Abstract
Objective.
To estimate trends of annual antipsychotic medication use by privately insured US young children (2–7 years) and to describe clinical and treatment characteristics of these children.
Method.
The study population included young children from a nationwide commercial claims database (2007–2017). We estimated annual antipsychotic use by age and sex, defined as the number of children dispensed an antipsychotic per year divided by the number enrolled. We described clinical diagnoses and mental health services utilization in those with prescription antipsychotic use in 2009 and 2017.
Results.
Annual antipsychotic use in young children was 0.27% in 2007, peaked at 0.29% in 2009, and statistically significantly declined to 0.17% by 2017 (linear trend: −0.017% per year, 95% CI:−0.018 to −0.016). Antipsychotic use was higher in boys than girls. A greater proportion of antipsychotic users received a mental disorder diagnosis in 2017 (89%) than 2009 (86%, p<.01). The most common clinical diagnoses in antipsychotic users, under a hierarchical classification, were pervasive developmental disorder (2009=27%, 2017=38%, p<.01), conduct or disruptive behavior disorder (2009=15%, 2017=21%, p<.01), and ADHD (2009=24%, 2017=18%, p<.01). Among 2017 antipsychotic users, 32% had 4+ psychotherapy claims, 43% had a psychiatrist visit, and the majority used another psychotropic medication, most commonly a stimulant (boys=57%, girls=50%).
Conclusion.
In privately insured young children, antipsychotic use declined from 2009 to 2017, with shifts towards indications with some supporting evidence. Nevertheless, a majority of use remains off-label and for conditions lacking effectiveness and safety data. Improving antipsychotic prescribing in young children remains a challenge.
Keywords: antipsychotic agents, child, trends, off-label use, drug utilization
Introduction
Antipsychotics are frequently prescribed to children and adolescents in the United States, with annual use estimated at 1.7% for children (0–17 years) insured by Medicaid in 2010 and 0.8% for privately insured children in 2013.1 Several antipsychotics are approved by the Food and Drug Administration (FDA) for schizophrenia, bipolar disorder, tic disorders, and severe irritability in autism spectrum disorders in children of various ages,2 and these drugs are prescribed off-label to children with conduct disorder, attention-deficit/hyperactivity disorder (ADHD), anxiety, and depression.3–6 Almost three-quarters of youth treated with antipsychotics in 2004 were diagnosed with conditions for which there was no FDA indication.5
Antipsychotic prescribing increased from late 1990s into the early 2000s in youth.5,7,8 The increase corresponded to rising use of antipsychotics for the treatment of ADHD, conduct disorders, and mood disorders.8,9 Even in preschool aged children (2–5 years), antipsychotic prescribing rates increased from 0.08% in 1999–2001 to 0.16% in 2007.10 Increases in antipsychotic prescribing have provoked concern given the paucity of evidence for effectiveness in several conditions for which they are prescribed as well as safety concerns, with treatment guidelines recommending caution in prescribing antipsychotics to young children.11–13 In youth, antipsychotic medication has been associated with risk of weight gain, sedation, diabetes, hyperlipidemia, cardiovascular effects, extrapyramidal side effects, and unexpected death.12,14–17 These concerns are especially salient in very young children in whom antipsychotics have unknown developmental and other long-term adverse effects.12,14,18
In an effort to curtail inappropriate antipsychotic prescribing to young people, states began to enact policy initiatives surrounding the prescribing of antipsychotics to young children insured by Medicaid. By 2014, 31 states had implemented prior authorization policies for atypical antipsychotic prescribing to children enrolled in Medicaid, with the majority of state policies adopted for children younger than 5, 6, or 7 years.19 Atypical antipsychotic prescribing to young children substantially declined in Medicaid in states that adopted peer review prior authorization policies.20,21 Other studies observed declines in antipsychotic prescribing to children enrolled in Medicaid into the early 2010s.22,23
Declines in annual antipsychotic medication use have also occurred in young, privately insured children from 0.16% (2008) to 0.11% (2010), including among young boys (0.24% to 0.16%) and girls (0.09% to 0.06%).6 A recent analysis observed declines in antipsychotic use in privately insured children under age 12 following the Medicaid antipsychotic prior authorization policies in nine US states, suggesting spillover effects to privately insured populations.24 It remains unknown whether trends in antipsychotic prescribing in very young, privately insured children stabilized, declined, or increased through 2017 following declines observed in the early 2010s.
Given pronounced sex differences in antipsychotic prescribing to young children, with more young boys receiving antipsychotic prescriptions than girls,6,9,25 and underlying variation in the prevalence of common psychiatric diagnoses among young antipsychotic users by sex,26 examining trends separately in boys and girls can inform efforts to improve antipsychotic prescribing. Therefore, we estimated trends and patterns in annual antipsychotic use in a national sample of privately insured US young children aged 2–7 years from 2007 to 2017 overall and separately for boys and girls and we described characteristics of young children recently prescribed antipsychotic medication. In light of Medicaid prior authorization policies and the American Psychiatric Association and the American Academy of Child and Adolescent Psychiatry urging caution when prescribing these medications to young children,11–13 we hypothesized that there would be an overall decline in the rate of antipsychotic medication use in young children during the study period.
Method
Data source and study population
We used MarketScan Commercial Claims and Encounters data from Truven Health Analytics from 2007 through 2017.27 This database covers individuals with employer-sponsored insurance and their dependents across the United States, with an overrepresentation of large employers.27 In recent years, the database covered over 40 million persons. The data were originally collected for administrative purposes and are constructed from patient-level claims for healthcare contacts, which are linked by a unique patient ID. That data includes patient-level details on insurance enrollment, outpatient and inpatient services, and records of dispensed prescriptions. Diagnostic codes (ICD-9-CM, ICD-10-CM) and procedures codes are available for inpatient and outpatient service visits. Racial and ethnic data are not unavailable in the dataset.
The study population included privately insured children aged 2–7 years. We identified antipsychotic prescriptions dispensed to children aged 2 to 7 years from 2007 to 2017. We included prescriptions for second-generation antipsychotics (risperidone, aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, ziprasidone) and first-generation antipsychotics (chlorpromazine, chlorprothixene, fluphenazine, haloperidol, loxapine, mesoridazine, molindone, perphenazine, pimozide, promazine, thioridazine, thiothixene, trifluoperazine, triflupromazine).
Estimating annual use
In calculating the annual use of antipsychotic prescriptions in young children, the numerator was defined as the number of children filling at least one antipsychotic prescription during that calendar year. The denominator was defined as the total number of children in the target age group enrolled in a plan covered by the database in July of that year with prescription drug coverage. Annually, the number of children aged 2–7 years with prescription drug coverage and enrolled in July ranged from 1.4 to 2.8 million across the study period. Using the number of total enrollees on July approximates the number of subjects eligible to contribute to the numerator that year, without requiring periods of continuous insurance enrollment. This approach has been used in prior trend analyses of claims data.28
For a sensitivity analysis, we estimated annual antipsychotic use among children with continuous insurance enrollment with prescription drug coverage. The denominator in this analysis included children with continuous insurance enrollment for the full calendar year and the numerator included the children who also had an antipsychotic prescription that year.
Prescription details and child characteristics
We examined antipsychotic prescription details including the agent, days supply, quantity, and, for common antipsychotic agents, the dose-per-day. For children prescribed antipsychotics, we identified patient characteristics from inpatient and outpatient records and records of dispensed prescriptions from any point in that calendar year. These included sex, geographical area, mental health diagnoses, psychotropic medication prescriptions, and mental health services: psychotherapy visits, visit with a psychiatrist or other mental health provider, psychiatric-related emergency department visit, or psychiatric-related (primary diagnosis) inpatient admission.
To discern reasons for which antipsychotics were prescribed to young children, children were assigned to diagnostic categories using a hierarchical, mutually exclusive, classification system similar to that used in previous research of child antipsychotic use.1,5 The hierarchy began with conditions that have a stronger clinical rationale for antipsychotic medication use. Each child was assigned the highest-listed diagnostic group for which he or she had a diagnosis code in that calendar year. For example, children with a pervasive developmental disorder (PDD) diagnosis and a conduct disorder diagnosis in 2017 were classified under PDD. Definitions of mental health diagnoses appear in Table S1, available online; the category “PDD included diagnoses for intellectual disabilities.
Analysis
We estimated annual antipsychotic use by age (2–3; 4–5; 6–7 years) and sex from 2007 to 2017. In the primary analysis, trends were calculated by dividing the number of persons dispensed at least one antipsychotic medication during that study year by the total number of the target group enrolled with prescription coverage in July of that year. We estimated the slope of antipsychotic use from the observed peak (highest annual use) in 2009 through 2017, using linear regression and associated 95% confidence intervals (CI). Estimates of antipsychotic use were standardized across geographical divisions. Standardization accounts for geographical shifts in the MarketScan base population to prevent trends from being influenced by these shifts. For a sensitivity analysis, annual antipsychotic use estimates were calculated among children with continuous insurance enrollment for that calendar year.
We estimated the number of antipsychotic prescription fills per child for each calendar year. We described the diagnoses, medication, and mental health services use in the subset of young children receiving antipsychotic medication in 2017, the most recent year available, and in 2009, the highest annual antipsychotic use in young children. For a sensitivity analyses, we examined characteristics of antipsychotic users in 2009 and 2017 with at least 9 months enrollment in that calendar year.
Results
Trends in antipsychotic use in young children
In privately insured young children (2–7 years), prescription antipsychotic use was 0.27% (27 per 10,000 children) in 2007, was highest in 2009 at 0.29% and declined to 0.17% by 2017 (Table 1). The linear trend of antipsychotic use from 2009 to 2017 declined by 0.017% per year (95% CI: −0.018 to −0.016, p<0.01).
Table 1.
Annual Prescription Antipsychotic Use in Privately Insured Young Children (2–7 Years), 2007–2017
| Year | Persons with antipsychotic prescription | Antipsychotic usea,b | Antipsychotic use standardized by divisionc | ||
|---|---|---|---|---|---|
| Total | Boys | Girls | |||
| 2007 | 3,778 | 0.27% | 0.26% | 0.40% | 0.12% |
| 2008 | 7,368 | 0.29% | 0.29% | 0.44% | 0.13% |
| 2009 | 8,035 | 0.29% | 0.29% | 0.44% | 0.13% |
| 2010 | 6,960 | 0.27% | 0.27% | 0.41% | 0.12% |
| 2011 | 7,182 | 0.26% | 0.26% | 0.39% | 0.12% |
| 2012 | 7,067 | 0.26% | 0.26% | 0.39% | 0.12% |
| 2013 | 4,981 | 0.23% | 0.24% | 0.37% | 0.11% |
| 2014 | 4,322 | 0.20% | 0.21% | 0.31% | 0.10% |
| 2015 | 2,964 | 0.18% | 0.19% | 0.28% | 0.09% |
| 2016 | 2,771 | 0.17% | 0.17% | 0.26% | 0.08% |
| 2017 | 2,501 | 0.17% | 0.16% | 0.25% | 0.08% |
Note:
Denominator: Count of children aged 2–7 years enrolled in a covered insurance plan with prescription drug coverage in July of that year
Antipsychotic prevalence was highest in 2009. Prevalence overall, and stratified in boys and girls, was not statistically significantly higher in 2009 than in 2008; antipsychotic prevalence in 2009 was significantly (p<0.01) higher than in 2010. The linear trend of antipsychotic use from 2009 to 2017 declined by 0.017% (95% CI: −0.018 to −0.016, p<0.01) per year.
Antipsychotic use estimates standardized by geographical division (reference year = 2011, unknown division excluded); categories included: New England, Middle Atlantic, South Atlantic, East North Central, East South Central, West North Central, West South Central, Mountain, or Pacific
Trends were similar across age and sex categories, with higher antipsychotic use in boys than girls and with older age (Figure 1). Antipsychotic use was highest in boys aged 6–7 years, rising from 2007 (0.85%) to peak in 2009 (1.01%) and declining through 2017 (0.59%), an absolute change of −0.42% from 2009 to 2017 and linear trend of −0.058% (95% CI: −0.061 to −0.054) per year from 2009 to 2017. In girls aged 6–7 years, antipsychotic use was 0.27% in 2007, peaked at 0.30% in 2009 and declined to 0.18%, a linear trend of −0.015% (95% CI: −0.017 to −0.013) per year from 2009 to 2017. Boys aged 2–3 years had the greatest relative change in antipsychotic use (0.07% in 2007 to 0.03% in 2017, p<0.01).
Figure 1. Antipsychotic Use in Privately Insured Young Children (2–7 Years) by Age and Sex.
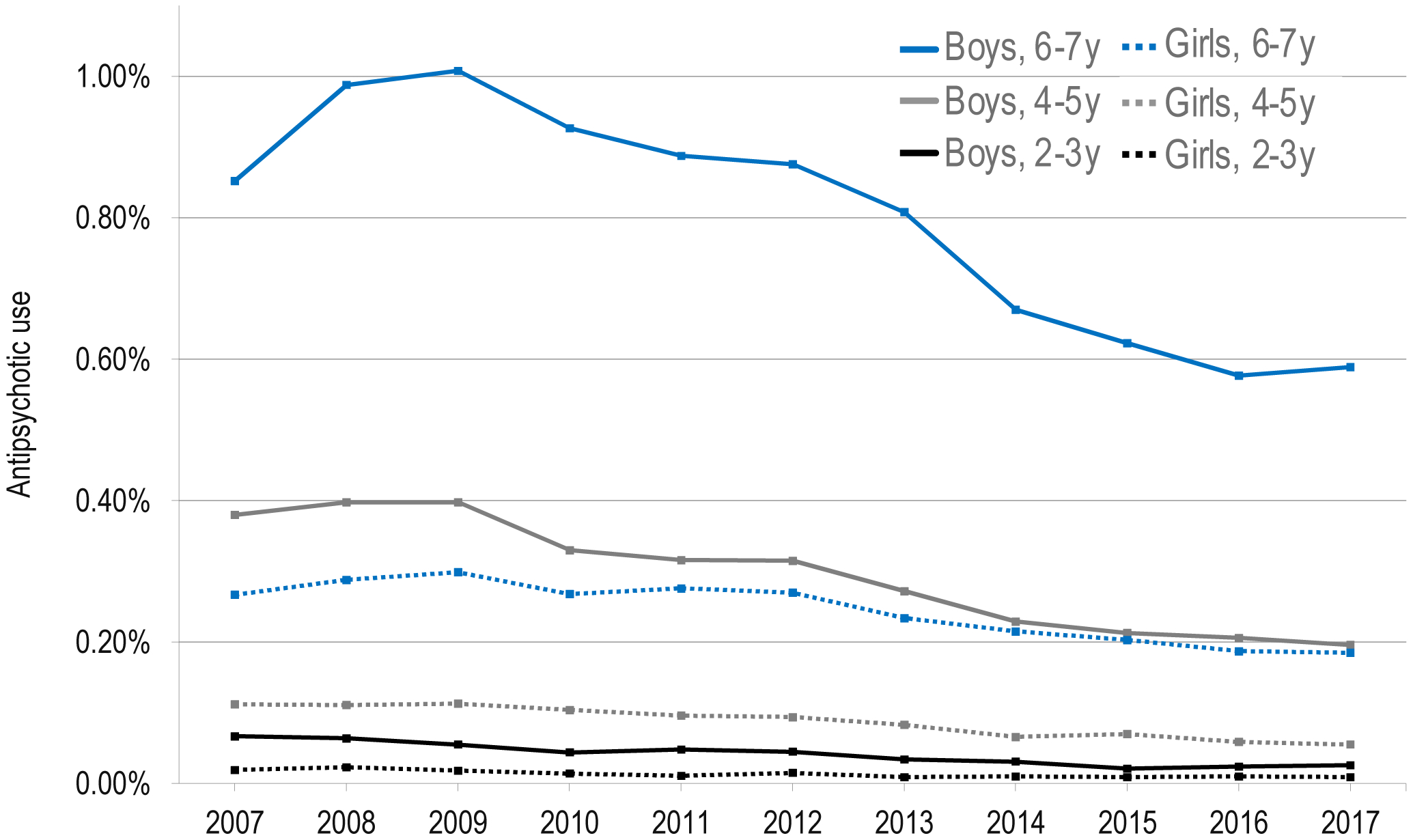
Note: Denominator is the count of children aged 2–7 years enrolled in July of that year in a plan with prescription drug coverage
Trends and annual antipsychotic use estimates were consistent when standardized by geographical division (Table 1) and when restricting the sample to children with continuous insurance enrollment for the entire calendar year (Table S2, available online).
Antipsychotic prescription details
Between 2007 and 2017, there were 301,311 antipsychotic prescriptions filled for children aged 2–7 years; the majority were risperidone (69%) or aripiprazole (20%) (Table S3, available online). The median number of fills for each child was consistent across years at 4 fills/year. Prescriptions were most commonly dispensed with a 30-day supply and the median dose-per-day for prescriptions dispensed in tablet form was 0.75 mg/day for risperidone and 5.0 mg/day for aripiprazole.
Diagnoses associated with antipsychotic medication
We identified 2,501 young children (2–7 years) who filled an antipsychotic prescription in 2017 and 8,035 in 2009. In 2017, under the hierarchical classification, the most common diagnoses were PDD (38%), conduct or disruptive behavior disorder (21%), or ADHD (18%) (Table 2). Among children treated with antipsychotics, diagnoses were similar by sex, with slightly more PDD diagnoses (39% vs. 33%, p=0.01) and proportionately fewer depression and anxiety disorder diagnoses (p<0.01) in boys.
Table 2.
Mental Health Diagnoses of Young Children (2–7 Years) Filling Antipsychotic Prescriptions in 2009 or 2017
| 2009 antipsychotic users No. (column %) | 2017 antipsychotic users No. (column %) | Total 2017 vs. 2009 P | ||||||
|---|---|---|---|---|---|---|---|---|
| Total n=8,035 | Boys n=6,256 | Girls n=1,779 | Total n=2,501 | Boys n=1,929 | Girls n=572 | Girl vs. boy p-value | ||
| Diagnostic group, hierarchical classification | ||||||||
| Schizophrenia, psychotic-related disorder (1) | 119 (1.5) | 91 (1.5) | 28 (1.6) | 42 (1.7) | 29 (1.5) | 13 (2.3) | 0.21 | 0.48 |
| PDD; Intellectual disability (2) | 2,185 (27.2) | 1,853 (29.6) | 332 (18.7) | 940 (37.6) | 751 (38.9) | 189 (33.0) | 0.01 | <0.01 |
| Bipolar disorder (3) | 773 (9.6) | 556 (8.9) | 217 (12.2) | 119 (4.8) | 84 (4.4) | 35 (6.1) | 0.08 | <0.01 |
| Conduct or disruptive behavior disorder, no ADHD (4) | 415 (5.2) | 293 (4.7) | 122 (6.9) | 92 (3.7) | 64 (3.3) | 28 (4.9) | 0.08 | <0.01 |
| Conduct or disruptive behavior disorder and ADHD (5) | 819 (10.2) | 643 (10.3) | 176 (9.9) | 431 (17.2) | 352 (18.2) | 79 (13.8) | 0.01 | <0.01 |
| ADHD (6) | 1,946 (24.2) | 1,543 (24.7) | 403 (22.7) | 440 (17.6) | 333 (17.3) | 107 (18.7) | 0.43 | <0.01 |
| Depression, anxiety, adjustment disorder (7)a | 336 (4.2) | 209 (3.3) | 127 (7.1) | 85 (3.4) | 53 (2.7) | 32 (5.6) | <0.01 | 0.08 |
| Other, unspecified mood disorder (8) | 158 (2.0) | 110 (1.8) | 48 (2.7) | (1.7)b | (1.8)b | (1.6)b | 0.76 | 0.43 |
| Other mental health diagnosis (9) | 181 (2.3) | 123 (2.0) | 58 (3.3) | 44 (1.8) | 27 (1.4) | 17 (3.0) | 0.01 | 0.14 |
| No mental health diagnosis (10) | 1,103 (13.7) | 835 (13.3) | 268 (15.1) | 265 (10.6) | 202 (10.5) | 63 (11.0) | 0.71 | <0.01 |
| Any mental health diagnosis | 6,932 (86.3) | 5,421 (86.7) | 1,511 (84.9) | 2,236 (89.4) | 1,727 (89.5) | 509 (89.0) | 0.71 | <0.01 |
Note: ADHD = attention-deficit/hyperactivity disorder; PDD = pervasive developmental disorder
Includes post-traumatic stress disorder (PTSD) and obsessive–compulsive disorder (OCD)
Numbers not displayed due to low cell count in one of the strata
Comparing young children prescribed antipsychotics in 2009 vs. 2017, a significantly higher proportion of antipsychotic users in 2017 had a PDD diagnosis or a conduct or disruptive behavior disorder diagnosis. The proportion initiating antipsychotic medication for ADHD, depression, anxiety, adjustment disorder, unspecified mood disorder, or the residual “other mental disorder” group declined from 33% in 2009 users to 24% in 2017 users (p<0.01). A greater proportion of young children with antipsychotic use received a mental disorder diagnosis in 2017 (89%) than 2009 (86%, p<.01). Clinical diagnoses were consistent when restricting to children with at least 9 months of insurance enrollment (Table S4, available online).
Characteristics of antipsychotic users
A majority of boys and girls receiving antipsychotics filled a prescription for another class of psychotropic medications in the same year (Table 2). In 2017, the most commonly prescribed other psychotropic medications were stimulants (57% boys, 50% girls), clonidine or guanfacine (55% boys, 52% girls), and antidepressants (31% boys, 35% girls). Estimates considering non-mutually exclusive mental health diagnoses highlight comorbidity in young children receiving antipsychotics (Table S5, available online). Among children treated with antipsychotics in 2017, 65% had an ADHD diagnosis as a primary or comorbid condition and 26% had an anxiety disorder, 17% a sleep disorder, and 7% a depression diagnosis as a primary or comorbid condition, with some variation by sex.
Mental health service use was similar among boys and girls who received antipsychotic medication, with 43% having a recorded visit with a psychiatrist and 46% having at least one psychotherapy claim in 2017 (Table 2). One-third (32%) had 4+ psychotherapy visits and 17% had 10+ visits in 2017, compared to 29% (p<0.01) and 14% (p<0.01), respectively, of antipsychotic users in 2009. Of those with a psychotherapy visit, the median number of visits was 6 visits (interquartile range: 3–14) in 2017 compared to 5 visits (interquartile range: 2–11) in 2009. Approximately one fourth (26%) of antipsychotic users without psychotherapy and without a visit with a mental health provider had no mental health diagnosis recorded compared with only 3% in antipsychotic users who had psychotherapy or contact with a mental health provider (p<0.01).
Discussion
From 2007 to 2017 there was an overall decline in antipsychotic prescribing rates in privately insured children aged 2 to 7 years, with prescribing highest in 2009 and then declining through 2017. This decline occurred in boys and girls and across age groups and may reflect a trend towards more cautious prescribing. Along with a decline in antipsychotic prescribing, there were shifts in the most common indications for antipsychotic medications; a higher proportion of recent antipsychotic users had a diagnosis with some clinical evidence. Nevertheless, most use in this very young population remains off-label for conditions lacking effectiveness and safety data and only about half of young children treated with antipsychotics received psychotherapy and a similar proportion had contact with psychiatrist.
Declining use of antipsychotic medications in privately insured young children mirrors, and extends, declines observed in other populations. Studies of very young US children observed antipsychotic use declining after peaking in late 2000s.1,6,23 In one state’s Medicaid program, for example, antipsychotic use among very young children (0–5 years) declined from 0.91% (2008) to 0.38% (2013)22 and in another state from 0.16% (2006) to 0.02% (2012) in children aged 0–4 years.23
These changes may reflect the cumulative impact of provider-education and prior-authorization initiatives. Policy initiatives to improve antipsychotic prescribing in US children included state peer-review authorization programs for children insured by Medicaid.20,21 While these initiatives are not targeted to privately insured children, many providers treat children across insurance types and prior authorization programs have been associated with reductions in antipsychotic prescribing in privately insured children.24 Similarly, in other medical contexts, Medicaid prescribing policies had spillover effects on prescribing practices for privately insured patients.29,30 Additionally, guidance from organizations urging caution prescribing antipsychotics to young children and providing quality measures11–13,31 may have contributed to the declines we observed.
Consistent with prior research, PDD, conduct or disruptive behavior disorder, and ADHD were the most common diagnoses in boys and girls receiving antipsychotics.6,10,22 A minority of antipsychotics were prescribed for psychotic disorders,26 with a decline in the proportion of recent antipsychotic users with bipolar disorder. PDD was the most common indication and accounted for a larger percentage of the antipsychotic prescribing in recent years. There is some evidence supporting use of antipsychotics in young children with PDD or intellectual disabilities for target symptoms or comorbid conditions32,33 with some antipsychotics (risperidone and aripiprazole) having FDA approval for irritability associated with autism spectrum disorders for children as young as 5 years. In children with autism, pharmacotherapies, such as antipsychotics, may increase the benefits that children receive from behavioral and educational interventions.33
By contrast, antipsychotics are not FDA approved for conduct disorders or ADHD. Despite continued prescribing, there is limited evidence for the efficacy of antipsychotics for conduct or disruptive behavior disorders in very young children and long-term outcomes remain poorly understood.34–36 In a recent Cochrane review, for example, risperidone was associated with a reduction in conduct problems in youth with disruptive disorders compared to placebo; however, there were no studies with children under age 5 and many side effects were not evaluated.34
The proportion of antipsychotic users initiating treatment with ADHD but no higher-listed diagnosis in the hierarchical classification was lower in 2017 (18%) than in 2009 (24%) and in earlier estimates.10 While antipsychotics continued to be prescribed to children for ADHD, including often prescribed concurrently with stimulants,37 the evidence of efficacy for antipsychotics in pediatric ADHD is limited and not established.3,38 Clinical practice guidelines for the treatment of pediatric ADHD suggest that adjunctive medication therapy may be considered when stimulants are not fully effective.39 However, guidelines recommend medications such as extended-release guanfacine and clonidine40 and atomoxetine;41 antipsychotics are not recommended.39
Guidelines recommend careful assessment before children initiate antipsychotics and recommend psychosocial services before antipsychotic treatment or combining pharmacological and psychosocial treatments when possible.11,13,36,42 Yet, fewer than half of young children receiving antipsychotic treatment had a visit with a psychiatrist or a psychotherapy claim, a finding consistent with reports in privately insured children from a decade earlier.10 In Medicaid-enrolled young children (0 to 5 years), 62% of young children on antipsychotics were prescribed an antipsychotic by a psychiatrist22 and only 39% received a psychosocial service before starting antipsychotic treatment.43
The low rate of use of safer first-line psychosocial treatments potentially puts children at unnecessary risks associated with antipsychotic treatment.31 Parent-child interaction therapy has shown positive outcomes for young children with externalizing behaviors problems.44 Trials of parent management training and cognitive-behavioral therapy and other psychosocial interventions such as school-based social skills training have also demonstrated success for children with disruptive behaviors and in reducing aggression.42,45 In older children, treatments such as multisystemic therapy have been successful in improving externalizing behaviors.46 Increasing accessibility to safer, evidence-based psychosocial interventions may reduce the need for antipsychotic medications in young children.
There is a substantial burden of comorbid mental health problems in young children treated with antipsychotic medications26 and many receive other psychotropic prescriptions,6,10 raising potential concerns over polypharmacy. Consistent with prior research, pharmaceutical treatments for ADHD were common in young children prescribed antipsychotics6 and mood disorders were more common in girls and PDD was more common in boys.26 In the case of comorbidity, we cannot determine the condition or conditions for which antipsychotics were prescribed.
Given that young children continue to be prescribed antipsychotics, research on their effectiveness and safety in young children, particularly long-term effects, is essential.2,12,18,34 Even for indications in which antipsychotics have demonstrated efficacy in randomized trial settings, evidence for safety and effectiveness in the youngest children is scarce. Pharmacoepidemiological research with observational data could inform benefit-harm evaluations; comparative effectiveness and safety studies will be particularly valuable in informing decisions made in clinical practice.18,47 Further, given scant evidence on heterogeneity in antipsychotic efficacy and safety across populations,3 this will be another important avenue of research to guide treatment decisions.
Limitations of the study should be considered. This study examined trends in privately insured children with prescription drug coverage; our findings likely do not generalize to uninsured children or children enrolled in Medicaid. The datasource over-represents large-employers and may not generalize to all individuals with private insurance; we did not require periods of continuous enrollment in our primary analysis, as doing so would have restricted the sample to children in households with more stable employment. Antipsychotic prescribing likely varies across regions in the US. Diagnoses were based on diagnostic codes recorded anytime in that calendar year; diagnoses are not validated against structured psychiatric interviews and we cannot determine the intended indication of antipsychotic treatment. Further, we cannot determine the clinical appropriateness or effectiveness of the antipsychotic treatment. Variation in diagnoses between 2009 and 2017 could be related to the shift from ICD-9-CM to ICD-10-CM coding; additionally, increases in diagnosed comorbidities may be due to changes in diagnostic and recording practices. We do not distinguish between new and continuing antipsychotic use and we do not account for the temporal ordering of antipsychotic prescriptions and diagnoses, medication, or mental health services use. We may underestimate mental illnesses in the population as our estimates are limited to those diagnosed and recorded. Mental health services not covered by insurance, such as services paid for out-of-pocket, are not captured in the database. In our examination of other prescription medications, we cannot identify the indication for use; some medications of interest may have been used for non-psychiatric disorders (e.g., anticonvulsants for seizure disorders). We cannot reliably distinguish between child and adolescent psychiatrists from other psychiatrists and we lacked prescriptions dispensed in hospital settings, although our focus was on community treatment.
Lastly, data were unavailable on race and ethnicity in this private insurance dataset, an area that should be addressed in future research as few studies have examined antipsychotic prescribing trends by race and ethnicity in young children. In non-foster care children (<18 years) enrolled in Medicaid, the antipsychotic prescribing rate was higher in white children (2.5%) compared to Black (1.3%) and Hispanic (0.4%) children.1 This is similar to other studies of children enrolled in Medicaid.7,48 In children diagnosed with schizophrenia, slightly more Black than white children were prescribed antipsychotics49 and in a privately insured population with recent-onset psychosis, antipsychotic use was similar across racial-ethnic groups with higher outpatient mental health services in white youth.50 Efforts to examine antipsychotic prescribing trends by race and ethnicity in young children are needed to identify disparities and improve antipsychotic prescribing.
Overall, the prescribing of antipsychotic medications declined in the past decade among very young boys and girls who are privately insured, which may reflect a trend toward more-cautious prescribing. Results suggest broad shifts away from prescribing antipsychotics for conditions with less supporting clinical evidence in young children along with slight increases in psychotherapy use among young children prescribed antipsychotic treatment. Despite these encouraging trends, however, much antipsychotic use in young children continues to take place in children diagnosed only with conditions lacking effectiveness and safety data. These findings, and the remaining substantial number of children treated with antipsychotics who do not receive psychosocial mental health interventions, suggest that there remains room for improvement in the community treatment of young children with antipsychotic medications.
Supplementary Material
Table 3.
Medication Use and Mental Health Services in Young Children (2–7 Years) Filling Antipsychotic Prescriptions in 2017
| 2009 antipsychotic users No. (column %) | 2017 antipsychotic users No. (column %) | Total 2017 vs. 2009 P | ||||||
|---|---|---|---|---|---|---|---|---|
| Total n=8,035 | Boys n=6,256 | Girls n=1,779 | Total n=2,501 | Boys n=1,929 | Girls n=572 | Girl vs. boy p-value | ||
| Prescription psychotropic use, year | 6,564 (81.7) | 5,139 (82.1) | 1,425 (80.1) | 2,162 (86.4) | 1,680 (87.1) | 482 (84.3) | 0.08 | <0.01 |
| Stimulant | 4,545 (56.6) | 3,656 (58.4) | 889 (50.0) | 1,375 (55.0) | 1,090 (56.5) | 285 (49.8) | <0.01 | 0.16 |
| Clonidine/guanfacine | 2,539 (31.6) | 2,015 (32.2) | 524 (29.5) | 1,363 (54.5) | 1,067 (55.3) | 296 (51.7) | 0.13 | <0.01 |
| Antidepressant | 1,979 (24.6) | 1,480 (23.7) | 499 (28.0) | 788 (31.5) | 589 (30.5) | 199 (34.8) | 0.05 | <0.01 |
| Anticonvulsant | 1,355 (16.9) | 1,027 (16.4) | 328 (18.4) | 396 (15.8) | 299 (15.5) | 97 (17.0) | 0.40 | 0.23 |
| Atomoxetine | 761 (9.5) | 605 (9.7) | 156 (8.8) | 171 (6.8) | 134 (6.9) | 37 (6.5) | 0.69 | <0.01 |
| Hydroxyzine | 213 (2.7) | 161 (2.6) | 52 (2.9) | 165 (6.6) | 124 (6.4) | 41 (7.2) | 0.53 | <0.01 |
| Benzodiazepine | 304 (3.8) | 211 (3.4) | 93 (5.2) | 147 (5.9) | 104 (5.4) | 43 (7.5) | 0.06 | <0.01 |
| Other anxiolytic, sedative | 124 (1.5) | 92 (1.5) | 32 (1.8) | 67 (2.7) | 53 (2.7) | 14 (2.4) | 0.70 | <0.01 |
| Lithium | 155 (1.9) | 117 (1.9) | 38 (2.1) | 37 (1.5) | 26 (1.3) | 11 (1.9) | 0.32 | 0.14 |
| Mental health services, year | ||||||||
| Psychotherapy claim | 3,546 (44.1) | 2,725 (43.6) | 821 (46.1) | 1,146 (45.8) | 872 (45.2) | 274 (47.9) | 0.26 | 0.14 |
| Visit with mental health provider | 4,128 (51.4) | 3,211 (51.3) | 917 (51.5) | 1,494 (59.7) | 1,153 (59.8) | 341 (59.6) | 0.95 | <0.01 |
| Psychiatrist | 3,400 (42.3) | 2,669 (42.7) | 731 (41.1) | 1,074 (42.9) | 824 (42.7) | 250 (43.7) | 0.67 | 0.58 |
| Other mental health provider | 2,179 (27.1) | 1,644 (26.3) | 535 (30.1) | 971 (38.8) | 754 (39.1) | 217 (37.9) | 0.62 | <0.01 |
| Inpatient psychiatric visita | 351 (4.4) | 285 (4.6) | 66 (3.7) | 129 (5.2) | 100 (5.2) | 29 (5.1) | 0.91 | 0.10 |
| ED visit, psychiatric-related | 722 (9.0) | 578 (9.2) | 144 (8.1) | 303 (12.1) | 229 (11.9) | 74 (12.9) | 0.49 | <0.01 |
Note: ED = emergency department
Inpatient admission with primary diagnosis for psychiatric-related diagnosis
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health under Award Number T32MH013043, the Patient-Centered Outcomes Research Institute IHS-1409-23194, the Agency for Healthcare Research and Quality R01HS026001 and 1R18HS023258, and the National Institutes of Health (NIH) R01DA047347 and UL1TR003017. This content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Financial Disclosures. Dr. Bushnell received support from the National Institute of Mental Health under Award Number T32MH013043. The authors have no conflicts of interest relevant to this article to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Bushnell, Crystal, and Olfson have reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Crystal S, Mackie T, Fenton MC, et al. Rapid Growth Of Antipsychotic Prescriptions For Children Who Are Publicly Insured Has Ceased, But Concerns Remain. Health Aff (Millwood). 2016;35(6):974–982. [DOI] [PubMed] [Google Scholar]
- 2.Christian R, Saavedra L, Gaynes BN, et al. Future Research Needs for First- and Second-Generation Antipsychotics for Children and Young Adults. Rockville (MD)2012. [PubMed] [Google Scholar]
- 3.Maglione M, Maher AR, Hu J, et al. Off-Label Use of Atypical Antipsychotics: An Update. Rockville (MD)2011. [PubMed] [Google Scholar]
- 4.Centers for Medicare & Medicaid Services (CMS) Medicaid Integrity Group (MIG). Atypical Antipsychotic Medications: Use in Pediatric Patients. 2015. [Google Scholar]
- 5.Crystal S, Olfson M, Huang C, Pincus H, Gerhard T. Broadened use of atypical antipsychotics: safety, effectiveness, and policy challenges. Health Aff (Millwood). 2009;28(5):w770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olfson M, King M, Schoenbaum M. Treatment of Young People With Antipsychotic Medications in the United States. JAMA Psychiatry. 2015;72(9):867–874. [DOI] [PubMed] [Google Scholar]
- 7.Zito JM, Burcu M, Ibe A, Safer DJ, Magder LS. Antipsychotic use by medicaid-insured youths: impact of eligibility and psychiatric diagnosis across a decade. Psychiatr Serv. 2013;64(3):223–229. [DOI] [PubMed] [Google Scholar]
- 8.Cooper WO, Hickson GB, Fuchs C, Arbogast PG, Ray WA. New users of antipsychotic medications among children enrolled in TennCare. Arch Pediatr Adolesc Med. 2004;158(8):753–759. [DOI] [PubMed] [Google Scholar]
- 9.Patten SB, Waheed W, Bresee L. A review of pharmacoepidemiologic studies of antipsychotic use in children and adolescents. Can J Psychiatry. 2012;57(12):717–721. [DOI] [PubMed] [Google Scholar]
- 10.Olfson M, Crystal S, Huang C, Gerhard T. Trends in antipsychotic drug use by very young, privately insured children. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(1):13–23. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Choosing Wisely: Five Things Physicians and Patients Should Question. 2015. [Google Scholar]
- 12.Findling RL, Drury SS, Jensen PS, Rapoport JL, AACAP Committee on Quality Issues. Practice Parameter for the Use of Atypical Antipsychotic Medications in Children and Adolescents. 2011; https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_Antipsychotic_Medications_Web.pdf. [Google Scholar]
- 13.Kealey E, Scholle SH, Byron SC, et al. Quality concerns in antipsychotic prescribing for youth: a review of treatment guidelines. Acad Pediatr. 2014;14(5 Suppl):S68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen PS, Buitelaar J, Pandina GJ, Binder C, Haas M. Management of psychiatric disorders in children and adolescents with atypical antipsychotics: a systematic review of published clinical trials. Eur Child Adolesc Psychiatry. 2007;16(2):104–120. [DOI] [PubMed] [Google Scholar]
- 15.Ray WA, Stein CM, Murray KT, et al. Association of Antipsychotic Treatment With Risk of Unexpected Death Among Children and Youths. JAMA Psychiatry. 2019;76(2):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solmi M, Fornaro M, Ostinelli EG, et al. Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry. 2020;19(2):214–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galling B, Roldan A, Nielsen RE, et al. Type 2 Diabetes Mellitus in Youth Exposed to Antipsychotics: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2016;73(3):247–259. [DOI] [PubMed] [Google Scholar]
- 18.Seida JC, Schouten JR, Boylan K, et al. Antipsychotics for children and young adults: a comparative effectiveness review. Pediatrics. 2012;129(3):e771–784. [DOI] [PubMed] [Google Scholar]
- 19.Schmid I, Burcu M, Zito JM. Medicaid prior authorization policies for pediatric use of antipsychotic medications. JAMA. 2015;313(9):966–968. [DOI] [PubMed] [Google Scholar]
- 20.Zito JM, Burcu M, McKean S, Warnock R, Kelman J. Pediatric Use of Antipsychotic Medications Before and After Medicaid Peer Review Implementation. JAMA Psychiatry. 2018;75(1):100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennap D, Burcu M, Safer DJ, Zito JM. The Impact of a State Medicaid Peer-Review Authorization Program on Pediatric Use of Antipsychotic Medications. Psychiatr Serv. 2018;69(3):293–299. [DOI] [PubMed] [Google Scholar]
- 22.Edelsohn GA, Karpov I, Parthasarathy M, et al. Trends in Antipsychotic Prescribing in Medicaid-Eligible Youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2017;56(1):59–66. [DOI] [PubMed] [Google Scholar]
- 23.Kalverdijk LJ, Bachmann CJ, Aagaard L, et al. A multi-national comparison of antipsychotic drug use in children and adolescents, 2005–2012. Child Adolesc Psychiatry Ment Health. 2017;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spence O, Reeves G, dosReis S. Spillover effects of state medicaid antipsychotic prior authorization policies in US commercially insured youth. Pharmacoepidemiol Drug Saf. 2020. [DOI] [PubMed] [Google Scholar]
- 25.Curtis LH, Masselink LE, Ostbye T, et al. Prevalence of atypical antipsychotic drug use among commercially insured youths in the United States. Arch Pediatr Adolesc Med. 2005;159(4):362–366. [DOI] [PubMed] [Google Scholar]
- 26.Penfold RB, Stewart C, Hunkeler EM, et al. Use of antipsychotic medications in pediatric populations: what do the data say? Curr Psychiatry Rep. 2013;15(12):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.IBM Watson Health™. IBM MarketScan Research Databases for Health Services Researchers. White Paper 2019; https://www.ibm.com/downloads/cas/6KNYVVQ2. [Google Scholar]
- 28.Raman SR, Man KKC, Bahmanyar S, et al. Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet Psychiatry. 2018;5(10):824–835. [DOI] [PubMed] [Google Scholar]
- 29.Wang YR, Pauly MV, Lin YA. Impact of Maine’s Medicaid drug formulary change on non-Medicaid markets: spillover effects of a restrictive drug formulary. Am J Manag Care. 2003;9(10):686–696. [PubMed] [Google Scholar]
- 30.Virabhak S, Shinogle JA. Physicians’ prescribing responses to a restricted formulary: the impact of Medicaid preferred drug lists in Illinois and Louisiana. Am J Manag Care. 2005;11 Spec No:SP14–20. [PubMed] [Google Scholar]
- 31.Healthcare Effectiveness Data and Information Set (HEDIS). Use of First-Line Psychosocial Care for Children and Adolescents on Antipsychotics (APP). HEDIS Measures and Technical Resources 2020; https://www.ncqa.org/hedis/measures/use-of-first-line-psychosocial-care-for-children-and-adolescents-on-anti-psychotics/. [Google Scholar]
- 32.Siegel M, McGuire K, Veenstra-VanderWeele J, Stratigos K, King B, American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI). Practice Parameter for the Assessment and Treatment of Psychiatric Disorders in Children and Adolescents with Intellectual Disability (Intellectual Developmental Disorder). Journal of the American Academy of Child and Adolescent Psychiatry. 2020;59(4):468–496. [DOI] [PubMed] [Google Scholar]
- 33.Volkmar F, Siegel M, Woodbury-Smith M, et al. Practice parameter for the assessment and treatment of children and adolescents with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(2):237–257. [DOI] [PubMed] [Google Scholar]
- 34.Loy JH, Merry SN, Hetrick SE, Stasiak K. Atypical antipsychotics for disruptive behaviour disorders in children and youths. The Cochrane database of systematic reviews. 2017;8:CD008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKinney C, Renk K. Atypical antipsychotic medications in the management of disruptive behaviors in children: safety guidelines and recommendations. Clin Psychol Rev. 2011;31(3):465–471. [DOI] [PubMed] [Google Scholar]
- 36.Scotto Rosato N, Correll CU, Pappadopulos E, et al. Treatment of maladaptive aggression in youth: CERT guidelines II. Treatments and ongoing management. Pediatrics. 2012;129(6):e1577–1586. [DOI] [PubMed] [Google Scholar]
- 37.Kamble P, Chen H, Johnson ML, Bhatara V, Aparasu RR. Concurrent use of stimulants and second-generation antipsychotics among children with ADHD enrolled in Medicaid. Psychiatr Serv. 2015;66(4):404–410. [DOI] [PubMed] [Google Scholar]
- 38.Catala-Lopez F, Hutton B, Nunez-Beltran A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS One. 2017;12(7):e0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolraich ML, Hagan JF Jr., Allan C, et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics. 2019;144(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirota T, Schwartz S, Correll CU. Alpha-2 agonists for attention-deficit/hyperactivity disorder in youth: a systematic review and meta-analysis of monotherapy and add-on trials to stimulant therapy. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(2):153–173. [DOI] [PubMed] [Google Scholar]
- 41.Treuer T, Gau SS, Mendez L, et al. A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability. J Child Adolesc Psychopharmacol. 2013;23(3):179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knapp P, Chait A, Pappadopulos E, Crystal S, Jensen PS, Group TMS. Treatment of maladaptive aggression in youth: CERT guidelines I. Engagement, assessment, and management. Pediatrics. 2012;129(6):e1562–1576. [DOI] [PubMed] [Google Scholar]
- 43.Finnerty M, Neese-Todd S, Pritam R, et al. Access to Psychosocial Services Prior to Starting Antipsychotic Treatment Among Medicaid-Insured Youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2016;55(1):69–76 e63. [DOI] [PubMed] [Google Scholar]
- 44.Thomas R, Abell B, Webb HJ, Avdagic E, Zimmer-Gembeck MJ. Parent-Child Interaction Therapy: A Meta-analysis. Pediatrics. 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 45.Sukhodolsky DG, Smith SD, McCauley SA, Ibrahim K, Piasecka JB. Behavioral Interventions for Anger, Irritability, and Aggression in Children and Adolescents. J Child Adolesc Psychopharmacol. 2016;26(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zajac K, Randall J, Swenson CC. Multisystemic Therapy for Externalizing Youth. Child Adolesc Psychiatr Clin N Am. 2015;24(3):601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birnbaum ML, Saito E, Gerhard T, et al. Pharmacoepidemiology of antipsychotic use in youth with ADHD: trends and clinical implications. Curr Psychiatry Rep. 2013;15(8):382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cataife G, Weinberg DA. Racial and Ethnic Differences in Antipsychotic Medication Use Among Children Enrolled in Medicaid. Psychiatr Serv. 2015;66(9):946–951. [DOI] [PubMed] [Google Scholar]
- 49.Sleath B, Domino ME, Wiley-Exley E, Martin B, Richards S, Carey T. Antidepressant and antipsychotic use and adherence among Medicaid youths: differences by race. Community Ment Health J. 2010;46(3):265–272. [DOI] [PubMed] [Google Scholar]
- 50.van der Ven E, Susser E, Dixon LB, Olfson M, Gilmer TP. Racial-Ethnic Differences in Service Use Patterns Among Young, Commercially Insured Individuals With Recent-Onset Psychosis. Psychiatr Serv. 2020;71(5):433–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


