Abstract
The infectious microscopic viruses invade living cells to reproduce themselves, and causes chronic infections such as HIV/AIDS, hepatitis B and C, flu, etc. in humans which may lead to death if not treated. Different strategies have been utilized to develop new and superior antiviral drugs to counter the viral infections. The FDA approval of HIV nucleoside reverse transcriptase inhibitor, zidovudine in 1987 boosted the development of antiviral agents against different viruses. Currently, there are a number of combination drugs developed against various viral infections to arrest the activity of same or different viral macromolecules at multiple stages of its life cycle; among which majority are targeted to interfere with the replication of viral genome. Besides these, other type of antiviral molecules includes entry inhibitors, integrase inhibitors, protease inhibitors, interferons, immunomodulators, etc. The antiviral drugs can be toxic to human cells, particularly in case of administration of combination drugs, and on the other hand viruses can grow resistant to the antiviral drugs. Furthermore, emergence of new viruses like Ebola, coronaviruses (SARS-CoV, SARS-CoV-2) emphasizes the need for more innovative strategies to develop better antiviral drugs to fight the existing and the emerging viral infections. Hence, we reviewed the strategic enhancements in developing antiviral drugs for the treatment of different viral infections over the years.
Keywords: Antiviral drugs, NRTIs, NNRTIs, Protease inhibitors, Entry inhibitors, Combination therapies, Virus pandemics
1. Introduction
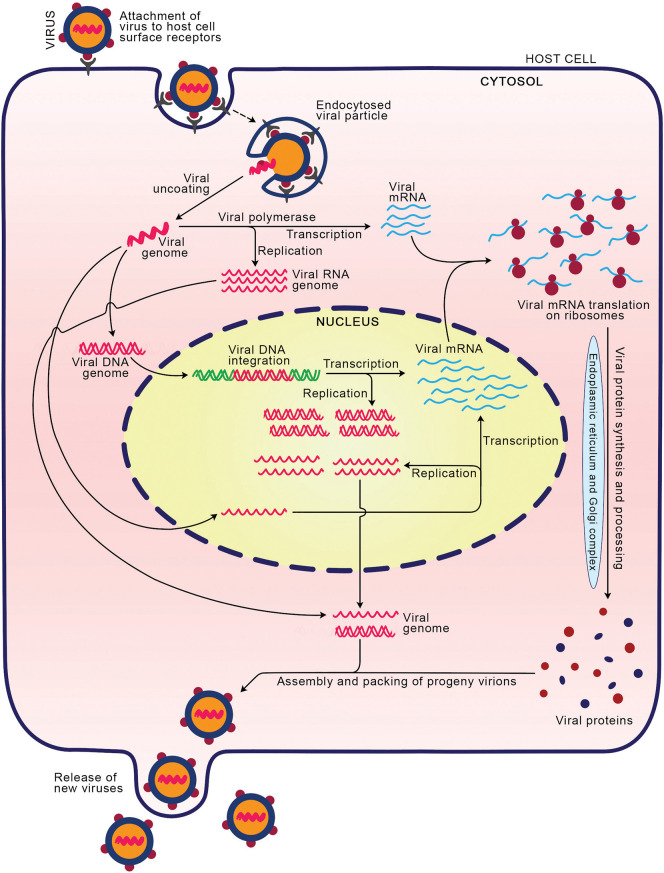
A virus is a microscopic organism that cannot reproduce by itself outside the host body. The viruses carry either ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) as their genetic material which can be single-stranded or double-stranded [1]. The infections by viruses in humans caused millions of deaths around the globe and are accountable for the human diseases like HIV/AIDS, hepatitis, influenza, herpes simplex, common cold, etc. [[2], [3], [4]]. The infection process varies among viral species; however, they follow certain common steps in infecting the animal hosts. The typical stages of virus infection include (i) attachment and entry: initially, the glycoproteins on the envelope of virus attach to the receptor/co-receptor molecules on the host cell membrane, which facilitates the entry of virus inside the host cell through endocytosis [5,6]. (ii) Virus uncoating: the capsid of internalized virus is degraded by the host cell enzymes and the viral components (genetic material and proteins) released into the host cytosol. (iii) Replication and transcription of viral genome: the viral genome comprising DNA or RNA is transported into the nucleus where its replication and transcription occurs to produce the multiple copies of genome and messenger RNA (mRNA) molecules, respectively (Fig. 1 ). The replication mechanism varies based on the genome type such as DNA/RNA, single/double-stranded, positive/negative sense [[6], [7], [8], [9]]. The RNA genome can replicate in the cytoplasm itself [5,6]. (iv) Protein synthesis: the viral mRNAs are translated into structural and regulatory proteins in the cytoplasm utilizing the host cell protein synthetic machinery. (v) Assembly: successful replication and expression of the viral genome produces the components that are essential for the survival of the progeny virus after release from host cell. Thus, all the necessary components are packed together to produce new viruses. (vi) Release: the assembled progeny viruses are released into the extracellular fluid through lysis of the host cell or budding. The lysis process causes death of the host cell whereas budding may not [5,6]. The infectious nature of viruses enforced the scientific community to develop antiviral drug molecules to limit the survival and spreading of viruses through blocking any one or combination of above stages of virus life cycle. Antiviral drug is an agent (small or large molecule(s), synthetic or natural) that can reduce the infectious disease caused by a virus. In 1963, the US Food and Drug Administration (FDA) agency approved the first antiviral drug, idoxuridine to treat the infection caused by herpes simplex virus [10,11]. The upsurge in chronic viral infections such as HIV, HCV, HBV, etc. and the emergence of new viruses like severe acute respiratory syndrome (SARS) coronaviruses [[12], [13], [14], [15]] emphasize the growing need for novel strategies to develop the antiviral agents. Particularly, emergence of human immunodeficiency virus (HIV) associated acquired immune deficiency syndrome (AIDS) epidemic around the world during 1980s, improved the efforts leading to advancements in therapeutic innovations as well as basic science. This led to development of antiviral inhibitors against not only HIV but also included other viruses (Fig. 2a).
Fig. 1.
The life cycle of viruses. The virus initially attaches to the host cell receptors via its surface proteins which facilitates its internalization. The internalized virus releases its genome into the cytosol to be replicated (RNA in cytosol and DNA in nucleus), transcribed and translated, to produce essential viral proteins. The viral components assemble together to develop into progeny virions which are released into extracellular space through budding or lysis of host cell.
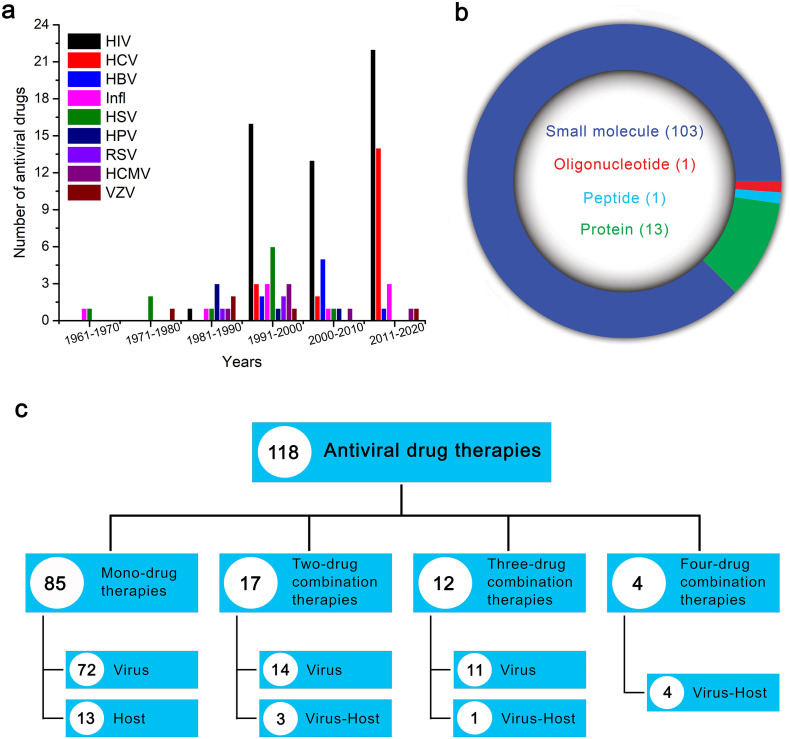
Fig. 2.
Trends in antiviral drugs development. (a) Comparison of number of antiviral drugs developed against different viral diseases since 1960s. Major breakthrough in development of antiviral therapeutics for different viral infections occurred after the emergence of HIV (1991–2020). (b) Among the FDA approved antiviral therapies, majority are small molecules and the smaller fraction oligomeric molecules include proteins, peptides and oligonucleotides. (c) Division of the 118 antiviral therapies based on number of their drug components and target of action. HIV - human immunodeficiency virus, HCV - hepatitis C virus, HBV - hepatitis B virus, Infl - influenza virus, HSV - herpes simplex virus, HPV - human papillomavirus, RSV - respiratory syncytial virus, HCMV - human cytomegalovirus, VZV - varicella-zoster virus.
Among the FDA approved antiviral compounds, majority are small molecules (Fig. 2b) with diverse roles in clinical use. The large molecules approved as antiviral drugs include proteins (interferons, monoclonal antibodies), peptides, and oligonucleotides. Most of these FDA-approved antiviral drugs target the virus cellular machinery, on the other hand very few of them target the host cells/cellular mechanisms (Fig. 2c). The antiviral drugs are administered as mono and combination therapies. In mono-therapies, the antiviral agents target either virus or host systems, whereas in case of combination therapies, although major number of therapies target viral proteins, few targets both the viral and host proteins (Fig. 2c). The FDA approved drug molecules have different mechanisms of antiviral activity and based on their structure and/or function could be grouped into structural analogues (nucleoside analogues, non-nucleoside pyrophosphate analogues, 5-substituted 2′-deoxyuridine analogues, acyclic nucleoside phosphonate analogues, acyclic guanosine analogues), entry inhibitors, integrase inhibitors, nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors, inhibitors specific to certain viruses (influenza virus inhibitors, and hepatitis C virus NS5A protein and NS5B polymerase inhibitors), and interferons, immunomodulators, antimitotic inhibitors and oligonucleotides.
In this review, we summarized all the antiviral agents that are developed since the approval of first drug molecule by FDA in 1963, against the major infectious viruses. The development of diverse drug molecules with the underlying strategies, against each viral infection are described. Additionally, we included brief description on the pandemics of 21st century and their treatment, with emphasis on coronaviruses.
2. FDA-approved drugs against viral infections in humans
2.1. Human immunodeficiency virus infections
Human immunodeficiency virus which causes AIDS was discovered in 1983 [16,17]. HIV belongs to retroviridae family, containing a linear, single-stranded RNA (ssRNA) genome. HIV exists in two major types viz., HIV-1 (most common) and HIV-2 (uncommon and less infectious), and the high genetic variations in its genome makes HIV a fastest-evolving organism [18]. The blood or body fluids contaminated with HIV are the source of its transmission. According to the WHO, 770000 people died from HIV-related causes in 2018 and approximately 37.9 million people are living with HIV at the end of 2018.
Zidovudine (Retrovir) was the first drug molecule developed against HIV [19], approved for treatment in 1987. It is a pyrimidine analogue, thus inhibits nucleoside reverse transcriptase activity and HIV-DNA replication. Following this, other RT inhibitors such as didanosine (Videx) [20], zalcitabine (Hivid) [20], stavudine (Zerit) [21,22], lamivudine (Epivir) [23], abacavir sulfate (Ziagen) [24], etc. (Table 1 ) were developed to treat HIV infections. Saquinavir mesylate (Invirase) was the first approved protease inhibitor (PI) that blocks the activity of HIV protease by binding to its active site which result in unprocessed viral proteins thereby prevents HIV multiplication. In 2007, first integrase inhibitor raltegravir (Isentress) was approved as HIV inhibitor which blocks the integration of its DNA into host genome. In 1997, the first combination therapy was approved with a fixed dose of the reverse transcriptase inhibitors, lamivudine and zidovudine (Combivir) for the treatment of HIV-infected patients. Kaletra, another combination drug constituted of a HIV protease inhibitor, lopinavir and ritonavir which prevents lopinavir metabolism in the host cell by inhibiting host cytochrome protein CYP3A was approved in 2000 for HIV infections treatment. CYP3A is a subfamily of cytochrome P450 (CYP) enzymes, are essential for metabolism of many drugs. CYP3A4 is the most important isoform and located abundantly in liver and small intestine. The low substrate specificity of CYP3A4 makes it susceptible to reversible or irreversible inhibition by a variety of drugs utilizing NADPH, in time- and dose-dependent manner [25]. Inhibition of CYP3A increases the bioavailability of combination drug molecules helping in their preferred therapeutic action. Following Kaletra, Trizivir, a fixed dose tablet of three components (abacavir sulfate, lamivudine and zidovudine) was approved against HIV infection that targets viral replication by inhibiting its reverse transcriptase enzyme. Later, there have been a number of combination therapies approved for HIV infections treatment. The combination therapies are chiefly aimed to stop or slow down the development of drug resistance in viruses by arresting their replication and/or other critical cellular processes at multiple points of life cycle (Fig. 3 ).
Table 1.
List of drugs approved by US Food and Drug Administration (FDA) agency for the treatment of viral infections in humans.
| Year | Trade name | Generic name | Molecule type | Therapy type | Hostb/viral target(s) |
|---|---|---|---|---|---|
| Human immunodeficiency virus (HIV) | |||||
| 1987 | Retrovir | Zidovudine (AZT) | Small molecule | Mono | RT |
| 1991 | Videx | Didanosine (ddI) | Small molecule | Mono | RT |
| 1992 | Hivida | Zalcitabine (ddC) | Small molecule | Mono | RT |
| 1994 | Zerit | Stavudine (d4T) | Small molecule | Mono | RT |
| 1995 | Epivir | Lamivudine (3TC) | Small molecule | Mono | RT |
| 1995 | Invirase | Saquinavir mesylate (SQV) | Small molecule | Mono | Protease |
| 1996 | Crixivan | Indinavir sulfate (IDV) | Small molecule | Mono | Protease |
| 1996 | Norvir | Ritonavir (RTV) | Small molecule | Mono | Protease |
| 1996 | Viramune | Nevirapine (NVP) | Small molecule | Mono | RT |
| 1997 | Combivir | Lamivudine (3TC)/zidovudine (AZT) | Small molecule | Combo | RT/RT |
| 1997 | Rescriptora | Delavirdine mesylate (DLV) | Small molecule | Mono | RT |
| 1997 | Viracept | Nelfinavir mesylate (NFV) | Small molecule | Mono | Protease |
| 1998 | Sustiva | Efavirenz (EFV) | Small molecule | Mono | RT |
| 1998 | Ziagen | Abacavir sulfate (ABC) | Small molecule | Mono | RT |
| 1999 | Agenerasea | Amprenavir (APV) | Small molecule | Mono | Protease |
| 2000 | Kaletra | Lopinavir (LPV)/ritonavir (RTV) | Small molecule | Combo | Protease/CYP3Ab |
| 2000 | Trizivir | Abacavir sulfate (ABC)/lamivudine (3TC)/zidovudine (AZT) | Small molecule | Combo | RT/RT/RT |
| 2001 | Viread | Tenofovir disoproxil fumarate (TDF) | Small molecule | Mono | RT |
| 2003 | Emtriva | Emtricitabine (FTC) | Small molecule | Mono | RT |
| 2003 | Fuzeon | Enfuvirtide (T20) | Peptide | Mono | GP41 glycoprotein |
| 2003 | Lexiva | Fosamprenavir calcium (FPV) | Small molecule | Mono | Protease |
| 2003 | Reyataz | Atazanavir sulfate (ATV) | Small molecule | Mono | Protease |
| 2004 | Epzicom | Abacavir sulfate (ABC)/lamivudine (3TC) | Small molecule | Combo | RT/RT |
| 2004 | Truvada | Emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | RT/RT |
| 2005 | Aptivus | Tipranavir (TPV) | Small molecule | Mono | Protease |
| 2006 | Atripla | Efavirenz (EFV)/emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | RT/RT/RT |
| 2006 | Prezista | Darunavir ethanolate (DRV) | Small molecule | Mono | Protease |
| 2007 | Isentress | Raltegravir potassium (RAL) | Small molecule | Mono | Integrase |
| 2007 | Selzentry | Maraviroc (MVC) | Small molecule | Mono | Host CCR5 receptorb |
| 2008 | Intelence | Etravirine (ETR) | Small molecule | Mono | RT |
| 2011 | Complera | Emtricitabine (FTC)/rilpivirine hydrochloride (RPV)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | RT/RT/RT |
| 2011 | Edurant | Rilpivirine hydrochloride (RPV) | Small molecule | Mono | RT |
| 2012 | Stribild | Cobicistat (COBI)/elvitegravir (EVG)/emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | CYP3Ab/integrase/RT/RT |
| 2013 | Tivicay | Dolutegravir sodium (DTG) | Small molecule | Mono | Integrase |
| 2014 | Triumeq | Abacavir sulfate (ABC)/dolutegravir sodium (DTG)/lamivudine (3TC) | Small molecule | Combo | RT/integrase/RT |
| 2014 | Vitektaa | Elvitegravir (EVG) | Small molecule | Mono | Integrase |
| 2015 | Dutrebisa | Lamivudine (3TC)/raltegravir (RAL) | Small molecule | Combo | RT/integrase |
| 2015 | Evotaz | Atazanavir sulfate (ATV)/cobicistat (COBI) | Small molecule | Combo | Protease/CYP3Ab |
| 2015 | Genvoya | Cobicistat (COBI)/elvitegravir (EVG)/emtricitabine (FTC)/tenofovir alafenamide fumarate (TAF) | Small molecule | Combo | CYP3Ab/integrase/RT/RT |
| 2015 | Prezcobix | Cobicistat (COBI)/darunavir ethanolate (DRV) | Small molecule | Combo | CYP3Ab/protease |
| 2016 | Descovy | Emtricitabine (FTC)/tenofovir alafenamide fumarate (TAF) | Small molecule | Combo | RT/RT |
| 2016 | Odefsey | Emtricitabine (FTC)/rilpivirine hydrochloride (RPV)/tenofovir alafenamide fumarate (TAF) | Small molecule | Combo | RT/RT/RT |
| 2017 | Juluca | Dolutegravir (DTG)/rilpivirine (RPV) | Small molecule | Combo | Integrase/RT |
| 2018 | Biktarvy | Bictegravir (BIC)/emtricitabine (FTC)/tenofovir alafenamide fumarate (TAF) | Small molecule | Combo | Integrase/RT/RT |
| 2018 | Cimduo | Lamivudine (3TC)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | RT/RT |
| 2018 | Delstrigo | Doravirine (DOR)/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | RT/RT/RT |
| 2018 | Pifeltro | Doravirine (DOR) | Small molecule | Mono | RT |
| 2018 | Symfi | Efavirenz (EFV)/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | RT/RT/RT |
| 2018 | Symfi Lo | Efavirenz (EFV)/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF) | Small molecule | Combo | RT/RT/RT |
| 2018 | Symtuza | Darunavir (DRV)/cobicistat (COBI)/emtricitabine (FTC)/tenofovir alafenamide (TAF) | Small molecule | Combo | Protease/CYP3Ab/RT/RT |
| 2018 | Trogarzo | Ibalizumab-uiyk (IBA) | Protein | Mono | Host CD4+ T cellsb |
| 2019 | Dovato | Dolutegravir (DTG)/lamivudine (3TC) | Small molecule | Combo | Integrase/RT |
| Hepatitis C virus (HCV) | |||||
| 1991 | Intron A | Interferon alfa-2b (INT2B) | Protein | Mono | Host immune systemb |
| 1997 | Infergena | Interferon alfacon-1 (CIFN) | Protein | Mono | Host immune systemb |
| 1998 | Rebetol | Ribavirin (RBV) | Small molecule | Mono | RNA polymerase |
| 2001 | Pegintron | Peginterferon alfa-2b (PEG2b) | Protein | Mono | Host immune systemb |
| 2002 | Pegasys | Peginterferon alfa-2a (PEG2a) | Protein | Mono | Host immune systemb |
| 2011 | Victrelisa | Boceprevir (BOC) | Small molecule | Mono | NS3/4A protease |
| 2011 | Inciveka | Telaprevir (TPV) | Small molecule | Mono | NS3/4A protease |
| 2013 | Olysioa | Simeprevir sodium (SIM) | Small molecule | Mono | NS3/4A protease |
| 2013 | Sovaldi | Sofosbuvir (SOF) | Small molecule | Mono | NS5B polymerase |
| 2014 | Harvoni | Ledipasvir (LED)/sofosbuvir (SOF) | Small molecule | Combo | NS5A protein/NS5B polymerase |
| 2014 | Viekira Pak | Dasabuvir sodium (DAS)/ombitasvir (OMB)/paritaprevir (PAR)/ritonavir (RTV) | Small molecule | Combo | NS5B polymerase/NS5A protein/NS3/4A protease/CYP3Ab |
| 2014 | Daklinza/Sunvepra (Japan) | Daclatasvir dihydrochloride (DCV)/asunaprevir (ASV) | Small molecule | Combo | NS5A protein/NS3/4A protease |
| 2014 | Vanihep (Japan) | Vaniprevir (VPV)/peginterferon alpha-2a (PEG2a)/ribavirin (RBV) | Small molecule | Combo | NS3/4A protease/immunomodulationb/RNA polymerase |
| 2015 | Techniviea | Ombitasvir (OMB)/paritaprevir (PAR)/ritonavir (RTV) | Small molecule | Combo | NS5A protein/NS3/4A protease/CYP3Ab |
| 2015 | Daklinza/Sovaldi | Daclatasvir dihydrochloride (DCV)/sofosbuvir (SOF) | Small molecule | Combo | NS5A protein/NS5B polymerase |
| 2016 | Zepatier | Elbasvir (ELB)/grazoprevir (GZR) | Small molecule | Combo | NS5A protein/NS3/4A protease |
| 2016 | Epclusa | Sofosbuvir (SOF)/velpatasvir (VEL) | Small molecule | Combo | NS5B polymerase/NS5A protein |
| 2017 | Vosevi | Sofosbuvir (SOF)/velpatasvir (VEL)/voxilaprevir (VOX) | Small molecule | Combo | NS5B polymerase/NS5A protein/NS3/4A protease |
| 2017 | Mavyret | Glecaprevir (GLE)/pibrentasvir (PIB) | Small molecule | Combo | NS3/4A protease/NS5A protein |
| Hepatitis B virus (HBV) | |||||
| 1992 | Intron A | Interferon alfa-2b (INT2B) | Protein | Mono | Host immune systemb |
| 1998 | Epivir-HBV | Lamivudine (3TC) | Small molecule | Mono | DNA polymerase |
| 2002 | Hepsera | Adefovir dipivoxil (ADE) | Small molecule | Mono | DNA polymerase |
| 2005 | Baraclude | Entecavir (ENT) | Small molecule | Mono | DNA polymerase |
| 2005 | Pegasys | Peginterferon alfa-2a (PEG2a) | Protein | Mono | Host immune systemb |
| 2006 | Tyzekaa | Telbivudine (LdT) | Small molecule | Mono | DNA polymerase |
| 2008 | Viread | Tenofovir disoproxil fumarate (TDF) | Small molecule | Mono | DNA polymerase |
| 2016 | Vemlidy | Tenofovir alafenamide fumarate (TAF) | Small molecule | Mono | DNA polymerase |
| Influenza virus | |||||
| 1966 | Symmetrela | Amantadine | Small molecule | Mono | M2 channel protein |
| 1985 | Virazole | Ribavirin (RBV) | Small molecule | Mono | RNA polymerase |
| 1993 | Flumadine | Rimantadine (RIM) | Small molecule | Mono | M2 channel protein |
| 1999 | Relenza | Zanamivir (ZAN) | Small molecule | Mono | Neuraminidase |
| 1999 | Tamiflu | Oseltamivir (OTV) | Small molecule | Mono | Neuraminidase |
| 2010 | Inavir (Japan) | Laninamivir octanoate (LO) | Small molecule | Mono | Neuraminidase |
| 2014 | Rapivab | Peramivir (PER) | Small molecule | Mono | Neuraminidase |
| 2014 | Avigan (Japan) | Favipiravir (FPV) | Small molecule | Mono | RNA polymerase |
| 2018 | Xofluza | Baloxavir marboxil (BXM) | Small molecule | Mono | Endonuclease |
| Herpes simplex virus (HSV) | |||||
| 1963 | Dendrida | Idoxuridine (IDU) | Small molecule | Mono | DNA polymerase |
| 1976 | Vira-Aa | Vidarabine (VDR) | Small molecule | Mono | DNA polymerase |
| 1980 | Viroptic | Trifluridine (TFT) | Small molecule | Mono | DNA polymerase |
| 1982 | Zovirax | Acyclovir (ACY) | Small molecule | Mono | DNA polymerase |
| 1991 | Foscavir | Foscarnet sodium (PFA) | Small molecule | Mono | DNA polymerase |
| 1994 | Famvir | Famciclovir (FAM) | Small molecule | Mono | DNA polymerase |
| 1995 | Valtrex | Valacyclovir hydrochloride (VAL) | Small molecule | Mono | DNA polymerase |
| 1996 | Denavir | Penciclovir (PEN) | Small molecule | Mono | DNA polymerase |
| 2000 | Abreva | Docosanol (DOC) | Small molecule | Mono | Fusion/entry of viral particles |
| 2000 | Zostex | Brivudine (BVDU) | Small molecule | Mono | DNA polymerase |
| 2009 | Xerese | Acyclovir/hydrocortisone (ACY) | Small molecule | Combo | DNA polymerase |
| Human papillomavirus (HPV) | |||||
| 1988 | Intron A | Interferon alfa-2b (INT2B) | Protein | Mono | Host immune systemb |
| 1989 | Alferon N | Interferon alfa-N3 (INTN3) | Protein | Mono | Host immune systemb |
| 1990 | Condylox | Podofilox (PDX) | Small molecule | Mono | Cell divisionb |
| 1997 | Aldara | Imiquimod (IMQ) | Small molecule | Mono | Host immune systemb |
| 2006 | Veregen | Sinecatechins (SIN) | Small molecule | Mono | Host immune systemb |
| Respiratory syncytial virus (RSV) | |||||
| 1985 | Virazole | Ribavirin (RBV) | Small molecule | Mono | RNA polymerase |
| 1996 | RespiGama | RSV-IGIV | Protein | Mono | Glycoproteins G and F |
| 1998 | Synagis | Palivizumab (PZ) | Protein | Mono | Glycoprotein F |
| Human cytomegalovirus (HCMV) | |||||
| 1989 | Cytovene | Ganciclovir sodium (GCV) | Small molecule | Mono | DNA polymerase |
| 1991 | Foscavir | Foscarnet sodium (PFA) | Small molecule | Mono | DNA polymerase |
| 1996 | Vistidea | Cidofovir (CID) | Small molecule | Mono | DNA polymerase |
| 1998 | Vitravenea | Fomivirsen sodium (FMV) | Oligo-nucleotide | Mono | Replication |
| 2001 | Valcyte | Valganciclovir hydrochloride (VALG) | Small molecule | Mono | DNA polymerase |
| 2017 | Prevymis | Letermovir (LET) | Small molecule | Mono | DNA terminase complex |
| Varicella-zoster virus (VZV) | |||||
| 1976 | Vira-Aa | Vidarabine (VDR) | Small molecule | Mono | DNA polymerase |
| 1981 | VZIGa | Varicella-zoster immunoglobulin (VZIG) | Protein | Mono | Viral particles |
| 1982 | Zovirax | Acyclovir (ACY) | Small molecule | Mono | DNA polymerase |
| 2000 | Zostex (Europe) | Brivudine (BVDU) | Small molecule | Mono | DNA polymerase |
| 2012 | VariZIG | Varicella-zoster immune globulin (VariZIG) | Protein | Mono | Viral particles |
RT - reverse transcriptase enzyme of HIV; common antiviral drugs: Foscavir for HCMV and HSV; Intron A for HBV, HCV and HPV; Pegasys for HBV and HCV; Vira A for HSV and VZV; Zostex for HSV and VZV.
Discontinued antiviral drugs.
Antiviral drugs targeting host system.
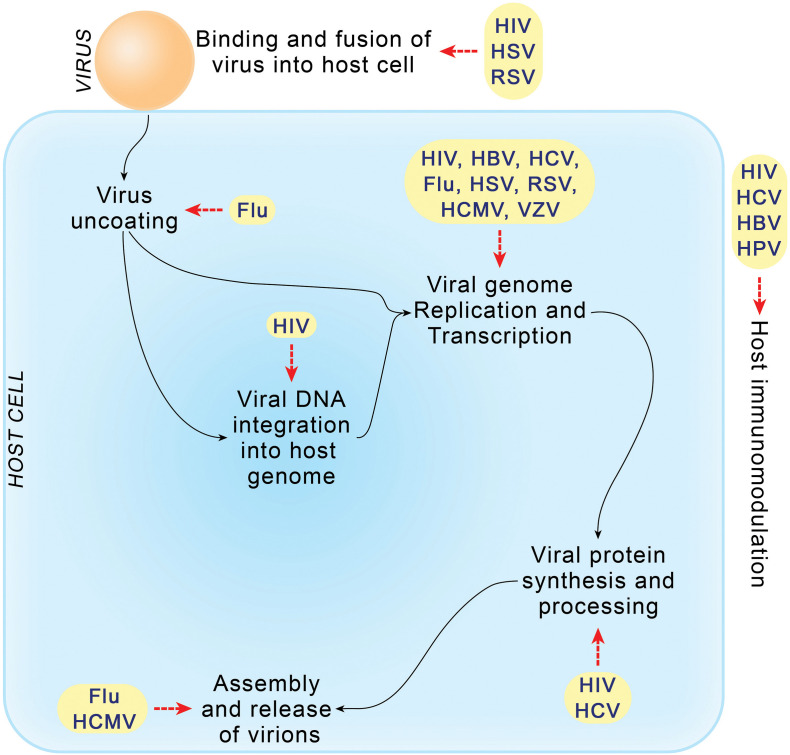
Fig. 3.
Antiviral drugs developed to target different stages of the life cycle of infectious viruses. HIV is targeted at nearly all stages of its multiplication, while viral genome replication and transcription stages of all viruses are targeted to prevent viral replication.
Enfuvirtide (Fuzeon) was the first peptide (36 amino acids) - HIV inhibitor approved in 2003 [26]. Enfuvirtide blocks the fusion of HIV with host cell membrane by mimicking the helix in heptad region 2 of the viral glycoprotein 41 (GP41) [27]. However, subcutaneous administration of this drug limited its usage for long-term application. In 2007, FDA approved another entry inhibitor that specifically targets host cellular protein. Maraviroc (Selzentry) was the first antagonist that binds host chemokine receptor (CCR5) located on the surface of T-lymphocytes and phagocytic macrophages and prevents HIV fusion into host cells. Targeting CCR5 with maraviroc in cell lines and clinical trials showed promising antiviral activity [[28], [29], [30]]. Interestingly, the human clinical trials showed that maraviroc is an efficient suppressor of R5 HIV-1 infections but not R4 HIV-1 infections [31] indicating its selective phenotype based antiviral activity.
In 2018, ibalizumab (Trogarzo), a monoclonal antibody which binds to CD4 receptors on the surface of T-cells and prevents the entry and reproduction of HIV was approved. Ibalizumab was approved for use in combination therapies for the treatment of patients infected with multidrug resistant HIV-1 [32]. The combination drug Cimduo comprising NRTIs, lamivudine and tenofovir disoproxil fumarate (TDF) was approved to treat the HIV-1 infections [33]. Doravirine (Pifeltro), a NNRTI was prescribed for the treatment of HIV-1 infection in adult patients. In addition, doravirine was also approved for the treatment with combination of the NRTIs - lamivudine, and tenofovir disoproxil fumarate (Delstrigo) [[34], [35], [36]].
In addition, bictegravir, an integrase strand transfer inhibitor (INSTI), also known as integrase inhibitor, prevents the integration of viral DNA into human genome was approved to use as a combination drug along with two NRTIs - emtricitabine and tenofovir alafenamide (Biktarvy) [37,38]. Symtuza was approved as a fixed-dose, single tablet of four drug molecules, darunavir - a protease inhibitor, cobicistat - a host CYP3A inhibitor, and two NRTIs - emtricitabine and tenofovir alafenamide [39,40]. Further FDA approved Symfi Lo and Symfi as anti-HIV-1 drugs for patients weighing at least 35 and 40 kg, respectively. These are single tablets of fixed-dose of reverse transcriptase inhibitors, efavirenz - a NNRTI, and NRTIs - lamivudine and tenofovir disoproxil fumarate [33,41]. In April 2019, the FDA approved a two-drug regimen of dolutegravir and lamivudine (Dovato) for the treatment of HIV-1 infection. Dolutegravir is an INSTI and lamivudine is a NRTI, which together blocks the protease activity and HIV-1 multiplication [42].
In addition, FDA approved the herbal tablet, Fulyzaq (crofelemer) for the treatment of non-infectious diarrhea associated with HIV/AIDS patients on anti-retroviral therapy. Each tablet contains Crofelemer (125 mg), composed of (+)-catechin, (−)-epicatechin, (+)-gallocatechin, and (−)-epigallocatechin monomers in random order, derived from the red latex of Croton lechleri Müll. Arg. [43].
2.2. Hepatitis C virus infections
Hepatitis C virus (HCV) was discovered in 1989 consisting a linear, positive-sense, single-stranded RNA genome. HCV belongs to Flaviviridae family and classified into seven major genotypes, among which genotypes 1 and 2 are most prevalent in infecting human populations [44]. The transmission of HCV occurs through transfusion of infected blood and blood products, needle sharing, sexual practices, etc. According to WHO, 71 million people were estimated to have chronic HCV infection, and nearly 399,000 people died globally from hepatitis C virus infection in 2016.
HCV employs serine proteases to process the precursor polyproteins. Currently, there are nine antiviral drugs approved for clinical use as HCV protease inhibitors among which majority are combination drugs. The FDA approved small drug molecules, glecaprevir, grazoprevir, vaniprevir, asunaprevir, paritaprevir, simeprevir, boceprevir, telaprevir, and voxilaprevir (Table 1), efficiently inhibits HCV NS3/4A protease activity [[45], [46], [47]]. Boceprevir (Victrelis) and telaprevir (Incivek), approved in 2011 were withdrawn from market for commercial reasons in 2014. In May 2018, another protease inhibitor, simeprevir (Olysio) was also discontinued from market. The combination drug tablets Viekira Pak (dasabuvir sodium, ombitasvir, paritaprevir and ritonavir) and Technivie (ombitasvir, paritaprevir and ritonavir) were approved to treat infections caused by HCV genotypes 1 and 4, respectively. Interestingly, Viekira Pak is still prescribed for the treatment while Technivie was discontinued in January 2019.
In December 2013, a novel direct-acting antiviral drug, sofosbuvir (Sovaldi) was permitted to treat the infections of HCV genotypes 1, 2, 3 and 4 [48]. Sofosbuvir binds to nonstructural NS5B polymerase and inhibits HCV replication [45]. Based on HCV genotype, ribavirin and peginterferon may be co-administered along with sofosbuvir. Soon after, a fixed-dose combination drug of sofosbuvir and ledipasvir (Harvoni) was approved to use against HCV genotypes 1 and 4 infections with or without cirrhosis [49,50]. It does not require co-administration with ribavirin or peginterferon. Epclusa (sofosbuvir and velpatasvir), a combination tablet was approved (June 2016) to treat all six hepatitis C genotypes, including patients with cirrhosis [51]. Furthermore, Vosevi (sofosbuvir, velpatasvir, voxilaprevir), another combination tablet of sofosbuvir was approved in July 2017 to treat all genotypic infections of HCV [[52], [53], [54]]. Vosevi is primarily intended for patients who did not achieve viral clearance on sofosbuvir treatment.
In Japan, the combination drugs, asunaprevir and daclatasvir dihydrochloride (Sunvepra plus Daklinza); and Vanihep (vaniprevir, ribavirin and pegylated interferon alpha-2a) were approved for treatment of HCV-1 [55]. In July 2015, daclatasvir dihydrochloride (Daklinza) which specifically binds to HCV replication associated nonstructural protein NS5A, was approved in combination with sofosbuvir for the treatment of HCV-3 infection [56,57]. In January 2016, another fixed-dose combination drug, Zepatier (elbasvir and grazoprevir) was approved for the treatment of HCV-1 or HCV-4 infection with or without cirrhosis [58,59]. Elbasvir inhibits NS5A protein activity while grazoprevir inhibits NS3/4A protease activity.
In August 2017, FDA approved the use of Mavyret, a combination drug of glecaprevir and pibrentasvir against all six genotypes of HCV, for eight to twelve weeks duration [[60], [61], [62]]. Glecaprevir and pibrentasvir blocks replication/multiplication of HCV by inhibiting the activities of NS3/4A protease and NS5A protein, respectively. Mavyret is very effective and three tablets a day with food is recommended.
2.3. Hepatitis B virus infections
Hepatitis B virus (HBV) belongs to Hepadnaviridae family, and exists in eight major genotypes with a double-stranded, circular DNA (dsDNA) genome [7]. HBV is also transmitted through direct contact with infected blood or other body fluids i.e., sharing needles, syringes, or sexual contact, or from mother to child at birth. In 2016, WHO reported that 27 million people worldwide are living with hepatitis B viral infection.
The following drugs were approved by FDA to treat HBV infections, viz., (pegylated) interferons (Intron A, Pegasys), adefovir dipivoxil (Hepsera), entecavir (Baraclude), telbivudine (Tyzeka), lamivudine (Epivir-HBV), tenofovir disoproxil fumarate (TDF) (Viread) and tenofovir alafenamide fumarate (TAF) (Vemlidy) (Table 1). The nucleoside analogues, entecavir and telbivudine were exclusively prescribed for the treatment of HBV infections unlike lamivudine and TDF which are also used for HIV inhibition. The biochemical, histological and virological analysis in HBV patients showed entecavir has higher efficacy and less drug resistance on long-term use than lamivudine [[63], [64], [65]]. In addition, the other nucleoside analogue, telbivudine also showed better inhibition of HBV DNA polymerase than lamivudine in the clinical trials [[66], [67], [68]]. Further, entecavir is strongly recommended to use over telbivudine, mainly for children between 2 and 12 years of age, on terms of its safety. However, considering the high costs of the drugs, lamivudine - the reverse transcriptase inhibitor, is generally used in first-line remedy against HBV infections irrespective of its higher pace of drug resistance [69]. Currently there are no combination drug therapies are available for the treatment of HBV infections.
2.4. Influenza virus infections
The influenza viruses belong to Orthomyxoviridae family with a linear, negative-sense ssRNA genome [70] and are divided into three types: A, B and C. The flu pandemics such as Spanish flu (1918), Asian flu (1957), Hong Kong flu (1968) [71] and Swine flu (2009) [72] were caused by Influenza A viruses.
Till April 2020, FDA approved nine antiviral drugs for the treatment of influenza infections, which include two matrix 2 (M2) ion channels inhibitors, four neuraminidase inhibitors, two polymerase inhibitors and one endonuclease inhibitor (Fig. 3) (Table 1). M2 transmembrane proteins forms proton channels in the viral envelope to maintain pH across the viral membrane during cell entry and across the trans-Golgi membrane of infected cells during viral maturation [73,74]. Neuraminidase assists the maturation stage of influenza infection by cleaving sialic acids from the host cell receptors and from hemagglutinin and neuraminidase on the surface of nascent virions. This process prevents virion aggregation and helps the release of progeny virions by stopping virus binding back to the dying host cell [75,76]. Amantadine (Symmetrel) and rimantadine (Flumadine) targets virus uncoating inside the endosomes by blocking the H+ ions passage into the viral particles through M2 channels [77,78]. The prescription of amantadine was discontinued due to high resistance viruses against its activity.
The viral neuraminidase inhibitors include zanamivir (Relenza), oseltamivir (Tamiflu), laninamivir octanoate (Inavir), and peramivir (Rapivab). Inhalation of zanamivir interestingly, prevents the release of viral particles from host cells by targeting viral neuraminidase [79]. Oseltamivir phosphate is recommended for oral intake to treat acute, uncomplicated influenza [80]. Peramivir which is administered as intravenous injection [81] shows similar efficacy as that of oseltamivir, and prescribed as a therapy for severe seasonal influenza [82]. On the other hand, inhalation of laninamivir octanoate exhibited much effectiveness in seasonal influenza treatment, even including the oseltamivir-resistant adult patients [83].
The triphosphate form of ribavirin (Virazole) and favipiravir (Avigan) efficiently inhibits influenza RNA polymerase activity [84]. Favipiravir was approved in Japan for the treatment of infections by influenza A, B, and C viruses as it can inhibit RNA polymerases of diverse influenza viruses, including the highly pathogenic H5N1 viruses [[85], [86], [87]]; and several other positive/negative-sense RNA viruses [88]. Further, baloxavir marboxil (Xofluza) approved in October 2018 selectively inhibits the cap-dependent endonuclease of viruses that in turn prevents RNA polymerase activity of influenza virus and its mRNA replication [89,90].
2.5. Herpes simplex virus infections
Herpes simplex virus belongs to the Simplex virus genus in the Herpesviridae family. HSV contains a linear dsDNA genome and classified into HSV-1 and HSV-2 [91]. HSV-1 is a highly prevalent pathogen which causes oral herpes infections [92] while HSV-2 is sexually transmitted and causes genital herpes infections [93]. According to WHO, in 2017, 3.7 billion people under 50 years and 417 million people aged between 15 and 49 years are living with HSV-1 and HSV-2 infection, respectively.
Idoxuridine (Dendrid) was the first drug reported to possess antiviral properties [94] and approved to treat hepatic eye infections caused by HSV in 1963 (Table 1) [95]. In 1980, trifluridine (Viroptic) was used for HSV infections treatment [96]. The phosphorylated forms of these deoxyuridine analogues inhibit viral and cellular DNA replication, thus the multiplication of HSV. After 20 years, brivudine, a thymidine analogue with high specific activity against HSV-1 and VZV than idoxuridine and trifluridine was approved for HSV infections treatment [[97], [98], [99]]. The 5′-phosphorylated brivudine inhibits viral DNA synthesis by targeting viral DNA polymerase. Brivudine is used as eye drops to treat epithelial keratitis caused by HSV-1 infection. Vidarabine (Vira-A), a nucleoside analogue inhibiting viral DNA polymerase was withdrawn from the market.
Docosanol (Abreva), a topical cream was approved in 2000 to treat HSV infections. The clinical studies showed that treatment with 10% docosanol cream is safe and effective; and reduces curing time of herpes labialis [100]. Currently, docosanol remains the only non-prescription medication to treat cold sores and fever blisters. In addition, 1% penciclovir (Denavir) and 5% acyclovir (Zovirax) creams are the two available prescription topical agents [101]. The acyclic guanosine analogues, famciclovir (Famvir), valacyclovir hydrochloride (Valtrex) and penciclovir (Denavir) were the oral antiviral drugs approved for the treatment of orolabial herpes and genital herpes infections [[101], [102], [103]].
2.6. Human papillomavirus infections
Human papillomavirus belongs to Papillomaviridae family and comprises closed circular dsDNA as genome [104]. HPV is classified into >100 types based on the sequence variation in L1 protein [105]. HPV infections account for nearly 5% of the cancers in human which include cervical cancer, anal cancer, penile cancer, etc. Further, 71% of global cervical cancer cases are caused by two of the HPV strains viz., HPV-16 and HPV-18 [106,107]. In 2012, 630,000 new HPV-related cancers were reported in women, of which 530,000 (84%) were suffering with cervical cancer. It was estimated that 266,000 (8%) deaths occurred worldwide.
Interferon alpha-2b (Intron A) was the first FDA approved large molecule for antiviral therapy (Table 1). It is a mixture of human interferon alpha proteins used in the treatment of genital warts for its immunomodulatory, antiproliferative and antiviral properties since 1988. The recombinant interferon alpha N3 (Alferon N) was recommended for HPV infections in 1997. The antimitotic compound podofilox (Condylox) was recommended for the treatment of external genital warts [108]. Podofilox is a cytotoxic drug that interrupts the viral cell division by inhibiting the mitotic spindle formation at metaphase [109,110].
Imiquimod 5% (Aldara) cream was approved in 1997 for the topical treatment of HPV-associated infections i.e., genital and perianal warts, superficial basal cell carcinoma, and actinic keratosis [111,112]. Imiquimod induces macrophages to secrete cytokines i.e., INF-α, TNF-α, IL-1, IL-6 and IL-8 to clear the external warts [111,113]. In 2006, sinecatechin 15% (Veregen) ointment, a botanical drug (catechins purified from Chinese green tea) was approved for the treatment of external genital warts [114,115].
2.7. Respiratory syncytial virus infections
Respiratory syncytial virus (RSV) belongs to the Paramyxoviridae family, containing a linear, single-stranded negative-sense RNA genome [116], with two antigenic subtypes: A and B.
The FDA approved RSV-IGIV (RespiGam), a human immunoglobulin to treat RSV in 1996. These antibodies prevent binding of RSV particles to host cells by inhibiting the viral surface glycoproteins G and F [117]. However, it was discontinued from clinical use due to high cost and strict guidelines of usage. Later in 1998, a cost-effective monoclonal antibody, Palivizumab (Synagis) was licensed to treat the infants at risk of contracting severe RSV infections (Table 1). Palivizumab targets the epitope in the A antigenic site of RSV fusion (F) protein and prevents binding to host cells [118,119].
FDA approved the broad-spectrum antiviral agent, ribavirin (Virazole) in 1985 to target the viral RNA polymerase activity by inhibiting inosine-5′-monophosphate (IMP) dehydrogenase which is needed for de novo synthesis of GTP [120].
2.8. Human cytomegalovirus infections
Human cytomegalovirus (HCMV) contains a linear double-stranded DNA genome and classified into four genotypes based on the variation in the sequence of glycoprotein B encoding gene UL55 [121,122].
Ganciclovir (Cytovene) was the first drug approved for the treatment of HCMV associated infections [123]. Another acyclic guanosine analogue, valganciclovir (Valcyte) showed better recovery than ganciclovir [124]. Foscarnet (Foscavir) although shows good response, its usage is limited due to the high toxicity levels during long term treatment [125]. All these drugs including the discontinued drug cidofovir (Vistide) reduces viral infection by blocking the viral DNA synthesis through inhibition of its DNA polymerase.
Fomivirsen (Vitravene) is the only antiviral oligonucleotide (Table 1) approved for the treatment of HCMV-induced retinitis in the patients who are infected with AIDS by administering as an intravitreal injection [126,127]. This antisense drug binds to the DNA of HCMV and inhibits the expression of crucial proteins [128]. This drug was discontinued for the commercial reasons. Approved in 2017, Letermovir (Prevymis) inhibits the DNA terminase complex (pUL51, pUL56, and pUL89) of HCMV [129,130]. This inhibition interferes with viral DNA processing, packaging and virion maturation.
2.9. Varicella-zoster virus infections
Varicella-zoster virus (VZV) belongs to the Varicellovirus genus in the Herpesviridae family. VZV carries a linear, double-stranded DNA genome [131] and classified into five clades, 1–5, which comprises of 9 genotypes. The transmission of VZV is airborne, occurs mostly through aerosols and droplets or lesions (cell-free airborne virus). VZV infections primarily cause chickenpox (varicella) and as immunity declines, it causes shingles or herpes zoster (a painful skin rash) [132].
The viral DNA polymerase inhibitor, brivudine (Zostex) was approved for the treatment of herpes zoster (shingles) in 2000 [97,98]. Vidarabine, a nucleoside analogue, also inhibits VZV DNA polymerase activity [133].
In 1981, FDA approved VZIG, immunoglobulin antibodies drug to be used for treatment of patients with VZV infections [134] and it was discontinued in 2004 after 2 decades of use. In 2012, VariZIG, another human plasma monoclonal antibodies drug (Table 1) was approved for the treatment of VZV-infected patients with compromised immunity, through intramuscular injection [135,136].
3. Major viral pandemics in 21st century
3.1. Swine flu
Swine flu is a respiratory disease caused by influenza type A virus, the subtypes include H1N1, H1N2, H2N1, H3N1, H3N2 and H2N3 [137,138]. The 2009 influenza pandemic was caused by a novel H1N1 strain, which was first recognized at the border between Mexico and United States in April 2009. This pandemic affected >214 countries and caused over 18,449 deaths [139].
Currently, four different antiviral drugs - oseltamivir, peramivir, zanamivir, and baloxavir marboxil are recommended for the treatment of influenza [[140], [141], [142]]. The mechanism of antiviral function of these drugs are discussed above (Section 2.4 Influenza virus infections).
3.2. Ebola virus
Ebola virus disease (EVD) is a deadly disease with occasional outbreaks that mostly affects people and other primates such as monkeys, gorillas, and chimpanzees. Ebola virus was discovered in 1976 near the Ebola River in the Democratic Republic of Congo (DRC). The virus is believed to be animal-borne, and transmitted to humans initially through direct contact with the blood, body fluids and tissues of animals; among humans, it spreads through contact with infected body fluids, and gets in through broken skin or mucous membranes in the eyes, nose, or mouth. The outbreak of EVD during 2014–2016 in West Africa [143,144] was the largest and most complex since the virus was first discovered, with more cases and deaths compared to all other outbreaks combined.
Currently, there is no antiviral drug approved by the FDA to treat Ebola virus infected people. The nucleoside analogues, favipiravir was considered for the treatment of EVD, however, the drug trials suggested its failure to counter the virus, particularly, in patients with very high viral loads [145]. ZMapp, a cocktail of three monoclonal antibodies was tested during West Africa Ebola outbreak. The outcome of this randomized, controlled trial did not show required efficacy [146]. Remdesivir (GS-5734), a nucleotide analogue RNA polymerase inhibitor was identified to possess antiviral activity against multiple variants of Ebola virus [147].
During the 2018 outbreak in eastern Democratic Republic of the Congo, four investigational drugs viz., ZMapp (triple monoclonal antibody), remdesivir (antiviral agent), MAb114 (single monoclonal antibody) and REGN-EB3 (triple monoclonal antibody), were used for the treatment of EVD. Two of these drugs, REGN-EB3 and MAb114, proved to be superior therapeutic agents against Ebola virus disease, with much higher survival of patients [148]. These two antiviral drugs currently remain in use for the treatment of Ebola patients [149].
On 19 December 2019, FDA approved a single dose vaccine rVSV-ZEBOV (Ervebo) which is safe and protective against only Zaire ebolavirus species [150]. Another investigational vaccine comprising two components Ad26.ZEBOV and MVA-BN-Filo was developed and introduced to combat Ebola outbreak (Zaire ebolavirus only) in the DRC. This vaccine was prescribed to be administered in two doses, with initial dose followed by a second “booster” dose after 56 days [151,152].
3.3. Coronaviruses
Till today, seven coronavirus species are reported to infect humans. The human coronaviruses include 229E, OC43, NL63, HKU1, SARS-CoV, MERS-CoV and SARS-CoV-2. The viral species 229E and NL63 belong to alpha-coronavirus genera while OC43, HKU1, SARS-CoV, MERS-CoV and SARS-CoV-2 are beta-coronavirus. The coronaviruses 229E, OC43, NL63 and HKU1 have been infecting human populations for hundreds of years causing common cold and mild respiratory illness [153]. On the other hand, SARS-CoV, MERS-CoV and SARS-CoV-2 are recently emerged (21st century) and causes severe respiratory illness that might lead to death, if not treated [14,154,155].
3.3.1. Severe acute respiratory syndrome coronavirus
Severe acute respiratory syndrome (SARS) is the first pandemic of the 21st century. In late 2002, people with a life-threatening respiratory disease were reported from Guangdong Province, China [154,156]. Later the same was reported from Vietnam, Canada, Hong Kong and worldwide where severe respiratory illness spread among the household members and health care workers [[157], [158], [159]]. In March 2003, this disease was designated as severe acute respiratory syndrome (SARS) which caused >8000 infections, with ~10% mortality. The causative agent of SARS was characterized as a novel coronavirus [[160], [161], [162], [163]]. Severe acute respiratory syndrome coronavirus (SARS-CoV) is most likely originated in wild bats with palm civets and raccoon dogs acting as zoonotic intermediates in transmission to humans [164,165]. Human-to-human transmission occurs through respiratory secretions.
The 2002–2003 SARS-CoV outbreak was controlled solely by using public-health measures, such as wearing surgical masks, washing hands well, and quarantining the infected people. Human-to-human transmission of SARS-CoV was prevented by July 2003. However, as the virus lives in wild bats and civets, future outbreaks are still possible [166].
3.3.2. Middle East respiratory syndrome coronavirus
The Middle East respiratory syndrome (MERS) virus was first isolated from a patient and reported in September 2012, who died of severe pneumonia and multi-organ failure in June 2012 in Jeddah, Saudi Arabia [155,167,168]. Since 2012, MERS-CoV spread beyond the Middle East, across 27 countries [169]. According to WHO, till January 2020, 2519 people were confirmed with MERS-CoV infection and 866 people were dead, globally [170]. Like SARS-CoV, MERS-CoV is also zoonotic in origin with camels and bats acting as its reservoirs [[171], [172], [173], [174]]. Human-to-human transmission of MERS-CoV occurred in health-care settings and family clusters due to close contact and not following adequate practices for prevention and control of infections [[175], [176], [177]].
3.3.3. Treatment of SARS and MERS
Treatment practices for coronaviral diseases, SARS and MERS, included the repurposing of some of the safe antiviral drugs such as remdesivir, lopinavir-ritonavir, interferons. Remdesivir (GS-5734), a nucleoside analogue that inhibits viral RNA polymerase showed antiviral activity against different viral families such as Filoviridae, Pneumoviridae, Paramyxoviridae and Coronaviridae [147,178,179]. Studies using human airway epithelial (HAE) cell cultures demonstrated that remdesivir could decrease the growth of SARS-CoV and MERS-CoV as it reduced the viral titers and viral RNA in in vitro models [178]. Additionally, remdesivir had similar effects against other diverse CoVs including HCoV-NL63 and Mouse Hepatitis Virus (MHV) [180]. Further, treatment of MA15 SARS-CoV infected mice with remdesivir showed ameliorated disease signs (weight loss, lung viral titers) [178]. These results support the use of remdesivir as a therapeutic drug against coronaviruses.
The HIV-1 protease inhibitor, lopinavir-ritonavir (Kaletra) showed inhibitory activity against 3CLpro of SARS-CoV [181]. Lopinavir-ritonavir in combination with ribavirin decreased viral load and clinical manifestations of death in SARS patients [182]. The oral administration of lopinavir-ritonavir in marmoset model of MERS-CoV infection improved the disease condition [[183], [184], [185]]. Based on in vitro and in vivo studies, a clinical trial (MIRACLE trial) is under progress to investigate the efficacy of the combination therapy of lopinavir/ritonavir and recombinant Interferon-β1b in laboratory confirmed, hospitalized MERS patients [186,187]. Studies involving monoclonal antibodies (mAb) as therapeutics suggested the need of targeting several conserved viral epitopes using multiple mAbs, as the mutations help viral escape [188].
Further, host mechanisms that are used by coronaviruses are targeted. The host proteases such as furin, cathepsins and TMPRSS2, process the S-glycoproteins on the virus surface to help its entry into host cells. Inhibition of these host proteins activity blocked the entry of SARS-CoV and MERS-CoV into host cells [[189], [190], [191]]. However, as different coronaviruses vary in their S-glycoprotein, they require different host proteases for the entry into host cells. So, the treatment regimens should comprise combination of host protease inhibitors, in particular to counter the emergence of new coronaviruses. Furthermore, the immunomodulatory interferons (IFNs) are employed for the treatment of coronavirus infection. Type I interferons treatment against SARS-CoV and MERS-CoV proved to be effective in in vitro and in vivo primate models [[192], [193], [194]]. Often, IFNs will be administered in combination therapies along with ribavirin and lopinavir-ritonavir [193,195].
3.3.4. Severe acute respiratory syndrome coronavirus 2
In December 2019, the World Health Organization (WHO) was informed of occurrence of cluster of pneumonia cases with unknown cause in Wuhan, Hubei province of China [14,15]. The causative agent of this disease condition was identified as a new strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), on 07 January 2020. SARS-CoV-2 is highly contagious and currently it has infected people throughout the world [15,196,197]. On 30 January 2020, following the recommendations of the Emergency Committee, the Director-General of WHO declared that the SARS-CoV-2 outbreak constitutes a Public Health Emergency of International Concern (PHEIC). Later, on 11 March 2020, WHO declared COVID-19 as a pandemic based on the reported illness caused by SARS-CoV-2 in over 110 countries and territories around the world and the risk of further global spread. As on 8 January 2021, 86,749,940 people were infected in 235 countries and 1,890,342 deaths occurred globally [198].
There are different levels of research activities from computational investigations to clinical trials using FDA approved drugs, and development of vaccines to block SARS-CoV-2 infection and its multiplication. In order to tackle the immediate global challenge, studies have focused on drug repurposing, an emerging strategy where existing safe medicines, are redeployed [199,200]. Repurposing the existing drugs can be less risky, more cost effective and time savior. The polypharmacology nature of a drug i.e., an off-target in addition to the biological target may present prospects in treating other disorders. Systems biology linked with polypharmacological assessments helps in identifying beneficial off-target activity that can be utilized for drug repurposing. Chloroquine, an anti-malarial drug, is effective against SARS-CoV-2 infection by interfering the virus entry step [201] and clinical trials are in progress to test the efficacy and safety of chloroquine phosphate [202]. The nucleotide analogue remdesivir inhibits the RNA-dependent RNA polymerase activity of SARS-CoV-2 and blocks viral genome replication [201,203]. A single case study was reported where treatment with remdesivir [203] for 7 days showed improvement in a patient health without adverse effects; and the viral PCR was negative for the virus after one day of therapy. Later, Japan approved the use of remdesivir for the treatment of SARS-CoV-2 patients which showed shortened recovery time of the infected patients [204,205]. Besides, the American pharmaceutical company Gilead Sciences announced the clinical trials for the remdesivir with positive response in the recovery time of the patient [206]. Interestingly, recent studies suggested the possible resistance of SASRS-CoV-2 to remdesivir. Exploration of Ebola virus resistance to remdesivir showed the mutation F548S in the F-motif of viral polymerase active site confers resistance. Homology modeling of F-motif showed that it is similar to structural motif containing resistance mutations in coronaviruses [180,207]. Another study using a rational ligand-based interface design complemented with mutational mapping revealed the effect of mutations in nsp12 subunit of RdRp on its function. The results suggested that very few mutations at the remdesivir-binding site of nsp12 could cause resistance in SARS-CoV-2 against remdesivir [208]. Interestingly, the results of interim WHO solidarity clinical trial showed that the repurposed antiviral drugs – remdesivir, hydroxychloroquine, lopinavir and interferon β1α have little or no effect on hospitalized patients with Covid-19, as indicated by overall mortality, initiation of ventilation, and duration of hospital stay [209].
There have been diverse strategies in application to develop potential inhibitors of SARS-CoV-2, however, there is no drug approved to treat SARS-CoV-2 infection, globally. The host binding SARS-CoV-2 S-glycoprotein which shares high homology with that of SARS-CoV, found to be a potent therapeutic target for inhibition with CR3022, a SARS-CoV antibody [210]. However, >85% variation in receptor binding domain (RBD) epitopes of S-glycoprotein suggest the need for the development of new monoclonal antibodies against SARS-CoV-2 [211]. The entry receptor angiotensin-converting enzyme 2 (ACE2) on host cells was also targeted [[212], [213], [214]]. The clinical study planned to investigate the effect of recombinant human ACE2 (rhACE2) on SARS-CoV-2 infected patients is now withdrawn without CDE approval [215]. Camostat mesylate against the host serine protease TMPRSS2 significantly reduced SARS-CoV-2 infection in lung cell line [216]. The clathrin-mediated virus endocytosis regulating host kinase, AP-2-associated protein kinase 1 (AAK1) [217] was targeted with baricitinib (Janus kinase inhibitor). Baricitinib was expected to be a suitable drug candidate as standard doses are efficient in inhibiting AAK1 [218]. Arbidol which inhibits the fusion of virus and host cell membranes, is used as SARS-CoV-2 inhibitor. Additionally, the main protease (3CLpro or Mpro) which performs the proteolytic processing of viral polyproteins is also targeted with lopinavir and ritonavir [219].
Further, development of therapies under progress to counter the hyperinflammatory condition in some SARS-CoV-2 infected patients. Although low-dose corticosteroid treatment in a subset of critically ill patients showed potential benefits [220], more studies are required on corticosteroids usage. Inhibition of interleukin 6 (IL-6) which is overexpressed during inflammation, with tocilizumab (an IL-6 receptor-specific antibody) is under clinical study (ChiCTR2000029765, NCT04324021, TOCOVID-19). Recently, the anti-inflammatory corticosteroid dexamethasone showed to reduce the effect of SARS-CoV-2 in seriously ill persons [[221], [222], [223]]. Furthermore, a recent study [224] identified 66 druggable human proteins in SARS-CoV-2 and study the effectiveness of 69 reported FDA drugs, drugs in clinical trials and/or preclinical compounds, in live SARS-CoV-2 infection assays. Currently, there are several other drugs are in clinical trials as monotherapies and combination therapies for the treatment of SARS-CoV-2 infection [[225], [226], [227], [228], [229]]. In addition, the convalescent plasma from recovered patients, which serves as source of specific human antibodies against SARS-CoV-2 is under clinical investigation to determine its efficacy and safety in transfusion to SARS-CoV-2 patients (ChiCTR2000030010, ChiCTR2000030179 and ChiCTR2000030381).
Furthermore, several research works are in progress to develop potent vaccines [230]. Development of a vaccine involves antigen identification and development of an appropriate delivery system to achieve robust cellular and humoral immunity. Currently, few vaccines are authorized/approved against SARS-CoV-2 in some countries. BioNTech and Pfizer developed lipid nanoparticle formulated, nucleoside modified mRNA-based vaccine, BNT162b2 was authorized/approved in United Kingdom, Bahrain, Canada, Mexico, US, Singapore, Oman, Saudi Arabia, Kuwait, European Union. BNT162b2 is injected intramuscularly in two doses 21 days apart, to induce immune response against SARS-CoV-2, by encoding a mutated form of the full spike protein of the virus. The Phase 3 clinical trials on 43,448 participants showed that BNT162b2 is 95% effective [231]. mRNA-1273 is another lipid nanoparticle-encapsulated mRNA-based vaccine, expressing the prefusion-stabilized spike glycoprotein, was developed by Moderna and the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID). It is a two-dose vaccine administered intramuscularly 28 days apart and showed 94.1% efficacy in preventing Covid-19 illness [232]. mRNA-1273 vaccine was authorized/approved in the US and Canada.
The Health Ministry of the Russian Federation approved Sputnik V as the first vaccine for COVID-19. Sputnik V is a non-replicating adenoviral vector vaccine, currently in Phase 3 trial in Russia and internationally (NCT04530396, NCT04564716) and also approved its use in Bolivia, Argentina, Serbia and Belarus [233,234]. China approved the use of inactivated vaccines CoronaVac developed by Sinovac Biotech, and BBIBP-CorV developed by Sinopharm for high-risk individuals such as health care workers and essential personnel. Currently Phase 3 trials are in progress (NCT04456595, NCT04582344, ChiCTR2000034780, NCT04560881) [235,236]. AZD1222 is a non-replicating vaccine based on chimpanzee adenovirus called ChAdOx1 that expresses SARS-CoV-2-5 surface glycoprotein, developed by the University of Oxford and AstraZeneca [[237], [238], [239], [240]]. The United Kingdom approved the use of this vaccine on 30 December 2020 [241]. On January 3, 2021, India approved Covaxin developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV). Covaxin is the Indigenous, inactivated vaccine currently in Phase 3 clinical trials in 26,000 participants [242].
4. Conclusions
This article provides information about the strategic developments of different antiviral agents that have been used/using to inhibit the growth of viral infections in humans, to provide comprehensive idea on the up-to-date FDA approved antiviral drugs. Although these drugs show effective inhibitory activities on the viral infections, research should be focused on developing clinical strategies to completely cure the infections. The efficient antiviral drugs i) should resist the drug resistance developed by viruses on long-term application, ii) should tackle the effects of integrated viral DNA in the human genome, iii) should be able to treat co-infections by different viruses, iv) should avoid interactions between drugs in the combination drug treatments to prevent adverse effects, and v) should be cost-effective and cause low-toxicity in patients. The cases like resistance of coronaviruses to remdesivir can be overcome by incorporating nucleos(t)ide analogue triphosphates (NA-TPs) by RdRp faster than the excision rate of nucleos(t)ide analogue monophosphates (NA-MPs) by exonuclease (ExoN). Studies analysing the difference in mechanism of RdRp and ExoN activity in recognition, incorporation of different NA-TPs and excision of NA-MPs would provide crucial insights to design novel NAs. Further, coupling the inhibitors of ExoN with NAs may be a better option to reduce the potential of viral escape. Furthermore, the multitudinous virus population that infects humans across the globe emphasizes the need for extensive and effective research to develop novel antiviral therapeutics to counter the existing viral infections, newly emerging infections like SARS-CoV-2 and the outbreak of new viruses in future.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
Saraboji Kadhirvel gratefully acknowledges Science and Engineering Research Board (SERB), Government of India (Sanction Numbers: CVD/2020/000604 & EMR/2017/002841/BBM) for financial support.
References
- 1.H. Lodish, A. Berk, S.L. Zipursky, P. Matsudaira, D. Baltimore, J. Darnell, Molecular Cell Biology. 4 ed., W.H. Freeman & Co. Ltd, New York, 2000.
- 2.Greub G., Ledergerber B., Battegay M., Grob P., Perrin L., Furrer H., Burgisser P., Erb P., Boggian K., Piffaretti J.C., Hirschel B., Janin P., Francioli P., Flepp M., Telenti A. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV cohort study. Lancet. 2000;356(9244):1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 3.CDC, HIV/AIDs Surveillance Report, US Department of Health and Human Services, Public Health Service . Center for Infectious Diseases; Division of HIV/AIDS: 1990. Centers for Disease Control; pp. 1–18. [Google Scholar]
- 4.A.S. Evans, Viral Infections of Humans: Epidemiology and Control, 3 ed., Springer Science & Business Media2013.
- 5.Soderstrom K. In: xPharm: The Comprehensive Pharmacology Reference. Enna S.J., Bylund D.B., editors. Elsevier; New York: 2007. Viral replication; pp. 1–5. [Google Scholar]
- 6.Fenner F., Bachmann P.A., Gibbs E.P.J., Murphy F.A., Studdert M.J., White D.O. Viral replication. Veterinary Virology. 1987:55–88. doi: 10.1016/B978-0-12-253055-5.50008-6. [DOI] [Google Scholar]
- 7.Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- 8.Rice C.M., Lenches E.M., Eddy S.R., Shin S.J., Sheets R.L., Strauss J.H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- 9.Strauss E.G., Strauss J.H. In: Current Topics in Microbiology and Immunology: Volume 105. Cooper M., Hofschneider P.H., Koprowski H., Melchers F., Rott R., Schweiger H.G., Vogt P.K., Zinkernagel R., editors. Springer; Berlin Heidelberg, Berlin, Heidelberg: 1983. Replication strategies of the single stranded RNA viruses of eukaryotes; pp. 1–98. [DOI] [PubMed] [Google Scholar]
- 10.Evaluation of idoxuridine (IDU): (Dendrid, Herplex, Stoxil), JAMA 190(6) (1964) 535–536. doi: 10.1001/jama.1964.03070190055009. [DOI] [PubMed]
- 11.Thomas C.I., Purnell E.W., Rosenthal M.S. Treatment of hepatic keratitis with IDU and corticosteroids; report of 105 cases. Am J. Ophthalmol. 1965;60:204–217. doi: 10.1016/0002-9394(65)90919-0. [DOI] [PubMed] [Google Scholar]
- 12.A chronicle on the SARS epidemic, Chinese Law & Government 36(4) (2003) 12–15. doi: 10.2753/CLG0009-4609360412. [DOI]
- 13.J.-F. He, G.-W. Peng, J. Min, D.-W. Yu, W.-J. Liang, S.-Y. Zhang, R.-H. Xu, H.-Y. Zheng, X.-W. Wu, J. Xu, Z.-H. Wang, L. Fang, X. Zhang, H. Li, X.-G. Yan, J.-H. Lu, Z.-H. Hu, J.-C. Huang, Z.-Y. Wan, J.-L. Hou, J.-Y. Lin, H.-D. Song, S.-Y. Wang, X.-J. Zhou, G.-W. Zhang, B.-W. Gu, H.-J. Zheng, X.-L. Zhang, M. He, K. Zheng, B. Wang, G. Fu, X.-N. Wang, S.-J. Chen, Z. Chen, P. Hao, H. Tang, S.-X. Ren, Y. Zhong, Z.-M. Guo, Q. Liu, Y.-G. Miao, X.-Y. Kong, W.-Z. He, Y.-X. Li, C.-I. Wu, G.-P. Zhao, R.W.K. Chiu, S.S.C. Chim, Y.-k. Tong, P.K.S. Chan, J.S. Tam, Y.M.D. Lo, Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China, Science 303(5664) (2004) 1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed]
- 14.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China, Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo R.C., Montagnier L. The discovery of HIV as the cause of AIDS. N. Engl. J. Med. 2003;349(24):2283–2285. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- 17.Barre-Sinoussi F., Chermann J.C., Rey F., Nugeyre M.T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vezinet-Brun F., Rouzioux C., Rozenbaum W., Montagnier L. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 18.Rambaut A., Posada D., Crandall K.A., Holmes E.C. The causes and consequences of HIV evolution. Nat. Rev. Genet. 2004;5(1):52–61. doi: 10.1038/nrg1246. [DOI] [PubMed] [Google Scholar]
- 19.Mitsuya H., Weinhold K.J., Furman P.A., Clair M.H. St, Lehrman S.N., Gallo R.C., Bolognesi D., Barry D.W., Broder S. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc. Natl. Acad. Sci. U. S. A. 1985;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsuya H., Broder S. Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2′,3′-dideoxynucleosides. Proc. Natl. Acad. Sci. U. S. A. 1986;83(6):1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baba M., Pauwels R., Herdewijn P., De Clercq E., Desmyter J., Vandeputte M. Both 2′,3′-dideoxythymidine and its 2′,3′-unsaturated derivative (2′,3′-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem. Biophys. Res. Commun. 1987;142(1):128–134. doi: 10.1016/0006-291x(87)90460-8. [DOI] [PubMed] [Google Scholar]
- 22.Hamamoto Y., Nakashima H., Matsui T., Matsuda A., Ueda T., Yamamoto N. Inhibitory effect of 2′,3′-didehydro-2′,3′-dideoxynucleosides on infectivity, cytopathic effects, and replication of human immunodeficiency virus. Antimicrob. Agents Chemother. 1987;31(6):907–910. doi: 10.1128/aac.31.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soudeyns H., Yao X.I., Gao Q., Belleau B., Kraus J.L., Nguyen-Ba N., Spira B., Wainberg M.A. Anti-human immunodeficiency virus type 1 activity and in vitro toxicity of 2′-deoxy-3′-thiacytidine (BCH-189), a novel heterocyclic nucleoside analog. Antimicrob. Agents Chemother. 1991;35(7):1386–1390. doi: 10.1128/aac.35.7.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daluge S.M., Good S.S., Faletto M.B., Miller W.H., Clair M.H. St, Boone L.R., Tisdale M., Parry N.R., Reardon J.E., Dornsife R.E., Averett D.R., Krenitsky T.A. 1592U89, a novel carbocyclic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob. Agents Chemother. 1997;41(5):1082–1093. doi: 10.1128/AAC.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S.F. Potential strategies for minimizing mechanism-based inhibition of cytochrome P450 3A4. Curr. Pharm. Des. 2008;14(10):990–1000. doi: 10.2174/138161208784139738. [DOI] [PubMed] [Google Scholar]
- 26.Matthews T., Salgo M., Greenberg M., Chung J., DeMasi R., Bolognesi D. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat. Rev. Drug Discov. 2004;3(3):215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 27.Wild C., Greenwell T., Matthews T. A synthetic peptide from HIV-1 gp41 is a potent inhibitor of virus-mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 1993;9(11):1051–1053. doi: 10.1089/aid.1993.9.1051. [DOI] [PubMed] [Google Scholar]
- 28.Dorr P., Westby M., Dobbs S., Griffin P., Irvine B., Macartney M., Mori J., Rickett G., Smith-Burchnell C., Napier C., Webster R., Armour D., Price D., Stammen B., Wood A., Perros M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005;49(11):4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatkenheuer G., Pozniak A.L., Johnson M.A., Plettenberg A., Staszewski S., Hoepelman A.I., Saag M.S., Goebel F.D., Rockstroh J.K., Dezube B.J., Jenkins T.M., Medhurst C., Sullivan J.F., Ridgway C., Abel S., James I.T., Youle M., van der Ryst E. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 2005;11(11):1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 30.Gulick R.M., Lalezari J., Goodrich J., Clumeck N., DeJesus E., Horban A., Nadler J., Clotet B., Karlsson A., Wohlfeiler M., Montana J.B., McHale M., Sullivan J., Ridgway C., Felstead S., Dunne M.W., van der Ryst E., Mayer H., Teams M.S. Maraviroc for previously treated patients with R5 HIV-1 infection. N. Engl. J. Med. 2008;359(14):1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.S. Raymond, A. Maillard, C. Amiel, G. Peytavin, M.A. Trabaud, D. Desbois, P. Bellecave, C. Delaugerre, C. Soulie, A.G. Marcelin, D. Descamps, J. Izopet, A.A.C.R.S.G. on behalf the, Virological failure of patients on maraviroc-based antiretroviral therapy, J. Antimicrob. Chemother. 70(6) (2015) 1858–64. doi: 10.1093/jac/dkv026. [DOI] [PubMed]
- 32.Markham A. Ibalizumab: first global approval. Drugs. 2018;78(7):781–785. doi: 10.1007/s40265-018-0907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dionne B. Key principles of antiretroviral pharmacology. Infect. Dis. Clin. N. Am. 2019;33(3):787–805. doi: 10.1016/j.idc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Deeks E.D. Doravirine: first global approval. Drugs. 2018;78(15):1643–1650. doi: 10.1007/s40265-018-0993-4. [DOI] [PubMed] [Google Scholar]
- 35.R. Talwani, Z. Temesgen, Doravirine: a new non-nucleoside reverse transcriptase inhibitor for the treatment of HIV infection, Drugs of today (Barcelona, Spain: 1998) 56(2) (2020) 113–124. doi: 10.1358/dot.2020.56.2.3109966. [DOI] [PubMed]
- 36.Sharp M., Corp Dohme. Merck & Co, Inc.; Whitehouse Station, NJ: 2018. Delstrigo (Doravirine, Lamivudine, and Tenofovir Disoproxil Fumarate) Prescribing Information. [Google Scholar]
- 37.Tsiang M., Jones G.S., Goldsmith J., Mulato A., Hansen D., Kan E., Tsai L., Bam R.A., Stepan G., Stray K.M., Niedziela-Majka A., Yant S.R., Yu H., Kukolj G., Cihlar T., Lazerwith S.E., White K.L., Jin H. Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob. Agents Chemother. 2016;60(12):7086–7097. doi: 10.1128/AAC.01474-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markham A. Bictegravir: first global approval. Drugs. 2018;78(5):601–606. doi: 10.1007/s40265-018-0896-4. [DOI] [PubMed] [Google Scholar]
- 39.Luis Casado J., Fontecha M., Monsalvo M., Vizcarra P. Symtuza® in clinical practice. Enferm. Infecc. Microbiol. Clin. 2018;36(Suppl. 2):31–36. doi: 10.1016/s0213-005x(18)30395-1. [DOI] [PubMed] [Google Scholar]
- 40.Gómez Ayerbe C., Santos González J., Palacios Muñoz R. Symtuza® (DRV/c/FTC/TAF) in the management of treatment-naive HIV-patients. Enferm. Infecc. Microbiol. Clin. 2018;36(Suppl. 2):17–21. doi: 10.1016/s0213-005x(18)30393-8. [DOI] [PubMed] [Google Scholar]
- 41.Sebaaly J.C., Kelley D. HIV clinical updates: new single-tablet regimens. Ann. Pharmacother. 2019;53(1):82–94. doi: 10.1177/1060028018793252. [DOI] [PubMed] [Google Scholar]
- 42.Scott L.J. Dolutegravir/lamivudine single-tablet regimen: a review in HIV-1 infection. Drugs. 2020;80(1):61–72. doi: 10.1007/s40265-019-01247-1. [DOI] [PubMed] [Google Scholar]
- 43.RxList, Fulyzaq. https://www.rxlist.com/fulyzaq-drug.htm#description.
- 44.Wilkins T., Malcolm J.K., Raina D., Schade R.R. Hepatitis C: diagnosis and treatment. Am. Fam. Physician. 2010;81(11):1351–1357. [PubMed] [Google Scholar]
- 45.De Clercq E. Current race in the development of DAAs (direct-acting antivirals) against HCV. Biochem. Pharmacol. 2014;89(4):441–452. doi: 10.1016/j.bcp.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 46.De Clercq E. Development of antiviral drugs for the treatment of hepatitis C at an accelerating pace. Rev. Med. Virol. 2015;25(4):254–267. doi: 10.1002/rmv.1842. [DOI] [PubMed] [Google Scholar]
- 47.McCauley J.A., McIntyre C.J., Rudd M.T., Nguyen K.T., Romano J.J., Butcher J.W., Gilbert K.F., Bush K.J., Holloway M.K., Swestock J., Wan B.L., Carroll S.S., DiMuzio J.M., Graham D.J., Ludmerer S.W., Mao S.S., Stahlhut M.W., Fandozzi C.M., Trainor N., Olsen D.B., Vacca J.P., Liverton N.J. Discovery of vaniprevir (MK-7009), a macrocyclic hepatitis C virus NS3/4a protease inhibitor. J. Med. Chem. 2010;53(6):2443–2463. doi: 10.1021/jm9015526. [DOI] [PubMed] [Google Scholar]
- 48.T. McQuaid, C. Savini, S. Seyedkazemi, Sofosbuvir, a significant paradigm change in HCV treatment, Journal of clinical and translational hepatology 3(1) (2015) 27–35. doi: 10.14218/jcth.2014.00041. [DOI] [PMC free article] [PubMed]
- 49.M. Bourliere, J.P. Bronowicki, V. de Ledinghen, C. Hezode, F. Zoulim, P. Mathurin, A. Tran, D.G. Larrey, V. Ratziu, L. Alric, R.H. Hyland, D. Jiang, B. Doehle, P.S. Pang, W.T. Symonds, G.M. Subramanian, J.G. McHutchison, P. Marcellin, F. Habersetzer, D. Guyader, J.D. Grange, V. Loustaud-Ratti, L. Serfaty, S. Metivier, V. Leroy, A. Abergel, S. Pol, Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS), Lancet Infect. Dis. 15(4) (2015) 397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed]
- 50.S. Naggie, C. Cooper, M. Saag, K. Workowski, P. Ruane, W.J. Towner, K. Marks, A. Luetkemeyer, R.P. Baden, P.E. Sax, E. Gane, J. Santana-Bagur, L.M. Stamm, J.C. Yang, P. German, H. Dvory-Sobol, L. Ni, P.S. Pang, J.G. McHutchison, C.A. Stedman, J.O. Morales-Ramirez, N. Brau, D. Jayaweera, A.E. Colson, P. Tebas, D.K. Wong, D. Dieterich, M. Sulkowski, I.O.N. Investigators, Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1, N. Engl. J. Med. 373(8) (2015) 705–13. doi: 10.1056/NEJMoa1501315. [DOI] [PMC free article] [PubMed]
- 51.Mir F., Kahveci A.S., Ibdah J.A., Tahan V. Sofosbuvir/velpatasvir regimen promises an effective pan-genotypic hepatitis C virus cure. Drug Des. Devel. Ther. 2017;11:497–502. doi: 10.2147/DDDT.S130945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.FDA U. 2017. FDA Approves Vosevi for Hepatitis C. [Google Scholar]
- 53.Kilaru S.M., Jacobson I.M. Sofosbuvir/velpatasvir/voxilaprevir in the treatment of chronic hepatitis C infection. Future Virol. 2019;14(2):61–71. doi: 10.2217/fvl-2018-0134. [DOI] [Google Scholar]
- 54.J.G. Taylor, S. Zipfel, K. Ramey, R. Vivian, A. Schrier, K.K. Karki, A. Katana, D. Kato, T. Kobayashi, R. Martinez, M. Sangi, D. Siegel, C.V. Tran, Z.Y. Yang, J. Zablocki, C.Y. Yang, Y. Wang, K. Wang, K. Chan, O. Barauskas, G. Cheng, D. Jin, B.E. Schultz, T. Appleby, A.G. Villasenor, J.O. Link, Discovery of the pan-genotypic hepatitis C virus NS3/4A protease inhibitor voxilaprevir (GS-9857): a component of Vosevi((R)), Bioorg. Med. Chem. Lett. 29(16) (2019) 2428–2436. doi: 10.1016/j.bmcl.2019.03.037. [DOI] [PubMed]
- 55.Hayashi N., Nakamuta M., Takehara T., Kumada H., Takase A., Howe A.Y., Ludmerer S.W., Mobashery N. Vaniprevir plus peginterferon alfa-2b and ribavirin in treatment-naive Japanese patients with hepatitis C virus genotype 1 infection: a randomized phase III study. J. Gastroenterol. 2016;51(4):390–403. doi: 10.1007/s00535-015-1120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.L. Margusino-Framinan, P. Cid-Silva, A. Mena-de-Cea, I. Rodriguez-Osorio, B. Pernas-Souto, M. Delgado-Blanco, S. Pertega-Diaz, I. Martin-Herranz, A. Castro-Iglesias, Effectiveness and safety of daclatasvir/sofosbuvir with or without ribavirin in genotype 3 hepatitis C virus infected patients. Results in real clinical practice, Rev. Esp. Quimioter. 32(2) (2019) 137–144. [PMC free article] [PubMed]
- 57.Cheema S.U.R., Rehman M.S., Hussain G., Cheema S.S., Gilani N. Efficacy and tolerability of sofosbuvir and daclatasvir for treatment of hepatitis C genotype 1 & 3 in patients undergoing hemodialysis-a prospective interventional clinical trial. BMC Nephrol. 2019;20(1):438. doi: 10.1186/s12882-019-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.E. Lawitz, E. Gane, B. Pearlman, E. Tam, W. Ghesquiere, D. Guyader, L. Alric, J.P. Bronowicki, L. Lester, W. Sievert, R. Ghalib, L. Balart, F. Sund, M. Lagging, F. Dutko, M. Shaughnessy, P. Hwang, A.Y. Howe, J. Wahl, M. Robertson, E. Barr, B. Haber, Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial, Lancet 385(9973) (2015) 1075–86. doi: 10.1016/S0140-6736(14)61795-5. [DOI] [PubMed]
- 59.Zeuzem S., Ghalib R., Reddy K.R., Pockros P.J., Ben Ari Z., Zhao Y., Brown D.D., Wan S., DiNubile M.J., Nguyen B.Y., Robertson M.N., Wahl J., Barr E., Butterton J.R. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann. Intern. Med. 2015;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 60.Asselah T., Kowdley K.V., Zadeikis N., Wang S., Hassanein T., Horsmans Y., Colombo M., Calinas F., Aguilar H., de Ledinghen V., Mantry P.S., Hezode C., Marinho R.T., Agarwal K., Nevens F., Elkhashab M., Kort J., Liu R., Ng T.I., Krishnan P., Lin C.W., Mensa F.J. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin. Gastroenterol. Hepatol. 2018;16(3):417–426. doi: 10.1016/j.cgh.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 61.Kwo P.Y., Poordad F., Asatryan A., Wang S., Wyles D.L., Hassanein T., Felizarta F., Sulkowski M.S., Gane E., Maliakkal B., Overcash J.S., Gordon S.C., Muir A.J., Aguilar H., Agarwal K., Dore G.J., Lin C.-W., Liu R., Lovell S.S., Ng T.I., Kort J., Mensa F.J. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1–6 without cirrhosis. J. Hepatol. 2017;67(2):263–271. doi: 10.1016/j.jhep.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 62.Gane E., Lawitz E., Pugatch D., Papatheodoridis G., Brau N., Brown A., Pol S., Leroy V., Persico M., Moreno C., Colombo M., Yoshida E.M., Nelson D.R., Collins C., Lei Y., Kosloski M., Mensa F.J. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N. Engl. J. Med. 2017;377(15):1448–1455. doi: 10.1056/NEJMoa1704053. [DOI] [PubMed] [Google Scholar]
- 63.Chang T.T., Gish R.G., de Man R., Gadano A., Sollano J., Chao Y.C., Lok A.S., Han K.H., Goodman Z., Zhu J., Cross A., DeHertogh D., Wilber R., Colonno R., Apelian D., B.E.A.S. Group A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2006;354(10):1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 64.Lai C.L., Shouval D., Lok A.S., Chang T.T., Cheinquer H., Goodman Z., DeHertogh D., Wilber R., Zink R.C., Cross A., Colonno R., Fernandes L., B.E.A.S. Group Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2006;354(10):1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 65.Tenney D.J., Rose R.E., Baldick C.J., Pokornowski K.A., Eggers B.J., Fang J., Wichroski M.J., Xu D., Yang J., Wilber R.B., Colonno R.J. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49(5):1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 66.Lai C.L., Gane E., Liaw Y.F., Hsu C.W., Thongsawat S., Wang Y., Chen Y., Heathcote E.J., Rasenack J., Bzowej N., Naoumov N.V., Di Bisceglie A.M., Zeuzem S., Moon Y.M., Goodman Z., Chao G., Constance B.F., Brown N.A., Globe Study G. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 2007;357(25):2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 67.Lai C.L., Leung N., Teo E.K., Tong M., Wong F., Hann H.W., Han S., Poynard T., Myers M., Chao G., Lloyd D., Brown N.A., Telbivudine Phase I.I.I.G. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology. 2005;129(2):528–536. doi: 10.1016/j.gastro.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 68.Liaw Y.F., Gane E., Leung N., Zeuzem S., Wang Y., Lai C.L., Heathcote E.J., Manns M., Bzowej N., Niu J., Han S.H., Hwang S.G., Cakaloglu Y., Tong M.J., Papatheodoridis G., Chen Y., Brown N.A., Albanis E., Galil K., Naoumov N.V., G.S. Group 2-year GLOBE trial results: telbivudine is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology. 2009;136(2):486–495. doi: 10.1053/j.gastro.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 69.Lok A.S. Hepatitis B: 50 years after the discovery of Australia antigen. J. Viral Hepat. 2016;23(1):5–14. doi: 10.1111/jvh.12444. [DOI] [PubMed] [Google Scholar]
- 70.Medina R.A., Garcia-Sastre A. Influenza A viruses: new research developments. Nat. Rev. Microbiol. 2011;9(8):590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kilbourne E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006;12(1):9–14. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Franco-Paredes C., Hernandez-Ramos I., Del Rio C., Alexander K.T., Tapia-Conyer R., Santos-Preciado J.I. H1N1 influenza pandemics: comparing the events of 2009 in Mexico with those of 1976 and 1918–1919. Arch. Med. Res. 2009;40(8):669–672. doi: 10.1016/j.arcmed.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 73.Pielak R.M., Chou J.J. Influenza M2 proton channels. Biochim. Biophys. Acta. 2011;1808(2):522–529. doi: 10.1016/j.bbamem.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cady S.D., Luo W., Hu F., Hong M. Structure and function of the influenza A M2 proton channel. Biochemistry. 2009;48(31):7356–7364. doi: 10.1021/bi9008837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palese P., Tobita K., Ueda M., Compans R.W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- 76.McAuley J.L., Gilbertson B.P., Trifkovic S., Brown L.E., McKimm-Breschkin J.L. Influenza virus neuraminidase structure and functions. Front. Microbiol. 2019;10:39. doi: 10.3389/fmicb.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cady S.D., Mishanina T.V., Hong M. Structure of amantadine-bound M2 transmembrane peptide of influenza A in lipid bilayers from magic-angle-spinning solid-state NMR: the role of Ser31 in amantadine binding. J. Mol. Biol. 2009;385(4):1127–1141. doi: 10.1016/j.jmb.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Clercq E. Antiviral agents active against influenza A viruses. Nat. Rev. Drug Discov. 2006;5(12):1015–1025. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russell R.J., Haire L.F., Stevens D.J., Collins P.J., Lin Y.P., Blackburn G.M., Hay A.J., Gamblin S.J., Skehel J.J. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature. 2006;443(7107):45–49. doi: 10.1038/nature05114. [DOI] [PubMed] [Google Scholar]
- 80.McKimm-Breschkin J., Trivedi T., Hampson A., Hay A., Klimov A., Tashiro M., Hayden F., Zambon M. Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir. Antimicrob. Agents Chemother. 2003;47(7):2264–2272. doi: 10.1128/aac.47.7.2264-2272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McLaughlin M.M., Skoglund E.W., Ison M.G. Peramivir: an intravenous neuraminidase inhibitor. Expert. Opin. Pharmacother. 2015;16(12):1889–1900. doi: 10.1517/14656566.2015.1066336. [DOI] [PubMed] [Google Scholar]
- 82.Yoo J.W., Choi S.H., Huh J.W., Lim C.M., Koh Y., Hong S.B. Peramivir is as effective as oral oseltamivir in the treatment of severe seasonal influenza. J. Med. Virol. 2015;87(10):1649–1655. doi: 10.1002/jmv.24232. [DOI] [PubMed] [Google Scholar]
- 83.Watanabe A., Chang S.C., Kim M.J., Chu D.W., Ohashi Y., M.S. Group Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin. Infect. Dis. 2010;51(10):1167–1175. doi: 10.1086/656802. [DOI] [PubMed] [Google Scholar]
- 84.Eriksson B., Helgstrand E., Johansson N.G., Larsson A., Misiorny A., Noren J.O., Philipson L., Stenberg K., Stening G., Stridh S., Oberg B. Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob. Agents Chemother. 1977;11(6):946–951. doi: 10.1128/aac.11.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kiso M., Takahashi K., Sakai-Tagawa Y., Shinya K., Sakabe S., Le Q.M., Ozawa M., Furuta Y., Kawaoka Y. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc. Natl. Acad. Sci. U. S. A. 2010;107(2):882–887. doi: 10.1073/pnas.0909603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Furuta Y., Takahashi K., Kuno-Maekawa M., Sangawa H., Uehara S., Kozaki K., Nomura N., Egawa H., Shiraki K. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 2005;49(3):981–986. doi: 10.1128/AAC.49.3.981-986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smee D.F., Hurst B.L., Egawa H., Takahashi K., Kadota T., Furuta Y. Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. J. Antimicrob. Chemother. 2009;64(4):741–746. doi: 10.1093/jac/dkp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Clercq E. Highlights in antiviral drug research: antivirals at the horizon. Med. Res. Rev. 2013;33(6):1215–1248. doi: 10.1002/med.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Hanlon R., Shaw M.L. Baloxavir marboxil: the new influenza drug on the market. Curr. Opin. Virol. 2019;35:14–18. doi: 10.1016/j.coviro.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Heo Y.-A. Baloxavir: first global approval. Drugs. 2018;78(6):693–697. doi: 10.1007/s40265-018-0899-1. [DOI] [PubMed] [Google Scholar]
- 91.Whitley R.J., Kimberlin D.W., Roizman B. Herpes simplex viruses. Clin. Infect. Dis. 1998;26(3):541–553. doi: 10.1086/514600. quiz 554-5. [DOI] [PubMed] [Google Scholar]
- 92.Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357(9267):1513–1518. doi: 10.1016/s0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 93.D.M. Koelle, A. Wald, Herpes simplex virus: the importance of asymptomatic shedding, J. Antimicrob. Chemother. 45 Suppl T3 (2000) 1–8. doi: 10.1093/jac/45.suppl_4.1. [DOI] [PubMed]
- 94.Prusoff W.H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim. Biophys. Acta. 1959;32(1):295–296. doi: 10.1016/0006-3002(59)90597-9. [DOI] [PubMed] [Google Scholar]
- 95.Herrmann E.C. Plaque inhibition test for detection of specific inhibitors of DNA containing viruses. Proc. Soc. Exp. Biol. Med. 1961;107(1):142–145. doi: 10.3181/00379727-107-26560. [DOI] [PubMed] [Google Scholar]
- 96.Kaufman H.E., Heidelberger C. Therapeutic antiviral action of 5-trifluoromethyl-2′-deoxyuridine in herpes simplex keratitis. Science. 1964;145(3632):585–586. doi: 10.1126/science.145.3632.585. [DOI] [PubMed] [Google Scholar]
- 97.De Clercq E. Discovery and development of BVDU (brivudin) as a therapeutic for the treatment of herpes zoster. Biochem. Pharmacol. 2004;68(12):2301–2315. doi: 10.1016/j.bcp.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 98.Andrei G., Sienaert R., McGuigan C., De Clercq E., Balzarini J., Snoeck R. Susceptibilities of several clinical varicella-zoster virus (VZV) isolates and drug-resistant VZV strains to bicyclic furano pyrimidine nucleosides. Antimicrob. Agents Chemother. 2005;49(3):1081–1086. doi: 10.1128/AAC.49.3.1081-1086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maudgal P.C., De Clercq E. Bromovinyldeoxyuridine treatment of herpetic keratitis clinically resistant to other antiviral agents. Curr. Eye Res. 1991;10(Suppl):193–199. doi: 10.3109/02713689109020379. [DOI] [PubMed] [Google Scholar]
- 100.Sacks S.L., Thisted R.A., Jones T.M., Barbarash R.A., Mikolich D.J., Ruoff G.E., Jorizzo J.L., Gunnill L.B., Katz D.H., Khalil M.H., Morrow P.R., Yakatan G.J., Pope L.E., Berg J.E. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: a multicenter, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 2001;45(2):222–230. doi: 10.1067/mjd.2001.116215. [DOI] [PubMed] [Google Scholar]
- 101.Modi S., Van L., Gewirtzman A., Mendoza N., Bartlett B., Tremaine A.M., Tyring S. Single-day treatment for orolabial and genital herpes: a brief review of pathogenesis and pharmacology. Ther. Clin. Risk Manag. 2008;4(2):409–417. doi: 10.2147/tcrm.s1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.M.A. Pue, L.Z. Benet, Pharmacokinetics of famciclovir in man, Antivir. Chem. Chemother. 4(6_suppl) (1993) 47–55. doi: 10.1177/09563202930040s602. [DOI]
- 103.M. Reitano, S. Tyring, W. Lang, C. Thoming, A.M. Worm, S. Borelli, L.O. Chambers, J.M. Robinson, L. Corey, Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International valaciclovir HSV study group, J. Infect. Dis. 178(3) (1998) 603–10. doi: 10.1086/515385. [DOI] [PubMed]
- 104.E.-M. de Villiers, C. Fauquet, T.R. Broker, H.-U. Bernard, H. zur Hausen, Classification of papillomaviruses, Virology 324(1) (2004) 17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed]
- 105.Zur Hausen H., De Villiers E.-M. Human papillomaviruses. Annu. Rev. Microbiol. 1994;48(1):427–447. doi: 10.1146/annurev.mi.48.100194.002235. [DOI] [PubMed] [Google Scholar]
- 106.K.A. Workowski, G.A. Bolan, C. Centers for Disease, Prevention, Sexually transmitted diseases treatment guidelines, 2015, MMWR Recomm. Rep. 64(RR-03) (2015) 1–137. [PMC free article] [PubMed]
- 107.M. Durst, L. Gissmann, H. Ikenberg, H. zur Hausen, A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions, Proc. Natl. Acad. Sci. U. S. A. 80(12) (1983) 3812–5. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed]
- 108.S. Tyring, L. Edwards, L.K. Cherry, W.M. Ramsdell, S. Kotner, M.D. Greenberg, J.C. Vance, G. Barnum, S.H. Dromgoole, F.P. Killey, T. Toter, Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts, Arch. Dermatol. 134(1) (1998) 33–8. doi: 10.1001/archderm.134.1.33. [DOI] [PubMed]
- 109.M.D. Greenberg, L.H. Rutledge, R. Reid, N.R. Berman, S.L. Precop, R.K. Elswick, Jr., A double-blind, randomized trial of 0.5% podofilox and placebo for the treatment of genital warts in women, Obstet. Gynecol. 77(5) (1991) 735–9. [PubMed]
- 110.Gunter J. Genital and perianal warts: new treatment opportunities for human papillomavirus infection. Am. J. Obstet. Gynecol. 2003;189(3 Suppl):S3–11. doi: 10.1067/s0002-9378(03)00789-0. [DOI] [PubMed] [Google Scholar]
- 111.Beutner K.R., Tyring S.K., Trofatter K.F., Jr., Douglas J.M., Jr., Spruance S., Owens M.L., Fox T.L., Hougham A.J., Schmitt K.A. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob. Agents Chemother. 1998;42(4):789–794. doi: 10.1128/AAC.42.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beutner K.R., Spruance S.L., Hougham A.J., Fox T.L., Owens M.L., Douglas J.M., Jr. Treatment of genital warts with an immune-response modifier (imiquimod) J. Am. Acad. Dermatol. 1998;38(2 Pt 1):230–239. doi: 10.1016/s0190-9622(98)70243-9. [DOI] [PubMed] [Google Scholar]
- 113.Wiley D.J., Douglas J., Beutner K., Cox T., Fife K., Moscicki A.B., Fukumoto L. External genital warts: diagnosis, treatment, and prevention. Clin. Infect. Dis. 2002;35(Suppl. 2):S210–S224. doi: 10.1086/342109. [DOI] [PubMed] [Google Scholar]
- 114.Chen S.T., Dou J., Temple R., Agarwal R., Wu K.M., Walker S. New therapies from old medicines. Nat. Biotechnol. 2008;26(10):1077–1083. doi: 10.1038/nbt1008-1077. [DOI] [PubMed] [Google Scholar]
- 115.Tatti S., Swinehart J.M., Thielert C., Tawfik H., Mescheder A., Beutner K.R. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet. Gynecol. 2008;111(6):1371–1379. doi: 10.1097/AOG.0b013e3181719b60. [DOI] [PubMed] [Google Scholar]
- 116.Simoes E.A. Respiratory syncytial virus infection. Lancet. 1999;354(9181):847–852. doi: 10.1016/S0140-6736(99)80040-3. [DOI] [PubMed] [Google Scholar]
- 117.Robinson R.F., Nahata M.C. Respiratory syncytial virus (RSV) immune globulin and palivizumab for prevention of RSV infection. Am. J. Health Syst. Pharm. 2000;57(3):259–264. doi: 10.1093/ajhp/57.3.259. [DOI] [PubMed] [Google Scholar]
- 118.Cardenas S., Auais A., Piedimonte G. Palivizumab in the prophylaxis of respiratory syncytial virus infection. Expert Rev. Anti-Infect. Ther. 2005;3(5):719–726. doi: 10.1586/14787210.3.5.719. [DOI] [PubMed] [Google Scholar]
- 119.Scott L.J., Lamb H.M. Palivizumab. Drugs. 1999;58(2):305–311. doi: 10.2165/00003495-199958020-00009. [DOI] [PubMed] [Google Scholar]
- 120.Wray S.K., Gilbert B.E., Noall M.W., Knight V. Mode of action of ribavirin: effect of nucleotide pool alterations on influenza virus ribonucleoprotein synthesis. Antivir. Res. 1985;5(1):29–37. doi: 10.1016/0166-3542(85)90012-9. [DOI] [PubMed] [Google Scholar]
- 121.Vogelberg C., Meyer-Konig U., Hufert F.T., Kirste G., von Laer D. Human cytomegalovirus glycoprotein B genotypes in renal transplant recipients. J. Med. Virol. 1996;50(1):31–34. doi: 10.1002/(SICI)1096-9071(199609)50:1<31::AID-JMV7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 122.Chou S. Comparative analysis of sequence variation in gp116 and gp55 components of glycoprotein B of human cytomegalovirus. Virology. 1992;188(1):388–390. doi: 10.1016/0042-6822(92)90771-g. [DOI] [PubMed] [Google Scholar]
- 123.Crumpacker C.S. Ganciclovir. N. Engl. J. Med. 1996;335(10):721–729. doi: 10.1056/nejm199609053351007. [DOI] [PubMed] [Google Scholar]
- 124.D.W. Kimberlin, P.M. Jester, P.J. Sanchez, A. Ahmed, R. Arav-Boger, M.G. Michaels, N. Ashouri, J.A. Englund, B. Estrada, R.F. Jacobs, J.R. Romero, S.K. Sood, M.S. Whitworth, M.J. Abzug, M.T. Caserta, S. Fowler, J. Lujan-Zilbermann, G.A. Storch, R.L. DeBiasi, J.Y. Han, A. Palmer, L.B. Weiner, J.A. Bocchini, P.H. Dennehy, A. Finn, P.D. Griffiths, S. Luck, K. Gutierrez, N. Halasa, J. Homans, A.L. Shane, M. Sharland, K. Simonsen, J.A. Vanchiere, C.R. Woods, D.L. Sabo, I. Aban, H. Kuo, S.H. James, M.N. Prichard, J. Griffin, D. Giles, E.P. Acosta, R.J. Whitley, Valganciclovir for symptomatic congenital cytomegalovirus disease, N. Engl. J. Med. 372(10) (2015) 933–43. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed]
- 125.Minces L.R., Nguyen M.H., Mitsani D., Shields R.K., Kwak E.J., Silveira F.P., Abdel-Massih R., Pilewski J.M., Crespo M.M., Bermudez C., Bhama J.K., Toyoda Y., Clancy C.J. Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens. Antimicrob. Agents Chemother. 2014;58(1):128–135. doi: 10.1128/AAC.00561-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Smet M.D., Meenken C.J., van den Horn G.J. Fomivirsen - a phosphorothioate oligonucleotide for the treatment of CMV retinitis. Ocul. Immunol. Inflamm. 1999;7(3–4):189–198. doi: 10.1076/ocii.7.3.189.4007. [DOI] [PubMed] [Google Scholar]
- 127.Orr R.M. Technology evaluation: fomivirsen, Isis Pharmaceuticals Inc/CIBA vision. Curr. Opin. Mol. Ther. 2001;3(3):288–294. [PubMed] [Google Scholar]
- 128.L.R. Grillone, R. Lanz, Fomivirsen, Drugs of today (Barcelona, Spain : 1998) 37(4) (2001) 245–255. doi: 10.1358/dot.2001.37.4.620590. [DOI] [PubMed]
- 129.Goldner T., Hewlett G., Ettischer N., Ruebsamen-Schaeff H., Zimmermann H., Lischka P. The novel anticytomegalovirus compound AIC246 (Letermovir) inhibits human cytomegalovirus replication through a specific antiviral mechanism that involves the viral terminase. J. Virol. 2011;85(20):10884–10893. doi: 10.1128/JVI.05265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bowman L.J., Melaragno J.I., Brennan D.C. Letermovir for the management of cytomegalovirus infection. Expert Opin. Investig. Drugs. 2017;26(2):235–241. doi: 10.1080/13543784.2017.1274733. [DOI] [PubMed] [Google Scholar]
- 131.Arvin A.M. Varicella-zoster virus. Clin. Microbiol. Rev. 1996;9(3):361–381. doi: 10.1128/CMR.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zerboni L., Sen N., Oliver S.L., Arvin A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Microbiol. 2014;12(3):197–210. doi: 10.1038/nrmicro3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.R.J. Whitley, L.T. Ch'ien, R. Dolin, G.J. Galasso, C.A. Alford, Jr., Adenine arabinoside therapy of herpes zoster in the immunosuppressed. NIAID collaborative antiviral study, N. Engl. J. Med. 294(22) (1976) 1193–9. doi: 10.1056/NEJM197605272942201. [DOI] [PubMed]
- 134.Zaia J.A., Levin M.J., Preblud S.R., Leszczynski J., Wright G.G., Ellis R.J., Curtis A.C., Valerio M.A., LeGore J. Evaluation of Varicella-Zoster immune globulin: protection of immunosuppressed children after household exposure to varicella. J. Infect. Dis. 1983;147(4):737–743. doi: 10.1093/infdis/147.4.737. [DOI] [PubMed] [Google Scholar]
- 135.CDC, FDA approval of an extended period for administering VariZIG for postexposure prophylaxis of varicella, MMWR Morb. Mortal. Wkly. Rep. 61(12) (2012) 212. [PubMed]
- 136.CDC, Updated recommendations for use of VariZIG-United States, 2013, MMWR Morb. Mortal. Wkly. Rep. 62(28) (2013) 574–576. [PMC free article] [PubMed]
- 137.Schnitzler S.U., Schnitzler P. An update on swine-origin influenza virus A/H1N1: a review. Virus Genes. 2009;39(3):279–292. doi: 10.1007/s11262-009-0404-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Neumann G., Noda T., Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459(7249):931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheng V.C., To K.K., Tse H., Hung I.F., Yuen K.Y. Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin. Microbiol. Rev. 2012;25(2):223–263. doi: 10.1128/cmr.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.CDC Swine flu: variant influenza virus treatment. 2019. https://www.cdc.gov/flu/swineflu/variant-treatment.htm
- 141.Rewar S., Mirdha D., Rewar P. Treatment and prevention of pandemic H1N1 influenza. Ann Glob Health. 2015;81(5):645–653. doi: 10.1016/j.aogh.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 142.FDA Influenza (Flu) antiviral drugs and related information. 2019. https://www.fda.gov/drugs/information-drug-class/influenza-flu-antiviral-drugs-and-related-information
- 143.Breman J.G., Heymann D.L., Lloyd G., McCormick J.B., Miatudila M., Murphy F.A., Muyembe-Tamfun J.J., Piot P., Ruppol J.F., Sureau P., van der Groen G., Johnson K.M. Discovery and description of Ebola Zaire virus in 1976 and relevance to the West African epidemic during 2013–2016. J. Infect. Dis. 2016;214(Suppl. 3):S93–S101. doi: 10.1093/infdis/jiw207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baseler L., Chertow D.S., Johnson K.M., Feldmann H., Morens D.M. The pathogenesis of Ebola virus disease. Annu. Rev. Pathol. 2017;12:387–418. doi: 10.1146/annurev-pathol-052016-100506. [DOI] [PubMed] [Google Scholar]
- 145.D. Sissoko, C. Laouenan, E. Folkesson, A.-B. M'Lebing, A.-H. Beavogui, S. Baize, A.-M. Camara, P. Maes, S. Shepherd, C. Danel, S. Carazo, M.N. Conde, J.-L. Gala, G. Colin, H. Savini, J.A. Bore, F. Le Marcis, F.R. Koundouno, F. Petitjean, M.-C. Lamah, S. Diederich, A. Tounkara, G. Poelart, E. Berbain, J.-M. Dindart, S. Duraffour, A. Lefevre, T. Leno, O. Peyrouset, L. Irenge, N.F. Bangoura, R. Palich, J. Hinzmann, A. Kraus, T.S. Barry, S. Berette, A. Bongono, M.S. Camara, V. Chanfreau Munoz, L. Doumbouya, H. Souley, P.M. Kighoma, F.R. Koundouno, L. Réné, C.M. Loua, V. Massala, K. Moumouni, C. Provost, N. Samake, C. Sekou, A. Soumah, I. Arnould, M.S. Komano, L. Gustin, C. Berutto, D. Camara, F.S. Camara, J. Colpaert, L. Delamou, L. Jansson, E. Kourouma, M. Loua, K. Malme, E. Manfrin, A. Maomou, A. Milinouno, S. Ombelet, A.Y. Sidiboun, I. Verreckt, P. Yombouno, A. Bocquin, C. Carbonnelle, T. Carmoi, P. Frange, S. Mely, V.-K. Nguyen, D. Pannetier, A.-M. Taburet, J.-M. Treluyer, J. Kolie, R. Moh, M.C. Gonzalez, E. Kuisma, B. Liedigk, D. Ngabo, M. Rudolf, R. Thom, R. Kerber, M. Gabriel, A. Di Caro, R. Wölfel, J. Badir, M. Bentahir, Y. Deccache, C. Dumont, J.-F. Durant, K. El Bakkouri, M. Gasasira Uwamahoro, B. Smits, N. Toufik, S. Van Cauwenberghe, K. Ezzedine, E. Dortenzio, L. Pizarro, A. Etienne, J. Guedj, A. Fizet, E. Barte de Sainte Fare, B. Murgue, T. Tran-Minh, C. Rapp, P. Piguet, M. Poncin, B. Draguez, T. Allaford Duverger, S. Barbe, G. Baret, I. Defourny, M. Carroll, H. Raoul, A. Augier, S.P. Eholie, Y. Yazdanpanah, C. Levy-Marchal, A. Antierrens, M. Van Herp, S. Günther, X. de Lamballerie, S. Keïta, F. Mentre, X. Anglaret, D. Malvy, J.S. Group, Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea, PLoS Med. 13(3) (2016) e1001967. doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed]
- 146.Davey R.T., Jr., Dodd L., Proschan M.A., Neaton J., Neuhaus Nordwall J., Koopmeiners J.S., Beigel J., Tierney J., Lane H.C., Fauci A.S., Massaquoi M.B.F., Sahr F., Malvy D. A randomized, controlled trial of ZMapp for Ebola virus infection. N. Engl. J. Med. 2016;375(15):1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.T.K. Warren, R. Jordan, M.K. Lo, A.S. Ray, R.L. Mackman, V. Soloveva, D. Siegel, M. Perron, R. Bannister, H.C. Hui, N. Larson, R. Strickley, J. Wells, K.S. Stuthman, S.A. Van Tongeren, N.L. Garza, G. Donnelly, A.C. Shurtleff, C.J. Retterer, D. Gharaibeh, R. Zamani, T. Kenny, B.P. Eaton, E. Grimes, L.S. Welch, L. Gomba, C.L. Wilhelmsen, D.K. Nichols, J.E. Nuss, E.R. Nagle, J.R. Kugelman, G. Palacios, E. Doerffler, S. Neville, E. Carra, M.O. Clarke, L. Zhang, W. Lew, B. Ross, Q. Wang, K. Chun, L. Wolfe, D. Babusis, Y. Park, K.M. Stray, I. Trancheva, J.Y. Feng, O. Barauskas, Y. Xu, P. Wong, M.R. Braun, M. Flint, L.K. McMullan, S.S. Chen, R. Fearns, S. Swaminathan, D.L. Mayers, C.F. Spiropoulou, W.A. Lee, S.T. Nichol, T. Cihlar, S. Bavari, Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys, Nature 531(7594) (2016) 381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed]
- 148.S. Mulangu, L.E. Dodd, R.T. Davey, Jr., O. Tshiani Mbaya, M. Proschan, D. Mukadi, M. Lusakibanza Manzo, D. Nzolo, A. Tshomba Oloma, A. Ibanda, R. Ali, S. Coulibaly, A.C. Levine, R. Grais, J. Diaz, H.C. Lane, J.J. Muyembe-Tamfum, P.W. Group, B. Sivahera, M. Camara, R. Kojan, R. Walker, B. Dighero-Kemp, H. Cao, P. Mukumbayi, P. Mbala-Kingebeni, S. Ahuka, S. Albert, T. Bonnett, I. Crozier, M. Duvenhage, C. Proffitt, M. Teitelbaum, T. Moench, J. Aboulhab, K. Barrett, K. Cahill, K. Cone, R. Eckes, L. Hensley, B. Herpin, E. Higgs, J. Ledgerwood, J. Pierson, M. Smolskis, Y. Sow, J. Tierney, S. Sivapalasingam, W. Holman, N. Gettinger, D. Vallee, J. Nordwall, P.C.S. Team, A randomized, controlled trial of Ebola virus disease therapeutics, N. Engl. J. Med. 381(24) (2019) 2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed]
- 149.CDC Ebola (Ebola virus disease): treatment. 2019. https://www.cdc.gov/vhf/ebola/treatment/index.html
- 150.FDA, U.S. Food and Drug Administration. First FDA-approved Vaccine for the Prevention of Ebola Virus Disease, Marking a Critical Milestone in Public Health Preparedness and Response, 2019.
- 151.CDC Ebola (Ebola virus disease): prevention and vaccine. 2019. https://www.cdc.gov/vhf/ebola/prevention/index.html
- 152.NIAID Ebola vaccines. 2020. https://www.niaid.nih.gov/diseases-conditions/ebola-vaccines
- 153.Davis B.M., Foxman B., Monto A.S., Baric R.S., Martin E.T., Uzicanin A., Rainey J.J., Aiello A.E. Human coronaviruses and other respiratory infections in young adults on a university campus: prevalence, symptoms, and shedding. Influenza Other Respir. Viruses. 2018;12(5):582–590. doi: 10.1111/irv.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhong N., Zheng B., Li Y., Poon L., Xie Z., Li P., Tan S., Chang Q., Xie J., Liu X. Epidemiological and aetiological studies of patients with severe acute respiratory syndrome (SARS) from Guangdong in February 2003. Lancet. 2003;362(9393):1353–1358. doi: 10.1016/s0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 156.Peiris J.S., Yuen K.Y., Osterhaus A.D., Stohr K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 157.Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M., Ahuja A., Yung M.Y., Leung C.B., To K.F., Lui S.F., Szeto C.C., Chung S., Sung J.J. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348(20):1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- 158.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348(20):1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 159.CDC, Update: outbreak of severe acute respiratory syndrome-worldwide, 2003, MMWR Morb. Mortal. Wkly. Rep. 52(12) (2003) 241–6, 248. [PubMed]
- 160.LeDuc J.W., Barry M.A. SARS, the first pandemic of the 21st century. Emerging Infect. Dis. 2004;10(11):e26. doi: 10.3201/eid1011.040797_02. [DOI] [Google Scholar]
- 161.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 162.Drosten C., Günther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguière A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 163.Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stöhr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362(9380):263–270. doi: 10.1016/s0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kan B., Wang M., Jing H., Xu H., Jiang X., Yan M., Liang W., Zheng H., Wan K., Liu Q., Cui B., Xu Y., Zhang E., Wang H., Ye J., Li G., Li M., Cui Z., Qi X., Chen K., Du L., Gao K., Zhao Y.T., Zou X.Z., Feng Y.J., Gao Y.F., Hai R., Yu D., Guan Y., Xu J. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J. Virol. 2005;79(18):11892–11900. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang M., Yan M., Xu H., Liang W., Kan B., Zheng B., Chen H., Zheng H., Xu Y., Zhang E., Wang H., Ye J., Li G., Li M., Cui Z., Liu Y.F., Guo R.T., Liu X.N., Zhan L.H., Zhou D.H., Zhao A., Hai R., Yu D., Guan Y., Xu J. SARS-CoV infection in a restaurant from palm civet. Emerg. Infect. Dis. 2005;11(12):1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gompf S.G. Severe Acute Respiratory Syndrome (SARS) 2020. https://www.medicinenet.com/severe_acute_respiratory_syndrome_sars/article.htm
- 167.de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L., Fouchier R.A.M., Galiano M., Gorbalenya A.E., Memish Z.A., Perlman S., Poon L.L.M., Snijder E.J., Stephens G.M., Woo P.C.Y., Zaki A.M., Zambon M., Ziebuhr J. Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J. Virol. 2013;87(14):7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hijawi B., Abdallat M., Sayaydeh A., Alqasrawi S., Haddadin A., Jaarour N., Alsheikh S., Alsanouri T. Novel coronavirus infections in Jordan, April 2012: epidemiological findings from a retrospective investigation. East Mediterr. Health J. 2013;19(Suppl. 1):S12–S18. [PubMed] [Google Scholar]
- 169.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) 2019. https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov
- 170.WHO Middle East respiratory syndrome coronavirus (MERS-CoV) – the kingdom of Saudi Arabia. 2020. https://www.who.int/csr/don/24-february-2020-mers-saudi-arabia/en/
- 171.Chan J.F., Lau S.K., K.K. To, Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28(2):465–522. doi: 10.1128/cmr.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.C.B. Reusken, B.L. Haagmans, M.A. Müller, C. Gutierrez, G.J. Godeke, B. Meyer, D. Muth, V.S. Raj, L. Smits-De Vries, V.M. Corman, J.F. Drexler, S.L. Smits, Y.E. El Tahir, R. De Sousa, J. van Beek, N. Nowotny, K. van Maanen, E. Hidalgo-Hermoso, B.J. Bosch, P. Rottier, A. Osterhaus, C. Gortázar-Schmidt, C. Drosten, M.P. Koopmans, Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study, Lancet Infect. Dis. 13(10) (2013) 859–66. doi: 10.1016/s1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed]
- 173.Azhar E.I., El-Kafrawy S.A., Farraj S.A., Hassan A.M., Al-Saeed M.S., Hashem A.M., Madani T.A. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 174.Wang Q., Qi J., Yuan Y., Xuan Y., Han P., Wan Y., Ji W., Li Y., Wu Y., Wang J., Iwamoto A., Woo P.C., Yuen K.Y., Yan J., Lu G., Gao G.F. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe. 2014;16(3):328–337. doi: 10.1016/j.chom.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Assiri A., McGeer A., Perl T.M., Price C.S., Al Rabeeah A.A., Cummings D.A.T., Alabdullatif Z.N., Assad M., Almulhim A., Makhdoom H., Madani H., Alhakeem R., Al-Tawfiq J.A., Cotten M., Watson S.J., Kellam P., Zumla A.I., Memish Z.A. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013;369(5):407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Al-Abdallat M.M., Payne D.C., Alqasrawi S., Rha B., Tohme R.A., Abedi G.R., Al Nsour M., Iblan I., Jarour N., Farag N.H., Haddadin A., Al-Sanouri T., Tamin A., Harcourt J.L., Kuhar D.T., Swerdlow D.L., Erdman D.D., Pallansch M.A., Haynes L.M., Gerber S.I. Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin. Infect. Dis. 2014;59(9):1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Memish Z.A., Zumla A.I., Al-Hakeem R.F., Al-Rabeeah A.A., Stephens G.M. Family cluster of Middle East respiratory syndrome coronavirus infections. N. Engl. J. Med. 2013;368(26):2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 178.T.P. Sheahan, A.C. Sims, R.L. Graham, V.D. Menachery, L.E. Gralinski, J.B. Case, S.R. Leist, K. Pyrc, J.Y. Feng, I. Trantcheva, R. Bannister, Y. Park, D. Babusis, M.O. Clarke, R.L. Mackman, J.E. Spahn, C.A. Palmiotti, D. Siegel, A.S. Ray, T. Cihlar, R. Jordan, M.R. Denison, R.S. Baric, Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses, Sci. Transl. Med. 9(396) (2017) eaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed]
- 179.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O., Mackman R.L., Hui H.C., Perron M., Ray A.S., Cihlar T., Nichol S.T., Spiropoulou C.F. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017;7(1):43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.M.L. Agostini, E.L. Andres, A.C. Sims, R.L. Graham, T.P. Sheahan, X. Lu, E.C. Smith, J.B. Case, J.Y. Feng, R. Jordan, A.S. Ray, T. Cihlar, D. Siegel, R.L. Mackman, M.O. Clarke, R.S. Baric, M.R. Denison, Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease, mBio 9(2) (2018) e00221–18. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed]
- 181.Wu C.Y., Jan J.T., Ma S.H., Kuo C.J., Juan H.F., Cheng Y.S., Hsu H.H., Huang H.C., Wu D., Brik A., Liang F.S., Liu R.S., Fang J.M., Chen S.T., Liang P.H., Wong C.H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101(27):10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L., Li F., Xiao C., Gao H., Yu P., Cai J.P., Chu H., Zhou J., Chen H., Qin C., Yuen K.Y. Treatment with lopinavir/ritonavir or interferon-beta1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kim U.J., Won E.J., Kee S.J., Jung S.I., Jang H.C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-alpha for Middle East respiratory syndrome. Antivir. Ther. 2016;21(5):455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- 185.Bin S.Y., Heo J.Y., Song M.S., Lee J., Kim E.H., Park S.J., Kwon H.I., Kim S.M., Kim Y.I., Si Y.J., Lee I.W., Baek Y.H., Choi W.S., Min J., Jeong H.W., Choi Y.K. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin. Infect. Dis. 2016;62(6):755–760. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Y.M. Arabi, A. Alothman, H.H. Balkhy, A. Al-Dawood, S. AlJohani, S. Al Harbi, S. Kojan, M. Al Jeraisy, A.M. Deeb, A.M. Assiri, F. Al-Hameed, A. AlSaedi, Y. Mandourah, G.A. Almekhlafi, N.M. Sherbeeni, F.E. Elzein, J. Memon, Y. Taha, A. Almotairi, K.A. Maghrabi, I. Qushmaq, A. Al Bshabshe, A. Kharaba, S. Shalhoub, J. Jose, R.A. Fowler, F.G. Hayden, M.A. Hussein, Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial, Trials 19(1) (2018) 81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed]
- 187.Y.M. Arabi, A.Y. Asiri, A.M. Assiri, H.A. Aziz Jokhdar, A. Alothman, H.H. Balkhy, S. AlJohani, S. Al Harbi, S. Kojan, M. Al Jeraisy, A.M. Deeb, Z.A. Memish, S. Ghazal, S. Al Faraj, F. Al-Hameed, A. AlSaedi, Y. Mandourah, G.A. Al Mekhlafi, N.M. Sherbeeni, F.E. Elzein, A. Almotairi, A. Al Bshabshe, A. Kharaba, J. Jose, A. Al Harthy, M. Al Sulaiman, A. Mady, R.A. Fowler, F.G. Hayden, A. Al-Dawood, M. Abdelzaher, W. Bajhmom, M.A. Hussein, Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial, Trials 21(1) (2020) 8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed]
- 188.Sui J., Deming M., Rockx B., Liddington R.C., Zhu Q.K., Baric R.S., Marasco W.A. Effects of human anti-spike protein receptor binding domain antibodies on severe acute respiratory syndrome coronavirus neutralization escape and fitness. J. Virol. 2014;88(23):13769–13780. doi: 10.1128/JVI.02232-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhou Y., Vedantham P., Lu K., Agudelo J., Carrion R., Jr., Nunneley J.W., Barnard D., Pöhlmann S., McKerrow J.H., Renslo A.R., Simmons G. Protease inhibitors targeting coronavirus and filovirus entry. Antivir. Res. 2015;116:76–84. doi: 10.1016/j.antiviral.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291(17):9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ströher U., DiCaro A., Li Y., Strong J.E., Aoki F., Plummer F., Jones S.M., Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J. Infect. Dis. 2004;189(7):1164–1167. doi: 10.1086/382597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci. Rep. 2013;3(1):1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Haagmans B.L., Kuiken T., Martina B.E., Fouchier R.A., Rimmelzwaan G.F., van Amerongen G., van Riel D., de Jong T., Itamura S., Chan K.H., Tashiro M., Osterhaus A.D. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10(3):290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Falzarano D., de Wit E., Rasmussen A.L., Feldmann F., Okumura A., Scott D.P., Brining D., Bushmaker T., Martellaro C., Baseler L., Benecke A.G., Katze M.G., Munster V.J., Feldmann H. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat. Med. 2013;19(10):1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.WHO, Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. (Accessed 8 January 2021).
- 199.Andersen P.I., Ianevski A., Lysvand H., Vitkauskiene A., Oksenych V., Bjoras M., Telling K., Lutsar I., Dumpis U., Irie Y., Tenson T., Kantele A., Kainov D.E. Discovery and development of safe-in-man broad-spectrum antiviral agents. Int. J. Infect. Dis. 2020;93:268–276. doi: 10.1016/j.ijid.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020;38(4):379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 201.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 203.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295(15):4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.J.H. Beigel, K.M. Tomashek, L.E. Dodd, A.K. Mehta, B.S. Zingman, A.C. Kalil, E. Hohmann, H.Y. Chu, A. Luetkemeyer, S. Kline, D. Lopez de Castilla, R.W. Finberg, K. Dierberg, V. Tapson, L. Hsieh, T.F. Patterson, R. Paredes, D.A. Sweeney, W.R. Short, G. Touloumi, D.C. Lye, N. Ohmagari, M.-d. Oh, G.M. Ruiz-Palacios, T. Benfield, G. Fätkenheuer, M.G. Kortepeter, R.L. Atmar, C.B. Creech, J. Lundgren, A.G. Babiker, S. Pett, J.D. Neaton, T.H. Burgess, T. Bonnett, M. Green, M. Makowski, A. Osinusi, S. Nayak, H.C. Lane, Remdesivir for the treatment of Covid-19 — final report, N. Engl. J. Med. 383(19) (2020) 1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed]
- 205.J. Grein, N. Ohmagari, D. Shin, G. Diaz, E. Asperges, A. Castagna, T. Feldt, G. Green, M.L. Green, F.-X. Lescure, E. Nicastri, R. Oda, K. Yo, E. Quiros-Roldan, A. Studemeister, J. Redinski, S. Ahmed, J. Bernett, D. Chelliah, D. Chen, S. Chihara, S.H. Cohen, J. Cunningham, A. D'Arminio Monforte, S. Ismail, H. Kato, G. Lapadula, E. L'Her, T. Maeno, S. Majumder, M. Massari, M. Mora-Rillo, Y. Mutoh, D. Nguyen, E. Verweij, A. Zoufaly, A.O. Osinusi, A. DeZure, Y. Zhao, L. Zhong, A. Chokkalingam, E. Elboudwarej, L. Telep, L. Timbs, I. Henne, S. Sellers, H. Cao, S.K. Tan, L. Winterbourne, P. Desai, R. Mera, A. Gaggar, R.P. Myers, D.M. Brainard, R. Childs, T. Flanigan, Compassionate use of remdesivir for patients with severe Covid-19, N. Engl. J. Med. 382(24) (2020) 2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed]
- 206.Gilead, Gilead announces results from phase 3 trial of remdesivir in patients with moderate COVID-19, 2020.
- 207.H. Balfour, Researchers identify a potential remdesivir resistance mechanism in SARS-CoV-2. https://www.drugtargetreview.com/news/73959/researchers-identify-a-potential-remdesivir-resistance-mechanism-in-sars-cov-2/#:~:text=Researchers%20have%20examined%20how%20Ebola,for%20these%20specific%20resistance%20mutations. (Accessed 8 January 8 2021).
- 208.A.K. Padhi, R. Shukla, P. Saudagar, T. Tripathi, High-throughput rational design of the remdesivir binding site in the RdRp of SARS-CoV-2: implications for potential resistance, iScience (2020) 101992. doi: 10.1016/j.isci.2020.101992. [DOI] [PMC free article] [PubMed]
- 209.Repurposed antiviral drugs for Covid-19 — interim WHO Solidarity Trial results, (2020) doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed]
- 210.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9(1):382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zheng M., Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell. Mol. Immunol. 2020;17(5):536–538. doi: 10.1038/s41423-020-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lei C., Fu W., Qian K., Li T., Zhang S., Ding M., Hu S. 2020. Potent neutralization of 2019 novel coronavirus by recombinant ACE2-Ig, bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.R.L. Kruse, Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China, F1000Res 9 (2020) 72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed]
- 215.ClinicalTrials.gov, Recombinant human angiotensin-converting enzyme 2 (rhACE2) as a treatment for patients with COVID-19. https://clinicaltrials.gov/ct2/show/study/NCT04287686. (Accessed 30 June 2020).
- 216.M. Hoffmann, H. Kleine-Weber, S. Schroeder, N. Krüger, T. Herrler, S. Erichsen, T.S. Schiergens, G. Herrler, N.-H. Wu, A. Nitsche, M.A. Müller, C. Drosten, S. Pöhlmann, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell 181(2) (2020) 271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed]
- 217.Neveu G., Ziv-Av A., Barouch-Bentov R., Berkerman E., Mulholland J., Einav S. AP-2-associated protein kinase 1 and cyclin G-associated kinase regulate hepatitis C virus entry and are potential drug targets. J. Virol. 2015;89(8):4387–4404. doi: 10.1128/JVI.02705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Rawling M., Savory E., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhou W., Liu Y., Tian D., Wang C., Wang S., Cheng J., Hu M., Fang M., Gao Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5(1):18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.P. Horby, W.S. Lim, J. Emberson, M. Mafham, J. Bell, L. Linsell, N. Staplin, C. Brightling, A. Ustianowski, E. Elmahi, B. Prudon, C. Green, T. Felton, D. Chadwick, K. Rege, C. Fegan, L.C. Chappell, S.N. Faust, T. Jaki, K. Jeffery, A. Montgomery, K. Rowan, E. Juszczak, J.K. Baillie, R. Haynes, M.J. Landray, Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report, medRxiv (2020) 2020.06.22.20137273. doi: 10.1101/2020.06.22.20137273. [DOI]
- 222.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582(7813):469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 223.ClinicalTrials.gov, Efficacy and safety of corticosteroids in COVID-19. https://clinicaltrials.gov/ct2/show/record/NCT04273321. (Accessed 30 June 2020).
- 224.D.E. Gordon, G.M. Jang, M. Bouhaddou, J. Xu, K. Obernier, K.M. White, M.J. O'Meara, V.V. Rezelj, J.Z. Guo, D.L. Swaney, T.A. Tummino, R. Hüttenhain, R.M. Kaake, A.L. Richards, B. Tutuncuoglu, H. Foussard, J. Batra, K. Haas, M. Modak, M. Kim, P. Haas, B.J. Polacco, H. Braberg, J.M. Fabius, M. Eckhardt, M. Soucheray, M.J. Bennett, M. Cakir, M.J. McGregor, Q. Li, B. Meyer, F. Roesch, T. Vallet, A. Mac Kain, L. Miorin, E. Moreno, Z.Z.C. Naing, Y. Zhou, S. Peng, Y. Shi, Z. Zhang, W. Shen, I.T. Kirby, J.E. Melnyk, J.S. Chorba, K. Lou, S.A. Dai, I. Barrio-Hernandez, D. Memon, C. Hernandez-Armenta, J. Lyu, C.J.P. Mathy, T. Perica, K.B. Pilla, S.J. Ganesan, D.J. Saltzberg, R. Rakesh, X. Liu, S.B. Rosenthal, L. Calviello, S. Venkataramanan, J. Liboy-Lugo, Y. Lin, X.-P. Huang, Y. Liu, S.A. Wankowicz, M. Bohn, M. Safari, F.S. Ugur, C. Koh, N.S. Savar, Q.D. Tran, D. Shengjuler, S.J. Fletcher, M.C. O'Neal, Y. Cai, J.C.J. Chang, D.J. Broadhurst, S. Klippsten, P.P. Sharp, N.A. Wenzell, D. Kuzuoglu-Ozturk, H.-Y. Wang, R. Trenker, J.M. Young, D.A. Cavero, J. Hiatt, T.L. Roth, U. Rathore, A. Subramanian, J. Noack, M. Hubert, R.M. Stroud, A.D. Frankel, O.S. Rosenberg, K.A. Verba, D.A. Agard, M. Ott, M. Emerman, N. Jura, M. von Zastrow, E. Verdin, A. Ashworth, O. Schwartz, C. d'Enfert, S. Mukherjee, M. Jacobson, H.S. Malik, D.G. Fujimori, T. Ideker, C.S. Craik, S.N. Floor, J.S. Fraser, J.D. Gross, A. Sali, B.L. Roth, D. Ruggero, J. Taunton, T. Kortemme, P. Beltrao, M. Vignuzzi, A. García-Sastre, K.M. Shokat, B.K. Shoichet, N.J. Krogan, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature 583(7816) (2020) 459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed]
- 225.ClinicalTrials.gov, Efficacy and safety of Darunavir and Cobicistat for treatment of COVID-19 (DC-COVID-19). https://clinicaltrials.gov/ct2/show/NCT04252274?term=DARUNAVIR&draw=2&rank=4. (Accessed 30 June 2020).
- 226.ClinicalTrials.gov, Favipiravir combined with tocilizumab in the treatment of corona virus disease 2019. https://clinicaltrials.gov/ct2/show/NCT04310228?term=tocilizumab&cond=covid-19+OR+coronavirus&draw=2. (Accessed 30 June 2020).
- 227.ClinicalTrials.gov, A prospective/retrospective, randomized controlled clinical study of antiviral therapy in the 2019-nCoV pneumonia. https://clinicaltrials.gov/ct2/show/NCT04255017?cond=2019-ncov&draw=2. (Accessed 30 June 2020).
- 228.ClinicalTrials.gov, Tocilizumab for SARS-CoV2 (COVID-19) severe pneumonitis. https://clinicaltrials.gov/ct2/show/NCT04315480?term=tocilizumab&cond=covid-19+OR+coronavirus&draw=2. (Accessed 30 June 2020).
- 229.ClinicalTrials.gov, COVID-19. https://clinicaltrials.gov/ct2/results?cond=COVID-19. (Accessed 30 June 2020).
- 230.Craven J. COVID-19 vaccine tracker. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
- 231.F.P. Polack, S.J. Thomas, N. Kitchin, J. Absalon, A. Gurtman, S. Lockhart, J.L. Perez, G. Pérez Marc, E.D. Moreira, C. Zerbini, R. Bailey, K.A. Swanson, S. Roychoudhury, K. Koury, P. Li, W.V. Kalina, D. Cooper, R.W. Frenck, L.L. Hammitt, Ö. Türeci, H. Nell, A. Schaefer, S. Ünal, D.B. Tresnan, S. Mather, P.R. Dormitzer, U. Şahin, K.U. Jansen, W.C. Gruber, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine, 383(27) (2020) 2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed]
- 232.L.R. Baden, H.M. El Sahly, B. Essink, K. Kotloff, S. Frey, R. Novak, D. Diemert, S.A. Spector, N. Rouphael, C.B. Creech, J. McGettigan, S. Khetan, N. Segall, J. Solis, A. Brosz, C. Fierro, H. Schwartz, K. Neuzil, L. Corey, P. Gilbert, H. Janes, D. Follmann, M. Marovich, J. Mascola, L. Polakowski, J. Ledgerwood, B.S. Graham, H. Bennett, R. Pajon, C. Knightly, B. Leav, W. Deng, H. Zhou, S. Han, M. Ivarsson, J. Miller, T. Zaks, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, (2020) doi: 10.1056/NEJMoa2035389. [DOI]
- 233.Palagin A. Bolivia has registered Sputnik V vaccine based on Russian clinical trial data. https://sputnikvaccine.com/newsroom/pressreleases/bolivia-has-registered-sputnik-v-vaccine-based-on-russian-clinical-trial-data/
- 234.Palagin A. RDIF to supply 2 million doses of Sputnik V vaccine to Serbia. https://sputnikvaccine.com/newsroom/pressreleases/rdif-to-supply-2-million-doses-of-sputnik-v-vaccine-to-serbia/
- 235.Chinese Covid vaccine CoronaVac 78% effective in Brazil trials, claim officials. https://www.indiatoday.in/coronavirus-outbreak/story/chinese-covid-vaccine-coronavac-78-effective-in-brazil-trials-claim-officials-1756958-2021-01-08. (Accessed 8 January 2021).
- 236.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., Chen X., Hu Y., Liu X., Jiang C., Li J., Yang M., Song Y., Wang X., Gao Q., Zhu F. 2020. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial, The Lancet Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.ClinicalTrials.gov, Investigating a vaccine against COVID-19. https://clinicaltrials.gov/ct2/show/NCT04400838. (Accessed 8 January 2021).
- 238.M. Voysey, S.A.C. Clemens, S.A. Madhi, L.Y. Weckx, P.M. Folegatti, P.K. Aley, B. Angus, V.L. Baillie, S.L. Barnabas, Q.E. Bhorat, S. Bibi, C. Briner, P. Cicconi, A.M. Collins, R. Colin-Jones, C.L. Cutland, T.C. Darton, K. Dheda, C.J.A. Duncan, K.R.W. Emary, K.J. Ewer, L. Fairlie, S.N. Faust, S. Feng, D.M. Ferreira, A. Finn, A.L. Goodman, C.M. Green, C.A. Green, P.T. Heath, C. Hill, H. Hill, I. Hirsch, S.H.C. Hodgson, A. Izu, S. Jackson, D. Jenkin, C.C.D. Joe, S. Kerridge, A. Koen, G. Kwatra, R. Lazarus, A.M. Lawrie, A. Lelliott, V. Libri, P.J. Lillie, R. Mallory, A.V.A. Mendes, E.P. Milan, A.M. Minassian, A. McGregor, H. Morrison, Y.F. Mujadidi, A. Nana, P.J. O'Reilly, S.D. Padayachee, A. Pittella, E. Plested, K.M. Pollock, M.N. Ramasamy, S. Rhead, A.V. Schwarzbold, N. Singh, A. Smith, R. Song, M.D. Snape, E. Sprinz, R.K. Sutherland, R. Tarrant, E.C. Thomson, M.E. Török, M. Toshner, D.P.J. Turner, J. Vekemans, T.L. Villafana, M.E.E. Watson, C.J. Williams, A.D. Douglas, A.V.S. Hill, T. Lambe, S.C. Gilbert, A.J. Pollard, M. Aban, F. Abayomi, K. Abeyskera, J. Aboagye, M. Adam, K. Adams, J. Adamson, Y.A. Adelaja, G. Adewetan, S. Adlou, K. Ahmed, Y. Akhalwaya, S. Akhalwaya, A. Alcock, A. Ali, E.R. Allen, L. Allen, T.C.D.S.C. Almeida, M.P.S. Alves, F. Amorim, F. Andritsou, R. Anslow, M. Appleby, E.H. Arbe-Barnes, M.P. Ariaans, B. Arns, L. Arruda, P. Azi, L. Azi, G. Babbage, C. Bailey, K.F. Baker, M. Baker, N. Baker, P. Baker, L. Baldwin, I. Baleanu, D. Bandeira, A. Bara, M.A.S. Barbosa, D. Barker, G.D. Barlow, E. Barnes, A.S. Barr, J.R. Barrett, J. Barrett, L. Bates, A. Batten, K. Beadon, E. Beales, R. Beckley, S. Belij-Rammerstorfer, J. Bell, D. Bellamy, N. Bellei, S. Belton, A. Berg, L. Bermejo, E. Berrie, L. Berry, D. Berzenyi, A. Beveridge, K.R. Bewley, H. Bexhell, S. Bhikha, A.E. Bhorat, Z.E. Bhorat, E. Bijker, G. Birch, S. Birch, A. Bird, O. Bird, K. Bisnauthsing, M. Bittaye, K. Blackstone, L. Blackwell, H. Bletchly, C.L. Blundell, S.R. Blundell, P. Bodalia, B.C. Boettger, E. Bolam, E. Boland, D. Bormans, N. Borthwick, F. Bowring, A. Boyd, P. Bradley, T. Brenner, P. Brown, C. Brown, C. Brown-O'Sullivan, S. Bruce, E. Brunt, R. Buchan, W. Budd, Y.A. Bulbulia, M. Bull, J. Burbage, H. Burhan, A. Burn, K.R. Buttigieg, N. Byard, I. Cabera Puig, G. Calderon, A. Calvert, S. Camara, M. Cao, F. Cappuccini, J.R. Cardoso, M. Carr, M.W. Carroll, A. Carson-Stevens, Y.d.M. Carvalho, J.A.M. Carvalho, H.R. Casey, P. Cashen, T. Castro, L.C. Castro, K. Cathie, A. Cavey, J. Cerbino-Neto, J. Chadwick, D. Chapman, S. Charlton, I. Chelysheva, O. Chester, S. Chita, J.-S. Cho, L. Cifuentes, E. Clark, M. Clark, A. Clarke, E.A. Clutterbuck, S.L.K. Collins, C.P. Conlon, S. Connarty, N. Coombes, C. Cooper, R. Cooper, L. Cornelissen, T. Corrah, C. Cosgrove, T. Cox, W.E.M. Crocker, S. Crosbie, L. Cullen, D. Cullen, D.R.M.F. Cunha, C. Cunningham, F.C. Cuthbertson, S.N.F. Da Guarda, L.P. da Silva, B.E. Damratoski, Z. Danos, M.T.D.C. Dantas, P. Darroch, M.S. Datoo, C. Datta, M. Davids, S.L. Davies, H. Davies, E. Davis, J. Davis, J. Davis, M.M.D. De Nobrega, L.M. De Oliveira Kalid, D. Dearlove, T. Demissie, A. Desai, S. Di Marco, C. Di Maso, M.I.S. Dinelli, T. Dinesh, C. Docksey, C. Dold, T. Dong, F.R. Donnellan, T. Dos Santos, T.G. dos Santos, E.P. Dos Santos, N. Douglas, C. Downing, J. Drake, R. Drake-Brockman, K. Driver, R. Drury, S.J. Dunachie, B.S. Durham, L. Dutra, N.J.W. Easom, S. van Eck, M. Edwards, N.J. Edwards, O.M. El Muhanna, S.C. Elias, M. Elmore, M. English, A. Esmail, Y.M. Essack, E. Farmer, M. Farooq, M. Farrar, L. Farrugia, B. Faulkner, S. Fedosyuk, S. Felle, S. Feng, C. Ferreira Da Silva, S. Field, R. Fisher, A. Flaxman, J. Fletcher, H. Fofie, H. Fok, K.J. Ford, J. Fowler, P.H.A. Fraiman, E. Francis, M.M. Franco, J. Frater, M.S.M. Freire, S.H. Fry, S. Fudge, J. Furze, M. Fuskova, P. Galian-Rubio, E. Galiza, H. Garlant, M. Gavrila, A. Geddes, K.A. Gibbons, C. Gilbride, H. Gill, S. Glynn, K. Godwin, K. Gokani, U.C. Goldoni, M. Goncalves, I.G.S. Gonzalez, J. Goodwin, A. Goondiwala, K. Gordon-Quayle, G. Gorini, J. Grab, L. Gracie, M. Greenland, N. Greenwood, J. Greffrath, M.M. Groenewald, L. Grossi, G. Gupta, M. Hackett, B. Hallis, M. Hamaluba, E. Hamilton, J. Hamlyn, D. Hammersley, A.T. Hanrath, B. Hanumunthadu, S.A. Harris, C. Harris, T. Harris, T.D. Harrison, D. Harrison, T.C. Hart, B. Hartnell, S. Hassan, J. Haughney, S. Hawkins, J. Hay, I. Head, J. Henry, M. Hermosin Herrera, D.B. Hettle, J. Hill, G. Hodges, E. Horne, M.M. Hou, C. Houlihan, E. Howe, N. Howell, J. Humphreys, H.E. Humphries, K. Hurley, C. Huson, A. Hyder-Wright, C. Hyams, S. Ikram, A. Ishwarbhai, M. Ivan, P. Iveson, V. Iyer, F. Jackson, J. De Jager, S. Jaumdally, H. Jeffers, N. Jesudason, B. Jones, K. Jones, E. Jones, C. Jones, M.R. Jorge, A. Jose, A. Joshi, E.A.M.S. Júnior, J. Kadziola, R. Kailath, F. Kana, K. Karampatsas, M. Kasanyinga, J. Keen, E.J. Kelly, D.M. Kelly, D. Kelly, S. Kelly, D. Kerr, R.d.Á. Kfouri, L. Khan, B. Khozoee, S. Kidd, A. Killen, J. Kinch, P. Kinch, L.D.W. King, T.B. King, L. Kingham, P. Klenerman, F. Knapper, J.C. Knight, D. Knott, S. Koleva, M. Lang, G. Lang, C.W. Larkworthy, J.P.J. Larwood, R. Law, E.M. Lazarus, A. Leach, E.A. Lees, N.-M. Lemm, A. Lessa, 3S. Leung, Y. Li, A.M. Lias, K. Liatsikos, A. Linder, S. Lipworth, S. Liu, X. Liu, A. Lloyd, S. Lloyd, L. Loew, R. Lopez Ramon, L. Lora, V. Lowthorpe, K. Luz, J.C. MacDonald, G. MacGregor, M. Madhavan, D.O. Mainwaring, E. Makambwa, R. Makinson, M. Malahleha, R. Malamatsho, G. Mallett, K. Mansatta, T. Maoko, K. Mapetla, N.G. Marchevsky, S. Marinou, E. Marlow, G.N. Marques, P. Marriott, R.P. Marshall, J.L. Marshall, F.J. Martins, M. Masenya, M. Masilela, S.K. Masters, M. Mathew, H. Matlebjane, K. Matshidiso, O. Mazur, A. Mazzella, H. McCaughan, J. McEwan, J. McGlashan, L. McInroy, Z. McIntyre, D. McLenaghan, N. McRobert, S. McSwiggan, C. Megson, S. Mehdipour, W. Meijs, R.N.Á. Mendonça, A.J. Mentzer, N. Mirtorabi, C. Mitton, S. Mnyakeni, F. Moghaddas, K. Molapo, M. Moloi, M. Moore, M.I. Moraes-Pinto, M. Moran, E. Morey, R. Morgans, S. Morris, S. Morris, H.C. Morris, F. Morselli, G. Morshead, R. Morter, L. Mottal, A. Moultrie, N. Moya, M. Mpelembue, S. Msomi, Y. Mugodi, E. Mukhopadhyay, J. Muller, A. Munro, C. Munro, S. Murphy, P. Mweu, C.H. Myasaki, G. Naik, K. Naker, E. Nastouli, A. Nazir, B. Ndlovu, F. Neffa, C. Njenga, H. Noal, A. Noé, G. Novaes, F.L. Nugent, G. Nunes, K. O'Brien, D. O'Connor, M. Odam, S. Oelofse, B. Oguti, V. Olchawski, N.J. Oldfield, M.G. Oliveira, C. Oliveira, A. Oosthuizen, P. O'Reilly, P. Osborne, D.R.J. Owen, L. Owen, D. Owens, N. Owino, M. Pacurar, B.V.B. Paiva, E.M.F. Palhares, S. Palmer, S. Parkinson, H.M.R.T. Parracho, K. Parsons, D. Patel, B. Patel, F. Patel, K. Patel, M. Patrick-Smith, R.O. Payne, Y. Peng, E.J. Penn, A. Pennington, M.P. Peralta Alvarez, J. Perring, N. Perry, R. Perumal, S. Petkar, T. Philip, D.J. Phillips, J. Phillips, M.K. Phohu, L. Pickup, S. Pieterse, J. Piper, D. Pipini, M. Plank, J. Du Plessis, S. Pollard, J. Pooley, A. Pooran, I. Poulton, C. Powers, F.B. Presa, D.A. Price, V. Price, M. Primeira, P.C. Proud, S. Provstgaard-Morys, S. Pueschel, D. Pulido, S. Quaid, R. Rabara, A. Radford, K. Radia, D. Rajapaska, T. Rajeswaran, A.S.F. Ramos, F. Ramos Lopez, T. Rampling, J. Rand, H. Ratcliffe, T. Rawlinson, D. Rea, B. Rees, J. Reiné, M. Resuello-Dauti, E. Reyes Pabon, C.M. Ribiero, M. Ricamara, A. Richter, N. Ritchie, A.J. Ritchie, A.J. Robbins, H. Roberts, R.E. Robinson, H. Robinson, T.T. Rocchetti, B.P. Rocha, S. Roche, C. Rollier, L. Rose, A.L. Ross Russell, L. Rossouw, S. Royal, I. Rudiansyah, S. Ruiz, S. Saich, C. Sala, J. Sale, A.M. Salman, N. Salvador, S. Salvador, M. Sampaio, A.D. Samson, A. Sanchez-Gonzalez, H. Sanders, K. Sanders, E. Santos, M.F.S. Santos Guerra, I. Satti, J.E. Saunders, C. Saunders, A. Sayed, I. Schim van der Loeff, A.B. Schmid, E. Schofield, G. Screaton, S. Seddiqi, R.R. Segireddy, R. Senger, S. Serrano, R. Shah, I. Shaik, H.E. Sharpe, K. Sharrocks, R. Shaw, A. Shea, A. Shepherd, J.G. Shepherd, F. Shiham, E. Sidhom, S.E. Silk, A.C. da Silva Moraes, G. Silva-Junior, L. Silva-Reyes, A.D. Silveira, M.B.V. Silveira, J. Sinha, D.T. Skelly, D.C. Smith, N. Smith, H.E. Smith, D.J. Smith, C.C. Smith, A. Soares, T. Soares, C. Solórzano, G.L. Sorio, K. Sorley, T. Sosa-Rodriguez, C.M.C.D.L. Souza, B.S.D.F. Souza, A.R. Souza, A.J. Spencer, F. Spina, L. Spoors, L. Stafford, I. Stamford, I. Starinskij, R. Stein, J. Steven, L. Stockdale, L.V. Stockwell, L.H. Strickland, A.C. Stuart, A. Sturdy, N. Sutton, A. Szigeti, A. Tahiri-Alaoui, R. Tanner, C. Taoushanis, A.W. Tarr, K. Taylor, U. Taylor, I.J. Taylor, J. Taylor, R. te Water Naude, Y. Themistocleous, A. Themistocleous, M. Thomas, K. Thomas, T.M. Thomas, A. Thombrayil, F. Thompson, A. Thompson, K. Thompson, A. Thompson, J. Thomson, V. Thornton-Jones, P.J. Tighe, L.A. Tinoco, G. Tiongson, B. Tladinyane, M. Tomasicchio, A. Tomic, S. Tonks, J. Towner, N. Tran, J. Tree, G. Trillana, C. Trinham, R. Trivett, A. Truby, B.L. Tsheko, A. Turabi, R. Turner, C. Turner, M. Ulaszewska, B.R. Underwood, R. Varughese, D. Verbart, M. Verheul, I. Vichos, T. Vieira, C.S. Waddington, L. Walker, E. Wallis, M. Wand, D. Warbick, T. Wardell, G. Warimwe, S.C. Warren, B. Watkins, E. Watson, S. Webb, A. Webb-Bridges, A. Webster, J. Welch, J. Wells, A. West, C. White, R. White, P. Williams, R.L. Williams, R. Winslow, M. Woodyer, A.T. Worth, D. Wright, M. Wroblewska, A. Yao, R. Zimmer, D. Zizi, P. Zuidewind, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, The Lancet 397(10269) (2021) 99–111. doi: https://doi.org/10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed]
- 239.K.J. Ewer, J.R. Barrett, S. Belij-Rammerstorfer, H. Sharpe, R. Makinson, R. Morter, A. Flaxman, D. Wright, D. Bellamy, M. Bittaye, C. Dold, N.M. Provine, J. Aboagye, J. Fowler, S.E. Silk, J. Alderson, P.K. Aley, B. Angus, E. Berrie, S. Bibi, P. Cicconi, E.A. Clutterbuck, I. Chelysheva, P.M. Folegatti, M. Fuskova, C.M. Green, D. Jenkin, S. Kerridge, A. Lawrie, A.M. Minassian, M. Moore, Y. Mujadidi, E. Plested, I. Poulton, M.N. Ramasamy, H. Robinson, R. Song, M.D. Snape, R. Tarrant, M. Voysey, M.E.E. Watson, A.D. Douglas, A.V.S. Hill, S.C. Gilbert, A.J. Pollard, T. Lambe, A. Ali, E. Allen, M. Baker, E. Barnes, N. Borthwick, A. Boyd, C. Brown-O'Sullivan, J. Burgoyne, N. Byard, I.C. Puig, F. Cappuccini, J.-S. Cho, P. Cicconi, E. Clark, W.E.M. Crocker, M.S. Datoo, H. Davies, S.J. Dunachie, N.J. Edwards, S.C. Elias, J. Furze, C. Gilbride, S.A. Harris, S.H.C. Hodgson, M.M. Hou, S. Jackson, K. Jones, R. Kailath, L. King, C.W. Larkworthy, Y. Li, A.M. Lias, A. Linder, S. Lipworth, R.L. Ramon, M. Madhavan, E. Marlow, J.L. Marshall, A.J. Mentzer, H. Morrison, A. Noé, D. Pipini, D. Pulido-Gomez, F.R. Lopez, A.J. Ritchie, I. Rudiansyah, H. Sanders, A. Shea, S. Silk, A.J. Spencer, R. Tanner, Y. Themistocleous, M. Thomas, N. Tran, A. Truby, C. Turner, N. Turner, M. Ulaszewska, A.T. Worth, L. Kingham-Page, M.P.P. Alvarez, R. Anslow, L. Bates, K. Beadon, R. Beckley, A. Beveridge, E.M. Bijker, L. Blackwell, J. Burbage, S. Camara, M. Carr, R. Colin-Jones, R. Cooper, C.J. Cunningham, T. Demissie, C.D. Maso, N. Douglas, R. Drake-Brockman, R.E. Drury, K.R.W. Emary, S. Felle, S. Feng, K.J. Ford, E. Francis, L. Gracie, J. Hamlyn, B. Hanumunthadu, D. Harrison, T.C. Hart, S. Hawkins, J. Hill, E. Howe, N. Howell, E. Jones, J. Keen, S. Kelly, D. Kerr, L. Khan, J. Kinch, S. Koleva, E.A. Lees, A. Lelliott, X. Liu, S. Marinou, J. McEwan, E. Morey, G. Morshead, J. Muller, C. Munro, S. Murphy, P. Mweu, E. Nuthall, K. O'Brien, D. O'Connor, P.J. O'Reilly, B. Oguti, P. Osborne, N. Owino, K. Parker, K. Pfafferott, S. Provstgaard-Morys, H. Ratcliffe, T. Rawlinson, S. Rhead, H. Roberts, K. Sanders, L. Silva-Reyes, C.C. Smith, D.J. Smith, A. Szigeti, T.M. Thomas, A. Thompson, S. Tonks, R. Varughes, I. Vichos, L. Walker, C. White, R. White, X.L. Yao, C.P. Conlon, J. Frater, L. Cifuentes, I. Baleanu, E. Bolam, E. Boland, T. Brenner, B.E. Damratoski, C. Datta, O.E. Muhanna, R. Fisher, P. Galian-Rubio, G. Hodges, F. Jackson, S. Liu, L. Loew, R. Morgans, S.J. Morris, V. Olchawski, C. Oliveria, H. Parracho, E.R. Pabon, A. Tahiri-Alaoui, K. Taylor, P. Williams, D. Zizi, E.H. Arbe-Barnes, P. Baker, A. Batten, C. Downing, J. Drake, M.R. English, J.A. Henry, P. Iveson, A. Killen, T.B. King, J.P.J. Larwood, G. Mallett, K. Mansatta, N. Mirtorabi, M. Patrick-Smith, J. Perring, K. Radia, S. Roche, E. Schofield, R.t.W. Naude, J. Towner, N. Baker, K.R. Bewley, E. Brunt, K.R. Buttigieg, S. Charlton, N.S. Coombes, M.J. Elmore, K. Godwin, B. Hallis, D. Knott, L. McInroy, I. Shaik, K. Thomas, J.A. Tree, C.L. Blundell, M. Cao, D. Kelly, D.T. Skelly, A. Themistocleous, T. Dong, S. Field, E. Hamilton, E. Kelly, P. Klenerman, J.C. Knight, Y. Lie, C. Petropoulos, C. Sedik, T. Wrin, G. Meddaugh, Y. Peng, G. Screaton, E. Stafford, C.V.T.G. the Oxford, T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial, Nat. Med. (2020) doi: 10.1038/s41591-020-01194-5. [DOI]
- 240.J.R. Barrett, S. Belij-Rammerstorfer, C. Dold, K.J. Ewer, P.M. Folegatti, C. Gilbride, R. Halkerston, J. Hill, D. Jenkin, L. Stockdale, M.K. Verheul, P.K. Aley, B. Angus, D. Bellamy, E. Berrie, S. Bibi, M. Bittaye, M.W. Carroll, B. Cavell, E.A. Clutterbuck, N. Edwards, A. Flaxman, M. Fuskova, A. Gorringe, B. Hallis, S. Kerridge, A.M. Lawrie, A. Linder, X. Liu, M. Madhavan, R. Makinson, J. Mellors, A. Minassian, M. Moore, Y. Mujadidi, E. Plested, I. Poulton, M.N. Ramasamy, H. Robinson, C.S. Rollier, R. Song, M.D. Snape, R. Tarrant, S. Taylor, K.M. Thomas, M. Voysey, M.E.E. Watson, D. Wright, A.D. Douglas, C.M. Green, A.V.S. Hill, T. Lambe, S. Gilbert, A.J. Pollard, J. Aboagye, J. Alderson, A. Ali, E. Allen, L. Allen, R. Anslow, C.V. Arancibia-Cárcamo, E.H. Arbe-Barnes, M. Baker, P. Baker, N. Baker, I. Baleanu, E. Barnes, L. Bates, A. Batten, K. Beadon, R. Beckley, A. Beveridge, K.R. Bewley, E.M. Bijker, L. Blackwell, C.L. Blundell, E. Bolam, E. Boland, N. Borthwick, A. Boyd, T. Brenner, P. Brown, C. Brown-O'Sullivan, E. Brunt, J. Burbage, K.R. Buttigieg, N. Byard, I. Cabrera Puig, S. Camara, M. Cao, F. Cappuccini, M. Carr, M.W. Carroll, J. Chadwick, I. Chelysheva, J.-S. Cho, L. Cifuentes, E. Clark, R. Colin-Jones, C.P. Conlon, N.S. Coombes, R. Cooper, W.E.M. Crocker, C.J. Cunningham, B.E. Damratoski, M.S. Datoo, C. Datta, H. Davies, T. Demissie, C. Di Maso, D. DiTirro, T. Dong, F.R. Donnellan, N. Douglas, C. Downing, J. Drake, R. Drake-Brockman, R.E. Drury, S.J. Dunachie, O.E. Muhanna, S.C. Elias, M.J. Elmore, K.R.W. Emary, M.R. English, S. Felle, S. Feng, C.F. Da Silva, S. Field, R. Fisher, K.J. Ford, J. Fowler, E. Francis, J. Frater, J. Furze, P. Galian-Rubio, H. Garlant, K. Godwin, G. Gorini, L. Gracie, G. Gupta, E. Hamilton, J. Hamlyn, B. Hanumunthadu, S.A. Harris, D. Harrison, T.C. Hart, S. Hawkins, J.A. Henry, G. Hodges, S.H.C. Hodgson, M.M. Hou, E. Howe, N. Howell, B. Huang, H. Humphries, P. Iveson, S. Jackson, F. Jackson, S. Jauregui, K. Jeffery, E. Jones, K. Jones, R. Kailath, J. Keen, S. Kelly, D. Kelly, E. Kelly, D. Kerr, L. Khan, B. Khozoee, A. Killen, J. Kinch, T.B. King, L. King, L. Kingham-Page, P. Klenerman, J.C. Knight, D. Knott, S. Koleva, C.W. Larkworthy, J.P.J. Larwood, E.A. Lees, A. Lelliott, S. Leung, Y. Li, A.M. Lias, S. Lipworth, S. Liu, L. Loew, R. Lopez Ramon, G. Mallett, K. Mansatta, N.G. Marchevsky, S. Marinou, E. Marlow, J.L. Marshall, P. Matthews, J. McEwan, J. McGlashan, L. McInroy, G. Meddaugh, A.J. Mentzer, N. Mirtorabi, E. Morey, R. Morgans, S.J. Morris, H. Morrison, G. Morshead, R. Morter, N. Moya, E. Mukhopadhyay, J. Muller, C. Munro, S. Murphy, P. Mweu, A. Noé, F.L. Nugent, E. Nuthall, K. O'Brien, D. O'Connor, D. O'Donnell, B. Oguti, V. Olchawski, C. Oliveria, P.J. O'Reilly, P. Osborne, N. Owino, K. Parker, H. Parracho, M. Patrick-Smith, Y. Peng, E. Penn, M.P. Peralta Alvarez, J. Perring, C. Petropoulos, K. Pfafferott, D. Pipini, D. Phillips, P. Proud, S. Provstgaard-Morys, D. Pulido, K. Radia, D. Rajapaksa, F. Ramos Lopez, H. Ratcliffe, T. Rawlinson, E.R. Pabon, S. Rhead, A.J. Ritchie, H. Roberts, S. Roche, I. Rudiansyah, S. Salvador, H. Sanders, K. Sanders, I. Satti, A. Schmid, E. Schofield, G. Screaton, C. Sedik, I. Shaik, H.R. Sharpe, A. Shea, S. Silk, L. Silva-Reyes, D.T. Skelly, C.C. Smith, D.J. Smith, A.J. Spencer, E. Stafford, A. Szigeti, A. Tahiri-Alaoui, R. Tanner, I.J. Taylor, K. Taylor, R. te Water Naude, Y. Themistocleous, A. Themistocleous, M. Thomas, T.M. Thomas, A. Thompson, L. Tinh, A. Tomic, S. Tonks, J. Towner, N. Tran, J.A. Tree, A. Truby, C. Turner, N. Turner, M. Ulaszewska, R. Varughese, I. Vichos, L. Walker, M. Wand, C. White, R. White, P. Williams, A.T. Worth, C.V.T.G. the Oxford, Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses, Nat. Med. (2020) doi: 10.1038/s41591-020-01179-4. [DOI]
- 241.Pharmaphorum, COVID-19 vaccine cleared in UK, dosing to start next week. https://pharmaphorum.com/news/az-covid-19-vaccine-cleared-in-uk-dosing-to-start-next-week/. (Accessed 8 January 2021).
- 242.Covaxin - India's first indigenous COVID-19 vaccine. https://www.bharatbiotech.com/covaxin.html. (Accessed 8 January 2021).