Abstract
Context.
Children and adolescents with cancer experience treatment-related, subjective adverse events (AEs). Identifying distinct groups of patients who predictably experience higher prevalence of AEs could guide patient care.
Objectives.
Study aims were to 1) identify groups of children and adolescents reporting AEs using the Pediatric Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (Ped-PRO-CTCAE); 2) determine whether demographic and clinical characteristics predict AE group membership; and 3) examine whether AE group membership was related to the distal outcome of psychological stress.
Methods.
Four hundred seventy-seven patients self-reported AEs via the Ped-PRO-CTCAE at T1 (beginning of treatment) and the PROMIS Pediatric Psychological Stress measure at T2 (7–28 days later). Latent class analysis was conducted to identify groups of patients and the relationships of the groups with demographic and clinical characteristics, and with stress.
Results.
Three distinct a priori unknown AE groups were identified (high AE prevalence, moderate AE prevalence, and low AE prevalence). Females, blacks, patients with high psychological stress, and patients more recently diagnosed were more likely to be in the high AE prevalence group. Gender, age, race, and time since diagnosis were associated with psychological stress.
Conclusion.
Children with cancer are heterogeneous in experiencing subjective AEs. Gender, race, and time since diagnosis were significantly associated with higher subjective AE prevalence that may lead to psychological stress.
Keywords: Pediatric oncology, latent class analysis, symptom cluster, PROMIS, PRO-CTCAE
Introduction
Children with cancer experience multiple symptoms throughout treatment1–3 but most do not report subjective, treatment-related toxicities unless directly asked by clinicians.4 When questioned, most pediatric oncology patients report the presence of five or more subjective adverse events (AEs)5 while those receiving myelosuppressive chemotherapy report on average 10.6 subjective AEs.6 Because AEs are experienced concurrently and synergistically, they exert an exponential effect4,7 on patients’ overall symptom burden, quality of life, and psychological stress.8–11 The established relationship between number of AEs and patients’ psychological stress can serve to validate a new application of a statistical method to measure the impact of subjective AEs on pediatric oncology patients’ quality of life.
Two different data reduction statistical approaches have been applied to patient-reported subjective AE data. The first is a variable-centered symptom cluster approach that groups symptoms based on clinical observation of symptom co-occurrence, research hypotheses, findings of qualitative data analysis, or statistical modeling (e.g., factor analysis).11–17
The second approach, finite mixture model, includes latent class analysis (LCA),18 latent profile analysis (LPA), and latent transition analysis (LTA).19 These approaches are person-centered and group patients by patterns of symptoms. These approaches yield distinct profiles of children with subjective symptom suffering or AEs during cancer treatment, thereby helping to place a child within a profile and perhaps allowing clinicians to tailor supportive care to match a specific child’s symptom profile.
Previously, our research team applied latent profile analysis and latent transition analysis to data from the PROMIS (Patient-Reported Outcomes Measurement Information System) Pediatric Fatigue, Pain Interference, Anxiety and Depressive Symptom measures reported by pediatric oncology patients aged 8 to 18 years and identified two to four profiles of subjective symptom suffering.18,19 Dominant profiles included high and low symptom groups. In those previous studies, we did not include a clinically relevant, distal outcome of the symptom groups that could have helped to validate the groups. In the present study, we employed LCA with AEs using the newly validated Pediatric Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (Ped-PRO-CTCAE) and we included psychological stress as a distal outcome.
The objectives of our study were to 1) identify groups of children and adolescents with cancer experiencing similar patterns of subjective AEs, 2) determine whether demographic and clinical characteristics predict AE group membership, and 3) examine whether AE group membership was associated with the distal outcome of psychological stress.
Methods
Participants
Eligible patients were 7–18 years, receiving frontline cancer therapy (chemotherapy, surgery, radiation, or combination), had completed at least one month of therapy, were three to six weeks from surgery, able to read and understand English, not experiencing clinically significant cognitive impairments, and agreed to participate. Parents/caregivers gave consent for self and permission for their child to participate. Exclusion criteria were patients participating in any phase I trial, treated with surgery alone or receiving end-of-life care.
Settings
Nine oncology centers participated: Children’s Healthcare of Atlanta, Children’s Hospital Los Angeles, UPMC Children’s Hospital of Pittsburgh, Children’s National Hospital (Washington, DC), Dana-Farber Cancer Institute/Boston Children’s Hospital, Duke Cancer Institute/Duke University (Durham, NC), the Hospital for Sick Children (Toronto, Ontario, Canada), St. Jude Children’s Research Hospital (Memphis, TN), and the University of North Carolina at Chapel Hill.
Ethics
The study was approved by the institutional review boards at each site in accord with an assurance approved by the Department of Health and Human Services.
Design
Patients self-reported 15 “core” (i.e., frequently occurring) AEs via the Ped-PRO-CTCAE using a tablet at a time when fewer subjective AEs (T1) were anticipated. The psychological stress measurement was purposefully administered at a time that more subjective AEs (T2) were anticipated to determine if AE group membership at T1 predicted psychological stress at T2. T1 occurred within 72 hours of beginning a course of treatment; T2 occurred 7 to 17 days later for patients receiving chemotherapy and four weeks later for patients receiving radiation.
Measures
Demographic Forms.
Parents/caregivers completed two sociodemographic questionnaires, one for themselves (birthdate, gender, ethnicity/race, relationship status, highest completed grade, occupational status, and household income) and one for their child (birthdate, gender, ethnicity/race, current and highest completed school grade).
Pediatric-PRO-CTCAE (Ped-PRO-CTCAE).
The Ped-PRO-CTCAE is a validated set of items to determine the presence, severity, and interference with daily activities of subjective cancer treatment AEs as reported by children aged 7–18 years.20–24 Items use a seven- day reference period with 4 response options per item consistent with CTCAE grading. Clinicians select AEs from the Ped-PRO-CTCAE library for inclusion in a clinical trial or for assessing clinical concerns. For this study, patients completed items for the 15 most frequently occurring AEs. Presence was measured as a dichotomous variable of the AE symptom (1 – if the symptom was present; 0 – otherwise).
PROMIS Pediatric Psychological Stress Measure.
This measure, developed from literature and interviews with children, parents, and health professionals, assesses children’s cognitive, psychological, and somatic states.25 Psychometric testing established strong reliability and construct validity of the child-report items of perceived stress during the past seven days in pediatric oncology patients aged 7 to 18 years (those reporting anxiety or depression reported higher stress and were more likely to be taking medicines to treat mood)23 and in children and adolescents experiencing chronic pain (those with higher pain reported higher stress).26 The 5-point Likert response options range from never1 to always;5 higher scores indicate higher stress.27 Computerized-adaptive testing (CAT) methods were used to tailor data collected from each child.
Analysis.
We used LCA28–31 to identify groups of patients with respect to presence of the 15 AEs at T1. We estimated and compared several LCA models with an increasing number of groups (e.g., 2, 3, 4). We used information criterion indices (for example, Akaike’s information criterion, Bayesian information criterion, and the adjusted Bayesian information criterion) and likelihood ratio (LR) tests (for example, the Lo-Mendell-Rubin likelihood ratio [LMR LR] test, the adjusted LMR LR [ALMR LR] test, and the bootstrap likelihood ratio test] for model fit comparisons. A smaller information criterion index indicates better model fit; a significant LR test (P < 0.05) indicates that the higher group number (e.g., 3 groups) model fit data better than the model with fewer groups (e.g., 2 groups). The relationship of group membership with demographic factors (age, gender, education, race) and clinical measures (time since diagnosis, hemoglobin) was assessed. Finally, we examined the effects of demographic and clinical variables on psychological stress and assessed whether such effects were moderated by group membership.
We applied a 3-step method to examine relationships between the latent groups, covariates, and stress and to minimize measurement error.32 Associations of latent group membership with demographic and clinical variables were examined with automatic implementation of the 3-step method.33 The effects of demographic and clinical variables on the distal outcome (i.e., stress score at T2) were examined by group simultaneously in an auxiliary structural equation model where the 3-step method was performed manually.32,34
Results
Sample
Mean age among the 477 participants was 13.5 years (SD = 3.4); the majority were male (n = 253, 53.7%), white (n = 268, 56.2%), and receiving leukemia treatment (n = 264, 55.1%). Almost all (n = 437, 91.2%) received chemotherapy (Table 1). Most parents had completed at least some college education (78%).
Table 1.
Sample Statistics (N = 477)
| Variable | Statistics |
|---|---|
| Child age (yrs) | |
| Mean (SD) | 13.47 (3.4) |
| Duration since diagnosis | |
| Mean (SD) | 0.44 (0.7) |
| Hemoglobin (HGB) | |
| Mean (SD) | 10.59 (1.6) |
| Gender, N (%) | |
| Male | 253 (53.7) |
| Female | 218 (46.3) |
| Race, N (%) | |
| White | 268 (56.2) |
| Black | 77 (16.1) |
| Hispanic | 65 (13.6) |
| Others | 67 (14.0) |
| Parent education, N (%) | |
| Elementary/primary school | 6 (1.3) |
| Secondary/high school | 96 (20.5) |
| Some college/university | 125 (26.7) |
| College/university | 169 (36.1) |
| Postgraduate degree | 72 (15.4) |
| Cancer Type, N (%) | |
| Leukemia/lymphoma | 264 (55.1) |
| Solid tumor | 135 (28.2) |
| Neuro-oncology | 71 (14.8) |
| Bone marrow transplant (BMT) | 9 (1.9) |
| Cancer treatment, N (%) | |
| Chemotherapy | 437 (91.2) |
| Radiation | 33 (6.9) |
| Bone marrow transplant | 9 (1.9) |
Frequencies of some variables may not sum up to N = 477 due to missing values.
Descriptive Findings
Ped-PRO-CTCAE.
The highest prevalence AEs at T1 were fatigue (68.3%), insomnia (52.6%), and pain (50.7%) (Table 2). The remaining 12 AEs were reported at rates from 22.4% (vomiting) to 45.9% (nausea). Nearly all participants (97%) experienced ≥ 1 of the core AEs and 66% experienced ≥ 5 AEs (Table 2).
Table 2.
Prevalence of PED-PRO-CTCAE Core AEs at T1 (N = 477)
| AE Symptom | N (%) |
|---|---|
| Fatigue | 326 (68.3) |
| Insomnia | 251 (52.6) |
| Pain | 242 (50.7) |
| Nausea | 219 (45.9) |
| Anxiety | 217 (45.5) |
| Abdominal pain | 216 (45.3) |
| Anorexia | 212 (44.4) |
| Headache | 207 (43.4) |
| Depression | 185 (38.8) |
| Constipation | 178 (37.3) |
| Cough | 173 (36.3) |
| Diarrhea | 145 (30.4) |
| Neuropathy | 142 (29.8) |
| Mucositis | 141 (29.6) |
| Vomiting | 107 (22.4) |
| Total number of AEs reported | |
| 0 | 15 (3.1) |
| 1 | 39 (8.2) |
| 2 | 31 (6.5) |
| 3 | 35 (7.3) |
| 4 | 43 (9.0) |
| 5+ | 314 (65.8) |
An AE toxicity was defined “Yes” if any of its attribute (frequency, severity, or interference) scores was ≥1.
PROMIS Pediatric Psychological Stress.
The mean score of the PROMIS Pediatric Psychological Stress measure was 46.2 (SE = 9.6).
LCA Model Fit Findings.
All information criterion indices and LR tests support the 3-group model (Appendix Table 1) as did interpretability from a clinical perspective. Classification probabilities for high, moderate, and low AE prevalence groups were 0.90, 0.92, and 0.87, respectively, with an entropy statistic of 0.75, indicating adequate classification quality.
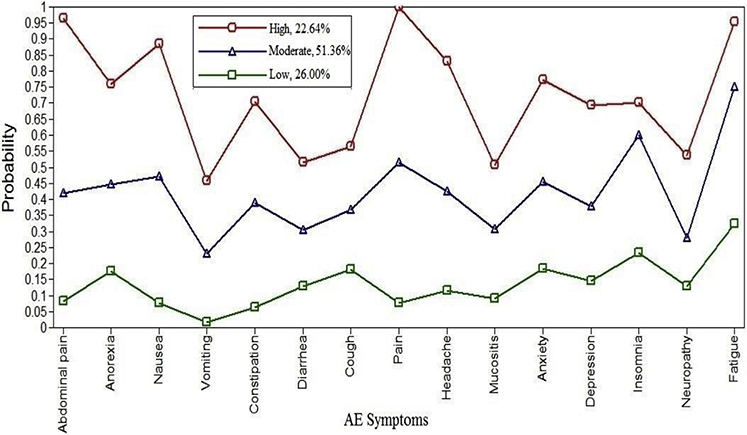
The AE groups denoted by the 3-group model showed distinct patterns of presence of subjective AEs (Fig. 1). Patterns were consistent in sensitivity analyses that excluded patients who received radiation treatment (N = 33) or bone marrow transplant (N = 9). We labeled the AE groups as high prevalence, moderate prevalence, and low prevalence. Approximately 22.6% of patients were classified into high prevalence, 51.4% into moderate prevalence, and 26.0% into low prevalence. In the high prevalence group, patients (n = 108) reported an average of 11 AEs (SD = 1.6; range, 8 to 15 AEs); moderate prevalence (n = 245) an average of 6.3 AEs (SD = 1.7, range, 3 to 11); and low prevalence (n = 124) an average of 1.8 AEs (SD = 1.1, range, 0 to 4) (Table 2).
Fig. 1.
Adverse event (AE) subjective symptom groups resulting from the 3-class latent class analysis model.
Demographic and Clinical Characteristics.
Mean age and hemoglobin values were similar across groups (Table 3). Parent education level, race/ethnicity composition, and time since diagnosis varied slightly across groups. Gender composition was significantly different across the 3 groups (Table 4). The percentage of males was lower in the high prevalence group (37.0%), compared with moderate prevalence (57.6%) and low prevalence (58.1%) groups. Patients with shorter time since diagnosis were more likely to be in the high prevalence group (Table 4).
Table 3.
Demographic and Clinical Characteristics by Group (N = 477)a
| Latent Group |
|||
|---|---|---|---|
| High Prevalence (N = 108, 22.6%) |
Moderate Prevalence (N = 245, 51.4%) |
Low Prevalence (N = 124, 26.0%) |
|
| Variable | Mean (SD) | Mean (SD) | Mean (SD) |
| Age | 13.52 (3.4) | 13.52 (3.4) | 13.31 (3.5) |
| χ2 = 0.341, P = 0.843 | |||
| Hemoglobin | 10.35 (1.4) | 10.67 (1.7) | 10.64 (1.5) |
| χ2 = 3.131, P = 0.209 | |||
| Gender, N (%) | |||
| Female | 68 (63.0) | 104 (42.5) | 52 (41.9) |
| Male | 40 (37.0) | 141 (57.6) | 72 (58.1) |
| χ2 = 14.36, P = 0.001 | |||
| Parent education, N (%) | |||
| < College | 18 (17.0) | 49 (20.4) | 35 (28.7) |
| College/university | 69 (65.1) | 155 (64.6) | 70 (57.4) |
| Postgraduate | 19 (17.9) | 36 (15.0) | 17 (13.9) |
| χ2 = 5.41, P = 0.247 | |||
| Race/ethnicity, N (%) | |||
| White | 60 (55.6) | 136 (55.5) | 72 (58.1) |
| Black | 17 (15.7) | 33 (13.5) | 27 (21.8) |
| Hispanic | 19 (17.6) | 35 (14.3) | 11 (8.9) |
| Others | 12 (11.1) | 41 (16.7) | 14 (11.3) |
| χ2 = 9.61, P = 0.142 | |||
| Time since diagnosis, N (%) | |||
| ≤Median (0.30) | 72 (66.7) | 137 (55.9) | 65 (52.4) |
| >Median (0.30) | 36 (33.3) | 108 (44.1) | 59 (47.6) |
| χ2 = 5.27, P = 0.071 | |||
Sample size slightly varies by variables due to missing values.
Table 4.
Effects of Demographic and Clinical Measures on Latent Group Classificationa
| Latent Group |
|||
|---|---|---|---|
| Covariate | High vs. Low Prevalence, β (P-value) | Moderate vs. Low Prevalence, β (P-value) | High vs. Moderate Prevalence, β (P-value) |
| Age | −0.01 (0.838) | −0.00 (0.935) | −0.01 (0.885) |
| Gender | |||
| Female | — | — | — |
| Male | −0.78 (0.022) | −0.04 (0.898) | −0.74 (0.024) |
| Parent education | |||
| <High school | — | — | — |
| High school | 0.58 (0.174) | 0.38 (0.286) | 0.19 (0.654) |
| College+ | 0.99 (0.064) | 0.58 (0.257) | 0.41 (0.423) |
| Time since diagnosis | |||
| ≤ Median | — | — | — |
| > Median | −1.46 (0.239) | −0.29 (0.094) | −1.17 (0.357) |
| Race | |||
| Othersb | — | — | — |
| White | −0.53 (0.214) | −0.52 (0.176) | −0.02 (0.968) |
| Black | −0.87 (0.118) | −1.11 (0.025) | 0.24 (0.679) |
| Hemoglobin | −0.09 (0.444) | −0.01 (0.952) | −0.08 (0.461) |
Estimated using AUXILIARY statement with R3STEP option in Mplus 8.4.33
Including Hispanics.
LCA and PROMIS Pediatric Psychological Stress.
The mean psychological stress score was highest in the high prevalence group (53.7), followed by moderate prevalence (46.4), and lowest in the low prevalence group (40.1) (Table 5). The mean score in the high prevalence group is in the median percentile for this measure; the other two mean scores are below the median percentile.35 The effects of demographic and clinical measures on stress vary by group (Appendix Table 2). The stress score was higher (β = 12.98, P < 0.01) in black patients than other racial groups in the high prevalence group. However, a race effect was not statistically significant in the other two AE prevalence groups. Age and gender had significant effects on stress only in the moderate prevalence group, that is, older patients reported higher stress (β = 0.59, P = 0.03) and males reported lower stress (β = −3.83, P < 0.0.48). More time since diagnosis had a significant positive effect on stress in the moderate group (β = 3.75, P = 0.02) (Appendix Table 2).
Table 5.
| Subgroup | Mean | SE |
| High (N = 108, 22.64%) | 53.7 | 1.5 |
| Moderate (N = 245, 51.36%) | 46.4 | 0.8 |
| Low (N = 124, 26.00%) | 40.1 |
0.6 |
| Chi-Square |
P-Value |
|
| Overall test | 109.3 | <0.001 |
| High vs. moderate | 14.8 | <0.001 |
| High vs. low | 68.3 | <0.001 |
| Moderate vs. low | 42.1 | <0.001 |
PROMIS Pediatric Psychological Stress score at T2.
Estimated using AUXILIARY statement with DE3STEP option in Mplus 8.4.33
Discussion
In this large pediatric oncology sample, 477 children and adolescents on therapy self-reported core subjective AEs using the Ped-PRO-CTCAE at two time points: before a scheduled treatment course (T1), and the PROMIS Pediatric Psychological Stress measure later (T2) when symptom burden was expected to be higher. Most (65.8%) reported ≥ 5 AEs, similar to reports of pediatric oncology patients experiencing multiple, concurrent subjective AEs even during periods of expected stability.1–7,12–17 We identified three distinct groups of pediatric oncology patients according to their reports of subjective treatment toxicities which indicated that the study sample was heterogeneous with respect to the 15 AEs. The finding of three distinct patient groups based on prevalence of subjective AEs is significant, as it is similar to previous findings from similar patient groups but based on more established (well validated) pediatric symptom and function measures. Importantly, the number of prevalence groups was not determined a priori but derived from statistical results.
In our group’s previous cross-sectional study of 200 pediatric patients who completed four PROMIS Pediatric measures during chemotherapy (n = 97) or in survivorship (n = 103), four latent groups emerged.18 Similarly, in our prior longitudinal study involving 96 pediatric patients assessed before and during a chemotherapy cycle, three distinct groups emerged with high and low symptom suffering subgroups dominating.19 In the two prior studies, a limited number4 of subjective symptoms were studied, whereas in this study, the much larger sample of pediatric oncology patients on active therapy reported on 15 AEs with three distinct groups emerging.
The number of groups identified across these three studies using LCA and patient-reported outcomes is consistently small (three to four).18,19 This suggests that the number of clinically meaningful groups of pediatric oncology patients experiencing subjective AEs is limited and therefore translatable into clinical care guidelines specific to each subgroup. Clinicians could be alerted to the patient’s group membership to help trigger use of group-specific support resources and inform personalized symptom management. Perhaps most importantly, across studies, there exists a group of high symptom suffering or high AE prevalence that can be identified.18,19
High AE Prevalence Subgroup
Our study identified 22.6% of patients in the high prevalence group; our prior two studies found 16%–45.8% of patients in the high symptom suffering group at T1.18,19 The high symptom suffering group is relatively small across studies and may even decrease during therapy. This group is distinct in terms of probabilities of AEs (Fig. 1), raw number of AEs (8 to 15 AEs per group member compared to 3 to 11 AEs for the moderate group, and 0 to 4 AEs for the low prevalence group) and mean number of AEs (11.0 [SD = 1.56] compared with 6.3 [SD = 1.74] and 1.8 [SD = 1.15] in the moderate and low AE groups]. Identifying patients in this smaller, high suffering group may be especially relevant as this group includes patients likely to need enhanced supportive care.
Moderate AE Prevalence Group
This group at 51.36% was nearly twice the size of the other two groups. Its AE pattern (Fig. 1) was similar to that in the high prevalence group but its prevalence rates were half that of the same AE in the high prevalence group. Insomnia was the exception as it is similar in prevalence between the two groups. These patients’ subjective AE experience cannot be discounted given that each reports 3 to 11 AEs.
Low Prevalence AE Group
In each study using the PROMIS Pediatric measures, a low-symptom burden, and in some studies, a high-functioning cohort emerged.12,18,35 A proportion of children in this current study reported experiencing no (3.1%),1 (8.2%), or 2 (6.5%) AEs at T1. Insomnia and pain were reported by about half (52.6% for insomnia and 50.7% for pain), while over two-third of patients reported fatigue. The possibility that there may be a select cohort of patients for whom symptom burden starts low and remains low across treatments has been demonstrated in one study across a one-month period.19 Longer-term examination of the groups throughout treatment is merited.
Influence of Gender and Race
A limited number of differences related to gender and race are noted, including a greater proportion of females and a greater proportion of blacks reporting higher stress levels in the high AE prevalence group compared to the other two groups (Table 5). Females in the moderate prevalence group reported significantly higher levels of psychological stress than males (Appendix Table 2). However, similar to previous studies, these patient characteristics and other demographic variables are rarely found to influence psychological and other outcomes in pediatric oncology patients.18,19,35–37 When measured, it may be socioeconomic status more than race or gender that is associated with such outcomes.36 Although gender and racial differences in AE prevalence as well as cultural, clinical, or biological etiologies for these differences need further exploration, the concurrent measurement of socioeconomic status is recommended.
Influence of Time
More time since diagnosis was associated with lower levels of psychological stress in the moderate AE group only. Prior studies of pediatric patient reports during chemotherapy indicate the symptom burden decreased over time.1,3 Whether this is because children adapt over time, receive more intense therapies earlier, or access personalized supportive care interventions over time is an underexplored but important research question.
Limitations
We did not study the existence, size, and distinctive features of patient groups by diagnosis and oncologic treatment across extended time periods to observe AE patterns of prevalence, interference, and AE severity. We also did not study pediatric self-reported measurements of function (i.e., physical, cognitive, social) but doing so would provide important dimensions to clinical meaningfulness.
Conclusion
Co-occurring subjective AEs occur during pediatric oncology therapies. Using different patient-reported items and in a notably large sample of pediatric patients aged 7–18 years with diverse oncologic diagnoses, our study confirms that clinically meaningful groups exist among pediatric oncology patients based on their experiences with AEs. Using direct AE reports to identify membership in an AE subgroup has clinical utility, such as alerting clinicians to the need to enhance or reduce planned symptom management strategies, particularly as they may relate to psychological stress. As such, our findings have important implications for better understanding AE experiences and preparing personalized supportive care approaches for patients.
Key Message.
Three distinct groups of children and adolescents exist regarding their experience with prevalence of subjective adverse events (AEs) during cancer treatment: high, moderate, and low AE prevalence. Clinicians could be alerted to patients’ group membership to help trigger use of specific support resources to personalize AE management.
Disclosures and Acknowledgments
The authors sincerely acknowledge the contributions to this study from Sharon Dibridge, Mary Petrany, Dr. Mia Waldron, Dr. Catriona Mowbray, Courtney Mann, and Nicole Lucas. The authors have no conflicts of interest to report. The study was funded by National Cancer Institute (R01CA175759), National Institute of Arthritis and Musculoskeletal and Skin Diseases (U19AR069522), Alex’s Lemonade Stand Foundation, the Jonathan M. Houy Memorial Fund and the A. J. and Sigismunda Palumbo Charitable Trust.
Appendix
Appendix Table 1.
Latent Group Model Fit Comparison (N = 477)
| Model | AIC | BIC | aBIC | LMR LR P-value | ALMR LR P-value | BLRT P-value |
|---|---|---|---|---|---|---|
| 1-Group | 9297.67 | 9343.35 | 9292.57 | — | — | — |
| 2-Group | 8601.74 | 8735.10 | 8633.54 | <0.0001 | <0.0001 | <0.0001 |
| 3-Group | 8526.26 | 8726.30 | 8573.95 | <0.0001 | <0.0001 | <0.0001 |
| 4-Group | 8493.70 | 8760.42 | 8557.30 | 0.4855 | 0.4902 | <0.0001 |
AIC = Akaike information criterion; BIC = Bayesian information criterion; aBIC = adjusted BIC; LMR LR = Lo-Mendell-Rubin likelihood ratio; ALMR LR = adjusted LMR LR; BLRT = bootstrap likelihood ratio test; — = not applicable.
Appendix Table 2.
The Effects of Demographic and Clinical Measures on PROMIS Pediatric Psychological Stressa by Latent Groupb
| Covariates | β | P-value |
|---|---|---|
| High AE prevalence | ||
| Age | −0.39 | 0.485 |
| Gender | ||
| Female | — | — |
| Male | −4.24 | 0.331 |
| Parent education | ||
| < College | — | — |
| College/university | −5.14 | 0.640 |
| Postgraduate | 0.44 | 0.959 |
| Race | ||
| Othersc | — | — |
| White | −1.07 | 0.886 |
| Black | 12.98 | 0.005 |
| Time since diagnosis | ||
| ≤ Median | — | — |
| > Median | −4.05 | 0.292 |
| Hemoglobin | 1.82 | 0.311 |
| Moderate AE Prevalence | ||
| Age | 0.59 | 0.034 |
| Gender | ||
| Female | — | — |
| Male | −3.83 | 0.048 |
| Parent education | ||
| < College | — | — |
| College/university | 1.01 | 0.708 |
| Postgraduate | −0.56 | 0.822 |
| Racec | ||
| Others | — | — |
| White | −1.30 | 0.637 |
| Black | −3.75 | 0.132 |
| Time since diagnosis | ||
| ≤ Median | — | — |
| > Median | 3.75 | 0.015 |
| Hemoglobin | −0.35 | 0.453 |
| Low AE prevalence | ||
| Age | 0.09 | 0.633 |
| Gender | ||
| Female | — | — |
| Male | 0.19 | 0.633 |
| Parent education | ||
| < College | — | — |
| College/university | −0.82 | 0.668 |
| Postgraduate | −0.56 | 0.738 |
| Racec | ||
| Others | — | — |
| White | 1.14 | 0.602 |
| Black | −2.06 | 0.276 |
| Time since diagnosis | ||
| ≤ Median | — | |
| > Median | 0.61 | 0.429 |
| Hemoglobin | −0.45 | 0.452 |
— = reference group.
PROMIS Pediatric Psychological Stress score at T2.
Estimated by manual 3-step estimation procedure in Mplus 8.4.33
Including Hispanics.
Contributor Information
Pamela S. Hinds, Department of Nursing Science, Professional Practice and Quality, Children’s National Hospital, Washington, District of Columbia; Department of Pediatrics, The George Washington University, Washington, District of Columbia.
Meaghann S. Weaver, Division of Pediatric Palliative Care, Children’s Hospital and Medical Center, Omaha, Nebraska; Division of Pediatric Oncology, Children’s Hospital and Medical Center, Omaha, Nebraska.
Janice S. Withycombe, School of Nursing, Clemson University, Clemson, South Carolina.
Justin N. Baker, St. Jude Children’s Research Hospital, Memphis, Tennessee.
Shana S. Jacobs, Department of Pediatrics, Children’s National Hospital, The George Washington University, Washington, District of Columbia.
Jennifer W. Mack, Department of Pediatric Oncology and Center for Population Sciences, Dana-Farber Cancer Institute and Boston Children’s Hospital, Boston, Massachusetts.
Scott H. Maurer, Department of Pediatrics, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania; Division of Palliative Medicine and Supportive Care, UPMC Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania.
Molly McFatrich, Population Health Sciences, Duke University School of Medicine, Durham, North Carolina.
Laura C. Pinheiro, Department of Medicine, Weill Cornell Medicine, New York, New York.
Bryce B. Reeve, Department of Population Health Sciences, Duke Cancer Institute, Duke University School of Medicine, Durham, North Carolina; Department of Pediatrics, Duke Cancer Institute, Duke University School of Medicine, Durham, North Carolina.
Jichuan Wang, Division of Biostatistics and Study Methodology, Children’s National Hospital, Washington, District of Columbia; Epidemiology and Biostatistics, The George Washington University School of Medicine and Health Sciences, Washington, District of Columbia, USA.
References
- 1.Ruland CM, Hamilton GA, Schjodt-Osmo B. The complexity of symptoms and problems experienced in children with cancer: a review of the literature. J Pain Symptom Manage 2009;37:403–418. [DOI] [PubMed] [Google Scholar]
- 2.Kestler SA, LoBiondo-Wood G. Review of symptom experiences in children and adolescents with cancer. Cancer Nurs 2012;35:E31–E49. [DOI] [PubMed] [Google Scholar]
- 3.Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol Nurs Forum 2011;38:E382–E393. [DOI] [PubMed] [Google Scholar]
- 4.Weaver MS, Reeve BB, Baker JN, et al. Concept-elicitation phase for the development of the pediatric patient-reported outcome version of the common terminology criteria for adverse events. Cancer 2016;122:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madden K, Magno Charone M, Mills S, et al. Systematic symptom reporting by pediatric palliative care patients with cancer: a preliminary report. J Palliat Med 2019;22:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggott C, Dodd M, Kennedy C, et al. Changes in children’s reports of symptom occurrence and severity during a course of myelosuppressive chemotherapy. J Pediatr Oncol Nurs 2010;27:307–315. [DOI] [PubMed] [Google Scholar]
- 7.Docherty SL. Symptom experiences of children and adolescents with cancer. Annu Rev Nurs Res 2003;21:123–149. [PubMed] [Google Scholar]
- 8.Harper FWK, Albrecht TL, Trentacosta CJ, Taub JW, Phipps S, Penner LA. Understanding differences in the long-term psychosocial adjustment of pediatric cancer patients and their parents: an individual differences resources model. Transl Behav Med 2019;9:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olagunju AT, Sarimiye FO, Olagunju TO, Habeebu MY, Aina OF. Child’s symptom burden and depressive symptoms among caregivers of children with cancers: an argument for early integration of pediatric palliative care. Ann Palliat Med 2016;5:157–165. [DOI] [PubMed] [Google Scholar]
- 10.Heden L, Poder U, von Essen L, Ljungman G. Parents’ perceptions of their child’s symptom burden during and after cancer treatment. J Pain Symptom Manage 2013;46: 366–375. [DOI] [PubMed] [Google Scholar]
- 11.Trentacosta CJ, Harper FW, Albrecht TL, Taub JW, Phipps S, Penner LA. Pediatric cancer patients’ treatment-related Distress and Longer-term anxiety: an individual differences Perspective. J Dev Behav Pediatr 2016;37:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh CH, Chiang YC, Chien LC, et al. Symptom clustering in older Taiwanese children with cancer. Oncol Nurs Forum 2008;35:273–281. [DOI] [PubMed] [Google Scholar]
- 13.Hockenberry MJ, Hooke MC, Gregurich M, et al. Symptom clusters in children and adolescents receiving cisplatin, doxorubicin, or ifosfamide. Oncol Nurs Forum 2010;37: E16–E27. [DOI] [PubMed] [Google Scholar]
- 14.Hockenberry MJ, Hooke MC, McCarthy K, et al. Sickness behavior clustering in children with cancer. J Pediatr Oncol Nurs 2011;28:263–272. [DOI] [PubMed] [Google Scholar]
- 15.Baggott C, Cooper BA, Marina N, et al. Symptom cluster analyses based on symptom occurrence and severity ratings among pediatric oncology patients during myelosuppressive chemotherapy. Cancer Nurs 2012;35:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atay S, Conk Z, Bahar Z. Identifying symptom clusters in paediatric cancer patients using the Memorial Symptom Assessment Scale. Eur J Cancer Care 2012;21:460–468. [DOI] [PubMed] [Google Scholar]
- 17.Hooke MC, Rodgers C, Taylor O, et al. Physical activity, the childhood cancer symptom cluster-leukemia, and cognitive function: a longitudinal Mediation analysis. Cancer Nurs 2018;41:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckner TW, Wang J, DeWalt DA, et al. Patterns of symptoms and functional impairments in children with cancer. Pediatr Blood Cancer 2014;61:1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Jacobs S, Dewalt DA, et al. A longitudinal study of PROMIS pediatric symptom clusters in children undergoing chemotherapy. J Pain Symptom Manage 2018;55: 359–367. [DOI] [PubMed] [Google Scholar]
- 20.Reeve BB, McFatich M, Pinheiro L, et al. Eliciting child’s voice in adverse event reporting in oncology trials: cognitive interview findings from pediatric patient-reported outcomes version of the common terminology criteria for adverse events initiative. Pediatr Blood Cancer 2017;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeve BB, McFatrich M, Pinheiro LC, et al. Cognitive interview-based validation of the patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) in adolescents with cancer. J Pain Symptom Manage 2017;53:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McFatrich M, Brondon J, Lucas NR, et al. Mapping child and adolescent self-reported symptom data to clinician-reported adverse event grading to improve pediatric oncology care and research. Cancer 2020;126:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeve BB, McFatrich M, Mack JW, et al. Validity and reliability of the pediatric patient-reported outcomes – Common Terminology criteria for adverse events. JNCI 2020. 10.1093/jnci/djaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Withycombe JS, McFatrich M, Pinheiro L, et al. The association of age, literacy and race on completing patient-reported outcome measures in pediatric oncology. Qual Life Res 2019;28:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bevans KB, Gardner W, Pajer K, et al. Qualitative development of the PROMIS pediatric stress response item banks. J Pediatr Psychol 2013;38:173–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson S, Burns M, Logan D. The clinical utility of a Brief psychological stress measure (patient-reported outcomes measurement information system) in youth with chronic pain. Pain Med 2020. 10.1093/pm/pnaa263. [DOI] [PubMed] [Google Scholar]
- 27.Bevans KB, Gardner W, Pajer KA, et al. Psychometric evaluation of the PROMIS pediatric psychological and physical stress experience measures. J Pediatr Psychol 2018;43: 678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarsfeld PF, Henry NW. Latent structure analysis. Boston: Houghton Mill, 1968. [Google Scholar]
- 29.McLachlan GJ, Peel D. Finite mixture models. New York: Wiley, 2000. [Google Scholar]
- 30.Collins LM, Lanza ST. Latent class and latent transition analysis with applications in the social behavioral, and health sciences. Hoboken, NJ: Wiley, 2010. [Google Scholar]
- 31.Muthén BO. Beyond SEM: general latent variable modeling. Behaviormetrika 2002;29:81–117. [Google Scholar]
- 32.Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: three-step approaches using Mplus. Struct EQU Model 2014;21:329–341. [Google Scholar]
- 33.Muthén L, Muthén B. 1998–2017. Mplus User’s Guide. Los Angeles, CA: Muthen & Muthen, 2017. [Google Scholar]
- 34.Wang J, Wang X. Structural equation modeling: Applications using Mplus, 2nd ed. New York: John Wiley, 2020. [Google Scholar]
- 35.Hinds PS, Wang J, Cheng YI, et al. PROMIS pediatric measures validated in a longitudinal study design in pediatric oncology. Pediatr Blood Cancer 2019;66:e27606. [DOI] [PubMed] [Google Scholar]
- 36.Ramsey LH, Graves PE, Howard Sharp KM, Seals SR, Collier AB, Karlson CW. Impact of race and socioeconomic status on psychologic outcomes in childhood cancer patients and caregivers. J Pediatr Hematol Oncol 2019;41:433–437. [DOI] [PubMed] [Google Scholar]
- 37.Li HCW, Williams PD, Lopez V, Chung JO, Chiu SY. Relationships among therapy-related symptoms, depressive symptoms, and quality of life in Chinese children hospitalized with cancer: an exploratory study. Cancer Nurs 2013; 36:346–354. [DOI] [PubMed] [Google Scholar]