Abstract
Ferroptosis is a form of iron-dependent regulated cell death. Evidence of its existence and the effects of its inhibitors on subarachnoid hemorrhage (SAH) is still lacking. In the present study, we found that liproxstatin-1 protected HT22 cells against hemin-induced injury by protecting mitochondrial functions and ameliorating lipid peroxidation. In in vivo experiments, we demonstrated the presence of characteristic shrunken mitochondria in ipsilateral cortical neurons after SAH. Moreover, liproxstatin-1 attenuated the neurological deficits and brain edema, reduced neuronal cell death, and restored the redox equilibrium after SAH. The inhibition of ferroptosis by liproxstatin-1 was associated with the preservation of glutathione peroxidase 4 and the downregulation of acyl-CoA synthetase long-chain family member 4 as well as cyclooxygenase 2. In addition, liproxstatin-1 decreased the activation of microglia and the release of IL-6, IL-1β, and TNF-α. These data enhance our understanding of cell death after SAH and shed light on future preclinical studies.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00620-5) contains supplementary material, which is available to authorized users.
Keywords: Subarachnoid hemorrhage, Ferroptosis, Inflammation, Liproxstatin-1
Introduction
Subarachnoid hemorrhage (SAH) is a devastating type of stroke with high mortality and disability rates [1, 2]. The sharply increased intracranial pressure after hemorrhage causes global ischemia and hypoxia, which eventually leads to brain tissue damage and neurological dysfunction [3–5]. Apart from the elevated intracranial pressure, neurotoxins released from the hematoma also contribute to cell death. Released from hemoglobin/hemin, iron has been demonstrated to be an important toxin that threatens neuronal viability [6, 7]. Iron overload triggers the generation of reactive oxygen species (ROS) through the Fenton reaction and attacks the inner membrane of mitochondria, subsequently inducing energy deficiency and cellular dysfunction [8, 9]. Ferroptosis, a recently identified iron-dependent non-apoptotic form of cell death, is morphologically characterized by shrunken mitochondria with condensed membrane density, a reduction of mitochondrial cristae, and outer membrane rupture [10]. Ferroptosis has been reported in kidney failure [11], cardiomyopathy [12], liver cancer [13], intracerebral hemorrhage (ICH) [14], and neurodegeneration [15]. However, whether ferroptosis occurs in SAH remains to be elucidated.
Previously, Dixon and colleagues [10] demonstrated that iron-dependent lipid peroxidation plays a pivotal role in erastin-induced ferroptosis in HT-1080 fibrosarcoma cells and lipid ROS have been posited to be the executioner of cell death. Inactivation of the lipid repair enzyme glutathione (GSH) peroxidase 4 (Gpx4) or system x-c light chain (xCT) by RSL3 and erastin triggers ferroptosis. Moreover, acyl-CoA synthetase long-chain family member 4 (ACSL4) has been reported to facilitate the esterification of arachidonic acid (AA) into phosphatidylethanolamine, and the enhanced expression of ACSL4 sensitizes AU565 cells to RSL3-induced ferroptosis [16]. Interestingly, cell death was rescued by the selective ferroptosis inhibitor liproxstatin-1 (Lip-1) but not the RIP kinase inhibitor necrostatin-1 or the caspase inhibitor Z-VAD-fmk in the above study. Lip-1 has been reported to mitigate intestinal ischemia/reperfusion (I/R) injury [17] and ischemic stroke [18], yet whether it has neuroprotective effects after SAH remains unclear.
Although the essence of ferroptosis is lipid peroxidation, it is also related to inflammatory responses in conditions such as ICH [19], non-alcoholic steatohepatitis [20], and intestinal I/R injury [17]. In fact, ferroptosis occurs independently of necrosome components, yet it manifests as a necrotic morphotype and is potentially associated with the consistent release of pro-inflammatory factors. Notably, PTGS2, a gene that is heavily involved in inflammatory processes, is upregulated in Gpx4-deficient mice and is considered to be a crucial factor in ferroptosis [21]. Furthermore, cyclooxygenase 2 (Cox 2), which is encoded by PTGS2, has been identified as a marker of ferroptosis that is induced by ICH and suppressed by ferrostatin-1 (Fer-1) [14]. The goal of this study was to explore whether ferroptosis occurs after SAH and whether the inhibition of ferroptosis has neuroprotective effects. In addition, the effect of Lip-1 on neuroinflammation was examined to enhance our understanding of ferroptosis and provide new therapeutic targets for preclinical SAH studies.
Materials and Methods
Cell Culture
HT22 cells were cultured at 37 °C with 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, Sigma-Aldrich, St. Louis, USA) with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin. To simulate the pathological process of SAH, cells were exposed to different concentrations of hemin (100–400 μmol/L, Sigma-Aldrich) for 24 h. Co-treatment with 200 nmol/L Lip-1 (Selleck, China) was used to evaluate the protective effects of Lip-1 against hemin exposure.
Cell Viability
Cell counting kit-8 (CCK-8, Dojindo, Tokyo, Japan) was used to evaluate cell viability according to the instructions. Briefly, 6000 cells per well were plated in 96-well plates and allowed to grow overnight. After the cells were exposed to treatment for 24 h, 100 μL of medium containing 10 μL of CCK-8 solution was applied. Then, the plates were incubated at 37 °C for 1 h, after which the optical density at 450 nm was measured.
Mitochondrial Functions
The level of ATP in HT22 cells was determined by the ATP Bioluminescence Assay Kit (Beyotime, Shanghai, China). Briefly, cells were lysed with lysis buffer, and the harvested cells were centrifuged at 12,000 × g for 5 min at 4 °C. After addition of 20 μL of supernatant or standards to 100 μL of luciferase reagent in a light-proof 96-well plate, the concentration of ATP was measured by a microplate luminometer.
Dissipation of the mitochondrial membrane potential (Δψm) is a biochemical feature of ferroptosis [22]. A JC-1 kit (Beyotime, Shanghai, China) was used to analyze Δψm changes in HT22 cells after hemin challenge. Cells were rinsed with PBS and subsequently incubated with JC-1 solution at 37 °C for 20 min. After washing with JC-1 staining buffer, the cells were imaged under a fluorescence microscope (Olympus, Tokyo, Japan) using the same parameters. Images were analyzed using NIH ImageJ software, and the average red/green fluorescence intensity ratio was calculated.
We evaluated mitochondrial ferrous ion (Fe2+) using Mito-FerroGreen (Dojindo, Tokyo, Japan). Briefly, cells were washed three times with serum-free DMEM after hemin exposure and subsequently incubated with 5 μmol/L Mito-FerroGreen working solution at 37 °C for 30 min in a 5% CO2 incubator. Images were captured on a confocal laser scanning microscope (TCS SP5II, Leica, Wetzlar, Germany) through a 63 × 1.4 NA oil immersion objective. Average green fluorescence intensity was determined using ImageJ.
Lipid Peroxidation
Lipid peroxidation was assessed using an Image-iTTM Lipid Peroxidation Kit (Invitrogen, Karlsruhe, Germany). Cells were plated in 6-well plates and incubated with 10 μmol/L BODIPY 581/591 C11 reagent for 30 min at 37 °C. Upon oxidation, the fluorescence emission peak of the reagent shifted from ~ 590 nm (red) to ~ 510 nm (green). Images were captured under a fluorescence microscope with the same parameters. ImageJ was used to determine the average red/green fluorescence intensity ratio.
Animals
Male C57BL/6 mice (22–25 g) were obtained from SLAC Laboratory Animal Co., Ltd (Shanghai, China). A total of 324 male mice were used in this study. Mice were randomly distributed into sham, sham + Lip-1, SAH + vehicle, and SAH + Lip-1 groups. Mice were housed in a temperature- and humidity- controlled environment under a standard 12-h dark/light cycle and had free access to food and water. The animal protocol was approved by the Institutional Ethics Committee of the Second Affiliated Hospital, Zhejiang University School of Medicine. All procedures were conducted according to the ARRIVE guidelines.
In Vivo SAH Model
An endovascular perforation model of SAH was established as previously reported with minor revision [23]. In short, mice were anesthetized with 40 mg/kg 1% pentobarbital sodium via intraperitoneal injection. Under a microscope, a 5–0 monofilament was inserted via the left external carotid artery into the internal carotid artery and perforated the bifurcation of the anterior and middle cerebral arteries. Sham-operated mice underwent the same procedures without perforation.
Drug Administration
The selective ferroptosis inhibitor Lip-1 was dissolved in dimethyl sulfoxide (DMSO) and diluted with 0.9% normal saline to obtain a final concentration of 10 mg/kg. Lip-1 or vehicle (200 μL of normal saline containing 5% DMSO) was administered via intraperitoneal injection. For the tests at 1 day after SAH, a single dose of Lip-1 was administered 4 h after SAH. For the tests at 3 days after SAH, Lip-1 was administered daily beginning 4 h after SAH for a total of three doses. The dosage and timing of Lip-1 injection was based on a previous study [14].
SAH Grade, Weight Loss, and Neurological Evaluation
Three consecutive doses of 10 mg/kg per day Lip-1 were administered to the mice after SAH, and SAH grade, weight loss, and neurological deficits were evaluated. The severity of SAH was blindly evaluated using the previously-reported SAH grading scale after transcardiac perfusion. Body weight, a sensitive and objective indicator of the overall status of injured animals, was recorded for three consecutive days, and the average weight loss was calculated.
Neurological impairments were examined daily with the Garcia scale and Rotarod test [24, 25]. Briefly, the Garcia scale consists of six tests that evaluate motor and sensory functions, including spontaneous activity, symmetry of movement of the four limbs, forepaw outstretching, climbing, body proprioception, and response to vibrissae touch. Physical strength, balance, and coordination were assessed by the rotarod test using the Rotamex-5 apparatus (Columbus Instruments, Columbus, OH). The mice were pre-trained for 3 consecutive days, and baseline performance was evaluated 1 day before surgery. Acclimatization consisted of 3 trials in which the speed was increased from 4–40 r/min over 5 min. The mean duration on the device was recorded.
Fluoro-Jade C (FJC) Staining
An FJC staining kit (Biosensis, USA) was used to identify degenerating neurons as previously reported [26]. Sections were dried at 50–60 °C for 30 min and then immersed in 1% sodium hydroxide for 5 min. After rinsing with 70% ethanol and distilled water for 2 min, the sections were transferred to 0.06% potassium permanganate for 10 min with gentle shaking. Afterwards, the sections were incubated in 0.0002% FJC for 10 min in the dark. Images were captured under a fluorescence microscope (Olympus, Tokyo, Japan), and degenerating neurons were counted in six fields (200 ×) per section.
Brain Water Content
The wet-dry method was used to evaluate brain water content at 1 and 3 days post-surgery. Brains were collected immediately after euthanasia and separated into the right hemisphere, left hemisphere, cerebellum, and brain stem. Each part of the brain was weighed after removal (wet weight) and after drying at 105 °C for three days. The percentage of brain water was calculated as (wet weight – dry weight)/wet weight.
GSH, GPx Activity, and MDA Measurement
Coronal sections (4 mm) centered around the perforation point were obtained using a mouse brain slicer matrix (Zivic Instruments) as previously described [27], and the ipsilateral cortex was separated to measure the levels of GSH and MDA and the activity of GPx at 1 or 3 days after SAH. The assay kits were from Beyotime (Shanghai, China). The protein concentrations in the samples were determined using a BCA protein assay kit (Bio‐Rad Laboratories, Hercules, CA, USA). To ensure the comparability of results from different groups, the GSH and MDA levels and GPx activity were normalized to protein content.
Elisa
Mouse IL-6 and TNF-α ELISA kits (Boster Biological Technology, Wuhan, China) were used to explore the effect of Lip-1 on changes in inflammatory cytokines according to the manufacturer’s instructions. ACSL4 was identified as a biomarker of ferroptosis, and 5-hydroxyicosatetraenoic acid (5-HETE) is an important AA oxidation product mediated by ACSL4 [28]. We assessed the activity of ACSL4 using a 5-HETE ELISA kit (Uscnlife, Hubei, China) according to the manufacturer’s instructions. In addition, the content of 4-hydroxynonenal (4-HNE), a marker of lipid peroxidation, was determined using a competitive ELISA kit (Cell Biolabs, San Diego, CA, USA).
Western blot Analysis
Mice were sacrificed at 1 or 3 days after SAH or the sham procedure. Coronal sections (4 mm) centered around the perforation point were collected, and the ipsilateral cortex was carefully separated. The protein concentration was determined by a BCA protein assay kit (Thermo Fisher Scientific, WA, USA). Equal amounts of protein (40 μg/lane) were separated on a 10% sodium dodecyl sulfate polyacrylamide gel and transferred to polyvinylidene fluoride membranes (Millipore, Burlington, MA, USA). The membranes were blocked in 5% nonfat milk for 1 h at room temperature and probed with primary antibodies against Gpx4 (1:1000, ab125066, Abcam), xCT (1:500, ab37185, Abcam), ACSL4 (1:1000, ab155282, Abcam), Cox 2 (1:1000, #12282, Cell Signaling Technology), MMP-9 (1:1000, ab58803, Abcam), ZO-1 (1:500, 14-9776-80, Invitrogen), albumin (1:500, ab106582, Abcam), IL-1β (1:500, ab9722, Abcam), and β-actin (1:1000, ab8227, Abcam) at 4 °C overnight. The membranes were washed with TBST and subsequently incubated with anti-rabbit or anti-chicken secondary antibodies for 1 h. The intensity of the protein signal was quantified by ImageJ and normalized to the mean value of the sham group.
Immunofluorescence
Brains were fixed and dehydrated at 3 days after SAH, and consecutive coronal frozen sections (8 μm thick) were cut at 100-μm intervals and placed on slides. The bifurcation of the anterior and middle cerebral arteries was used for positioning. Sections approximately –2 mm to +2 mm from the bifurcation were collected for staining. The brain tissue was blocked and permeabilized with 10% BSA and 0.3% Triton X-100 for 1 h. Then, the slides were incubated at 4 °C overnight with anti-ACSL4 (1:200, ab155282, Abcam), anti-NeuN (1:500, ab104224, Abcam), anti-CD68 (1:100, ab31630, Abcam), and anti-Iba-1 (1:1000, 019-19741, Wako) antibodies. After washing with 0.01 mol/L PBS, the sections were incubated with secondary antibodies for 1 h at 37 °C in the dark. Three sections around the bifurcation were used for each mouse and three random fields (× 40) in the basal cortex were acquired under a fluorescence microscope (Olympus, Tokyo, Japan), and the images were analyzed using ImageJ.
Quantitative Real-Time PCR
Cortical samples were collected in the same way as those used for Western blot. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA) and reverse transcription was performed using 5 × PrimeScript RT Master Mix (TakaraBio, Shiga, Japan). Amplification was conducted on an Applied Biosystems Quant StudioTM 5 system (Thermo Fisher Scientific, WA, USA) using TB Green Premix Ex Taq (TakaraBio, Shiga, Japan). The 2-△△Ct method was applied to determine the relative mRNA expression levels. The data are expressed as the fold change relative to sham. The primers are listed in Table S1.
Transmission Electron Microscopy (TEM)
Mice were sacrificed after three days of recovery and perfused with normal saline and 4% paraformaldehyde. Tissues were processed as previously described [29]. Briefly, ~ 1-mm3 blocks of cortex were immersed in 2.5% glutaraldehyde at 4 °C overnight and postfixed in 1% osmium tetroxide for 1 h. Then, the samples were stained in 2% uranyl acetate and dehydrated in graded ethanols. After embedding in 100% acetone overnight, the samples were cut into 100-nm sections and stained with 2% uranyl acetate and lead citrate. Images were captured with a Philips Tecnai 10 TEM at Zhejiang University.
Statistical Analysis
The data are presented as the mean ± SD. Statistical analysis of SAH grade was performed using Student’s t test. Statistical comparisons between groups were performed using one-way ANOVA followed by Bonferroni’s multiple comparisons test. P < 0.05 was considered statistically significant. Calculations were conducted using GraphPad Prism (version 5; GraphPad Software, San Diego, CA, USA) and SPSS (version 22.0; IBM SPSS Statistics, Armonk, NY, USA).
Results
Lip-1 Attenuates Hemin-induced Neuronal Injury In Vitro Through Inhibiting Ferroptosis
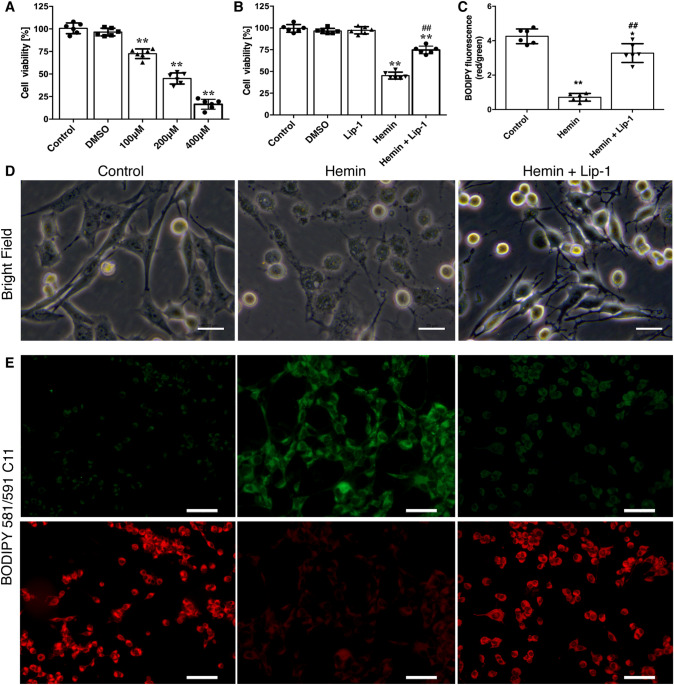
To determine whether hemin induces neuronal death and whether the selective ferroptosis inhibitor Lip-1 protects cells against hemin-induced injury, we exposed HT22 cells to different concentrations of hemin (100–400 μmol/L) for 24 h and assessed cell viability by the CCK-8 assay. The results showed that hemin induced cell death in a concentration-dependent manner. Hemin significantly decreased cell viability in all groups (100 μmol/L, 72.51 ± 5.32%; 200 μmol/L, 44.5 ± 5.01%; 400 μmol/L, 14.17 ± 3.61%; all P < 0.01 vs control, Fig. 1A). Thereafter, 200 μmol/L hemin was used to evaluate the protective effects of Lip-1 in vitro. Co-treatment with 200 nmol/L Lip-1 significantly increased cell viability at the end point (Fig. 1B; morphological changes are shown in Fig. 1D).
Fig. 1.
Lip-1 increases cell viability and inhibits lipid peroxidation at 24 h after hemin exposure. A Hemin decreases cell viability in a concentration-dependent manner (n = 6). B CCK-8 results depicting a significant decrease in cell viability after exposure to hemin (200 μmol/L); this was rescued by Lip-1 (200 nmol/L; n = 6). C Lip-1 inhibits hemin-induced lipid peroxidation, as indicated by the increased red/green fluorescence ratio of BODIPY 581/591 C11 (n = 6). D Morphological changes of HT22 cells exposed to hemin with/without Lip-1 (scale bars, 20 μm). E Representative images of BODIPY 581/591 C11 stained cells (scale bars, 50 μm). Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 vs control group; ## P < 0.01 vs hemin-treated group.
Lipid peroxidation and mitochondrial impairment are landmark changes in cells undergoing ferroptosis. Upon oxidation, the fluorescence of BODIPY 581/591 C11 is converted from red to green. Hemin triggered the production of large amounts of lipid ROS, and Lip-1 treatment significantly increased the red/green fluorescence ratio compared with vehicle (Fig. 1C, E), indicating that the disturbance in redox homeostasis was mitigated.
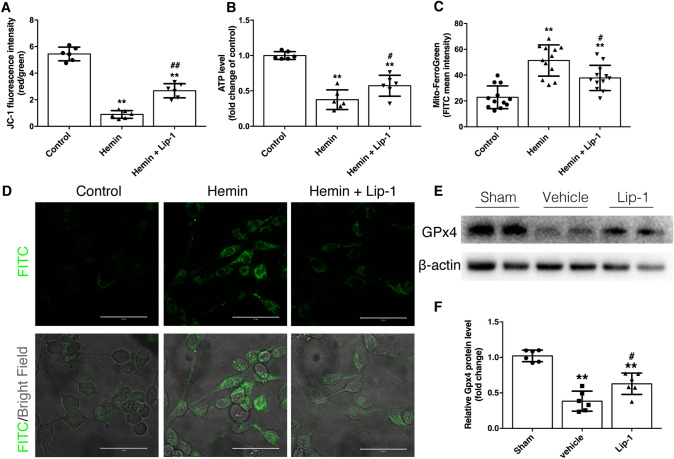
We found that the Δψm and ATP level were remarkably decreased by exposure to 200 μmol/L hemin, and were partially rescued by Lip-1 treatment (Fig. 2A, B). Accumulation of Fe2+ in the mitochondria disturbs the Fenton reaction, thereby not only stimulating ROS generation but also causing cellular dysfunction and cell death. Hemin exposure remarkably increased the level of Fe2+ in the mitochondria in both the hemin- and hemin + Lip-1-treated groups, and compared with vehicle, Lip-1 massively reduced the accumulation of mitochondrial Fe2+ (Fig. 2C, D).
Fig. 2.
Lip-1 ameliorates hemin-induced mitochondrial damage and downregulation of Gpx4. A, B JC-1 staining and ATP measurement showing that Lip-1 improves mitochondrial function upon hemin challenge (n = 6). C, D Mito-FerroGreen staining indicates that Lip-1 treatment decreases the hemin-induced accumulation of Fe2+ in mitochondria (scale bars, 50 μm; n = 12). E, F Lip-1 prevents the decrease of Gpx4, a sensitive marker of ferroptosis, after hemin exposure (n = 6). Data are expressed as the mean ± SD (**P < 0.01 vs control group; #P < 0.05, ##P < 0.01 vs hemin-treated group).
Recently, ferroptosis has been categorized as a type of regulated necrosis [30]. Flow cytometry revealed that only the number of Annexin V-/PI+ cells was significantly reduced by Lip-1 treatment upon hemin challenge (Fig. S1A and B). Considering that Lip-1 prevented the decrease of Gpx4 (Fig. 2E, F) but not the upregulation of cleaved caspase-3 or mixed lineage kinase domain-like protein (Fig. S1C–E), these data collectively suggested that Lip-1 attenuates neuronal injury by inhibiting ferroptosis but not apoptosis or necroptosis.
Administration of Lip-1 Reduces Weight Loss, Neurological Deficits, and Neuronal Degeneration after SAH In Vivo
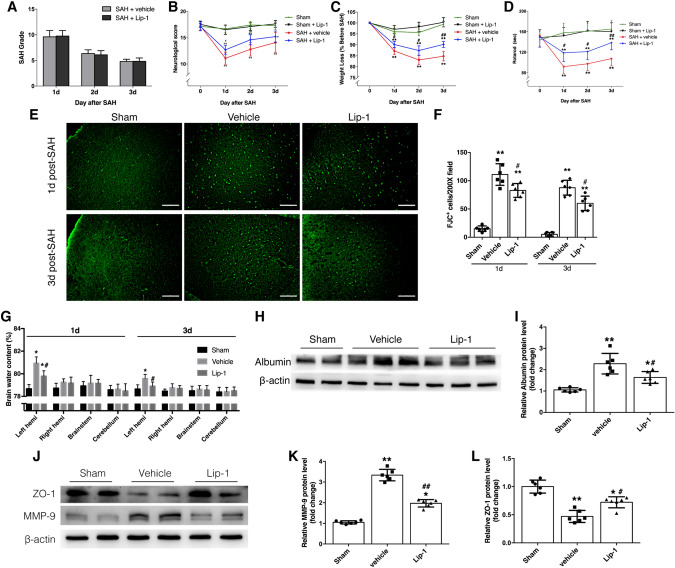
Subarachnoid hematoma was evident after SAH, and blood clots were located mainly in the basal cistern and on the surface of the brainstem. The clots resolved, and the SAH grade decreased gradually over time. No significant difference was found between the vehicle- and Lip-1-treated groups up to 3 days after SAH (Fig. 3A).
Fig. 3.
Effects of Lip-1 on body weight, neurological functions, and brain edema after SAH in mice. A Evaluation of SAH grade reveals no significant difference between the vehicle- and Lip-1-treated groups (n = 12). B Lip-1 increases the Garcia score at 1 and 2 days after SAH (n = 12). C, D Weight loss is reduced and latency on the rotarod is increased by Lip-1 administration at all three time points (n = 12). E, F FJC staining and quantification of degenerating neurons (scale bars, 100 μm; n = 6). G –L Lip-1 treatment protects BBB integrity after SAH by upregulating ZO-1 expression and downregulating MMP-9 expression (n = 6). Data are expressed as the mean ± SD (*P < 0.05, **P < 0.01 vs sham group; #P < 0.05, ##P < 0.01 vs vehicle-treated group).
An 18-point scale was used to evaluate the spontaneous activity, motor, and sensory functions of the mice following SAH. Compared with vehicle, Lip-1 treatment significantly improved neurological function on the first 2 days after SAH, but did not affect the performance 3 days after SAH (Fig. 3B).
To further verify the protective effects of Lip-1 against SAH, we consecutively recorded body weight changes and the latency to fall off the rotarod. Body weight fluctuated over time after SAH, but the sham group did not significantly differ from baseline. Therefore, the mean weight of the sham group was used as the standard for analyzing weight loss after SAH. The most severe weight loss occurred at 2 days post-surgery, and the most remarkable discrepancy between the Lip-1-treated and vehicle-treated groups occurred at 3 days after SAH (Fig. 3C). Similar baseline performance on the rotarod was recorded among the three groups after 3 days of acclimatization. Compared with sham, SAH caused severe impairments in balance and coordination up to 3 days after injury, regardless of treatment. However, compared with vehicle, Lip-1 administration significantly increased the time spent on the rotarod, the most prominent improvement occurring at 3 days after SAH (Fig. 3D). FJC staining showed that the number of degenerating neurons in the cortex peaked at 1 day after SAH, and the number of FJC-positive cells was remarkably reduced by Lip-1 at both 1 and 3 days after SAH (Fig. 3E, F).
Taken together, these data indicated that Lip-1 exerts protective effects by alleviating early brain injury. Although both one dose and three doses were efficacious, mice may benefit more from the longer therapeutic strategy.
Lip-1 Attenuates Brain Edema Via Protecting the Integrity of the Blood-Brain Barrier (BBB)
Other than the hematoma caused by vascular rupture, brain edema is another major cause of elevated intracranial pressure and cerebral hypoxia and ischemia. Mice exhibited remarkably increased brain water content after SAH, yet Lip-1 had a protective effect against brain edema compared with that of vehicle (Fig. 3G). More importantly, brain water content returned to normal after daily administration of Lip-1 for 3 days. As evidenced by assessment of the albumin content in the cortex, we found that BBB integrity was damaged up to 3 days after SAH, and compared with vehicle, Lip-1 significantly decreased the leakage of albumin from vessels (Fig. 3H, I).
Next, we explored the mechanism underlying the effect of Lip-1 on BBB protection. The expression of MMP-9 was significantly upregulated, while the expression of ZO-1 was markedly suppressed at 3 days after SAH (Fig. 3J–L). Lip-1 treatment partially reversed these changes, indicating that Lip-1 protects BBB integrity by reducing extracellular matrix degradation and stabilizing tight junctions.
Lip-1 Suppresses Lipid Peroxidation by Regulating the Expression of Gpx4, But Not xCT
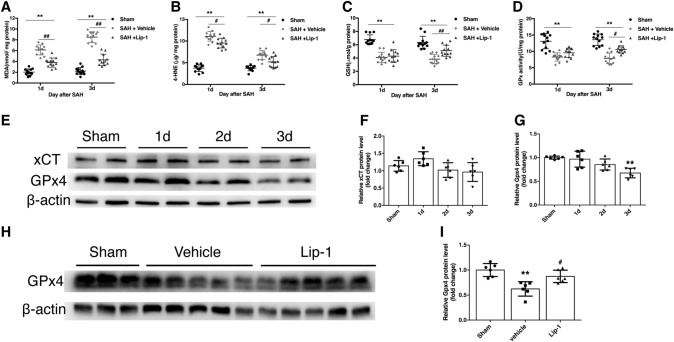
Since lipid peroxidation is a hallmark of cells undergoing ferroptosis, we estimated its level and the content of antioxidants after SAH. Subarachnoid hematoma triggered the production of large amounts of MDA and 4-HNE in the ipsilateral cortex, while Lip-1 treatment, compared with vehicle, markedly decreased their levels at both 1 and 3 days after injury (Fig. 4A, B). The content of GSH and the activity of GPx were significantly reduced after SAH at both time points. Interestingly, both a single dose and three consecutive doses of Lip-1 had a protective effect by restoring antioxidative ability, but significant improvement was achieved only after the daily administration of Lip-1 for three days (Fig. 4C, D).
Fig. 4.
Lip-1 suppresses SAH-induced lipid peroxidation and the decrease of Gpx4 expression. A, B MDA and 4-HNE content at 1 and 3 days after SAH confirming that Lip-1 strongly inhibits lipid peroxidation (n = 12). C, D GSH and GPx activity assays revealing the protective effects of Lip-1 in restoring antioxidant capability at 3 days after SAH (n = 12). E–G Western blots and analysis showing non-significant changes in xCT expression after SAH and time-dependent decreases in the expression of Gpx4 (n = 6). H, I Western blots and analysis revealing relatively higher expression of Gpx4 at 3 days post-SAH in the Lip-1-treated group (n = 6). Data are expressed as the mean ± SD (*P < 0.05, ** P < 0.01 vs sham group; #P < 0.05, ##P < 0.01 vs vehicle-treated group).
Gpx4 and xCT are not only important enzymes for GSH metabolism but are also thought to be biomarkers of ferroptosis, so we examined their expression following SAH. While the expression of xCT did not change significantly after SAH, the expression of Gpx4 decreased gradually and remained significantly downregulated at 3 days after SAH (Fig. 4E–G). Compared with vehicle, the administration of Lip-1 significantly promoted the expression of Gpx4 at 3 days after SAH (Fig. 4H, I).
Lip-1 Inhibits the Expression of ACSL4 and Reduces the Production of 5-HETE
To further verify the effect of Lip-1 on inhibiting ferroptosis after SAH, we examined the role of Lip-1 in regulating the expression of ACSL4, another important contributor to ferroptosis. ACSL4 was significantly upregulated after SAH and reached a peak at 3 days after SAH (Fig. 5A, B). Compared with that in the vehicle-treated group, the expression of ACSL4 was significantly suppressed in the Lip-1-treated group (Fig. 5C, D). ACSL4-mediated AA metabolism is closely associated with 5-HETE, which is involved in the induction of ferroptosis. Similarly, the production of 5-HETE was dramatically decreased by the administration of Lip-1 (Fig. 5E). However, unlike ACSL4, the level of 5-HETE did not return to normal, indicating that other mechanisms of the accumulation of 5-HETE exist. Immunofluorescence staining revealed that the number of cells co-stained for ACSL4 and NeuN was increased after SAH and was significantly reduced by Lip-1 compared with vehicle (Fig. 5F, G).
Fig. 5.
Lip-1 downregulates ACSL4 expression and activity in neurons 3 days after SAH. A, B Time-dependent changes in ACSL4 expression after SAH (n = 6). C, D Western blots and analysis revealing decreased expression of ACSL4 in the Lip-1-treated group at 3 days post-SAH (n = 6). E Lip-1 decreases the level of 5-HETE at 3 days after SAH (n = 6). F, G Representative immunofluorescence images of ACSL4 in neurons and the quantification of ACSL4-positive neurons (scale bars, 50 μm; n = 6). Data are expressed as the mean ± S.D (*P < 0.05, **P < 0.01 vs sham group; #P < 0.05, ##P < 0.01 vs vehicle-treated group).
Expression of Ferroptosis-related Genes and Mitochondrial Changes after SAH
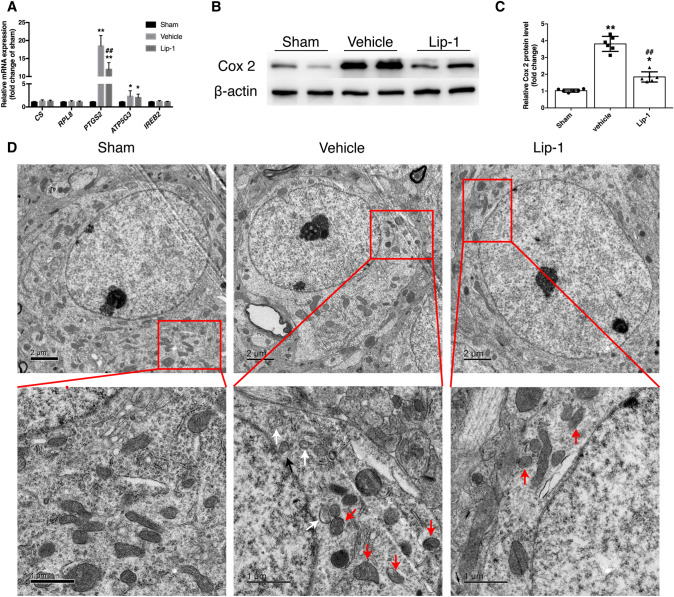
To explore whether specific ferroptosis-related gene sets are changed in the in vivo SAH model, we assessed the mRNA levels of CS, RPL8, PTGS2, ATP5G3, and IREB2 [10]. We found that the mRNA expression of PTGS2 and ATP5G3 was upregulated 3 days after SAH, but only the increase in PTGS2 expression was decreased by Lip-1 treatment (Fig. 6A). Consistent with the mRNA results, the protein level of Cox 2, which is encoded by PTGS2, was significantly decreased by the administration of Lip-1 (Fig. 6B, C).
Fig. 6.
SAH induces upregulation of PTGS2 and Cox 2 as well as mitochondrial damage at 3 days post-SAH. A Ferroptosis-related gene expression assessed by qRT-PCR (n = 6). B, C Lip-1 prevents the SAH-induced upregulation of Cox 2 compared with that in the vehicle-treated group (n = 6). D Representative TEM images of mitochondria in the sham, vehicle-treated, and Lip-1-treated groups (red arrowheads, classical shrunken mitochondria in the soma; white arrowheads, reduced/vanishing cristae; black arrowheads, outer membrane rupture; scale bars, upper panel, 2 μm, lower panel, 1 μm). Data are expressed as the mean ± SD (*P < 0.05, **P < 0.01 vs sham group; ##P < 0.01 vs vehicle-treated group).
Morphological changes in mitochondria are the most representative feature of ferroptosis. We used TEM to verify the existence of ferroptosis at 3 days after SAH. The results showed that shrunken mitochondria with condensed membrane density, reduction of cristae, and outer membrane rupture were more prominent in sections from the vehicle-treated group. In contrast, relatively normal mitochondria with clear cristae were observed at 3 days after SAH in the Lip-1-treated group (Fig. 6D).
Collectively, these findings confirmed the involvement of ferroptosis in the in vivo SAH model and revealed that Lip-1 protects against brain injury after SAH, at least partly by inhibiting ferroptosis.
Lip-1 Reduces the Activation of Microglia and Alleviates Neuroinflammation After SAH
Ferroptosis is a pro-inflammatory process caused by the release of damage-associated molecular pattern molecules [31, 32]. Thus, we investigated whether the protective effects of Lip-1 against SAH are related to reduced neuroinflammation. The microglia in the sham group were highly ramified with a small soma, while the microglia in the SAH group transformed into an amoeboid shape with a larger soma and shorter protrusions. In addition, the number of CD 68/Iba-1-positive microglia increased significantly after SAH, and this was markedly reduced by Lip-1 administration (Fig. 7A, B). The levels of IL-6 and TNF-α and the expression of IL-1β in the ipsilateral cortex were quantified by ELISA and Western blot analysis, respectively. The levels of these inflammatory cytokines induced by SAH were significantly upregulated at 3 days after injury, and Lip-1 strongly restrained the elevation of IL-6, TNF-α, and IL-1β expression (Fig. 7C–F).
Fig. 7.
Lip-1 reduces microglial activation and alleviates neuroinflammation at 3 days after SAH. A, B Representative immunofluorescence images of microglial activation and the quantification of CD68/Iba-1-positive cells in the ipsilateral cortex (scale bars, 50 μm; n = 12). C, D ELISA results showing that, compared with vehicle, Lip-1 reduces the expression of IL-6 and TNF-α (n = 6). E, F Western blots and analysis demonstrating that the increase in the expression of IL-1β induced by SAH is reduced by Lip-1 administration (n = 6). Data are expressed as the mean ± SD (*P < 0.05, **P < 0.01 vs sham group; #P < 0.05 vs vehicle-treated group).
Discussion
Ferroptosis is a recently discovered form of regulated cell death that has been identified in Parkinson’s disease [33], ICH [14], brain ischemia [18], and traumatic brain injury [34]. However, to the best of our knowledge, whether ferroptosis occurs after SAH and whether inhibiting ferroptosis protects neurological functions have not been studied so far. The major findings of our study are: (1) Lip-1 inhibited hemin-induced neuronal injury at least partly through inhibiting ferroptosis; (2) ferroptosis occurred after SAH and neurological deficits were alleviated by Lip-1; (3) Lip-1 preserved the expression of Gpx4 and decreased the expression of ACSL4 and Cox2 after SAH; and (4) Lip-1 attenuated SAH-induced microglial activation and neuroinflammation.
ICH and SAH are the main types of hemorrhagic stroke that cause high mortality and morbidity. Released from the degradation of hemoglobin, excessive Fe2+ impairs mitochondrial function and results in increased production of lipid-associated radicals and peroxides [8]. Zille and colleagues [35] found that incubation of primary neurons with 100 μmol/L hemin leads to a 50% reduction in cell survival, which is alleviated by the canonical ferroptosis inhibitor Fer-1 and the necroptosis inhibitor necrostatin-1, but not inhibitors of autophagy or apoptosis. These findings suggested that neuronal death after hemorrhagic stroke shares features of ferroptosis and necroptosis. In a collagenase-induced mouse ICH model, increased frequency of shrunken mitochondria at both 3 and 6 days after surgery has been reported, while swollen mitochondria are largely present at 28 days post-ICH [36]. This discrepancy indicates that ferroptosis may be an acute or sub-acute process after ICH, and the form of cell death may change along with the lysis and absorption of the hematoma. Furthermore, intracerebral ventricular administration of Fer-1 reduces neuronal degeneration and attenuates the neurological deficits after ICH in mice [14]. Similar to these previous studies, we noted that mice treated with Lip-1 after SAH showed marked brain protection through alleviated mitochondrial stress and reduced lipid peroxidation. Notably, while several studies have claimed that selective ferroptosis inhibitors inhibit the accumulation of iron [14, 19], others argue that these inhibitors are potent radical-trapping antioxidants but not iron chelators [11, 37]. In our study, Lip-1 decreased the level of Fe2+ in the mitochondria in HT22 cells, yet the expression of proteins related to iron storage and transport remained unchanged (Fig. S2). Collectively, these findings indicate that ferroptosis presents a novel target in dealing with hemorrhagic strokes, though the underlying mechanism remains to be further elucidated.
In a recent study, intranasal Lip-1 administration attenuated the functional deficits both immediately after cerebral I/R and 6 h after reperfusion, as evidenced by rotarod performance up to 7 days after injury in mice [18]. It is worth noting that Lip-1 rescued neurological deterioration in a dose-dependent manner in the above study, since the higher dose of 10 mg/kg, but not the 5 mg/kg dose, was protective against cerebral I/R injury. Similarly, we found that, although both a single dose and multiple doses reduced the weight loss, alleviated brain edema, and improved the neurological deficits after SAH, mice tended to benefit more from multiple doses. This can be explained by the ADME-Tox profile conducted in the above study, which suggested that Lip-1 has a short half-life of 4.6 h [11], and daily administration might be beneficial for maintaining an effective blood concentration. Furthermore, the efficacy of Lip-1 after a 2-h delay in administration may pave the way for future clinical applications.
As key regulators of ferroptosis, Gpx4 converts reduced GSH to oxidized GSSG to reduce lipid hydroperoxides [38] and xCT is a glutamate/cysteine antiporter that takes up cysteine for GSH synthesis [39]. The inhibition of Gpx4 and xCT by RSL3 and erastin sensitized HT22 cells to ferroptosis, which was rescued by Lip-1 (Fig. S3) [40, 41]. In the present study, we found that the expression of Gpx4 in the ipsilateral cortex was downregulated after SAH, and this change was accompanied by ferroptotic mitochondrial changes in neurons. Considering that an exclusive biomarker of ferroptosis and the most representative signaling pathway have yet to be discovered, TEM remains the best way to identify ferroptosis [42]. We observed typical shrunken mitochondria with condensed membrane density and a reduction in the number of mitochondrial cristae after SAH. More importantly, shrunken mitochondria with outer membrane rupture, which was previously induced by the genetic or pharmacological inactivation of Gpx4 [11], was also observed in our study, indicating that the level of Gpx4 decreased in neurons in a ferroptosis-related manner. Similar to a study that focused on the effects of Lip-1 on intestinal I/R injury [17], we found that Lip-1 attenuated the decrease in Gpx4 expression and Gpx activity following SAH. Knowing that Gpx4 is a membrane-anchored glycoprotein and that Lip-1 is an excellent radical-trapping antioxidant in the phospholipid bilayer [37], we speculated that Gpx4 is protected due to reduced lipid peroxidation and stabilized membrane phospholipids.
Encoded by PTGS2, Cox 2 has been identified as a mediator of ferroptosis. A previous study confirmed that Cox 2 is expressed in neurons and astrocytes 1 day after ICH but in astrocytes only 3 days after ICH [43]. Consistent with previous studies [14, 17], we found that Lip-1 downregulated the mRNA level of PTGS2 and the expression of Cox 2. ACSL4 enriches cellular membranes with long polyunsaturated ω6 fatty acids, a substrate of inflammatory lipid mediators, and in turn sensitizes cells to ferroptosis [16]. In particular, lipid oxidation upon Gpx4 inhibition also requires ACSL4. However, ACSL4-related ferroptosis has not been investigated in SAH to date. In the present study, we showed that ACSL4 was strongly expressed in neurons after SAH, and Lip-1 inhibited the expression of not only ACSL4 but also 5-HETE, the ACSL4-mediated oxidation of polyunsaturated fatty acids. Unfortunately, we failed to discover a more detailed mechanism underlying the regulation of ACSL4 by Lip-1. Notably, Cox 2 is tightly regulated by ACSL4 since knocking down ACSL4 decreases the expression of Cox 2 in MCF7 cells [28]. These data highlight the interaction between ACSL4 and Cox 2 in regulating ferroptosis, and future studies are needed to develop a more potent and viable therapeutic strategy based on the inhibition of ACSL4.
Neuronal membranes are abundant in phospholipid bilayer, in which AA and phosphatidylethanolamine can be oxidized into lipid mediators that regulate not only ferroptosis but also a diverse set of inflammatory processes [44]. Neuroinflammation has been reported to contribute to the brain damage in models of multiple diseases, such as ICH [45], SAH [46], ischemic stroke [47], and neurodegeneration [48, 49]. It is worth noting that iron also accumulates in microglia and changes their polarization state, resulting in the release of a large amount of pro-inflammatory cytokines. In fact, the relationship between ferroptosis and inflammation in brain injury has been investigated before. Lip-1 has been reported to reduce ferroptosis as well as serum inflammation mediators in intestinal I/R injury [17]. The intraventricular administration of RSL3 and GPx4 small interfering RNAs aggravates neuroinflammation in ICH rats, while genetically promoting the expression of Gpx4 decreases the level of IL-1β and TNF-α in the serum and cerebrospinal fluid [19]. Consistent with these studies, we demonstrated that Lip-1 was able to restrain the activation of microglia and reduce the elevation of IL-6, IL-1β, and TNF-α in SAH in vivo. Interestingly, inflammatory mediators, particularly IL-1β, are considered to be the main activators of MMP-9 [50, 51]. This also explains how Lip-1 protects the brain against edema.
Considering that other forms of cell death such as necroptosis and pyroptosis are also pro-inflammatory, whether ferroptosis works in coordination with them and whether the combined inhibition of different forms of cell death is more effective remains unclear and requires intensive study. Furthermore, it is important to better understand how neurons, astrocytes, and microglia interact during ferroptosis and the mutual effects among them. Besides, mice are prone to smaller hemorrhage and quicker hematoma clearance, as well as relatively mild neurological deficits compared with rats in endovascular perforation models. The scoring criteria of some of the subsets in the Garcia scale are not objective in that some of the variables cannot be quantified precisely. It has been reported that modifying the Garcia scale to include vestibulomotor function tests (e.g., beam walking test, Rotarod) may increase its diagnostic accuracy. It is critical to develop a more sensitive and accurate scoring system to identify functional deficits after SAH in mice.
Conclusions
In conclusion, we demonstrated for the first time that ferroptosis occurs after SAH and demonstrated that Lip-1 administration reduces neuronal death and microglial activation, as well as improving the overall well-being and neurological function of injured mice. Targeting the inhibition of ferroptosis may be a novel therapeutic strategy for ameliorating brain injury after SAH in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2018YFC1312600 and 2018YFC1312603), the National Science Foundation of China (81870910 and 81870908), the Natural Science Foundation of Zhejiang Province, China (LY18H090007), and the Scientific Research Fund of Zhejiang Provincial Education Department, China (Y201941838).
Conflicts of interest
All authors claim that there are no conflicts of interest.
Footnotes
Yang Cao and Yin Li have contributed to this work equally.
References
- 1.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 2.Lawton MT, Vates GE. Subarachnoid hemorrhage. N Engl J Med. 2017;377:257–266. doi: 10.1056/NEJMcp1605827. [DOI] [PubMed] [Google Scholar]
- 3.Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, an evolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–446. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 5.Fang YJ, Mei SH, Lu JN, Chen YK, Chai ZH, Dong X, et al. New risk score of the early period after spontaneous subarachnoid hemorrhage: for the prediction of delayed cerebral ischemia. CNS Neurosci Ther. 2019;25:1173–1181. doi: 10.1111/cns.13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Xi G, Keep RF, Hua Y. Iron enhances the neurotoxicity of amyloid beta. Transl Stroke Res. 2012;3:107–113. doi: 10.1007/s12975-011-0099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Keep RF, He Y, Sagher O, Hua Y, Xi G. Hemoglobin and iron handling in brain after subarachnoid hemorrhage and the effect of deferoxamine on early brain injury. J Cereb Blood Flow Metab. 2010;30:1793–1803. doi: 10.1038/jcbfm.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garton T, Keep RF, Hua Y, Xi G. Brain iron overload following intracranial haemorrhage. Stroke Vasc Neurol. 2016;1:172–184. doi: 10.1136/svn-2016-000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song N, Xie J. Iron, Dopamine, and alpha-synuclein interactions in at-risk dopaminergic neurons in Parkinson’s disease. Neurosci Bull. 2018;34:382–384. doi: 10.1007/s12264-018-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Du L, Qiao Y, Zhang X, Zheng W, Wu Q, et al. Ferroptosis is governed by differential regulation of transcription in liver cancer. Redox Biol. 2019;24:101211. doi: 10.1016/j.redox.2019.101211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2:e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Wu Y, Yuan S, Zhang P, Zhang J, Li H, et al. Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage. Brain Res. 2018;1701:112–125. doi: 10.1016/j.brainres.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Tsurusaki S, Tsuchiya Y, Koumura T, Nakasone M, Sakamoto T, Matsuoka M, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10:449. doi: 10.1038/s41419-019-1678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JR, Xu HZ, Nie S, Peng YC, Fan LF, Wang ZJ, et al. Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subrachnoid hemorrhage in rats. J Neuroinflammation. 2017;14:186. doi: 10.1186/s12974-017-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao S, Shrestha S, Li J, Yu X, Chen J, Yan F, et al. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci Rep. 2017;7:2417. doi: 10.1038/s41598-017-02679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J, Sun Z, Fang Y, Zheng J, Xu S, Xu W, et al. Melatonin suppresses microglial necroptosis by regulating deubiquitinating enzyme A20 after intracerebral hemorrhage. Front Immunol. 2019;10:1360. doi: 10.3389/fimmu.2019.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R, Liu W, Yin J, Chen Y, Guo S, Fan H, et al. TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J Neuroinflammation. 2018;15:231. doi: 10.1186/s12974-018-1279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang CF, Cho S, Wang J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol. 2014;1:258–271. doi: 10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 29.Fan LF, He PY, Peng YC, Du QH, Ma YJ, Jin JX, et al. Mdivi-1 ameliorates early brain injury after subarachnoid hemorrhage via the suppression of inflammation-related blood-brain barrier disruption and endoplasmic reticulum stress-based apoptosis. Free Radic Biol Med. 2017;112:336–349. doi: 10.1016/j.freeradbiomed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 31.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Do B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, et al. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Xie BS, Wang YQ, Lin Y, Mao Q, Feng JF, Gao GY, et al. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci Ther. 2019;25:465–475. doi: 10.1111/cns.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis. Stroke. 2017;48:1033–1043. doi: 10.1161/STROKEAHA.116.015609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Weiland A, Chen X, Lan X, Han X, Durham F, et al. Ultrastructural characteristics of neuronal death and white matter injury in mouse brain tissues after intracerebral hemorrhage: coexistence of ferroptosis, autophagy, and necrosis. Front Neurol. 2018;9:581. doi: 10.3389/fneur.2018.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zilka O, Shah R, Li B, Friedmann Angeli JP, Griesser M, Conrad M, et al. On the mechanism of cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of lipid peroxidation in ferroptotic cell death. ACS Cent Sci. 2017;3:232–243. doi: 10.1021/acscentsci.7b00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507–515. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi S, Sato M, Kasakoshi T, Tsutsui T, Sugimoto M, Osaki M, et al. Cystathionine is a novel substrate of cystine/glutamate transporter: implications for immune function. J Biol Chem. 2015;290:8778–8788. doi: 10.1074/jbc.M114.625053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jelinek A, Heyder L, Daude M, Plessner M, Krippner S, Grosse R, et al. Mitochondrial rescue prevents glutathione peroxidase-dependent ferroptosis. Free Radic Biol Med. 2018;117:45–57. doi: 10.1016/j.freeradbiomed.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 41.Neitemeier S, Jelinek A, Laino V, Hoffmann L, Eisenbach I, Eying R, et al. BID links ferroptosis to mitochondrial cell death pathways. Redox Biol. 2017;12:558–570. doi: 10.1016/j.redox.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu T, Wu H, Wang J, Wang J. Expression and cellular localization of cyclooxygenases and prostaglandin E synthases in the hemorrhagic brain. J Neuroinflammation. 2011;8:22. doi: 10.1186/1742-2094-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 45.Zheng J, Sun Z, Liang F, Xu W, Lu J, Shi L, et al. AdipoRon attenuates neuroinflammation after intracerebral hemorrhage through AdipoR1-AMPK pathway. Neuroscience. 2019;412:116–130. doi: 10.1016/j.neuroscience.2019.05.060. [DOI] [PubMed] [Google Scholar]
- 46.Hu HM, Li B, Wang XD, Guo YS, Hui H, Zhang HP, et al. Fluoxetine is neuroprotective in early brain injury via its anti-inflammatory and anti-apoptotic effects in a rat experimental subarachnoid hemorrhage model. Neurosci Bull. 2018;34:951–962. doi: 10.1007/s12264-018-0232-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, et al. Dual functions of microglia in ischemic stroke. Neurosci Bull. 2019;35:921–933. doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panaro MA, Aloisi A, Nicolardi G, Lofrumento DD, De Nuccio F, La Pesa V, et al. Radio electric asymmetric conveyer technology modulates neuroinflammation in a mouse model of neurodegeneration. Neurosci Bull. 2018;34:270–282. doi: 10.1007/s12264-017-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He L, Chen L, Li L. The TBK1-OPTN axis mediates crosstalk between mitophagy and the innate immune response: a potential therapeutic target for neurodegenerative diseases. Neurosci Bull. 2017;33:354–356. doi: 10.1007/s12264-017-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang J, Chen Y, Kuai L, Yang P, Peng J, Wu Y, et al. Inhibition of blood-brain barrier disruption by an apolipoprotein E-mimetic peptide ameliorates early brain injury in experimental subarachnoid hemorrhage. Transl Stroke Res. 2017;8:257–272. doi: 10.1007/s12975-016-0507-1. [DOI] [PubMed] [Google Scholar]
- 51.Qin W, Lu W, Li H, Yuan X, Li B, Zhang Q, et al. Melatonin inhibits IL1beta-induced MMP9 expression and activity in human umbilical vein endothelial cells by suppressing NF-kappaB activation. J Endocrinol. 2012;214:145–153. doi: 10.1530/JOE-12-0147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.