Abstract
The natural capacity of plants to endure salt stress is largely regulated by multifaceted structural and physio-biochemical modulations. Salt toxicity endurance mechanism of six ecotypes of Typha domingensis Pers. was evaluated by analyzing photosynthesis, ionic homeostasis, and stomatal physiology under different levels of salinity (0, 100, 200 and 300 mM NaCl). Typha populations were collected across different areas of Punjab, an eastern province in Pakistan. All studied attributes among ecotypes presented differential changes as compared to control. Different salt treatments not only affected gas exchange attributes but also shown significant modifications in stomatal anatomical changes. As compared to control, net photosynthetic rate, transpiration rate, total chlorophyll contents and carotenoids were increased by 111%, 64%, 103% and 171% respectively, in Sahianwala ecotype among all other ecotypes. Similarly, maximum water use efficiency (WUE), sub stomatal CO2 concentration, sodium (Na+) and chloride (Cl−) contents were observed in Sahianwala (191%, 93%, 168%, 158%) and Knotti (162%, 75%, 146%, 182%) respectively, as compared to the others ecotypes. Adaxial and abaxial stomatal areas remained stable in Sahianwala and Knotti. The highest abaxial stomatal density was observed in Gatwala ecotype (42 mm2) and maximum adaxial stomatal density was recorded in Sahianwala ecotype (43 mm2) at 300 mM NaCl salinity. The current study showed that Typha ecotypes responded varyingly to salinity in terms of photosynthesis attributes to avoid damages due to salinity. Overall, differential photosynthetic activity, WUE, and changes in stomatal attributes of Sahianwala and Knotti ecotypes contributed more prominently in tolerating salinity stress. Therefore, Typha domingensis is a potential species to be used to rehabilitate salt affected lands for agriculture and aquatic habitat.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-00963-x.
Keywords: Typha populations, Stomatal regulation, Structural modifications, Transpiration rate, Water-use efficiency
Introduction
Soil salinity is one of the most injurious environmental constraints that rigorously impede plant growth, development, and productivity. Salt stress usually increase disorders in plants (Acosta-Motos et al. 2017). Among these salinity associated perturbations in plants, reduced leaf area, declined photosynthesis (Agarwal et al. 2013), stomatal closure and over-production of Reactive Oxygen Species (ROS) (Hussain et al. 2016) are well-known and cause alarming reduction in plant growth and productivity (Phang et al. 2008). Imbalances in ionic homeostasis coupled with osmotic stress due to Na+ ions enhance toxicity (Horie et al. 2012). Besides, unequal ionic concentration in plant parts leads to irregularity in stomatal functions (Nilson and Assmann 2007). Such changes in stomatal functions directly influence crucial plant processes such as transpiration, gaseous exchange and photosynthesis. Salt tolerant plants mainly use three types of tolerance mechanisms. These include toxic ion exclusion, limitation/deposition of ions into specific plant cells/tissues as well as subcellular organelles and shoot ion-independent salinity tolerance (Munns and Tester 2008). Other physiological processes i.e. photosynthesis, transpiration, ionic homeostasis, antioxidant defense are also parallel contributors in these mechanisms for making plant tolerant to salt stress (Negrão et al. 2017). These facts suggest a strong and positive correlation between physiological and anatomical attributes in plants that collectively determine the survival of a plant species growing in saline soil.
Different plant populations such as grasses and macrophytes naturally show better tolerance to salinity stress (Hameed and Ashraf 2008). For instance, salt tolerant plants i.e. halophytes showed tolerance by adjusting their histo-physiological and biochemical attributes in their life cycle and such endurance to stress conditions is reflected in terms of growth and productivity (Khan and Gul 2002). Typha domingensis Pers. can inhabit variety of salt and metal affected lands e.g. salt range, wetlands, and coastal marshes. This plant is native to South America and found as invasive macrophyte in different regions of the world. Typha has shown high potential for phytoremediation (Oliveira et al. 2018) and its tolerance to deteriorated rooting medium is due to the evolution of specific histological traits (Wu 1981). Survival of Typha plants in salinity affected rhizosphere would provide mechanistic insight into working relationship between anatomical and physiological characteristics. It also supports the notion that interaction between plants and soil is not limited to single feature. Although much work has been done on physiological aspects of salinity tolerance in plants yet other aspects including morphological changes in plant tissues with respect to salt stress needs extensive investigations (Noreen et al. 2019; Yoo et al. 2009; Zulfiqar et al. 2020a). Previously, Typha plants have been evaluated for their tolerance to heavy metals and other nutrient uptake studies (da Cunha Cruz et al. 2020; Oliveira et al. 2018).
It was hypothesized that histological modifications in stomatal characteristics were directly linked to physiological modulations. Therefore, primary objective of this study was to assess the changes in stomatal attributes and their link to different physiological attributes in T. domingensis Pers. affecting their distribution and tolerance to salinity. Intra-species differences among Typha populations inhabiting fresh water or saline rhizosphere were also focused to determine the most tolerant ecotype in relation to different sites. It was revealed that salinity levels significantly affected the stomatal density on epidermal surface and indicated correlated physiological responses across the Typha domingensis Pers. populations.
Materials and methods
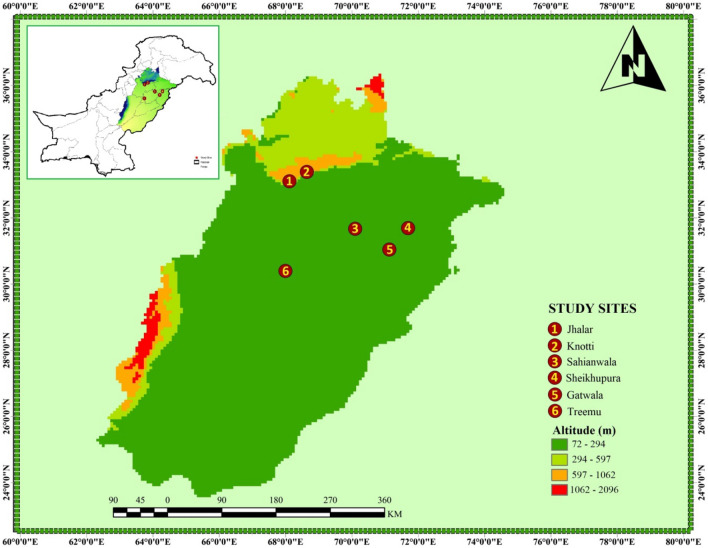
Six Typha domingensis Pers. ecotypes were collected from different areas of Punjab province in Pakistan; viz. Sahianwala (saline waterlogged), Knotti (salt range), Jahlar (hyper saline), Gatwala (Fresh water), Treemu (fresh water), and Sheikhupura (industrially polluted) (Fig. 1, Table 1). These plants were individually grown in plastic pots of size 10 × 10 × 10 (L × W × H) and 500 g weight filled clayey-loam soils and sand in 1:1 ratio, of pH:6.8 and then filled with 10 L irrigation water in the Botanic Garden at University of Agriculture, Faisalabad under natural environmental conditions for a period of 2 months. Then the acclimatized plants were treated with salt treatment for 3 months (Naseer et al. 2017). The average temperatures of day and night were 38–41 °C and 24–26 °C, respectively and light period ranged from 13 to 16 h. The relative humidity alternate from 46.8 to 59.3% (Zulfiqar et al. 2020b).
Fig. 1.
The map of the Punjab, Pakistan showing six different collection sites for Typha domingensis Pers. ecotypes
Table 1.
Data about Typha ecotypes, their habitats and collection sites
| Ecotype name | Accession number | Date of collection | Place name | Collection area | Geographic coordinates of location | No. of plants | Stage of sample |
|---|---|---|---|---|---|---|---|
| Jahlar | 17001 | 12-04-2017 | Jahlar lake, hypersaline, Punjab, Pakistan | Lake |
32° 56′ N, 72° 41′ E |
15 | Vegetative |
| Sheikhupura | 17002 | 22-04-2017 | Sheikhupura road, industrial polluted area, Punjab, Pakistan | Road side |
31° 72′ N, 73° 91′ E |
15 | Vegetative |
| Sahianwala | 17003 | 02-05-2017 | Sahianwala area, water-logged wetlands Punjab, Pakistan | Urban |
31° 43′ N, 73° 63′ E |
15 | Vegetative |
| Gatwala | 17004 | 10-05-2017 | Gatwala fresh water lake Punjab, Pakistan | Canal bank |
31° 71′ N, 73° 12′ E |
15 | Vegetative |
| Treemu | 17005 | 14-05-2017 | Treemu head, fresh water,Punjab, Pakistan | River |
31° 08′ N, 72° 08′ E |
15 | Vegetative |
| Knotti | 17006 | 20-05-2017 | Knotti garden,salt range, Punjab, Pakistan | Wetland |
32° 42′ N, 72° 14′ E |
15 | Vegetative |
Establishment of saline levels
The levels of salt used in this experiment were 0, 100, 200, and 300 mM NaCl with half-strength Hoagland’s nutrient solution (Hoagland and Arnon 1950) applied as rooting medium to all the Typha (Typha domingensis Pers.) ecotypes. Salt treatment was give once in week along with all media. After 15 days of treatment tissues were harvested for further measurements.
Plant pigments
Chlorophyll a, b, and carotenoids were determined according to the method of Arnon (1949), and Davies (1976). The chlorophyll a, b, and carotenoids were calculated using following formula:
Determination of photosynthetic activity
Net CO2 assimilation rate (PN), transpiration rate (E), and stomatal conductance (gs) were measured on one fully expanded flag leaf from each plant using an open system LCA-4 ADC portable infrared gas analyzer (Analytical Development Company, Hoddesdon, England). The data were recorded for different parameters with molar flow of air per unit leaf area 403.3 mmol m−2 s−1, atmospheric pressure 99.9 kPa, maximum light intensity (PPFD) at leaf surface 1,711 μmol m−2 s−1, leaf temperature ranging from 28.4 to 32.4 °C, ambient temperature from 22.4 to 27.9 °C, and ambient CO2 concentration of 352 μmol mol−1 (Aqeel et al. 2021; Noreen et al. 2018).
Digestion of plant material
For the estimation of nutrients firstly plant material was digested according to the prescribed methodology (Wolf 1982; Ali 2018). The dried, well ground plant material (0.5 g in each tube) was placed in digestion tubes and 5 mL of concentrated H2SO4 was added to each tube. All tubes were incubated overnight at room temperature. Then 0.5 mL of H2O2 (35% A. R. Grade extra pure) was poured down the sides of the digestion tube, ported the tubes in a digestion block and heated at 350 °C until fumes were produced. The heating was continued for another 30 min. The digestion tubes were removed from the block and cooled. 0.5 mL of H2O2 was slowly added to each tube and tubes were placed back into the digestion block. The above step was repeated until the cooled digested material was colorless. The volume of the extract was brought up to 50 mL. The extract was filtered and used for determining ionic contents.
Estimation of ionic contents
Na+ and K+ were determined with a flame photometer (Jenway, PFP-7, Burlington, NJ, USA). A graded series of standards (ranging from 5 to 25 mg L−1) of Na+ and K+ were prepared and standard curves were plotted. The values from flame photometer were compared with standard curves and total quantities were computed. (Horneck and Hanson 1998; Jiang et al. 2019; Ditta et al. 2018; Jackson 1962). P content was measured by following the protocol of Jackson (1962) by using Spectrophotometer (Hitachi-220, Tokyo, Japan). Magnesium (Mg2+) and calcium (Ca2+) contents in shoot were determined by EDTA titration according to the method of Tucker and his co-worker (Tucker and Kurtz 1961; Yang et al. 2020). Cl− contents of leaf were determined by following the method described by Tavakkoli et al. (2010). Shoot samples (100 mg each) were ground and heated in 10 ml of H2O at 80 °C until the volume became half. The volume was brought to 10 mL again by adding distilled water. Cl− content was determined with a chloride meter (Model 926, Sherwood Scientific Ltd., Cambridge, UK).
Anatomical attributes
For stomatal studies, 1 cm piece from the leaf used for gas-exchange measurements was taken and fixed in FAA (formalin 5%, acetic acid 10%, ethanol 50%, and water 35%) solution for 48 h. The material was then transferred to acetic-alcohol (v/v, acetic acid 25%, and ethanol 75%) solution for long-term storage and till for further analysis (Johansen 1940; de Andrade Silva et al. 2020). The paradermic piece of leaf from adaxial and abaxial sides of leaf were taken using steel blades and further sections were stained with 0.1% safranin according to (Kraus and Arduin 1997; de Andrade Silva et al. 2020). Stomatal density, area, and orientation were studied by scratching the leaf surface. The basal portion of the leaf (1 cm long) was selected for stomatal studies. Measurements and photographs were taken with the help of a camera equipped light microscope (Nikon 104, Japan).
Statistical design
The experiment was planned in a Completely Randomized Design (CRD) with two factors (plant species and salinity) and three replications. The data was subjected to statistical analysis using Minitab statistical software (Version: Minitab 19.1.1) for analysis of variance (ANOVA) and LSD for comparison of mean values (Steel et al. 1997; Kim 2014). Graphs were prepared using Origin Pro 9.1 (OriginLab Corporation 2014), and statistical program “R (v 4.0.1)” was used for estimating Pearson's correlation co-efficient and for Principal component analysis among various measured variables (R Development Core Team 2017).
Results
Physiological parameters
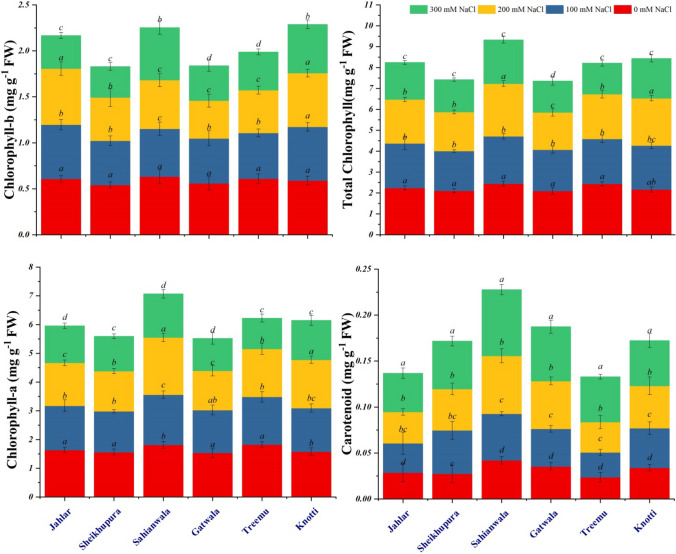
Plant chlorophyll-a content reduced significantly in Gatwala ecotype in response to increase in salt levels. A profound decline was recorded in Typha collected from Sheikhupura, Sahianwala, Treemu, and Knotti only at 300 mM NaCl. In comparison, a minor reduction in Chl. a content was observed in Jahlar ecotype in response to increased salinity in growth medium (Fig. 2). A prominent and significant reduction was recorded in Chl. b content in ecotypes from Sheikhupura, Gatwala and Treemu in response to progressive increase in salt levels. Chl. b value remained stable up to 200 mM salinity in the Jahlar and Knotti ecotypes and showed significant reduction at 300 mM salt level. In the Sahianwala ecotype, this parameter showed significant reduction at moderate levels of salt stress (100 and 200 mM NaCl). However, high dose of salt (300 mM NaCl) yielded non-significant variation. Total chlorophyll contents were significantly reduced in Jahlar, Sheikhupura, Gatwala, and Treemu region Typha plants. However, ecotypes from Knotti and Sahianwala showed non-significant changes up to 200 mM salt level, but a significant reduction was recorded at the 300 mM salinity. Carotenoids invariably increased in Typha ecotypes in response to salt stress, particularly at the highest salt level (Fig. 2). Carotenoid content however, was significantly higher in the Sahianwala ecotype as compared to all other ecotypes. In short, Sahianwala ecotype was tolerant in term of chlorophyll a, b, and carotenoids content as least reduction was observed in these pigments as compared to control. On the other hand, Typha ecotypes from Sheikhupura and Gatwala were sensitive that showed greater reduction in chlorophyll a, b and total chlorophyll as compared to control.
Fig. 2.
Plant pigments of six ecotypes of Typha domingensis Pers. under different levels of salt stress. After 15 days of treatment, tissues were harvested for pigment measurements.
Photosynthetic parameters
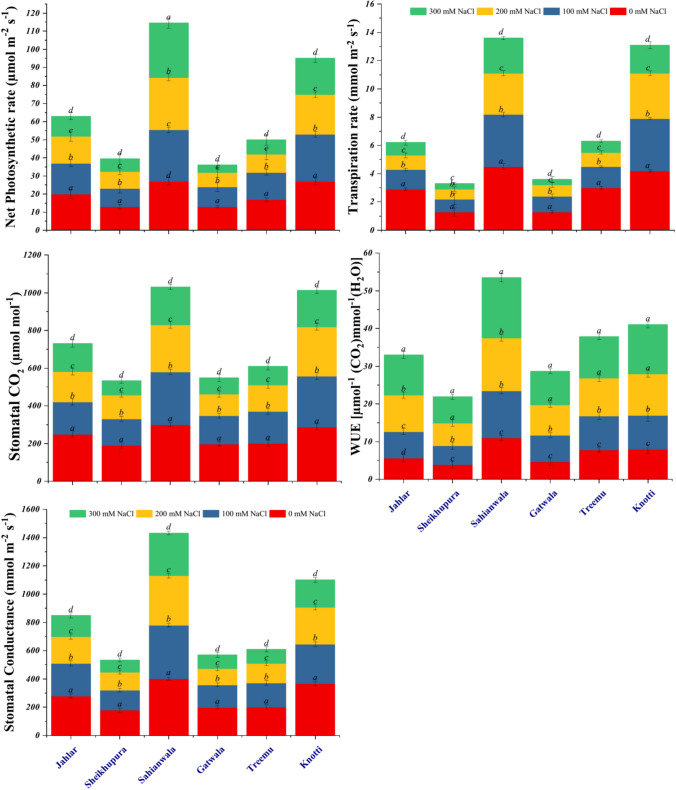
Transpiration rate (E) was decreased in all populations of Typha domingensis Pers. under salinity stress as compared to that of control. Sheikhupura and Gatwala populations showed lowest transpiration rate. Reducing transpiration is a good indicator of stress tolerance. PN (net CO2 assimilation) was also recorded to reduce under salt stress in all populations except Sahianwala, a population from high saline habitat, which showed marked increase in net CO2 assimilation in response to gradual increase of salt stress levels. Substomatal CO2 concentration was less affected due to salinity stress. This may be correlated to an increase in stomatal density which reduces loss of CO2 under saline conditions. Sahianwala populations showed marked enhancement in sub-stomatal CO2 concentration along with increased salinity levels (Fig. 3). Various levels of salinity influenced stomatal conductance (gs) adversely. Populations from fresh water and industrially polluted areas; Gatwala, Treemu and Sheikhupura showed more sensitivity as compared to Sahianwala, Knotti and Jahlar populations from highly saline habitat. WUE was progressively increased under higher salinity levels in all populations which depict their salt tolerance mechanism. Sahianwala and Knotti populations from high saline soils showed more WUE as compared to Jahlar population from saline-waterlogged habitat, Gatwala, Treemu and Sheikhupura populations from fresh water to industrially polluted habitats. Typha ecotypes from Sahianwala and Knotti showed better adaptation as compared to other ecotypes. Tolerance behavior was recorded in Sahianwala ecotype that showed the highest net photosynthetic rate, high WUE and least effected stomatal conductance and sub-stomatal CO2 activities. The Sheikhupura ecotype was sensitive in term of decrease photosynthetic rate, transpiration rate, sub-stomatal CO2 concentration and stomatal conductance as compared to control.
Fig. 3.
Gas exchange characteristics of six ecotypes of Typha domingensis Pers. under different levels of salt stress
Ionic contents
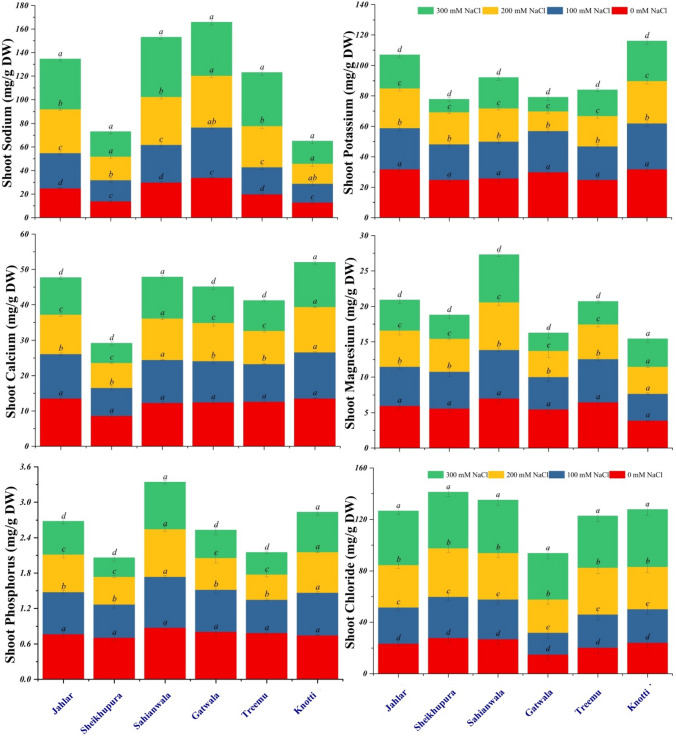
Shoot sodium (Na+) content in all ecotypes of Typha showed progressive and significant increase along with increased salt levels of growth medium. Ecotype from Gatwala accumulated highest shoot Na+ content as compared to all other ecotypes. On the other hand, ecotype from Knotti revealed least increase in this parameter in response to salt stress as compared to control. There was a profound and significant reduction in shoot K+ in all ecotypes with increase in salt conc. of external growth medium. The greatest reduction in shoot K+ was observed in Sheikhupura ecotype at 300 mM NaCl as compared to other ecotypes (Fig. 4). Shoot P value was gradually and prominently reduced in the Sheikhupura, Gatwala and Treemu ecotypes in response to increase salinity levels of growth medium. Ecotypes from Jahlar, Sahianwala and Knotti showed non-significant differences among P content variations. Shoot Cl− content progressively and significantly increased in all Typha domingensis ecotypes as compared to control in response to salt stress. Highest increase was observed at 300 mM NaCl. Data relevant to shoot Mg2+ content was gradually and significantly decrease in all ecotypes except Knotti site Typha ecotype. Shoot Ca2+ value showed a significant decrease in ecotypes from Jahlar, Sheikhupura, Gatwala and Treemu. However, in ecotypes from Sahianwala and Knotti, the pattern of variation of Ca2+ was stable and non-significant changes were observed under salinity stress (Fig. 4). Overall, Sahianwala ecotype showed high Na+ and Cl− contents and least reduction in Ca2+, Mg2+ and P. Tolerant behavior was observed in Sahianwala ecotype as compared to all other Typha (Typha domingensis Pers.) ecotypes. The sensitive ecotype was Sheikhupura that showed reduced values of Na+, K+, Ca2+, Mg2+ and P.
Fig. 4.
Shoot ionic contents of six ecotypes of Typha domingensis Pers. under different levels of salt stress
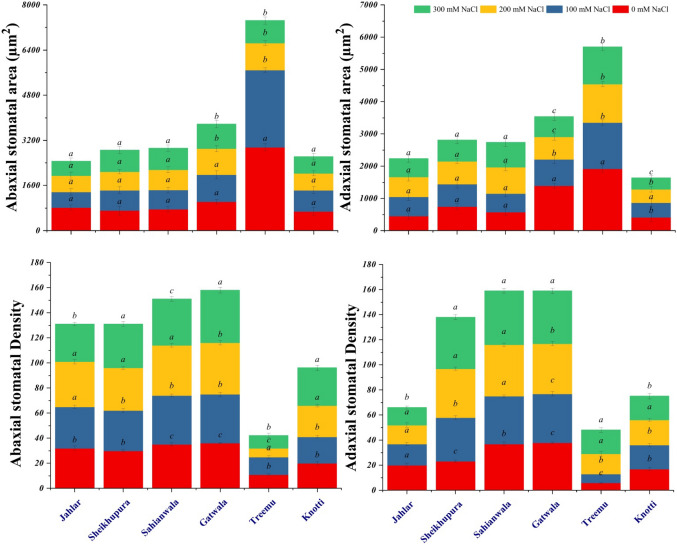
Stomatal area and density
Stomatal area refers to the area occupied by stomatal pore as per unit surface of leaf (abaxial and adaxial) and density is the count of number of stomata on either abaxial or adaxial leaf surface (Figs. 5 and 6). Both these characteristics have inverse relation to each area, as increase density will provide less area to stomata. Under salinity stress Treemu and Gatwala populations showed reduced stomatal area of abaxial and adaxial leaf surfaces (Fig. 7). However, Sahianwala, Knotti and Jahlar populations showed non-significant variations in adaxial and abaxial stomatal area. Adaxial stomatal area of Jahlar population was increased under different salinity levels. Salinity induced decrease in stomatal area, transpiration rate E, which ultimately enhance WUE, is an effective strategy under stress condition. Net CO2 assimilation increased with decreasing stomatal area. Stomatal density however, showed non-significant pattern of variation among all populations, overall enhanced abaxial stomatal density was observed.
Fig. 5.
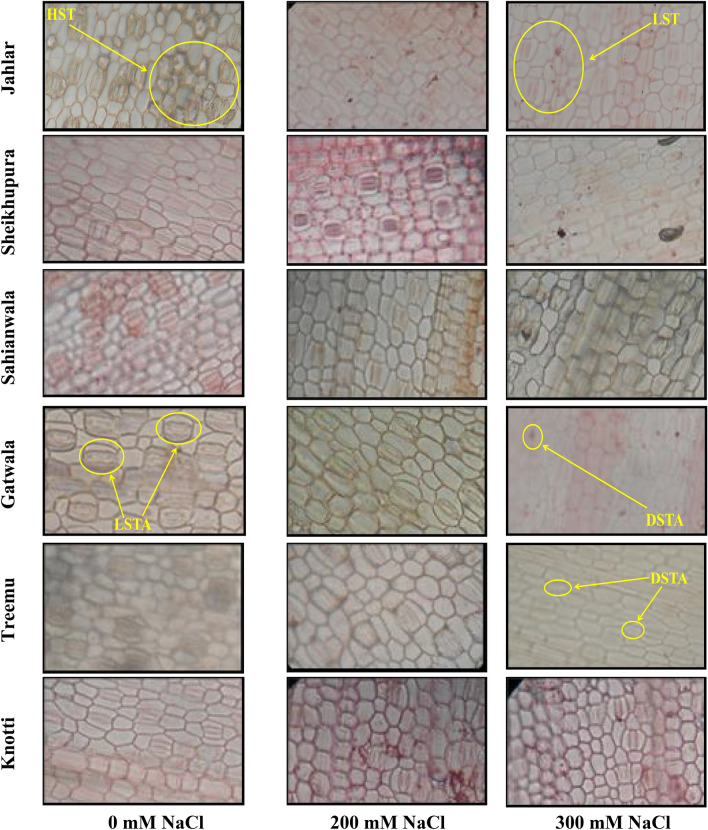
Adaxial epidermis of six ecotypes of Typha domingensis Pers. under different levels of salt stress. HST high stomatal density, LST low stomatal density, LSTA large stomatal area, DSTA decrease stomatal area
Fig. 6.
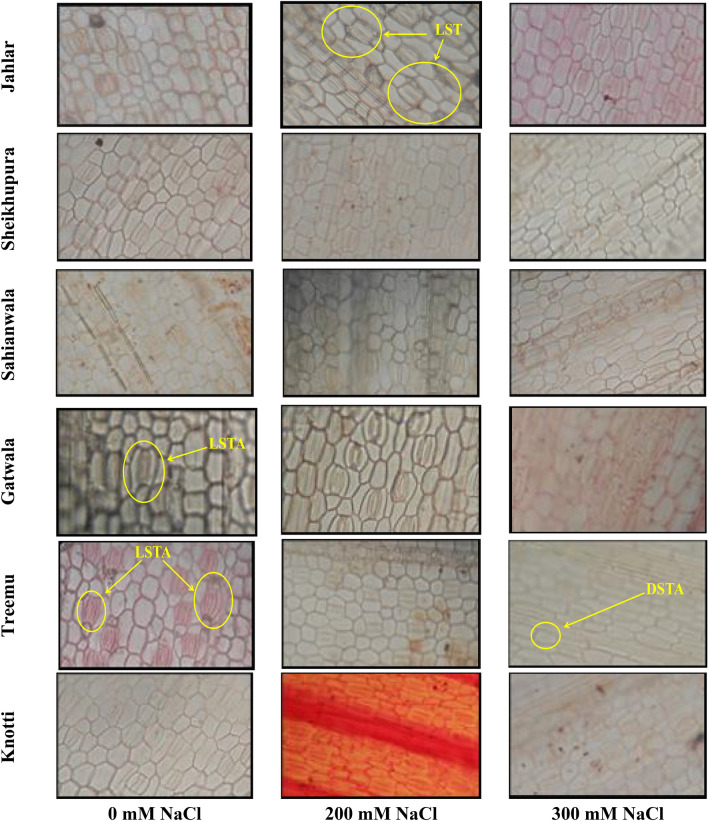
Abaxial epidermis of six ecotypes of Typha domingensis Pers. under different levels of salt stress. LST low stomatal density, LSTA large stomatal area, DSTA decrease stomatal area. All images were taken with light microscope.
Fig. 7.
Stomatal area and density of six ecotypes of Typha domingensis Pers. under different levels of salinity stress. These results are average of data obtained in three replicates
In Jahlar population decrease in adaxial stomatal density was recorded under increase salt stress, but in other populations this parameter increased progressively under salt stress. Lowest stomatal density was recorded in Treemu population which showed increase in adaxial stomatal density, but abaxial side showed increase in stomatal density only at 100 mM NaCl, thereafter gradual decrease was recorded. Increased stomatal density provide better stomatal conductance which could be an effective precautionary measure under water limiting conditions (Figs. 5, 6, and 7). The high abaxial and adaxial stomatal density, and adaxial stomatal area was recorded in Sahianwala ecotype. Sahianwala ecotype also showed least reduction in abaxial stomatal area as compared to other Typha domingensis Pers. ecotypes. So, Sahianwala ecotype showed tolerance in comparison with other ecotypes.
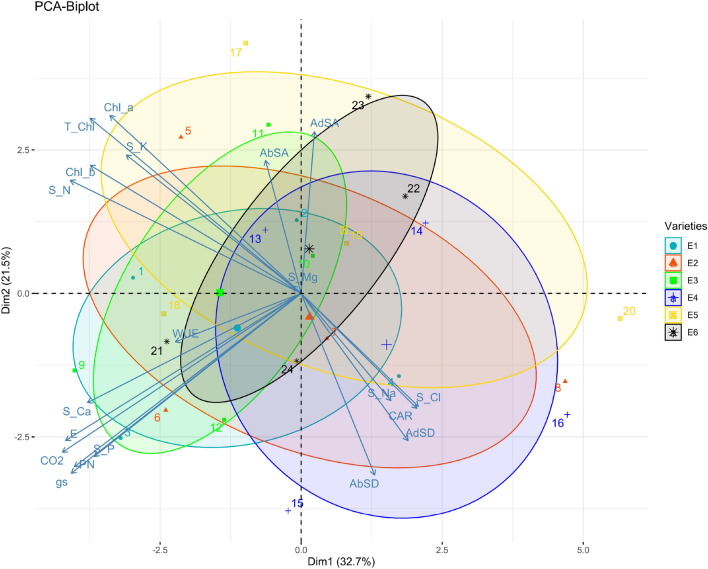
PCA and Pearson correlation
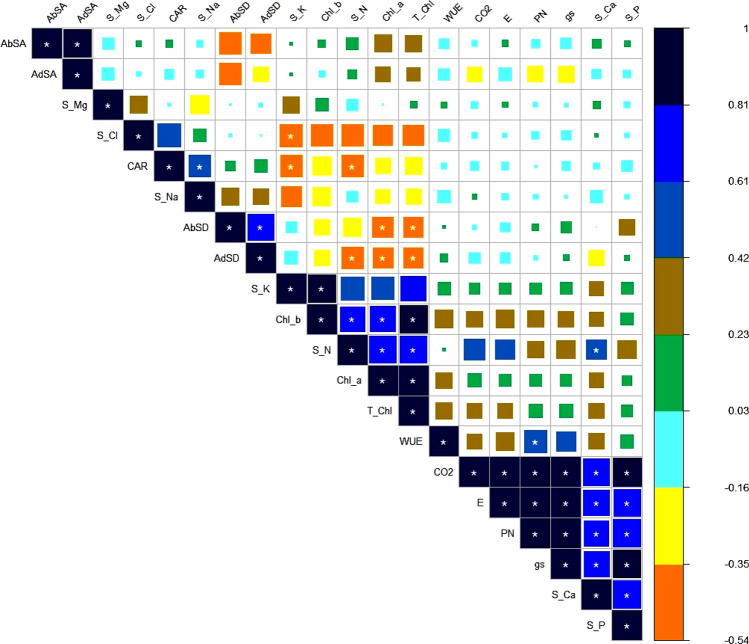
For assessment of inter-relationship among various attributes of Typha ecotypes and salinity levels, the principal component analysis and Pearson correlation was executed (Figs. 8 and 9). Pearson’s correlation showed a significant positive correlation of salinity levels with transpiration rate, WUE, sub-stomatal CO2 concentration, Shoot P, Ca2+ and stomatal modifications. Adaxial and abaxial stomatal area is highly correlated but negatively correlated with stomatal density. Shoot Na+ and Cl− is negatively correlated with K+. Furthermore, a significant negative correlation is evident for Cl− concentrations with Chl a, b and total chlorophyll. This correlation represented a close connection and relationship between salinity and plant attributes (Fig. 8).
Fig. 8.
Pearson Correlation between different attributes in Typha (Typha domingensis Pers.) reveal relationship to support ecological survival of different ecotypes subjected to multiple salinity regimes. AbSA: Abaxial Stomatal Area; AbSD: Abaxial Stomatal Density; AdSA: Adaxia Stomatal Area; AdSD: Adaxial Stomatal Density; CAR: Cartenoid; Chl_a: Chlorophyll-a; Chl_b: Chlorophyll-b; CO2: Internal Carbon dioxide; E: Transpiration rate; gs: Stomatal Conductance; PN: Net Photosynthetic Rate; S_Ca: Shoot Calcium; S_Cl: Shoot Chloride; S_K: Shoot Potassium; S_Mg: Shoot Magnesium; S_N: Shoot Nitrogen; S_Na: Shoot Sodium; S_P: Shoot Phosphorus; T_Chl: Total chlorophyll; WUE: Water Use Efficiency
Fig. 9.
Principal component analysis showing relationship between different attributes and Typha ecotypes under salinity stress
On a factor plane in PCA, the directions of the cases with the Bi-plot were imaged. A clear separation of Typha (Typha domingensis Pers.) ecotypes attributes in response to different salinity levels were shown by PC1. Of the total variation, contribution of PC1 was 32.7% as compared to 21.5% contribution by PC2. We detected a clear separation among different attributes studied in Typha ecotypes. Although we observed a strong overlap yet there is a clear bias between the ecotypes. The maximum variation in PC1 was elaborated by gs, Pn, adaxial stomatal area, abaxial and adaxial stomatal density, Na+ and Cl− (Fig. 9).
Discussion
Naturally growing monocot species are best adapted to grow in habitats facing environmental stresses such as salinity. Such successful adaptation depict evolution in their genetic makeup and certain structural as well as functional modifications of certain tissues and organs (Hameed et al. 2010). Major processes like photosynthesis, respiration and transpiration show functional modifications that make plants able to compete stress. Stomata are the vital organs to regulate these processes by maintaining water and CO2 supply under limiting environmental conditions (Naz et al. 2010; Tufail et al. 2020; Yang et al. 2004). In present study, all ecotypes of Typha domingensis Pers. exhibited declined transpiration rate (E) and stomatal conductance (gs) with increasing salinity levels. This is depiction of the fact that plants decrease transpiration rate and stomatal conductance as an effective strategy to prevent water loss and dehydration for maintaining normal functioning of photosynthetic machinery under saline conditions (Naz et al. 2010; Robinson et al. 1998; Tufail et al. 2020).
In ecotype Sahianwala, larger adaxial and abaxial stomatal area along with increased stomatal density under salt stress contributed in maximum CO2 supply for photosynthesis. This attribute correlated with increased sub-stomatal CO2 in the same ecotype and emerged as an evidence supporting process of photosynthesis. Besides, certain invaginations of epidermal tissues in the Sahianwala ecotype also elaborated a direct relationship between increased stomatal area and corresponding stomatal conductance to prevent transpirational losses. Our stance is supported by the mechanistic explanation presented by Naz and co-workers (Naz et al. 2010). According to them, in Aeluropus lagopoides controlled transpiration rate and high water-use efficiency were major players in salinity tolerance that was tightly controlled by fewer and smaller stomata on adaxial and abaxial leaf surfaces as well as stomatal encryption due to epidermal invaginations (Naz et al. 2010).
Furthermore, Jahlar ecotype showed an increase in adaxial stomtal area. Increased stomatal area and the size of stomatal pore may be decreased by epidermal appendages that act as limiting factors to reduce transpiration. However, CO2 could diffuse through discrete stomatal pore (Parkhurst 1994). This is a beneficial aspect of architectural adjustments for survival. Such managed size, presence and number of stomata are critical traits not only for regulation of stomatal function but also for photosynthetic attributes. Contrary to these findings in ecotypes i.e. Sahianwala and Jahlar, freshwater ecotypes i.e. Gatwala and Treemu displayed marked diminution of abaxial and adaxial stomatal area due to salinity which leads to dropped transpiration rate under saline conditions and marked their sensitivity to salinity. Sheikhupura ecotype from industrially polluted sites presented unaffected stomatal area under salinity.
Under salinity, transpiration rate is reduced to minimize water loss and increase WUE in many macrophytes (Ashraf 2004). Decreased stomatal area contributes to maintain maximum water use efficiency thus enhancing photosynthesis (Franks and Farquhar 2007). Increased stomatal density on both abaxial and adaxial surfaces of leaf was an adaptive response to salinity in all ecotypes. Increased stomatal density enhanced number of stomata. Consequently, it reduces the diameter of stomatal pore to tackle stressed conditions that damage the plant and avoid direct contact with external harsh conditions (Jordan et al. 2008). High WUE-water use efficiency is the major determinant among other salinity tolerance indicators observed in all ecotypes.
Higher WUE was correlated to increased stomatal density that projected greater number of sunken stomata to prevent water loss and ensuring maximum water availability for photosynthetic activity under osmotic stress due to higher salinity treatments. Sunken stomata are complementary support in survival process that prevent their direct contact with environmental harshness (Jordan et al. 2008). The considerable WUE and other photosynthetic attributes in Sahianwala and Knotti ecotypes along with enhanced stomatal attributes are adaptive components of salinity tolerance. Unaffected or least affected stomatal behavior point out successful adaptation of these ecotypes related to the modified photosynthetic parameters. This can be due to the effectiveness of stomatal closure instead of stomatal density and area (Parkhurst 1994). The salinity affected chlorophyll contents but managed photosynthetic activity in Sahianwala and Knotti ecotypes is sufficient proof of supplementary aid to avoid much metabolic loss. Similarly, ionic homeostasis in terms of different concentrations of various ions was among the prominent supporters to the survival along with WUE, gs and stomatal density. The same had been recorded by Zhu and co-workers (Zhu et al. 2016).
Different ions significantly contributed for tolerance and this is exhibited by the correlation between ionic homeostasis and stomatal functions. Maintenance of appropriate balance between ions and their optimal concentration in plants is a key player in salinity tolerance (Cuin et al. 2009). Hence, plant's capacity to transport K+ to the shoot and its retention in mesophyll in exchange with Na+ would be a chief participant for maintenance of optimal K+/Na+ ratio in leaf that influence salinity tolerance in plants. It is on record that salinity tolerant plants maintain a balance between ionic concentrations (Shabala et al. 2013). Our findings are consistent with this fact. Overall, osmotic stress due to high salt content in root zone decreased stomatal conductance (Munns 2002) and this constriction may govern for many weeks, until explicit ion toxicity in leaves begins to play a main role. Noteworthy correlations were recorded between salinity tolerance and stomatal conductance as well as stomatal density. Ecotypes with high stomatal conductance and stomatal density have ability to show better tolerance against salt stress.
Decrease in stomatal area and vice versa for stomatal density plays crucial role in stress tolerance as decreased area provide minimum water loss and increased density gives better stomatal conductance as well as diffusion of CO2 for photosynthesis. These adaptations in macrophytes like T. domingensis allow this species to survive in areas facing lots of environmental stresses like salinity, drought, heavy metals, light and temperature that induces modifications in gas exchange characteristics to regulate photosynthesis and transpiration (Naz et al. 2010; Noman et al. 2017, 2012; Aqeel et al. 2021; Khalid et al. 2021).
Conclusion
In summary, the competitive superiority and survival of T. domingensis i.e. Sahianwala and Knotti ecotypes was due to its better capacity in terms of anatomical and physiological modulations. The considerable WUE, other photosynthetic pigments and attributes in Sahianwala and Knotti ecotypes along with enhanced stomatal attributes are adaptive components of salinity tolerance. Stomatal behavior in connection with physiological modulations points out successful adaptation of these ecotypes. Such anatomical and physiological indices as observed in these ecotypes are best indicators of survival. The histo-physiological and functional relationship contributed in enhanced capability of Sahianwala and Knotti ecotypes in stress tolerance and survival, which deserves further study at molecular level.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors extend their appreciation to the deanship of scientific research, King Khalid University for funding work through research group program under Grant R.G.P. 2/11/42
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Noreen Akhter and Muhammad Aqeel have contributed equally to this work.
References
- Acosta-Motos JR, Ortuño MF, Bernal-Vicente A, Diaz-Vivancos P, Sanchez-Blanco MJ, Hernandez JA. Plant responses to salt stress: adaptive mechanisms. Agronomy. 2017;7(1):18. [Google Scholar]
- Agarwal PK, Shukla PS, Gupta K, Jha B. Bioengineering for salinity tolerance in plants: state of the art. Mol Biotechnol. 2013;54(1):102–123. doi: 10.1007/s12033-012-9538-3. [DOI] [PubMed] [Google Scholar]
- Ali AM. Nutrient sufficiency ranges in mango using boundary-line approach and compositional nutrient diagnosis norms in El-Salhiya, Egypt. Commun Soil Sci Plant Anal. 2018;49(2):188–201. doi: 10.1080/00103624.2017.1421651. [DOI] [Google Scholar]
- Aqeel M, Khalid N, Tufail A, Ahmad RZ, Akhter MS, Luqman M, Javed MT, Irshad MK, Alamri S, Hashem M, Noman A. Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L.) varieties. Environ Sci Pollut Res. 2021 doi: 10.1007/s11356-021-12579-5. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. Some important physiological selection criteria for salt tolerance in plants. Flora Morphol Distrib Funct Eco Plants. 2004;199(5):361–376. [Google Scholar]
- Cuin TA, Tian Y, Betts SA, Chalmandrier R, Shabala S. Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct Plant Biol. 2009;36(12):1110–1119. doi: 10.1071/FP09051. [DOI] [PubMed] [Google Scholar]
- da Cunha Cruz Y, Scarpa ALM, Pereira MP, de Castro EM, Pereira FJ. Root anatomy and nutrient uptake of the cattail Typha domingensis Pers. (Typhaceae) grown under drought condition. Rhizosphere. 2020;16:100253. [Google Scholar]
- Davies B. Chemistry and biochemistry of plant pigments. Carotenoids. 1976;2:38–165. [Google Scholar]
- de Andrade Silva FK, dos Santos Magalhães C, Sá RD, Carolina F, da Silva L, Randau KP (2020) Anatomical and histochemical study of Sechium edule (Jacq.) Sw. In: Anales de Biología. Servicio de Publicaciones de la Universidad de Murcia, pp 173–181
- Ditta A, Imtiaz M, Mehmood S, Rizwan MS, Mubeen F, Aziz O, Qian Z, Ijaz R, Tu S. Rock phosphate-enriched organic fertilizer with phosphate-solubilizing microorganisms improves nodulation, growth, and yield of legumes. Commun Soil Sci Plant Anal. 2018;49(21):2715–2725. [Google Scholar]
- Franks PJ, Farquhar GD. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiol. 2007;143(1):78–87. doi: 10.1104/pp.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed M, Ashraf M. Physiological and biochemical adaptations of Cynodon dactylon (L.) Pers. from the salt range (Pakistan) to salinity stress. Flora Morphol Distrib Funct Eco Plants. 2008;203(8):683–694. [Google Scholar]
- Hameed M, Ashraf M, Naz N, Al-Qurainy F. Anatomical adaptations of Cynodon dactylon (L.) Pers. from the salt range Pakistan to salinity stress. I. Root and stem anatomy. Pak J Bot. 2010;42(1):279–289. [Google Scholar]
- Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. In: Circular. California agricultural experiment station, vol 347, 2nd edn. College of Agriculture, University of California, Berkeley
- Horie T, Karahara I, Katsuhara M. Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice. 2012;5(1):1–18. doi: 10.1186/1939-8433-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horneck D, Hanson D. Determination of potassium and sodium by flame emission spectrophotometry. In: Karla YP, editor. Handbook of reference methods for plant analysis. Washington: CRC Press; 1998. pp. 153–155. [Google Scholar]
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson ML (1962) Soil chemical analysis-advanced course, vol 497. Dept. of Soil Science, Univ. of Wisconsin Madison, Wisconsin
- Jiang J-L, Tian Y, Li L, Yu M, Hou R-P, Ren X-M. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front Plant Sci. 2019 doi: 10.3389/fpls.2019.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen DA. Plant microtechnique. London: McGraw-Hill Book Company Inc; 1940. p. 530p. [Google Scholar]
- Jordan F, Waugh WJ, Glenn EP, Sam L, Thompson T, Thompson TL. Natural bioremediation of a nitrate-contaminated soil-and-aquifer system in a desert environment. J Arid Environ. 2008;72(5):748–763. [Google Scholar]
- Khalid N, Rizvi ZF, Yousaf N, Khan SM, Noman A, Aqeel M, Latif K, Rafique A. Rising metals concentration in the environment: a response to effluents of leather industries in Sialkot. Bull Environ Contam Toxicol. 2021 doi: 10.1007/s00128-021-03111-z. [DOI] [PubMed] [Google Scholar]
- Khan MA, Gul B (2002) Some ecophysiological aspects of seed germination in halophytes. Halophyte Util Reg Sustain Dev Agric 56–68
- Kim H-Y. Analysis of variance (ANOVA) comparing means of more than two groups. Restor Dent Endod. 2014;39(1):74. doi: 10.5395/rde.2014.39.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JE, Arduin M. Manual básico de métodos em morfologia vegetal (basic manual of methods in plant morphology) Seropedica: Edur Rio de Janeiro; 1997. [Google Scholar]
- Munns R (2002) Salinity, growth and phytohormones. In: Salinity: environment-plants-molecules. Springer, pp 271–290
- Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Naseer M, Hameed M, Zahoor A, Ahmad F, Fatima S, Ahmad MSA, Ahmad KS, Iftikhar M. Photosynthetic response in buttonwood (Conocarpus erectus L.) to salt stress. Pak J Bot. 2017;49(3):847–856. [Google Scholar]
- Naz N, Hameed M, Ashraf M, Al-Qurainy F, Arshad M. Relationships between gas-exchange characteristics and stomatal structural modifications in some desert grasses under high salinity. Photosynthetica. 2010;48(3):446–456. [Google Scholar]
- Negrão S, Schmöckel S, Tester M. Evaluating physiological responses of plants to salinity stress. Ann Bot. 2017;119(1):1–11. doi: 10.1093/aob/mcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilson SE, Assmann SM. The control of transpiration. Insights from Arabidopsis. Plant Physiol. 2007;143(1):19–27. doi: 10.1104/pp.106.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman A, Hameed M, Ali Q, Aqeel M. Foliar tissue architectural diversity among three species of genus Hibiscus for better adaptability under industrial environment. Int J Envrion Sci. 2012;2(4):2212–2222. [Google Scholar]
- Noman A, Aqeel M, Javed M, Zafar S, Ali Q, Islam W, Irshad M, Buriro M, Kanwal H, Khalid N. Histological changes in Hibiscus rosa-sinensis endorse acclimation and phytoremediation of industrially polluted sites. J Anim Plant Sci. 2017;27(5):1637–1648. [Google Scholar]
- Noreen S, Akhter MS, Yaamin T, Arfan M. The ameliorative effects of exogenously applied proline on physiological and biochemical parameters of wheat (Triticum aestivum L.) crop under copper stress condition. J Plant Interact. 2018;13(1):221–230. [Google Scholar]
- Noreen S, Faiz S, Akhter MS, Shah KH. Influence of foliar application of osmoprotectants to ameliorate salt stress in sunflower (Helianthus annuus L.) Sarhad J Agric. 2019;35(4):1316–1325. [Google Scholar]
- Oliveira J, Pereira M, Duarte V, Corrêa F, Castro E, Pereira F. Cadmium tolerance of Typha domingensis Pers. (Typhaceae) as related to growth and leaf morphophysiology. Braz J Biol. 2018;78(3):509–516. doi: 10.1590/1519-6984.171961. [DOI] [PubMed] [Google Scholar]
- OriginLab Corporation (2014) OriginPro 9.1: scientific data analysis and graphing software. Northampton, MA 01060, United States. https://www.originlab.com. Assessed 15 Nov 2020
- Parkhurst DF. Diffusion of CO2 and other gases inside leaves. New Phytol. 1994;126(3):449–479. doi: 10.1111/j.1469-8137.1994.tb04244.x. [DOI] [PubMed] [Google Scholar]
- Phang TH, Shao G, Lam HM. Salt tolerance in soybean. J Integr Plant Biol. 2008;50(10):1196–1212. doi: 10.1111/j.1744-7909.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. Accessed 15 Nov 2020
- Robinson MF, Heath J, Mansfield T. Disturbances in stomatal behaviour caused by air pollutants. J Exp Bot. 1998;49:461–469. [Google Scholar]
- Shabala S, Hariadi Y, Jacobsen S-E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J Plant Physiol. 2013;170(10):906–914. doi: 10.1016/j.jplph.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Steel RG, Torrie JH, Dickey DA. Principles and procedures of statistics. New York: McGraw-Hill; 1997. [Google Scholar]
- Tavakkoli E, Rengasamy P, McDonald GK. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot. 2010;61(15):4449–4459. doi: 10.1093/jxb/erq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B, Kurtz L. Calcium and magnesium determinations by EDTA titrations. Soil Sci Soc Am J. 1961;25(1):27–29. [Google Scholar]
- Tufail A, Aqeel M, Khalid N, Ahsan M, Khalil S, Ahmad F, Hameed M, Noman A, Alamri S, Hashem M. Salt toxicity in a natural habitat induces structural and functional modifications and modulate metabolism in bermuda grass (Cynodon dactylon [L.] Pers.) Appl Ecol Environ Res. 2020;18(5):6569–6588. [Google Scholar]
- Wolf B. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Commun Soil Sci Plant Anal. 1982;13(12):1035–1059. [Google Scholar]
- Wu L. The potential for evolution of salinity tolerance in Agrostis stolonifera L. and Agrostis tenuis Sibth. New Phytol. 1981;89(3):471–486. [Google Scholar]
- Yang H, Zhang X, Wang G. Relationships between stomatal character, photosynthetic character and seed chemical composition in grass pea at different water availabilities. J Agric Sci. 2004;142:675. [Google Scholar]
- Yang G, Li F, Tian L, He X, Gao Y, Wang Z, Ren F. Soil physicochemical properties and cotton (Gossypium hirsutum L.) yield under brackish water mulched drip irrigation. Soil Till Res. 2020;199:104592. [Google Scholar]
- Yoo CY, Pence HE, Hasegawa PM, Mickelbart MV. Regulation of transpiration to improve crop water use. Crit Rev Plant Sci. 2009;28(6):410–431. [Google Scholar]
- Zhu M, Shabala S, Shabala L, Fan Y, Zhou M. Evaluating predictive values of various physiological indices for salinity stress tolerance in wheat. J Agron Crop Sci. 2016;202(2):115–124. [Google Scholar]
- Zulfiqar F, Akram NA, Ashraf M. Osmoprotection in plants under abiotic stresses: new insights into a classical phenomenon. Planta. 2020;251(1):3. doi: 10.1007/s00425-019-03293-1. [DOI] [PubMed] [Google Scholar]
- Zulfiqar F, Younis A, Riaz A, Mansoor F, Hameed M, Akram NA, Abideen Z. Morpho-anatomical adaptations of two Tagetes erecta L. cultivars with contrasting response to drought stress. Pak J Bot. 2020;52(3):801–810. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.