Highlights
-
•
A neural noise account on Tourette syndrome is conceptualized.
-
•
We outline how neurophysiological methods can be used to test this account.
-
•
The neural noise account may lead to novel treatment options.
Abbreviations: GTS, Gilles de la Tourette syndrome; LC, locus coeruleus; LFP, local field potential; NE, norepinephrine; NST, nucleus of the solitary tract; PFC, prefrontal cortex; PSD, power spectral density; SNR, signal-to-noise ratio; tVNS, transcutaneous vagus nerve stimulation
Keywords: Gilles de la Tourette syndrome, Signal-to-noise ratio, Dopamine, Cognition, tVNS
Abstract
Tics, often preceded by premonitory urges, are the clinical hallmark of Tourette syndrome. They resemble spontaneous movements, but are exaggerated, repetitive and appear misplaced in a given communication context. Given that tics often go unnoticed, it has been suggested that they represent a surplus of action, or motor noise. In this conceptual position paper, we propose that tics and urges, but also patterns of the cognitive profile in Tourette syndrome might be explained by the principle of processing of neural noise and adaptation to it during information processing. We review evidence for this notion in the light of Tourette pathophysiology and outline why neurophysiological and imaging approaches are central to examine a possibly novel view on Tourette syndrome. We discuss how neurophysiological data at multiple levels of inspections, i.e., from local field potentials using intra-cranial recording to scalp-measured EEG data, in combination with imaging approaches, can be used to examine the neural noise account in Tourette syndrome. We outline what signal processing methods may be suitable for that. We argue that, as a starting point, the analysis of 1/f neural noise or scale-free activity may be suitable to investigate the role of neural noise and its adaptation during information processing in Tourette syndrome. We outline, how the neural noise perspective, if substantiated by further neurophysiological studies and re-analyses of existing data, may pave the way to novel interventions directly targeting neural noise levels and patterns in Tourette syndrome.
1. Introduction
Gilles de la Tourette syndrome (GTS) is a multi-faceted neuropsychiatric disorder characterized by motor and phonic tics, often associated with preceding urges, with an onset before the age of 18 and a duration of at least one year (American Psychiatric Association, 2013). Tics, the clinical hallmark of GTS, resemble spontaneous movements. Even for GTS specialists, single tics may be difficult to discern from spontaneous movements occurring in healthy people since they often have the same or very similar phenomenology (Ganos et al., 2015, Paszek et al., 2010). However, tics are exaggerated, repetitive and appear misplaced in a given context (e.g. unintentional winking at a stranger) (Paszek et al., 2010). Therefore, usually within minutes, tics in GTS can easily be distinguished from spontaneous movements in non-ticcing individuals by a trained observer (Paszek et al., 2010). On the other hand, people with tics are often oblivious to their tics (Leckman, 2002, Pappert et al., 2003). This is particularly true for younger children and exemplified by the classical clinical scenario of worried parents having noted a spectrum of tics in their children, who do not seem to be aware of any or most of their tics (Leckman, 2002). This is also true, though, for adult GTS patients, who typically underestimate frequency and repertoire of their tics. For instance, most adults with a past history of tics considering themselves tic-free still have tics (Pappert et al., 2003). These clinical observations indicate that tics often represent non-conscious events that could be considered as a surplus of motor output or “motor noise” (Beste and Münchau, 2018). Undoubtedly, tics can be troublesome, uncomfortable or painful and can distract affected patients from other tasks. Also, they are noted and commented on by others, often with the implicit or explicit requests to stop them. A pre-requisite for successful inhibition of an action, i.e. a tic, is awareness of processes leading to its occurrence. Thus, to obey instructions by others and because of the wish not to attract attention, affected patients have to focus on tic-related processes including premonitory urges. This inevitably alters the valence of tics. Therefore, tics may evolve from at least partially unnoticed, non-consciously occurring noise to a contextually, socially relevant to-be processed signal.
Social media contributions (e.g. YouTube videos) with the topic “Tourette syndrome” have attracted massive public attention (Müller-Vahl et al., 2020). Although this has helped in some ways to relieve the burden of affected patients, it has also led to considerable problems and controversies. Of late, there is an increasing number of social media contributions showing adults with spectacular and at times outrageous behaviours resembling tics and labelled as “Tourette”, which in fact represent functional, and sometimes feigned, i.e. intentional, extra movements (Müller-Vahl et al., 2020). These are mis-conceived as “real tics” by a broad audience as reflected by followers’ comments and ‘likes’. Indeed, these “functional tics” are difficult to discern from classical tics (Ganos et al., 2019). Thus, whereas “real” tics (noise) are often mistaken as intentional (signal), intentional or functional movements (signal) shown on YouTube in turn are now mistaken as tics (noise). Here, we propose that tics and urges, but also patterns of the cognitive profile in GTS might be explained by an overarching neural principle – the processing of neural noise and adaptation of the signal-to-noise ratio (SNR) during information processing. We will review evidence for this notion in the light of GTS pathophysiology and outline why and how neurophysiological and neuroimaging methods are central for this novel perspective on GTS and could ultimately pave the way for novel treatments using brain stimulation approaches. From the neurophysiological perspective, it is important to note that although “noise” has the negative connotation of reflecting nuisance activity, noise has long been claimed to be functionally relevant and necessary (McDonnell and Ward, 2011); also, there are many definitions of noise. This is important, because we do not consider “noise” as meaningless signal or uninformative in Tourette syndrome. Considering neurophysiological data, we outline that specifically the quantification of “1/f noise” as an estimate for so-called ‘pink noise’ or scale-free neural activity (He, 2014), may be suitable to test the hypothesis that tics, urges, and the cognitive profile in GTS might be explained by the processing of neural noise and adaptation of the signal-to-noise ratio (SNR) during information processing.
2. GTS as a disorder of altered signal-to-noise ratio (SNR)
Bodily movements are intrinsically variable, likely because noise can arise at different stages of sensorimotor processing (Desmurget and Grafton, 2000), e.g. as “sensory noise”, “planning noise”, or “execution-noise”.
In addition to clinical reasoning outlined above that tics could, at least partly, be considered a sign of increased noise, there are also experimental data suggesting that noise in GTS is increased during sensorimotor processing (Buse et al., 2016, Kim et al., 2019). For instance, it has been shown that sensory and sensorimotor gating is reduced in GTS (Buse et al., 2016), which leads to increased sensorimotor noise. Also, testing double-step aiming movements it was demonstrated that GTS patients performed normally during aiming movements to the first target location, but showed greater movement variability when executing the second (return) movement. This has been interpreted as a reduction in the precision of forward model estimates due to increased sensorimotor noise in them (Kim et al., 2019). Results of another study where sequential button press movements with different levels of advance information were tested in GTS demonstrating that these patients relied more on external visual cues to execute a motor programme than healthy controls (Georgiou et al., 1995), could be interpreted along similar lines. Tying up with these findings, symptoms and signs of GTS have been proposed to be conceptualized within a framework of action-oriented predictive processing (Rae et al., 2019), where the brain continuously engages in the minimisation of mismatches between sensory signals and prior expectations, i.e. sensory “prediction errors,” by updating perceptual priors and performing actions to change sensory signals. It is possible that increased activity in the direct pathway of the basal ganglia with a concomitant disinhibition of thalamo-cortical projections found in GTS (Kalanithi et al., 2005, Kataoka et al., 2010), leads to the spontaneous occurrence of priors inducing an action (tic) not predicted by higher-level cortical motor regions (Rae et al., 2019). When the intention to execute a movement is weak, the specific moment it occurs is largely determined by spontaneous subthreshold fluctuations in neuronal activity (Schurger et al., 2012) affecting higher cortical centers. Accordingly, enhanced random fluctuations will then not only lead to spontaneous tic movements (Ganos et al., 2014) but will also interfere with the decision “when” to carry out a voluntary action (Schurger et al., 2012). It is therefore possible that in GTS, tics are (mis-)interpreted as relevant action-related information (i.e. a relevant signal) that needs to be gated/controlled (Buse et al., 2016, Kim et al., 2019), even though it reflects noise. Thus, striatal priors leading to tics could be viewed as noise increasing prediction errors in higher cortical centers in feedforward basal ganglia-prefrontal loops. Such alterations in feedforward processing between basal ganglia and neocortical structures are corroborated by findings showing that volitional proactive and reactive inhibition were normal in GTS patients, whereas inhibition depending on feedforward mechanisms such as priming (Eimer and Schlaghecken, 2003), was impaired (Rawji et al., 2020). Importantly, if tics resembling spontaneous movements represent noise, then problems for GTS should arise especially during processes related to movement preparation and the perception of the intentionality of movements. This is indeed the case since patients have problems in judging the intention to act, e.g. in the Libet experiment (Ganos et al., 2018, Ganos et al., 2015, Moretto et al., 2011) and could be tested examining “1/f noise” as an estimate for so-called ‘pink noise’ or scale-free neural activity using neurophysiological data recorded during ticcing, tic suppression and experimental paradigms examining volitional movements in GTS.
The view that tics might represent noise has also been framed in a way that tics reflect a 'surplus' of movements that emerge because of an increased propensity in GTS to form bindings or associations between sensory antecedents including urges and movements (Beste and Münchau, 2018). This has just recently been corroborated by experimental evidence showing that motor or sensory processes alone are less relevant for the understanding of GTS than cognitive processes engaged in linking and restructuring of perception–action associations (Buse et al., 2016, Kleimaker et al., 2020a, Kleimaker et al., 2020b, Kleimaker et al., 2020c, Weissbach et al., 2020). It is an overly strong binding between perception and motor responses that relates to tic severity (M. Kleimaker et al., 2020c). The finding that GTS patients have a tendency to create stronger stimulus–response associations compared to healthy controls (Kleimaker et al., 2020c) is in keeping with previous work showing that they also have an increased tendency to form habits (Delorme et al., 2016). Likely, tics representing bindings between sensory processes and motor responses are spontaneously established and released in GTS (Beste and Münchau, 2018, Kleimaker et al., 2020c). These pre-fabricated, but insufficiently controllable and contextually inappropriate sensory-motor bindings (tics), compete with contextually relevant sensory-motor bindings, i.e. voluntary actions. The similarity of tics and voluntary actions both phenomenologically and with respect to their inner structure as stimulus–response associations (Beste and Münchau, 2018; M. Kleimaker et al., 2020b) may render their distinction particularly difficult (Ganos et al., 2018, Ganos et al., 2015). This is in keeping with clinical findings that even GTS specialists have difficulties to discern tics from spontaneously occurring movements in healthy people (Ganos et al., 2015, Paszek et al., 2010). Thus, it is possible that a suboptimal distinction between tics as noise and other response options might be a core neurobiological problem in GTS. If so, then alterations in cognitive functioning in GTS should also be explainable within a SNR framework. Through the examination of parameters reflecting noise in neurophysiological signals, these questions can directly be tested.
Of note, in GTS patients some cognitive functions are compromised, whereas others are superior compared to healthy controls. For example, cognitive persistence requiring the distinction between relevant and irrelevant information such as attentional control (Johannes et al., 2001), the integration of irrelevant stimulus features (Beste et al., 2016), and the maintenance of memory information (Jeter et al., 2015) are impaired in GTS. On the other hand, GTS patients show enhanced performance in cognitive flexibility, defined as the ability to adapt behaviours in reaction to changes in the environment. This becomes evident in switching between tasks (Brandt et al., 2017, Mueller et al., 2006), strategies (Takács et al., 2018), and behaviours (Güler et al., 2015). There is evidence (Beste et al., 2018, Hommel and Colzato, 2017) that the mentioned spectrum of cognitive functions showing compromised and superior levels in GTS is an emerging property of the interaction of systems promoting cognitive persistence including focusing on one goal and on relevant information, and systems promoting cognitive flexibility that is needed, for instance, for switching to other plans, opening up for other opportunities, and considering a broader range of possibilities. This is often referred to as metacontrol (Hommel, 2015, Hommel and Colzato, 2017) and describes how information is processed (Hommel and Wiers, 2017); i.e. it can bias information processing towards a more focused or a flexible state (Hommel, 2015). A more focused information processing is equivalent to a high signal-to-noise ratio (Desimone and Duncan, 1995). In GTS, the inability to disentangle signals from noise might compromise cognitive persistence, but may foster tendencies to engage in exploration and uncommon behaviour, and may also lead to greater behavioural variability (see Fig. 1). As such the cognitive profile of GTS may also be explained by a signal-to-noise ratio account.
Fig. 1.

Metacontrol can be conceptualized as a scale which is assumed to determine the balance between cognitive persistence and cognitive flexibility. (A) Given a decreased signal-to-noise ratio, people with Tourette syndrome are expected to display enhanced cognitive flexibility but decreased cognitive persistence. (B) We expect transcutaneous vagus nerve stimulation (tVNS) to decrease sensorimotor noise by increasing the signal-to-noise ratio ameliorating cognitive persistence and normalizing cognitive flexibility.
From an overarching neuroscientific perspective, the modulation of the SNR is captured by ‘gain control/modulation principles’ (Servan-Schreiber et al., 1990, Yousif et al., 2016). Increasing gain control can be conceived as amplifying an information processing system’s responsivity to input signals and increasing its ability to dissociate signal from noise. High gain control is, hence, associated with less noise and better/more stable cognitive performance across various domains. Negative consequences of low SNR are obvious: every decision that relies on the distinction between relevant (signal) and irrelevant (noise) information necessarily suffers from increased noise. However, higher noise levels might also trigger more behavioural variability (Gureckis and Love, 2009), which might be beneficial when cognitive flexibility is important. Indeed, the exploration of multiple solutions benefits from higher noise levels and larger neural variability (McIntosh et al., 2008). Gain control mechanisms and its impact on the SNR are closely linked to actions of the dopaminergic and norepinephrine (NE) system. High activity in these systems enhances the SNR in neural networks (Servan-Schreiber et al., 1990, Yousif et al., 2016). In GTS, the neural basis of SNR alterations are probably functional and structural changes in fronto-striatal networks and altered dopaminergic transmission (Maia and Conceição, 2018). Dopaminergic drugs decrease internal noise, enhance the SNR and support cognitive persistence (Cools, 2016, Zink et al., 2019). Given that tics in GTS patients are probably related to a hyper-dopaminergic state with the core finding of increases in stimulus-dependent dopamine releases in these patients (Buse et al., 2013) and the fact that anti-psychotic/anti-dopaminergic medication is the mainstay of treatment in GTS (Buse et al., 2013) one might, at first sight, expect SNR to be increased rather than decreased in GTS. However, a decreased SNR in GTS can be explained by the inverted U-shape curve of dopamine functioning signifying optimal dopamine action and (motor) performance at the peak of the curve, but suboptimal functioning both on the up-slope and the down-slope of the curve (Seamans and Yang, 2004). Given excessive dopamine release in GTS, these patients may be on a suboptimal down slope and hence a state of reduced SNR. Thus, toning down dopamine activity by means of pharmacological interventions is expected to not only improve tics but also to increase the SNR by shifting GTS patients back to an ‘optimal’ dopamine level. As we detail below, neurophysiological metrics allowing to examine 1/f noise” as an estimate for so-called ‘pink noise’ or scale-free neural activity using neurophysiological data are sensitive to changes in neurobiological parameters modulating the information processing system’s responsivity to input signals and increasing its ability to dissociate signal from noise (i.e. factors modulating gain control).
3. Measuring neural noise in relation to GTS pathophysiology
Above, we argued that may facets of GTS, particularly seemingly contradictive findings, may be related to an altered processing of neural “noise”. To test this novel hypothesis in GTS, neurophysiological and/or neuroimaging methods are of central importance. As already mentioned, the term “noise” often has the negative connotation that it reflects nuisance activity. Importantly, however, the above line of arguments assumes that “noise” is not meaningless, random or unstructured neural activity that carries no information. In fact, noise has long been claimed to be functionally relevant and necessary (McDonnell and Ward, 2011). This needs to be considered when trying to define noise and to examine the neural noise hypothesis using neurophysiological and/or imaging methods.
There are many definitions of noise and several distinct classes of noise are known, e.g., ‘white noise’, ‘brown/red noise’ or ‘pink noise’. Brown/red noise is produced by Brownian motion; white noise can be considered as a random signal with equal intensity at different frequencies, which is why the power spectral density (PSD) is constant across all frequencies in white noise. Since brown/red or white noise definitions thus imply random signals not important for information processing, they do not seem useful to examine this sort of noise in neural signals and considering the above line of arguments that in GTS “noise” is not meaningless, random or unstructured neural activity that carries no information. Generally, it is assumed that a neurophysiological time series signal [s(t)] consists of evoked (phase-locked) [e(t)] and induced (non-phase-locked) [i(t)] signal components and contains noise [n(t)] as an independent component; s(t) = e(t) + i(t) + n(t) (Graichen et al., 2009). In fact, noise in neural signals does not obey to brown/red or white noise definitions. The reason is that due to the power law principle, the PSD is not constant for brain neurophysiological activity, e.g., as measured using EEG. Rather, there is a prominent decrease in power with increasing frequency, i.e., power in the theta frequency band is higher than in beta or gamma frequency bands following a power-law function: P ~ 1/fβ. P is power, f is frequency and β is a to-be estimated parameter (usually in the range between 0 and 3) called the “power-law exponent” (He, 2014). This is why the quantification of “noise” to examine the neural noise hypothesis of GTS must relate to the PSD. As outlined by Buzsáki (2006), several approaches can be used to examine noise in a PSD-varying signal. One way to do so is to calculate the “1/f noise” as an estimate for so-called ‘pink noise’ in neurophysiological time series data. Pink noise is ubiquitously found in nature and is also generated in the central nervous system (Buzsáki, 2006). 1/f noise is also often referred to as scale-free activity (He, 2014), because the power-law function is indicative for scale invariance; i.e. no specific timescale or frequency clearly dominates the dynamics (He, 2014). Importantly, several lines of evidence suggest that 1/f noise or scale-free activity is not meaningless unstructured noise (He, 2014). Rather, it contains specific organizations relevant to information processing and brain functioning (Bassett et al., 2006, He, 2014, Voytek and Knight, 2015); i.e. it is also affected by brain activation during task processing (Ouyang et al., 2020, Pertermann et al., 2019a, Pertermann et al., 2019b, Podvalny et al., 2015). This is why we suggest that 1/f noise may reflect a suitable metric to examine the neural noise hypothesis of GTS. To calculate 1/f noise, the distribution of neural activation based on the power-law scaling is approximated by calculating the logarithm of PSD across the frequency spectrum, revealing a negative linear relationship (i.e. slope) (Dave et al., 2018, He, 2014, He et al., 2010, Miller et al., 2009, Voytek and Knight, 2015) (for critique see Touboul and Destexhe, 2017). Therefore, noise is evident in every recorded (EEG) signal, which is why the EEG signal may be a good starting point to examine/calculate the degree of pink noise and use this to examine the neural noise hypothesis in GTS. This can e.g. be done for every EEG sensor thus yielding scalp topography maps of noise activity (Pertermann et al., 2019a, Pertermann et al., 2019b, Voytek et al., 2015b). The 1/f noise approach is physiologically plausible since power-law distributions are characteristic for neurophysiological processes at multiple levels of inspection, i.e. at the level of membrane potentials, local field potentials (LFPs) on the basis of invasive recordings, electro-corticography, EEG as well as fMRI signals (He, 2014). Consequently, the 1/f noise metric has been applied in the past using EEG data (Dave et al., 2018, Pertermann et al., 2019a, Pertermann et al., 2019b, Voytek et al., 2015b, Voytek and Knight, 2015). A steeper slope of the 1/f noise functions indicates less noise in the neurophysiological data, while a flatter slope indicates more 1/f noise in the neural time series data (cf. Fig. 2).
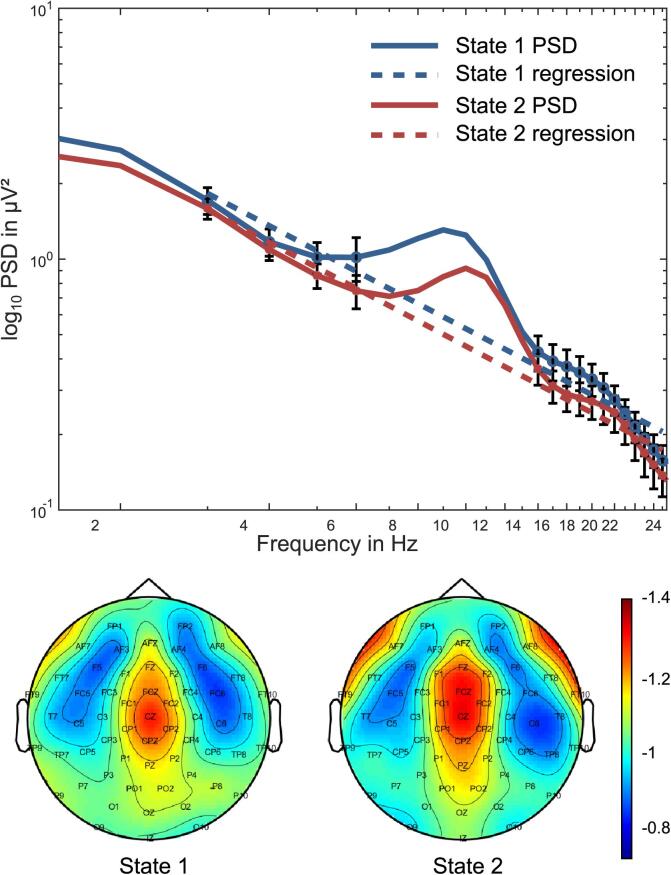
Fig. 2.
Schematic illustration of the 1/n noise method. (Top) Power spectral density (PSD) is shown in a log–log plots; i.e. the x-axis shows the log of the frequency and the y-axis the log of the PSD in µV2. Two different “states” are plotted for illustration. These states can refer to experimental condition, patient groups etc. In this example the included frequencies range between 1 and 25 Hz, however, broader spectrum and also smaller band-widths are possible. To estimate 1/f noise, and linear function is fit to the PSD. During the fit, the alpha frequency is not considered since the alpha frequency band does not obey to the 1/f metric (Voytek et al., 2015b, Voytek and Knight, 2015). The linear function is show in dashed lines. The slope of the linear function denotes the estimator of 1/f noise. Generally, 1/f noise is stronger the steeper the slope of the linear function fit the PSD log–log plot. (Bottom) The 1/f noise parameter can be calculated on a single electrode level and as an average of all electrodes. In the case 1/f noise is calculated for EEG electrode separately, 1/f noise scalp topography maps can be generated. The colour codings the scalp topography plots can then illustrate differences in the degree of 1/f noise.
The reason behind this is as follows. It is assumed that the slope of the 1/f noise function is determined by the level of neuronal population spiking activity as measured by LFPs (Voytek and Knight, 2015). This population spiking activity contributing to LFPs is what is actually measured using the EEG signal (Katzner et al., 2009, Musall et al., 2014). Synchronized neuronal spiking activity is associated with reduced neuronal noise. On the contrary, asynchronous spiking, related to increased neural noise levels, is associated with a flatter slope (Podvalny et al., 2015, Voytek et al., 2015b). The 1/f neural noise parameter can therefore be seen as an estimator of noise occurring in neurophysiological systems. However, it is important that the 1/f noise metric has mainly been applied to measure broadband noise. In fact, it has been shown that particularly higher frequency band activity (e.g., in the gamma frequency band) contributes to 1/f noise. Nevertheless, 1/f noise can also be detected in lower frequency band activity, e.g., in the theta or beta frequency band. The reason is that low-frequency oscillations are co-modulated with higher frequencies by phase-amplitude coupling (Buzsáki and Draguhn, 2004, Canolty and Knight, 2010, Fries, 2005, Voytek et al., 2010, Voytek et al., 2015a). There is evidence that 1/f-like dynamics is evident in narrow-band amplitude fluctuations (Gireesh and Plenz, 2008, Palva et al., 2013). On that basis, Voytek et al. (2015b) proposed to estimate 1/f neural noise based on low-frequency activity including EEG theta and beta frequency bands. The 1/f noise parameter thus provides a neurophysiological valid estimator of noise or scale free activity. Since this activity can be captured in a single parameter, this metric can easily be used in different study setting to examine the role of neural noise in GTS. Especially the possibility to measure 1/f noise in specific frequency bands (e.g. using EEG data) is of particular relevance in the context of the neural noise hypothesis is GTS for a number of reasons:
Frist, the relevance of the 1/f noise metric for the understanding of GTS and its use to examine the neural noise hypothesis of GTS becomes clear from a neurobiological perspective. As mentioned, it is assumed that the slope is determined by the level of neuronal population spiking activity as measured by LFPs (Voytek and Knight, 2015). The generation of LFPs is modulated by multiple neurobiological factors, one of which is the GABAergic system (Kardos, 1999). The GABAergic system is of importance in GTS given the importance of basal ganglia neuropathology (Kalanithi et al., 2005, Kataoka et al., 2010), e.g., showing a reduced number and altered distribution of inhibitory GABAergic parvalbumin-expressing and tonically active cholinergic interneurons, predominantly in the sensorimotor and associative areas of the striatum (Kalanithi et al., 2005, Kataoka et al., 2010) likely resulting in excitatory imbalances between the partially segregated sensorimotor and associative cortico-striato-thalamo-cortical loops (Kataoka et al., 2010). This is corroborated by data on tic-associated neuronal activity in a primate model where jerky stereotypic facial movements resembling tics were induced by injecting the GABAA-antagonist bicuculline in the striatum (Bronfeld and Bar-Gad, 2011). Since some data suggest that the 1/f metric is very sensitive to pharmacological interventions affecting the GABA-system (Muthukumaraswamy and Liley, 2018), the 1/f metric seems very useful to provide further insights into the pathophysiology of GTS. It is conceivable that the slope of the 1/f function changes in response to medication administered in the treatment of GTS. Considering the above example that tic-associated neuronal activity in a primate model can be induced by injecting the GABAA-antagonist bicuculline in the striatum (Bronfeld and Bar-Gad, 2011) it is possible that also the 1/f metric of tic-associated neuronal activity changes. If tics do indeed represent neural noise and if this specific pattern of noise is captured by the 1/f metric, the slope of the 1/f noise function should be flatter in a drug-induced ticcing state, compared to a state, in which less tics occur. Importantly, and considering patients, examining the impact of the GABAergic system on 1/noise processes it will be possible to combine MR imaging approaches with EEG-based approaches. Using EEG-measures, 1/f noise can be quantified in GTS in different states, e.g., at rest, before tics, or while performing a cognitive task. As argued above, if tics do indeed represent neural noise and if this specific pattern of noise is captured by the 1/f metric, the slope of the 1/f noise function should vary between rest, ticcing states and/or states in which patients actively suppress tics. Using GABA-edited MR-spectroscopy it is possible to examine neuroanatomical structure specific GABA concentrations (Mikkelsen et al., 2019, Mikkelsen et al., 2017) (e.g. in the basal ganglia), which can then be related to scalp-recorded EEG data (Haag et al., 2015, Quetscher et al., 2015, Takacs et al., 2020, Yildiz et al., 2014). Considering that GABA-edited MR-spectroscopy has been shown to yield valuable insights into the pathophysiology of GTS and tics (Draper et al., 2014, Jackson et al., 2015, Martino et al., 2018, Puts et al., 2015) it seems relevant to combine this neurobiochemical imaging approach with neurophysiological estimates of 1/f noise activity.
Second, as outlined above, the 1/f noise metric is determined by the level of neuronal population spiking activity as measured by LFPs (Voytek and Knight, 2015). During deep brain stimulation in GTS, especially LFPs reflecting neural population activity have been recorded directly from subcortical target regions. In a number of case series, theta oscillations in LFPs have emerged as characteristic in these patients both for the internal segment of the globus pallidus (Alam et al., 2015, Giorni et al., 2017, Jimenez-Shahed et al., 2016) and thalamic nuclei (Maling et al., 2012, Marceglia et al., 2010, Molina et al., 2018, Priori et al., 2013, Shute et al., 2016). Also, synchronized oscillations in the theta range have been recorded across deep brain stimulation targets (Priori et al., 2013). In the study by Neumann et al. (2018) in addition to confirming peaks of activity in the theta range in pallidal and thalamic recordings in GTS patients, also beta activity was reported in these targets. Moreover, there were synchronized pallidal and thalamic theta oscillations in GTS patients, which were functionally coupled between the pallidum and the thalamus (Neumann et al., 2018). Importantly, in this study, theta power and theta burst length correlated significantly with preoperative motor tic severity suggesting that longer theta bursts may be related to tics (Neumann et al., 2018). In addition, recordings of oscillatory activity from the centro-median nucleus of the thalamus in GTS patients revealed power increases in the theta band time-locked to the onset of tics but not during voluntary movements in these patients (Cagle et al., 2020). Collectively, these data suggest that pallido-thalamic oscillations in the theta range are relevant in the pathophysiology of GTS. What is missing thus far are analyses examining 1/noise using LFP data recorded from striatal structures and in relation to tics. However, this will be crucial when examining the neural noise hypothesis in GTS assuming that noise in fronto-striatal circuits is central for the understanding of the pathophysiology in GTS. The 1/f metric can easily be applied to existing deep brain stimulation recordings to elucidate this further. Given the specific importance of theta band activity in tic-related states in fronto-striatal circuits, it is possible that the slope of the 1/f function shows frequency band specific modulations and that particularly theta band activity may be subject to modulations of the 1/f metric. Particularly given the evidence that 1/f-like dynamics are evident in narrow-band amplitude fluctuations (Gireesh and Plenz, 2008, Palva et al., 2013), such a frequency-specific analysis is relevant.
Third, the possibility to measure 1/f noise in specific frequency bands (Gireesh and Plenz, 2008, Palva et al., 2013) is of importance to examine the above outlined conundrum of compromised and superior cognitive functions in GTS patients giving rise to the neural noise hypothesis in GTS and which we suggest may relate to the concept of meta-control (Hommel, 2015, Hommel and Colzato, 2017) describing how information is processed (Hommel and Wiers, 2017). As outlined above, the mentioned spectrum of cognitive functions showing compromised and superior levels in GTS is an emerging property of the interaction of systems promoting cognitive persistence and systems promoting cognitive flexibility. Several lines of evidence suggest that for cognitive persistence and flexibility, processes in the theta frequency band are relevant (Cavanagh and Frank, 2014, Cohen, 2014) and there is direct evidence showing that for cognitive control functions depending on cognitive persistence the 1/f noise metric is modulated in a neurobiologically meaningful way (Pertermann et al., 2019a, Pertermann et al., 2019b). Especially findings that metacontrol processes seem to depend on fronto-striatal networks (Beste et al., 2018) underline that the 1/f noise metric in the theta frequency, connected to the metacontrol framework, may yield meaningful insights into cognitive functions in GTS. Since the 1/noise metric can be applied to the theta frequency band it will provide the opportunity to examine neural noise during cognitive processes shown to be concomitantly enhanced and compromised. As mentioned, in GTS patients some cognitive functions are compromised, whereas others are superior compared to healthy controls. We argued that this paradox can be explained on the ground of metacontrol processes (Hommel, 2015, Hommel and Colzato, 2017). In GTS, the inability to disentangle signals from noise might compromise cognitive persistence, but may foster tendencies to engage in exploration and uncommon behaviour, and may also lead to greater behavioural variability (see Fig. 1). It is, therefore, possible that patients with GTS show relatively constant levels of elevated neural noise (i.e., the slope of the slope of the 1/f function does not change strongly depending on task requirements). This can be associated with advantages in situations requiring flexibility in cognitive processes, but may lead to difficulties on other occasions requiring persistence. The analysis of noise in lower-frequency band activity in GTS is all the more relevant considering evidence that motor or sensory processes alone are less relevant for the understanding of GTS than cognitive processes engaged in linking and restructuring of perception–action associations (Kleimaker et al., 2020a, Kleimaker et al., 2020b, Kleimaker et al., 2020c). Of note, low-frequency, high-amplitude oscillations (e.g. theta) are relevant to integrate information across spatial distances (Buzsáki and Draguhn, 2004, Cavanagh and Frank, 2014) and it has been shown that the organization of theta frequency network activity is essential for perception–action associations (Takacs et al., 2020) shown to be abnormally strong in GTS. Given this relevance of theta activity for processes that are also central for the understanding of clinical facets of GTS, it seems reasonable that scale-free activity (or 1/f noise) is central in the analysis of neurophysiological data recorded from GTS patients and to examine whether GTS can be framed within the neural noise hypothesis.
Fourth, aside the insights probably gained from the analysis of 1/f noise using intra-cranial recordings, or EEG approaches in combination with MRI approaches outlined above, the analysis of 1/f noise activity may also be central from the viewpoint that dopaminergic drugs are still a mainstay of pharmacological interventions in GTS (Buse et al., 2013, Roessner et al., 2013). Dopaminergic drugs are known to decrease internal noise, (Cools, 2016, Zink et al., 2019). Tics in GTS patients are probably related to a hyper-dopaminergic state (Buse et al., 2013) and targeted by anti-psychotic/anti-dopaminergic medication (Buse et al., 2013). In fact, it has been shown that scale-free or 1/f noise activity is modulated by dopaminergic drugs with 1/noise becoming reduced in the theta frequency band after increasing dopaminergic as well as norepinephrinergic concentrations (Pertermann et al., 2019a). This pattern is very well in line with the previously mentioned gain control framework (Servan-Schreiber et al., 1990, Yousif et al., 2016), where it has been shown that high activity in these systems enhances the SNR in neural networks (Servan-Schreiber et al., 1990, Yousif et al., 2016) and which may also reflect an underlying mechanism of metacontrol. Since in GTS, the neural basis of SNR alterations are probably functional and structural changes in fronto-striatal networks and altered dopaminergic transmission (Maia and Conceição, 2018). 1/f noise should be directly affected by neuromodulatory treatments targeting the dopaminergic system and tics as a major facet of GTS. Evidence suggesting that pharmacological treatment effects targeting the catecholaminergic system can be evaluated using the 1/noise metric (Pertermann et al., 2019a) suggest that the 1/f metric may also be useful as a novel neurophysiological outcome measures in pharmacological interventions in GTS. If tics do indeed represent neural noise and if this specific pattern of noise is captured by the 1/f metric, the slope of the 1/f noise function should be flatter in a drug-induced ticcing state, compared to a state in which less tics occur. The 1/f metric may therefore reflect a possible endpoint in future clinical studies in GTS.
4. New routes for the modulation of noise in GTS
As already mentioned in the previous section, in addition to current first-line pharmacological treatments targeting the dopamine system, 1/f activity may also be modulated by other neurotransmitter systems that are of relevance in GTS and the 1/f metric may be a useful outcome measure in future studies. In this respect, the NE and GABAergic systems are of particular interest and the 1/noise metric possibly useful to examine the neural noise hypothesis of GTS may even suggest novel brain-stimulation based treatment approaches targeting the NE and GABAergic system. Brain stimulation approaches have seen an increase in popularity to modulate GTS symptoms (Kleimaker et al., 2020b).
Modulations are directly dependent on the locus coeruleus NE system (Pertermann et al., 2019b). The NE and the GABAergic system influence each other (Salgado et al., 2016), and this reciprocal influence depends on which noradrenergic receptor type is activated (Berridge and Spencer, 2016). While α2 receptor stimulation increases cognitive functions such as the processing of sensory stimuli and long-term memory, activation of α1 receptors and lower affinity β-receptors seem to compromise them (Robbins and Arnsten, 2009). The α2 agonist clonidine has been shown to improve tics (Leckman et al., 1991), an effect that might be mediated through an improved SNR. GABA, particularly in the supplementary motor is considered to be relevant for successful tic suppression (Draper et al., 2014, Jackson et al., 2015). A means to modulate both NE and GABA-related (cognitive functions) is auricular transcutaneous vagus nerve stimulation (atVNS), as reviewed recently (Colzato and Beste, 2020) (see Fig. 3). tVNS has attracted considerable interest in cognitive and clinical neuroscience in recent years (Farmer et al., 2021).
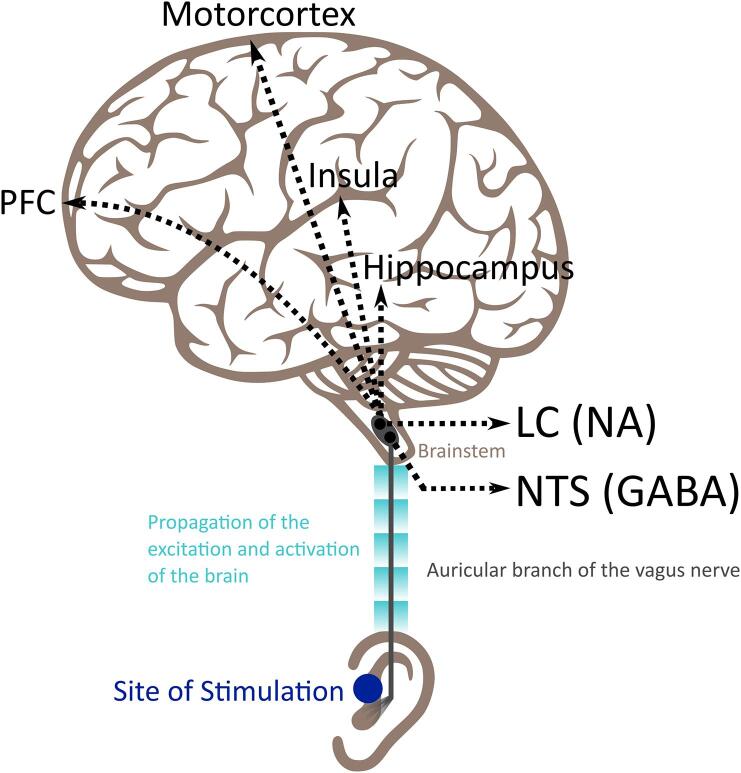
Fig. 3.
Schematic representation of the main brain areas activated following afferent stimulation of the auricular branch of the vagus nerve. Auricular transcutaneous vagus nerve stimulation (tVNS) is administered through a special earplug electrode to the outer ear, usually the cymba conchae or the tragus, which sends electrical impulses to the auricular branch of the vagus nerve and activates the vagal afferent pathway including the locus coeruleus (LC) and the nucleus of the solitary tract (NTS) in the brainstem (Yakunina et al., 2017), which are noradrenergic (NA) and GABAergic nuclei, respectively(Aston-Jones et al., 1991). From there, the activation propagates to cortical structures including the insula, the prefrontal cortex (PFC) and the motor cortex (Shiozawa et al., 2014).
Given that auricular tVNS may influence the NE system predominantly via α2 receptors, it is possible that tVNS increases the SNR (Colzato and Beste, 2020). Considering tics and urges as “noisy” phenomena (Buse et al., 2016, Ganos et al., 2015, Ganos et al., 2014, Kim et al., 2019) it is reasonable to assume that increasing the SNR and gain control will also lead to decreases of tics and urges. In fact, in a single case study (Diamond et al., 2006) of a patient with GTS with co-morbid medically-refractory epilepsy invasive VNS led to reductions of motor and phonic tic frequency. This was corroborated by another small study obtaining evidence for positive effects of tVNS on tic severity (Sperling et al., 2008) and was reviewed by Hawksley et al. (2015). It is possible that concomitant administration of tVNS and clonidine will boost effects on tics and urges.
Assuming that the neurobiological mechanisms underlying potential beneficial effects of tVNS on tics and urges in GTS are an adjustment in the SNR and optimization of gain control processes by jointly acting on NA and GABA (Colzato and Beste, 2020), tVNS is expected to also affect cognitive functions in GTS and is likely to affect processes captured by the 1/f metric. More specifically, we would expect tVNS to improve cognitive persistence in GTS requiring a clear distinction between relevant and irrelevant information. This may be associated with a steeper slope of the 1/f neural noise function calculated using neurophysiological data recordings during tasks examining cognitive persistence. Considering that in animals (Clark et al., 1995) and epileptic patients (Clark et al., 1999) the relationship between cognitive performance and tVNS stimulation intensities (or neural enhancement) (Colzato et al., 2020) follows an inverted U-shaped function akin to dopamine, and that this principle is relevant for the NE and GABA systems (Introini-Collison et al., 1994), we expect increased cognitive flexibility in GTS, such as switching between tasks, strategies, and behaviours to be normalized at intermediate or higher stimulation intensities. It is possible that also the slope of the 1/f neural noise function follows an inverted U-shaped pattern in that only optimal stimulation intensities will modulate neural noise to a level ‘optimal’ for the task at hand. Similar to a possibly synergistic effect of tVNS combined with clonidine, combined application of tVNS and cognitive training might also affect cognitive functions in GTS. An ideal cognitive training to be combined with tVNS might be the Tonic and Phasic Alertness Training (Van Vleet et al., 2016). This specific training is relevant because it trains functions related to NE, such as alertness, and to GABA, such as response selection. The goal of this training procedure is to foster prolonged focused task engagement. People with GTS could practice sustained response monitoring in the training task (tonic alertness) and response inhibition when presented with rare no-go, target trials (phasic alertness). The effects of such approaches can then be evaluated on a neurophysiological level using the 1/f noise metric outlined above in section 3.
5. Conclusions
If the SNR-hypothesis of GTS is correct, spontaneously generated tics become problematic because such motor noise is misinterpreted by the brain (or the environment) as apparently relevant (to be controlled) signal that needs to be gated/controlled (Buse et al., 2016, Ganos et al., 2015, Ganos et al., 2014, Kim et al., 2019). Accordingly, tVNS brain stimulation, via increasing gain control and the SNR, might attenuate noise including tics and might be a potential tool in GTS to ameliorate cognitive persistence and at the same time normalize cognitive flexibility. We recommend that future studies shall examine the role of the SNR and gain control in GTS. We encourage the use of well-defined neurophysiological methods, particularly EEG, to examine the neural hypothesis using the 1/f metric of GTS and tVNS studies in combination with pharmacological challenges to clarify whether tVNS is a valuable treatment in GTS.
Acknowledgement
This work was supported by the Deutsche Forschungsgemeinschaft (FOR 2698). We thank Benjamin Teufert for help in designing the Figures.
References
- Alam M., Schwabe K., Lütjens G., Capelle H.H., Manu M., von Wrangel C., Müller-Vahl K., Schrader C., Scheinichen D., Blahak C., Heissler H.E., Krauss J.K. Comparative characterization of single cell activity in the globus pallidus internus of patients with dystonia or Tourette syndrome. J. Neural Trans. (Vienna) 2015;122:687–699. doi: 10.1007/s00702-014-1277-0. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. ed. American Psychiatric Association. 10.1176/appi.books.9780890425596.
- Aston-Jones G., Shipley M.T., Chouvet G., Ennis M., van Bockstaele E., Pieribone V., Shiekhattar R., Akaoka H., Drolet G., Astier B. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog. Brain Res. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Meyer-Lindenberg A., Achard S., Duke T., Bullmore E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19518–19523. doi: 10.1073/pnas.0606005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge C.W., Spencer R.C. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 2016;1641:189–196. doi: 10.1016/j.brainres.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beste C., Moll C.K.E., Pötter-Nerger M., Münchau A. Striatal microstructure and its relevance for cognitive control. Trends Cogn. Sci. (Regul. Ed.) 2018;22:747–751. doi: 10.1016/j.tics.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Beste C., Münchau A. Tics and Tourette syndrome — surplus of actions rather than disorder? Mov. Disord. 2018;33:238–242. doi: 10.1002/mds.27244. [DOI] [PubMed] [Google Scholar]
- Beste C., Tübing J., Seeliger H., Bäumer T., Brandt V., Stock A.-K., Münchau A. Altered perceptual binding in Gilles de la Tourette syndrome. Cortex. 2016;83:160–166. doi: 10.1016/j.cortex.2016.07.015. [DOI] [PubMed] [Google Scholar]
- Brandt V.C., Stock A.-K., Münchau A., Beste C. Evidence for enhanced multi-component behaviour in Tourette syndrome - an EEG study. Sci. Rep. 2017;7:7722. doi: 10.1038/s41598-017-08158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfeld M., Bar-Gad I. Loss of specificity in Basal Ganglia related movement disorders. Front. Syst. Neurosci. 2011;5:38. doi: 10.3389/fnsys.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse J., Beste C., Herrmann E., Roessner V. Neural correlates of altered sensorimotor gating in boys with Tourette Syndrome: a combined EMG/fMRI study. World J. Biol. Psychiatry. 2016;17:187–197. doi: 10.3109/15622975.2015.1112033. [DOI] [PubMed] [Google Scholar]
- Buse J., Schoenefeld K., Münchau A., Roessner V. Neuromodulation in Tourette syndrome: dopamine and beyond. Neurosci. Biobehav. Rev. 2013;37:1069–1084. doi: 10.1016/j.neubiorev.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Oxford University Press; 2006. Rhythms of the Brain. [Google Scholar]
- Buzsáki G., Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Cagle J.N., Okun M.S., Opri E., Cernera S., Molina R., Foote K.D., Gunduz A. Differentiating tic electrophysiology from voluntary movement in the human thalamocortical circuit. J. Neurol. Neurosurg. Psychiatry. 2020;91:533–539. doi: 10.1136/jnnp-2019-321973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty R.T., Knight R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010;14:506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. (Regul. Ed.) 2014;18:414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K.B., Krahl S.E., Smith D.C., Jensen R.A. Post-training Unilateral Vagal Stimulation Enhances Retention Performance in the Rat. Neurobiol. Learn. Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- Clark K.B., Naritoku D.K., Smith D.C., Browning R.A., Jensen R.A. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Cohen M.X. A neural microcircuit for cognitive conflict detection and signaling. Trends Neurosci. 2014;37:480–490. doi: 10.1016/j.tins.2014.06.004. [DOI] [PubMed] [Google Scholar]
- Colzato L., Beste C. A literature review on the neurophysiological underpinnings and cognitive effects of transcutaneous vagus nerve stimulation: challenges and future directions. J. Neurophysiol. 2020;123:1739–1755. doi: 10.1152/jn.00057.2020. [DOI] [PubMed] [Google Scholar]
- Colzato, L.S., Hommel, B., Beste, C., 2020. The Downsides of Cognitive Enhancement. Neuroscientist 1073858420945971. doi: 10.1177/1073858420945971. [DOI] [PMC free article] [PubMed]
- Cools R. The costs and benefits of brain dopamine for cognitive control. Wiley Interdiscip. Rev. Cogn. Sci. 2016;7:317–329. doi: 10.1002/wcs.1401. [DOI] [PubMed] [Google Scholar]
- Dave S., Brothers T.A., Swaab T.Y. 1/f neural noise and electrophysiological indices of contextual prediction in aging. Brain Res. 2018;1691:34–43. doi: 10.1016/j.brainres.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme C., Salvador A., Valabrègue R., Roze E., Palminteri S., Vidailhet M., de Wit S., Robbins T., Hartmann A., Worbe Y. Enhanced habit formation in Gilles de la Tourette syndrome. Brain. 2016;139:605–615. doi: 10.1093/brain/awv307. [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Desmurget M., Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn. Sci. (Regul. Ed.) 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Diamond A., Kenney C., Jankovic J. Effect of vagal nerve stimulation in a case of Tourette’s syndrome and complex partial epilepsy. Mov. Disord. 2006;21:1273–1275. doi: 10.1002/mds.20949. [DOI] [PubMed] [Google Scholar]
- Draper A., Stephenson M.C., Jackson G.M., Pépés S., Morgan P.S., Morris P.G., Jackson S.R. Increased GABA contributes to enhanced control over motor excitability in tourette syndrome. Curr. Biol. 2014;24:2343–2347. doi: 10.1016/j.cub.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M., Schlaghecken F. Response facilitation and inhibition in subliminal priming. Biol. Psychol. 2003;64:7–26. doi: 10.1016/s0301-0511(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Farmer A.D., Strzelczyk A., Finisguerra A., Gourine A.V., Gharabaghi A., Hasan A., Burger A.M., Jaramillo A.M., Mertens A., Majid A., Verkuil B., Badran B.W., Ventura-Bort C., Gaul C., Beste C., Warren C.M., Quintana D.S., Hämmerer D., Freri E., Frangos E., Tobaldini E., Kaniusas E., Rosenow F., Capone F., Panetsos F., Ackland G.L., Kaithwas G., O’Leary G.H., Genheimer H., Jacobs H.I.L., Van Diest I., Schoenen J., Redgrave J., Fang J., Deuchars J., Széles J.C., Thayer J.F., More K., Vonck K., Steenbergen L., Vianna L.C., McTeague L.M., Ludwig M., Veldhuizen M.G., De Couck M., Casazza M., Keute M., Bikson M., Andreatta M., D’Agostini M., Weymar M., Betts M., Prigge M., Kaess M., Roden M., Thai M., Schuster N.M., Montano N., Hansen N., Kroemer N.B., Rong P., Fischer R., Howland R.H., Sclocco R., Sellaro R., Garcia R.G., Bauer S., Gancheva S., Stavrakis S., Kampusch S., Deuchars S.A., Wehner S., Laborde S., Usichenko T., Polak T., Zaehle T., Borges U., Teckentrup V., Jandackova V.K., Napadow V., Koenig J. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020) Front. Hum. Neurosci. 2021;14 doi: 10.3389/fnhum.2020.568051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cognit. Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Ganos C., Asmuss L., Bongert J., Brandt V., Münchau A., Haggard P. Volitional action as perceptual detection: predictors of conscious intention in adolescents with tic disorders. Cortex. 2015;64:47–54. doi: 10.1016/j.cortex.2014.09.016. [DOI] [PubMed] [Google Scholar]
- Ganos C., Kühn S., Kahl U., Schunke O., Feldheim J., Gerloff C., Roessner V., Bäumer T., Thomalla G., Haggard P., Münchau A. Action inhibition in Tourette syndrome. Mov. Disord. 2014;29:1532–1538. doi: 10.1002/mds.25944. [DOI] [PubMed] [Google Scholar]
- Ganos C., Martino D., Espay A.J., Lang A.E., Bhatia K.P., Edwards M.J. Tics and functional tic-like movements: can we tell them apart? Neurology. 2019 doi: 10.1212/WNL.0000000000008372. [DOI] [PubMed] [Google Scholar]
- Ganos C., Rothwell J., Haggard P. Voluntary inhibitory motor control over involuntary tic movements: voluntary inhibition of tics. Mov. Disord. 2018;33:937–946. doi: 10.1002/mds.27346. [DOI] [PubMed] [Google Scholar]
- Georgiou N., Bradshaw J.L., Phillips J.G., Bradshaw J.A., Chiu E. Advance information and movement sequencing in Gilles de la Tourette’s syndrome. J. Neurol. Neurosurg. Psychiatry. 1995;58:184–191. doi: 10.1136/jnnp.58.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorni A., Windels F., Stratton P.G., Cook R., Silberstein P., Coyne T., Silburn P.A., Sah P. Single-unit activity of the anterior Globus pallidus internus in Tourette patients and posterior Globus pallidus internus in dystonic patients. Clin. Neurophysiol. 2017;128:2510–2518. doi: 10.1016/j.clinph.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Gireesh E.D., Plenz D. Neuronal avalanches organize as nested theta- and beta/gamma-oscillations during development of cortical layer 2/3. Proc. Natl. Acad. Sci. 2008;105:7576–7581. doi: 10.1073/pnas.0800537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graichen U., Witte H., Haueisen J. Analysis of induced components in electroencephalograms using a multiple correlation method. Biomed. Eng. Online. 2009;8:21. doi: 10.1186/1475-925X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler A.S., Berkem M., Yazgan Y., Kalaça S. Cognitive flexibility and social responsiveness in children and adolescents with tourette syndrome. Child Psychiatry Hum. Dev. 2015;46:940–950. doi: 10.1007/s10578-015-0533-3. [DOI] [PubMed] [Google Scholar]
- Gureckis T.M., Love B.C. Learning in noise: dynamic decision-making in a variable environment. J. Math. Psychol. 2009;53:180–193. doi: 10.1016/j.jmp.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag L., Quetscher C., Dharmadhikari S., Dydak U., Schmidt-Wilcke T., Beste C. Interrelation of resting state functional connectivity, striatal GABA levels, and cognitive control processes. Hum. Brain Mapp. 2015;36:4383–4393. doi: 10.1002/hbm.22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksley J., Cavanna A.E., Nagai Y. The role of the autonomic nervous system in Tourette Syndrome. Front. Neurosci. 2015;9 doi: 10.3389/fnins.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free brain activity: past, present, and future. Trends Cognit. Sci. 2014;18:480–487. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Zempel J.M., Snyder A.Z., Raichle M.E. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66:353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B. Chapter Two - Between Persistence and Flexibility: The Yin and Yang of Action Control. Advances in Motivation Science. Elsevier; 2015. pp. 33–67. [DOI] [Google Scholar]
- Hommel B., Colzato L.S. The social transmission of metacontrol policies: mechanisms underlying the interpersonal transfer of persistence and flexibility. Neurosci. Biobehav. Rev. 2017;81:43–58. doi: 10.1016/j.neubiorev.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Hommel B., Wiers R.W. Towards a unitary approach to human action control. Trends Cogn. Sci. (Regul. Ed.) 2017;21:940–949. doi: 10.1016/j.tics.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Introini-Collison I.B., Castellano C., McGaugh J.L. Interaction of GABAergic and beta-noradrenergic drugs in the regulation of memory storage. Behav. Neural Biol. 1994;61:150–155. doi: 10.1016/s0163-1047(05)80068-8. [DOI] [PubMed] [Google Scholar]
- Jackson G.M., Draper A., Dyke K., Pépés S.E., Jackson S.R. Inhibition, disinhibition, and the control of action in tourette syndrome. Trends Cognit. Sci. 2015;19:655–665. doi: 10.1016/j.tics.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Jeter C.B., Patel S.S., Morris J.S., Chuang A.Z., Butler I.J., Sereno A.B. Oculomotor executive function abnormalities with increased tic severity in Tourette syndrome. J. Child Psychol. Psychiatry. 2015;56:193–202. doi: 10.1111/jcpp.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Shahed J., Telkes I., Viswanathan A., Ince N.F. GPi oscillatory activity differentiates tics from the resting state, voluntary movements, and the unmedicated parkinsonian state. Front. Neurosci. 2016;10:436. doi: 10.3389/fnins.2016.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes S., Wieringa B.M., Nager W., Müller-Vahl K.R., Dengler R., Münte T.F. Electrophysiological measures and dual-task performance in Tourette syndrome indicate deficient divided attention mechanisms. Eur. J. Neurol. 2001;8:253–260. doi: 10.1046/j.1468-1331.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- Kalanithi P.S.A., Zheng W., Kataoka Y., DiFiglia M., Grantz H., Saper C.B., Schwartz M.L., Leckman J.F., Vaccarino F.M. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. PNAS. 2005;102:13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardos J. Recent advances in GABA research. Neurochem. Int. 1999;34:353–358. doi: 10.1016/s0197-0186(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Kataoka Y., Kalanithi P.S.A., Grantz H., Schwartz M.L., Saper C., Leckman J.F., Vaccarino F.M. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J. Comp. Neurol. 2010;518:277–291. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S., Nauhaus I., Benucci A., Bonin V., Ringach D.L., Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Jackson G.M., Dyke K., Jackson S.R. Impaired forward model updating in young adults with Tourette syndrome. Brain. 2019;142:209–219. doi: 10.1093/brain/awy306. [DOI] [PubMed] [Google Scholar]
- Kleimaker A., Kleimaker M., Bäumer T., Beste C., Münchau A. Gilles de la tourette syndrome-a disorder of action-perception integration. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.597898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleimaker M., Kleimaker A., Weissbach A., Colzato L.S., Beste C., Bäumer T., Münchau A. Non-invasive brain stimulation for the treatment of gilles de la tourette syndrome. Front. Neurol. 2020;11 doi: 10.3389/fneur.2020.592258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleimaker M., Takacs A., Conte G., Onken R., Verrel J., Bäumer T., Münchau A., Beste C. Increased perception-action binding in Tourette syndrome. Brain. 2020 doi: 10.1093/brain/awaa111. [DOI] [PubMed] [Google Scholar]
- Leckman J.F. Tourette’s syndrome. Lancet. 2002;360:1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [DOI] [PubMed] [Google Scholar]
- Leckman J.F., Hardin M.T., Riddle M.A., Stevenson J., Ort S.I., Cohen D.J. Clonidine treatment of Gilles de la Tourette’s syndrome. Arch. Gen. Psychiatry. 1991;48:324–328. doi: 10.1001/archpsyc.1991.01810280040006. [DOI] [PubMed] [Google Scholar]
- Maia T.V., Conceição V.A. Dopaminergic disturbances in tourette syndrome: an integrative account. Biol. Psychiatry. 2018;84:332–344. doi: 10.1016/j.biopsych.2018.02.1172. [DOI] [PubMed] [Google Scholar]
- Maling N., Hashemiyoon R., Foote K.D., Okun M.S., Sanchez J.C. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette’s syndrome. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0044215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceglia S., Servello D., Foffani G., Porta M., Sassi M., Mrakic-Sposta S., Rosa M., Barbieri S., Priori A. Thalamic single-unit and local field potential activity in Tourette syndrome. Mov. Disord. 2010;25:300–308. doi: 10.1002/mds.22982. [DOI] [PubMed] [Google Scholar]
- Martino D., Ganos C., Worbe Y. Neuroimaging applications in tourette’s syndrome. Int. Rev. Neurobiol. 2018;143:65–108. doi: 10.1016/bs.irn.2018.09.008. [DOI] [PubMed] [Google Scholar]
- McDonnell M.D., Ward L.M. The benefits of noise in neural systems: bridging theory and experiment. Nat. Rev. Neurosci. 2011;12:415–426. doi: 10.1038/nrn3061. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Kovacevic N., Itier R.J. Increased brain signal variability accompanies lower behavioral variability in development. PLoS Comput. Biol. 2008;4 doi: 10.1371/journal.pcbi.1000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M., Barker P.B., Bhattacharyya P.K., Brix M.K., Buur P.F., Cecil K.M., Chan K.L., Chen D.Y.-T., Craven A.R., Cuypers K., Dacko M., Duncan N.W., Dydak U., Edmondson D.A., Ende G., Ersland L., Gao F., Greenhouse I., Harris A.D., He N., Heba S., Hoggard N., Hsu T.-W., Jansen J.F.A., Kangarlu A., Lange T., Lebel R.M., Li Y., Lin C.-Y.E., Liou J.-K., Lirng J.-F., Liu F., Ma R., Maes C., Moreno-Ortega M., Murray S.O., Noah S., Noeske R., Noseworthy M.D., Oeltzschner G., Prisciandaro J.J., Puts N.A.J., Roberts T.P.L., Sack M., Sailasuta N., Saleh M.G., Schallmo M.-P., Simard N., Swinnen S.P., Tegenthoff M., Truong P., Wang G., Wilkinson I.D., Wittsack H.-J., Xu H., Yan F., Zhang C., Zipunnikov V., Zöllner H.J., Edden R.A.E. Big GABA: edited MR spectroscopy at 24 research sites. Neuroimage. 2017;159:32–45. doi: 10.1016/j.neuroimage.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M., Rimbault D.L., Barker P.B., Bhattacharyya P.K., Brix M.K., Buur P.F., Cecil K.M., Chan K.L., Chen D.Y.-T., Craven A.R., Cuypers K., Dacko M., Duncan N.W., Dydak U., Edmondson D.A., Ende G., Ersland L., Forbes M.A., Gao F., Greenhouse I., Harris A.D., He N., Heba S., Hoggard N., Hsu T.-W., Jansen J.F.A., Kangarlu A., Lange T., Lebel R.M., Li Y., Lin C.-Y.E., Liou J.-K., Lirng J.-F., Liu F., Long J.R., Ma R., Maes C., Moreno-Ortega M., Murray S.O., Noah S., Noeske R., Noseworthy M.D., Oeltzschner G., Porges E.C., Prisciandaro J.J., Puts N.A.J., Roberts T.P.L., Sack M., Sailasuta N., Saleh M.G., Schallmo M.-P., Simard N., Stoffers D., Swinnen S.P., Tegenthoff M., Truong P., Wang G., Wilkinson I.D., Wittsack H.-J., Woods A.J., Xu H., Yan F., Zhang C., Zipunnikov V., Zöllner H.J., Edden R.A.E. Big GABA II: water-referenced edited MR spectroscopy at 25 research sites. Neuroimage. 2019;191:537–548. doi: 10.1016/j.neuroimage.2019.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.J., Sorensen L.B., Ojemann J.G., den Nijs M. Power-law scaling in the brain surface electric potential. PLoS Comput. Biol. 2009;5 doi: 10.1371/journal.pcbi.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina R., Okun M.S., Shute J.B., Opri E., Rossi P.J., Martinez-Ramirez D., Foote K.D., Gunduz A. Report of a patient undergoing chronic responsive deep brain stimulation for Tourette syndrome: proof of concept. J. Neurosurg. 2018;129:308–314. doi: 10.3171/2017.6.JNS17626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto G., Schwingenschuh P., Katschnig P., Bhatia K.P., Haggard P. Delayed experience of volition in Gilles de la Tourette syndrome. J. Neurol. Neurosurg. Psychiatry. 2011;82:1324–1327. doi: 10.1136/jnnp.2010.221143. [DOI] [PubMed] [Google Scholar]
- Mueller S.C., Jackson G.M., Dhalla R., Datsopoulos S., Hollis C.P. Enhanced cognitive control in young people with Tourette’s syndrome. Curr. Biol. 2006;16:570–573. doi: 10.1016/j.cub.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Müller-Vahl K., Roessner V., Münchau A. Tourette-syndrom: häufig eine fehldiagnose. Dtsch Arztebl. 2020;7:A332–A333. [Google Scholar]
- Musall S., von Pföstl V., Rauch A., Logothetis N.K., Whittingstall K. Effects of neural synchrony on surface EEG. Cereb. Cortex. 2014;24:1045–1053. doi: 10.1093/cercor/bhs389. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Liley D.T. 1/f electrophysiological spectra in resting and drug-induced states can be explained by the dynamics of multiple oscillatory relaxation processes. NeuroImage. 2018;179:582–595. doi: 10.1016/j.neuroimage.2018.06.068. [DOI] [PubMed] [Google Scholar]
- Neumann W.-J., Huebl J., Brücke C., Lofredi R., Horn A., Saryyeva A., Müller-Vahl K., Krauss J.K., Kühn A.A. Pallidal and thalamic neural oscillatory patterns in tourette’s syndrome. Ann. Neurol. 2018;84:505–514. doi: 10.1002/ana.25311. [DOI] [PubMed] [Google Scholar]
- Ouyang G., Hildebrandt A., Schmitz F., Herrmann C.S. Decomposing alpha and 1/f brain activities reveals their differential associations with cognitive processing speed. NeuroImage. 2020;205 doi: 10.1016/j.neuroimage.2019.116304. [DOI] [PubMed] [Google Scholar]
- Palva J.M., Zhigalov A., Hirvonen J., Korhonen O., Linkenkaer-Hansen K., Palva S. Neuronal long-range temporal correlations and avalanche dynamics are correlated with behavioral scaling laws. PNAS. 2013;110:3585–3590. doi: 10.1073/pnas.1216855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappert E.J., Goetz C.G., Louis E.D., Blasucci L., Leurgans S. Objective assessments of longitudinal outcome in Gilles de la Tourette’s syndrome. Neurology. 2003;61:936–940. doi: 10.1212/01.wnl.0000086370.10186.7c. [DOI] [PubMed] [Google Scholar]
- Paszek J., Pollok B., Biermann-Ruben K., Müller-Vahl K., Roessner V., Thomalla G., Robertson M.M., Orth M., Schnitzler A., Münchau A. Is it a tic?–Twenty seconds to make a diagnosis. Mov. Disord. 2010;25:1106–1108. doi: 10.1002/mds.23053. [DOI] [PubMed] [Google Scholar]
- Pertermann M., Bluschke A., Roessner V., Beste C. The modulation of neural noise underlies the effectiveness of methylphenidate treatment in attention-deficit/hyperactivity disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4:743–750. doi: 10.1016/j.bpsc.2019.03.011. [DOI] [PubMed] [Google Scholar]
- Pertermann M., Mückschel M., Adelhöfer N., Ziemssen T., Beste C. On the interrelation of 1/f neural noise and norepinephrine system activity during motor response inhibition. J. Neurophysiol. 2019;121:1633–1643. doi: 10.1152/jn.00701.2018. [DOI] [PubMed] [Google Scholar]
- Podvalny E., Noy N., Harel M., Bickel S., Chechik G., Schroeder C.E., Mehta A.D., Tsodyks M., Malach R. A unifying principle underlying the extracellular field potential spectral responses in the human cortex. J. Neurophysiol. 2015;114:505–519. doi: 10.1152/jn.00943.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priori A., Giannicola G., Rosa M., Marceglia S., Servello D., Sassi M., Porta M. Deep brain electrophysiological recordings provide clues to the pathophysiology of Tourette syndrome. Neurosci. Biobehav. Rev. 2013;37:1063–1068. doi: 10.1016/j.neubiorev.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Puts N.A.J., Harris A.D., Crocetti D., Nettles C., Singer H.S., Tommerdahl M., Edden R.A.E., Mostofsky S.H. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J. Neurophysiol. 2015;114:808–817. doi: 10.1152/jn.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quetscher C., Yildiz A., Dharmadhikari S., Glaubitz B., Schmidt-Wilcke T., Dydak U., Beste C. Striatal GABA-MRS predicts response inhibition performance and its cortical electrophysiological correlates. Brain Struct. Funct. 2015;220:3555–3564. doi: 10.1007/s00429-014-0873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C.L., Critchley H.D., Seth A.K. A bayesian account of the sensory-motor interactions underlying symptoms of tourette syndrome. Front. Psychiatry. 2019;10:29. doi: 10.3389/fpsyt.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawji V., Modi S., Latorre A., Rocchi L., Hockey L., Bhatia K., Joyce E., Rothwell J.C., Jahanshahi M. Impaired automatic but intact volitional inhibition in primary tic disorders. Brain. 2020;143:906–919. doi: 10.1093/brain/awaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W., Arnsten A.F.T. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu. Rev. Neurosci. 2009;32:267–287. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner V., Schoenefeld K., Buse J., Bender S., Ehrlich S., Münchau A. Pharmacological treatment of tic disorders and Tourette Syndrome. Neuropharmacology. 2013;68:143–149. doi: 10.1016/j.neuropharm.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Salgado H., Treviño M., Atzori M. Layer- and area-specific actions of norepinephrine on cortical synaptic transmission. Brain Res. 2016;1641:163–176. doi: 10.1016/j.brainres.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Schurger A., Sitt J.D., Dehaene S. An accumulator model for spontaneous neural activity prior to self-initiated movement. Proc. Natl. Acad. Sci. 2012;109:E2904–E2913. doi: 10.1073/pnas.1210467109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans J.K., Yang C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D., Printz H., Cohen J.D. A network model of catecholamine effects: gain, signal-to-noise ratio, and behavior. Science. 1990;249:892–895. doi: 10.1126/science.2392679. [DOI] [PubMed] [Google Scholar]
- Shiozawa P., da Silva M.E., de Carvalho T.C., Cordeiro Q., Brunoni A.R., Fregni F. Transcutaneous vagus and trigeminal nerve stimulation for neuropsychiatric disorders: a systematic review. Arq. Neuropsiquiatr. 2014;72:542–547. doi: 10.1590/0004-282x20140061. [DOI] [PubMed] [Google Scholar]
- Shute J.B., Okun M.S., Opri E., Molina R., Rossi P.J., Martinez-Ramirez D., Foote K.D., Gunduz A. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. Neuroimage Clin. 2016;12:165–172. doi: 10.1016/j.nicl.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W., Reulbach U., Maihöfner C., Kornhuber J., Bleich S. Vagus nerve stimulation in a patient with Gilles de la Tourette syndrome and major depression. Pharmacopsychiatry. 2008;41:117–118. doi: 10.1055/s-2008-1062698. [DOI] [PubMed] [Google Scholar]
- Takács Á., Kóbor A., Chezan J., Éltető N., Tárnok Z., Nemeth D., Ullman M.T., Janacsek K. Is procedural memory enhanced in Tourette syndrome? Evidence from a sequence learning task. Cortex. 2018;100:84–94. doi: 10.1016/j.cortex.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Takacs A., Zink N., Wolff N., Münchau A., Mückschel M., Beste C. Connecting EEG signal decomposition and response selection processes using the theory of event coding framework. Hum. Brain Mapp. 2020;41:2862–2877. doi: 10.1002/hbm.24983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touboul J., Destexhe A. Power-law statistics and universal scaling in the absence of criticality. Phys. Rev. E. 2017;95 doi: 10.1103/PhysRevE.95.012413. [DOI] [PubMed] [Google Scholar]
- Van Vleet T.M., DeGutis J.M., Merzenich M.M., Simpson G.V., Zomet A., Dabit S. Targeting alertness to improve cognition in older adults: a preliminary report of benefits in executive function and skill acquisition. Cortex. 2016;82:100–118. doi: 10.1016/j.cortex.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Kayser A.S., Badre D., Fegen D., Chang E.F., Crone N.E., Parvizi J., Knight R.T., D’Esposito M. Oscillatory dynamics coordinating human frontal networks in support of goal maintenance. Nat. Neurosci. 2015;18:1318–1324. doi: 10.1038/nn.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Knight R.T. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry Cortical Oscillations Cognit. Circuit Dysfunction Psychiatric Disord. 2015;77:1089–1097. doi: 10.1016/j.biopsych.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Kramer M.A., Case J., Lepage K.Q., Tempesta Z.R., Knight R.T., Gazzaley A. Age-related changes in 1/f neural electrophysiological noise. J. Neurosci. 2015;35:13257–13265. doi: 10.1523/JNEUROSCI.2332-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B., Secundo L., Bidet-Caulet A., Scabini D., Stiver S.I., Gean A.D., Manley G.T., Knight R.T. Hemicraniectomy: a new model for human electrophysiology with high spatio-temporal resolution. J. Cognt. Neurosci. 2010;22:2491–2502. doi: 10.1162/jocn.2009.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A., Kleimaker M., Bäumer T., Beste C., Münchau A. Electro-myo-stimulation induced tic exacerbation - increased tendencies for the formation of perception-action links in tourette syndrome. Tremor Other Hyperkinet Mov (N Y) 2020;10:41. doi: 10.5334/tohm.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunina N., Kim S.S., Nam E.-C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. 2017;20:290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- Yildiz A., Quetscher C., Dharmadhikari S., Chmielewski W., Glaubitz B., Schmidt-Wilcke T., Edden R., Dydak U., Beste C. Feeling safe in the plane: neural mechanisms underlying superior action control in airplane pilot trainees–a combined EEG/MRS study. Hum. Brain Mapp. 2014;35:5040–5051. doi: 10.1002/hbm.22530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif, N., Fu, R.Z., Abou-El-Ela Bourquin, B., Bhrugubanda, V., Schultz, S.R., Seemungal, B.M., 2016. Dopamine Activation Preserves Visual Motion Perception Despite Noise Interference of Human V5/MT. J. Neurosci. 36, 9303–9312. 10.1523/JNEUROSCI.4452-15.2016. [DOI] [PMC free article] [PubMed]
- Zink, N., Bensmann, W., Arning, L., Colzato, L.S., Stock, A.-K., Beste, C., 2019. The role of DRD1 and DRD2 receptors for response selection under varying complexity levels - implications for metacontrol processes. Int. J. Neuropsychopharmacol. Doi: 10.1093/ijnp/pyz024. [DOI] [PMC free article] [PubMed]