Abstract
Purpose
To investigate risk factors for instrumentation failure (IF) in titanium (Ti) mesh reconstruction for thoracic and lumbar tumors.
Patients and Methods
The clinical data of patients with thoracic or lumbar tumors who received Ti mesh reconstruction via the posterior approach in our hospital from 2013 to 2018 were analyzed retrospectively. The observation indexes included sex, age, BMI, the vertebra resection mode, the number of resected vertebral segments, application of bone cement, radiotherapy, chemotherapy, revision or primary surgery, and primary tumor metastasis. Correlations between these factors and IF were analyzed by Kaplan–Meier survival and logistics regression analyses.
Results
The 178 patients included 108 males and 70 females with a mean age of 48.09±16.21 (6–78) years and a mean follow-up period of 51.18 (24–90) months. The data showed that 17 patients (9.55%) were inflicted with IF, involving the thoracic vertebra in 11 cases, thoracolumbar vertebrae (T12–L1) in 2 cases, and lumbar vertebrae in 4 cases. The mean interval between surgery to IF was 35.18±14.17 (14–59) months. Univariate analysis showed that total vertebral body resection, the number of resected vertebral segments, radiotherapy and multiple tumor resection were potential factors for IF, while multivariate analysis showed that only total vertebral body resection, the number of resected vertebral segments and radiotherapy were independent factors.
Conclusion
Total vertebra resection, the number of resected vertebral segments (≥2) and radiotherapy before and after operation were significant risk factors related to IF.
Keywords: instrumentation failure, titanium mesh, thoracic and lumbar tumors
Introduction
Spinal tumors include primary and metastatic tumors. Primary spinal tumors are relatively uncommon, accounting for less than 2% of all spinal tumors.1 Most spinal tumors are metastatic,2 affecting more than one-third of cancer patients.3 Surgical resection remains the mainstay of treatment for spinal vertebral tumors at present. However, once primary or secondary spinal tumors violate the vertebral column, the spine is easy to be destabilized and therefore robust instrumentation and/or anterior column reconstruction are often required.4 The current reconstruction materials of the anterior column include artificial vertebral bodies (AVBs), and titanium (Ti) meshes in particular, which has been more widely used in clinical practice because of potential effectiveness and relatively low costs.5
However, instrumentation failure (IF) in the surgical management of spinal tumors is not an uncommon occurrence,6–10 with a rate varying from 0% to 40%.7,9,11–14 Important factors of IF reported in the literature include body mass index (BMI), perioperative radiotherapy, the Ti mesh position, the excision site, and the number of fixed vertebral bodies.6–10 Li et al10 reported that perioperative radiotherapy, oblique TMC and BMI >28 were internal fixation failure factors. The purpose of the present study was to investigate the related elements of IF based on Ti mesh reconstruction for thoracic and lumbar tumors of patients with postoperative survival longer than two years by retrospectively analyzing risk factors in 178 patients who received Ti mesh reconstruction and fixation.
Patients and Methods
Patients and Data Collection
The clinical records of all patients with spinal tumors treated with internal fixation in our hospital from 2013 to 2018 were reviewed. The inclusion criteria were patients with thoracic and lumbar spinal tumors who received Ti mesh reconstruction via the posterior surgery. The exclusion criteria were patients with cervical, limb and trunk tumors who had undergone biopsies, artificial vertebral body implants or 3D printing, simple decompression laminectomy or decompression laminectomy plus pedicle screw fixation via the combined anterior and posterior approach, and patients with a follow-up period of less than 24 months. In addition, patients who had no complete medical records and radiographic images were also excluded. Imaging examination included X-ray radiography, CT and MRI. Imaging studies of all patients were reviewed for evidence of implant failure, which was defined as (1) displacement of the implant position, such as cage subsidence or pulling out of the screws; (2) change in spinal alignment, with an increase in sagittal angulation of the construct by more than 5°;15 (4) signs of implant loosening, such as development of halos around the screws; and (5) implant breakage. Finally, 178 patients met the inclusion criteria, of whom 17 patients (9.6%) suffered IF. The study was approved by the ethics committee of Shanghai Changzheng Hospital (Shanghai, China), and informed consent was from all participating patients.
Postoperative Evaluation
The patients were followed up first at three months after discharge by X-ray radiography, and then every 6 months by X-ray radiography, CT, and MRI. Patients who were unable to come for the clinic would be followed up by telephone interviews.
Statistical Analysis
Survival analysis was performed with SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA). The postoperative IF was estimated by the Kaplan-Meier method, and univariate analysis was performed on various possible prognostic factors (including sex, age≥60, BMI≥24, total vertebra resection, the number of resected vertebral segments (single or multiple segments), tumor resection location (T1-T11, T12-L1 or L2-L5), bone cement, radiotherapy, chemotherapy, revision, tumor nature, metastatic cancer, and multiple tumors) by using Log rank test. Survival of the internal implants was defined as the interval from the operation day (Day 0) to the occurrence of IF or the last follow-up date before that. Data that may have impact on failed internal fixation were analyzed. Factors with a P value ≤0.05 in univariate analysis were subjected to multivariate analysis Cox proportional hazards analysis. P values≤0.05 were considered statistically significant.
Results
Patient Data
The 178 patients included 108 males and 70 females ranging in age from 6 to 78 years with a mean of 48.09±16.21 years. The most common vertebra level in these patients was thoracic (n=107/60.11%), followed by thoracolumbar (n=28/15.73%) and lumbar (n=43/24.16%) (Table 1). The mean follow-up duration was 51.18 (24–90) months. At the last follow-up,139 patients survived.
Table 1.
Comparison of Parameters in Two Groups
| Risk Factor | N(%) | Failure | Chi-Square Value | P(Log Rank Test) |
|---|---|---|---|---|
| Gender | 0.615 | 0.433 | ||
| Male | 108 (39.33%) | 12 (11.11%) | ||
| Female | 70 (60.67%) | 5 (7.14%) | ||
| Age ≥60 | 1.444 | 0.229 | ||
| N | 50 (28.01%) | 15 (30.00%) | ||
| Y | 128 (71.91%) | 2 (1.56%) | ||
| BMI≥24 | 1.270 | 0.260 | ||
| N | 136 (76.40%) | 11 (8.19%) | ||
| Y | 42 (23.60%) | 6 (14.29%) | ||
| Total vertebral body resection | 12.119 | 0.000 | ||
| N | 86 (48.31%) | 1 (1.16%) | ||
| Y | 92 (51.69) | 16 (17.39%) | ||
| Vertebral resection segment | 19.838 | 0.000 | ||
| Single section | 130 (73.03%) | 4 (3.08%) | ||
| Multiple segments | 48 (26.97%) | 13 (27.08%) | ||
| Tumor resection location | 0.607 | 0.738 | ||
| Thoracic vertebrae | 107 (60.11%) | 11 (10.28%) | ||
| Thoracolumbar vertebrae | 28 (15.73%) | 2 (7.14%) | ||
| lumbar vertebra | 43 (24.16%) | 4 (9.30%) | ||
| Bone cement | 1.994 | 0.158 | ||
| N | 117 (65.73%) | 14 (11.97%) | ||
| Y | 61 (34.27%) | 3 (4.92%) | ||
| Radiotherapy | 15.279 | 0.000 | ||
| N | 150 (84.27%) | 9 (6.00%) | ||
| Y | 28 (15.73%) | 8 (28.57%) | ||
| Chemotherapy | 0.872 | 0.350 | ||
| N | 146 (82.02%) | 15 (10.27%) | ||
| Y | 32 (17.98%) | 2 (6.25%) | ||
| Revision | 2.820 | 0.093 | ||
| N | 142 (79.78%) | 10 (7.14%) | ||
| Y | 36 (20.22%) | 7 (19.44%) | ||
| Tumor nature | 2.146 | 0.143 | ||
| Benign tumor | 42 (23.60%) | 7 (16.67%) | ||
| Malignant tumor | 136 (76.40%) | 10 (7.35%) | ||
| Metastatic cancer | 3.338 | 0.068 | ||
| N | 103 (57.87%) | 14 (13.59%) | ||
| Y | 75 (42.13%) | 3 (4.00%) | ||
| Multiple tumors | 3.947 | 0.047 | ||
| N | 134 (75.28%) | 9 (6.72%) | ||
| Y | 44 (24.72%) | 8 (18.28%) |
Note: Bold values indicate statistically significant values.
IF
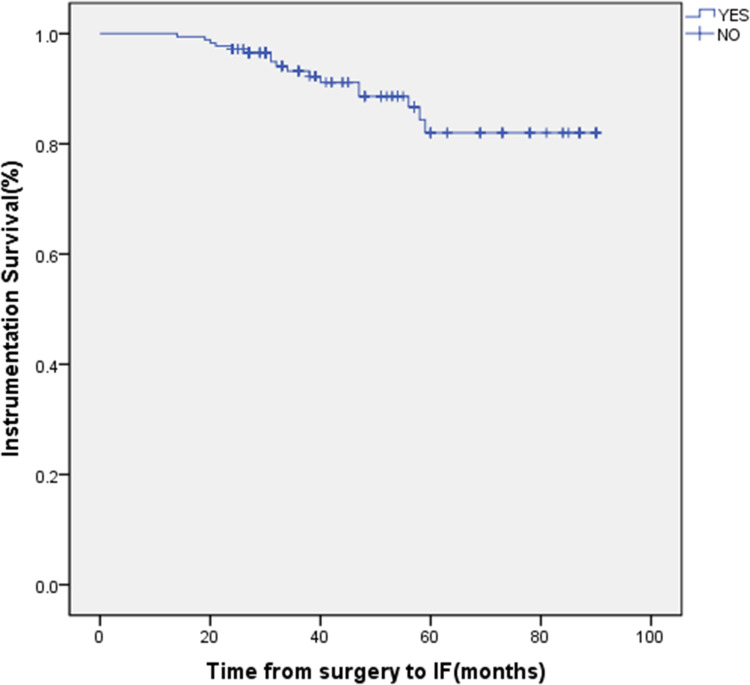
The interval time between surgery and IF occurrence of the 17 IF cases was 14–59 months with a mean of (35.18±14.17) months, including 12 cases (70.59%) receiving total en bloc spondylectomy (TES), and 5 cases receiving non-TES. IF included bilateral rod fracture in 8 cases, unilateral rod fracture in 4 cases, screw loosening in 2 cases, screw and nut loosening in 1 case, Ti mesh dislocation in 1 case, and Ti mesh fracture in 1 case. Rod fracture occurred in the lower edge of the Ti mesh in 12 cases (70.59%) and 2 cases in the upper edge of the Ti mesh in 2 cases (11.8%) (Tables 2 and 3). Kaplan-Meier analysis showed that the survival rate of IF was 96.84%, 89.69%, 77.42% and 43.44% at 24, 36, 48 and 60 months after operation respectively (Figure 1).
Table 2.
Information Table of Instrumentation Failure
| No. | Sex | Age | Weight | Height | BMI | Revision | DV | TVBR | TES | BC | RT | CT | MT | BT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 33 | 45 | 160 | 17.50 | N | T1-T4 | Y | N | N | Y | N | N | Y |
| 2 | M | 32 | 69 | 170 | 24.00 | Y | T3-T5 | Y | Y | N | N | N | N | N |
| 3 | M | 54 | 75 | 168 | 26.60 | Y | T4-T6 | Y | Y | N | Y | N | N | N |
| 4 | M | 17 | 65 | 173 | 21.70 | Y | T4-T5 | Y | Y | N | N | N | N | Y |
| 5 | M | 64 | 62 | 167 | 22.23 | N | T5-T7 | Y | Y | N | Y | N | N | N |
| 6 | M | 26 | 75 | 185 | 21.90 | N | T8-T9 | Y | Y | N | N | N | N | Y |
| 7 | M | 16 | 80 | 173 | 26.70 | N | T11 | Y | Y | N | N | N | N | Y |
| 8 | M | 54 | 60 | 165 | 22.00 | N | T12-L1 | Y | Y | N | N | N | Y | N |
| 9 | F | 65 | 37 | 155 | 15.40 | N | T8-T9 | N | N | Y | Y | Y | Y | N |
| 10 | M | 52 | 70 | 170 | 24.20 | N | L2 | Y | N | N | N | N | N | Y |
| 11 | M | 34 | 65 | 172 | 21.90 | N | L2 | Y | Y | N | Y | N | N | N |
| 12 | F | 26 | 43 | 167 | 15.40 | N | L1 | Y | N | N | N | N | N | Y |
| 13 | F | 46 | 60 | 160 | 23.40 | Y | L2-L3 | Y | Y | N | Y | N | Y | N |
| 14 | F | 34 | 50 | 160 | 19.50 | N | T9-T10 | Y | Y | N | Y | N | N | N |
| 15 | M | 40 | 65 | 165 | 23.80 | Y | T10-11 | Y | Y | Y | N | N | N | N |
| 16 | M | 58 | 84 | 178 | 26.50 | Y | L4-L5 | Y | N | Y | N | N | N | Y |
| 17 | M | 54 | 76 | 168 | 26.90 | Y | T4-7 | Y | Y | N | Y | Y | N | N |
Table 3.
Information Table of Instrumentation Failure
| No. | IF | TPORFOL | TR | IFT:(Mos.) | Diagnosis | Main Symptoms |
|---|---|---|---|---|---|---|
| 1 | Double rods | Lo | N | 21 | Giant cell tumor of bone | Fatigue of right lower limb with movement disturbance. |
| 2 | Double rods | Upp | N | 47 | Invasive osteoblastoma | No obvious symptoms |
| 3 | Left rod | Lo | N | 20 | Chondrosarcoma | Nocturnal pain, and aggravation of standing and walking, weakness of both lower limbs, hypothermia of pain and temperature below the level of bilateral nipples, chest bandage sensation. |
| 4 | Right rod | Lo | N | 40 | Hemangioma | Chest and back pain, weakness of both lower limbs. |
| 5 | Double rods | Lo | Y | 31 | Chondrosarcoma | Pain |
| 6 | Left rod | Lo | N | 14 | Hemangioma | The left chest and back pain when bending down. |
| 7 | Double rods | Lo | N | 34 | Aneurysmal bone cyst | Pain |
| 8 | Double rods | Lo | N | 19 | Spinal metastasis of renal cell carcinoma | Pain |
| 9 | Screw loose | Lo | N | 47 | Spinal metastasis of esophageal cancer | The affected skin is red, swollen and ulcerated. |
| 10 | Nut and pedicle screw Loose | N | N | 31 | Eosinophilic granuloma | Pain |
| 11 | Double rods | Lo | N | 38 | Invasive osteoblastoma | Pain |
| 12 | Double rods | Lo | N | 32 | Giant cell tumor of bone | Pain |
| 13 | Titanium mesh fracture | N | Y | 24 | Spinal metastasis of lung cancer | Pain |
| 14 | Double rods | Lo | N | 58 | Epithelioid osteoblastoma | No obvious symptoms. |
| 15 | Left rod | Lo | N | 59 | Multiple chondrosarcoma | Hear the sound of metal breaking on the back when walking. |
| 16 | Titanium mesh dislocation | Upp | N | 56 | Giant cell tumor of bone | Pain |
| 17 | Screw loose | N | N | 27 | Chondrosarcoma | No obvious symptoms. |
Abbreviations: Y, yes; N, no; IF, instrumentation failure; TPORFOL, the position of rod fracture or loosening; TR, tumor recurrence; IFT, IF time.
Figure 1.
Kaplan–Meier survival curve of overall survival in patients. The survival rate of IF was 96.84%, 89.69%, 77.42% and 43.44% at 24, 36, 48 and 60 months after operation in all patients studied, respectively.
In the 17 IF patients, clinical symptoms were observed in 8 cases including solitary pain at the lesion site, neurological disturbance in 3 cases, skin swelling and ulceration in 1 case, left thoracicolumbar pain on bending in 1 case, audible sound of metal fracture in the back when walking in 1 case, 3 cases of no apparent symptom in 3 cases, tumor recurrence during internal fixation failure in 2 cases (Table 3). All the IF patients received revision surgery, including extended resection for patients with tumor recurrence, and Ti mesh and double rod replacement for patients with broken rods. Whether to increase upper and lower segmental vertebral fixation was determined by intraoperative spinal stabilization (Figures 2 and 3). Patients with Ti fracture or displacement underwent artificial vertebral body replacement. In the 3 patients with loose screws, the loose screws were replaced by bone cement screws during the operation. At the same time, one adjacent vertebral body was instrumented to reinforce the stability of the spine. For the patients with Ti mesh displacement, revision surgery was performed, including replacement of the new Ti mesh and sagittal compression during operation (Tables 2 and 3). The above treatment programs were performed only after full communication with the patients and their families and obtainment of informed consent from the patients and their families. The iliac bone from the patient was implanted around the internal implant. Meanwhile, an intraoperative X-ray examination demonstrated that the sagittal and coronal planes of the implants were all in place. One patient with metastatic cancer died one year after operation.
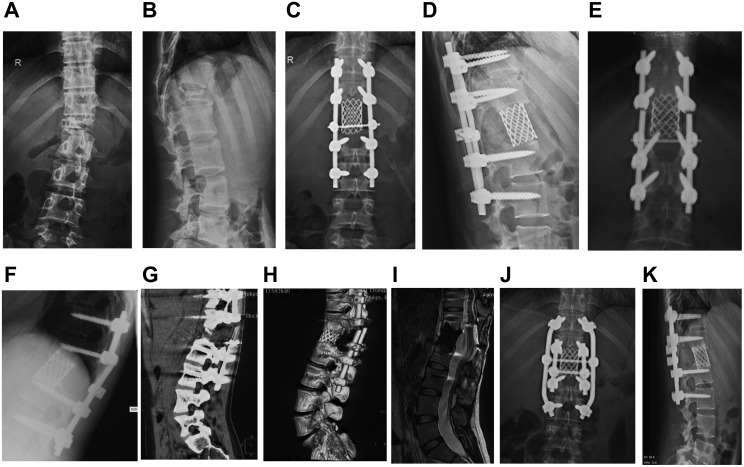
Figure 2.
Typical Case 1: A 26-year-old female patient with L1 giant cell tumor of bone. (A and B) Positive and lateral X-ray films of the lumbar spine when the patient was admitted for the first time. Pathological fracture of the lumbar vertebra, noticeable compression of the vertebral body, and spine instability were observed. (C and D) X-ray films of the positive and lateral positions of the lumbar vertebrae after tumor resection. The L1 vertebral body was resected intraoperatively, showing good positive and lateral positions of the instrumentation. (E and F) Positive and lateral X-ray films with double rods broken 32 months after operation. Broken rods occurred at the upper edge of the titanium mesh, and the titanium mesh was embedded into the upper vertebral body. (G and H) The coronal plane of lumbar CT and 3D reconstruction shows broken rods and spinal instability. (I) No tumor recurrence s found on T2-weighted MRI of the lumbar vertebrae. (J and K) The positive and lateral x-ray film after revision, fixed with double rods during the operation.
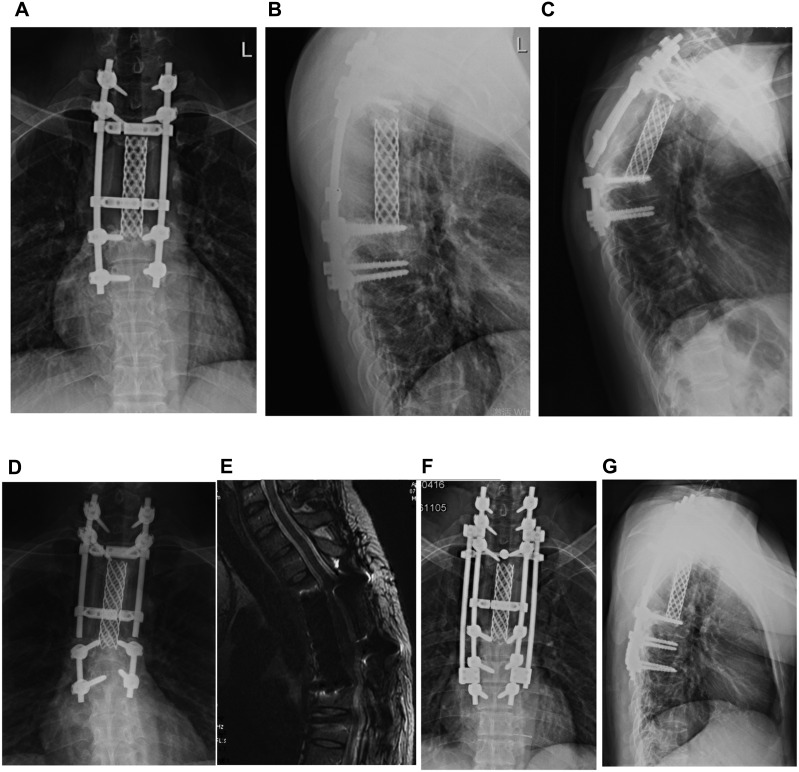
Figure 3.
Typical case 2: internal fixation failure occurred in a 32-year-old male patients 47 months after T3-5 invasive osteoblastoma operation. (A and B) The positive and lateral X-ray internal fixation was good before the rods are broken. (C and D) The positive and lateral X-ray of the broken rods. (E) No tumor recurrence found on T2-weighted MRI of the lumbar vertebrae. (F and G) The positive and lateral X-ray film after revision, fixed with double rods during the operation. Simultaneously, a pair of pedicle screws were added to the seventh cervical vertebra for fixation.
Risk Factor Analysis
Risk factors related to IF were analyzed using the Log rank test of Kaplan-Meier survivorship analysis. Among them, sex, age≥60, BMI≥24, total vertebra body resection, the number of resected vertebral segments, tumor resection location, bone cement, radiotherapy, chemotherapy, revision, tumor nature, metastatic cancer and multiple tumors were not significantly related to IF (Table 1). IF occurred in 16 (17.4%) of the 92 patients with total vertebral body resection vs 1 (1.2%) of the 86 patients with non-total vertebral resection; 4 (3.1%) of the 130 patients with single vertebral resection vs 13 (27.1%) of the 48 patients with non-single vertebral body resection; 8 (28.6%) of the 28 patients receiving radiotherapy vs 9 (6%) of the 150 receiving no radiotherapy; 8 (18.2%) of the 44 patients receiving multiple tumor resection vs 9 (6.7%) of the 134 patients receiving single tumor resection (Table 1).
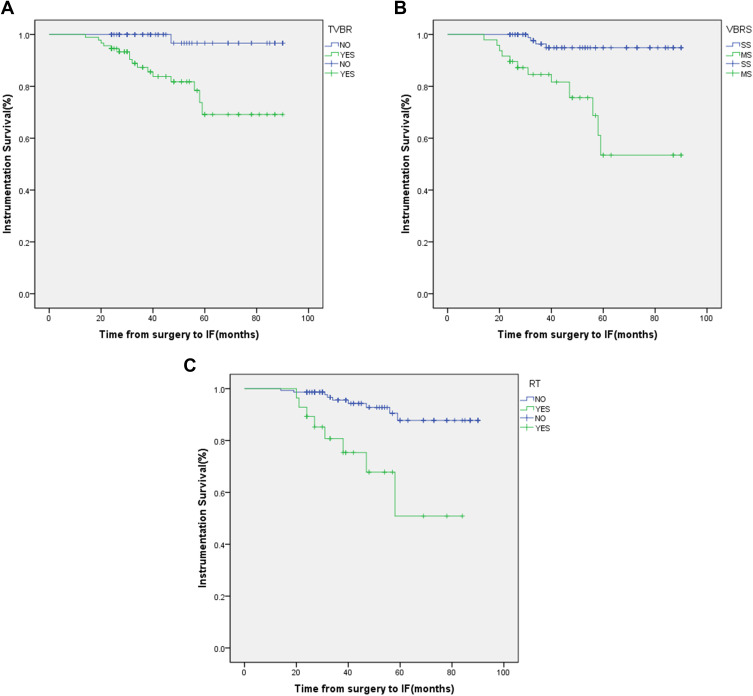
The result of univariate analysis suggested that total vertebral body resection (p=0.000), the number of resected vertebral segments (p=0.000), radiotherapy (p=0.000) and multiple tumor resection (p=0.047) were statistically significant factors related to IF (Table 1). The result of multivariate analysis showed that total vertebral body resection (p=0.040), the number of resected vertebral segments (p=0.015) and radiotherapy (p=0.007) were significant independent factors of IF (Table 4). Meanwhile, the Kaplan–Meier curves of total vertebral body resection, the number of resected vertebral segments and radiotherapy were significant influencing factors of IF (Figure 4A–C).
Table 4.
Cox Logistics Regression Analysis of Factors Related to Instrumentation Failure
| Risk Factor | B | HR(95% CI) | p-value |
|---|---|---|---|
| Total vertebral body resection | |||
| Y | 2.152 | 8.600 (1.101–67.197) | 0.040 |
| N | |||
| Vertebral resection segment | |||
| Single section | 1.479 | 4.389 (1.338–14.402) | 0.015 |
| Multiple segments | |||
| Radiotherapy | |||
| Y | 1.327 | 3.771 (1.429–9.952) | 0.007 |
| N | |||
| Multiple tumors | |||
| Y | 0.420 | 1.522 (0.567–4.083) | 0.404 |
| N | |||
Note: Bold values indicate statistically significant values.
Figure 4.
Kaplan-Meier curves. (A) By total vertebral body resection; (B) by the number of resected vertebral segments; (C) by radiotherapy.
Abbreviations: TVBR, total vertebral body resection; VBRS, vertebral body resection segment; SS, single section; MS, multiple segments; RT, radiotherapy.
Discussion
IF as a New Clinical Concern
Remarkable progress has been made in the treatment of cancer patients. But with prolonged survival of cancer patients, more metastatic cases are encountered in clinical practice and many of them need therapies for spinal involvement,8 IF has become a new clinical concern. In this study, we evaluated risk factors of IF based on Ti mesh reconstruction for thoracic and lumbar tumors. IF includes fracture or dislodgment of screws, rods, plates, hooks, and cages,2 among which rod fracture and screw loosening are the most common IF reported in the literature.6,7,16 Of the 178 IF cases in our series, rod fracture occurred in 12 cases (6.7%) and rod loosening in 3 cases (1.7%) (Table 3).
Clinical Incidence and Treatment of IF in Spinal Tumors
IF occurred in 17 (9.6%) of the 178 patients in our series during the mean of 35.18±14.17 (14–59) months follow-up periods. Of the 17 IF cases, 12 (70.6%) received TES (Table 2), which is similar to that in patients with spinal metastases but significantly lower than that in TES patients reported in other studies.14,15,17–20 Park et al reported 15 IF cases (12.1%) in 124 patients with spinal metastasis who underwent corpectomy with instrumentation.6 Sciubba et al reported IF occurrence in 9 (39.1%) of their 23 patients who underwent TES at the lumbar spine.14 According to Matsumoto et al,7 not all IF patients had clinical symptoms and therefore revision surgery was not a necessity in all IF patients. They found that implant failure caused patients to suffer moderate to severe back pain, but none experienced severe neurological deterioration. Bellato et al reported that none of their 9 implant failure patients needed revision surgery.18 There were three asymptomatic IF patients in our series who finally chose revision surgery because of their worry about more severe consequences caused by spinal instability. Two patients with broken rods were given double rods as shown in the typical cases (Figures 2 and 3), and the other patient with loose screws was given a substitute for bone cement screws (Table 3). Compared with the single-rod structure, the double-rod structure can not only provide better control strength but improve the initial correction with fewer complications.21–23
Three Factors Related to IF
It was found in our study that three factors (radiotherapy, total vertebra resection and the number (≥2) of resected vertebral segments were risk factors related to IF. Generally, the history of radiotherapy before and after operation is the most common reported factor related to IF, probably due to weakening of the surrounding normal bone, repair of the surrounding soft tissue, and the decreased bone healing ability caused by radiotherapy.24 To reduce the necessity for postoperative radiotherapy, it is particularly important to minimize intraoperative tumor contamination and get a negative resection margin.9 Of the 28 patients who received radiotherapy before and after the operation in our series, 8 patients had implant failure, with a failure rate of 28.6% (Table 1). The Kaplan–Meier curve and Cox regression analysis demonstrated a significant correlation between radiotherapy and IF (P=0.000 and P=0.007) (Tables 1 and 4). But some scholars believed that implant failure had nothing to do with radiotherapy. For example, Wong et al25 reported the occurrence of IF in 9(10.2%) of their 88 metastatic cancer cases and therefore believed that radiotherapy either before or after spinal operation was associated with a reduced incidence of implant failure. Some studies reported that the affected vertebra became recalcified 1–2 months after destruction of tumor cells by radiotherapy.16 Others argued that reconstitution of the bone stock after radiotherapy could increase the load-sharing ability of the vertebra and thereby reduce the implant failure rate after radiotherapy.17,18 They concluded that radiotherapy could improve implant stability in the early perioperative period but may give rise to late peri-construct failure over a prolonged period.26,27
Complete removal of the vertebral body is another factor affecting IF in our research. There is no doubt that spinal stability is closely related to the integrity of the three spinal columns.28 Since Denis and Ferguson perfected the Dmurf three-column theory in 1984,29,30 it has been widely used in the field of spine. Previous studies reported that anterior reconstruction could prevent the structure’s kyphosis in the early stage, in contrast, stable and successful anterior rebuilding depends on the bony endplate that can resist axial compression of the intact posterior longitudinal ligament, increasing flexion resistance.31 Hence, compared with complete vertebral resection, corpectomy retains part of the bony structure and soft tissue of the anterior column and middle column to a certain extent, which has more advantages in maintaining spinal stability and more dispersed force. That may be why the rate of IF of corpectomy is lower than that of total vertebra resection. Meanwhile, the Ti mesh for anterior reconstruction has a higher elastic modulus than the bone, and there is a tendency to sink or tilt after repeated cyclic loading, which may cause endophyte failure.32
Although Ti mesh and screw rods reconstruct the spinal stability for multi-segmental vertebra resection, spinal instability is more likely than in single vertebra resection before bony fusion and stability are completely achieved. On the other hand, multi-segmental resection can lead to insufficient blood supply to the surrounding tissue, so there is a need for a longer cage than in a single vertebral resection, as well as more difficulty in obtaining robust stabilization.8 So some researchers suggested performing additional anterior rod instrumentation and/or a longer posterior fixation after multilevel spondylectomy.7,33 Although a longer posterior fixation may prevent screw loosening, it does not prevent rod fracture. Katsuhito8 considered CoCr rods or additional rods (three or four rods) in the posterior instrumentation to reinforce the initial stabilization after a lower TES, while Amankulor et al16 found that patients with six adjacent vertebrae had a higher rate of plant failure, with a 2-year failure rate of 6.6%.
Limitations of This Study
Several limitations in this study design have to be taken into account when the results are interpreted. Firstly, this study mainly analyzed factors affecting IF in Ti mesh reconstruction via the posterior surgery for thoracic and lumbar tumors. The effects of some combined anterior and posterior operations on the final results were not discussed. Secondly, the process of cervical Ti mesh reconstruction is mostly performed with a combined anterior and posterior approach, and most of them are fixed merging with front column steel plate fixed, which is very different from that of thoracic and lumbar Ti mesh internal fixation. We did not include patients with cervical Ti mesh reconstruction in this study. Thirdly, this paper is a retrospective study, which means that there may be some differences in the treatment strategies. Fourthly, as this study was based on Ti mesh internal fixation of spinal tumors, including primary tumors and metastatic tumors, the survival status, survival time and bone quality may affect the statistical results. Finally, we did not perform stability test and bending test of instrumentation and lacked a control group. However, the purpose of this paper was to explore the influencing factors of IF based on Ti mesh reconstruction for thoracic and lumbar tumors in an attempt to provide more references for clinicians and reduce the failure rate of internal fixation, though the clinical significance of the results and conclusion of the present study need to be affirmed in more clinical trials.
Conclusion
To the best of our knowledge, this is the first study to address IF based on Ti mesh reconstruction of thoracic and lumbar tumors. We found that total vertebral body resection, the number of resected vertebral segments and radiotherapy were significant independent factors associated with IF, hoping that these finding could give clinicians more treatment references and help them reduce the probability of IF based on Ti mesh reconstruction of thoracic and lumbar tumors for the sake of reducing pain and economic burdens of the patients.
Acknowledgments
This study was supported by Sailing Talent Program of Navy Medical University, Shanghai Youth Science and Technology Talent Sailing Program (20YF1449100), National Natural Science Foundation of China (82002838), Shanghai Science and Technology Committee (Grant No. 17411950300, 17411950301).
Abbreviations
IF, instrumentation failure; Ti, titanium; BMI, body mass index; CT, computed tomography; BMI, magnetic resonance imaging; TES, total en bloc spondylectomy.
Data Sharing Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
All patients provided their written informed consent. This study was approved by the ethics committee of Shanghai Changzheng Hospital, and informed consent was obtained from all patients. This study complied with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the report has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Sohn S, Kim J, Chung CK, Lee NR, Sohn MJ, Kim SH. A nation-wide epidemiological study of newly diagnosed primary spine tumor in the adult Korean Population, 2009–2011. J Korean Neurosurg Soc. 2017;60(2):195–204. doi: 10.3340/jokes.2016.0505.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böhm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br. 2002;84(4):521–529. doi: 10.1302/0301-620x.84b4.12495 [DOI] [PubMed] [Google Scholar]
- 3.Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine. 1990;15(1):1–4. doi: 10.1097/00007632-199001000-00001 [DOI] [PubMed] [Google Scholar]
- 4.Kurisunkal V, Gulia A, Srinath G. Principles of management of spine metastasis. Indian J Orthop. 2020;54(2):181–193. doi: 10.1007/s43465-019-00008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medetbek A, Alexander A, Renat N, Anton S. The use of titanium mesh for defect closure after posterior spinal decompression. Coluna/Columna. 2019;18:322–326. doi: 10.1590/s1808-1851.20191804225594 [DOI] [Google Scholar]
- 6.Park SB, Kim KJ, Han S, et al. Instrumentation failure after partial corpectomy with instrumentation of a metastatic spine. J Korean Neurosurg Soc. 2018;61(3):415–423. doi: 10.3340/jokes.2017.0505.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto M, Watanabe K, Tsuji T, et al. Late instrumentation failure after total en bloc spondylectomy. J Neurosurg Spine. 2011;15(3):320–327. doi: 10.3171/2011.5.SPINE10813 [DOI] [PubMed] [Google Scholar]
- 8.Yoshioka K, Murakami H, Demura S, et al. Risk factors of instrumentation failure after multilevel total en bloc spondylectomy. Spine Surg Relat Res. 2017;1(1):31–39. doi: 10.22603/ssrr.1.2016-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SJ, Lee CS, Chang BS, et al; Korean Spine Tumor Study Group. Rod fracture and related factors after total en bloc spondylectomy. Spine J. 2019;19(10):1613–1619. doi: 10.1016/j.spinee.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Wei F, Liu Z, et al. Risk factors for instrumentation failure after total en bloc spondylectomy of thoracic and lumbar spine tumors using titanium mesh cage for anterior reconstruction. World Neurosurg. 2020;135:e106–e115. doi: 10.1016/j.wneu.2019.11.057 [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka K, Murakami H, Demura S, et al. Clinical outcome of spinal reconstruction after total en bloc spondylectomy at 3 or more levels. Spine. 2013;38(24):E1511–E1516. doi: 10.1097/BRS.0b013e3182a6427a [DOI] [PubMed] [Google Scholar]
- 12.Yanamadala V, Rozman PA, Kumar JI, et al. Vascularized fibular strut autografts in spinal reconstruction after resection of vertebral chordoma or chondrosarcoma: a retrospective series. Neurosurgery. 2017;81(1):156–164. doi: 10.1093/neurons/nyw057 [DOI] [PubMed] [Google Scholar]
- 13.Glennie RA, Rampersaud YR, Boriani S, et al. A systematic review with consensus expert opinion of best reconstructive techniques after osseous en bloc spinal column tumor resection. Spine. 2016;S205–S211. doi: 10.1097/BRS.0000000000001835 [DOI] [PubMed] [Google Scholar]
- 14.Sciubba DM, De la Garza Ramos R, Goodwin CR, et al. Total en bloc spondylectomy for locally aggressive and primary malignant tumors of the lumbar spine. Eur Spine J. 2016;25(12):4080–4087. doi: 10.1007/s00586-016-4641-y [DOI] [PubMed] [Google Scholar]
- 15.Kumar N, Patel R, Wadhwa AC, et al. Basic concepts in metalwork failure after metastatic spine tumor surgery. Eur Spine J. 2018;27(4):806–814. doi: 10.1007/s00586-017-5405-z [DOI] [PubMed] [Google Scholar]
- 16.Amankulor NM, Xu R, Iorgulescu JB, et al. The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J. 2014;14(9):1850–1859. doi: 10.1016/j.spinee.2013.10.028 [DOI] [PubMed] [Google Scholar]
- 17.Drakhshandeh D, Miller JA, Fabiano AJ. Instrumented spinal stabilization without fusion for spinal metastatic disease. World Neurosurg. 2018;111:e403–e409. doi: 10.1016/j.wneu.2017.12.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellato RT, Teixeira WG, Torelli AG, et al. Late failure of posterior fixation without bone fusion for vertebral metastases. Acta Ortop Bras. 2015;23(6):303–306. doi: 10.1590/1413-785220152306151402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedreira R, Abu-Bonsrah N, Karim Ahmed A, et al. Hardware failure in patients with metastatic cancer to the spine. J Clin Neurosci. 2017;45(undefined):166–171. doi: 10.1016/j.jocn.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 20.Park SJ, Lee KH, Lee CS, et al. Instrumented surgical treatment for metastatic spinal tumors: is fusion necessary? J Neurosurg Spine. 2019;1–9. doi: 10.3171/2019.8.SPINE19583 [DOI] [PubMed] [Google Scholar]
- 21.Teoh KH, Winson DM, James SH, et al. Magnetic controlled growing rods for early-onset scoliosis: a 4-year follow-up. Spine J. 2016;16:S34–S39. doi: 10.1016/j.spinee.2015.12.098 [DOI] [PubMed] [Google Scholar]
- 22.Thakar C, Kieser DC, Mardare M, Haleem S, Fairbank J, Nnadi C. Systematic review of the complications associated with magnetically controlled growing rods for treating early-onset scoliosis. Eur Spine J. 2018;27::2062–2071. doi: 10.1007/s00586-018-5590-4 [DOI] [PubMed] [Google Scholar]
- 23.Thompson GH, Akbarnia BA, Campbell RM. Growing rod techniques in early-onset scoliosis. J Pediatr Orthop. 2007;27(3):354–361. doi: 10.1097/BPO.0b013e3180333eea [DOI] [PubMed] [Google Scholar]
- 24.Kim TK, Cho W, Youn SM, Chang UK. The effect of perioperative radiation therapy on spinal bone fusion following spine tumor surgery. J Korean Neurosurg Soc. 2016;59(6):597–603. doi: 10.3340/jokes.2016.59.6.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong YC, Chau WW, Kwok KO, Law SW. Incidence and risk factors for implant failure in spinal metastasis surgery. Asian Spine J. 2020. doi: 10.31616/asj.2020.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunha SS, Sarmento VA, Ramalho LM, et al. Effects of radiotherapy on bone tissues. Radiol Bras. 2007;40:189–192. doi: 10.1590/s0100-39842007000300011 [DOI] [Google Scholar]
- 27.Boden SD. The biology of posterolateral lumbar spinal fusion. Orthop Clin North Am. 1998;29:603–619. doi: 10.1016/S0030-5898(05)70034-1 [DOI] [PubMed] [Google Scholar]
- 28.Schlenk RP, Stewart T, Benzel EC. The biomechanics of iatrogenic spinal destabilization and implant failure. Neurosurg Focus. 2003;15(3):E2. doi: 10.3171/foc.2003.15.3.2 [DOI] [PubMed] [Google Scholar]
- 29.Denis F. Spinal instability as defined by the three-column spine concept in acute spinal trauma. Clin Orthop Relat Res. 1984;undefined(189):65–76. [PubMed] [Google Scholar]
- 30.Ferguson RL, Allen BL. A mechanistic classification of thoracolumbar spine fractures. Clin Orthop Relat Res. 1984;undefined(189):77–88. [PubMed] [Google Scholar]
- 31.Krag MH. Biomechanics of thoracolumbar spinal fixation. A review. Spine. 1991;16(3 Suppl):S84–S99. doi: 10.1097/00007632-199103001-00014 [DOI] [PubMed] [Google Scholar]
- 32.Gercek E, Arlet V, Delisle J, Marchesi D. Subsidence of stand-alone cervical cages in anterior interbody fusion: warning. Eur Spine J. 2003;12(5):513–516. doi: 10.1007/s00586-003-0539-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Disch AC, Schaser KD, Melcher I, et al. Oncosurgical results of multilevel thoracolumbar en-bloc spondylectomy and reconstruction with a carbon composite vertebral body replacement system. Spine. 2011;36(10):E647–E655. doi: 10.1097/BRS.0b013e3181f8cb4e [DOI] [PubMed] [Google Scholar]