Abstract
Lim family members play an important role in the regulation of plant cell development and stress response. However, there are few studies on LIM family in quinoa. In this study, we identified nine LIMS (named cqlim01-cqlim09) from quinoa, which were divided into three subfamilies (α Lim1, γ lim2 and δ lim2) according to phylogeny. The differences in gene structure and motif composition among different subfamilies have been observed. In addition, we studied the repetitive events of the members of the family. The Ka/Ks (non synchronous substitution rate / synchronous substitution rate) ratio analysis showed that the repetitive CqLIMs probably experienced purifying selection pressure. Promoter analysis showed that the family genes contained a variety of hormones, stress and tissue-specific expression elements, and protein interactions showed that these genes had actin stabilizing effect. In addition, QRT PCR results showed that all CqLIM genes were positively regulated under three stresses (low temperature, salt and ABA). These results provide a theoretical basis of further study of LIM gene in quinoa.
Supplementary information
The online version contains supplementary material available at 10.1007/s12298-021-00988-2.
Keywords: Quinoa, LIM transcription factor family, Bioinformatics analysis, Gene expression
Introduction
Widely present in eukaryotes (Eliasson et al. 2000), LIM proteins are a class of cysteine rich proteins (CRPS) named after an acronym of the three LIM homologous domain proteins: LIN11, ISL1, and MEC3. LIN11 and MEC3 are from Caenorhabditis elegans and ISL1 is from rats (Way et al. 1988). LIM proteins’ homologous domains are related to DNA binding in the process of transcription. In eukaryotes, LIM proteins contain at least one LIM domain, which mediates interactions between proteins. In some cases, LIM proteins are associated with protein kinase domains or homologous domains. LIM proteins generally have two functions in plants: (1) as transcription factors, they regulate the expression of genes related to the phenylpropanoid biosynthesis pathway (Kawaoka and Ebinuma 2001) and (2) as binding proteins of actin, they regulate the actin cytoskeleton structure (Maul et al. 2003). The LIM domain is a cysteine- and histidine-rich zinc coordination domain (Dawid et al. 1998), which is generally composed of more than 50 amino acid residues. The LIM domain sequence (C-X2-C-X16-23-H-X2-C)-X2-(C-X16-23-Z-X2-C) (Dawid et al. 1995) is highly conserved in many species.
In animals, the LIM protein family has also been found to play a role in cell movement and thus it can participate in tumor occurrence and metastasis (Labalette et al. 2010). LIM protein was first found in the sunflower, where it was specifically expressed in pollen (Baltz et al. 1992). To date, LIM proteins have been identified in many plants including tobacco (Thomas et al. 2006), Arabidopsis (Papuga et al. 2009), cotton (Li et al. 2013), and tomato (Khatun et al. 2016). There are two different LIM domain protein subfamilies in plants: CRP and DAR. The CRP subfamily contains two conserved LIM domains similar to the members of animal cysteine rich protein (CRP) family (Arnaud et al. 2007). Plant LIM proteins in the CRP subfamily contain a short C-terminal and lack a glycine rich region (GRR) following the LIM domain. According to the expression patterns of CRP-like LIM domain proteins, they are divided into two groups: WLIMs (WLIM1 and WLIM2), which are widely expressed in different plant tissues. PLIMs (PLIM1 and PLIM2) have been observed as expressing extensively in pollen tubes (Arnaud et al. 2007). According to amino acid sequence similarity, plant CRP-like LIM proteins can be subdivided into four subgroups: αLIM1, βLIM1, γLIM2, and δLIM2. Among them, αLIM1 includes PLIM1, WLIM1, and FLIM, γLIM2 and δLIM2 include WLIM2 and PLIM2 respectively, and βLIM1 is a newly discovered LIM protein, which forms a new cluster in phylogenetic tree, and its specific function is unknown (Arnaud et al. 2007). The DAR/DA1 LIM subfamily proteins contain a conserved LIM domain, one or more ubiquitin interaction motif (UIM) domains, and a conserved C-terminal region. According to amino acid sequence similarity, DAR/DA1 class proteins can be divided into class I and class II. DAR/DA1 proteins are only found in terrestrial plants (Zhao et al., 2014).
Studies have shown that plant LIM proteins not only interact with actin proteins, but also participate in the phenylpropane secondary metabolism pathway and play a key role in the regulation of plant cell development and stress response (Srivastava et al. 2017). For example, the NtLIM1 protein of tobacco (Nicotiana tabacum) activates a key gene (PAL, phenylalanine ammonia lyase) involved in lignin metabolism by binding to the PAL box of the promoter region (Kawaoka et al. 2000). Furthermore, overexpression of GhWLIM1a in upland cotton not only increased the expression of 4CL (4-coumarate: Coenzyme A ligase), CCR (cinnamyl Coenzyme A reductase), and CAD (cinnamyl alcohol dehydrogenase), but also changed the structure of secondary cell wall of fibroblasts (Han et al. 2013). In addition, overexpression of PtaGLIMa can significantly change xylem development and lignin content of poplar (Yang et al. 2017). Recent studies have shown that the biological function of a LIM protein is related to its subcellular localization. There are three localization modes of LIM proteins: (1) cytoplasmic localization; (2) nuclear localization; and (3) cytoplasmic and nuclear colocalization (Sala et al. 2018). Nuclear localization of the LIM protein plays an important role in tissue-specific gene regulation and cell differentiation. For example, nuclear localization of LIM1 protein activates the expression of β-glucuronidase reporter gene in tobacco (Zheng and Zhao 2007). Moreover, in Arabidopsis thaliana, six LIM domain proteins are located on actin cytoskeleton and can bind with actin protein. This binding activity is regulated by pH and Ca2+ (Papuga et al. 2010). In recent years, studies have shown that LIM gene expression shifts in response to abiotic and biotic stresses, such as drought, salt, and hormone (Zhao et al., 2014). In many studies (Wasteneys et al. 2004; Wang et al. 2011a, b), plant cytoskeleton plays an important role in mediating plant stress response. The cytoskeleton participates in various life activities in cells, and recombination of cytoskeleton elements may also be one of the mechanisms of abiotic stress response. It has been observed that LIM genes may play an important role in plant response and resistance to abiotic stress (Zhao et al. 2014). Therefore, the LIM gene family is an exciting and important topic of study in plant research (Baltz et al. 1999; Moes et al. 2012).
The arid climate of Northwest China causes drought stress and cause serious effects on the yield and quality of crops. LIM proteins in many plants have been identified, including 9 in Arabidopsis thaliana, 6 in rice (Arnaud et al. 2007), 10 in Setaria italica (Yang et al. 2019), 14 in pear (Cheng et al. 2019), 12 in poplar (Arnaud et al. 2012), and 22 in Brassica rapa (Jong-In et al. 2014). Although the LIM gene family may play an important role in response to abiotic stress, to date, there has been no report on the LIM genes in quinoa. As a crop with large market prospects in China, quinoa has high economic value (Graf et al. 2015). Therefore, we have identified and analyzed the LIM gene family in quinoa. The nine CqLIM genes we identified were analyzed regarding basic physical and chemical properties, phylogeny, gene structure, cis-acting elements in the promoter region, and expression profiles. The aim of this study was to provide a framework for the study of LIM genes in plants and a strong foundation for further investigation of LIM genes in quinoa.
Materials and methods
Genome-wide identification of LIM gene members in quinoa
The genome, coding sequence, protein sequence, and gff annotation information of quinoa were obtained from the Phytozome database (https://phytozome.jgi.doe.gov/pz/portal.html). The protein sequences of the LIM family in Arabidopsis thaliana were downloaded from the PlantTFDB database (Tian et al. 2019). Quinoa LIM genes were predicted in the quinoa genome using alignment-based BLASTP search and hidden markov model based query (HMMER) with known sequences of Arabidopsis LIM genes. The quinoa genes’ LIM protein conserved domains were predicted with Pfam (http://pfam.xfam.org/family), NCBI-CDD (https://www.ncbi.nlm.nih.gov/cdd/), and SMART (http://smart.embl- heidelberg.de/).
Analysis of proteins’ basic physicochemical properties and construction of phylogenetic tree
The amino acid numbers, isoelectric points, molecular weights, and hydrophobicities of quinoa LIM proteins were analyzed with online software ExPASy (https://web.expasy.org/protparam/). Subcellular localization was determined with PSORT prediction (http://psort1.hgc.jp/form.html)( Gardy et al. 2005). MEGAX software’s ClustalW (version 2.1) method (Larkin et al. 2007) was used to align multiple LIM protein sequences identified in tomato, Arabidopsis, pepper, and quinoa. The resulting alignment was used to construct a neighbor-joining phylogenetic tree by MEGA7 (Kumar et al., 2006). Parameters in the tree construction were: poisson model, data deletion was set to pairwise deletion, the number of repeats was 1000, and all other settings were default. The evolutionary tree was visualized with Evolview (http://120.202.110.254:8280/evolview) (zhang et al. 2012).
Gene structure and conserved motif analysis
Using the annotations of the LIM family genes in the gff file, Gene Structure Display Server (GSDS) was used to analyze the gene structure characteristics (Hu et al. 2015) and the gene structure diagram was drawn with TBtools (Chen et al. 2020). Conserved motif analysis of amino acids was performed with Multiple Em for Motif Elicitation (MEME) online software (Steven et al. 1996), search number was set to 10 and all other parameters were set to default.
Analysis of chromosome distribution and gene duplication events of LIM genes in quinoa
According to quinoa gene annotations in the gff file, TBtools software (Chen et al. 2020) was used to illustrate the chromosome mapping of CqLIM genes. We detected duplicate gene pairs using the plant genome replication database server (http://chibba.agtec.uga.edu/duplication/index/locket). ClustalW software (Larkin et al. 2007) was used to predict the amino acid sequences of the partially repeated CqLIM genes. The program Codeml (http://www.bork.embl.de/PAL2NAL) in PAML (pal2nal) was used to estimate synonymous (Ks) and non-synonymous (Ka) replacement rates (Suyama et al. 2006). The following formula was used to estimate the timing (million years ago, Mya) of the divergence of each CqLIM gene: T = Ks/2λ (λ = 6.56E-9) (Lynch et al. 2003).
Analysis of upstream cis-acting elements and construction of protein interaction network
Using the information in the quinoa genome’s gff annotation file, we extracted the 2000 bp sequence upstream of the transcription start site of each LIM family gene with TBtools (Chen et al. 2020). Then, we searched and analyzed the cis-acting elements in these promoter regions of the LIM genes using PlantCARE (Lescot et al. 2002). To predict the protein–protein interaction network of the LIM family in quinoa, Arabidopsis LIM genes orthologous to those in quinoa were used. The LIM network was constructed with STRING software (Szklarczyk et al. 2015) (parameter of option value was greater than 0.8.)
Plant materials and treatments
L-2 (Longli No.2 from Gansu Academy of Agricultural Sciences) was used as material, quinoa seeds were sterilized in 75% ethanol for 30 s, washed twice with sterile water, then sterilized in 10% sodium hypochlorite for 15 min, then washed with sterile water for 5 times, then seeded on MS solid medium and cultured at 25 °C. The seedlings were cultured at 25 °C for 16/8 h (16 h light / 8 h darkness) until germination. The germinated seeds were then planted in a tray containing sand, perlite, and peat at a ratio of 1:1:1 and cultured in a growth chamber with conditions: relative humidity 60–70%, light time 12 h, and day and night temperatures 28 °C/18 °C. After the seedlings have grown for ~ 2 months, they are exposed to stress treatment. For low temperature, treatment, they are placed in the incubator at 4 °C. Under salt stress, the leaves were sprayed with 100 mmol/L NaCl. In ABA treatment, 200 µmol/L ABA was sprayed on the surface of plant leaves. The leaves were collected at 0, 2, 4, 8, and 12 h after treatment. The collected leaves were stored in the refrigerator at −80 °C for subsequent real-time fluorescence quantitative tests.
Expression analysis, RNA extraction, and real-time quantitative PCR of LIM family genes
Transcriptome data of different tissues and organs of quinoa (No: PRJNA394651), tissues from aboveground seedlings of quinoa under drought, high temperature, salt, and low phosphorus stress treatments (No: PRJNA306026) were downloaded from Bioproject database (www.ncbi.nlm.nih.gov/bioproject) and standardized using a Log2 method (Gao et al. 2018). Total RNA from each sample (100 mg) was extracted with Trizol reagent and cDNA was prepared with Superscript™ III reverse transcriptase kit (Perfect Real Time, Takara Biomedical Technology Co., Ltd. (Beijing, China)). The gene-specific primers were designed on Primer5.0 (Livak et al. 2001). RT-qPCR analysis was performed with the ABI-VIIA 7 real-time PCR system (American Applied Biosystems) using 2 × quantitect-sybr-green-pcr-mix (Qiagen). The procedure was as follows: 3 min denaturation at 95 °C, then 10 s denaturation at 95 °C for 40 cycles, and finally annealing/extension at 60 °C for 1 min (Zhang et al. 2013). The quinoa actin gene was used as the endogenous control (Genebank: LOC110715281, primer information in Table S4). The relative gene expression levels were calculated using the 2−∆∆Ct method (Livak et al. 2001). Each experiment was replicated three times with a separate RNA sample.
Results
Identification of LIM genes in quinoa
Briefly, using the known and charactierized LIM genes in Arabidopsis thaliana, the whole genome database of quinoa was compared and analyzed. Finally, nine LIM genes were identified in quinoa (Figure S1), which we designated CqLIM01-CqLIM09. LIM family members in quinoa contained the conserved LIM domain (CX2CX16-23HX2CX2CX2CX16-21CX2-3(C/H/D). The amino acid length of these proteins varied from 191 to 366 and, correspondingly, the molecular weights ranged from 21,216.14 Da to 41,024.32 Da (Table 1). The theoretical isoelectric point values were between 6.45 (CqLIM01) and 9.03 (CqLIM03), and average of hydrophobicity (GRAVY) values were below 0, which indicated that these proteins encoded by the CqLIM genes are composed of hydrophilic amino acids. Subcellular prediction revealed that seven CqLIM genes were located in the cytoplasm, one in the nucleus, and one in the mitochondrial matrix space.
Table 1.
Information of 9 LIM genes in quinoa
| Gene accession No | Gene | Size (aa) | Molecular weight (D) | Isoelectric point | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|
| AUR62004324-RA | CqLIM01 | 214 | 23,750.73 | 6.45 | − 0.503 | Cytoplasm |
| AUR62008273-RA | CqLIM02 | 219 | 23,921.94 | 8.75 | − 0.400 | Cytoplasm |
| AUR62012196-RA | CqLIM03 | 204 | 22,661.03 | 9.03 | − 0.403 | Cytoplasm |
| AUR62017249-RA | CqLIM04 | 285 | 32,621.68 | 5.23 | − 0.644 | Mitochondrial matrix space |
| AUR62022471-RA | CqLIM05 | 191 | 21,216.14 | 9.02 | − 0.661 | Cytoplasm |
| AUR62022829-RA | CqLIM06 | 204 | 22,628.97 | 9.03 | − 0.395 | Cytoplasm |
| AUR62031033-RA | CqLIM07 | 219 | 23,935.93 | 8.84 | − 0.470 | Cytoplasm |
| AUR62033338-RA | CqLIM08 | 218 | 24,193.29 | 6.70 | − 0.465 | Cytoplasm |
| AUR62037417-RA | CqLIM09 | 366 | 41,024.32 | 8.54 | − 0.731 | Nucleus |
Phylogenetic and evolutionary analysis of LIM proteins in quinoa
Arnaud (Arnaud et al. 2007) classified plant LIM proteins into four categories according to their phylogeny: αLIM1 (FLIM1, WLIM1 and PLIM1), βLIM1, γ LIM2 (WLIM2), and δLIM2 (PLIM2-I and PLIM2-II). In this study, we compared quinoa LIM proteins and investigated their evolutionary relationships with respect to other members of the LIM family from various plant species. To construct a phylogenetic tree, we used the LIM protein sequences of quinoa (9 protein), Arabidopsis (6 proteins), pepper (9 proteins), and tomato (10 proteins) (Table S1, Fig. 1.) The phylogeny revealed that the LIM proteins in quinoa could be clearly subdivided into three groups (no genes belong to βLIM1). CqLIM02, CqLIM04, CqLIM05, CqLIM07, and CqLIM09 belonged to αLIM1, CqLIM01 and CqLIM08 belonged to δLIM2, CqLIM03 and CqLIM06 belonged to γLIM2. In addition, four pairs of paralogous genes (CqLIM01 and CqLIM08, CqLIM02 and CqLIM07, CqLIM03 and CqLIM06, and CqLIM05 and CqLIM09) and one pair of orthologous genes (CqLIM04 and CaLIM09) were observed in the CqLIM genes.
Fig. 1.

Phylogenetic analysis of LIM proteins from quinoa and other plants. The phylogenetic tree was constructed using 6, 10, 9 and 9 LIM protein sequences from A. thaliana, S. lycopersicum, Capsicum annuum L and Chenopodium quinoa respectively. Plant LIM members were divided into 3 clades
Genomic structure, conserved domain, and motif analyses of LIM proteins in quinoa
To characterize the structural diversity of CqLIM proteins further, we examined the composition and organization of conserved motifs in CqLIM genes using MEME software (Steven et al. 1996). Ten conserved motifs were retrieved from the nine LIM genes in quinoa and were assigned motif 1 to motif 10 (Fig. 2a, Fig. 2c). We observed that each LIM gene has distinct motifs (four to seven motifs). However, the distribution and characterization of the conserved motifs were similar in each of the LIM subfamilies. Motif 1, motif 3, and motif 5 were common in CqLIM genes, and motif 2 and motif 4 were also present in eight of the CqLIM genes (except CqLIM04), indicating that these five motifs are particularly conserved and likely functionally similar in quinoa. Furthermore, some motifs only existed in individual CqLIM genes: motif 6 only exists in CqLIM02 and CqLIM07, motif 7 only exists in CqLIM03 and CqLIM06, motif 8 and motif 10 only exist in CqLIM01 and CqLIM08, and motif 9 only exists in CqLIM04, CqLIM05, and CqLIM09. These motifs may be the basis of their phylogenetic classification and may also play an important role in quinoa LIM genes.
Fig. 2.
Gene structure and conserved protein domains of CqLIM genes in quinoa showing exons, introns, and motif sequence organization. A: Conserved motif B: Intron exon structure C: Ten conserved motifs
Exon–intron gene structure plays a key role in the evolution of a gene family. Therefore, we investigated the number and location of exon and introns in CqLIM genes (Fig. 2b) and observed a low degree of structural divergence in the family. For example, six CqLIM genes (66.7%) had a UTR (untranslated region) at 5' and 3′ ends while CqLIM04 had no UTR. 77.8% of CqLIM genes had five exons, CqLIM04 had four exons, and CqLIM09 had seven exons. Most CqLIM genes falling within the same phylogenetic group displayed almost identical exon–intron organizations, both in terms of intron numbers and exon lengths.
Chromosome distributions and gene duplication events
In order to verify the relationship between genetic differentiation and gene replication in the evolutionary history of the LIM family, we generated a map showing the chromosomal positions of all CqLIM genes. This map showed that nine CqLIM genes were unevenly located on six chromosomes of quinoa (Fig. 3). Among them, CqLIM01, CqLIM06, and CqLIM07 genes were distributed on chromosomes 01(B), 04(A), and 08(A) respectively, and there are two CqLIM genes on chromosomes 03 (B), 10 (B), and 16 (B), respectively.
Fig. 3.
Distribution of CqLIM genes in quinoa chromosomes. Chromosome number suffixes A and B denote subgenome A and B in quinoa genome, respectively
Similarly, we analyzed the duplication events of quinoa LIM genes. While searching for high similarities in protein sequences, we have observed four pairs of homologous LIM genes in quinoa (Table 2). These LIM gene pairs are located in the same branch of the phylogenetic tree, and have a high degree of protein sequence homology (> 75%). In addition, these four pairs of homologous genes were located on different chromosomes, which provided strong evidence that the expansion of quinoa LIM genes might be primarily due to fragment replication rather than tandem replication; tandem duplications occur on the same chromosome while segmental duplications can occur on different chromosomes (Cannon et al. 2004). The Ka/Ks ratios (non synonymous and synonymous substitution ratios) of the duplicated LIM gene pair were less than 1, and according to the equation described in methods, the fragment repetition likely occurred in the range of 2.174 to 8.435 Mya (million years ago.)
Table 2.
CqLIM genes duplication event
| Duplicated LIM gene1 | Duplicated LIM gene2 | Ka | Ks | Ka/Ks | Date(mya)T = Ks/2λ | Selective pressure | Duplicate type |
|---|---|---|---|---|---|---|---|
| CqLIM01 | CqLIM08 | 0.018 | 0.073 | 0.253 | 4.288 | Purifying selection | Segmental |
| CqLIM02 | CqLIM07 | 0.012 | 0.143 | 0.083 | 8.435 | Purifying selection | Segmental |
| CqLIM03 | CqLIM06 | 0.004 | 0.037 | 0.115 | 2.174 | Purifying selection | Segmental |
| CqLIM05 | CqLIM09 | 0.024 | 0.122 | 0.199 | 7.213 | Purifying selection | Segmental |
Cis-acting element analysis and construction of protein interaction network
To illustrate the importance of cis-acting elements in the promoter region of CqLIM genes, we analyzed the 2000 bp sequence upstream of the transcription initiation site. A total of 43 elements related to plant hormones, stress response, tissue-specific expression, and light response were found (Table S2, Fig. 4). We have also observed that CqLIM genes contained multiple TATA boxes, and different genes had different types and numbers of cis-acting elements. Light response elements have the greatest numbers of cis-acting elements (21). Additionally, there were multiple copies of Box-4, G-box, and GT1 motif in several CqLIM genes. With regard to tissue-specific expression elements, ARE elements existed in the form of multiple copies in CqLIM genes. CqLIM04 and CqLIM09 contained five kinds of hormone response elements: abscisic acid, methyl jasmonate (MeJA), gibberellin (GA), salicylic acid (SA), and auxin. CqLIM01 only contained MeJA and ABA response elements, while CqLIM08 contained only MeJA and GA response elements. Thus, these regulatory motifs present in the upstream regions of LIM genes should be validated experimentally to verify if they are functional regulatory elements. These results indicate that different CqLIM genes may play a pivotal role in plant growth and development by responding to different hormone signals.
Fig. 4.
The number of stress-responsive cis-acting elements in the promoter region of each CqLIM gene
In order to explore the relationships among quinoa LIM proteins and their regulatory functions, we constructed a LIM family protein interaction network between Arabidopsis thaliana and quinoa using STRING software (Szklarczyk et al. 2015) (Fig. 5). According to the predicted interaction network, CqLIM proteins appear in the known interaction network of Arabidopsis LIM proteins. There is a close relationship between CqLIM and Arabidopsis LIM proteins, suggesting that we can predict the unknown function of CqLIM genes from the known function of AtLIM genes. We can observe that protein sequence of AtWLIM2a is highly similar to three CqLIM protein sequences (CqLIM03, CqLIM04, and CqLIM06), and that the AtWLIM1 protein sequence is similar to four CqLIM protein sequences (CqLIM02, CqLIM05, CqLIM07, and CqLIM09). Because AtWLIM2a and AtWLIM1 are GATA type zinc finger transcription factor family proteins, this close similarity suggests that these seven CqLIM genes may also be GATA type zinc finger transcription factor family proteins (Thomas et al. 2008); they can bind with actin filaments and promote cross-linking into thick bundles. The activity of actin protein was not affected by pH and Ca2+. AtPLIM2b is also a GATA type zinc finger transcription factor with actin stabilizing effects (Ye et al. 2012). Although its regulatory activity is not affected by Ca2+, its actin regulatory activity is inhibited when pH > 6.8 (Papuga et al. 2010). Therefore, CqLIM01 and CqLIM08 may have similar functions. Meanwhile, AtWLIM1 interacts with several proteins with known and unknown functions, which indicates that AtWLIM1 has a complex regulatory mechanism. These predictions based on gene homology are helpful in investigating the functions and mechanisms of quinoa LIM proteins.
Fig. 5.

The prediction of the interaction network of CqLIM proteins based on the interactions of their orthologs in Arabidopsis
Expression patterns of CqLIM at different tissues and treatments
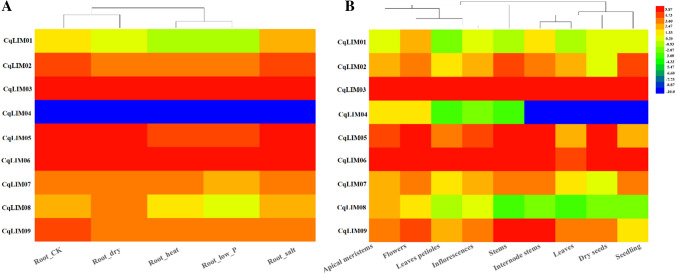
Analysis of organ-specific gene expression patterns can provide clues about the possible function of a gene over the course of development. Therefore, we investigated expression patterns with downloaded RNA sequence data from NCBI and measured it’s expression levels as FPKM (Fragments Per Kilobase per Million). We processed the data and generated a heat map (Fig. 6, Table S3). The analysis included three different tissues and organs (apical meristems, flowers, leaves, petioles, stems, internode stems, seedlings, inflorescences, and dry seeds) and five different treatments (cold, drought, low-Pi, salt, and heat). We found that three genes (CqLIM03, CqLIM05, and CqLIM06) were highly expressed in the nine tissues and five treatments, and two genes (CqLIM01 and CqLIM08) showed low expression in nine tissues and five treatments, CqLIM04 was not expressed in five treatments nor tissues (internode stems, seedlings, leaves, and dry seeds). In addition, some genes, such as CqLIM05 and CqLIM09, were highly expressed in stem.
Fig. 6.
Heatmap of CqLIM genes expression in different tissues and treatments. Data were obtained from a publicly available database, NCBI (Accession number: PRJNA394651, PRJNA306026)
Expression profiling of quinoa LIM genes under low temperature, NaCl, and ABA treatments
LIM genes play a critical role in response to abiotic and biotic stress. Therefore, we studied the expression patterns of CqLIM genes in leaves treated with low temperature, salt (NaCl), and ABA (Table S4, Table S5). The results showed that CqLIM genes’ expression was responsive to low temperature, salt (NaCl), and ABA treatments (Fig. 7). The expression level of CqLIM05 was particularly high under the three treatments, especially under ABA treatment. The relative expression of CqLIM05 was up to 61 flod at 2 h after treatment. Under ABA treatment, expression levels of nine CqLIM genes (CqLIM01-CqLIM09) showed significant (p < 0.05) up-regulation in the leaf with the highest expression levels of nine genes at 2 h. In the low temperature stress treatement, all CqLIM genes were up-regulated. Among them, two genes (CqLIM03 and CqLIM05) had their highest expression levels at 2 h, three genes (CqLIM01, CqLIM02, and CqLIM06) had their highest expression levels at 8 h, and four genes (CqLIM04, CqLIM07, CqLIM08, and CqLIM09) had their highest expression levels at 12 h. In addition, eight genes (except CqLIM09) had their the lowest expression levels at 4 h. In the NaCl stress treatment, all CqLIM genes were also up-regulated. Among which, the expression levels of five genes (CqLIM01, CqLIM04, CqLIM05, CqLIM06, and CqLIM07) increased steadily after treatment (at 2 h, 4 h, 8 h, 12 h), and four genes (CqLIM01, CqLIM02, CqLIM07, and CqLIM08) increased until 8 h and abruptly decreased at 12 h.
Fig. 7.
The expression levels of nine CqLIM genes in quinoa under low-temperature (4 °C), NaCl and ABA treatments. Bars represent the mean values of three replicates ± standard deviation (SD). All of the expression levels of the CqLIM genes were normalized by the expression levels of CqActin (LOC110715281). Untreated leaves (0 h) were normalized as “1” in each graph. Different lowercase letters indicate a significant difference compared to the control (0 h) at p < 0.05, respectively
Discussion
As plants grow and develop, they encounter a variety of biotic and abiotic stresses, and transcription factors are important for functioning in these adverse environments. The LIM transcription factor family genes in plants were induced by a variety of biotic and abiotic stresses. Although LIM gene family has been widely studied in many species, and 9 LIM genes have been identified in Arabidopsis thaliana, 6 in rice (Arnaud et al. 2007), 10 in Setaria italica L (Yang et al. 2019), 14 in pear (Cheng et al. 2019), 12 in poplar (Arnaud et al. 2012), and 22 in Brassica rapa (Jong-In et al. 2014). However, to date, a detailed investigation of LIM proteins has been lacking quinoa. Therefore, in this study, a genome-wide analysis of the LIM family was performed and 9 LIM genes were identified in quinoa, which were the homologous to the 9 in Arabidopsis thaliana. These results showed that the number of LIM genes in distinct plant species differs slightly. Moreover, eight of the identified CqLIM genes contained double LIM domains and one (CqLIM04) contained a single LIM domain. The single LIM domain protein only retained the N-terminal domain, which might be a result of evolutionary processes driving sequence variation at the C-terminal LIM domain ultimately leading to a new highly conserved domain at the C-terminus. Although, the function of this new highly conserved domain at the C-terminus is unknown, it is still rich in cysteine residues, and thus it might be analogous to other LIM domains. A previous study (Cai et al. 2011) demonstrated that regardless of the fact that LIM genes contain a double LIM domain or a single LIM domain, if the domain contains 60–61 amino acid residues, then it has complete LIM protein function. Therefore, we hypothesize that the 9 CqLIM genes in this study on quinoa have complete LIM protein function. Previous studies showed that the LIM proteins located in the nucleus are mainly involved in the regulation of gene transcription, while LIM proteins located in the cytoplasm regulate actin proteins and thus participate in formation of the cytoskeleton (Tran et al. 2005). Tobacco NtWLIM1 and NtWLIM2, sunflower WLIM1, and Arabidopsis protein families show simultaneous localization of the nucleus and cytoplasm (Brière et al. 2003). This study shows that the CqLIM family proteins are mainly located in the cytoplasm, and it is hypothesized that they may mainly act on the formation of the cytoskeleton, which differs from the above conclusions.
Accurate understanding of a species’ evolutionary history is the first step for understanding the evolutionary relationships of genes, which may be useful for studying their functions and the ways in which they have evolved. In a phylogenetic analysis, the closer the gene clustering relationship on the tree, the more similar the gene function (Shen et al. 2016). In order to further understand the relationships between the LIM genes of quinoa and other species, we constructed a phylogenetic tree of LIM proteins from quinoa, Arabidopsis, pepper, and tomato. The results showed that the LIM proteins of quinoa are more closely related to pepper LIM proteins than to tomato LIM proteins, which recapitulates the evolutionary history of quinoa and pepper; both originated in the same place (Peru). Moreover, Gabaldón and Koonin (2013) have indicated that the orthologous genes often maintain similar functions in different species during evolutionary processes. Therefore, we hypothesize that CqLIM04 and CaLIM09 have similar biological functions.
In the processes of gene evolution and differentiation, gene duplication (tandem replication and fragment duplication) plays an important role in gene expansion and gene function differentiation (Vision et al. 2000). In this study, four pairs of homologous CqLIM genes are located in the same branch of the phylogenetic tree and are located on different chromosomes, therefore indicating that they are four pairs of fragment repeat genes. The evidence that the expansion of CqLIM genes is mainly caused by fragment duplication rather than tandem replication, could be caused by quinoa retaining a large number of repeated chromosome segments during the chromosome doubling process (Wang et al. 2012). This evolutionary history is consistent with a study of the calmodulin binding activator of transcription (CAMTA) in Gossypium (Pant et al. 2018). The history of the selection acting on the encoding sequence can be measured by the Ka/Ks ratio. If Ka/Ks < 1, this is evidence of negative or purifying selection; if Ka/Ks = 1, this is evidence of neutral selection or drift; if Ka/Ks > 1, this is evidence of positive selection (Chen et al. 2014). The Ka/Ks ratios of the four segmental duplicated gene pairs in this study were all less than 0.5. Therefore, quinoa LIM genes have primarily experienced purifying selection in their evolutionary histories, with slight variation after duplication (Zhang et al. 2006). Moreover, we have observed that it took between 2.174 and 8.435 Mya for these genes to replicate, which indicates that the evolution of LIM gene family in quinoa genome is relatively slow and highly conserved.
The function of a gene is closely related to its structure. For example, introns can increase the transcription level by affecting the transcription rate, nuclear output, and transcriptional stability (Shaul et al. 2017). In this study, through the analysis of gene structure, it has been observed that the genes clustered in the same phylogenetic tree branch are highly similar in structure or in conservative motifs, which not only validates the phylogenetic tree construction, but also further supports the conserved evolutionary characteristics of the LIM gene family. Moreover, it also illustrated that LIM genes share functional similarity in the same subgroup. For example, LIM03 and LIM06 (γLIM2) can bind with actin filaments (Thomas et al. 2008). Simultaneously, there is a slight variation in the number of exons in CqLIM genes (four to seven), which may be a result of replication events conserving the structure of CqLIM genes. Previous studies (Arnaud et al. 2007) showed that most LIM genes in plants contained four to five introns, and Arnaud observed that exons one, two, and four of the LIM gene family in poplar, Arabidopsis, and rice were highly conserved. Our results are consistent with Arnaud et al. (2007); the number of exons in eight of the CqLIM genes ranged from four to five, and exons one, two, and four were highly conserved), indicating that these genes were particularly conserved during evolutionary processes (Kaufmann et al. 2005).
Plants have evolved a variety of ways to protect themselves from biotic and abiotic stressors, which is mainly manifested in the expression of stress response genes, and the expression of genes is regulated by their promoters or transcription factors. Therefore, the analysis of promoter cis-regulatory elements is essential for studying the function of a specific gene (Wang et al. 2020). We analyzed the cis-acting elements in the promoter region of CqLIM genes and observed that the number of some elements in CqLIM genes promoter regions with single and double LIM domains differed slightly, which was consistent with previous studies (Cai et al. 2011). This illustrates that LIM genes with single and double domains are closely related at the gene level. Meanwhile, we have also observed elements related to hormones (ABA, MeJA, GA, SA, and auxin). These elements play a key role in the plant response to biotic and abiotic stresses and plant signal transduction (Wang et al. 2011a, b). For example, b-ZIP transcription factors AREB1, AREB2, and ABF3 interact with the ABRE motif in the DREB2A promoter to jointly regulate plant response to osmotic stress (Kim et al. 2011). In addition, under the induction of exogenous SA, the TCA element can activate the expression of the pVqMYB15 gene, but in the absence of the ABRE element, the activity of the pVqMYB15 promoter induced by ABA was significantly decreased (Li et al. 2020). In this study, nine CqLIM genes were observed as having light responsive elements. Light is the basic element controlling many important biological processes of plant growth and development. Studies have shown that the ZmRXO1 promoter may be regulated by light, and its promoter sequence contains multiple light responsive elements, such as BOXI and AE-box (Hen et al. 2015).
Genes achieve their biological functions and signal transduction pathways through interaction networks. Therefore, studying the potential interaction networks related to gene families is helpful to understand gene functions (Papuga et al. 2010; Tohge et al. 2010). In this study, AtWLIM2a was observed to be highly homologous with three CqLIM protein sequences (CqLIM03, CqLIM04, and CqLIM06.) It is highly expressed in the vascular system of root and leaf and regulates actin protein independent of pH or Ca2+. The AtWLIM1 protein sequence was similar to the four CqLIM protein sequences (CqLIM02, CqLIM05, CqLIM07, and CqLIM09), its activity is regulated by pH and Ca2+, and AtWLIM can interact with AtDAR7 to regulate developmental processes. AtPLIM2b (CqLIM01 and CqLIM08) is expressed in pollen. Therefore, we speculate that nine CqLIM genes (genes homologous to Arabidopsis LIM genes) have similar functions.
There is a close relationship between the tissue-specific expression of genes and their functions (Fan et al. 2015). Previous works have shown that LIM family genes play an important role in lignin synthesis, pollen tube growth, repair to mechanical damage, organ development, and plant morphogenesis (Mundel et al. 2000; Kawaoka et al. 2000). Generally, the family of genes may be involved in important activities in cells, including cell replication, morphogenesis, signal induction, and resistance to external stresses (Wang et al. 2011a, b). Moreover, it has been reported that NtLIM genes participate in the regulation of cell proliferation and cell cycle processes through modulation of gene expression (Moes et al. 2012). In this study, eight CqLIM genes were expressed in flower, leaves, internode stems, and stems of quinoa, and their expression levels were different in different tissues, suggesting that CqLIM genes might be involved in the development of different organs, further reflecting that this family of genes may control cell proliferation and pollen growth. In addition, the expression levels of CqLIM03, CqLIM06, and CqLIM09 were higher in stems, demonstrating that LIM genes have tissue-specific expression (Eliasson et al. 2000), and suggesting that these three genes might be significantly involved in in lignin metabolism. In addition, LIM genes have been observed as induced by various stresses and hormones in many plants (Khatun et al. 2016). This is likely due to the fact that LIM proteins (AtLIM) regulate filamentous actin bundle assembly, protect their depolymerization, and control actin dynamics (Day et al. 2011). Actin is a vital signaling molecule; the actin cytoskeleton can recognize various abiotic and biotic stimuli. Research has shown that ABA can significantly induce the expression of PbLIMs in pear (Cheng et al. 2019). Similarly, in tomato, ABA, cold, drought, NaCl and heat treatments can induce the expression of LIM genes in tomato (Khatun et al. 2016). The LIM genes of grape can be induced by low temperature, ABA, and pH (pH 5, pH 7 and pH 9 treatments) (Park et al. 2014). Similar results were also reported in this study; under treatments of low temperature, salt, and hormone (ABA), the nine LIM genes measured using RT-qPCR were upregulated, indicating that these genes play an important role in regulating plant growth under abiotic and biotic stress. Interestingly, replicative gene pairs (CqLIM01/CqLIM08, CqLIM02/CqLIM07, and CqLIM03/CqLIM06) shared expression patterns. This might mean that the expression of one of the gene is no longer dominant, or it may be a redundant phenomenon, which is common in the biological world. There was also one replicative gene pair (CqLIM05/CqLIM09) in which each gene had a distinct expression pattern, which might indicate that members of this gene family are constantly dividing. This research may indicate that LIM genes have diverse functions in different species.
Conclusion
In conclusion, we identified nine LIM genes in quinoa, and comprehensively and systematically investigated these LIM family members regarding their basic physical and chemical properties, phylogenetic relationships, gene structures of exons/introns, conserved motif compositions, gene replication events, promoter elements, and expression profiles. The expression patterns of these family members under abiotic stress treatments (low temperature, salt, and ABA) were investigated using RT-qPCR. This study provides comprehensive information on the LIM family of quinoa and a deeper understanding of the structural and functional relationships among its members. These results present a robust framework for the identification and study of molecular evolution of the LIM family as well as lay an important foundation for future functional research on CqLIM genes.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all contributors to this research for their help. The research was financially supported by National Natural Science Foundation of China, Gansu Provincial Key Laboratory of Aridland Crop Science.
Author contributions
Data curation, X.W; Funding acquisition, X.W. and C.Z.; Methodology, C.Z., X.W., and X.Z.; Project administration,X.W.; Resources, L.H.; Supervision,X.Z and X.W.; Writing—original draft, B.W.; Writing—review and editing, X.Z and X.W.
Funding
The research was financially supported by National Natural Science Foundation of China (no. 32060401).
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaolin Zhu, Email: 2428498183@qq.com.
Baoqiang Wang, Email: 1839699160@qq.com.
Xian Wang, Email: 1146459429@qq.com.
Chaoyang Zhang, Email: 2087927552@qq.com.
Xiaohong Wei, Email: weixh@gsau.edu.cn.
References
- Arnaud D, Dejardin A, Leple JC, Lesage-Descauses MC, Pilate G. Genome-wide analysis of LIM gene family in populus trichocarpa, arabidopsis thaliana, and oryza sativa. DNA Res. 2007;14:103–116. doi: 10.1093/dnares/dsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud D, Déjardin A, Leplé JC. Expression analysis of LIM gene family in poplar, toward an updated phylogenetic classification. BMC Res Notes. 2012;5(1):102. doi: 10.1186/1756-0500-5-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R, Evrard JL, Domon C, Steinmetz A. A LIM motif is present in a pollen-specific protein. Plant Cell. 1992;9:264–268. doi: 10.1105/tpc.4.12.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R, Schmit AC, Kohnen M, Hentges F, Steinmetz A. Differential localization of the LIM domain protein PLIM-1 in microspores and mature pollen grains from sunflower. Sex Plant Reprod. 1999;12(1):60–65. [Google Scholar]
- Brière C, Bordel AC, Barthou H, Jauneau A, Steinmetz A, Alibert G, Petitprez M. Is the LIM-domain protein Ha WLIM1 associated with cortical microtubules in sun flower protoplasts. Plant Cell Physiol. 2003;44(10):105–1063. doi: 10.1093/pcp/pcg126. [DOI] [PubMed] [Google Scholar]
- Cai XH. Genome-wide analysis of LIM Genes and evolution pattens in Zea mays L. Hefei: Shandong Agriculture University; 2011. [Google Scholar]
- Cannon SB, Mitra A, Baumgarten A, Young ND, May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4(1):1–21. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Chen Z, Zhao Y, Cheng BJ, Xiang Y. Genome-wide analysis of soybean HD-Zip gene family and expression profiling under salinity and drought treatments. PLoS ONE. 2014;9:e87156. doi: 10.1371/journal.pone.0087156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH. TBtools, a toolkit for biologists integrating various HTS-data handling tools with a user-friendly interface. Mol Plant. 2020;13(8):1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Cheng X, Li G, Muhammad A, Zhang J, Jiang T, Jin Q, Zhao H, Cai Y, Lin Y. Molecular identification, phylogenomic characterization and expression patterns analysis of the LIM (LIN-11, Isl1 and MEC-3 domains) gene family in pear (Pyrus bretschneideri) reveal its potential role in lignin metabolism. Gene. 2019;686:237–249. doi: 10.1016/j.gene.2018.11.064. [DOI] [PubMed] [Google Scholar]
- Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–162. doi: 10.1016/s0168-9525(98)01424-3. [DOI] [PubMed] [Google Scholar]
- Dawid IB, Toyama R, Taira M. Lim domain proteins. Comptes Rendus de l'Académie des Sciences de Paris. 1995;318:295–306. [PubMed] [Google Scholar]
- Day B, Henty JL, Porter KJ, Staiger CJ. The pathogen-actin connection: a platform for defense signaling in plants. Annu. Rev. Phytopathol. 2011;49(1):483–506. doi: 10.1146/annurev-phyto-072910-095426. [DOI] [PubMed] [Google Scholar]
- Eliasson A, Gass N, Mundel C, Baltz R, Krauter R, Evrard JL, Steinmetz A. Molecular and expression analysis of a LIM protein gene family from flowering plants. Mol Gen Genet. 2000;264:257–267. doi: 10.1007/s004380000312. [DOI] [PubMed] [Google Scholar]
- Fan B, Liu MX, Li HJ, Zhang X, Wang XM, Cui XY. Bioinformatics analysis of AAP gene family in Arabidopsis thaliaha. Chem Life. 2015;36(3):372–378. [Google Scholar]
- Gabaldón T, Koonin EV. Functional and evolutionary implications of gene orthology. Nat Rev Genet. 2013;14:360–366. doi: 10.1038/nrg3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen Y, Chen M, Wang S, Wen X, Zhang S. Identification of key candidate genes and biological pathways in bladder cancer. PeerJ. 2018;6(4):e6036. doi: 10.7717/peerj.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardy JL, Laird MR, Chen F, Rey S, Walsh CJ, Ester M. PSORTb v.2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics. 2005;21(5):617–623. doi: 10.1093/bioinformatics/bti057. [DOI] [PubMed] [Google Scholar]
- Graf BL, Rojas-Silva P, Rojo LE, Delatorre-Herrera J, Baldeón ME, Raskin I. Innovations in health value and functional food development of Quinoa (Chenopodium quinoa Willd.) Compr Rev Food Sci Food Saf. 2015;14(4):431–445. doi: 10.1111/1541-4337.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L-B, Li Y-B, Wang H-Y, Wu X-M, Li C-L, Luo M, Wu S-J, Kong Z-S, Pei Y, Jiao G-L, Xia G-X. The dual functions of WLIM1a in cell elongation and secondary wall formation in developing cotton fibers. Plant Cell. 2013;25:4421–4438. doi: 10.1105/tpc.113.116970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen X, Song R, Yu M, Sui J, Wang J, Qiao L. Cloning and functional analysis of the chitinase gene promoter in peanut. Genet Mol Res. 2015;14(4):12710. doi: 10.4238/2015.October.19.15. [DOI] [PubMed] [Google Scholar]
- Hu B, Jin J, Guo AY, Zhang H, Luo J, Gao G. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics. 2015;31(8):1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong-In P, Nasar UA, Hee-Jeong J. Identification and characterization of LIM gene family in Brassica rapa. BMC Genom. 2014;15(1):641. doi: 10.1186/1471-2164-15-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka A, Ebinuma H. Transcriptional control of lignin biosynthesis by tobacco LIM protein. Phytochemistry. 2001;57(7):1149–1157. doi: 10.1016/s0031-9422(01)00054-1. [DOI] [PubMed] [Google Scholar]
- Kawaoka A, Kaothien P, Yoshida K, Endo S, Yamada K, Ebinuma H. Functional analysis of tobacco LIM protein NtLIM1 involved in lignin biosynthesis. Plant J. 2000;22:289–301. doi: 10.1046/j.1365-313x.2000.00737.x. [DOI] [PubMed] [Google Scholar]
- Kaufmann K, Melzer R, Theiβen G. MIKC-type MADS-domain proteins: structural modularity, protein interactions and network evolution in land plants. Gene. 2005;347(2):183–198. doi: 10.1016/j.gene.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mole Biol Evol. 2006;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun K, Robin AK, Park JI, Ahmed NU, Kim CK, Lim KB, Kim MB, Lee DJ, Nou IS, Chung MY. Genome-wide identification, characterization and expression profiling of LIM family genes in Solanum lycopersicum L. Plant Physiol Biochem. 2016;108:177–190. doi: 10.1016/j.plaphy.2016.07.006. [DOI] [PubMed] [Google Scholar]
- Kim JS, Mizoi J, Yoshida T, Fujita Y, Nakajima J, Ohori T, Todaka D, Nakashima K, Hirayama T, Shinozaki K, Yamaguchi-Shinozaki K. An ABRE promoter sequence is involved in osmotic stress-responsive expression of the DREB2A gene, which encodes a transcription factor regulating drought-inducible genes in Arabidopsis. Plant Cell Physiol. 2011;52:2136–2146. doi: 10.1093/pcp/pcr143. [DOI] [PubMed] [Google Scholar]
- Labalette C, Nouet Y, Levillayer F, Colnot S, Chen J, Claude V, Huerre M, Perret C, Buendia MA, Wei Y. Deficiency of the LIM-only protein FHL2 reduces intestinal tumorigenesis in Apc mutant mice. PLoS ONE. 2010;5:e10371. doi: 10.1371/journal.pone.0010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van PY. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucl Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RX, Zhu FD, Duan D. Function analysis and stress-mediated cis-element identification in the promoter region of VqMYB15. Plant Signal Behav. 2020;15(7):1773664. doi: 10.1080/15592324.2020.1773664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jiang J, Li L, Wang XL, Wang NN, Li DD, Li XB. A cotton LIM domain-containing protein (ghwlim5) is involved in bundling actin filaments. Plant Physiol Biochem. 2013;66:34–40. doi: 10.1016/j.plaphy.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR. Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lynch M, Conery JS. The evolutionary demography of duplicate genes. J Struct Funct Genom. 2003;3:35–44. [PubMed] [Google Scholar]
- Maul RS, Song Y, Amann KJ, Gerbin SC, Pollard TD, Chang DD. EPLIN regulates actin dynamics by cross-linking and stabilizing filaments. J Cell Biol. 2003;160(6):399–407. doi: 10.1083/jcb.200212057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moes D, Gatti S, Hoffmann C, Dieterle M, Moreau F, Neumann K, Schumacher M, Diederich M, Grill E, Shen WH. A LIM domain protein from tobacco involved in actin-bundling and histone gene transcription. Mole Plant. 2012;6(2):483–502. doi: 10.1093/mp/sss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel C, Baltz R, Eliasson A, Bronner R, Grass N, Kruter R, Evrard JL, Steinmetz A. A LIM-domain protein from sunflower is localized to the cytoplasm and/or nucleus in a wide variety of tissues and is associated with the phragmoplast in dividing cells. Plant Mol Biol. 2000;42(2):291–302. doi: 10.1023/a:1006333611189. [DOI] [PubMed] [Google Scholar]
- Pant P, Iqbal Z, Pandey BK, Sawant SV. Genome-wide comparative and evolutionary analysis of calmodulin-binding transcription activator (CAMTA) family in Gossypium species. Sci Rep. 2018;8(1):5573. doi: 10.1038/s41598-018-23846-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papuga J, Hoffmann C, Dieterle M, Moes D, Moreau F, Tholl S, Steinmetz A, Thomas C. Arabidopsis LIM proteins: a family of actin bundlers with distinct expression patterns and modes of regulation. Plant Cell. 2010;22:3034–3052. doi: 10.1105/tpc.110.075960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papuga J, Thomas C, Dieterle M, Moreau F, Steinmetz A. Arabidopsis LIM domain proteins involved in actin bundling exhibit different modes of regulation. FEBS J. 2009;276:245. [Google Scholar]
- Park JI, Ahmed NU, Jung HJ, Arasan SK, Chung MY, Cho YG, Watanabe M, Nou IS. Identification and characterization of LIM gene family in brassica rapa. BMC Genom. 2014;15:641. doi: 10.1186/1471-2164-15-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala S, Ampe C. An emerging link between lim domain proteins and nuclear receptors. Cell Mol Life Sci. 2018;75:1959–1971. doi: 10.1007/s00018-018-2774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O. How introns enhance gene expression. Int J Biochem Cell Biol. 2017;91:145–155. doi: 10.1016/j.biocel.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Shen XX, Salichos L, Rokas A. A genome-scale investigation of how sequence, function, and tree-based gene properties influence phylogenetic inference. Genome Biol Evol. 2016;8:2565–2580. doi: 10.1093/gbe/evw179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V, Verma PK. The plant LIM proteins: unlocking the hidden attractions. Planta. 2017;246:365–375. doi: 10.1007/s00425-017-2715-7. [DOI] [PubMed] [Google Scholar]
- Suyama M, Torrents D, Bork P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucl Acids Res. 2006;34:W609–W612. doi: 10.1093/nar/gkl315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucl Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven DC. Molecular genetic studies confirm the role of brassinosteroids in plant growth and development. Plant J. 1996;10(1):1–8. doi: 10.1046/j.1365-313x.1996.10010001.x. [DOI] [PubMed] [Google Scholar]
- Tian F, Yang DC, Meng YQ, Jin JP, Gao G. PlantRegMap: charting functional regulatory maps in plants. Nucl Acids Res. 2019 doi: 10.1093/nar/gkz1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Dieterle M, Gatti S, Hoffmann C, Moreau F, Papuga J, Steinmetz A. Actin bundling via LIM domains. Plant Signal Behav. 2008;3(5):3200–3211. doi: 10.4161/psb.3.5.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Hoffmann C, Dieterle M, Van Troys M, Ampe C, Steinmetza A. Tobacco wlim1 is a novel f-actin binding protein involved in actin cytoskeleton remodeling. Plant Cell. 2006;18:2194–2206. doi: 10.1105/tpc.106.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohge T, Fernie AR. Combining genetic diversity, informatics and metabolomics to facilitate annotation of plant gene function. Nat Protoc. 2010;5:1210–1227. doi: 10.1038/nprot.2010.82. [DOI] [PubMed] [Google Scholar]
- Tran TC, Singleton C, Fraley TS, Greenwood JA. Cysteine-rich protein 1(CRP1) regulates actin filament bundling. BMC Cell Biol. 2005;6:45. doi: 10.1186/1471-2121-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vision TJ, Brown DG, Tanksley SD. The origins of genomic duplications in Arabidopsis. Science. 2000;290:2114–2117. doi: 10.1126/science.290.5499.2114. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang L, Huang R. Cytoskeleton and plant salt stress tolerance. Plant Signal Behav. 2011;6(1):29–31. doi: 10.4161/psb.6.1.14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu GJ, Yan XF, Wei ZG, Xu ZR. MeJA-inducible expression of the heterologous jaz2 promoter from arabidopsis in populus trichocarpa protoplasts. Plant Dis. 2011;118:69–74. [Google Scholar]
- Wang Y, Wang C, Rajaofera M, Jayne N, Zhu L, Liu WB, Zheng FC, Miao WG. WY7 is a newly identified promoter from the rubber powdery mildew pathogen that regulates exogenous gene expression in both monocots and dicots. PLoS ONE. 2020;15(6):e0233911. doi: 10.1371/journal.pone.0233911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Paterson AH. Genome and gene duplications and gene expression divergence: a view from plants. Ann N Y Acad Sci. 2012;1256(1):1–14. doi: 10.1111/j.1749-6632.2011.06384.x. [DOI] [PubMed] [Google Scholar]
- Wasteneys GO, Yang Z. The cytoskeleton becomes multidisciplinary. Plant Physiol. 2004;136(4):3853–3854. doi: 10.1104/pp.104.900130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way JC, Chalfie M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell. 1988;54:5–16. doi: 10.1016/0092-8674(88)90174-2. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhu G, Wang C, Chen L, Song Y, Wang J. Regulation of secondary xylem formation in young hybrid poplars by modifying the expression levels of the PtaGLIMa gene. Mol Breed. 2017;37:37–40. [Google Scholar]
- Yang R, Chen M, Sun JC. Genome-Wide Analysis of LIM Family Genes in Foxtail Millet (Setaria italica L.) and Characterization of the Role of SiWLIM2b in Drought Tolerance. Int J Mol Sci. 2019;20(6):1303–1309. doi: 10.3390/ijms20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Xu M. Actin bundler PLIM2s are involved in the regulation of pollen development and tube growth in Arabidopsis. J Plant Physiol. 2012;169(5):516–522. doi: 10.1016/j.jplph.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cheng T, Wang G, Yan Y. Cloning and evolutionary analysis of tobacco MAPK gene family. Mol Biol Rep. 2013;40:1407–1415. doi: 10.1007/s11033-012-2184-9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li J, Zhao XQ, Wang J, Wong GKS, Yu J. KaKs_calculator: calculating Ka and Ks through model selection and model averaging. Genom Proteom Bioinform. 2006;4:259–263. doi: 10.1016/S1672-0229(07)60007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gao S, Lercher MJ, Hu S, Chen WH. EvolView, an online tool for visualizing, annotating and managing phylogenetic trees. Nucl Acids Res. 2012;4:W569–W572. doi: 10.1093/nar/gks576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, He L, Gu Y, Wang Y, Chen Q, He C. Genome-wide analyses of a plant-specific LIM-domain gene family implicate its evolutionary role in plant diversification. Genome Biol Evol. 2014;6:1000–1012. doi: 10.1093/gbe/evu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, Zhao Y. The diverse biofunctions of LIM domain proteins: determined by subcellular localization and protein-protein interaction. Biol Cell. 2007;99:489–502. doi: 10.1042/BC20060126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.