Dear Editor,
Anesthetic-induced unconsciousness is accompanied by decreased information exchange among relevant cortical functional modular areas that support normal cognitive function [1]. Using the electroencephalogram (EEG) signal, evidence suggest that the feedback and feedforward connectivity among cortical regions may be asymmetrically affected during anesthetic-induced anesthesia [2]. In particular, the feedback connectivity originating from the frontal cortex to other cortical regions, especially the parietal cortex, is suppressed by anesthetic agents with distinct molecular targets [3, 4]. Therefore, the breakdown of frontal-parietal connectivity has been proposed as the final common pathway underlying the anesthetic-induced unconsciousness with possible clinical utilization, such as quantifying the depth of anesthesia [5].
However, results regarding the changes of frontal-parietal connectivity during anesthesia have been inconsistent. The frontal-parietal connectivity in the frequency band 1–12 Hz quantified by the partial directed coherence significantly increased after propofol-induced unconsciousness was achieved [6]. With Granger causality, bidirectional connections between the anterior and posterior cingulate cortex were significantly increased in the frequency ranges 12–25 Hz and 25–40 Hz [7]. Using phase-amplitude coupling (phase providing frequency band: 0.1–1 Hz, amplitude providing frequency band: 14–25 Hz), the connectivity strength among cortical regions between the frontal eye field and lateral intra-parietal area demonstrated a 27-fold increment during propofol-induced unconsciousness in non-human primates [8].
The studies described above investigated the frontal-parietal functional connectivity using different analytical methods with different spatial resolutions and found inconsistent changing patterns in different frequency bands. These results, combined with other results showing decreased connectivity strength, lead to the question of whether the proposed breakdown of frontal-parietal feedback connectivity during anesthesia is a universal phenomenon or a special case related to the analytical methods and the frequency band of interest.
Functional connectivity refers to the statistical dependence between a pair of neural recordings. The mechanism underling the statistical dependence is that the neural populations work in coordination and exhibit synchronized activity [9]. To facilitate efficient information transmission, the incoming signal needs to arrive at a particular phase of the oscillatory activity that corresponds to the maximum excitability of the receiving neural assembly [10]. This synchronization pattern can be quantified by using the phase relationship between a pair of EEG recordings. Furthermore, the synchronization among different neural populations can also be realized through cross-frequency phase amplitude coupling in which the phase of low-frequency activity modulates the amplitude of high-frequency activity. Apart from the connectivity measures that rely on phase relationships, the statistical dependence can also be directly estimated by using information-theoretical measures such as normalized symbolic transfer entropy (NSTE). Yet, no study has investigated the functional connectivity alternation during anesthetic-induced unconsciousness using different analytical methods in the same study population.
In the present study, we investigated the changes of functional connectivity among cortical regions by using different analytical methods: cross-frequency phase-amplitude coupling (PAC), direct phase lag index (dPLI), and NSTE. We first identified a significant decrement in functional connectivity by using cross-frequency PAC, and then we validated the obtained decrement in functional connectivity by using dPLI and NSTE.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. EEG data from 19 male participants were recorded before and during propofol-induced unconsciousness to achieve three distinct states—the resting state, and light and deep levels of unconsciousness—each with a 5-min EEG recording. The light and deep anesthesia stages were defined as the bispectrum (BIS) index within a 5-point interval at 60 and 40. The details of the anesthesia protocol are given in the supplementary material.
After pre-processing, the cortical activity at 68 cortical regions according to the Desikan-Killiany atlas were estimated by using EEG source imaging. Then, we grouped these 68 regions into 7 regions of interest: frontal, prefrontal, central, parietal, occipital, cingulate, and temporal cortices (detailed parcellation in Table S1). With the obtained cortical activity, we estimated the cross-frequency PAC, dPLI, and NSTE for each of the consciousness levels separately. The PAC was quantified by using the modulation index (MI) due to its better tolerance to noise [11]. The details of implementation of EEG signal pre-processing, EEG source imaging, and connectivity estimation methods are provided in the supplementary material.
Both light and deep levels of anesthesia were achieved in all participants. The anesthesia level of each participant was further confirmed by using both the BIS index and spectrum analysis. The group-averaged spectrum is shown in Figure S2. When compared with the baseline, the power increased in the frequency ranges 0.1–4 Hz (P < 0.0001; t = − 12.50) and 8–15 Hz (P < 0.0001; t = − 9.37) during light anesthesia. During deep anesthesia (BIS 40), the low-frequency power at 0.1–4 Hz (P < 0.001; t = − 6.81) and the power in the frequency range 5–10 Hz (P = 0.001; t = − 3.85) further increased. The spectrum changes during the light and deep levels of anesthesia were consistent with the trend of the BIS index, confirming that two distinct levels of anesthesia were achieved.
Given that the pre-defined anesthesia level was achieved, we then estimated the connectivity by using PAC for each of the consciousness levels. The strength of PAC was quantified using the MI, which was estimated by first identifying the ranges of the phase providing frequency band and the amplitude providing frequency band. We estimated MI between the frontal and parietal cortex over a large range of frequency bands. As shown in Figure S3, there was a clear maximum in MI value when choosing the phase providing frequency at 0.1–1 Hz, and the modulated-amplitude in the frequency range 5–15 Hz. Hence, these two frequency bands were selected for further PAC analysis.
The group-level MIs among cortices at baseline, and the light and deep levels of anesthesia are shown in Figure 1A. There were large differences in the MI values across different consciousness states, especially for the prefrontal, frontal, and parietal cortices (details in Tables S2–S4). The statistically significant differences in PAC strength among different depths of anesthesia are showed in Figure 1B–D. We found that the PAC strength from the prefrontal cortex to the central (baseline vs. BIS 60: P = 0.017, t = 2.86; baseline vs. BIS 40: P < 0.001, t = 7.11), parietal (baseline vs. BIS 60: P < 0.001, t = 6.63; baseline vs. BIS 40: P < 0.001, t = 6.35), and temporal cortices (baseline vs. BIS 60: P = 0.031, t = 2.49; baseline vs. BIS 40: P = 0.007, t = 3.81) decreased during propofol-induced anesthesia when compared to baseline, while there were no significant differences between light and deep anesthesia (Fig. 1B). Likewise, there were decreases in PAC strength from the frontal cortex to the central (baseline vs. BIS 60: P = 0.019, t = 2.78; baseline vs. BIS 40: P = 0.001; t = 4.74), parietal (baseline vs. BIS 60: P < 0.001, t = 6.33; baseline vs. BIS 40: P = 0.0101; t = 4.84), and temporal (baseline vs. BIS 60: P = 0.273, t = 1.22; baseline vs. BIS 40: P = 0.024; t = 3.05) cortices during anesthesia, compared to baseline (Fig. 1C).
Fig. 1.
Inter-areal phase-amplitude coupling. A Mean of the modulation index (MI) between the frontal, prefrontal, central, parietal, occipital, cingulate, and temporal cortex at the consciousness levels of baseline, BIS 60, and BIS 40. B–D Box-plots showing phase-amplitude coupling among cortices at different consciousness levels. Y-axis, the value of phase-amplitude coupling quantified by MI; X-axis, the consciousness levels of baseline, BIS 60, and BIS 40. The MI difference between two consciousness levels was compared using a two-tailed paired Student t-test and corrected for multiple comparisons using the false discovery rate (FDR) method (***P < 0.001, **P < 0.01, *P < 0.05; FDR correction).
On the other hand, there were cortical regions showing an increase in PAC strength during propofol-induced anesthesia. As shown in Fig. 1D, with increasing levels of anesthesia, there was an increment in PAC strength from the occipital cortex (baseline vs. BIS 60: P = 0.067, t = − 2.08; baseline vs. BIS 40: P = 0.001, t = − 4.81) and parietal cortex (baseline vs. BIS 60: P = 0.060, t = − 2.15; baseline vs. BIS 40: P = 0.007, t = − 3.87) to the temporal cortex, and from the cingulate cortex to the prefrontal cortex (baseline vs. BIS 60: P = 0.006, t = − 3.54; baseline vs. BIS 40: P = 0.017, t = − 3.29). The decreases in PAC strength from prefrontal and frontal to central, parietal, and temporal cortices represented a disruption of information communication and integration among cortices, which are essential for the support of consciousness [12]. Hence, our results verified the hypothesis that anesthetic drugs suppress consciousness by disrupting the information integrative process among cortices [3].
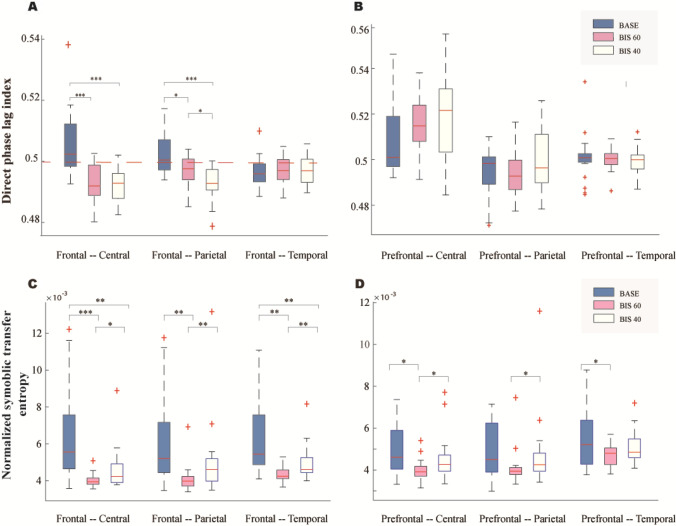
The above results showed that the differences of PAC strength across different consciousness states were mainly located from the frontal and prefrontal cortices to other cortices (parietal, central, and temporal), confirming that suppression of the feedback connectivity is responsible for the anesthetic-induced unconsciousness [12–14]. To investigate whether such differences can be replicated using other connectivity measures, we estimated the dPLI among cortices with a significant change in PAC. As shown in Figure 2A and Table S5, the alteration of dPLI was mainly located between the frontal cortex to the central (baseline vs. BIS 60: P < 0.001, t = 4.43; baseline vs. BIS 40: P < 0.001, t = 5.10) and parietal (baseline vs. BIS 60: P < 0.001, t = 4.70; baseline vs. BIS 40: P = 0.016, t = 3.03) cortices. Moreover, we found that the dPLI values from the frontal cortex to the central and parietal cortices were > 0.5 at baseline, indicating a frontal-lead functional connectivity pattern, while during light and deep anesthesia, the dPLI values from the frontal cortex to the central and parietal cortices dropped below 0.5, indicating a frontal-lag functional connectivity pattern. The phase relationship between the frontal and temporal cortex did not show any statistically significant changes while transiting from wakefulness to anesthesia; neither did the dPLI values from the prefrontal cortex to the lower order cortices (Fig. 2B). Numerous studies have suggested that both GABAergic and glutamatergic (NMDA) anesthetic drugs disrupt frontal-to-parietal functional communication [15]. Consistent with previous studies, our findings support the hypothesis that the top-down (frontal-parietal) communication rather than the feedforward connectivity was suppressed during anesthetic-induced unconsciousness.
Fig. 2.
Connectivity strength estimated by dPLI and NSTE. A, B dPLI from the frontal cortex (A) and from the prefrontal cortex (B) to the central, parietal, and temporal cortex at three consciousness levels. C, D NSTE from the frontal cortex (C) and from the prefrontal cortex (D) to the central, parietal and temporal cortex at three consciousness levels (***P < 0.001, **P < 0.01, *P < 0.05).
We then estimated the NSTE among cortical regions demonstrating a significant decrease in PAC. As shown in Fig. 2C and Table S6, significant changes of NSTE were found in the connectivity between the frontal cortex and the central (baseline vs. BIS 60: P < 0.001, t = 4.4613; baseline vs. BIS 40: P < 0.01, t = 3.895), parietal (baseline vs. BIS 60: P < 0.01, t = 3.6774; baseline vs. BIS 40: P = 0.118, t = 1.644), and temporal (baseline vs. BIS 60: P < 0.01, t = 3.9396; baseline vs. BIS 40: P < 0.01, t = 3.1664) cortices. NSTE was significantly lower during deep and light anesthesia than in the resting state, indicating that normal information flow was disrupted among regions under the influence of the anesthetic. The changes in connectivity strength from prefrontal to central, parietal, and temporal cortices are shown in Fig. 2D and Table S7. The connectivity from prefrontal to central and temporal cortices was also decreased at BIS 60, compared to baseline. There were no significant and uniform changes from the prefrontal to the parietal cortices. Moreover, we found that the trend of decreased connectivity in the NSTE was not uniform, which is in contrast to previous work [3] reporting that the NSTE is higher at a deep than at a light level of anesthesia. However, this previous work [3] only required a loss of behavioral responses to be qualified as unconsciousness. It has been shown that the loss of behavioral responses usually occurs at BIS of 60 [16]. Hence, the change in NSTE at deep levels of anesthesia remained unknown. Our results showed that the change of NSTE during unconsciousness is not monotonic. After an initial drop during light anesthesia, the NSTE rebounded at deep levels.
To delineate the relationship between the estimated functional connectivity strength using PAC, dPLI, and NSTE and the depth of anesthesia, we conducted a correlation analysis (Fig. S4), which showed a significant correlation between the strength of functional connectivity estimated using PAC and the level of anesthesia in all feedback connectivity originating from the frontal and prefrontal cortex (Fig. S4A). In the cases of dPLI and NSTE, a significant correlation was found in the connectivity originating from the frontal cortex. Combing the results shown in Figs. 1, 2 and S4, we found that the PAC from the prefrontal and frontal-central cortex, and the dPLI from the frontal-parietal cortex were promising candidates to form an index of the depth of anesthesia. However, to obtain a clinically usable index of anesthetic depth, these candidate connectivity measures must be evaluated in a carefully designed experiment with step-wise titration of anesthetic targeting different behavioral endpoints. Furthermore, using EEG-based connectivity estimation to form an index of anesthetic depth also requires the EEG electrodes to be precisely located at the same cortical regions of interest across participants. Such a requirement is hard to meet in practice, as the head shape differs considerably among participants and their posture during surgery could also cause changes in the location of EEG electrodes. Hence, further research is required to investigate the impact of electrode displacement on the connectivity strength.
In conclusion, by estimating connectivity measures PAC, dPLI, and NSTE on the same group of participants, we supported the hypothesis that the disconnection of frontal-parietal connectivity is a signature of propofol-induced anesthesia, and this does not depend on the analytical method. However, our results also suggest that connectivity strength alone may not be sufficient for assessing the level of consciousness, and further investigation is required to fully depict the association between changes in functional connectivity and levels of consciousness.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81701787, 81671778, 31271063 and U1401255) and the Natural Science Basic Research Plan in Shaanxi Province, China (2019JQ-138).
Conflict of interest
All authors declared no conflict of interest.
Footnotes
Xue Zhao and Yubo Wang contributed equally to this work.
Contributor Information
Qiang Wang, Email: dr.wangqiang@139.com.
Liyu Huang, Email: huangly@mail.xidian.edu.cn.
References
- 1.Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, et al. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–E1151. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee H, Mashour GA, Noh GJ, Kim S, Lee U. Reconfiguration of network hub structure after propofol-induced unconsciousness. Anesthesiology. 2013;119:1347–1359. doi: 10.1097/ALN.0b013e3182a8ec8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–1275. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal D, Silverstein BH, Sharba L, Li D, Hambrecht-Wiedbusch VS, Hudetz AG, et al. Propofol, sevoflurane, and ketamine induce a reversible increase in delta-gamma and theta-gamma phase-amplitude coupling in frontal cortex of rat. Front Syst Neurosci. 2017;11:00041. doi: 10.3389/fnsys.2017.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranft A, Golkowski D, Kiel T, Riedl V, Kohl P, Rohrer G, et al. Neural correlates of sevoflurane-induced unconsciousness identified by simultaneous functional magnetic resonance imaging and electroencephalography. Anesthesiology. 2016;125:861–872. doi: 10.1097/ALN.0000000000001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maksimow A, Silfverhuth M, Långsjö J, Kaskinoro K, Georgiadis S, Jääskeläinen S, et al. Directional connectivity between frontal and posterior brain regions is altered with increasing concentrations of propofol. PLoS One. 2014;9:e113616. doi: 10.1371/journal.pone.0113616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolaou N, Hourris S, Alexandrou P, Georgiou J. EEG-based automatic classification of 'awake' versus 'anesthetized' state in general anesthesia using Granger causality. PLoS One. 2012;7:e33869. doi: 10.1371/journal.pone.0033869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma L, Liu W, Hudson AE. Propofol anesthesia increases long-range frontoparietal corticocortical interaction in the oculomotor circuit in macaque monkeys. Anesthesiology. 2019;130:560–571. doi: 10.1097/ALN.0000000000002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koenig T, Studer D, Hubl D, Melie L, Strik WK. Brain connectivity at different time-scales measured with EEG. Philos Trans R Soc Lond B Biol Sci. 2005;360:1015–1023. doi: 10.1098/rstb.2005.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Tort AB, Komorowski R, Eichenbaum H, Kopell N. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol. 2010;104:1195–1210. doi: 10.1152/jn.00106.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mashour GA, Alkire MT. Consciousness, anesthesia, and the thalamocortical system. Anesthesiology. 2013;118:13–15. doi: 10.1097/ALN.0b013e318277a9c6. [DOI] [PubMed] [Google Scholar]
- 13.Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601–628. doi: 10.1146/annurev-neuro-060909-153200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malekmohammadi M, Price CM, Hudson AE, DiCesare JAT, Pouratian N. Propofol-induced loss of consciousness is associated with a decrease in thalamocortical connectivity in humans. Brain. 2019;142:2288–2302. doi: 10.1093/brain/awz169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder KE, Irwin ZT, Gaidica M, Nicole Bentley J, Patil PG, Mashour GA, et al. Disruption of corticocortical information transfer during ketamine anesthesia in the primate brain. Neuroimage. 2016;134:459–465. doi: 10.1016/j.neuroimage.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Oliveira GS, Jr., Kendall MC, Marcus RJ, McCarthy RJ. The relationship between the Bispectral Index (BIS) and the Observer Alertness of Sedation Scale (OASS) scores during propofol sedation with and without ketamine: a randomized, double blinded, placebo controlled clinical trial. J Clin Monit Comput. 2016;30:495–501. doi: 10.1007/s10877-015-9745-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.