfusion while Sequential Organ Failure Assessment (SOFA) score, oxygen saturation, and inflammatory parameters (CRP, procalcitonin, and IL-6) improved after 10 days post-treatment [10]. High levels of IL-6 and CRP are reported to be associated with mortality in COVID-19 [10]. PE could decrease the inflammatory factors through plasma clearance, while CP therapy could afford neutralizing antibodies against SARS-CoV-2. Recent reports have provided recommendations regarding CP therapy in COVID-19 [33,34]. Noteworthy, it has been proposed that CP therapy is better to combine with PE to achieve a better result [36]. Thrombocytopenia, organ failure and a high level of LDH in COVID-19 patients are in common with thrombocytopenia-associated multiple organ failure (TAMOF) in which rapid PE could significantly improve the outcomes [37]. Kesici et al. recommended that performing PE along with CP transfusion could provide clearance of pro-inflammatory cytokines as well as the positive effects of CP transfusion [36].
In the present study, we performed PE twice consecutively to reduce the inflammatory mediators and alleviate acute inflammation. As expected, both first and second PE decreased the concentration of inflammatory mediators. These results were parallel to the findings of other studies and suggested the benefit of PE to mitigate the hyperinflammatory conditions [18,36,38]. Thereafter, to further decrease the inflammatory mediators as well as supplement the patients’ blood with plasma proteins and specific anti-SARS-CoV-2 antibodies, in the third cycle of PE, the FFP was substituted by CP. The blood group-matched donors were evaluated carefully to confirm the negative SARS-CoV-2 infection for at least two weeks and also having high titers of IgG antibody against SARS-CoV-2. Interestingly, the patients that have been chosen for PE/CP therapy had either A+ (three patients) and O+ (two patients) blood group. Studies about the association of ABO blood group with the susceptibility to COVID-19 and its clinical outcomes are controversial. Generally, it has been stated that the blood group A+ might be associated with higher COVID-19 susceptibility and more unfavorable outcomes [[39], [40], [41]]. On the other hand, blood group O is believed to have the lowest risk of COVID-19 [38,40]. However, evidence suggests that the COVID-19 severity is not associated with the ABO blood group [40]. Regarding the high frequency of blood groups, A+ and O+ in the Iranian population [42], it seems normal to have a high frequency of A+ and O+ patients. As the results illustrated, the inflammatory factors such as CRP, LDH, D-dimer, and IL-6 are decreased following the PE/CP therapy cycles indicating the inflammation subsidence. IL-6, CRP, and coagulation parameters such as D-dimer are reported to be hallmarks of COVID-19, and reducing these factors correlates with better prognosis of patients [17,43,44]. Moreover, the body temperature and oxygen saturation which are the two most critical parameters in COVID-19 were improved after PE/CP therapy showing the efficacy of the treatment. Patient 4 manifested alleviated fever after PE. However, her body temperature raised several days after CP therapy, which might be due to the sHLH and fungal infection. Regarding the received steroids and antipyretic medications, the exact role of PE/CP therapy in the management of fever is difficult to interpret. However, PE/CP therapy might reduce the fever by decreasing the pyrogenic mediators such as IL-6 and inflammatory molecules [45]. Parallel to the laboratory findings, the lung CT scans showed considerable improvements in lesions after PE/CP therapy. The resolution of lesions in the lung occurs gradually as we can see in the Fig. 3 that the CT scans which has been taken beyond one week post-CP therapy (patient 1 and 3) showed more lesion healing and less involvement in the lung.
One patient (patient 4) did not respond well to the treatment and expired due to the extensive lung fungal infection. One explanation for her unresponsiveness could be the late onset of treatment (one-month post-admission). Although PE is recommended for sHLH, the right time of treatment onset is a critical factor to determine the response of the patient. As can be seen in results, the initial response of the patients post-PE was promising, but the fungal infection that spread throughout the lung despite anti-fungal treatment led to respiratory distress and death. PE was performed within 10 days post-admission (1–9 days), except patient 4 for which the onset of PE was one month after her admission. One possible reason for the unresponsiveness of patient 4 might be the delay in the onset of PE/CP therapy. It has been reported that PE/CP therapy is more effective when used at the early stages of the disease [34]. Accordingly, several clinical and laboratory findings of patient 4 contrasted the others. Putting patient 4 aside, the other patients showed satisfactory responses to the treatment resulted in negative PCR results and discharge of patients even one week after CP therapy (patient 5). This study has several limitations. The small sample size and uncontrolled method of the study are the most important limitations. The result that comes from such a small sample size could not be conclusive enough. However, Regarding the fact that the overall result is parallel to the findings of other studies, it could be indicative of the beneficial effects of PE/CP therapy in COVID-19 patients. The other limitation was the missing data that was too many for such a small data set. Regarding the emergency condition of the COVID-19 pandemic and the expiration of patient 4, missing data was increased. There were confounding factors such as concurrent antiviral, antibacterial, and steroid therapies that made the actual effects of PE/CPT unclear. Due to the necessity of receiving such medications, cutting them could have threatened the lives of COVID-19 patients.
Despite the promising result of PE/CP therapy in COVID-19 patients, the question is: Can this method be widely used for COVID-19 patients? Although PE/CP therapy is capable of resolving inflammation, its potential survival benefit is still a matter of debate [31,13]. PE/CP therapy is a laborious strategy that might not worth to be used in regular COVID-19 patients. It might be a method of choice for those patients who are refractory to less laborious conventional therapies.
5. Conclusion
To sum up, our study is in line with the previous findings and is suggestive of possible benefit of CP therapy in COVID-19 patients. It also suggests PE cycles prior to CP transfusion as a possible complementary treatment in critical patients to reduce the inflammation and then replenish the patients’ plasma with SARS-CoV-2-specific antibody-containing plasma. Further controlled investigations with larger sample size are required to prove the efficacy of the suggested protocol compared to the other conventional therapies.
Authorship contributors
A.P., S.S., E.R., A.H.; Participated in designed experiments and critical revision of the manuscript; G.S, M.S., A.J., A.S; performed process treatment. S.M.H., M.R.M.; data collection and analyzed the data. All authors revised the manuscript and approved the final paper
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgment
The authors would like to thank the staff of the Hematopoietic Stem Cell Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran for providing the possibility of performing the study.
References
- 1.Yi C.W., Ching S.C., Yu J.C. 2020. The outbreak of COVID-19: an overview. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P., et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatipoğlu N. The" new" problem of humanity: new coronavirus (2019-nCoV/COVID-19) disease. Medical J. Bakirkoy. 2020;16 doi: 10.5222/BMJ.2020.22931. [DOI] [Google Scholar]
- 5.Chen Z.M., Fu J.F., Shu Q., Chen Y.H., Hua C.Z., Li F.B., et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020:1–7. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yi Y., Lagniton P.N., Ye S., Li E., Xu R.-H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadevall A., Pirofski L.-a. The convalescent sera option for containing COVID-19. J Clin Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakhshaei P., Kazemi M.H., Golara M., Abdolmaleki S., Khosravi-Eghbal R., Khoshnoodi J., et al. Investigation of the cellular immune response to recombinant fragments of filamentous hemagglutinin and pertactin of Bordetella pertussis in BALB/c mice. J Interferon Cytokine Res. 2018;38:161–170. doi: 10.1089/jir.2017.0060. [DOI] [PubMed] [Google Scholar]
- 10.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roback J.D., Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020 doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan A.A. Therapeutic plasma exchange: a technical and operational review. J Clin Apher. 2013;28:3–10. doi: 10.1002/jca.21257. [DOI] [PubMed] [Google Scholar]
- 13.Lu W., Kelley W., Fang D.C., Joshi S., Kim Y., Paroder M., et al. The use of therapeutic plasma exchange as adjunctive therapy in the treatment of coronavirus disease 2019: a critical appraisal of the current evidence. J Clin Apher. 2021;12(February) doi: 10.1002/jca.21883. doi: 10.1002/jca.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves H.M., Winters J.L. The mechanisms of action of plasma exchange. Br J Hematol. 2014;164:342–351. doi: 10.1111/bjh.12629. [DOI] [PubMed] [Google Scholar]
- 15.Winters J.L. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines, Hematology 2010. Book. 2012;2012:7–12. doi: 10.1182/asheducation.V2012.1.7.3797920. [DOI] [PubMed] [Google Scholar]
- 16.Pusey C., Dash C., Garrett M., Gascoigne E., Gesinde M., Gillanders K., et al. Experience of using human albumin solution 4· 5% in 1195 therapeutic plasma exchange procedures. Transfus Med. 2010;20:244–249. doi: 10.1111/j.1365-3148.2010.00999.x. [DOI] [PubMed] [Google Scholar]
- 17.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi S., Kim Mc, Kwon Js, Kim Jy, Lee Kh, Kim S.H. Case report: use of plasma exchange followed by convalescent plasma therapy in a critically ill patient with severe fever and thrombocytopenia syndrome–associated encephalopathy: cytokine/chemokine concentrations, viral loads, and antibody responses. Am J Trop Med. 2018;99:1466–1468. doi: 10.4269/ajtmh.17-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt J.J., Asper F., Einecke G., Eden G., Hafer C., Kielstein J.T. Therapeutic plasma exchange in a tertiary care center: 185 patients undergoing 912 treatments-a one-year retrospective analysis. BMC Nephrol. 2018;19(1):1–7. doi: 10.1186/s12882-017-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan A.A. A simple and accurate method for prescribing plasma exchange. ASAIO J. 1990;36(3) M597-9. PMID: 2252761. [PubMed] [Google Scholar]
- 21.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khorramdelazad H., Kazemi M.H., Najafi A., Keykhaee M., Emameh R.Z., Falak R. Immunopathological similarities between COVID-19 and influenza: investigating the consequences of Co-infection. Microb Pathog. 2020 doi: 10.1016/j.micpath.2020.104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander V., Zachariah U., Goel A., Kandasamy S., Chacko B., Punitha J.V., et al. Low-volume plasma exchange and low-dose steroid to treat secondary hemophagocytic lymphohistiocytosis: a potential treatment for severe COVID-19? Current Med. 2020;18:77. doi: 10.4103/cmi.cmi_48_20. [DOI] [Google Scholar]
- 26.Matsuda K., Moriguchi T., Oda S., Hirasawa H. Efficacy of continuous hemodiafiltration with a cytokine-adsorbing hemofilter in the treatment of acute respiratory distress syndrome. Acute Blood Purif. 2010:83–92. doi: 10.1159/000314856. [DOI] [PubMed] [Google Scholar]
- 27.Geri G., Terrier B., Heshmati F., Moussaoui H., Massot J., Mira J.P., et al. Effect of plasma exchange in acute respiratory failure due to Anti-neutrophil cytoplasmic antibody-associated vasculitis. Crit Care. 2018;22:1–3. doi: 10.1186/s13054-018-2264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel P., Nandwani V., Vanchiere J., Conrad S.A., Scott L.K. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A—an associated respiratory failure and hemodynamic shock. Crit Care Med. 2011;12:e87. doi: 10.1097/PCC.0b013e3181e2a569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Focosi D., Anderson A.O., Tang J.W., Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin Microbiol Rev. 2020;33(4) doi: 10.1128/CMR.00072-20. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieto-Aristizábal I., Vivas A.J., Ruiz-Montaño P., Aragon C.C., Posso-Osorio I., Quiñones J., et al. Therapeutic plasma exchange as a treatment for autoimmune neurological disease. Autoimmune Dis. 2020 doi: 10.1155/2020/3484659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khamis F., Al-Zakwani I., Al Hashmi S., Al Dowaiki S., Al Bahrani M., Pandak N., et al. Therapeutic plasma exchange in adults with severe COVID-19 infection. Int J Infect Dis. 2020;99:214–218. doi: 10.1016/j.ijid.2020.06.064). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaiswal V., Nasa P., Raouf M., Gupta M., Dewedar H., Mohammad H., Al Rais Z., Baqer M.A., Alsabbah A., Ibrahim Y., Salem M. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19—an exploratory study. Int J Infect Dis. 2020;102(January):332–334. doi: 10.1016/j.ijid.2020.10.085. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seghatchian J., Lanza F. Convalescent plasma, an apheresis research project targeting and motivating the fully recovered COVID 19 patients: a rousing message of clinical benefit to both donors and recipients alike. Transfus Apher Sci. 2020;59(June 3) doi: 10.1016/j.transci.2020.102794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanza F., Seghatchian J. Reflection on passive immunotherapy in those who need most: some novel strategic arguments for obtaining safer therapeutic plasma or autologous antibodies from recovered COVID‐19 infected patients. Br J Haematol. 2020;190(July 1):e27–29. doi: 10.1111/bjh.16814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. PNAS. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kesici S., Yavuz S., Bayrakci B. Get rid of the bad first: therapeutic plasma exchange with convalescent plasma for severe COVID-19. PNAS. 2020 doi: 10.1073/pnas.2006691117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortenberry J.D., Nguyen T., Grunwell J.R., Aneja R.K., Wheeler D., Hall M., et al. Therapeutic plasma exchange in children with thrombocytopenia-associated multiple organ failure: the thrombocytopenia-associated multiple organ failure network prospective experience. Crit Care Med. 2019;47:e173–e181. doi: 10.1097/CCM.0000000000003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiswal V., Nasa P., Raouf M., Gupta M., Dewedar H., Mohammad H., Al Rais Z., Baqer M.A., Alsabbah A., Ibrahim Y., Salem M. Therapeutic plasma exchange followed by convalescent plasma transfusion in critical COVID-19—An exploratory study. Int J Infect Dis. 2020;102:332–334. doi: 10.1016/j.ijid.2020.10.085. Int J Infect Dis. 2021 Jan;102:332-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu N., Zhang T., Ma L., Zhang H., Wang H., Wei W., et al. The impact of ABO blood group on COVID-19 infection risk and mortality: a systematic review and meta-analysis. Blood Rev. 2020;8(December) doi: 10.1016/j.blre.2020.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Göker H., Karakulak E.A., Demiroğlu H., Ceylan ÇM., Büyükaşik Y., Inkaya A.Ç, et al. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J Med Sci. 2020;50(June 4):679–683. doi: 10.3906/sag-2005-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muñiz-Diaz E., Llopis J., Parra R., Roig I., Ferrer G., Grifols J., et al. Relationship between the ABO blood group and COVID-19 susceptibility, severity and mortality in two cohorts of patients. Blood Transfus. 2021;19(January 1):54–63. doi: 10.2450/2020.0256-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammadali F., Pourfathollah A. Association of ABO and Rh blood groups to blood-borne infections among blood donors in Tehran-Iran. Iran J Public Health. 2014;43(July 7):981–989. PMID: 25909065. [PMC free article] [PubMed] [Google Scholar]
- 43.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eskilsson A., Mirrasekhian E., Dufour S., Schwaninger M., Engblom D., Blomqvist A. Immune-induced fever is mediated by IL-6 receptors on brain endothelial cells coupled to STAT3-dependent induction of brain endothelial prostaglandin synthesis. J Neurosci. 2014;34(November 48):15957–15961. doi: 10.1523/JNEUROSCI.3520-14. [DOI] [PMC free article] [PubMed] [Google Scholar]