Abstract
This study analyzes the content and chemical profile of extractives present in the young phloem of mature trees of maritime pine (Pinus pinaster) and stone pine (P. pinea) in three sites in Portugal located in different climatic environments.
The cross-sites average of extractives was similar in both pines with 38.5% in P. pinea and 37.7% in P. pinaster phloem. The hydrophilic fraction represented 82% and 70% of P. pinea and P. pinaster total extractives respectively, with large contents of phenolic compounds, flavonoids and tannins, and showed very high oxygen scavenging and reducing ability. Lipophilic extractives were present in higher proportion in P. pinaster phloem than in P. pinea phloem, and showed a large content of resin acids, with the predominance of abietic acid in P. pinaster, and dehydroabietic acid in P. pinea phloems, and of alkanoic acids.
P. pinaster and P. pinea have specific defences related to phloem production of resin and phenolic compounds with the ratio phenolic-to-oleoresin compounds higher for P. pinea (4.7 vs 2.3 for P. pinaster) and constant in the three sites. The phytochemical content and composition of the young phloem of P. pinaster and P. pinea showed site differences highlighting the relationship between environment and metabolic production.
Keywords: Pinus pinaster, Pinus pinea, Secondary metabolites, Extractives, Phenolics
Pinus pinaster, Pinus pinea, Secondary metabolites, Extractives, Phenolics.
1. Introduction
Maritime pine (Pinus pinaster Aiton) and stone or Portuguese pine (Pinus pinea L.) are the two most represented pine species in Portugal, and have great socio-economic impact: maritime pine is directed mainly for timber and stone pine for production of pine nuts. Both species are adapted to a Mediterranean climate, frequently coexisting in mixed stands in the west of the Mediterranean basin. Nonetheless, they differ greatly in their susceptibility to pinewood nematode, with P. pinaster considered as very susceptible to pine wilt disease (Vicente et al., 2012; Pimentel et al. 2016, 2017) while P. pinea is quite resistant (Franco et al., 2011; Santos et al., 2012; Pimentel et al. 2016, 2017) even if it may also be a host plant for the nematode (EFSA PLH Panel 2013).
The phloem, located in the inner part of tree barks, is the primary transport tissue for photosynthates, crucial in plant carbon–water interactions, carbon fluxes and signals, and regulates a variety of physiological processes from growth to reproduction in plants (Savage et al., 2016). The phloem is responsible for the distribution and release of many secondary metabolites that act as defense compounds (e.g. alkaloids, flavonoids and glucosinolates) (Heil and Ton, 2008; Turgeon and Wolf, 2009).
The major defenses of pines (Pinus spp) against insects, pests and fungi include both mechanical and chemical mechanisms that can be present constitutively or be induced upon challenge. They may comprise many specialized chemicals such as terpenoids in oleoresin and phenolics, as well as structural features such as thick bark, large resin ducts size and density, and specialized phloem parenchyma cells (Nunes da Silva et al., 2015; Franceschi et al., 2005; Bonello et al., 2006; Wallis et al., 2011; Celedon and Bohlmann, 2019). Concentration of constitutive chemicals (Lattanzio et al., 2006) or of nutrients and stored mobile carbon and nitrogen compounds (that are a potential food source for invading fungi) may also play an important role in disease resistance or tolerance mechanisms (Lahr and Krokene, 2013; Menéndez-Gutiérrez et al., 2018).
The chemical characterization of phloems was reported for several species e.g. chemical analysis of phloem roughly separated from other bark tissues was studied for some hardwood species (e.g., Thornber and Northcote, 1961; Eyles et al., 2007; Şen et al., 2010; Dou et al., 2016) as well as for softwoods (Cardoso et al., 2018), but only a few studies were made for pine species (Pimentel et al., 2016). Some of the chemical studies on phloem target a characterization needed for their valorization (e.g. Dou et al., 2016; Şen et al., 2010).
The role of terpenoids, stilbenes and other phenolic groups on preventing the reproduction and dispersion of nematodes in the plant has been recognized by several authors (Kuroda et al., 2011; Nunes da Silva et al., 2015). Environmental factors may be responsible for modification on the synthesis and accumulation of phenolics and terpenes in conifers, which may be related with host disease susceptibility (Viiri et al., 2001; Blodgett et al., 2005; Wallis et al., 2011; Ramakrishna and Ravishankar, 2011; Sampaio et al., 2016).
We hypothesize that P. pinaster and P. pinea interact differently with the pinewood nematode because of chemical differences in their specific constitutive defense compounds, which will partially determine nematode development or migration through stems.
This study analyzes the content and chemical composition of extractives present in the young phloem of mature trees of maritime pine (P. pinaster) and stone pine (P. pinea) in three sites in Portugal located in different climatic environments and aims at contributing to an understanding of the defense strategies of conifers and the site variation, of secondary metabolites content and composition.
2. Material and methods
2.1. Field sites and phloem sampling
For this work, three sea level coastal forests were selected, with similar well drained sandy soils and located in a climatic gradient from south to north: 1) Site 1, Alentejo (Aberta Nova), the southern location (38°10′39N, 8° 46′75″W); 2) Site 2, 50 km north of Site 1, in Setúbal peninsula (Herdade da Apostiça) (38°32′38″N, 9°8′40″W); Site 3, in Leiria National Pine Forest, the northern location (39°8′50″N, 8°43′45″W). The three forests are dominated by planted and natural regenerated P. pinaster trees with a few plots of natural regenerated P. pinea trees. There is a considerable north-south increase in the average annual temperature, and an important variation in total annual precipitation, with the southernmost area being the driest one, as follows: Site 1, 17.2 ± 0.5 °C and 443.5 ± 160.8 mm; Site 2, 16.3 ± 0.7 °C and 795.7 ± 235.5 mm; and Site 3, 15.9 ± 0.6 °C and 684.6 ± 196.8 mm (1980–2016 annual averages, obtained from http://www.isa.ulisboa.pt/proj/clipick/.data source HadRM3Q0_A1B, Palma, 2017). The three areas have a Mediterranean climate with wet mild winters and a characteristic summer dry season (typically from June to August), with Site 1 and Site 2 classified as Csa -Hot-summer Mediterranean climate, while the northern (Koppen classification; Cs--Summer Drought “Mediterranean; a - hot summer) Site 3 is classified as Csb - Cool-summer Mediterranean climate (Koppen classification; b - warm summer). Therefore, more severe hot and drought conditions during the dry season occur as we move south, and cooler and wetter conditions during the winter as we move north (Köppen climate classification). Site 3 is located in a region considered optimal for growth of P. pinaster, while the region encompassing Site 1 and Site 2 is considered optimal for P. pinea.
The P. pinaster forest in Site 1 has faced considerable decline in previous years, with up to 30 % standing death or dying trees, and a high incidence of the pinewood nematode B. xylophilys and its insect vector (Pimentel et al., 2017).
During the spring of 2013, six trees of each species in each of the three sites (36 trees in total) were randomly selected for this study. A large branch was cut from the south aspect in the lower crown of each tree. Twig portions corresponding to the 2nd and 3rd year of growth were cut from each branch, and their bark was manually separated from the wood with a chisel. The extracted pine bark at this very young age (2-3-year-old) are constituted by phloem with a very thin external epidermal layer and still without visible periderm formation (Nunes da Silva et al., 2015). Subsequently these bark samples will be subsequently called phloem.
2.2. Determination of extractives content
The phloem samples were dehydrated in a freeze-dryer (Sanvac Coolsafe 95-80) and ground individually with a small laboratorial cutting mil.
The samples were extracted successively with dichloromethane for 6 h, ethanol and water for 16 h each, in a Soxhlet apparatus. The extractives content was determined from the mass difference of the solid after drying at 105 °C and reported as a percentage of the dry phloem mass. The experiments were performed in duplicate samples.
2.3. Lipophilic extracts composition
Previous to GC-MS analysis, 2 mg of each dried extract were derivatized in 120 μL of pyridine, and then 80 μL of bis(trimethylsily)-trifluoroacetamide (BSTFA) were added. The mixture was heated at 60 °C for 30 min in an oven. The hydroxyl and carboxyl groups of the components of the extracts were converted into trimethylsilyl (TMS) ethers and esters, respectively (Ferreira et al., 2017).
The derivatized extracts (1 μL) were analysed by GC-MS (EMIS, Agilent 5973 MSD, Palo Alto, CA). The electron energy was 70 eV, and the MS source kept at 220 °C.GC conditions: Zebron 7HGG015-02 column (30 m, 0.25 mm; ID, 0.1 μm film thickness), injector 280 °C. The oven temperature was held initially at 50 °C min−1, ramped to 150 °C at 10 °C min−1, increased to 300 °C at 4 °C min−1, to 370 °C at 5 °C min−1, and finally increased to 380 °C at 8 °C min−1, followed by an isothermal period of 5 min. For quantitative analysis, the characteristic ions of each compound were identified as their TMS derivatives by matching their mass spectra with a GC-MS spectral library (Wiley, NIST) and by comparing their fragmentation profiles with published data (Kolattukudy and Agrawal, 1974; Eberhardt, 2012; Ferreira et al., 2017).
2.4. Composition of polar bioactive compounds
The extracts in ethanol and water obtained successively by Soxhlet extraction were combined and their total phenolic, flavonoid and tannin contents were determined.
The total phenolic content was determined using the Folin–Ciocalteu method (Singleton and Rossi, 1965). Aliquots (100 μL) of extract (0.1–1.0 mg/mL) solutions were mixed with 4 mL of the Folin–Ciocalteu reagent. After 6 min, 4 mL of Na2CO3 (7%) was added to the mixture and incubated for 15 min in a bath at 45 OC. Afterwards the absorbances were taken at 760 nm against a prepared blank. A calibration curve was built using gallic acid as standard (0–150 μg/mL). The total phenolic content was expressed as milligram of gallic acid equivalents (GAE) per gram of dry extract. Triplicate measurements were carried out.
The flavonoid content was determined using the aluminum chloride methodology (Zhishen et al., 1999). Aliquots (1 mL) of the extract (0.1–1.0 mg/mL) solutions, or the catechin standard solution (0.10–1.0 mg/mL), were mixed with 4 ml water and 0.3 ml NaNO2 solution (5 % w/v), and kept for 5 min in the dark. Then 0.3 ml AlCl3 solution (10%) were added, followed by 2 ml NaOH solution (4%) after 6 min. Water (2.4 mL) was added sequentially and the mixture was vigorously shaken. Absorbances were taken at 510 nm after 30 min incubation, against water A standard calibration plot was generated with concentration from 0.10 to 1.0 mg/mL. The concentrations of flavonoids were expressed as mg of catechin (CA) equivalent per gram of extract.
Condensed tannins were determined by the vanillin-H2SO4 method (Abdalla et al., 2014). Aliquots (1.0 mL) of the extract (0.1–1.0 mg/mL) solutions were mixed with 2.5 mL of 1.0% (w/v) vanillin in absolute methanol and then with 2.5 mL of 25% (v/v) sulphuric acid in absolute methanol. A blank solution was prepared with the same procedure without vanillin. Absorbance were taken at 500 nm after 15 min. The tannin content was calculated from a calibration curve using catechin as standard, and expressed as milligram of catechin equivalents (CE) per gram of the extract. Triplicate measurements were carried out.
2.5. Antioxidant activity of polar bioactive compounds
The antioxidant activity of the extracts was determined by two methods: 2,2-diphenyl-1-picryhydrazyl (DPPH), which measures the free radical scavenging capacity, and ferric reducing antioxidant power (FRAP), which measures the ferric reducing power of the sample, by Cai et al. (2004) and Benzie and Strain (1996), respectively.
DPPH (2,2-diphenyl-1-picrylhydrazyl hydrate) is a stable free radical and it is used to test compounds containing antioxidant potential by acting as radical scavengers. The DPPH results were expressed as IC50 value and as mg Trolox equivalents/g extract. A 1: 2 serial dilutions of the initial extracts and a stock solution of Trolox (0.2 mg/ml) in methanol were prepared. An aliquot of 100 μL of each methanolic solution was added to 3.9 ml of a DPPH methanolic solution (24 μg/ml). After 30 min incubation at room temperature in the dark, the absorbances were taken at 515 nm. The blank sample consisted of 100 μl of methanol added to 3.9 ml of DPPH solution.
The radical scavenging activity was calculated by the DPPH inhibition percentage: I % = [(Abs0-Abs1)/Abs0]×100, where Abs0,the absorbance of the blank and Abs1 the absorbance of the extract at different concentrations. The IC50 inhibiting concentration represents the concentration of a sample necessary to sequester 50% of the DPPH radicals and it was obtained by plotting the inhibition percentage against the extract concentrations.
The ferric reducing antioxidant power (FRAP) assay is based on the principle ofreduction of the ferrictripyridyltriazinecomplex (Fe3+-TPTZ) to ferrous tripyridyltriazine (Fe2+-TPTZ) by the presence of antioxidants. The FRAP reagent was obtained by mixing 300 mM sodium acetate buffer (pH 3.6), 10 mM TPTZ (tripyridyl triazine) solution and 20.0 mM FeCl3.6H2O solution in a ratio of 10:1:1 (vol). Aliquots (100 μL) of extract (0.1–1.0 mg/mL) solutions or standard solution was added to 3 ml of the FRAP reagent and the mixture incubated at 37 °C for 30 min. The absorbances were taken at 593 nm in comparison with a blank. Aqueous solutions of known Trolox concentrations in the range of 0–0.5 mMol/L were used for the calibration, and the results expressed as mMol Trolox equivalents/gram dry mass. Triplicate measurements were carried out.
2.6. Statistical analysis
The experimental results are given as mean and standard deviation (SD). The effect of the species, site and their interaction on the phloem chemical composition was determined using two-way ANOVA, followed by Student Newman-Keuls post hoc tests for pairwise comparisons. Statistical significance was set at p < 0.05. All statistical analyses were performed using the Scientific Statistical software the Sigmaplot® statistical software (version 11.0) from Jandal Corporation.
Principal Component Analysis (PCA) and Cluster Analysis (CA) were carried out to analyse the chemical composition of resin acids and alkaloid acids of the lipophilic extract and the composition of the hydrophilic extractives. By PCA, the hyperspace defined by the original variables is reduced to a smaller one defined by the significant principal components (new axes). Thus, the samples can be plotted onto a reduced space where similar samples can be grouped. Agglomerative hierarchical CA was also carried out to assess the existence of the groups of samples suggested by PCA. The Euclidean distance was used as coefficient of similarity between samples and the single linkage method as coefficient of comparison for sample grouping (Mirkin, 1996; Rencher, 2002). In the presented dendrograms, the height of the junctions of the branches, provided on the vertical axis, indicates the dissimilarity/distance between two objects/clusters e.g. the higher the height of the junctions of the branches, the less similar the objects are.
PCA and CA were performed with the Statistica™, version 6 software, from Statsoft, Tulsa, USA.
3. Results
3.1. Extractives content
The extraction yield of lipophilic and hydrophilic compounds of the phloems from P. pinaster and P. pinea growing in three sites of Portugal are presented in Table 1. Extractives represented on average 37.7% in P. pinaster phloem and 38.5% in P. pinea phloemand 38.5% in P. pinea phloem, and comprised mostly ethanol-soluble compounds (on average 19.5% and 22.4% of the phloem, respectively, corresponding to 52% and 58% of the total extractives). The water soluble compounds corresponded on average to 6.3% and 9.3% of the phloem, respectively, while the lipophilic compounds soluble in dichloromethane made up the remaining of the phloem extractives, representing 30% of the total extractives in P. pinaster phloem and 17.5% in P. pinea phloem.
Table 1.
Content (% of dry mass) of lipophilic and hydrophilic extractives in young phloems of Pinus pinaster and Pinus pinea from three sites in Portugal. Mean of six phloem samples and standard deviation.
| Total Extractives | Lipophilic extract. |
Hydrophilic extract. |
||
|---|---|---|---|---|
| Dichloromethane | Ethanol | Water | ||
| Pinus pinaster | ||||
| Site 1 | 38.1 ± 2.3 a | 11.1 ± 1.4 a | 19.4 ± 0.9 a | 7.6 ± 2.8 a |
| Site 2 | 33.7 ± 2.8 ab | 10.5 ± 1.5 a | 17.8 ± 1.7 ac | 5.4 ± 1.6 ac |
| Site 3 | 41.3 ± 2.8 a | 12.2 ± 2.3 a | 21.4 ± 2.7 a | 7.6 ± 1.6 a |
| Mean |
37.7 ± 2.8 |
11.3 ± 1.7 |
19.5 ± 2.0 |
6.9 ± 1.7 |
| Pinus pinea | ||||
| Site 1 | 39.3 ± 6.0 a | 7.0 ± 0.8 b | 21.5 ± 4.0 b | 10.8 ± 2.0 b |
| Site 2 | 34.6 ± 1.8 ab | 6.3 ± 0.7 b | 21.1 ± 1.0 bc | 7.2 ± 1.1 bc |
| Site 3 | 41.5 ± 3.3 a | 6.9 ± 0.7 b | 24.7 ± 1.3 b | 9.9 ± 2.6 b |
| Mean | 38.5 ± 4.6 | 6.7 ± 0.7 | 22.4 ± 2.9 | 9.3 ± 2.1 |
Within a column. estimates with a common letter were not significantly different (P = 0.05).
The differences of total extractives content between the two species were not statistically significant (P = 0.254) but the site had a highly significant effect (P < 0.001) with the trees of both species of site 2 being different from those of sites 1 and 3. The differences between the two species were significant for the lipophilic (P < 0.001) and hydrophilic extractives (P < 0.001). The site had a highly significant effect (P < 0.001) on the content of hydrophilic extractives but no significant effect on the content of lipophilic extractives (P = 0.147).
3.2. Chemical characterization of lipophilic extracts
The identified compounds in the lipophilic extracts of the phloems from P. pinaster and P. pinea are presented in Table 2 in proportion of the total chromatogram area and grouped by chemical family.
Table 2.
Chemical composition (% of all chromatogram peak areas) of the lipophilic extractives of young phloem of Pinus pinaster and Pinus pinea from three sites in Portugal (mean and standard deviation of three site per species).
| Chemical family/Compound |
Pinus pinaster |
Pinus pinea |
||||||
|---|---|---|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Average | Site 1 | Site 2 | Site 3 | Average | |
| Terpenic compounds | 36.1 | 44.7 | 43.1 | 41.3 ± 4.6 | 52.4 | 47.8 | 52.4 | 50.9 ± 2.7 |
| Pimarane-type resin acids | 8.2 | 8.7 | 11.9 | 9.6 ± 2.0 | 11.7 | 14.2 | 12.2 | 12.7 ± 1.3 |
| Pimaric acid | 6.1 | 7.0 | 10.2 | 7.8 ± 2.2 | 7.1 | 7.3 | 7.9 | 7.4 ± 0.4 |
| Isopimaric acid | 2.15 | 1.63 | 1.73 | 1.8 ± 0.3 | 4.60 | 6.89 | 4.2 | 5.2 ± 1.5 |
| Abietane-type resin acids | 27.9 | 36.4 | 31.2 | 31.8 ± 4.3 | 40.7 | 33.7 | 40.2 | 38.2 ± 3.9 |
| Di(dehydroabietc acid) | 1.5 | 3.9 | 2.4 | 2.6 ± 1.2 | nd | nd | nd | nd |
| Dehydroabietic acid | 11.4 | 6.9 | 11.4 | 9.9 ± 2.6 | 23.9 | 17.7 | 23.2 | 21.6 ± 3.4 |
| Abietic acid | 9.1 | 14.4 | 11.7 | 11.7 ± 2.7 | 0.9 | 10.0 | 12.2 | 10.4 ± 1.6 |
| 15-Hydroxy-dehydroabietic acid | 2.5 | 1.5 | 2.2 | 2.1 ± 0.5 | n d | nd | nd | nd |
| 7-Hydroxy-dehydroabietic acid | 3.4 | 2.3 | 3.7 | 3.1 ± 0.7 | 7.5 | 7.9 | 7.8 | 7.7 ± 0.2 |
| Fatty acids | 22.5 | 18.1 | 18.8 | 19.8 ± 2.4 | 14.2 | 20.7 | 13.9 | 16.3 ± 3.8 |
| Saturated fatty acids | 15.0 | 13.1 | 13.1 | 13.7 ± 1.1 | 8.9 | 13.2 | 8.3 | 10.1 ± 2.7 |
| Hexadecanoic acid (palmitic acid C16:0) | 3.0 | 0.3 | 3.4 | 2.2 ± 1.7 | 0.9 | nd | 0.2 | 0.6 ± 0.5 |
| Octadecanoic acid (stearic acid C18:0) | 1.0 | 0.4 | 0.6 | 0.7 ± 0.3 | 0.8 | 1.6 | 1.0 | 1.1 ± 0.4 |
| Eicosanoic acid (arachidic acid C20:0) | 6.1 | 8.9 | 5.6 | 6.9 ± 1.8 | 1.8 | 1.2 | 1.2 | 1.4 ± 0.3 |
| Docosanoic acid (behenic acid C22:0) | 2.0 | 1.4 | 1.4 | 1.6 ± 0.3 | 2.7 | 4.4 | 2.5 | 3.2 ± 1.0 |
| Tricosanoic acid (tricosylic acid C23:0) | 0.9 | 0.5 | 0.9 | 0.8 ± 0.2 | 0.7 | 0.5 | 0.7 | 0.6 ± 0.1 |
| Tetracosanoic acid (lignoceric acid C24:0) | 2.1 | 1.7 | 1.2 | 1.7 ± 0.5 | 2.11 | 5.6 | 2.7 | 3.5 ± 1.9 |
| Unsaturated fatty acids | 6.2 | 4.6 | 4.5 | 5.1 ± 1.0 | 4.5 | 6.8 | 5.1 | 5.5 ± 1.2 |
| 9-Octadecadienoic acid (oleic acid C18:1) | 4.0 | 2.4 | 2.3 | 2.9 ± 1.0 | 2.9 | 5.5 | 4.2 | 4.2 ± 1.3 |
| 9.12.15-Octadecatrienoic acid (linolenic acid C18:3) | 1.2 | 1.2 | 1.2 | 1.2 ± 0.0 | nd | nd | nd | nd |
| 10.16-Dihydroxyhexadecanoic acid (C16:2) | 1.0 | 1.0 | 1.0 | 1.0 ± 0.0 | 1.6 | 1.3 | 0.9 | 1.3 ± 0.4 |
| Methyl 16-hydroxyhexadecanoate | 0.6 | 0.5 | 0.5 | 0.5 ± 0.1 | 0.8 | 0.7 | 0.5 | 0.7 ± 0.2 |
| Methyl 18-hydroxy-9-octadecenoate | 0.9 | nd | 0.81 | 0.9 ± 0.1 | nd | nd | nd | nd |
| Flavonoids | 0.2 | nd | nd | 0.1 ± 0.1 | 3.4 | 0.1 | 1.0 | 1.5 ± 1.7 |
| Catechine (2R-cis) | 0.2 | nd | nd | 0.2 ± 0.0 | 3.4 | 0.1 | 1.0 | 1.5 ± 1.7 |
| Alkanes | 0.3 | nd | 0.3 | 0.2 ± 0.2 | nd | nd | nd | nd |
| Hexacosane | 0.3 | nd | 0.3 | 0.3 ± 0.0 | nd | nd | nd | nd |
| Sterols | 0.9 | 0.4 | 0.7 | 0.7 ± 0.3 | 0.8 | 0.7 | 1.0 | 0.8 ± 0.2 |
| β-Sitosterol | 0.8 | 0.4 | 0.6 | 0.6 ± 0.2 | 0.8 | 0.7 | 1.0 | 0.8 ± 0.2 |
| Stigmast-5-ene | 0.1 | nd | 0.09 | 0.1 ± 0.0 | nd | nd | nd | nd |
| Lignans | 3.0 | 0.7 | 2.2 | 2.0 ± 1.2 | nd | nd | nd | nd |
| Pinoresinol | 3.0 | 0.7 | 2.2 | 2.0 ± 1.2 | nd | nd | nd | nd |
nd - not detected.
Diterpenic resin acids were the main components of these lipophilic extracts, varying from 36.1 to 44.7% of the extracts in P. pinaster and 47.8–52.4% in P. pinea. Fatty acids were also found in considerable amounts: concentrations in the range of 17.5–21.1% of the lipophilic extracts in P. pinaster phloem and 13.4–20.0% in those of P. pinea. Sterols were identified in the lipophilic extractives, mainly β-sitosterol, representing between 0.6 and 1.2% of the total extractives.
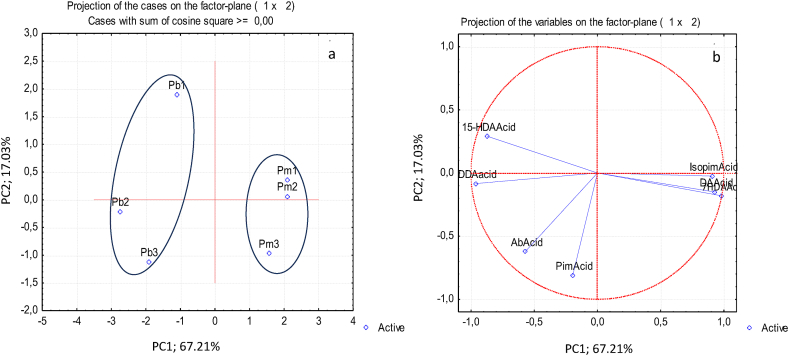
PCA was carried out for the different chemical families in the lipophilic extracts to understand eventual differences or similarities among samples. For the terpenic composition, PCA showed that 84% of the total variation could be explained by the first two principal components (PC1 and PC2) (Figure 1). The first component separates the two pine species: the P. pinea samples were characterized by higher contents of dehydroabietic acid, 7-hydroxy-dehydroabietic acid and isopimaric acid, while the P. pinaster samples contained higher contents of di-dehydroabietc acid and 15-hydroy-dehydroabietic acid. The P. pinea and P. pinaster samples from site 3 (Pm3 and Pb3) exhibited higher contents of abietic acid and pimaric acid.
Figure 1.
Principal component analysis of terpenic composition of phloem extracts from Pinus pinaster (Pb) and Pinus pinea (Pm) trees from 3 sites (a) distinction between the samples (scores) and (b) relation between the terpenic compounds (loadings). PimAcid- Pimaric acid; IsopimAcid- Isopimaric acid; DDAAcid - Di(dehydroabietc acid); DAAcid Dehydroabietic acid, AbAcid- Abietic acid; 15HDAAcid-15-Hydroxy-dehydroabietic acid; 7HDAAcid- 7Hydroxy-dehydroabietic acid.
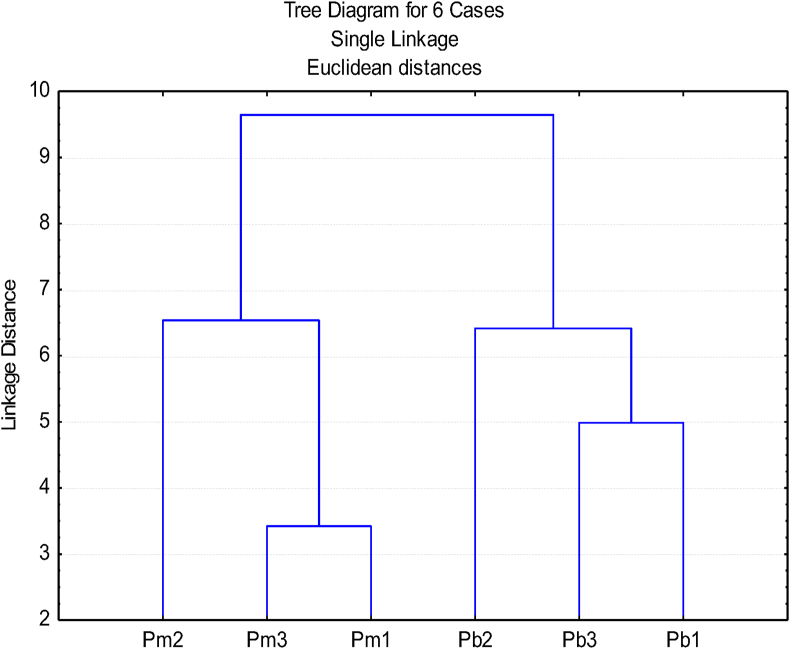
The dendrogram resulting from the agglomerative hierarchical CA (Figure 2) confirms the existence of the two species clusters separated by maximum dissimilarity, at a linkage distance of about 7. For both species, the terpenic composition of phloem extracts is more similar in sites 1 and 3 than in site 2.
Figure 2.
Dendrogram of the phloem lipophic extract from Pinus pinaster (Pb) and Pinus pinea (Pm) trees from 3 sites based on their terpenic composition.
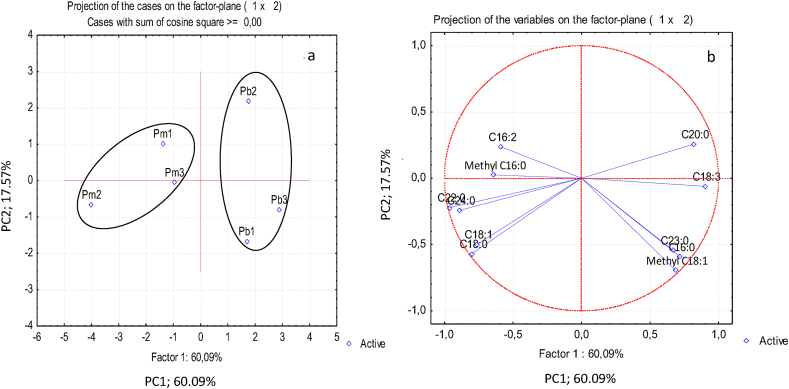
Concerning fatty acid composition, the two first principal components explain 77 % of the original information (Figure 3). The plot of the samples on this plane showed also a separation of the two species: P. pinea samples are richer in stearic (C18:0), oleic (C18:1), behenic (C22:0) and lignoceric (C24:0) acids, while P. pinaster samples have higher contents of palmitic (C16:0), arachidic (C20:0), tricosylic (C23:0), methyl oleic (methyl C18:1) and linolenic (C18:3) acids. The cluster analysis confirmed the two groups (P. pinea and P. pinaster) for a linkage distance higher than 4.5 (Figure 4). As observed for the terpenic fraction, the fatty acid composition of P. pinea or P. pinaster samples from sites 1 and 3 are more similar than those from site 2.
Figure 3.
Principal component analysis of fatty acid composition of phloem extracts from Pinus pinaster (Pb) and Pinus pinea (Pm) trees from 3 sites (a) distinction between the samples (scores) and (b) relation between the fatty acids (loadings).
Figure 4.
Dendrogram of the phloem lipophic extract from Pinus pinaster (Pb) and Pinus pinea (Pm) trees from 3 sites based on their fatty acid composition.
3.3. Chemical characterization of hydrophilic extractives
The phytochemical analysis regarding total polyphenol, flavonoid and tannin contents in the ethanol-water extracts (hydrophilic extractives) of the phloems of P. pinaster and P. pinea are presented in Table 3.
Table 3.
Composition (total phenolics, flavonoids and condensed tannins) and antioxidant capacity (radical scavenger activity, IC50 and reductive antioxidant power, FRAP) of ethanol-water extracts of young phloems of Pinus pinaster and Pinus pinea from three sites of Portugal. Mean of six phloem samples per site and standard deviation.
| Total phenolics (mg GAE/g extract) | Total flavonoids (mg CE/g extract) | Condensed tannins (mg CE/g extract) | IC50 values (μg extract/ml)∗ | FRAP (mM TEAC/g extract) | |
|---|---|---|---|---|---|
| Pinus pinaster | |||||
| Site 1 | 373.7 ± 63.8ab | 80.0 ± 18.9 | 84.4 ± 18.1 | 1.2 ± 0.2 | 3.5 ± 0.7 |
| Site 2 | 285.2 ± 60.6ac | 64.9 ± 37.6 | 95.2 ± 34.7 | 1.5 ± 0.3 | 4.1 ± 2.4 |
| Site 3 | 660.5 ± 178.6ad | 115.0 ± 36.9 | 125.1 ± 17.4 | 1.8 ± 0.2 | 2.8 ± 0.5 |
| Mean |
439.8 ± 160.2 |
86.6 ± 21.0 |
101.6 ± 17.2 |
1.5 ± 0.2 |
3.5 ± 0.5 |
| Pinus pinea | |||||
| Site 1 | 342.4 ± 70.2ba | 114.0 ± 30.9 | 97.5 ± 24.9 | 1.6 ± 0.4 | 3.1 ± 0.8 |
| Site 2 | 336.1 ± 34.4bc | 69.4 ± 14.6 | 107.9 ± 72.1 | 1.5 ± 0.5 | 5.4 ± 0.9 |
| Site 3 | 870.2 ± 294.3bd | 132.4 ± 65.2 | 128.1 ± 54.1 | 1.4 ± 0.2 | 2.4 ± 0.3 |
| Mean | 516.2 ± 250.3 | 105.3 ± 26.5 | 111.2 ± 12.7 | 1.5 ± 0.08 | 3.3 ± 1.3 |
Within a column. estimates with a common letter were not significantly different (P = 0.05).
IC50 Trolox in ethanol-water 3.81 μg Trolox/ml; GAE: gallic acid equivalents; CE: catechin equivalents; TEAC: Trolox equivalents antioxidant activity.
High quantities of phenolic compounds were present, representing 29%–66% of the ethanol-water extracts from P. pinaster phloem and 34%–87% of the extracts from P. pinea phloem. There were significant differences between species (P = 0.049) and between sites (P < 0.001). The total phenolic content was high, especially for P. pinea (mean value of 516.2 mg GAE/g extract in P. pinea and 439.8 mg GAE/g extract in P. pinaster) that contained also more flavonoids (105.3 mg CE/g extract in P. pinea and 86.6 mg CE/g extract in P. pinaster). The amount of condensed tannins in the phloem extracts was similar for both species (111.2 mg CE/g extract in P. pinea and 101.6 mg CE/g extract in P. pinaster).
The ethanol-water extracts from phloem of both species showed very high antioxidant activity and were able to effectively reduce the DPPH with IC50 values ranging from 1.2 to 1.8 μg/ml, which represent a stronger antioxidant activity than that of common antioxidant standards (e.g. Trolox, IC50 = 3.8 μg/ml and catechin IC50 = 2.2 μg/ml).
Their FRAP activity was comparable to that of Trolox (4.0 mM TEAC/g), and BHT (butylated hydroxytoluene, 2.6 mM TEAC/g), but lower than other standards (catechin, 25.4 mM TEAC/g and gallic acid, 43.5 mM TEAC/g) as previously reported (Olszewska et al., 2012).
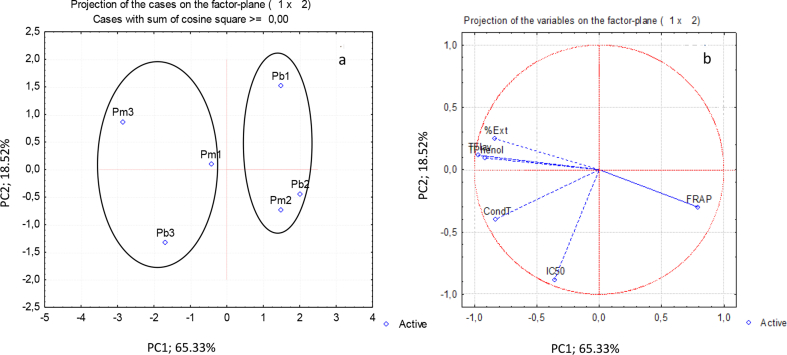
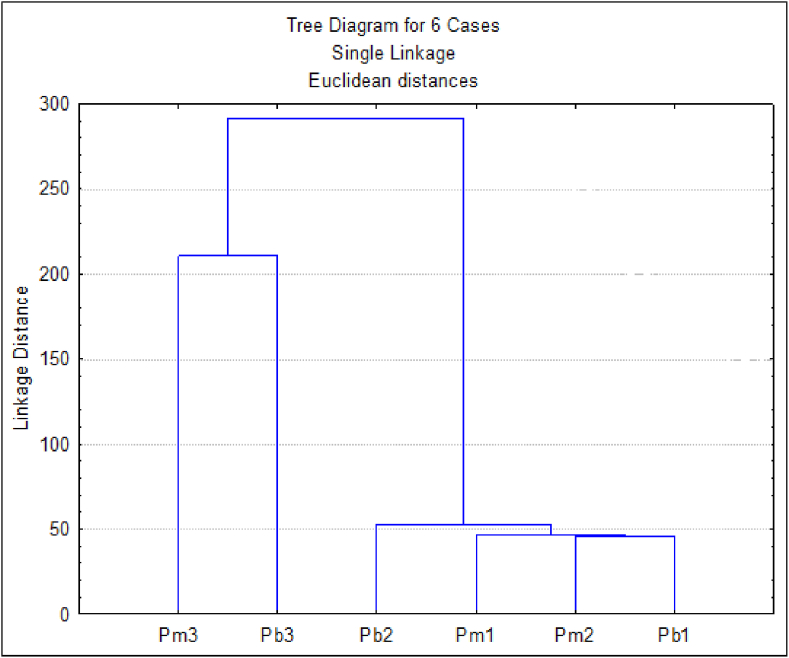
PCA and CA were applied to data of hydrophilic extract composition and antioxidant activity (Figures 5 and 6). The first two principal components could largely explain the variance of the original data (ca. 84 %). From the plot of the samples on the plane defined by PC1 and PC2, the samples seem to be grouped in function of sites and not of species (Figure 5). This was confirmed by the dendrogram (Figure 6) where one group with P. pinea and P. pinaster samples from sites 1 and 2 are grouped at a linkage distance of around 60, while another group with the two samples of site 3 is formed at a linkage distance of around 220. Samples of both species from sites 1 and 2 were characterized by higher antioxidant activity expressed as FRAP, while the samples from site 3 have higher values for the other variables (total phenolics, total flavonoids and condensed tannins). The P. pinaster samples of site 3 shows a considerably higher value for IC50 (Figure 5).
Figure 5.
Principal component analysis of the hydrophilic extract content and its phenolic composition of phloem from Pinus pinaster (Pb) and Pinus pinea (Pm) trees from 3 sites (a) distinction between the samples (scores) and (b) relation between the hydrophilic extract content and its phenolic composition (loadings). %Ext- % Hydrophilic extract; Tphenol- Total phenolic content TFlav- Total flavonoids content; CondT – Total condensed tannins.
Figure 6.
Dendrogram of the phloem hydrophilic extract from Pinus pinaster (Pb) and Pinus pinea (Pm) trees from 3 sites based on their fatty acid composition.
4. Discussion
4.1. Extractives content
We know that phloem is where most defence secondary metabolites are located in conifers and that these vary between species and are influenced by climate and environmental factors (Moreira et al., 2014).
In the present study, both pine species showed in their phloems a very high secondary metabolites content (on average 38.1%, Table 1) This is in accordance with extracts yields reported in an earlier work for phloems of eight mature pine species (P. pinaster 39.3%, P. pinea 40.5%, P. nigra 40.1%, P. taeda 36.6%, P. radiata 34.3%, P. halepensis 38.7%, P. strobus 32.1%, P. sylvestris 38.8%) (Pimentel et al., 2016).
P. pinaster and P. pinea differed in the chemical composition of their phloem metabolites, namely regarding the proportion of lipophilic and hydrophilic compounds (Table 1). The polar compounds (ethanol and water solubles) represent a larger proportion of total extractives in P. pinea phloem than in P. pinaster phloem (82.5% vs. 70% of the total). These results corrobortae the between-species phytochemical variation of phloems, as Pimentel et al. (2016) showed in their study of phloems from different Pinus spp. e.g. concentration of polyphenols was highest in P. pinea and P. halepensis and lowest in P. pinaster and P. taeda.
The higher levels of polar constitutive defences of P. pinea in relation to P. pinaster may be associated with growth differences (Endara and Coley, 2011) e.g. P. pinea is thermophilic and xerophytic and adapted to low-nutrient, highly drained sandy soils, while P. pinaster is a faster growing species adapted to richer soils (Lavery and Mead, 1998; Tapias et al., 2004). The trade-off of constitutive defences into induced defences has been associated with growth rate and may help explain variations in line with species-specific life history strategies and thus host susceptibility to diseases (Viiri et al., 2001; Strauss et al., 2002; Blodgett et al., 2005; Wallis et al., 2011; Moreira et al., 2014; Hood and Sala, 2015).
4.2. Lipophilic extractives composition
A special attention was given to the composition of the phloem lipophilic extracts of P. pinaster and P. pinea (Table 2). The two species differed with higher levels of fatty acids in P. pinaster phloem, and higher levels of diterpenic resin acids in P. pinea phloem. The most discriminatory resin acids in P. pinea were dehydroabietic, isopimaric and 7-hydroxydehydroabietic acids, while P. pinaster phloem only contained di-dehydroabietic acid and 15-hydroxy-dehydroabietic acid. These compounds were recently detected in the bark of P. pinaster and P. pinea, with lower concentrations in P. pinea bark (Sousa et al., 2018). These compounds play an important role in the chemical defence of conifers and a relationship between diterpenic acid content and tree resistance to herbivores and pathogens was reported (Keeling and Bohlmann, 2006). Resin acids, in combination with phenolic compounds, may show antifungal activity (Back and Allen, 2000). The presence of resin acids was reported for a number of pine species, including P. pinaster and P. pinea (Sousa et al., 2018), and in P. elliotii, P. oocarpa, P. caribeae, P. merkusii, P. montezumae and P. insularis (Masendra et al., 2018), and P. wallichiana, P. roxburghii and P. gerardiana (Willfӧr et al., 2009).
The two pine species also produced different fatty acids, with higher proportion of arachidic and palmitic acids in P. pinaster phloem. All the identified fatty acids were previously reported in the bark lipophilic extracts of P. pinaster and P. pinea (Sousa et al., 2018).
The phloem extracts also contained, other lipophilic compounds with low abundances, e.g. sterols and some aromatic compounds (Table 2). Previously, β-sitosterol was identified in bark extracts of P. pinaster and P. pinea (Sousa et al., 2018) and of P. elliotii, P. oocarpa, P. caribeae, P. merkusii, P. montezumae and P. insularis (Masendra et al., 2018), and P. wallichiana, P. roxburghii and P. gerardiana (Willfӧr et al., 2009).
4.3. Phenolic constituents
Polyphenols and flavonoid molecules are phytochemicals with high radical scavenging activity, and are therefore natural antioxidants functioning as antibiotics and natural pesticides (Franceschi et al., 2005). P. pinaster and P. pinea phloems contained a significant level of phenolic compounds with the extracts from P. pinea richer in flavonoids than those of P. pinaster (Table 3). Phenolic compounds such as catechin, epicatechin, taxifolin and phenolic acids were detected in bark extracts of P. pinea (Yesil-Celiktas et al., 2009) and P. pinaster (Navarrete et al., 2010).
Due to the high content in bioactive flavonoids and phenolic compounds, the phloem extracts revealed high antioxidant activity and reducing power that are above the values for known antioxidant standards (Table 3). Pimentel et al. (2016, 2017) showed that the levels of secondary metabolites clearly differentiate P. pinea from P. pinaster and other pines in relation to their susceptibility to pinewood nematode: more susceptible pine species tended to have low levels of total phenols, condensed tannins, flavonoids and lignin. Differences in susceptibility may therefore be related to differences in phytochemistry, as again shown here with the differences between P. pinea and P. pinaster.
4.4. Site effect
The influence of site and associated climatic conditions on P. pinaster and P. pinea investment in phytochemicals was addressed by the sampling in three areas of Portugal, where these two pine species coexist: a region on the north coast with mild climatic conditions (Site 3) and two regions on the southwest of the country one with less favorable environmental conditions and summer drought (Site 1) and the other with mild climatic conditions and the highest rainfall (Site 2).
Site had a highly significant effect on the content of hydrophilic extractives, suggesting that the geographic and climatic conditions, namely regarding annual precipitation, were important drivers on the accumulation of these metabolites, e.g. investment in phytochemicals was lower in P. pinaster and P. pinea trees from the Site 2 with the highest rainfall. P. pinea showed the same investment in phytochemicals in the three sites in agreement with the species adaptability to dry environments. Regarding the two major secondary chemicals (resin acids and polyphenolics), the observed responses were species specific, with the ratio phenolic-to-oleoresin constant for the trees of the three sites and much higher for P. pinea (4.7 for P. pinea and 2.3 for P. pinaster).
The results showed that environmental conditions seem to be as significant as the genetic differences between the two species regarding the secondary metabolites composition. When analysing the clusters projected for the lipophilic compounds (terpenic and fatty acid composition) the samples were very well discriminated by site (Figures 2 and 4). It can be observed that samples of the two species from Site 1 and 3 were clustered together, while the populations of the two species from the Site 2 had chemically distinct profiles. The two species in Site 1 and 3 were characterized by higher dehydroabietic acid content while the two species in Site 2 were characterized by higher arachidic and behenic acids (Figure 1 and 2).
Regarding content and composition of hydrophilic metabolites, the samples were perfectly separated into two groups: a group comprising Site 3 characterized by higher total phenolic flavonoids and condensed tannins and another comprising Sites 1 and 2 (Figures 5 and 6).
The influence of site in accumulation of various secondary plant products was also reported by Ramakrishna and Ravishankar (2011) who referred that the growing conditions have strong impact on the corresponding metabolic pathways. The association of the levels of phytochemicals with the abundance of the insect vector (Monochamus galloprovincialis) of the pinewood nematode was suggested by Pimentel et al. (2017) based on a study of P. pinaster and P. pinea from Sites 1 and 2. While the abundance of the insect vector was high in Site 1 were P. pinea had a slower growth and higher levels of constitutive defences, in Site 2 the abundance of the insect vector was much lower and P. pinea had clearly lower levels of secondary metabolites and higher growth rate (Pimentel et al., 2017).
5. Conclusions
The phytochemical profile of secondary metabolites in the young phloem of Pinus pinaster and Pinus pinea was analyzed in three areas of Portugal. Content and compositional profile of extractives were species specific. The hydrophilic extractives (ethanol and water extracts) were higher in P. pinea phloem, with a large content of phenolic compounds, flavonoids and tannins, and very high scavenging and reducing ability, while lipophilic extractives were higher in P. pinaster phloem and the ratio of phenolic-to-oleoresin compounds was substantially higher for P. pinea. These phytochemical features support the known differences between both species in relation to pinewood susceptibility with P. pinea showing the higher resistance.
The results also highlight the relationship between environment and the metabolic profile of P. pinaster and P. pinea phloems since production of some metabolites appeared to be a response to site differences e.g. precipitation and species specific growth conditions.
Declarations
Author contribution statement
Rita Simões: Performed the experiments.
Carla Pimentel: Conceived and designed the experiments.
Suzana Ferreira-Dias: Analyzed and interpreted the data.
Isabel Miranda: Conceived and designed the experiments; Wrote the paper.
Helena Pereira: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the PineChem: PTDC/ASP-SIL/29774/2017.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declarations of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdalla S., Pizzi A., Ayed N., Bouthoury F.C.-E., Charrier B., Bahabri F., Ganash A. MALDI-TOF analysis of Aleppo pine (Pinus halepensis) bark tannin. BioResources. 2014;9:3396–3406. [Google Scholar]
- Back E.L., Allen L.H. Tappi Press; Atlant: 2000. Pitch Control, wood Resin and Deresination. [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP Assay. Anal. Biochem. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Blodgett J.T., Herms D.A., Bonello P. Effects of fertilization on red pine defense chemistry and resistance to Sphaeropsis sapinea. For. Ecol. Manage. 2005;208:373–382. [Google Scholar]
- Bonello P., Gordon T.R., Herms D.A., Wood D.L., Erbilgin N. Nature and ecological implications of pathogen-induced systemic resistance in conifers: a novel hypothesis. Physiol. Mol. Plant Pathol. 2006;68:95–104. [Google Scholar]
- Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso S., Ferreira J., Miranda I., Pereira H. Age variation of Douglas-fir bark chemical composition. J. Wood Chem. Technol. 2018;38:385–396. [Google Scholar]
- Celedon J.M., Bohlmann J. Oleoresin defenses in conifers: chemical diversity, terpene synthases and limitations of oleoresin defense under climate change. New Phytol. 2019;224:1444–1463. doi: 10.1111/nph.15984. [DOI] [PubMed] [Google Scholar]
- Dou J., Galvis L., Holopainen-Mantila U., Reza M., Tamminen T., Vuorinen T. Morphology and overall chemical characterization of willow (Salix sp.) inner bark and wood: toward controlled deconstruction of willow biomass. ACS Sustain. Chem. Eng. 2016;4:3871–3876. [Google Scholar]
- Eberhardt T.L. Impact of industrial source on the chemical composition of loblolly pine bark. For. Prod. J. 2012;62:516–519. [Google Scholar]
- Endara M.J., Coley P.D. The resource availability hypothesis revisited: a meta-analysis. Funct. Ecol. 2011;25:389–398. [Google Scholar]
- EFSA Panel on Plant Health Scientific opinion on comments provided by Portugal on the phytosanitary risk associated with Pinus pinea for the spread of pine wood nematode. EFSA J. 2013;11:3163. [Google Scholar]
- Eyles A., Jones W., Riedl K., Cipollini D., Schwartz S., Chan K., Herms D.A., Bonello P. Comparative phloem chemistry of Manchurian (Fraxinus mandshurica) and two North American ash species (Fraxinus americana and Fraxinus pennsylvanica) J. Chem. Ecol. 2007;33:1430–1448. doi: 10.1007/s10886-007-9312-3. [DOI] [PubMed] [Google Scholar]
- Ferreira J., Quilhó T., Pereira H. Characterization of Betula pendula outer bark regarding cork and phloem components at chemical and structural levels in view of biorefinery integration. J. Wood Chem. Technol. 2017;37:10–25. [Google Scholar]
- Franceschi V.R., Krokene P., Christiansen E., Krekling T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005;167:353–375. doi: 10.1111/j.1469-8137.2005.01436.x. [DOI] [PubMed] [Google Scholar]
- Franco A.R., Santos C., Roriz M., Rodrigues R., Lima M.R.M., Vasconcelos M.W. Study of symptoms and gene expression in four Pinus species after pinewood nematode infection. Plant Genet. Resour. 2011;9:272–275. [Google Scholar]
- Heil M., Ton J. Long-distance signalling in plant defence. Trends Plant Sci. 2008;13:264–272. doi: 10.1016/j.tplants.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Hood S., Sala A. Ponderosa pine resin defenses and growth: metrics matter. Tree Physiol. 2015;35:1223–1235. doi: 10.1093/treephys/tpv098. [DOI] [PubMed] [Google Scholar]
- Keeling C.I., Bohlmann J. Diterpene resin acids in conifers. Phytochem. 2006;67:2415–2423. doi: 10.1016/j.phytochem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P., Agrawal V. Structure and composition of aliphatic constituents of potato tuber skin (suberin) Lipids. 1974;9:682–691. [Google Scholar]
- Kuroda H., Goto S., Kazumi E., Kuroda K. The expressed genes of Japanese red pine (Pinus densiflora) involved in the pine wilt disease severity. BMC Proc. 2011;5:P92. [Google Scholar]
- Lahr E.C., Krokene P. Conifer stored resources and resistance to a fungus associated with the spruce bark beetle Ips typographus. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0072405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio V., Lattanzio V.M.T., Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In: Imperato F., editor. Phytochemistry: Advances in Research. Research Signpost; Kerala: 2006. pp. 24–67. [Google Scholar]
- Lavery P.B., Mead D.J. Pinus radiata: a narrow endemic from North America takes on the world. In: Richardson D.M., editor. Ecology and Biogeography of Pinus. Cambridge University Press; Cambridge, UK: 1998. pp. 432–449. [Google Scholar]
- Masendra, Ashitani T., Takahashi K., Lukmandaru G. Lipophilic extractives of the inner and outer barks from six different Pinus species grown in Indonesia. J. For. Res. 2018;29:1329–1336. [Google Scholar]
- Menéndez-Gutiérrez M., Alonso M., Jinénez E., Toval G., Mansilla P., Abelleira A., Abelleira-Sanmartín A., Diaz R. Interspecific variation of constitutive chemical compounds in Pinus spp. xylem and susceptibility to pinewood nematode (Bursaphelenchus xylophilus) Eur. J. Plant Pathol. 2018;150:939–953. [Google Scholar]
- Mirkin B. Springer US; Boston, MA: 1996. Mathematical Classification and Clustering, Nonconvex Optimization and its Applications. [Google Scholar]
- Moreira X., Mooney K.A., Rasmann S., Petry W.K., Carrillo-Gavilán A., Zas R., Sampedro L. Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecol. Lett. 2014;17:537–546. doi: 10.1111/ele.12253. [DOI] [PubMed] [Google Scholar]
- Navarrete P., Pizzi A., Pasch H., Rode K., Delmotte L. MALDI-TOF and 13C NMR characterization of maritime pine industrial tannin extract. Ind. Crop. Prod. 2010;32:105–110. [Google Scholar]
- Nunes da Silva M., Solla A., Sampedro L., Zas R., Vasconcelos M.W. Susceptibility to the pinewood nematode (PWN) of four pine species involved in potential range expansion across Europe. Tree Physiol. 2015;35:987–999. doi: 10.1093/treephys/tpv046. [DOI] [PubMed] [Google Scholar]
- Olszewska M.A., Presler A., Michel P. Profiling of phenolic compounds and antioxidant activity of dry extracts from the selected Sorbus species. Molecules. 2012;17:3093–3113. doi: 10.3390/molecules17033093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma J.H.N. CliPick – climate change web picker. A tool bridging daily climate needs in process based modelling in forestry and agriculture. Forest Syst. 2017;26(1) [Google Scholar]
- Pimentel C.S., Firmino P.N., Calvão T., Ayres M.P., Miranda I., Pereira H. Pinewood nematode population growth in relation to pine phloem chemical composition. Plant Pathol. 2016;66:856–864. [Google Scholar]
- Pimentel C.S., Gonçalves E.V., Firmino P.N., Calvão T., Fonseca L., Abrantes I., Correia O., Máguas C. Differences in constitutive and inducible defences in pine species determining susceptibility to pinewood nematode. Plant Pathol. 2017;66:131–139. [Google Scholar]
- Ramakrishna A., Ravishankar G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011;6:1720–1731. doi: 10.4161/psb.6.11.17613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rencher A.C. J. Wiley; 2002. Methods of Multivariate Analysis. [Google Scholar]
- Sampaio B.L., Edrada-Ebel R., Batista da Costa F. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: a model for environmental metabolomics of plants. Sci. Rep. 2016;6:29265. doi: 10.1038/srep29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C.S., Pinheiro M., Silva A.I., Egas C., Vasconcelos M.W. Searching for resistance genes to Bursaphelenchus xylophilus using high throughput screening. BMC Genom. 2012;13:599. doi: 10.1186/1471-2164-13-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J.A., Clearwater M.J., Haines D.F., Klein T., Mencuccini M., Sevanto S., Turgeon R., Zhang C. Allocation, stress tolerance and carbon transport in plants: how does phloem physiology affect plant ecology? Plant Cell Environ. 2016;39:709–725. doi: 10.1111/pce.12602. [DOI] [PubMed] [Google Scholar]
- Şen A., Miranda I., Santos S., Graça J., Pereira H. The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind. Crop. Prod. 2010;31:417–422. [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- Sousa J.L.C., Ramos P.A.B., Freire C.S.R., Silva A.M.S., Silvestre A.J.D. Chemical composition of lipophilic bark extracts from Pinus pinaster and Pinus pinea cultivated in Portugal. Appl. Sci. 2018;8:2575. [Google Scholar]
- Strauss S.Y., Rudgers J.A., Lau J.A., Irwin R.E. Direct and ecological costs of resistance to herbivory. Trends Ecol. Evol. 2002;17:278–285. [Google Scholar]
- Tapias R., Climent J., Pardos J.A., Gil L. Life histories of Mediterranean pines. Plant Ecol. 2004;171:53–68. [Google Scholar]
- Thornber J.P., Northcote D.H. Changes in the chemical composition of a cambial cell during it is differentiation into xylem and phloem tissue in trees. II. Carbohydrate constituents of each main component. Biochem. J. 1961;81:455–464. doi: 10.1042/bj0810455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R., Wolf S. Phloem transport: cellular pathways and molecular trafficking. Annu. Rev. Plant Biol. 2009;60:207–221. doi: 10.1146/annurev.arplant.043008.092045. [DOI] [PubMed] [Google Scholar]
- Vicente C., Espada M., Vieira P., Mota M. Pine wilt disease: a threat to European forestry. Eur. J. Plant Pathol. 2012;133:89–99. [Google Scholar]
- Viiri H., Annila E., Kitunen V., Niemela P. Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus, Ceratocystis polonica. Trees (Berl.) 2001;15:112–122. [Google Scholar]
- Wallis C., Eyles A., Chorbadjian R., Reidl K., Schwartz S., Hansen R., Cipollini D., Herms D.A., Bonello P. Differential effects of nutrient availability on the secondary metabolism of Austrian pine (Pinus nigra) phloem and resistance to Diplodia pinea. For. Pathol. 2011;41:52–58. [Google Scholar]
- Willför S., Ali M., Karonen M., Reunanen M., Arfan M., Harlamow R. Extractives in bark of different conifer species growing in Pakistan. Holzforschung. 2009;63:551–558. [Google Scholar]
- Yesil-Celiktas O., Ganzera M., Akgun I., Sevimli C., Kormaz K.S., Bedir E. Determination of polyphenolic constituents and biological activities of bark extracts from different Pinus species. J. Sci. Food Agric. 2009;89:1339–1345. [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.