Abstract
Objective:
An arteriovenous fistula (AVF) is the preferred vascular access for chronic hemodialysis, yet the rates of AVF maturation failure and reintervention remain high. We investigated the AVF geometric parameters and their associations with AVF physiologic maturation and reintervention in a prospective multi-center study.
Methods:
From 2011 to 2016, patients undergoing a vein end-to-artery side upper-extremity AVF creation surgery were recruited. Contrast-free dark-blood and phase-contrast magnetic resonance imaging (MRI) scans were performed using 3.0T scanners to obtain AVF lumen geometry and flow rates, respectively, at postoperative Day 1, Week 6, and Month 6. Arterial-venous anastomosis angle, nonplanarity, and tortuosity of the fistula were calculated based on lumen centerlines. AVFs were considered physiologically matured if, based on the Week 6 MRI data, the flow rate was at least 500 mL/min, and the minimum vein lumen diameter was at least 5 mm. Associations of these geometric parameters with AVF maturation and reintervention due to peri-anastomotic and mid-vein stenosis within one year were assessed.
Results:
There were 111 patients with usable Day 1 scan, with the majority being upper-arm AVFs (n = 73). Compared to forearm AVFs, upper-arm AVFs had greater anastomosis angles (P < .001), larger deviations from a plane (nonplanarity, P = .002), and more prominent tortuosity (P = .038) at Day 1. These parameters significantly increased between Day 1 and Week 6 in upper-arm AVFs; in contrast, significant changes in these parameters in forearm AVFs were not observed. The rate of maturation was 54% and 86% for forearm and upper-arm AVFs, respectively, with none of the geometric parameters at Day 1 being associated with maturation for AVFs in either location. The rate of reintervention was 24% and 30% for forearm and upper-arm AVFs, respectively, with a larger nonplanarity angle at Day 1 being associated with less reintervention (30° ± 15° vs. 21° ± 10°, P = .034) in upper-arm AVFs only. This relationship was unchanged after adjusting for age, sex, race, dialysis status, or diabetes.
Conclusions:
Upper-arm fistulas have a larger anastomosis angle, are more nonplanar, and have more tortuous veins than forearm fistulas. For upper-arm fistulas, a larger nonplanarity angle is associated with a lower rate of reintervention within one year. Once confirmed, vascular surgeons may consider increasing the nonplanarity angle by incorporating a tension-free gentle curvature in the proximal segment of the mobilized vein to reduce reinterventions when creating an upper-arm fistula.
Keywords: hemodialysis arteriovenous fistula, fistula maturation, fistula stenosis, anastomosis angle, nonplanarity angle
Table of Contents Summary
This prospective cohort study of 111 upper-extremity arteriovenous fistulas (AVFs) found that upper-arm AVFs had larger anastomosis angles, were more nonplanar and more tortuous than forearm fistulas. For upper-arm AVFs, a larger nonplanarity angle was associated with a lower rate of reintervention for peri-anastomotic and mid-vein stenosis within one year.
INTRODUCTION
An arteriovenous fistula (AVF), which is created by surgically connecting an artery to a vein directly, is the preferred vascular access for chronic hemodialysis in patients with end-stage renal disease due to its fewer complications and longer durability compared to AV grafts and central venous catheters.1 However, a newly created AVF needs to maturate before it can be used to support routine hemodialysis. AVF maturation requires a sufficient increase in both the lumen diameter and flow rate of the AVF vein. Yet approximately half of the AVFs created may not clinically maturate to the extent that can support actual use of hemodialysis without reinterventions,2 and early failure of matured AVFs is common. Therefore, improving AVF maturation and durability is crucial for achieving “the right access, in the right patient, at the right time, for the right reasons”.3 Currently, there are very limited noninvasive treatments to enhance AVF maturation and durability.4
Many factors contribute to the non-maturation of newly created AVFs and the early failure of matured AVFs. The exact pathomechanisms are not yet well understood and are most likely patient specific. Unfavorable hemodynamics, disturbed and complex blood flow induced by the abrupt change in flow direction at the arterial-venous anastomosis under a high flow condition, may adversely influence fistula remodeling and impede AVF maturation.5 Hemodynamics of an AVF is largely determined by its geometric configuration, which is patient specific and can be approximately described by the arterial-venous anastomosis angle, the level of nonplanarity, and tortuosity of each individual AVF. While quantification of detailed hemodynamics of an AVF through patient-specific computational fluid dynamics (CFD) simulations is computationally extensive and time-consuming, obtaining the geometric parameters is relatively quick and may provide a more rapid evaluation of clinically relevant information. Anastomosis angles have been associated with the values of lumen diameter and flow rate in an experimental porcine AVF model6 and with the rates of postoperative reintervention7 and maturation8 in patients. Augmented arterial nonplanarity has also been associated with a greater rate of maturation.8 However, these previous studies are limited in the number of patients and intensity of postoperative surveillance. We evaluated the hypothesis that AVF geometric parameters, specifically (anastomosis angle, nonplanarity, and tortuosity), are associated with AVF physiologic maturation and reintervention in a multi-center study, using high-resolution magnetic resonance imaging (MRI) to follow patients sequentially over time.
METHODS
Imaging
From 2011 to 2016, patients with end-stage renal disease undergoing a new upper-extremity AVF creation surgery were recruited at the University of Florida, University of Utah, and University of Cincinnati. Recruitment occurred only after actual AVF creation in patients who were at least 18 years old, had a life expectancy at least nine more months, and had no contraindications to MRI. The vein-end was manually sewn to the artery-side. Protocols were approved by the Institutional Review Board in each university. Written informed consent was obtained from each participant. Two types of MRI techniques, phase-contrast and double-inversion recovery, were performed at Day 1, Week 6, and Month 6 postoperatively using either a 3.0T General Electric or Siemens scanner9 and previously published imaging parameters.10 The scanned arm with the fistula was comfortably positioned at the side of the body with the palm facing the imaging table. Phase-contrast MRI sequences were used to obtain the blood velocities within the AVF vein, and flow rates were extracted using Segment (medviso.com/segment).10 A contrast agent-free double-inversion recovery sequence provided the AVF lumen images in dark blood.
Geometric parameter extraction
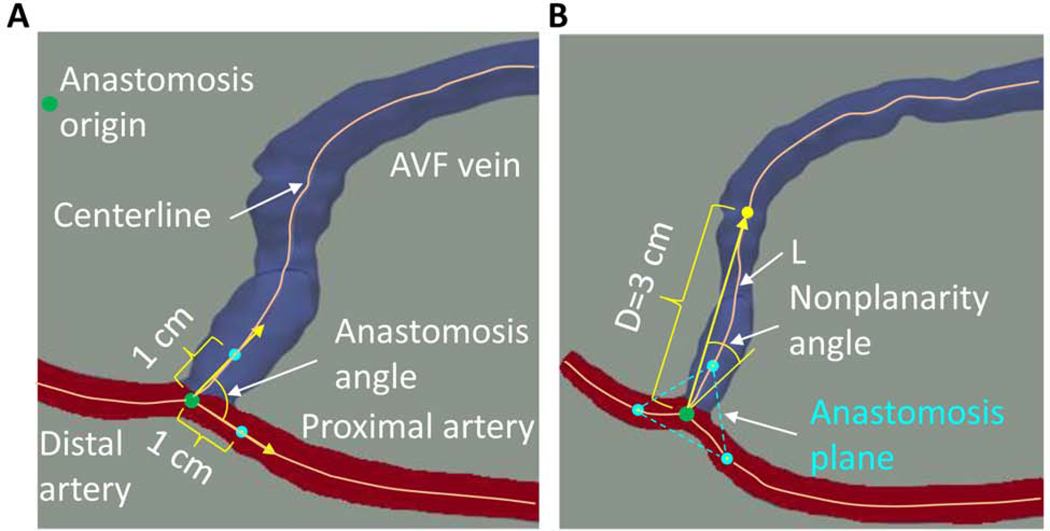
The three-dimensional (3D) AVF lumen geometry was reconstructed from the dark-blood images in Amira (Thermo Fisher Scientific, Waltham, MA). Three geometric parameters (anastomosis angle, nonplanarity angle, and tortuosity) were extracted based on the lumen centerlines and the anastomosis origin obtained using VMTK (www.vmtk.org) (Fig 1). The anastomosis origin was defined using an approach previously developed for the carotid artery bifurcation.11 The anastomosis angle was calculated as the angle between the straight lines connecting the anastomosis origin with the points at the respective centerlines of the proximal artery and the AVF vein that are 1 cm away from the origin (Fig 1, A). According to this definition, an anastomosis angle can vary between zero and 180 degrees. The anastomosis plane is needed for the definition of the nonplanarity angle and is formed by the three points on the centerlines of the proximal artery, AVF vein, and distal artery, each being 1 cm away from the anastomosis origin (Fig 1, B). The nonplanarity angle between the arteries and AVF vein was defined as the positive acute angle between the anastomosis plane and the straight line connecting the anastomosis origin and the point at the AVF vein centerline that is 3 cm away from the origin (Fig 1, B). Tortuosity of the vein was calculated as (), where L and D are the centerline curved length and straight-line length, respectively, between the anastomosis origin and an AVF vein centerline point with a value D (Fig 1, B). D was chosen to be 3 cm because at this distance the swing segment of the fistula vein has elevated to be close to the skin for most fistulas and therefore, most of the curved swing segment is included. In order to examine the sensitivity of the nonplanarity angle and tortuosity to the selection of distance D, these parameters were also determined for a D value of 3.5 cm.
Fig 1.
Definition of the geometric parameters. (A) Anastomosis angle; (B) Nonplanarity angle and tortuosity. The artery is color-coded red, the vein blue. AVF: arteriovenous fistula. D: the vein centerline straight-line length. L: the vein centerline curved length.
Statistical analysis
Continuous data were presented as mean ± standard deviation for normal distribution or median (interquartile range) for non-normal distribution data. Normality was evaluated using a Shapiro-Wilk test. T-tests were performed to compare geometric parameters between forearm and upper-arm fistulas at each time point if the data were normally distributed; otherwise, Wilcoxon rank-sum tests were performed. Equal variances were tested in t-tests; when the variances were not equal, the Satterthwaite method was used to adjust the degree of freedom of the t-distribution. Geometric parameter changes over time were examined using a paired t-test or a Wilcoxon signed-rank test, depending on data normality.
Unassisted physiologic maturation of a fistula was assumed to be achieved if the vein flow rate was at least 500 mL/min and the minimum vein lumen diameter was at least 5 mm, as determined from the Week 6 MRI data.8 Univariate logistic regression was used to examine the association of geometric parameters at Day 1 with fistula maturation or with reintervention due to peri-anastomotic and/or mid-vein (excluding central vein) stenosis within one year after fistula creation following standard-of-care of these patients. When an association was identified to be statistically significant, logistic regression models with the addition of one of the factors, including age, sex, race, dialysis status at fistula creation, and history of diabetes (Table I), were created to examine the potential confounding effects of these covariates on the association.4, 12, 13 Associations between other categorical variables were assessed using a chi-square test or a Fisher’s exact test (for n < 5). Fistulas were excluded from further analyses after reintervention. A P value less than .05 was considered as statistical significance. Statistical analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC).
Table I.
Baseline demographic and clinical characteristics
| Baseline factor | N (%) or Median (IQR) | |
|---|---|---|
| Recruited | With adequate MRI quality | |
| Total patients | 120 | 111 |
| Age (years) | 56.2 (19.0) | 57.8 (18.7) |
| Male | 91 (75.8) | 85 (76.6) |
| Race | ||
| Caucasian | 77 (64.2) | 71 (64.0) |
| Black | 34 (28.3) | 31 (27.9) |
| Other | 9 (7.5) | 9 (8.1) |
| On dialysis | 67 (55.8) | 60 (54.1) |
| Diabetes | 74 (61.7) | 68 (61.3) |
| Peripheral artery disease | 14 (11.7) | 14 (12.6) |
| Coronary artery disease | 35 (29.2) | 33 (29.7) |
| Forearm fistula | 39 (32.5) | 38 (34.2) |
| Two-stage fistula | 13 (10.8) | 11 (9.9) |
IQR: interquartile range
RESULTS
Patient cohort
One hundred and twenty patients were recruited, with 111 patients having usable Day 1 MRI scan. There were more upper-arm (65.8%) than forearm AVFs. The baseline demographic and clinical characteristics of these patients are provided in Table I. Among the 111 patients, 105 patients had a usable Week 6 scan, and 60 patients had a usable Month 6 scan. The detail of patient enrollment and retention is shown in Supplemental Fig 1. Nineteen surgeons placed the fistulas in this study, with the median number of cases performed by each surgeon being 5 (range 1 – 13).
Comparison of geometric parameters between forearm and upper-arm fistulas
This analysis used all of the fistulas that had usable MRI images (Supplemental Fig 1). The three geometric parameters of forearm and upper-arm fistulas at the three time points are shown in Fig 2. Upper-arm fistulas had larger anastomosis angles than the forearm fistulas at all time points (P < .001 for all, Fig 2, A). Compared to forearm fistulas, upper-arm fistulas also had larger nonplanarity angles at all time points (P = .002, <.001, and =.003 for Day 1, Week 6, and Month 6, respectively, Fig 2, B). Upper-arm fistulas had larger tortuosity than the forearm fistulas at all time points (P = .038, .020, and .018 for Day 1, Week 6, and Month 6, respectively, Fig 2, C).
Fig 2.
The three geometric parameters of forearm and upper-arm fistulas at the three time points. (A) Anastomosis angle; (B) Nonplanarity angle; (C) Tortuosity. The available numbers of fistulas for analysis are shown above the bars. Of note, since the length of the vein needs to be at least 3 cm to analyze nonplanarity angle and tortuosity, while only 1 cm is needed to analyze anastomosis angle, the number of fistulas for analysis of nonplanarity angle and tortuosity may be smaller than that of anastomosis angle.
Changes in geometric parameters over time
Only patients who had usable MRI images for both Day 1 and Week 6, or both Week 6 and Month 6, were selected for this pair-wise analysis. The comparisons of geometric parameters during the periods Day 1 – Week 6 and Week 6 – Month 6 are shown in Table II. For the anastomosis angle, only the upper-arm fistulas had a statistically significant change during Day 1 – Week 6, with the Week 6 mean being 2.5 degrees larger than Day 1, which was a 3.3% increase from Day 1; the upper-arm fistulas during Week 6 – Month 6 and the forearm fistulas during the two periods did not have a statistically significant change. Similarly, for the nonplanarity angle, only the upper-arm fistulas had a statistically significant change during Day 1 – Week 6, with the Week 6 mean being 3.2 degrees larger than that of Day 1, which was an 11.4% increase from Day 1; the upper-arm fistulas during Week 6 – Month 6 and the forearm fistulas during the two periods did not have a statistically significant change. For tortuosity, only the upper-arm fistulas had a statistically significant change during Day 1 – Week 6, with the Week 6 median value being 0.011 larger than that of Day 1, which was a 9.1% increase from Day 1. Therefore, the upper-arm fistulas had statistically significant increases in all three geometric parameters during the early period, but not during the later time period. The forearm fistulas did not have statistically significant increases in all three geometric parameters during both periods.
Table II.
Paired comparison of geometric parameters within fistula over time
| Day 1 - Week 6 | Week 6 - Month 6 | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | Location | Day 1 | Week 6 | P-value | Week 6 | Month 6 | P-value |
| Anastomosis angle (degree) | Forearm | 56.1 ± 15.4 | 56.9 ± 17.1 | .94 | 53.9 ± 12.4 | 57.9 ± 15.3 | .098 |
| Upper-arm | 75.6 ± 19.2 | 78.1 ± 17.8 | .040 | 78.1 ± 18.7 | 80.0 ± 20.3 | .46 | |
| Nonplanarity angle (degree) | Forearm | 18.8 ± 11.7 | 20.8 ± 13.9 | .18 | 17.3 ± 10.2 | 18.5 ± 12.7 | .64 |
| Upper-arm | 28.0 ± 13.6 | 31.2 ± 13.9 | .010 | 31.4 ± 13.5 | 34.1 ± 19.0 | .16 | |
| Tortuosity | Forearm | 0.079 (0.068) | 0.115 (0.113) | .23 | 0.087 (0.075) | 0.090 (0.079) | .17 |
| Upper-arm | 0.121 (0.099) | 0.132 (0.101) | .030 | 0.154 (0.121) | 0.145 (0.128) | .21 | |
Association of geometric parameters at Day 1 with fistula maturation at Week 6
The physiologic maturation status at Week 6 was determined for 106 fistulas based on the MRI-measured flow rate and minimal vein diameter. Twenty of the 37 (54.1%) forearm and 59 of the 69 (85.5%) upper-arm fistulas matured. None of the three geometric parameters was a statistically significant predictor of maturation for both forearm and upper-arm fistulas (Table III), so there was no evidence of any association between the three geometric parameters and fistula maturation.
Table III.
Geometric parameters at Day 1 stratified by maturation
| Parameter | Location | Matured | Not matured | P-value |
|---|---|---|---|---|
| Anastomosis angle (degree) | Forearm | 56.9 ± 16.7 | 58.0 ± 13.9 | .82 |
| Upper-arm | 76.3 ± 18.5 | 73.6 ± 23.9 | .68 | |
| Nonplanarity angle (degree) | Forearm | 16.7 ± 11.1 | 20.7 ± 12.2 | .35 |
| Upper-arm | 26.1 ± 13.8 | 31.5 ± 10.3 | .27 | |
| Tortuosity | Forearm | 0.081 (0.065) | 0.074 (0.079) | .46 |
| Upper-arm | 0.124 (0.105) | 0.096 (0.079) | .87 | |
Association of geometric parameters at Day 1 with fistula reintervention within one year after creation
Within one year after fistula creation, the status of reintervention due to peri-anastomotic and/or mid-vein (excluding central vein) stenosis was known for 99 fistulas. Nine of the 38 (23.7%) forearm and 18 of the 61 (29.5%) upper-arm fistula had at least one reintervention. The anastomosis angles at Day 1 were not statistically different between fistulas with and without reintervention for both forearm and upper-arm fistulas, although the mean anastomosis angles of the forearm fistulas with reintervention were 10.1 degrees larger than the value of fistulas without reintervention (P = .083, Table IV).
Table IV.
Geometric parameters at Day 1 stratified by reintervention
| Parameter | Location | Reintervention | No reintervention | P-value |
|---|---|---|---|---|
| Anastomosis angle (degree) | Forearm | 65.5 ± 14.7 | 55.4 ± 14.9 | .083 |
| Upper-arm | 80.1 ± 15.4 | 77.6 ± 19.5 | .63 | |
| Nonplanarity angle (degree) | Forearm | 24.8 ± 12.8 | 16.5 ± 10.8 | .095 |
| Upper-arm | 21.0 ± 10.4 | 29.6 ± 14.7 | .034 | |
| Tortuosity | Forearm | 0.100 (0.208) | 0.071 (0.070) | .32 |
| Upper-arm | 0.093 (0.105) | 0.125 (0.100) | .10 | |
For forearm fistulas, although the mean nonplanarity angle of the fistula with reintervention was 8.3 degrees larger than that of the fistula without reintervention, the nonplanarity angle was not a statistically significant predictor of reintervention [odds ratio of reintervention 1.069, 95% confidence interval (0.989 to 1.155) for every one-degree increase in nonplanarity angle, P = .095, Table IV]. Of note, all nine patients with forearm fistula reintervention were diabetic, and reintervention was significantly associated with diabetes (P = .036); the association between the nonplanarity angle and reintervention became even weaker when the status of diabetes was also in the regression model (P = .28). For upper-arm fistulas, the mean nonplanarity angle of the fistula with reintervention was 8.6 degrees smaller than that of the fistula without reintervention, and the nonplanarity angle was a statistically significant predictor of reintervention [odds ratio 0.952, 95% confidence interval (0.910 to 0.996) for every one-degree increase in nonplanarity angle, P = .034, Table IV].
Further investigation of five potential confounding factors found that when diabetes, sex, race, dialysis status, or age was also included in the logistic regression model as a predictor, the nonplanarity angle remained statistically significant (Table V, the Nonplanarity angle column). None of these five factors was a statistically significant predictor of reintervention when the nonplanarity angle was also in the model as a predictor (Table V, the Factor column) or when only one of the factors was the predictor (data not shown). Therefore, there was evidence to support that the nonplanarity angle of an upper-arm fistula was an independent predictor of undergoing reintervention. The effect of our selection of a 3-cm length in the determination of nonplanarity was evaluated by extending this distance to 3.5 cm. Using a length of 3.5 cm, similar results regarding the association of the nonplanarity angle with reintervention were obtained for both forearm and upper-arm fistulas. The nonplanarity angle remained statistically significant when diabetes, race, or age was also in the model as a predictor for upper-arm fistulas; however, when sex or dialysis status was also included in the logistic regression model as a predictor, the nonplanarity angle became marginally statistically nonsignificant (P = .054 and .053, respectively, Supplemental Table I).
Table V.
Association of reintervention with the nonplanarity angle and one of the five factors in upper-arm fistulas
| Nonplanarity angle | Factor | |||
|---|---|---|---|---|
| Odds ratio (95% CI) | P-value | Odds ratio (95% CI) | P-value | |
| Diabetes | 0.954 (0.912 to 0.998) | .043 | 0.775 (0.239 to 2.518) | .67 |
| Women | 0.954 (0.911 to 0.998) | .042 | 1.491 (0.416 to 3.337) | .54 |
| Black race | 0.950 (0.907 to 0.995) | .029 | 0.656 (0.189 to 2.279) | .51 |
| Dialysis status | 0.952 (0.909 to 0.998) | .039 | 2.532 (0.758 to 8.455) | .13 |
| Age | 0.952 (0.910 to 0.996) | .034 | 1.015 (0.972 to 1.060) | .50 |
Note: The odds ratio for age was for a one-year increase in age. CI: confidence interval
The tortuosity at Day 1 was not different between fistulas with and without reintervention for both forearm and upper-arm fistulas (Table IV).
DISCUSSION
Compared to forearm fistulas, we found that upper-arm fistulas had larger anastomosis angles, nonplanarity angles, and tortuosity up to 6 months after fistula creation. In contrast to forearm fistulas, these three geometric parameters within upper-arm fistulas increased during the initial stages of remodeling, as the fistulas adapted to the new hemodynamic environment. For upper-arm fistulas, the nonplanarity angle was associated with reintervention within one year after fistula creation due to peri-anastomotic and mid-vein stenosis.
Other investigators have performed a quantitative comparison of the geometric parameters between forearm and upper-arm fistulas. Although different definitions of the geometric parameters and earlier time points after AVF surgery were used [Day 1 (n=60) and Week 2 (n=20)],8 this study found that the brachiocephalic fistulas have a larger anastomosis angle and larger whole fistula nonplanarity compared to radiocephalic fistulas. Analogous to our use of tortuosity, they observed that brachiocephalic fistulas have a larger arterial curvature than the radiocephalic fistulas.
As a fistula adapts to the new biomechanical environment after its surgical creation, the resulting increase in diameter during the initial days to weeks following placement has been well documented;14 however, long-term temporal changes in the geometric configuration have not been reported. Our study is the first report to provide this information. Specifically, we found the anastomosis angle, nonplanarity angle, and tortuosity of only the upper-arm fistulas increased during Day 1 and Week 6, without significant changes in the later Week 6 to Month 6 timeframe. This observation is in agreement with the hemodynamic effect in other vascular conduits, where we observed that lower extremity vein bypass grafts had a robust remodeling response in the initial month following surgical placement.15 In contrast, significant temporal changes in the three-dimensional configuration of forearm AVFs were not observed. While the mechanism for this is unclear, it is interesting to speculate that the constraints imposed by the limited subcutaneous space in the forearm might hinder a more dramatic alteration in the geometry. Although these geometric parameters were not associated with our assigned threshold for physiologic maturation, this observation is in contrast to Corbett et al.,8 who determined that a larger anastomosis angle and greater nonplanarity were associated with maturation in a combined forearm and upper-arm AVF group. Although different imaging techniques (MRI versus ultrasound) and variations in the nonplanarity definition (entire anastomotic region versus inflow artery) were utilized, it is unlikely that these subtle differences were the primary drivers for these differing observations. Consistent in both studies, however, is the finding that fistula location is the dominant predictor of fistula physiologic maturation.8
Computational simulation studies have demonstrated that the anastomosis angle affects hemodynamics, especially at the peri-anastomotic region, where stenosis frequently occurs.16–22 Based on simulations of idealized coplanar fistulas employing differing anastomosis angles20 and in silico models incorporating nonplanarity and taper,22 a smaller anastomosis angle is recommended. However, this contradicts the finding by Sadaghianloo et al.,7 which reported that an anastomosis angle of less than 30 degrees was associated with increased reintervention in the juxta-anastomotic segment of radiocephalic fistulas. Furthermore, the current observations and the study by Corbett8 did not identify the anastomotic angle as an important determinant of forearm AVF reintervention. These disparate observations may be related to differences in the techniques used to determine anastomosis angle, where Sadaghianloo used an intra-operative protractor to estimate a two-dimensional angle. In contrast, we and Corbett used reconstructed cross-sectional images to determine this parameter in three-dimensional space. However, conclusions regarding the importance of anastomotic angle on brachiocephalic fistula reinterventions are in agreement, where Sadaghianloo, Corbett, and the current study failed to identify anastomosis angle as an important determinant of upper-arm fistula reintervention. It is possible that the difference in hemodynamics induced by the range of anastomosis angles in these patients was not sufficient to influence the rate of reintervention due to stenosis.
We found that a larger nonplanarity angle of the upper-arm fistula at Day 1was associated with less reintervention due to peri-anastomotic and mid-conduit vein stenosis within one year. This observation is consistent with our current understanding of the hemodynamic effect-associated variations in fistula geometry. A larger nonplanarity angle has been shown to induce a stronger helical flow,22 which also exists in the vasculature at physiological conditions.23, 24 Helical flow reduces the size and duration of low wall shear stress regions,25 inhibits flow separation and stagnation,22 enhances oxygen transport to the vessel wall,25 and reduces the luminal surface low-density lipoprotein concentration,23 all of which help reduce intimal hyperplasia and the stenosis that companies these lesions. In this spirit, helical-centerline stents have shown improved patency of treated superficial femoral arteries in a randomized trial.26 Interestingly, we found that a larger nonplanarity angle of the forearm fistula at Day 1was not significantly associated with more reintervention in a univariate logistic regression model. Compared with upper-arm fistulas, this statistical nonsignificance might be due to a smaller sample size of the forearm fistulas with reintervention (9 vs. 18). However, since all the nine patients with forearm fistula reintervention were diabetic, the association between nonplanarity and reintervention might be confounded by the status of diabetes while the upper-arm fistulas were not. Previous studies have shown that the effect of diabetes on fistula patency may be more prominent for a radiocephalic fistula, whose diameter is smaller than an upper-arm fistula.27, 28
This current study and its comparison to other published findings had several notable limitations. The precise parameters quantifying the geometric configuration of a fistula, and their corresponding definitions, vary among the different studies.7, 8 We used three parameters to describe the basic configuration; however, it is difficult to capture the inherent geometric complexity of a fistula using a limited number of parameters. Although our study is by far the largest study and uses the three-dimentional geometry of the fistulas spanning from 1 day to 6 months after their creation, there were fewer patients with forearm fistulas, and the number of forearm fistulas with reintervention was relatively small. There were also some missing data points, especially at Month 6; this is not uncommon for a longitudinal study of patients with a high rate of complications and diminished life expectancy, and might have affected the pairwise comparison of geometric parameters between Week 6 and Month 6. Also notable is that physiologic maturation, not clinical maturation, was utilized in this study to avoid the additional complexity induced by other factors that are not related to the configuration of the fistula.4 Some upper-arm AVFs had previous ipsilateral forearm AVFs, which might favor the physiologic maturation of upper-arm AVFs determined at Week 6.29
Conclusions
We have demonstrated that upper-arm fistulas have larger anastomosis angles, are more nonplanar and more tortuous than forearm fistulas. The quantitative geometric parameters of forearm and upper-arm fistulas and their alterations over time can be used to construct realistic models for systematically examining the detailed hemodynamics both experimentally and computationally. There is evidence to show the benefit of reducing reintervention due to peri-anastomotic and mid-vein stenosis by a larger nonplanarity angle in upper-arm fistulas. Due to its higher expense and more technically demanding than duplex scans and physical examination, we do not recommend MRI in routine clinical applications. However, if this is confirmed by future studies, it would support a general recommendation to the surgical community that increasing a nonplanarity angle by greater than 10 degrees may result in reduced peri-anastomotic narrowing and reintervention in upper-arm fistulas. The increase in a nonplanarity angle can be achieved by incorporating a tension-free gentle curvature in the proximal segment of the mobilized vein when creating a fistula.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research:
Multi-center prospective cohort study
Key Findings:
In this study of 111 arm arteriovenous fistulas (AVFs) upper-arm AVFs had a larger anastomosis angle, were more nonplanar, and had more tortuous veins than forearm AVFs. For upper-arm AVFs, a larger nonplanarity angle was associated with a lower rate of reintervention for peri-anastomotic and mid-vein stenosis within one year.
Take home Message:
Incorporating a gentle curvature in the proximal segment of the mobilized vein to increase the nonplanarity angle will reduce reinterventions for upper-arm AVFs.
Acknowledgments
Source of funding
This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK082222, U01DK082189, U01DK082218, and R01DK88777).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol 2013;24:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robbin ML, Greene T, Allon M, Dember LM, Imrey PB, Cheung AK, et al. Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the Hemodialysis Fistula Maturation study. J Am Soc Nephrol 2018;29:2735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, Abreo K, et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis 2020;75:S1–S164. [DOI] [PubMed] [Google Scholar]
- 4.Woodside KJ, Bell S, Mukhopadhyay P, Repeck KJ, Robinson IT, Eckard AR, et al. Arteriovenous fistula maturation in prevalent hemodialysis patients in the United States: a national study. Am J Kidney Dis 2018;71:793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunnane CV, Cunnane EM, Walsh MT. A review of the hemodynamic factors believed to contribute to vascular access dysfunction. Cardiovasc Eng Technol 2017;8:280–94. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamoorthy MK, Banerjee RK, Wang Y, Choe AK, Rigger D, Roy-Chaudhury P. Anatomic configuration affects the flow rate and diameter of porcine arteriovenous fistulae. Kidney Int 2012;81:745–50. [DOI] [PubMed] [Google Scholar]
- 7.Sadaghianloo N, Jean-Baptiste E, Rajhi K, François E, Declemy S, Dardik A, et al. Increased reintervention in radial-cephalic arteriovenous fistulas with anastomotic angles of less than 30 degrees. J Vasc Surg 2015;62:1583–9. [DOI] [PubMed] [Google Scholar]
- 8.Corbett RW, Grechy L, Iori F, Crane JS, Herbert PE, Di Cocco P, et al. Heterogeneity in the nonplanarity and arterial curvature of arteriovenous fistulas in vivo. J Vasc Surg 2018;68:152S–63S. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Terry CM, Nguyen C, Berceli SA, Shiu YT, Cheung AK. Serial analysis of lumen geometry and hemodynamics in human arteriovenous fistula for hemodialysis using magnetic resonance imaging and computational fluid dynamics. J Biomech 2013;46:165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, Shiu YT, Pike DB, Roy-Chaudhury P, Cheung AK, Berceli SA. Comparison of hemodialysis arteriovenous fistula blood flow rates measured by Doppler ultrasound and phase-contrast magnetic resonance imaging. J Vasc Surg 2018;68:1848–57 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas JB, Antiga L, Che SL, Milner JS, Steinman DA, Spence JD, et al. Variation in the carotid bifurcation geometry of young versus older adults: implications for geometric risk of atherosclerosis. Stroke 2005;36:2450–6. [DOI] [PubMed] [Google Scholar]
- 12.Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV. Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 2005;67:2462–9. [DOI] [PubMed] [Google Scholar]
- 13.Woo K, Ulloa J, Allon M, Carsten CG, 3rd, Chemla ES, Henry ML, et al. Establishing patient-specific criteria for selecting the optimal upper extremity vascular access procedure. J Vasc Surg 2017;65:1089–103 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbin ML, Greene T, Cheung AK, Allon M, Berceli SA, Kaufman JS, et al. Arteriovenous fistula development in the first 6 weeks after creation. Radiology 2015:150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y, DeSart K, Kubilis PS, Irwin A, Tran-Son-Tay R, Nelson PR, et al. Heterogeneous and dynamic lumen remodeling of the entire infrainguinal vein bypass grafts in patients. J Vasc Surg 2020;71:1620–8 e3. [DOI] [PubMed] [Google Scholar]
- 16.Carroll J, Varcoe RL, Barber T, Simmons A. Reduction in anastomotic flow disturbance within a modified end-to-side arteriovenous fistula configuration: results of a computational flow dynamic model. Nephrology 2019;24:245–51. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Kim S, Kim S-M, Song R, Kim HK, Park JS, et al. Assessing radiocephalic wrist arteriovenous fistulas of obtuse anastomosis using computational fluid dynamics and clinical application. J Vasc Access 2016;17:512–20. [DOI] [PubMed] [Google Scholar]
- 18.Stella S, Vergara C, Giovannacci L, Quarteroni A, Prouse G. Assessing the disturbed flow and the transition to turbulence in the arteriovenous fistula. J Biomech Eng 2019;141:101010. [DOI] [PubMed] [Google Scholar]
- 19.de Andrade Silva J, Karam-Filho J, Borges C. Computational analysis of anastomotic angles by blood flow conditions in side-to-end radio-cephalic fistulae used in hemodialysis. J Biomed Sci Eng 2015;08:131–41. [Google Scholar]
- 20.Ene-Iordache B, Cattaneo L, Dubini G, Remuzzi A. Effect of anastomosis angle on the localization of disturbed flow in side-to-end fistulae for haemodialysis access. Nephrol Dial Transpl 2013;28:997–1005. [DOI] [PubMed] [Google Scholar]
- 21.Van Canneyt K, Pourchez T, Eloot S, Guillame C, Bonnet A, Segers P, et al. Hemodynamic impact of anastomosis size and angle in side-to-end arteriovenous fistulae: a computer analysis. J Vasc Access 2010;11:52–8. [DOI] [PubMed] [Google Scholar]
- 22.Cunnane CV, Cunnane EM, Moran DT, Walsh MT. The presence of helical flow can suppress areas of disturbed shear in parameterised models of an arteriovenous fistula. Int J Numer Meth Bio 2019;35:e3259. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Pu F, Fan Y, Deng X, Li D, Li S. A numerical study on the flow of blood and the transport of LDL in the human aorta: the physiological significance of the helical flow in the aortic arch. Am J Physiol Heart Circ Physiol 2009;297:H163–70. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, Sun A, Fan Y, Deng X. Physiological significance of helical flow in the arterial system and its potential clinical applications. Ann Biomed Eng 2015;43:3–15. [DOI] [PubMed] [Google Scholar]
- 25.Coppola G, Caro C. Oxygen mass transfer in a model three-dimensional artery. J R Soc Interface 2008;5:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeller T, Gaines PA, Ansel GM, Caro CG. Helical centerline stent improves patency: two-year results from the randomized Mimics trial. Circ Cardiovasc Interv 2016;9:e002930. [DOI] [PubMed] [Google Scholar]
- 27.Zeebregts CJ, Tielliu IF, Hulsebos RG, de Bruin C, Verhoeven EL, Huisman RM, et al. Determinants of failure of brachiocephalic elbow fistulas for haemodialysis. Eur J Vasc Endovasc Surg 2005;30:209–14. [DOI] [PubMed] [Google Scholar]
- 28.Golledge J, Smith CJ, Emery J, Farrington K, Thompson HH. Outcome of primary radiocephalic fistula for haemodialysis. Br J Surg 1999;86:211–6. [DOI] [PubMed] [Google Scholar]
- 29.Zonnebeld N, Huberts W, van Loon MM, Delhaas T, Tordoir JHM. Natural vascular remodelling after arteriovenous fistula creation in dialysis patients with and without previous ipsilateral vascular access. Eur J Vasc Endovasc Surg 2020;59:277–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.