Abstract
The paper aimed to analyse the safety of drinking coffee by adult Poles in terms of Pb and Cd content. The degree to which Cd and Pb passed from coffee grounds into the coffee infusion was also examined. Twenty-three samples of natural coffee were examined. The content of metals was determined using the ICP method. On average, dry coffee contained ca. 0.004 μg Cd and 0.05 μg Pb per 1 g, and 95.5% Cd and 94% Pb passed into the infusion. Drinking coffee supplies these metals in the amount of less than 2% TWI (tolerable weekly intake) for Cd and BMDL (benchmark dose lower confidence limit) for Pb. In the presented studies, the values of CDI (chronic daily intake), THQ (target hazard quotient) and HI (hazard index) indicators were lower than 1, which means that the risk of developing diseases connected with chronic exposure to Cd and Pb consumed with coffee must be evaluated as very low. The content of Cd and Pb in the analysed coffee infusions was very low, so drinking coffee does not pose a risk for consumers in terms of the content of these metals. However, it must be remembered that no threshold limits for toxic metal consumption exist because these metals accumulate in the body for a long time. The studies presented here also showed a low (r = 0.26) but still a positive correlation between the content of Pb in coffee and the degree (%) to which Pb passed into the infusion. This problem should be thoroughly investigated.
Keywords: Coffee infusions, Cadmium, Lead, Risk assessment
Introduction
Coffee, next to tea, is one of the most popular drinks in the world [1]. It is a source of antioxidants including caffeine, phenolic compounds and diterpenes. Results of studies suggest that drinking coffee can increase the level of glutathione and improve the protection of the body against DNA damage, in particular, if consumed regularly [1]. It was demonstrated that drinking coffee decreased the risk of developing breast cancer, prostate and colorectal cancer, which is attributed to the presence of antioxidants [2, 3]. It is also suggested that drinking coffee decreases the risk of developing chronic diseases such as type 2 diabetes and Parkinson’s [4, 5]. According to surveys, 95.2% of adult Poles drink coffee compared with 61% of Italians and about 40% of Spaniards [6, 7]. Statistically, in 2017 in Poland, the consumption of coffee amounted to 2.16 kg per person [8]. In Poland, the most popular type of coffee is non-instant coffee (ground and grained). This is a choice of more than 50% of consumers, preceding instant coffee and coffee mixes [6]. Most often, Poles drink 1–3 cups of coffee a day [9, 10]. However, one should not drink more than 5 cups (1 cup = 150 ml = 80 mg caffeine) a day due to its possible negative effect on the cardiovascular system (increased LDL-chol and total cholesterol levels due to diterpenoid alcohols), problems falling asleep (caffeine), pregnancy (caffeine intake of > 300 mg per day proved negative effect on the duration of pregnancy and weight at birth) and increased secretion of gastric acid and bile, which exacerbates peptic ulcer disease and hyperacidity [1, 11–13].
Apart from antioxidants and other bioactive compounds, coffee contains carbohydrates, lipids, nitrogen compounds, vitamins and minerals, including toxic elements such as cadmium (Cd) and lead (Pb) [12, 14, 15]. The presence of toxic metals in food is a global problem. Their primary source for humans is the food of plant origin [16, 17]. Although, according to available literature and own studies, the content of Cd and Pb in food products normally does not exceed acceptable standard levels, due to the fact that these metals are capable of accumulating in tissues and have a long half-life: 5–30 years for Cd and from 30 days (in soft tissue) to 10 years (in bones) for Pb [18], their regular supply, even in small amounts, is dangerous. These metals display mutagenic, teratogenic, carcinogenic and embryotoxic effects [19]. In 2012, EFSA reduced the tolerable intake level for Cd and Pb. The TWI (tolerable weekly intake) for Cd was determined at the level of 2.5 μg kg−1 of body weight per week [16], whereas the BMDL (benchmark dose lower confidence limit) for Pb was BMDL01—10.5 μg kg−1 of body weight per week—and BMDL10—4.4 μg kg−1 of body weight per week [17]. The paper aimed at analysing the safety of drinking coffee by adult Poles in terms of Pb and Cd content. The degree to which Cd and Pb passed from coffee grounds into the coffee infusion was also examined. The presented results are a part of the project aiming to estimate the intake of minerals (toxic and essential) in the Polish population.
Material and methods
Study material
Twenty-three samples of natural coffee were examined (Table 1). The products were purchased in August 2017 from local groceries, still within their shelf life. Before the analyses, the coffee was stored in original, tightly sealed packages at room temperature.
Table 1.
Characteristic of the analysed products
| Coffee form | Coffee varieties | Trademark | Size of package, g | Annotation | Origin | Made in | |
|---|---|---|---|---|---|---|---|
| 1 | Beans | Arabica + Robusta | A | 1000 | South America, Asia, Africa | Poland | |
| 2 | Beans | No data | B-1 | 1000 | No data | Poland | |
| 3 | Ground | No data | B-2 | 250 | No data | Poland | |
| 4 | Ground | Robusta | C | 400 | India | Poland | |
| 5 | Beans | Arabica | D-1 | 500 | Brazil | Holland | |
| 6 | Beans | Arabica + Robusta | D-2 | 1000 | No data | Holland | |
| 7 | Ground | Arabica | D-1 | 500 | Columbia | Holland | |
| 8 | Ground | Arabica | D-3 | 500 | Brazil | Holland | |
| 9 | Ground | Arabica | G-1 | 250 | Bio, Fair Trade | Papua New Guinea, Peru, Mexico | Italy |
| 10 | Ground | Arabica | G-2 | 250 | Brazil | Italy | |
| 11 | Beans | Arabica + Robusta | G-3 | 1000 | South America, Indonesia | Italy | |
| 12 | Ground | Arabica + Robusta | I | 250 | South America, Indonesia | Germany | |
| 13 | Ground | Robusta | J-1 | 500 | Vietnam | Germany | |
| 14 | Ground | Robusta | J-2 | 250 | India | Germany | |
| 15 | Ground | Robusta | J-2 | 100 | India | Germany | |
| 16 | Ground | Arabica | J-2 | 500 | Brazil | Germany | |
| 17 | Beans | Arabica | J-3 | 1000 | Fair Trade | Bolivia, Peru, Ecuador, Nicaragua | Germany |
| 18 | Ground | Arabica | K-1 | 500 | Brazil | Germany | |
| 19 | Ground | Arabica | K-2 | 500 | South America | Germany | |
| 20 | Beans | Arabica | K-3 | 500 | South America | Germany | |
| 21 | Beans | Arabica + Robusta | K-4 | 500 | South America, Indonesia | Germany | |
| 22 | Ground | Arabica | L | 500 | Brazil | Germany | |
| 23 | Ground | Arabica | M | 227 | Fair Trade | Peru, Nicaragua | England |
Preparation of samples for analyses
Grained coffee was ground in a laboratory grinder with plastic blades. Ground coffee was mixed by hand. Coffee infusions were prepared as follows: 6 g of ground coffee was poured with 100 ml of drinking water with a temperature of 95–100 °C; after 10 min, the solutions were drained through the Whatman drain. The resulting coffee grounds were dried in a drier at a temperature of 65 °C for 24 h. Afterwards, they were pulverised in a laboratory grinder with plastic blades. The analyses covered both fresh ground coffee and coffee grounds remaining after coffee brewing.
Chemical analyses
The analysed material was manually mixed. Samples weighing ca. 3 g were weighed in 3 replications into previously heat sterilised china crucibles and then subjected to dry mineralisation in a muffle furnace at a temperature of 450 °C. The oxidant was hydrogen peroxide. The mineralisate was dissolved in 10 ml of 1 M HNO3 [20, 21]. The content of cadmium and lead was determined using ICP (inductively coupled plasma mass spectrometry) in a Varian 820 MS spectrometer (Varian, Melbourne, Australia). The parameters for determination and control of correct analyses were included in Table 2. The calibration curve was drawn using the models:
Cd: standard characterised by 99.999% purity used to prepare solutions with the concentration of 0.2; 0.4; 1; 2; 4; 10 μg of Cd L−1; the solutions were prepared in 1% ultra-pure nitric acid (V).
Pb: standard characterised by 99.999% purity used to prepare solutions with the concentration of 0.1; 0.2; 0.5; 1; 2; 5 μg of Pb L−1; the solutions were prepared in 1% ultra-pure nitric acid (V).
Table 2.
Measurement parameters and validation data for the determination of Cd and Pb levels by ICP-MS
| Cd | Pb | |
|---|---|---|
| Mass monitored | 114 | 206; 207; 208 |
| Plasma gas | Argon | Argon |
| Plasma gas flow, L min−1 | 18 | 18 |
| Nebuliser gas flow, L min−1 | 1 | 1 |
| Auxiliary gas flow, L min−1 | 1.70 | 1.70 |
| Sampling depth, mm | 5 | 5 |
| RF power, kW | 1.37 | 1.37 |
| Limit of detection LOD, μg kg−1 | 0.004 | 0.005 |
| Limit of quantification LOQ, μg kg−1 | 0.010 | 0.030 |
| Quality control | ||
| Blank sample | 1 M HNO3 | 1 M HNO3 |
| Certified reference material (1) | INCT-TL-1 (tea leaves) | INCT-TL-1 (tea leaves) |
| Certified reference material (2) | INCT-MPH-2 (mixed Polish herbs) | INCT-MPH-2 (mixed Polish herbs) |
| Certified element concentration in CRM 1 | ||
| Certified, mg kg−1 | 0.030 | 1.78 |
| Observed, mg kg−1 | 0.029 | 1.76 |
| Recovery rate, % | 98 | 99 |
| Certified element concentration in CRM 2 | ||
| Certified, mg kg−1 | 0.199 | 2.16 |
| Observed, mg kg−1 | 0.189 | 2.22 |
| Recovery rate, % | 95 | 103 |
| Precision, % | 6.04 | 6.07 |
| Replicates | 3 | 3 |
Each chemical analyses was repeated 3 times. The accuracy of determination was verified using a blind test (1 M HNO3) and two certified reference materials (CRM): INCT-TL-1 Tea leaves (containing 0.030 mg Cd and 1.78 mg Pb per 1 kg) and INCT-MPH-2 Mixed Polish herbs (containing 0.199 mg Cd and 2.16 mg Pb per 1 kg).
Reagents and reference materials
Hydrogen peroxide H2O2 (30% pure) and nitric acid HNO3 (65% ultra-pure) were purchased from POCH S.A. (Poland). Deionised water used for dilution was made in our laboratory (Hydrolab Poland, Gdańsk). The Cd and Pb standards were purchased from Merck (Germany). Certified reference materials INCT-TL-1 and INCT-MPH-2 were obtained from the Institute of Nuclear Chemistry and Technology (Warsaw, Poland).
Calculations
Based on the difference in the content of Cd and Pb in coffee grounds, the degree (%) to which those metals passed into the infusion was calculated prior to after coffee brewing.
The safety of drinking coffee for adult Poles was estimated on the grounds of (1) calculation of the percentage of Cd and Pb intake in comparison with the acceptable level proposed by EFSA [16, 17], (2) calculation of parameters describing the risk of development of cancer and (3) calculation of parameters describing the risk of development of non-carcinogenic diseases. Three consumption patterns were taken into account in the calculations: 1 cup, 2 cups or 3 cups a day for 365 days in a year because such amounts of coffee in Poland are drunk by ca. 80% of coffee drinkers [9, 10].
Percent of tolerable dose:
Estimated weekly intake (EWI) of Cd and Pb was calculated according to the formula [22]:
where MWC is the mean weekly consumption of coffee (one, two or three cups).
Tolerable weekly intake % (TWI) was calculated according to the formula [22]:
The value adopted for TWI was 2.5 μg Cd kg−1 of body weight per week [16].
Benchmark dose lower confidence limit % (BMDL) was calculated according to the formula [22]:
The value adopted for BMDL: two values suggested by the European Food Safety Authority (EFSA) were calculated per 1 week: BMDL01—10.5 μg Pb kg−1 of body weight per week—and BMDL10—4.4 μg Pb kg−1 of body weight per week [17].
The mean body weight was assumed as 70 kg.
-
(2)
Cancer risks parameters
Chronic daily intake (CDI) of Cd or Pb was calculated according to the formula [23, 24]:
where EDI is the estimated daily intake of Cd and Pb, calculated on the basis of the mean weekly consumption of coffee (one, two or three cups) and mean level of Cd and Pb; EFr is the days of exposure frequency (365 per year); EDtot is the exposure duration (years)—since in Poland regular coffee drinkers are adults only, it was assumed that the time of exposure was calculated from 18 to 74 years of age (74 years—average life span in Poland), which is 56 years; AT is the period of exposure (365 per year).
CSF is a cancer slope factor which is the risk produced by a lifetime average dose of 1 mg kg−1 BW per day and is contaminant specific.
-
(3)
Non-carcinogenic risks parameters
Target hazard quotient (THQ) was calculated according to the formula [23]:
where CDI is the chronic daily intake of Cd or Pb.
RfD (reference dose) for Cd is 1 μg kg−1 of body weight per day, whereas, for Pb, it is 3.5 μg kg−1 of body weight per day [25].
When THQ is higher than 1, it is assumed that there is a significant risk of developing negative effects on health resulting from chronic exposure to Cd and/or Pb [26].
Hazard index (HI) was calculated according to the formula [23]:
Statistical analysis
The mean content of Cd and Pb was calculated for each sample (three weighing replications × 3 replications of chemical analysis). A statistical analysis of the results (average value, minimum and maximum value, standard deviation, median, 75 and 25 percentile) was carried out using Statistica 13.1 software. Statistically significant differences (P < 0.05) were computed by single factor analysis of variance (ANOVA), using the Duncan test. The correlation between the content of Cd and Pb in coffee and the degree (%) to which they passed into the infusion was calculated using Pearson’s method (Statistica 6.0 software).
Results
Content of Cd and Pb in coffee
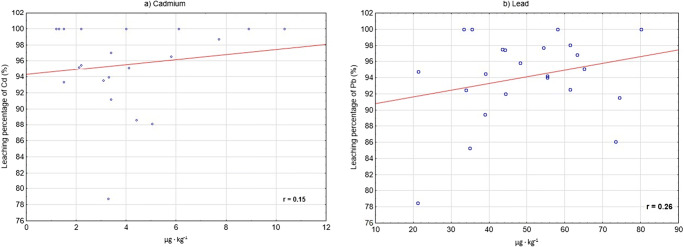
Dry coffee prior to brewing contained from 1.204 to 10.33 μg Cd per 1 kg (Tables 3 and 4). The mean content of Cd in the analysed samples was 3.784 μg (± 2.464) per 1 kg. Coffee grounds contained from < LOQ to 0.698 μg Cd per 1 kg; in 35% of samples, the level of Cd was lower than determinable with the applied method (LOQ = 0.01 μg kg−1). About 79 to 100% (on average 95.5%) of Cd present in the output material passed into the infusion; the infusion contained, on average 3.613 μg kg−1 (range 1.2–10.33 μg). A very low positive correlation r = 0.15 was identified between the content of Cd in coffee and the degree (%) to which Cd passed into the infusion (Fig. 1a). On average, dry coffee prior to brewing contained ca. 49.6 μg kg−1 Pb (range 21.22–80.06 μg kg−1), whereas coffee grounds < LOQ—10.2 μg kg−1. In 17% of coffee ground samples, the level of Pb was lower than determinable using the analytical method applied (LOQ = 0.03 μg kg−1). From nearly 79 to 100% (on average 94%) of Pb passed into the infusion, the infusion contained, on average, 46.86 μg kg−1 (range 16.66–80.06 μg). A low positive correlation r = 0.26 was identified between the content of Pb in coffee and the degree (%) to which Pb passed into the infusion (Fig. 1b).
Table 3.
Content of Cd and Pb in dry ground coffee (before brewing), dregs and infusions (n = 23), μg kg−1
| Dry coffee | Dregs | Infusions | Leaching percentages of Cd and Pb | |||||
|---|---|---|---|---|---|---|---|---|
| Cd | Pb | Cd | Pb | Cd | Pb | Cd | Pb | |
| 1 | 5.041E | 73.37F | 0.600F | 10.20I | 4.443D | 63.17F | 88B | 87A |
| 2 | 10.33I | 74.37F | < LOQA | 6.280H | 10.33H | 68.09F | 100C | 92A, B |
| 3 | 1.204A | 34.84B | < LOQA | 5.134G | 1.203A | 29.71C | 100C | 85A |
| 4 | 3.991D | 38.04B, C | < LOQA | 2.143C | 3.990D | 36.89C, D | 100C | 95B |
| 5 | 8.883H | 43.47C | < LOQA | 1.087B | 8.882G | 42.38D | 100C | 97B |
| 6 | 3.289C | 80.06G | 0.702G | < LOQA | 2.593B, C | 80.06G | 78A | 100B |
| 7 | 1.502A | 44.21C | < LOQA | 1.120B | 1.502A, B | 43.09D | 100C | 97B |
| 8 | 2.100B | 33.87B, C | 0.100B | 2.549C | 2.000B | 31.32C | 95B, C | 92A, B |
| 9 | 3.411C, D | 58.12D, E | 0.313D | < LOQA | 3.100C | 58.12E, F | 91B, C | 100B |
| 10 | 2.204B | 61.35E | 0.122B | 1.223B | 2.084B | 60.13F | 95B, C | 98B |
| 11 | 1.273A | 55.41D | < LOQA | 3.320E | 1.272A | 52.09E | 100C | 94A, B |
| 12 | 2.100B | 61.35E | < LOQA | 4.581F | 1.994B | 56.77E, F | 95B, C | 93A, B |
| 13 | 3.122C | 54.36D | 0.189C | 1.250B | 2.932C | 53.11E | 94B, C | 98B |
| 14 | 7.703G | 33.35B | 0.133B | < LOQA | 7.573F | 33.35C | 98B, C | 100B |
| 15 | 6.111F | 44.36C | < LOQA | 3.561D | 6.110E | 40.80D | 100C | 92A, B |
| 16 | 1.489A | 55.36D | 0.100B | 3.210D | 1.389A | 52.15E | 93B, C | 94A, B |
| 17 | 2.110B | 21.36A | 0.122B | 1.122B | 1.980B | 20.24B, C | 95B, C | 95A, B |
| 18 | 3.311C | 65.12E | 0.191C | 3.220D | 3.122C | 6.900A | 94B, C | 95A, B |
| 19 | 4.089D | 21.22A | 0.210C | 4.558E | 3.881C | 16.66B | 95B, C | 78A |
| 20 | 2.210B | 35.48B | < LOQA | < LOQA | 2.200B | 35.48C, D | 100C | 100B |
| 21 | 3.401C, D | 48.22C | 0.103B | 2.010C | 3.321C | 46.21D, E | 97B, C | 96B |
| 22 | 5.824F | 63.22E | 0.222C | 2.011C | 5.602E | 61.21F | 97B, C | 97B |
| 23 | 4.350D | 38.95B, C | 0.484E | 4.123F | 3.874C | 34.83C | 89B | 89A, B |
Average values for 3 replications
A, BMeans with different superscripts in the same column differs significantly at P < 0.05 by Duncan’s test; LOQ Cd = 0.010 μg kg−1; LOQ Pb = 0.030 μg kg−1
Table 4.
Results of coffee analysis (n = 23)
| Dry coffee | Dregs | Infusions | Leaching percentages of Cd and Pb | |
|---|---|---|---|---|
| Cd, μg kg-1 | ||||
| Mean | 3.784 | 0.156 | 3.613 | 95.49 |
| Maximum | 10.33 | 0.698 | 10.33 | 100.0 |
| Minimum | 1.204 | < LOQ | 1.200 | 78.79 |
| Median | 3.300 | 0.100 | 3.101 | 95.45 |
| SD | 2.464 | 0.197 | 2.471 | 5.162 |
| Variance analysis | 0.607 | 0.001 | 0.609 | 26.65 |
| Percentile | ||||
| 75% | 4.720 | 0.200 | 4.222 | 100.0 |
| 25% | 2.100 | < LOQ | 2.000 | 93.74 |
| Percent of samples < LOQ | 0% | 35% | ||
| Pb, μg kg-1 | ||||
| Mean | 49.59 | 2.726 | 46.86 | 94.07 |
| Maximum | 80.06 | 10.20 | 80.06 | 100.0 |
| Minimum | 21.22 | < LOQ | 16.66 | 78.51 |
| Median | 48.22 | 2.143 | 46.21 | 94.76 |
| SD | 16.27 | 2.412 | 15.85 | 5.332 |
| Variance analysis | 26.47 | 5.819 | 25.16 | 28.43 |
| Percentile | ||||
| 75% | 61.35 | 3.840 | 59.12 | 97.60 |
| 25% | 37.22 | 1.120 | 35.16 | 92.22 |
| Percent of samples < LOQ | 0% | 17% | ||
Average values for samples, each in 3 replications; SD, standard deviation; LOQ, limit of quantitation; LOQ Cd = 0.01 μg kg−1; LOQ Pb = 0.03 μg kg−1
Fig. 1.
Correlation between the content of Cd (a) and Pb (b) in coffee and the degree (%) to which they pass into the infusion
Coffee drinking safety
Data concerning the estimated safety of drinking coffee infusions, taking into account three consumption patterns (1, 2 or 3 cups of coffee a day), is presented in Table 5.
Table 5.
Safety of coffee for consumption
| Cd | Pb | |
|---|---|---|
| Pattern 1: drinking 1 cup of coffee a day | ||
| EWI, μg1 | 0.156 | 1.968 |
| % TWIA, B | 0.089 | |
| % BMDL01A, C | 0.268 | |
| % BMDL10A, D | 0.639 | |
| CD2 | 0.022 | 0.281 |
| THQ3 | 0.022 | 0.080 |
| HI4 | 0.103 | |
| Pattern 2: drinking 2 cups of coffee a day | ||
| EWI, μg1 | 0.312 | 3.936 |
| % TWIA, B | 0.178 | |
| % BMDL01A, C | 0.541 | |
| % BMDL10A, D | 1.278 | |
| CDI2 | 0.045 | 0.562 |
| THQ3 | 0.045 | 0.161 |
| HI4 | 0.205 | |
| Pattern 3: drinking 3 cups of coffee a day | ||
| EWI, μg1 | 0.468 | 5.715 |
| % TWIA, B | 0.267 | |
| % BMDL01A, C | 0.778 | |
| % BMDL10A, D | 1.856 | |
| CDI2 | 0.067 | 0.816 |
| THQ3 | 0.067 | 0.233 |
| HI4 | 0.300 | |
1EWI, estimated weekly intake calculated on the basis of the mean weekly consumption of coffee infusions and mean level of Cd and Pb
2Chronic daily intake calculated on the basis of the mean weekly consumption of coffee, mean level of Cd and Pb and exposure duration
3Target hazard quotient calculated on the basis of the chronic daily intake of Cd or Pb
4Hazard index is the sum of THQ for Cd and Pb
AMean body weight was assumed as 70 kg
BTWI, 2.5 μg Cd per kg of body weight per week [16]
CBMDL01, 10.5 μg Pb per kg of body weight per week [17]
DBMDL10, 4.4 μg Pb per kg of body weight per week [17]
Pattern 1: 1 cup of coffee a day
The estimated weekly intake (EWI) of Cd with coffee infusion is 0.156 μg, which accounts for about 0.09% TWI. The value of CDICd and THQCd indicators is identical and it amounts to 0.022. The estimated weekly intake of Pb is 1.968 μg, which corresponds to ca. 0.27% BMDL01 and ca. 0.64% BMDL10. The value of CDIPb = 0.281, whereas that of THQPb = 0.08. The HI risk factor (Cd + Pb) is 0.103.
Pattern 2: 2 cups of coffee a day
EWI of Cd with coffee is 0.312 μg, which accounts for 0.18% of TWI. The value of CDICd and THQCd indicators is 0.045 each. EWI of Pb is ca. 3.94, which corresponds to 0.54% BMDL01 and nearly 1.3% BMDL10. The value of CDIPb = 0.56, whereas THQPb = 0.16. The HI risk factor equals 0.205.
Pattern 3: 3 cups of coffee a day
EWI of Cd with infusion is less than 0.5 μg, which accounts for about 0.27% TWI. The values of CDICd and THQCd indicators are 0.067 each. EWI of Pb was equal to 5.715 μg, which corresponds to about 0.78% BMDL01 and about 1.86% BMDL10. The value of CDIPb = 816, whereas THQPb = 233. The HI risk factor equals 0.3.
Discussion
In the presented studies of this author, dry coffee contained on average nearly 3.8 μg Cd and ca. 50 μg Pb per 1 kg of the natural product, which accounts for ca. 0.004 μg Cd and 0.05 μg Pb per 1 g. As 95.5% Cd and 94% Pb passed into the infusion, the infusion contained on average 0.0037 μg Cd and ca. 0.047 μg Pb per 1 g. Considering the consumption of coffee infusion (1, 2 or 3 cups a day), an adult Pole consumes less than 0.5 μg Cd and nearly 6 μg Pb per day. Nędzarek et al. [14] examined the content of, among other elements, Pb in infusions of coffee purchased in Poland (n = 4), Lebanon (n = 1), Brazil (n = 3) and in Bosnia and Herzegovina (n = 3). These studies showed that infusions contained from 0.615 (Polish coffee) to 1.24 μg (Bosnia and Herzegovina) Pb per 1 g. According to those authors, in Poland, a person drinking 2.4 kg of coffee on an annual basis simultaneously consumes 1.48–2.43 mg Pb, which is slightly more than 4 μg Pb per day, whereas, in Bosnia and Herzegovina, it is ca. 33 μg Pb per day (12 mg per year).
Grembecka et al. [27] in 120 samples of different types of coffee, including 75 samples of ground coffee and 27 of instant coffee, found the presence of Cd and Pb in amounts lower than determinable using the applied method of analysis (LOD Cd = 0.003 mg 100 g−1, LOD Pb—0.01 mg 100 g−1). Gebretsadik et al. [28] in Ethiopian ground coffees found that the level of Cd was lower than 0.01 μg g−1, whereas that of Pb < 0.04 μg g−1. Similarly, Ashu and Chandravanshi [29] found that both in dry grains and infusions of Ethiopian coffee (n = 3), the level of Cd and Pb was lower than LOD. Studies in Brazil demonstrated that the level of Cd in roasted ground coffee (n = 15) was < 0.025 μg g−1, whereas the level of Pb ranged from 0.14 to 2.59 μg g−1 [15]. In as many as 8 samples, the level of Pb exceeded the maximum limit accepted by Brazilian legislation 0.5 μg g−1 [15]. According to other Brazilian studies [30], ground coffees contained 0.03–0.1 mg Cd and 0.025–1.58 mg Pb per 1 kg. The same authors found that the coffees were not safe in terms of the content of Pb; in 75% of 50 analysed samples, it contained more Pb than acceptable in Brazil, whereas, in 86% of samples, it exceeded the limit in the European Union (0.2 μg kg−1). In addition, da Silva et al. [30] found that the degree of extraction of Cd into the infusion is 26% and that of Pb is less than 47%; the values were considerably lower than measured by the present author. Instant coffees drunk in India contained 0.001–0.03 μg g−1 Cd and 0.02–0.2 μg g−1 Pb [31]. Studies carried out in Saudi Arabia showed that dry coffee contained 0.053 μg Pb g−1 [32]. In turn, Santos et al. [33] found that in coffee grains, the average content of Cd < 0.1 μg g−1, whereas that of Pb < 2.6 μg g−1. Turkish studies showed that green coffee grains contained on average 0.005 μg g−1 Cd (0.003–0.006 μg g−1) and 0.12 μg g−1 Pb (0.06–0.3 μg g−1) [34]. Those authors recount that up to 84% of Cd and up to 82.6% of Pb pass into the infusion, which is dependent on the coffee brewing method only (Turkish method—cooking—leaches more minerals, including toxic elements—except for Pb, than pouring with boiling water as practised in Poland), but it is not dependent on the type of coffee. Studies by Anderson et al. [35] also showed that Pb can be leached from vessels into the ground coffee infusion—the highest amount of Pb passes from ceramic cups.
In the presented studies, the values of CDI, THQ and HI indicators for all the assumed patterns were lower than 1, which means that the risk of developing diseases connected with chronic exposure to Cd and Pb consumed with coffee must be evaluated as very low. Coffee drinking safety is also confirmed by the degree of coverage of the tolerable intake level of Cd and Pb recommended by EFSA [16, 17]. According to the studies of the present author, drinking 3 cups of coffee a day contributes to supplying these metals in the amount of less than 0.3% TWI (Cd) and less than 2% BMDL (Pb). According to Şemen et al. [34], drinking 2 cups of coffee a day contributes to Cd intake amounting to 0.01–0.06% PTWI and Pb intake amounting to 0.03–0.38% PTWI, depending on the type of coffee and content of toxic metals. Suseela et al. [31] found that drinking instant coffee contributes to intake of Cd amounting to 1.1% and that of Pb amounting to 0.7% of the acceptable limit in India. Pigozzi et al. [15] recount that 1 cup (50 ml) of Brazilian ground coffee infusion contains maximum 2.835 μg Pb, which accounts for 0.21 to 4.54% of the acceptable limit (that is 25 μg kg−1 of body weight), while the content of Cd in those coffees was lower than LOD.
To sum up, the content of Cd and Pb in the analysed coffee infusions was very low. However, it must be remembered that no threshold limits for toxic metal consumption exist because these metals accumulate in the body for a long time; in the case of Cd and Pb, it is even 30 years [18], whereas their largest amounts accumulate in organs in charge of detoxicating processes (liver and kidneys) and in the brain [19, 36], leading to their damage and dysfunction. Nędzarek et al. [14] mention the level of Pb in coffee; despite it was low in their studies, those authors suggest that the content of Pb in coffee should be monitored regularly because it is higher than the content of Cd and can accumulate in tissues. Studies involving rats showed that during complex exposure (Cd + Pb), Pb accumulates in the organs to a higher degree than Cd (0.6% vs 0.48% in adults and 0.5% vs 0.7% in a younger population) [19, 36]. Lead is absorbed to a higher extent by the gastrointestinal tract than Cd after oral intake (10–50%, 1–8%) [37, 38]. In the presented study of this authors, an alarming signal is CDIPb close to 1. It must be taken into account that some authors found that the Pb level was higher than acceptable in 75% of the analysed samples [15, 30]. The studies presented here also showed a low (r = 0.26) but still, a positive correlation between the content of Pb in coffee and the degree (%) to which Pb passed into the infusion. This problem should be thoroughly investigated.
Conclusions
The content of Cd and Pb in the analysed coffee infusions was very low, so drinking coffee does not pose a risk for consumers in terms of the content of these metals. However, it must be remembered that no threshold limits for toxic metal consumption exist because these metals accumulate in the body for a long time; in the case of Cd and Pb, it is even 30 years.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martini D, Del Bo’ C, Tassotti M, Riso P, Del Rio D, Brighenti F, Porrini M. Coffee consumption and oxidative stress: a review of human intervention studies. Molecules. 2016;21:979. doi: 10.3390/molecules21080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nkondjock A. Coffee consumption and the risk of cancer: an overview. Cancer Lett. 2009;277:121–125. doi: 10.1016/j.canlet.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Cao S, Liu L, Yin X, Wang Y, Liu J, Lu Z. Coffee consumption and risk of prostate cancer: a meta-analysis of prospective cohort studies. Carcinogenesis. 2014;35:256–261. doi: 10.1093/carcin/bgt482. [DOI] [PubMed] [Google Scholar]
- 4.Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 5.Sääksjärvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Männistö S. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur J Clin Nutr. 2008;62:908–915. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 6.Chudy S. Development of coffee market and changes in coffee consumption among Poles. J Agribus Rural Dev. 2014;34:41–51. [Google Scholar]
- 7.Özen AE, Bibiloni Mdel M, Pons A, Tur JA. Consumption of functional foods in Europe; a systematic review. Nutr Hosp. 2014;29:470–478. doi: 10.3305/nh.2014.29.3.7148. [DOI] [PubMed] [Google Scholar]
- 8.Statistical Yearbook of the Republic of Poland (2018) Statistical Publishing Establishment, Warsaw, Poland.
- 9.Bartkowicz J. The selected behaviours of consumers in the market for natural coffee. Handel Wewn. 2015;355:45–57. [Google Scholar]
- 10.Kwiatkowska K, Winiarska-Mieczan A, Kwiecień M, Klebaniuk R, Krusiński R, Rusinek-Prystupa E, Sembratowicz I, Kamińska E, Danek-Majewska A, Cholewińska E. Analysis of coffee consumption among primary school teachers. Probl Hig Epidemiol. 2017;98:285–289. [Google Scholar]
- 11.EFSA Scientific opinion on the safety of caffeine. EFSA J. 2015;13(5):4102. doi: 10.2903/j.efsa.2015.4102. [DOI] [Google Scholar]
- 12.Gökcen BB, Şanlier N. Coffee consumption and disease correlations. Crit Rev Food Sci Nutr. 2017;59:336–348. doi: 10.1080/10408398.2017.1369391. [DOI] [PubMed] [Google Scholar]
- 13.Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ. 2017;359:j5024. doi: 10.1136/bmj.j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nędzarek A, Tórz A, Karakiewicz B, Clark JS, Laszczyńska M, Kaleta A, Adler G. Concentrations of heavy metals (Mn, Co, Ni, Cr, Ag, Pb) in coffee. Acta Biochim Pol. 2013;60:623–627. doi: 10.18388/abp.2013_2031. [DOI] [PubMed] [Google Scholar]
- 15.Pigozzi MT, Passos FR, Mendes FQ. Quality of commercial coffees: heavy metal and ash contents. J Food Qual. 2018;2018:5908463–5908467. doi: 10.1155/2018/5908463. [DOI] [Google Scholar]
- 16.EFSA Cadmium dietary exposure in the European population. EFSA J. 2012;10(1):2551. doi: 10.2903/j.efsa.2012.2551. [DOI] [Google Scholar]
- 17.EFSA Lead dietary exposure in the European population. EFSA J. 2012;10(7):2831. doi: 10.2903/j.efsa.2012.2831. [DOI] [Google Scholar]
- 18.Winiarska-Mieczan A, Grela ER. Content of cadmium and lead in raw, fried and baked commercial frozen fishery products consumed in Poland. J Sci Food Agric. 2017;97:2969–2974. doi: 10.1002/jsfa.8136. [DOI] [PubMed] [Google Scholar]
- 19.Winiarska-Mieczan A, Kwiecień M. The effect of exposure to Cd and Pb in the form of a drinking water or feed on the accumulation and distribution of these metals in the organs of growing Wistar rats. Biol Trace Elem Res. 2016;169:230–236. doi: 10.1007/s12011-015-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winiarska-Mieczan A. Cadmium, lead, copper and zinc in breast milk in Poland. Biol Trace Elem Res. 2014;157:36–44. doi: 10.1007/s12011-013-9870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winiarska-Mieczan A, Kwiatkowska K, Kwiecień M, Baranowska-Wójcik E, Wójcik G, Krusiński R. Analysis of the intake of sodium with cereal products by the population of Poland. Food Addit Contam Part A. 2019;36:884–892. doi: 10.1080/19440049.2019.1605209. [DOI] [PubMed] [Google Scholar]
- 22.Winiarska-Mieczan A, Florek M, Kwiecień M, Kwiatkowska K, Krusiński R. Cadmium and lead content in chosen commercial fishery products consumed in Poland and risk estimations on fish consumption. Biol Trace Elem Res. 2018;182:371–380. doi: 10.1007/s12011-017-1104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultana MS, Rana S, Yamazaki S, Aono T, Yoshida S. Health risk assessment for carcinogenic and noncarcinogenic heavy metal exposures from vegetables and fruits of Bangladesh. Cog Environ Sci. 2017;3:1291107. doi: 10.1080/23311843.2017.1291107. [DOI] [Google Scholar]
- 24.Issa AB, Yasin K, Loutfy N, Ahmed MT. Risk assessment of heavy metals associated with food consumption in Egypt: a pilot study. J Clin Exp Toxicol. 2018;2:15–24. [Google Scholar]
- 25.Song D, Zhuang D, Jiang D, Fu J, Wang Q. Integrated health risk assessment of heavy metals in Suxian County, South China. Int J Environ Res Public Health. 2015;12:7100–7117. doi: 10.3390/ijerph120707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.IRIS, Integrated Risk Information System (2015) U.S. Environmental Protection Agency. Chemical Assessment Summary. National Center for Environmental Assessment. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/subst/0141_summary.pdf (26.04.2019)
- 27.Grembecka M, Malinowska E, Szefer P. Differentiation of market coffee and its infusions in view of their mineral composition. Sci Total Environ. 2007;383:59–69. doi: 10.1016/j.scitotenv.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 28.Gebretsadik TA, Berhanu T, Kefarge B. Levels of selected essential and nonessential metals in roasted coffee beans of Yirgacheffe and Sidama, Ethiopia. Am J Environ Prot. 2015;4:188–192. doi: 10.11648/j.ajep.20150404.13. [DOI] [Google Scholar]
- 29.Ashu R, Chandravanshi BS. Concentration levels of metals in commercially available Ethiopian roasted coffee powders and their infusions. Bull Chem Soc Ethiop. 2011;25:11–24. doi: 10.4314/bcse.v25i1.63356. [DOI] [Google Scholar]
- 30.da Silva SA, Mendes FQ, Reis MR, Passos FR, de Carvalho AMX, de Oliveira Rocha KR, Pinto FG. Determination of heavy metals in the roasted and ground coffee beans and brew. Afr J Agric Res. 2017;12:221–228. doi: 10.5897/AJAR2016.11832. [DOI] [Google Scholar]
- 31.Suseela B, Bhalke S, Kumar AV, Tripathi RM, Sastry VN. Daily intake of trace metals through coffee consumption in India. Food Addit Contam. 2001;18:115–120. doi: 10.1080/02652030010008814. [DOI] [PubMed] [Google Scholar]
- 32.Al Othman ZA. Lead contamination in selected foods from Riyadh city market and estimation of the daily intake. Molecules. 2010;15:7482–7497. doi: 10.3390/molecules15107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos WPC, Hatje V, Lima LN, Trignano SV, Barros F, Castro JT, Korn MGA. Evaluation of sample preparation (grinding and sieving) of bivalves, coffee and cowpea beans for multi-element analysis. Microchem J. 2008;89:123–130. doi: 10.1016/j.microc.2008.01.003. [DOI] [Google Scholar]
- 34.Şemen S, Mercan S, Yayla M, Açıkkol M. Elemental composition of green coffee and its contribution to dietary intake. Food Chem. 2017;215:92–100. doi: 10.1016/j.foodchem.2016.07.176. [DOI] [PubMed] [Google Scholar]
- 35.Anderson GL, Garnick L, Fung MS, Gaffney SH. A pilot study to assess lead exposure from routine consumption of coffee and tea from ceramic mugs: comparison to California Safe Harbor Levels. Int J Food Contam. 2017;4:4. doi: 10.1186/s40550-017-0049-7. [DOI] [Google Scholar]
- 36.Winiarska-Mieczan A. Cumulative rate and distribution of Cd and Pb in the organs of adult male Wistar rats during oral exposure. Environ Toxicol Pharmacol. 2014;38:751–760. doi: 10.1016/j.etap.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 37.Ohta H, Yamauchi Y, Nakakita M, Tanaka H, Asami S, Seki Y, Yoshikawa H. Relationship between renal dysfunction and bone metabolism disorder in male rats after long-term oral quantitative cadmium administration. Ind Health. 2003;38:339–355. doi: 10.2486/indhealth.38.339. [DOI] [PubMed] [Google Scholar]
- 38.Järup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]