Abstract
Mature cystic teratomas are rare neoplasms composed of tissues from at least two of the germ layers. In the adult male pelvis, these tumors are exceptionally rare; only a small number of cases have been reported in the literature. We describe the case of a 76-year-old male with an extensive mature cystic teratoma in the rectovesical space, perineum, scrotum, and gluteal folds. This was misdiagnosed and managed as a chronic prostate abscess for six years. Few cases in the literature have reported mature cystic teratomas presenting as abscesses in male patients, and none in the male pelvis. This presentation should prompt physicians to consider the diagnosis of teratoma when managing similar cases, especially if cultures are negative and the symptoms recur despite treatment.
Keywords: Teratoma, Dermoid cyst, Abscess, Prostate, Pelvis
Introduction
Mature cystic teratomas (MCTs), also known as dermoid cysts, are rare tumors composed of tissues from at least two of the three germ layers: endoderm, ectoderm, and mesoderm. They are more frequently seen in the female pelvis and usually arise from the ovary. However, they are extremely rare in the male pelvis, especially in older patients. Only seven cases of MCT in the adult male pelvis have been reported [1], [2], [3], [4], [5], [6], [7].
MCTs are usually asymptomatic and discovered incidentally; however, patients may present with a palpable mass or symptoms related to mass effect on adjacent structures, such as urinary tract obstruction or constipation when in the pelvis [8]. Two cases have reported retroperitoneal teratomas presenting as abscesses in male patients [9,10], with no prior case to our knowledge of this in the male pelvis.
We describe for the first time a case of an extensive pelvic MCT in an adult male involving the rectovesical space, perineum, scrotum, and gluteal folds, which was misdiagnosed and managed as a chronic prostatic abscess for six years.
Case report
A 76-year-old male with a history of hypertension but no other significant medical problems initially presented at an outside institution in August 2014 with urinary retention which was attributed to benign prostatic hyperplasia. A transurethral resection of the prostate (TURP) was attempted, but the cystoscopy at the time of the procedure revealed a cystic prostatic area felt to be consistent with an abscess, and the operation was aborted. The patient was referred to interventional radiology (IR), who treated the collection with two percutaneously placed gluteal drains for eight weeks. The symptoms initially improved; however, the patient returned in July 2015 with similar symptoms. Computed tomography (CT) imaging showed a large multiloculated pelvic collection abutting and displacing the rectum and prostate (Fig. 1A), and the patient was diagnosed with a recurrent prostatic abscess. He was again managed by IR with two percutaneously placed gluteal drains, this time for twelve weeks. Symptoms again recurred in July 2016, and he was again treated by IR percutaneous drainage, with two gluteal drains and one perineal drain for twelve weeks. CT imaging in November 2016 showed marked reduction of size of the pelvic collection (Fig. 1B), and the drains were removed. Cultures from these procedures were negative, and the patient did not receive any antibiotics throughout the treatments.
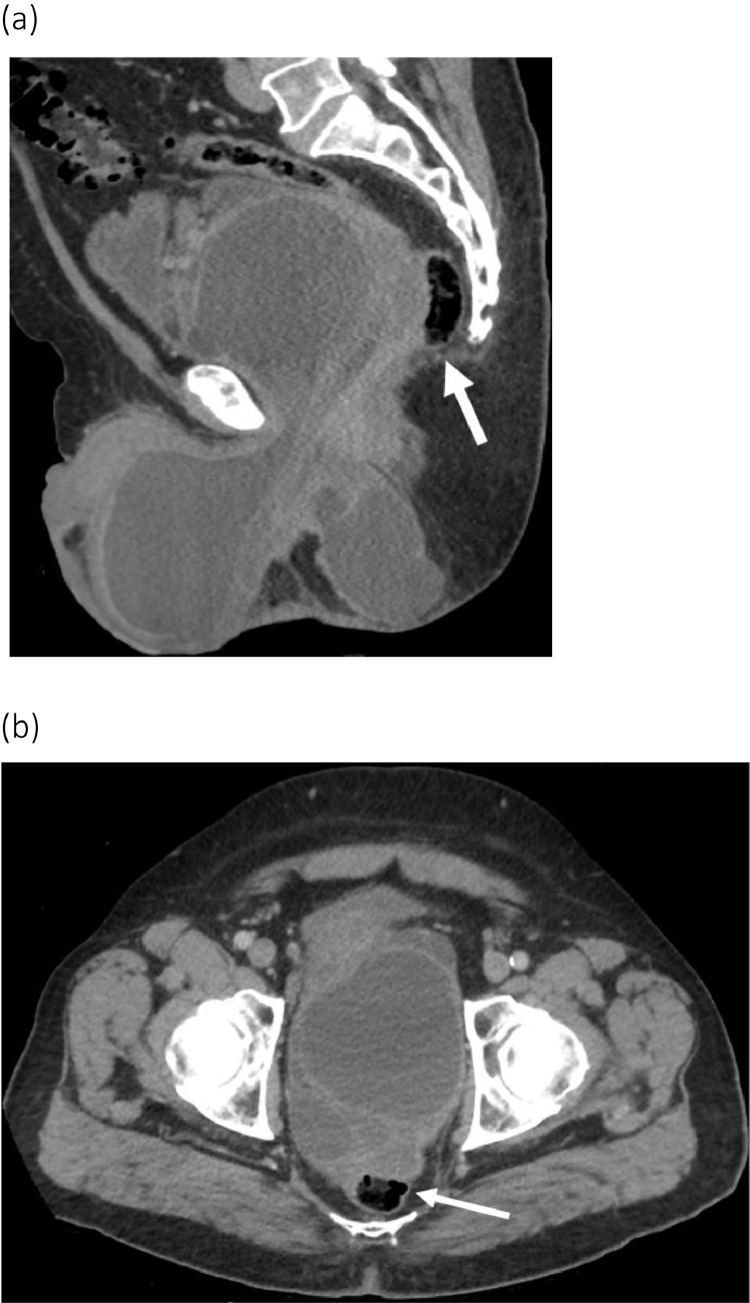
Fig. 1.
Axial contrast enhanced CT scan images of the pelvic MCT before and after drainage. (A) Recurrent rim-enhancing pelvic collection in July 2015 approximately one year after initial drainage measuring 13.8 × 9.6 cm, with mass effect on the rectum (arrowhead) and anteriorly displaced urinary bladder (double arrowhead). (B) After placement of bilateral percutaneous gluteal drains (arrows) in November 2016 in a third attempt at drainage, there is marked decrease size of the collection measuring 7.4 × 6.6 cm, with less mass effect on the rectum and urinary bladder.
In December 2016, the patient developed new symptoms of pain and swelling of the scrotum and the left buttock, in addition to worsening urinary retention and was referred to our institution for a “persistent sterile abscess of the prostate”. The patient was treated with IR drainage two times (December 2016 and March 2017), TURP and abscess unroofing in January 2017, endoscopic abscess unroofing in early March 2017 and prostate laser vaporization and abscess unroofing in late March 2017 with substantial improvement of symptoms. Pathological examination showed benign prostatic tissue with moderate chronic inflammation and fibrosis. Multiple CT scans were performed during this course of treatment and an example CT from March 2017 demonstrates a very large multiloculated rim-enhancing collection in the pelvis exerting mass effect on the prostate and rectum. The mass also extended into the scrotum, perineum, and gluteal folds (Fig. 2). No macroscopic fat was visible on the images. Cultures throughout this time were positive only in two anaerobic cultures in December 2016 and in one prostate tissue culture in January 2017 for Staphylococcus Epidermis from enrichment broth only, which has a strong possibility of contamination [11]. Numerous additional blood, body site and aspirate cultures were negative and the patient did not have a leukocytosis.
Fig. 2.
Recurrent extensive pelvic MCT in March 2017 despite four attempts at drainage and one abscess unroofing. Sagittal (a) and axial (b) contrast enhanced CT scan images contrast enhanced CT scan images showing a 25.4 × 13.5 cm multiloculated, rim-enhancing collection containing debris, extending through the pelvis and scrotum and perineum with mass effect on the rectum (arrow) and prostate.
For the remainder of 2017- 2019, the patient received intermittent aspirations of the pelvic fluid collection by IR at an outside hospital, with complete resolution of symptoms each time. In May 2019, a prostate magnetic resonance imaging (MRI) at the outside hospital showed an extensive multiloculated pelvic collection measuring approximately 12.5 × 9 cm in the rectovesical space intimately associated with the prostate, seminal vesicles, and rectum, and extending into the scrotum, perineum, and gluteal folds (Fig. 3). The mass had internal fluid and enhancing walls and septations, but no internal soft tissue nodularity or enhancement. There was no internal microscopic or macroscopic fat within the mass as assessed by spectral fat saturation and chemical shift imaging. The fluid from one of the aspirations was consistent with seminal fluid, suggesting a fistula to the seminal vesicles. He then returned to our institution in January 2020 for a robotic-assisted excision of the bilateral seminal vesicles, seminal vesicle cysts, and vas deferens by urology. During the surgery, the left seminal vesicle was inflamed and potentially fistulizing to the pelvic collection, which is in line with the drained fluid analysis results showing evidence of seminal fluid. Pathologic specimens of the cyst sac showed fragments of benign mature teratoma. The surgery was well tolerated and the patient was discharged without immediate complications.
Fig. 3.
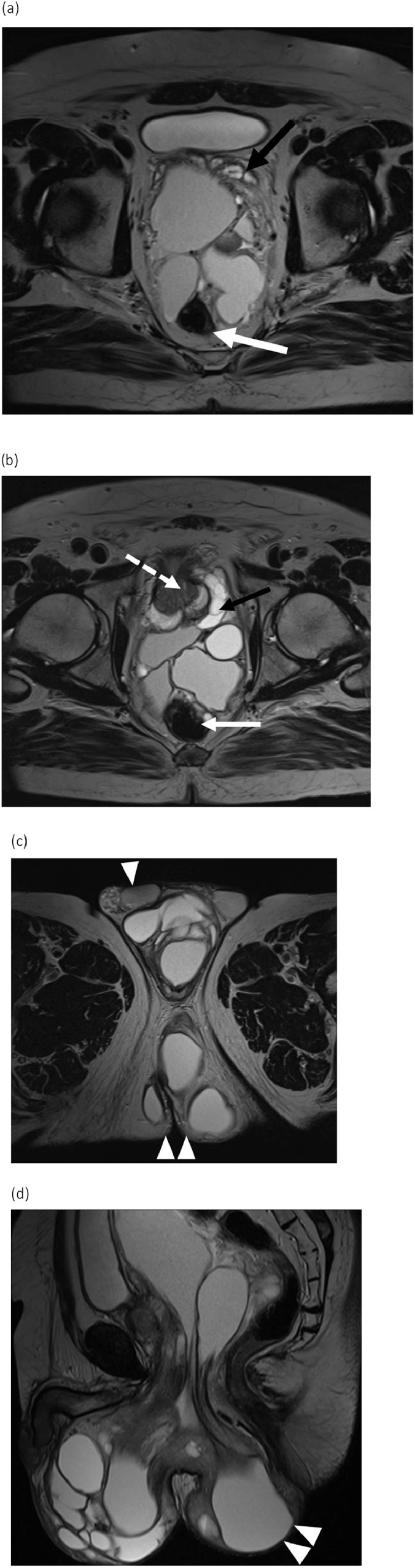
T2-weighted MR images of the pelvic MCT obtained In May 2019. Axial (A, B, C) and sagittal (D) images show a large multiloculated fluid collection in the pelvis extending into the perineum and bilateral gluteal tissues (C and D, double arrowheads) and the scrotal sac with anterior displacement of the testicles (C, arrowhead). The mass abuts and displaces the seminal vesicles (A, black arrow), rectum (A, white arrow) and prostate (B, dashed arrow), noting the prostate is normal in signal.
The patient's symptoms again recurred. In June 2020, surgical resection of the scrotal and perineal components of the teratoma was performed by urology. Two weeks later, the patient presented to the emergency department with worsening perineal swelling and severe pain. On laboratory work-up, the patient did not have a leukocytosis but did have a bandemia. CT imaging showed no significant change of the pelvic collection. To reduce the mass effect-related symptoms, IR placed a drain and the fluid sent for analysis. The fluid grew Escherichia coli, and the patient improved on antibiotics.
In August 2020, the patient underwent further surgery by a multi-disciplinary team of urology, colorectal surgery and plastic surgery. Excision of the pelvic portion of the teratoma including removal of the perirectal portion, dissection of the adherent teratoma off the posterior urethra and resection and closure of the complex perineal wound were performed. The pathological examination of the surgical specimen was again consistent with MCT (Figs. 4 and 5). No calcifications or fat components were present in any of the pathological specimens.
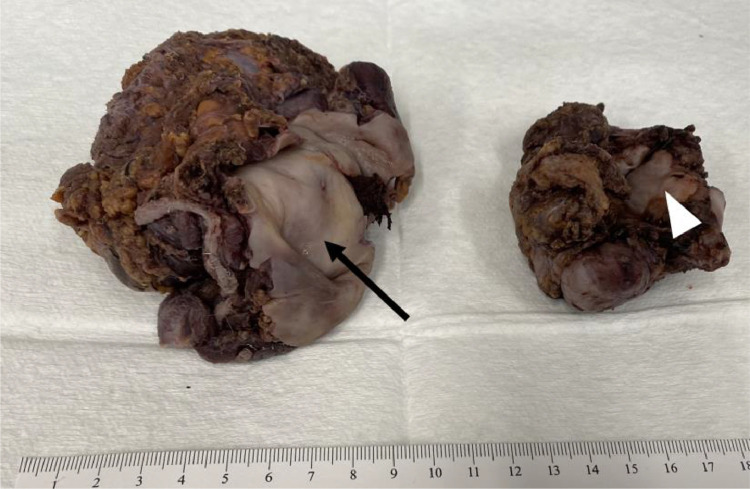
Fig. 4.
Gross pathology specimens of the pelvic mature cystic teratoma. Two pieces of fibrous soft tissue are shown, the larger one on the left measures 10.2 × 9.1 × 3.6 cm, and the smaller ring-shaped specimen on the right measures 7.3 × 5.5 × 4.0 cm. There are areas of tan-white, smooth, glistening tissue resembling colonic mucosa on one side of the first mass (arrow) and on the inner ring portion of the second mass (arrowhead).
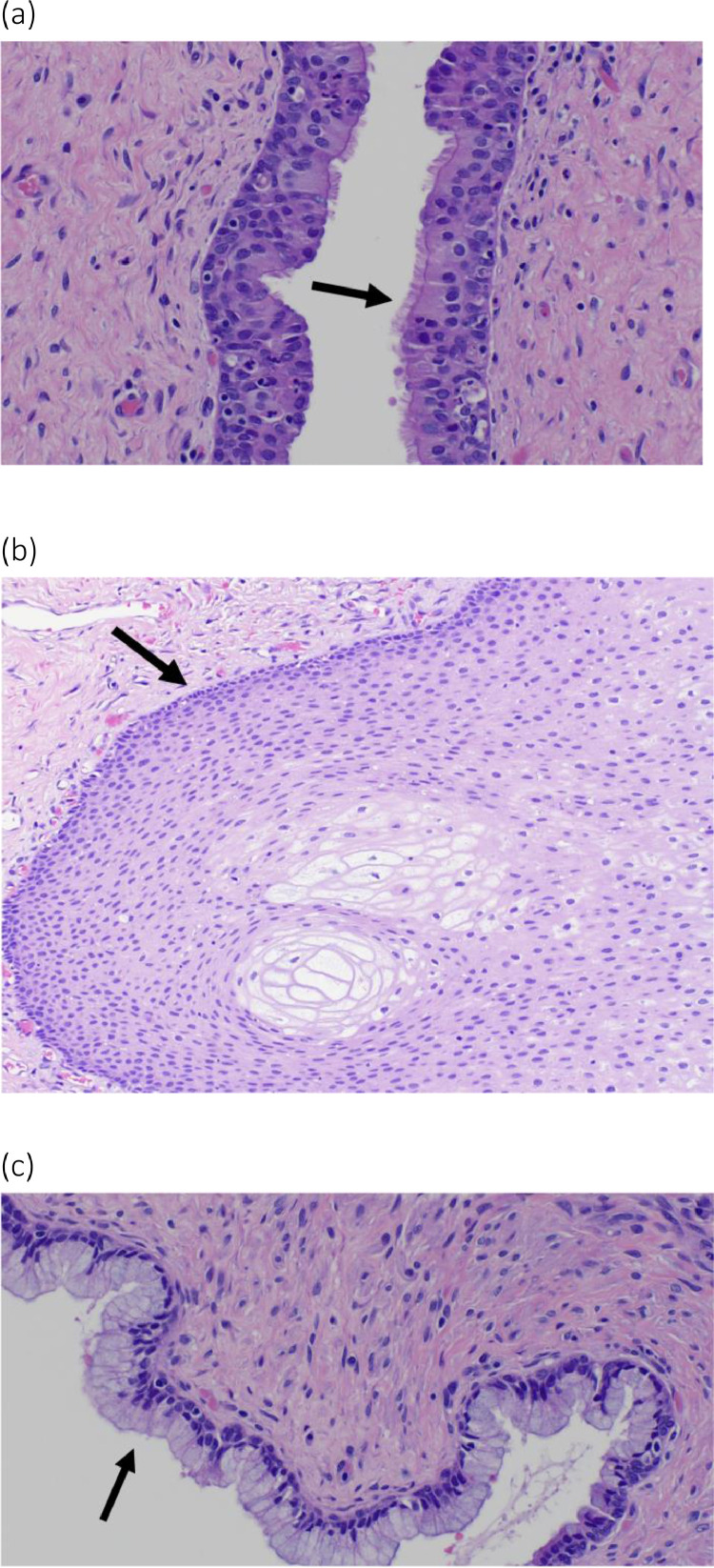
Fig. 5.
High power microscopic views of the pelvic MCT. Respiratory (A), squamous (B) and mucinous epithelium (C) were present (arrows), comprising the three different germ cell layers of the MCT.
On the first follow-up visit four weeks after the surgery, the patient was satisfied with the outcome and reported complete resolution of symptoms. The surgical team plans to continue following up with the patient to assess the surgery outcome both clinically and with diagnostic imaging.
Discussion
We presented the first reported case of a massive pelvic MCT in an adult male involving the rectovesical space, perineum, scrotum and gluteal folds, which was mistaken for and managed as a chronic prostatic abscess for six years.
Teratomas are rare tumors composed of tissues from at least two of the three germ layers: endoderm, ectoderm and mesoderm. Teratomas may be classified according to their epithelial lining into epidermoid, dermoid and teratoid teratomas. Dermoid teratomas contain dermoid elements in addition to the squamous epithelium seen in the epidermoid type. Teratoid teratomas contain cells derived from all three germ layers. Our case is an example of teratoid teratoma, as the surgical specimen contained squamous, respiratory, and mucinous epithelium (Figs. 5) [2,12].
Teratomas are thought to be related to abnormal germ cell migration during the embryogenesis process; this occurs along the embryonic fusion lines, which account for their midline and paramedian location [8] as seen in this case. These tumors have been reported in the intracranium, neck, mediastinum, intraperitoneum, retroperitoneum, presacral and coccygeal regions. In the retroperitoneum, teratomas account for 11% of primary retroperitoneal tumors and tend to occur in the pediatric age group [4,13]. MCTs are more often seen in the female pelvis and usually arise from the ovary, however in the male pelvis are extremely rare, especially in older patients [1]. Few cases in the adult male pelvis have been reported [[1], [2], [3], [4], [5], [6], [7]]. MCTs presenting in multiple locations in the body have been mentioned in a few reports, including four cases of double location and one case of MCT within three separate body compartments [14]. Colpan et al. previously reported a case of intracranial MCT in an 8-year-old male who also was found to have additional MCT in the chest and the right iliac region [14]. In our case, the presence of the dermoid tissue in the mesocolon space, scrotum, base of the penis, perineum, and gluteal clefts is believed to be an extension of the lesion rather than multiple locations.
MCTs are usually asymptomatic and discovered incidentally; however, patients may present with symptoms related to mass effect on adjacent structures, including urinary tract obstruction, as seen in this case. Patients also may present with chemical peritonitis related to dermoid rupture ([4,8]. Chronic infection of dermoid cysts is a known complication with associated local abscess and fistulous tract formation to adjacent structures, including the skin [12,15]. In this case, the patient was initially diagnosed with a chronic prostate abscess without consideration for an MCT even though he lacked infectious symptoms and had essentially negative cultures. Despite multiple percutaneous drainages over six years, the mass was recurrent and slowly increased in size. A fistula tract connecting the seminal vesicle to the MCT was also present in our case, noting that histopathologic examination of the resected portions of the prostate and of the entire seminal vesicles showed no evidence of MCT. Few cases in the literature have reported MCTs presenting as an abscess in male patients, and none in the male pelvis [4,10,16].
Imaging is essential for evaluation of MCTs, including diagnosis, assessing the degree of tumor extension such as invasion of adjacent structures and blood vessels, and predicting tumor aggressiveness to help with treatment planning [17]. That being said, a definitive diagnosis cannot be made without surgical resection since benign and malignant tissues can coexist within the teratoma [2,4]).
Imaging findings of MCTs are dependent on the tumor components, but there are several common features. Ultrasound (US) has a sensitivity of 58% and a specificity of 99% in diagnosing MCTs and can show echogenic shadowing components of the dermoid plug (a.k.a. Rokitansky nodule). CT is advantageous to US due to its ability to assess involvement of and mass effect on adjacent structures, macroscopic fat components found in 93% of cases [17], and calcification and ossification found in 50% of malignant tumors [4,18,19]. In our case, the lesion demonstrates cystic characteristics on CT scan, measuring 12-25 Hounsfield units, with no evidence of calcifications on imaging or in the surgical specimen.
MRI is superior to both US and CT scan in evaluating these tumors, which can be confidently diagnosed by identification of fat using fat suppression methods or chemical shift imaging. MRI can also easily identify other elements, including fluid and soft tissue, and detect the fibrous pseudocapsule when present [17,20]. In this case, there was no macroscopic or microscopic fat within the mass on imaging or pathology.
MCTs are treated with surgical resection, and the prognosis after surgery is excellent, with a five-year survival rate of 100%. The risk of malignant transformation of unresected tumors is between 6.8%- 36.3% [4,9]. In our case, the patient was satisfied with the outcome and reported a complete resolution of symptoms one month after his surgery.
Conclusion
MCTs are extremely rare in the male pelvis, with only a small number of reported cases in the literature. Imaging modalities, especially MRI, are useful tools in the diagnosis as well as management planning. Surgery is the treatment of choice and provides a definitive diagnosis for these tumors, which have a good prognosis after resection. MCTs that do not contain fat may be difficult to diagnose, and consideration to this entity should be given in pelvic fluid collections in men who do not have typical infectious symptoms and have recurrence despite multiple treatments.
References
- 1.Van Gelderen W, Al-Hindawi M, Archibald C, Merrie A, Cheng K. Radiological imaging of a massive dermoid in the male pelvis. Australas Radiol. 1995;39(4):408–410. doi: 10.1111/j.1440-1673.1995.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 2.Chalhoub K, Abou Zahr R, Mansour E, Aoun M, Jabbour M. Primary mature cystic teratoma compressing the prostate in a 28-year-old male: a case report and literature review. Urol Case Rep. 2019;2019 doi: 10.1155/2019/8970172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson RG. Dermoid cyst of the rectovesical space: report of a case. Dis Colon Rectum. 1973;16(6):530–531. doi: 10.1007/BF02588884. [DOI] [PubMed] [Google Scholar]
- 4.Tiu A, Sovani V, Khan N, Hooda S. Primary retroperitoneal mature cystic teratoma (dermoid cyst) in a 51-year-old male: case report and historical literature review. SAGE Open Med Case Rep. 2017;5 doi: 10.1177/2050313X17700745. 2050313X17700745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woussen S, De Backer A, Vanhoenacker F. Perineal dermoid cyst. Eurorad. 2015 doi: 10.1594/EURORAD/CASE.12843. [DOI] [Google Scholar]
- 6.Erden A, Ustuner E, Erden I, Kuzu M, Heper A. Retrorectal dermoid cyst in a male adult: case report. Abdom Imaging. 2003;28(5):725–727. doi: 10.1007/s00261-002-0093-4. [DOI] [PubMed] [Google Scholar]
- 7.Sloan M, Fantus RJ, Paner GP, Faris S. Perineal dermoid cyst in a young male. Urol Case Rep. 2020;33 doi: 10.1016/j.eucr.2020.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatcombe HG, Assikis V, Kooby D, Johnstone PA. Primary retroperitoneal teratomas: a review of the literature. J Surg Oncol. 2004;86(2):107–113. doi: 10.1002/jso.20043. [DOI] [PubMed] [Google Scholar]
- 9.Mathur P, Lopez-Viego MA, Howell M. Giant primary retroperitoneal teratoma in an adult: a case report. Case Rep Med. 2010 doi: 10.1155/2010/650424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen CT, Kratovil T, Edwards MJ. Retroperitoneal teratoma presenting as an abscess in childhood. J Pediatr Surg. 2007;42(11) doi: 10.1016/j.jpedsurg.2007.07.053. e21-e3. [DOI] [PubMed] [Google Scholar]
- 11.Morris AJ, Wilson SJ, Marx CE, Wilson ML, Mirrett S, Reller LB. Clinical impact of bacteria and fungi recovered only from broth cultures. J Clin Microbiol. 1995;33(1):161–165. doi: 10.1128/jcm.33.1.161-165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahan H, Arrivé L, Wendum D, le Pointe HD, Djouhri H, Tubiana J-M. Retrorectal developmental cysts in adults: clinical and radiologic-histopathologic review, differential diagnosis, and treatment. Radiographics. 2001;21(3):575–584. doi: 10.1148/radiographics.21.3.g01ma13575. [DOI] [PubMed] [Google Scholar]
- 13.Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush Jr WH, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics. 2011;31(4):949–976. doi: 10.1148/rg.314095132. [DOI] [PubMed] [Google Scholar]
- 14.Colpan M, Unlu A, Erden E, Kanpolat Y. Multilocated mature teratoma: a case report and review of the literature. Acta neurochirurgica. 2004;146(10):1145–1150. doi: 10.1007/s00701-004-0314-4. [DOI] [PubMed] [Google Scholar]
- 15.Erkan N, Agdeniz S, Polat AF, Yildirim M. Retrorectal dermoid cyst Visc Med. 2006;22(1):55–57. [Google Scholar]
- 16.Pandya J, Pai M, Muchhala S. Retroperitoneal teratoma presenting as acute abdomen in an elderly person. Indian J Gastroenterol. 2000;19(2):89. [PubMed] [Google Scholar]
- 17.Barka M, Mallat F, Hmida W, Ahmed KB, Chavey SO, Abdallah AB. Giant primary retroperitoneal teratoma in an adult male: a rare entity. Int J Case Rep Images. 2014;5(8):558–561. [Google Scholar]
- 18.Lewis WT, Nyguen D. Radiological case submission: mature presacral teratoma. Mil Med. 2009;174(2):214–216. doi: 10.7205/milmed-d-04-4207. [DOI] [PubMed] [Google Scholar]
- 19.Outwater EK, Siegelman ES, Hunt JL. Ovarian teratomas: tumor types and imaging characteristics. Radiographics. 2001;21(2):475–490. doi: 10.1148/radiographics.21.2.g01mr09475. [DOI] [PubMed] [Google Scholar]
- 20.Yang DM, Jung DH, Kim H, Kang JH, Kim SH, Kim JH. Retroperitoneal cystic masses: CT, clinical, and pathologic findings and literature review. Radiographics. 2004;24(5):1353–1365. doi: 10.1148/rg.245045017. [DOI] [PubMed] [Google Scholar]