Abstract
Background
Catecholamine is a typical index of exercise intensity, but it is difficult to detect. Plasma metanephrine (MN) and normethanephrine (NMN) levels are more stable than those of catecholamines. This study aimed to investigate plasma MN and NMN levels during acute exercise running in amateur runners.
Methods
Samples were collected from eight healthy male participants. They were either sedentary or running at low or high intensity for 30 min. Blood samples were collected under these conditions. Measurements taken included plasma adrenaline, noradrenaline, MN, and NMN.
Results
Plasma adrenaline levels increased after high-intensity exercise compared with sedentary subjects. Plasma noradrenaline, MN, and NMN levels increased after both low- and high-intensity exercise compared with sedentary subjects. In addition, these levels were also significantly higher at high intensity than at low intensity. Plasma adrenaline and noradrenaline levels were positively correlated with plasma free MN and NMN levels after acute running, respectively.
Conclusion
This study revealed that plasma MN and NMN levels transiently increased depending on exercise intensity in amateur runners. In addition, plasma NMN levels are better markers than plasma MN levels because of their stronger correlation with plasma catecholamine levels.
Keywords: Exercise, Running, Intensity, Ventilatory threshold
Introduction
Running has a significant positive effect on the body by improving cardiorespiratory function and by preventing and treating various diseases.1 As a result, and the number of people involved in running has increased worldwide. However, strenuous endurance exercises can cause immune system disorders and damage various tissues including muscles, as a result of metabolic and mechanical stress.2,3 Therefore, there is a need for biomarkers that can identify body conditions during and/or after endurance exercises. Moreover, biomarkers that can be measured via minimally invasive methods for continuous monitoring of internal conditions are important to prevent harmful effects.
To date, available biomarkers including lactate, free fatty acid, and glucose, have been investigated for various exercises.4,5 Among them, catecholamine is a typical index of exercise intensity.6 Catecholamine, including adrenaline and noradrenaline, regulates heart rate, blood pressure, and glycolysis.7 It is well recognized that adrenaline and noradrenaline increase with increasing exercise intensities.6 Epinephrine is almost completely synthesized and released from the adrenal medulla in response to adrenocorticotropic hormone of the sympathetic nervous system, while norepinephrine synthesis occurs primarily within the ends of sympathetic nerve fibers and in the adrenal glands to a lesser extent. It occurs within the chromaffin cells of the medulla.8 Catechol-O-methyltransferase (COMT), an enzyme that metabolizes catecholamines, occurs within the chromaffin cells of the medulla. COMT is abundantly present in the cytoplasm of liver and renal cells, changing blood adrenaline and noradrenaline to metanephrine (MN) and normethanephrine (NMN), which are inactivated by this enzyme. Subsequently, MN and NMN in the blood are rapidly sulfated and excreted from the kidney by phenol sulfotransferases (PST), which mainly distribute the hydroxyl group at the 4-position in the living body. As a result, free metanephrine is the form of the substance before undergoing conjugation, and it is in trace amounts compared to the conjugation type.9
Free MN and NMN are excreted from urine after acute exercise.10 It has been reported that blood MN and NMN are increased by acute exercises as well as urine.11, 12, 13, 14, 15 In addition, peaked plasma MN and NMN levels positively correlate with plasma adrenaline and noradrenaline after acute exercise, respectively. However, it is unclear whether these evaluations considered exercise intensity. The purpose of this study was to investigate plasma MN and NMN levels depending on exercise intensity.
Patients and methods subjects
Subjects
Eight healthy male subjects who underwent aerobic exercises at least twice per week were recruited. Subject characteristics are provided in Table 1. The subjects were instructed not to drink alcohol, ensure sufficient amounts of sleep, and avoid binge eating before the experiment. This study was approved by the Ethical Committee of the Faculty of Medicine at the University of Tsukuba (approval number: 274). All subjects received an explanation and written documentation in advance regarding the purpose of the experiment, its contents, and safety issues, and provided informed consent.
Table 1.
Participants’ characteristics in this study.
| Age |
Height |
Weight |
Body fat |
BMI |
O2max |
|
|---|---|---|---|---|---|---|
| (year) | (cm) | (kg) | (%) | – | (mL/kg・min) | |
| AVE | 21.6 | 173.0 | 61.8 | 9.9 | 20.6 | 64.3 |
| SD | 1.5 | 5.9 | 7.1 | 3.0 | 3.0 | 8.1 |
Experimental design
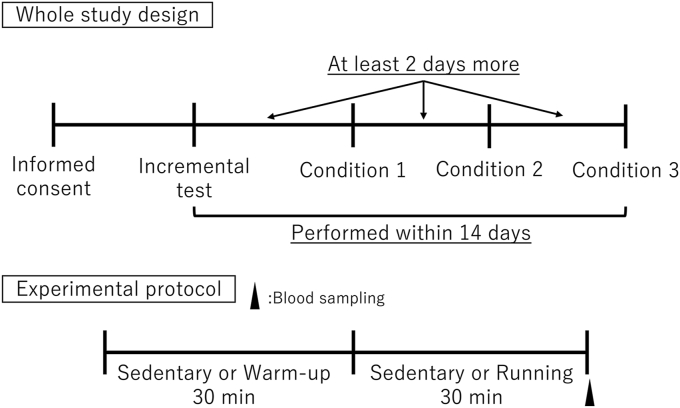
The experimental period is shown in Fig. 1. To set the exercise intensity level, the subjects performed exercises on a treadmill until exhaustion. In this study, subjects’ O2 was maximal because the respiratory expenditure rates were greater than 1.0. The maximal perceived exertion and observation plateau of O2 was assessed using an incremental test (data not shown), as described by Tokinoya et al. (2020).16 Subjects performed an incremental test in advance to calculate the ventilatory threshold (VT) intensity in each subject. VT was determined by an exercise physiologist. They were assessed under three conditions: sedentary, low intensity (85% VT), and high intensity (115% VT). After exercise, the subjects were each equipped with a watch that had a heart rate sensor and GPS, and they performed warm-up exercises before starting exercises under different conditions. They freely drank water (500mL) during the warm-up period on the day of the measurements. The heart rate (HR) sensor (HRM-RunTM, GARMIN, USA) within the exclusive watch (Fore Athlete® 920XTJ, GARMIN, America) recorded readings during exercise or sedentary periods. The subjects ran for 30 min around the 400 m track at a speed of 85% or 115% VT intensities. Blood samples were obtained immediately after entering the room after the exercise or sedentary period. For the sedentary condition, the subjects rested for 30 minutes in the building. All subjects fasted overnight, regardless of the testing conditions. Plasma samples with EDTA +2 Na were separated by centrifugation at 3000 rpm at 4 °C. These samples were stored at −80 °C until further analysis.
Fig. 1.
Experimental design. This is the entire study design. Conditions indicated sedentary, low-intensity exercise (85% VT), or high-intensity exercise (115% VT). All conditions were performed within a period of at least 2 days.
The experimental protocol is described as follows: The subjects were allowed to warm up for 30 min and then drank water (500 mL). After warming up, they ran for 30 min at VT speeds of 85% or 115%.
Plasma catecholamine, metanephrine (MN) and normethanephrine (NMN) levels
Plasma catecholamine levels were measured using high-performance liquid chromatography (HPLC). Plasma MN and NMN levels were measured using liquid chromatography mass spectrometry (LC/MS/MS) method. These analyzes were outsourced to Tsukuba i-Laboratory LLP (Tsukuba, Japan).
Statistical analysis
All results are presented as the mean ± standard deviation. GraphPad Prism 7 software (GraphPad, Inc., La Jolla, CA, USA). All data were analyzed using one-way analysis of variance (ANOVA) or t-test. All results were analyzed using the Bonferroni multiple comparison method after a one-way ANOVA analysis. Correlation analysis was conducted using the Pearson product-moment correlation coefficient method. The significance level was set at P < 0.05.
Results
Acute running for 30 min
The sedentary and exercise conditions were completed at a temperature of 14.9 ± 1.4 °C and 6.4 ± 1.5 °C, with a humidity level of 50.3 ± 5.9% and 72.0 ± 17.6%, using the WBGT measurement (KYOTO ELECTRONICS MANUFACTURING CO., LTD., Japan). The HR reserve (HRR) was calculated from the results of each exercise condition and all tests. HRR of low-intensity exercises was lower than that of high-intensity exercises (72.5 ± 9.3 vs. 88.6 ± 9.1, p < 0.01). The HRR during low-intensity exercise was lower than that during high-intensity exercise. Therefore, there was a significant difference between exercise intensities.
Plasma catecholamine, metanephrine (MN) and normethanephrine (NMN) levels
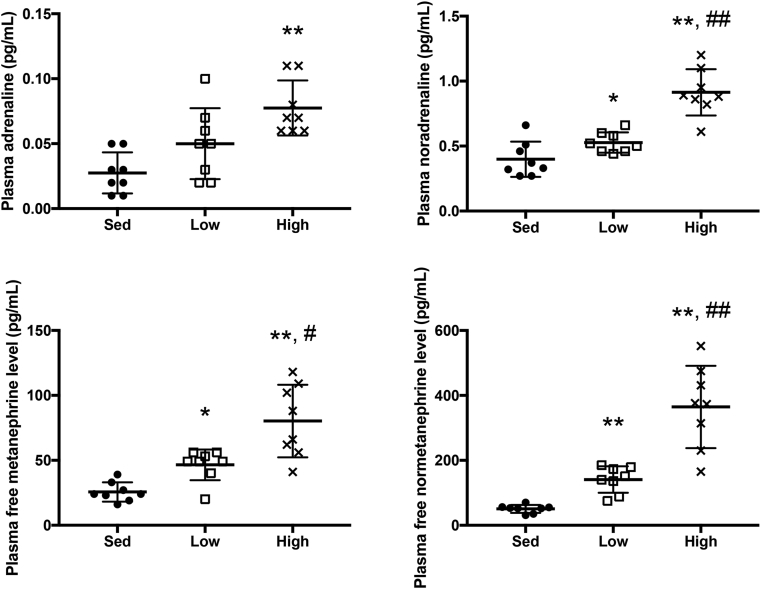
Plasma catecholamine, metanephrine (MN), and normethanephrine (NMN) levels are shown in Fig. 2. Plasma adrenaline levels only increased after high-intensity exercise compared with sedentary exercise (p < 0.01). Plasma noradrenaline, MN, and NMN levels increased after both low-and high-intensity exercises compared with sedentary subjects (p < 0.05, and p < 0.01, respectively). In addition, the subjects engaged in high-intensity exercises also showed significantly higher levels than those who engaged in low-intensity exercises (p < 0.05, and p < 0.01, respectively). Moreover, there were many significant correlations between plasma adrenaline and free MN levels after acute running (p < 0.01, Fig. 3). In addition, plasma noradrenaline levels were significantly correlated with plasma free NMN levels (p < 0.01).
Fig. 2.
Plasma adrenaline, noradrenaline, free metanephrine, and free normetanephrine levels depending on exercise intensities. Data are shown as the mean ± SD. n = 8 in each group. Data were analyzed using repeated one-way ANOVA. ∗,∗∗ statistical difference was set at p < 0.05 and p < 0.01 vs Sed. #,## statistical difference was set at p < 0.05 and p < 0.01 vs Low. Sed, sedentary; low, low intensity exercise; high, high-intensity exercise.
Fig. 3.
Correlation of plasma adrenaline and free metanephrine, noradrenaline and free normetanephrine levels on exercise. n = 8 in each group. Data were analyzed using the Pearson product-moment correlation coefficient. Statistical difference was set at p < 0.01 vs. Sed. Sed, sedentary; low, low intensity exercise; high, high-intensity exercise.
Discussion
This study investigated the plasma MN and NMN levels under high and low exercise intensities in amateur runners. It is important for us to research various biomarkers of exercise intensity because exercise intensities are different from effects on health benefits in previous studies.17 Our findings showed that plasma MN and NMN levels were increased by acute running for 30 min, depending on the exercise intensity. In addition, plasma adrenaline and noradrenaline levels were positively correlated with plasma MN and NMN levels, respectively.
Plasma catecholamines, involving adrenaline and noradrenaline, have been shown to decrease after exercise training.18 In other words, the baseline plasma catecholamine level in trained individuals tended to be lower than that in untrained individuals.19 Plasma catecholamine levels increased after 30 minutes of acute running. In this study, the target subjects were those who were regularly trained in aerobic exercises. In previous studies, plasma adrenaline and noradrenaline reportedly increased depending on exercise intensities.6 Therefore, these levels were significantly correlated with plasma MN and NMN in this study. In a previous study, blood MN and NMN levels were the most sensitive markers among the other markers, including blood and urine catecholamines and urine MN and NMN, in the diagnosis of pheochromocytoma.20 Moreover, and plasma NMN and noradrenaline levels were more correlated than plasma MN and adrenaline levels. Danese et al. (2018) reported that plasma NM levels were positively related to running performance.14 In addition, they found that the plasma NMN level was increased by acute cycling under various hypoxia conditions, while plasma MN level was not significantly increased under all conditions.12 Therefore, it is possible that NMN level is an index of exercise intensity under various conditions in addition to exercise performance.
In conclusion, plasma MN and NMN levels transiently increased after acute running in amateur runners. In addition, plasma catecholamine levels also increased depending on the exercise intensity. It is possible that plasma NMN level might be a better biomarker than plasma MN level because of its stronger correlation with plasma catecholamine levels in this study. In future studies, it will be necessary to investigate the effects of sex, age, and style of exercise on plasma MN and NMN levels to further investigate their suitability as exercise biomarkers.
Authorships
K Tokinoya, YS, YA and K Takekoshi contributed to the design. K Tokinoya, YS, NS, AA, YY, TS, YN, and K Takekoshi performed data acquisition. K Tokinoya, YS, TS, YN, and K Takekoshi analyzed and interpreted the data. K Tokinoya and K Takekoshi performed the statistical expertise. K Tokinoya drafted the manuscript. All authors supervised and edited the manuscript draft. All authors reviewed the manuscript. All authors provided final approval of this version of the manuscript for publication and agreed to be accountable for all aspects of the work.
Declaration of competing interest
There are no conflicts of interest to disclose.
Acknowledgments
All participants participated in this study. We appreciate Mr. Kai Aoki and Mr. Koki Yanazawa for helping with the experiments. We would also like to thank Editage (www.editage.com) for English language editing.
References
- 1.Lee D chul, Brellenthin A.G., Thompson P.D., Sui X., Lee I.M., Lavie C.J. Running as a key lifestyle medicine for longevity. Prog Cardiovasc Dis. 2017;60:45–55. doi: 10.1016/j.pcad.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Tokinoya K., Ishikura K., Ra S.G., Ebina K., Miyakawa S., Ohmori H. Relationship between early-onset muscle soreness and indirect muscle damage markers and their dynamics after a full marathon. J Exerc Sci Fit. 2020;18(3):115–121. doi: 10.1016/j.jesf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos V.C., Sierra A.P.R., Oliveira R. Marathon race affects neutrophil surface molecules: role of inflammatory mediators. PloS One. 2016;11(12):1–14. doi: 10.1371/journal.pone.0166687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hargreaves M., Spriet L.L. Skeletal muscle energy metabolism during exercise. Nat Metab. 2020 doi: 10.1038/s42255-020-0251-4. [DOI] [PubMed] [Google Scholar]
- 5.Soya H., Mukai A., Deocaris C.C. Threshold-like pattern of neuronal activation in the hypothalamus during treadmill running: establishment of a minimum running stress (MRS) rat model. Neurosci Res. 2007;58(4):341–348. doi: 10.1016/j.neures.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Katch V.L., McArdle W.D., Katch F.I. Hormones, Exercise, and Training. fourth ed. 2011. Essentials of exercise physiology; pp. 389–390. [Google Scholar]
- 7.Tank A.W., Wong D.L. Peripheral and central effects of circulating catecholamines. Comp Physiol. 2015;5(1):1–15. doi: 10.1002/cphy.c140007. [DOI] [PubMed] [Google Scholar]
- 8.Von Euler U.S., Hellner S. Excretion of noradrenaline and adrenaline in muscular work. Acta Physiol Scand. 1952;26(2-3):183–191. doi: 10.1111/j.1748-1716.1952.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 9.Eisenhofer G., Lenders J.W.M., Pacak K. Biochemical diagnosis of pheochromocytoma and paraganglioma. Front Horm Res. 2004;31:76–106. doi: 10.1016/j.ando.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Pequignot J.M., Peyrin L., Mayet M.H., Flandrois R. Metabolic adrenergic changes during submaximal exercise and in the recovery period in man. J Appl Physiol Respir Environ Exerc Physiol. 1979;47(4):701–705. doi: 10.1152/jappl.1979.47.4.701. [DOI] [PubMed] [Google Scholar]
- 11.Bracken R.M., Linnane D.M., Brooks S. Plasma catecholamine and nephrine responses to brief intermittent maximal intensity exercise. Amino Acids. 2009;36(2):209–217. doi: 10.1007/s00726-008-0049-2. [DOI] [PubMed] [Google Scholar]
- 12.Woods D.R., O’Hara J.P., Boos C.J. Markers of physiological stress during exercise under conditions of normoxia, normobaric hypoxia, hypobaric hypoxia, and genuine high altitude. Eur J Appl Physiol. 2017;117(5):893–900. doi: 10.1007/s00421-017-3573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allan R., Sharples A.P., Close G.L. Postexercise cold water immersion modulates skeletal muscle PGC-1α mRNA expression in immersed and nonimmersed limbs: evidence of systemic regulation. J Appl Physiol. 2017;123(2):451–459. doi: 10.1152/japplphysiol.00096.2017. [DOI] [PubMed] [Google Scholar]
- 14.Danese E., Tarperi C., Luca Salvagno G. Sympatho-adrenergic activation by endurance exercise. Effect on metanephrines spillover and its role in predicting athlete’s performance. Oncotarget. 2018;9(21):15650–15657. doi: 10.18632/oncotarget.24584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raber W., Raffesberg W., Waldhäusl W., Gasic S., Roden M. Exercise induces excessive normetanephrine responses in hypertensive diabetic patients. Eur J Clin Invest. 2003;33(6):480–487. doi: 10.1046/j.1365-2362.2003.01155.x. [DOI] [PubMed] [Google Scholar]
- 16.Tokinoya K., Ishikura K., Yoshida Y. LDH isoenzyme 5 is an index of early onset muscle soreness during prolonged running. J Sports Med Phys Fit. 2020;60(7):1020–1026. doi: 10.23736/S0022-4707.20.10278-0. [DOI] [PubMed] [Google Scholar]
- 17.Garber C.E., Blissmer B., Deschenes M.R. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 18.Katch V.L., McArdle W.D., Katch F.I. fourth ed. 2011. In the Cardiovascular System and Exercise; pp. 306–311. [Google Scholar]
- 19.Lehmann M., Dickhuth H.H., Schmid P. Plasma catecholamines, fl-adrenergic receptors, and isopro- terenol sensitivity in endurance trained and non-endurance trained volunteers. Eur J Appl Physiol. 1984;52(4):362–369. doi: 10.1007/BF00943364. [DOI] [PubMed] [Google Scholar]
- 20.Lenders J.W.M. Biochemical diagnosis of pheochromocytoma and paraganglioma. Ann Endocrinol. 2009;70(3):161–165. doi: 10.1016/j.ando.2009.02.008. [DOI] [PubMed] [Google Scholar]