Highlights
-
•
Neuroendocrine tumor metastasis to the brain is a rare entity that commonly originates from the lungs.
-
•
Neuroendocrine tumor grade 1, which is considered benign, can metastasize to the brain.
-
•
Neuroendocrine tumor can mimic other cerebral metastatic diseases in terms of hemorrhagic appearance, and thus, should be considered among the differential diagnosis.
Abbreviations: NET, neuroendocrine tumors; ECOG, Eastern cooperative oncology group; WBRT, whole-brain radiation therapy; CT, computed tomography; MRI, magnetic resonance imaging; TTF-1, thyroid transcription factor-1; GFAP, glial fibrillary acidic protein; MANEC, mixed adenoneuroendocrine carcinoma; SWI, susceptibility weighted imaging
Keywords: Neuroendocrine tumor, Low grade, Brain metastases, Natural history, Differential diagnosis
Abstract
Background
Neuroendocrine tumors (NET) are rare tumors with a low incidence of brain metastasis, especially in grade 1 NET. The most common source of brain metastasis is the lung. We present an unusual case of NET grade 1 with multiple hemorrhagic brain metastases.
Case description
A 46-year-old woman, who initially presented with a seizure, was diagnosed with multiple brain and lung lesions. She was offered a biopsy for diagnosis, but she refused and lost to follow up. Eighteen years later, she developed progressive quadriparesis and confusion. A biopsy of the left frontal lobe lesions showed NET grade 1. A lung biopsy of the left upper lobe was consistent with the same diagnosis. The patient’s functional status was poor with Eastern Cooperative Oncology Group (ECOG) grade 4. She only received palliative whole-brain radiation therapy (WBRT) and died 3 months after discharge.
Clinical discussion
NET is a spectrum that encompasses benign to malignant cells. There is a female predominance in lower grades and male predominance in higher grades. No effective management for brain metastases was described, and the prognosis remains poor.
Conclusion
Multiple brain metastases can be the first presentation of patients with NET. Early diagnosis and treatment may have a more favorable impact on the outcome of this disease. The longstanding numerous hemorrhagic NET brain metastases is exceedingly rare. The neuroimaging appearance is similar to other neoplastic and non-neoplastic lesions. An important differential diagnosis to consider is metastatic melanoma and choriocarcinoma, familial cavernous malformation, diffuse axonal injury, cerebral vasculitis, and amyloid angiopathy.
1. Introduction
NET originate from enterochromaffin cells, which are dispersed in most of the body’s organs [1]. The incidence based on the National Centre Institute’s surveillance, epidemiology, and end result program was 6.5% in African-Americans and 4.66% in Caucasians [2]. Similar to any tumor type, NET can be classified based on their histopathological appearance and proliferation rate (Ki-67 index) [3]. Brain metastasis from NET is extremely rare, with an incidence of < 5% [3]. Management can include steroids to alleviate symptoms of mass effect, WBRT, chemotherapy, such as alkylating agents, and surgical resection of large lesions. We report a case of NET grade 1 with multiple hemorrhagic metastases to the brain and multiple organs in a 46-year-old woman. This case is reported in line with the SCARE 2020 criteria [4].
2. Case report
The patient is a 46-year-old woman who had generalized tonic-clonic seizures that started in 1999. She was found to have multiple brain lesions in an outside hospital. Biopsy was not performed, and a provisional diagnosis of neurocysticercosis was made. She was empirically started on albendazole. Her seizures were controlled with carbamazepine (controlled release 800 mg twice a day) and topiramate (200 mg twice a day). In 2012, the patient was referred to our clinic due to simple partial seizures in the form of a left hemi-body clonic episode that lasted for 2 minutes. Computed tomography (CT) of the brain, chest, abdomen, and pelvis, followed by magnetic resonance imaging (MRI) of the brain showed multiple brain and lung lesions. The patient was offered a biopsy of one of the brain lesions. She refused the biopsy and was discharged against medical advice.
The patient did not follow up after discharge. In 2018, she developed progressive bilateral upper and lower limbs weakness of one-month duration. She had difficulty performing activities of daily living. Her vision in the left eye rapidly deteriorated, and she became confused and bedbound. She was admitted through the emergency department under the neurosurgery service. The patient was disoriented to time, place, and person, and she was dysarthric. Fundus exam revealed left retinal detachment. The muscle strength in the extremities was 0/5 on the left and 2/5 on the right.
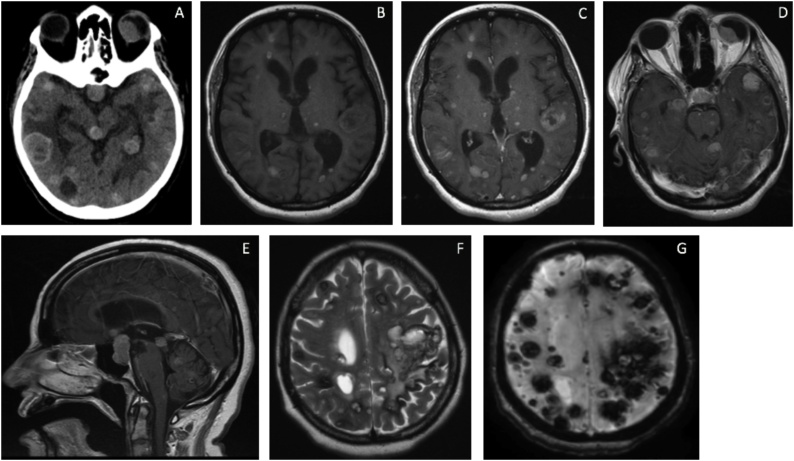
Brain CT followed by MRI (Fig. 1) showed multiple intra-axial supratentorial and infratentorial lesions. The lesions were of variable sizes with the small lesions showing a miliary pattern. Extra-axial lesions involved the sellar-suprasellar region and the left eye. The lesions were hemorrhagic, appearing as blooming artifacts on gradient echo MRI sequence, and resembling metastatic melanoma, metastatic choriocarcinoma, familial cavernous malformations, or amyloid angiopathy. Fluorodeoxyglucose-positron emission tomography CT showed bilateral lung, bilateral mediastinal lymph nodes, liver, and adrenal glands metastases. Craniotomy and biopsy of 3 brain lesions were done.
Fig. 1.
CT axial scan of the brain showing multiple mixed hyper- and hypodensity mass lesions within the supratentorial and infratentorial compartments, in addition to the left eye globe (Fig. 1A). Axial MRI T1 with gadolinium contrast reveals multiple enhancing lesions of supra- and infratentorial compartments, and the left eye globe (Fig. 1B–D). Sagittal MRI T1 with gadolinium demonstrates multiple midline lesions and a large sellar/suprasellar lesion (Fig. 1E). Axial T2 MRI of the supratentorial compartment shows multiple hyperintense lesions with a hypointense rim (Fig. 1F). GRE view of the supratentorial compartment demonstrates many blooming artifacts representing intra-tumoral hemorrhages (Fig. 1G).
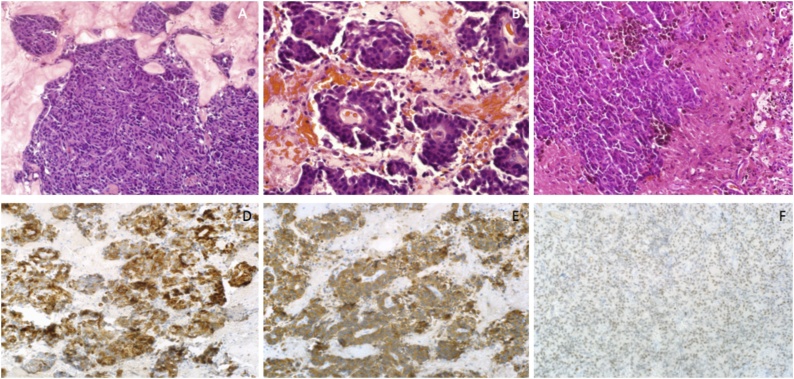
The histopathology diagnosis was neuroendocrine tumor grade 1 based on synaptophysin, cytokeratin-7, thyroid transcription factor-1 (TTF-1), glial fibrillary acidic protein (GFAP), and Ki-67 (< 3%) special stains (Fig. 2, Fig. 3). CT guided biopsy of the left upper lobe of the lung was consistent with the same diagnosis of a low-grade NET and implied the lung as the likely site of the primary tumor.
Fig. 2.
Hematoxylin and eosin stain of 20× magnification showing nesting pattern of mostly uniform cells (Fig. 2A). Pseudopapillary pattern (Fig. 2B) and adjacent brain with old hemorrhage areas and hemosiderin-laden macrophages (Fig. 2C). 20× magnification showing cytokeratin-7 (Fig. 2D), synaptophysin (Fig. 2E), and TTF-1 immunostains (Fig. 2F).
Fig. 3.
20× magnification showing positive GFAP in adjacent brain tissue and negative in tumor cells (Fig. 3A). Ki-67 proliferation index stain showing <3% proliferation (Fig. 3B).
Endocrinology consultation was obtained. The hormonal profile was normal, and the tumor was non-functional. Medical oncology did not recommend chemotherapy management because of the advanced disease and the poor functional status (ECOG 4) of the patient. Radiation oncology recommended palliative WBRT with 20 grays in 10 fractions. The radiation was completed while the patient was still in the hospital, then she was discharged without any improvement in her condition. The patient died three months after discharge due to disease progression.
3. Discussion
NET was first labeled as a carcinoid tumor in 1907, with a relative benign course and location in the pancreas [5]. It was found that NET exhibits variable metastatic features and should be considered malignant. NET was further divided into 5 types by the WHO in 2010: grade 1 NET (Ki-67 < 3%), grade 2 NET (Ki-67 3–20%), grade 3 neuroendocrine carcinoma (NEC, Ki-67 > 20%), mixed adenoneuroendocrine carcinoma (MANEC), and hyperplastic/neoplastic lesions [6]. A recent study found that the average age of patients who develop NET is 54 years (22–78), and these patients are predominantly male (56.3%) [7]. The most common primary site is the gastrointestinal (GI) tract, with the pancreas as the most common organ (25.2%); the liver was the most common initial metastatic site (74.6%) [7]. Grade 1 NET is the most common grade (33%), and grading is found to be the only statistically significant prognostic factor in general (P-value = 0.001) [7]. Almost 94.1% of NET are non-functional with normal hormonal profiles [3]. The median overall survival (OS) was 29.0 months (95% confidence interval, 25.0–33.0) in general [7].
The average age to develop NET-associated brain metastasis is 58 years (27–86), with male-to-female ratios varying between studies [3,8].Male patients with brain metastasis were found to have NEC (58.1%), while females had NET (70.6%) [3]. The most common primary site for brain metastasis is the lung (35.3% of NET and 54.8% of NEC brain metastasis) [3]. Other studies showed 45%–71% of lung-related NET brain metastasis [6]. Grade 1 NET brain metastasis is extremely rare, with only 5.9% of patients, compared to grade 2 NET (27.5%) and NEC (60.8%) [3]. There is an almost equal distribution among patients with the number of metastatic tumors to the brain (1–2 lesions were 43.1% in frequency; ≥ 3 lesions were 41.2% in frequency) [3]. Our patient had unique countless hemorrhagic brain metastases of grade 1 NET, in addition to multiple organ metastases. The incidence of metastasis to lymph nodes was 54.9% of patients in one study, liver 60.8%, and adrenal glands 9.8% [3]. The most likely primary site in our case is the lung. The period to develop brain metastasis after diagnosing patients with NET is 12.8 months in one study, and 18 months in another [8,9].
Prognostic factors for NET-associated brain metastasis are different from tumor metastasis to other organ systems. NET Univariate analysis for prognostic factors in one study showed poor prognosis in male patients (HR 2.7; 95% CI 1.2–5.9; P-value = 0.013), higher tumor grade NEC (HR 2.7; 95% CI 1.2–6.2; P-value = 0.022), and age ≥ 60 years (HR 2.1; 95% CI 1.0–4.3; P-value = 0.041) [3]. Overall survival in patients with brain metastasis and Ki-67 < 5%, 5%–20%, 21%–55%, > 55% was 15, 13, 9, and 7 months, respectively [3]. The survival of our patient exceeds 215 months.
The CT and MRI imaging appearance of the NET multiple cerebral hemorrhagic metastases may mimic other metastatic tumors, such as melanoma and choriocarcinoma. It is usually difficult to differentiate between these lesions based only on neuroimaging, so a tissue diagnosis is frequently needed. The blooming artifact of the susceptibility weighted imaging (SWI) that reflects the presence of paramagnetic elements like deoxyhemoglobin and hemosiderin or diamagnetic elements like calcification will add more diseases to the differential diagnosis in our case. The multiple hemorrhagic metastases, cavernomas, amyloid angiopathy, cerebral vasculitis, diffuse axonal injuries, and calcified neurocysticercosis all show similar SWI pictures. To narrow the radiologic differential diagnosis, we should consider the clinical background, other images’ modalities, and contrast enhancement. Phase-filtered imaging and CT scans can differentiate between hemorrhagic and calcified lesions. Contrast enhancement can be observed in the metastatic tumors, cerebral vasculitis, and some cases of neurocysticercosis. The lesions that usually do not show contrast enhancement are cavernomas, amyloid angiopathy, and diffuse axonal injuries.
Cerebral cavernous malformation, especially the familial type, can present with multiple hemorrhagic lesions. They appear as blooming artifacts with GRE or SWI, similar to NET [10]. Multiple choriocarcinoma metastases to the brain can be present with hemorrhagic lesions; MRI T1 with contrast may show enhancements in addition to blooming artifacts on GRE and SWI [11]. Metastatic melanoma is another differential diagnosis that can appear as hyperintense lesions with and without gadolinium administration on T1 MRI. Similarly, the presence of melanin and blood within the tumor can translate into blooming artifacts on GRE [12]. Diffuse axonal injury can be considered among the differential diagnoses, with small hemorrhagic foci on GRE [13]. Amyloid angiopathy can present with a multitude of hemorrhagic foci that looks similar to NET on GRE and SWI [14]. Neurocysticercosis, especially in its last stage, presents with intra-lesion calcifications and can imitate NET on GRE and SWI sequences [15].
Currently, no guideline consensus has been developed to manage NET-associated brain metastasis. Management options include surgical resection, WBRT, radiosurgery, or chemotherapy. None of the management options have proven effective in the overall survival of patients with brain metastasis [3]. Surgical resection followed by WBRT has been advocated by some authors [9,16].
4. Conclusion
NET brain metastases are rare tumors, especially in grade 1 type. When the number of metastases is extreme and hemorrhagic, and being associated with prolonged survival, the condition is considered extremely rare. A patient who initially presents with multiple cerebral hemorrhagic lesions without a known primary brings into play a neuroimaging dilemma of a group of disorders such as metastatic melanoma, metastatic choriocarcinoma, cavernous malformation, and amyloid angiopathy. Metastatic NET will be added to the differential diagnosis.
Metastatic NET carries a dismal prognosis with no effective management plan. Early screening and management of brain metastasis in newly diagnosed patients with NET may improve overall survival.
Declaration of Competing Interest
The authors report no declarations of interest.
Funding
Nothing to disclose.
Ethical approval
The case report was not submitted for ethical approval.
Consent
Written informed consent was obtained from the family for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
T.E: study design, writing the paper, revision.
O.B: Data collection, revision.
M.D: Data collection, revision.
M.H: Supervision, data analysis, revision.
Registration of research studies
Not applicable.
Guarantor
Maher Hassounah.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Tewari A., Palaniswamy S.S., Subramanyam P. Brain metastasis from neuroendocrine tumor of the gallbladder: a rare entity. South Asian J. Cancer. 2013;2(3):120. doi: 10.4103/2278-330X.114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauso O., Gustafsson B.I., Kidd M., Waldum H.L., Drozdov I., Chan A.K. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008;113:2655–2664. doi: 10.1002/cncr.23883. [DOI] [PubMed] [Google Scholar]
- 3.Krug S., Teupe F., Michl P., Gress T.M., Rinke A. Brain metastases in patients with neuroendocrine neoplasms: risk factors and outcome. BMC Cancer. 2019;19(1):362. doi: 10.1186/s12885-019-5559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A., SCARE group The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Baensch W.E., Karzinome Karzinoide. Sarkome und guitartige Tumoren des Dünndarms [Carcinomas, carcinoids, sarcomas and benign tumors of the small intestine] Rontgendiagnostik Ergeb. 1957;1952-1956:412–431. [PubMed] [Google Scholar]
- 6.Vinik A.I., Woltering E.A., Warner R.R., Caplin M., O’Dorisio T.M., Wiseman G.A. NANETS consensus guidelines for the diagnosis of neuroendocrine tumor. Pancreas. 2010;39(6):713–734. doi: 10.1097/MPA.0b013e3181ebaffd. [DOI] [PubMed] [Google Scholar]
- 7.Kim S.J., Kim J.W., Han S.W., Oh D.Y., Lee S.H., Kim D.W. Biological characteristics and treatment outcomes of metastatic or recurrent neuroendocrine tumors: tumor grade and metastatic site are important for treatment strategy. BMC Cancer. 2010;10:448. doi: 10.1186/1471-2407-10-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akimoto J., Fukuhara H., Suda T., Nagai K., Ichikawa M., Fukami S. Clinicopathological analysis in patients with neuroendocrine tumors that metastasized to the brain. BMC Cancer. 2016;16:36. doi: 10.1186/s12885-015-1999-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hlatky R., Suki D., Sawaya R. Carcinoid metastasis to the brain. Cancer. 2004;101(11):2605–2613. doi: 10.1002/cncr.20659. [DOI] [PubMed] [Google Scholar]
- 10.de Souza J.M., Domingues R.C., Cruz Jr L.C., Domingues F.S., Iasbeck T., Gasparetto E.L. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with t2-weighted fast spin-echo and gradient-echo sequences. AJNR Am. J. Neuroradiol. 2008;29(1):154–158. doi: 10.3174/ajnr.A0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn A.G., Salzman K.L., Jhaveri M.D. Diagnostic Imaging Brain. In: Osborn A.G., editor. Miscellaneous Malignant Germ Cell Neoplasm. Elsevier; Philadelphia, PA: 2016. pp. 586–589. [Google Scholar]
- 12.Gaviani P., Mullins M.E., Braga T.A., Hedley-White E.T., Halpern E.F., Schaefer P.S. Improved detection of metastatic melanoma by T2*-weighted imaging. AJNR Am. J. Neuroradiol. 2006;27(3):605–608. [PMC free article] [PubMed] [Google Scholar]
- 13.Ezaki Y., Tsutsumi K., Morikawa M., Nagata I. Role of diffusion-weighted magnetic resonance imaging in diffuse axonal injury. Acta Radiol. 2006;47(7):733–740. doi: 10.1080/02841850600771486. [DOI] [PubMed] [Google Scholar]
- 14.Charidimou A., Boulouis G., Gurol M.E., Ayata C., Bacskai B.J., Frosch M.P. Emerging concepts in sporadic cerebral amyloid angiopathy. Brain. 2017;140(7):1829–1850. doi: 10.1093/brain/awx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia H.H., Nash T.E., Del Brutto O.H. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13(12):1202–1215. doi: 10.1016/S1474-4422(14)70094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallory G.W., Fang S., Giannini C., Van Gompel J.J., Parney I.F. Brain carcinoid metastases: outcomes and prognostic factors. J. Neurosurg. 2013;118(4):889–895. doi: 10.3171/2013.1.JNS121556. [DOI] [PubMed] [Google Scholar]