Abstract
Brassinosteroids (BR) play diverse roles in the regulation of plant growth and development. BR promotes plant growth by triggering cell division and expansion. However, the effect of exogenous BR application on the leaf size and expansion of tobacco is unknown. Tobacco seedlings are treated with different concentrations of exogenous 2,4-epibrassinolide (EBL) [control (CK, 0 mol L−1), T1 (0.5 × 10−7 mol L−1), and T2 (0.5 × 10−4 mol L−1)]. The results show that T1 has 17.29% and T2 has 25.99% more leaf area than control. The epidermal cell area is increased by 24.40% and 17.13% while the number of epidermal cells is 7.06% and 21.06% higher in T1 and T2, respectively, relative to control. So the exogenous EBL application improves the leaf area by increasing cell numbers and cell area. The endogenous BR (7.5 times and 68.4 times), auxin (IAA) (4.03% and 25.29%), and gibberellin (GA3) contents (84.42% and 91.76%) are higher in T1 and T2, respectively, in comparison with control. Additionally, NtBRI1, NtBIN2, and NtBES1 are upregulated showing that the brassinosteroid signaling pathway is activated. Furthermore, the expression of the key biosynthesis-related genes of BR (NtDWF4), IAA (NtYUCCA6), and GA3 (NtGA3ox-2) are all upregulated under EBL application. Finally, the exogenous EBL application also upregulated the expression of cell growth-related genes (NtCYCD3;1, NtARGOS, NtGRF5, NtGRF8, and NtXTH). The results reveal that the EBL application increases the leaf size and expansion by promoting the cell expansion and division through higher BR, IAA, and GA3 contents along with the upregulation of cell growth-related genes. The results of the study provide a scientific basis for the effect of EBL on tobacco leaf growth at morphological, anatomical, biochemical, and molecular levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-00971-x.
Keywords: Brassinosteroids, Cell division and expansion, Gene expression, Hormones content, Leaf area and expansion, Tobacco leaf growth
Introduction
In plants, there are diverse studies that signify the role of hormones in the regulation of various processes during plant growth and development (Kumar et al. 2014). The phytohormones play a decisive role in the plant developmental processes (Alabadí et al. 2009). Brassinosteroids (BR) are growth-promoting steroid hormones which participated in various physiological process during the plant life cycle (Cheon et al. 2013). Brassinosteroids regulate germination, root growth, shoot growth, and bring adaptation under abiotic stresses (Steber and McCourt 2001; Müssig et al. 2003; Sirhindi et al. 2009; Divi et al. 2010; Yusuf et al. 2014; Jakubowska and Janicka 2017; Soliman et al. 2020).
Many studies highlighted that BR is involved in various biological processes as the genetic analysis revealed that BR-sensitive mutants (d2) in rice are dwarf, short internodes, erect leaves, and shortened grain (Hong et al. 2003). Clouse et al. (1996) proposed that BRI1 plays a critical role in BR signaling and perception and concluded that Arabidopsis bri1 mutant has many deficiencies like thick leaves of dark green in color, male sterility, and short stature. In rice, the severe mutant of BRI1 (d61-4) also shows phenotypes with short stature and twisted leaves (Nakamura et al. 2006). In Arabidopsis, the BR-insensitive mutants (bin2) are dark green in color, dwarf, and curly leaves (Li et al. 2001). In another study, the rice BR-deficient mutant (brd2) was dwarf, dark green, erect leaves, and shortened leaf sheaths (Hong et al. 2005). Similarly, BZR1 is the main transcription factor in the BR signaling pathway and necessary for anther and pollen development, as quadruple, pentuple, and hextuple bzr mutants were unable to produce seeds (Chen et al. 2019). The BR biosynthesis pathway comprises many genes in which DWF4, CPD, and CYP (P450) are the key enzymes biosynthesis genes (Ohnishi et al. 2012; Si et al. 2016; Zheng et al. 2018). Tanabe et al. (2005) characterized a dwarf and short seeds rice mutant which was associated with BR biosynthesis-related gene CYP724B1 (a novel cytochrome P450). The BR signalling pathway involves several genes in which BRI1 is the receptor kinase which further activated BAK1 and forms the BRI1/BAKI complex by transphosphorylation. Subsequently, phosphorylation of BSK and activation of BSU1, and nuclear accumulation of BZR1 and BES1 transcription factors occur. These transcription factors directly bind to the promoters of BR responsive genes for its regulation (Wang et al. 2006; Kim and Wang 2010).
Furthermore, BR play diverse roles throughout the plant life cycle. The exogenous application of BR significantly improves the growth and development of various crop plants. Foliar spraying of BR hamper leaf senescence, enhanced photosynthesis, number of kernels, improved source and sink capacity, and ultimately yield in maize (Gao et al. 2017). Tong et al. (2014) showed that BR regulates cell elongation in rice via gibberellin metabolism. In another study, BR application increases the plant height and delay senescence in older leaves than young leaves of papaya (de Assis-Gomes et al. 2018). The growth of apple tree is also regulated by foliar application of BR with the integration of auxin and gibberellin (Zheng et al. 2019a, b). Similarly, BR also plays an important role in adaptation and tolerance to various abiotic stresses. The exogenous application of BR confer tolerance to drought stress in maize (Anjum et al. 2011) and pepper plants (Kaya et al. 2019) and temperature stress in Brassica juncea (Kaur et al. 2018). Furthermore, the EBL foliar application also confers salt tolerance via modulation of osmolyte biosynthesis, enzymatic and non-enzymatic antioxidant defense system in apple (Soliman et al. 2020), soybean (Alam et al. 2019; Su et al. 2020), and tomato (Ahanger et al. 2020; Ahmad et al. 2018a, b). Moreover, the exogenous BR application also mitigates the heavy metal toxicity and confer tolerance via the activation of the antioxidant enzyme defense system, higher antioxidant contents, and more proline content accumulation in Brassica juncea (Soares et al. 2020), pepper (Kaya et al. 2020), tomato (Jan et al. 2020), pea (Jan et al. 2018), and chickpea (Ahmad et al. 2018b).
There are few studies in tobacco regarding the role of BR as it promotes seed germination (Leubner-Metzger 2001), confers tolerance to various abiotic stresses in Nicotiana benthamiana (Deng et al. 2015), enhance resistance to tobacco mosaic virus in Nicotiana benthamiana (Deng et al. 2016), and finally also enhance resistance in Nicotiana tabacum to a broad range of diseases (Nakashita et al. 2003). Leaves are central to the plant’s function. Leaves are initiated from the flanks of the shoot apical meristem and its development is flexible and adjustable according to species, developmental stage, and environmental responses which are regulated by various hormones, transcriptional regulators, and mechanical properties of the tissue (Bar and Ori 2014). The tobacco leaf axis arises from approximately 100 cells in the shoot apex and the pattern of cell growth in the lamina is complex (Poethig and Sussex 1985). For stem elongation and cambial proliferation in leaf is essential for GA accumulation and to send a mobile signal to the stem (Dayan et al. 2012). Therefore, a deeper understanding of leaf growth and development contributes to the overall comprehension of plant biology, and this understanding can be used to improve crop production. At present, keeping in view the role of BR, no such study has been done in the Nicotiana tabacum to elucidate the role of exogenously applied brassinosteroids on leaf growth and development. Therefore, this study is designed to clarify the role of exogenous EBL on tobacco leaf growth at morphological, biochemical, anatomical, and molecular levels.
Methods
Plant materials, growth conditions, and EBL treatment
Tobacco (Nicotiana tabacum) K326 variety was used in this study. This variety was selected by Northup King Seed Company (USA) and introduced by the Yunnan Branch of China Tobacco Company. K326 variety was kept under the national serial number “00002266” after the DUS test and certification from the National Tobacco Variety Certification Committee. This variety was provided by the National Infrastructure for Crop Germplasm Resources (Qingdao, China) as a test material to explore the role of brassinosteroids in the tobacco seedling leaf area and expansion. The 2,4-epibrassinolide (EBL) was purchased from Solarbio Science and Technology Company Limited (Beijing, China). The EBL was applied as, T1 (0.5 × 10−7 mol L−1), T2 (0.5 × 10−4 mol L−1) along with a control (CK, 0 mol L−1). The control plants were sprayed with ddH2O. After germination, the seedlings were planted in 5 cm × 7 cm (diameter × depth) plastic pots containing peat and vermiculite (V/V, 1:1) with one seedling per pot. The seedlings were held in a greenhouse under normal conditions (26 °C with 16 h light, 24 °C with 8 h dark, and 70% relative humidity). The tobacco seedlings grew to the four fully expanded leaves (length > 5 cm) and one new leaf (length < 1 cm) stage was used in this study. A total of 24 seedlings with no disease and pests and uniform growth were selected for each treatment. The EBL was sprayed once a day at 16:00 on the adaxial side of the leaves for consecutive five days. The sketch of the entire experimental setup is portrayed in Fig. 1.
Fig. 1.
Setup of the experiment. This schematic flow shows a brief introduction to the entire experiment from start to end. 2,4-epibrassinolide (EBL) was exogenously applied to the leaves of tobacco seedlings with different concentrations. CK symbolizes control (spraying of seedlings with ddH2O); T1 represents seedlings sprayed with EBL of 0.5 × 10−7 mol L−1 concentration; T2 denotes seedlings sprayed with EBL of 0.5 × 10−4 mol L−1 concentration; DAS shows days after sowing
Measurement of leaf morphological parameters
After exogenous EBL application for five days, ten seedlings with uniform growth were selected from each treatment. The fifth individual leaf of each seedling was excised with a razor blade. Leaf digital images were acquired by the camera (Nikon SMZ1000, Japan) and the leaf length, leaf width, and leaf area were measured using ImageJ software (http://rsbweb.nih.gov/ij/).
Scanning electron microscopy
Samples from the fifth leaf of seedlings from different treatments were detached and fixed in formalin acetic acid alcohol (FAA) solution [5% (v/v) formalin, (5% (v/v) acetic acid, and 70% (v/v) ethanol)] for 1 h. Following a brief rinse in 0.1 M sodium phosphate solution (PBS) (pH 7.4) (Servicebio, Beijing, China), the leaves were post-fixed by washing with 0.1 M PBS (pH 7.4) for 3 times, 15 min each. Then the leaf samples were transferred into 1% OsO4 (Ted Pella Inc. CA, USA) in 0.1 M PBS (pH 7.4) for 1–2 h at room temperature. After that, the samples were dehydrated in an ethanol series (30, 50, 70, 80, 90 and 95%) for 15 min at each gradation. Similarly, the samples were also dehydrated two times with 100% ethanol and in isoamyl acetate (Sigma, USA) for 15 min. The dehydrated samples were dried in a critical point dryer (Quorum, K850, England) with liquid CO2 as transitional fluid, after which the samples were sputter-coated with gold and examined in a scanning electron microscope (HITACHI, SU8100, Japan) (Kim et al. 2003). The area of the epidermal cells was determined using Image J software (http://rsbweb.nih.gov/ij/). Cell numbers were calculated by the total leaf area and the average cell number in 4.9152 mm2.
BR, IAA, and GA3 hormone contents
On the third day of EBL treatment, the fifth positioned-leaf from each treatment was collected and immediately frozen in liquid nitrogen and store at − 80 °C. Endogenous hormone content was extracted and purified using the modified procedure as described by Dobrev and Kamínek (2002). Approximately one g of fresh leaf weight for IAA and GA3 measurement and 2 g for BR measurement was firstly frozen in liquid nitrogen, grounded into fine powder. BRs were extracted with 80% methanol solution (4 °C) for 2 h and purified by Bond Elut column (Agilent Company, Beijing, China) and Strata-X column (Phenomenon Company, Cambridgeshire, England). Then the remaining methanol phase was evaporated to dryness by N2 and the residue was dissolved in methanol (200 µL). IAA and GA3 were extracted with 2-propanol/H2O/concentrated HCl extraction buffer (2:1:0.002, v/v/v, 10 mL) and internal standard solution (8 µL, 4 °C) vibrate for 30 min, and dissolved in 400 µL methanol (0.1% formic acid). The sample solution (2 µL) was injected into the reverse-phase C18 Gemini HPLC column for HPLC–ESI–MS/MS (Qtrap 6500, AB, USA) quantitative analysis (Ding et al. 2013). Hormone levels were measured using a high-performance liquid chromatography system. An external standard was used for quantitative analysis. BR, IAA, and GA3 standards were purchased from Sigma (USA).
Real time-qPCR (RT-qPCR) analysis
We examined BR signaling pathway-related genes, BR, IAA, GA3 biosynthesis-related genes in response to exogenous EBL application. Additionally, the expression levels of cell size- and cell division-related genes were also analyzed in leaves after EBL treatments. The National Centre for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/) for cDNA sequences of Arabidopsis thaliana genes were used as queries to identify tobacco homologs via a BLASTN search using e-value as < 1e − 5 of the NCBI. Only homologs with high scores, percentage nucleotide identity to the gene of interest, and high coverage of the query sequence were considered. The genes-specific primers were designed using Primer Premier 5.0. The gene-specific primers detail were provided in Additional file 1 (Table S1) and NtActin was used as the internal reference gene (Li et al. 2018). After 24 h of EBL treatments, the total RNA was extracted from the samples using MiniBEST Plant RNA Extraction Kit (TaKaRa, Shiga, Japan), according to the manufacturer's instructions. The concentration of the RNA samples was determined using the NanoPhotometer (IMPLEN, CA, USA) and its quality was evaluated on an agarose gel. The first-strand cDNA was synthesized from 1.0 µg of total RNA using the Prime Script 1st Strand cDNA Synthesis kit (TaKaRa, Shiga, Japan) following the manufacturer guidelines. The reaction volume was 20 µL, including cDNA 1 µL, SYBR RT-qPCR master mix 10 µL (TaKaRa, Shiga, Japan), forward primer 0.4 µL, reverse primer 0.4 µL, and ddH2O 8.2 µL. The relative expression of each gene was analyzed using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Statistical analysis
Statistical analysis was carried out using one-way ANOVA in SPSS 16.0 (SPSS Inc., Chicago, Ill., USA) and an LSD test (p < 0.05) was used to identify the significant differences in leaf dimensional parameters, cell size and cell area, hormones content, and gene expression under different EBL concentration treatments. Figures were generated using OriginPro 9.1 (OriginLab Corporation, Northampton, MA, USA).
Results
Changes in leaf morphology
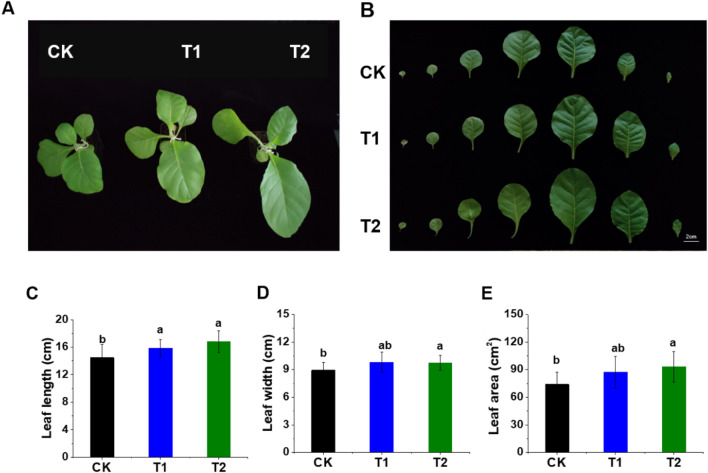
Exogenous application of EBL with different concentrations produces a prominent effect on tobacco seedlings and exhibit better growth performance than control (Fig. 2A). The leaf length, leaf width, and leaf area of the fifth leaf are significantly increased in response to EBL as compared with the control. The leaf is 9.47% and 16% longer in T1 and T2 compare with control, respectively (Fig. 2B & C), while it is 9.5% and 8.05% wider in T1 and T2 in comparison with control, respectively (Fig. 2B & D). Similarly, T1 results in 17.29% while T2 has 25.99% more leaf area compared with the control (Fig. 2B & E). Overall, the results show that the exogenous application of EBL promoted the leaf area via increment in the leaf length and width.
Fig. 2.
Effect of exogenous application of 2,4-epibrassinolide (EBL) on leaf growth and morphology. Growth performance under various concentrations of EBL along with a control (A and B). Leaf length, leaf width, and leaf area after EBL treatments (C, D and E, respectively). CK represents control seedlings sprayed with ddH2O, T1 represents seedlings sprayed with EBL of 0.5 × 10−7 mol L−1 concentration, and T2 represents seedlings sprayed with EBL of 0.5 × 10−4 mol L−1 concentration. Values represent the mean ± standard deviation (SD). Different letters indicate significant differences (LSD test; p < 0.05)
Changes in leaf anatomy
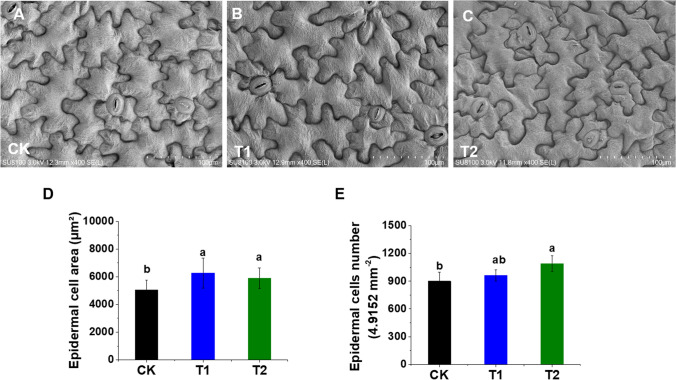
Leaf size is determined by cell proliferation and cell enlargement. The anatomical analysis reveals that exogenous EBL applications significantly affected the leaf structure (Fig. 3). The leaf epidermal cells are larger and more in number in the EBL-treated seedlings relative to control (Fig. 3A–C). 24.40% and 17.13% increment is observed in the epidermal cell area of T1 and T2, respectively, in comparison with control (Fig. 3D). Similarly, the number of epidermal cells is also increased with a 7.06% and 21.06% rise in T1 and T2, respectively, compared with control (Fig. 3E). In conclusion, the EBL application promoted the leaf area by an increase in the area and the number of epidermal cells.
Fig. 3.
Leaf structure changes in response to exogenous application of 2,4-epibrassinolide. Representative scanning electron microscopic images of the fifth leaf of control and EBL-treated seedlings (A, B and C). Epidermal cell area (D) and epidermal cell numbers (calculated in 4.9152 mm2) (E) in response to exogenous application of EBL. CK represents control seedlings sprayed with ddH2O, T1 represents seedlings sprayed with EBL of 0.5 × 10−7 mol L−1 concentration, and T2 represents seedlings sprayed with EBL of 0.5 × 10−4 mol L−1 concentration. Values represent the mean ± SD. Different letters indicate significant differences (LSD test; p < 0.05)
Changes in the hormones content
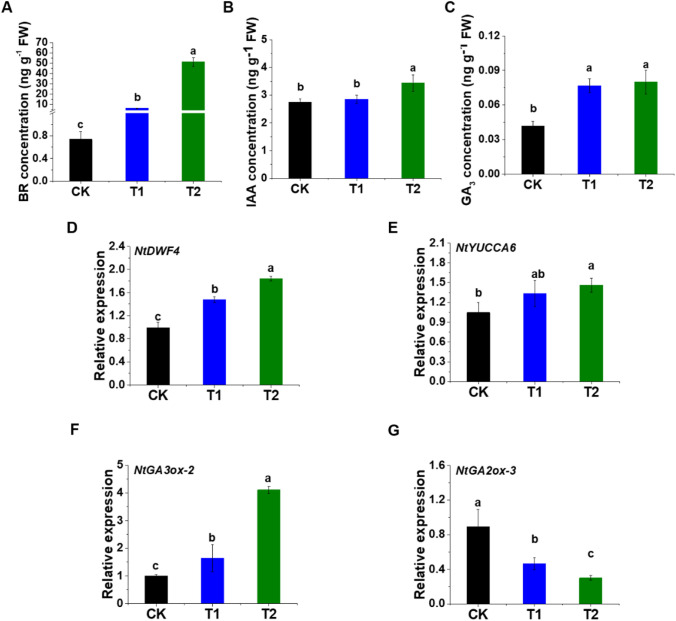
After EBL treatment, the various hormones like BR, IAA, and GA3 content are significantly affected and increased gradually with the increase in its concentration (Fig. 4). The BR content is 7.5 times and 68.4 times more in T1 and T2, respectively, in comparison with control (Fig. 4A). The IAA content is also elevated in the EBL-treated seedlings with 4.03% and 25.29% increment in T1 and T2, respectively, compared with control (Fig. 4B). Similarly, an 84.42% and 91.76% increase in the content of GA3 is observed in T1 and T2, respectively, compared with control (Fig. 4C).
Fig. 4.
The endogenous hormones content and its biosynthesis-related genes expression in response to EBL spraying. Brassinosteroids (BR) concentration (A), auxin (IAA) concentration (B), and gibberellin (GA3) concentration (C) under various EBL concentrations along with a control. NtDWF4 (BR biosynthesis gene) (D), NtYUCCA6 (IAA biosynthesis gene) (E), NtGA3-ox2 (GA3 biosynthesis gene) (F), and NtGA2-ox3 (GA3 inhibitor gene) (G) differentially expressed under various EBL treatments. Values represent mean ± SD. Different letters indicate significant differences (LSD test; p < 0.05). CK represents control seedlings sprayed with ddH2O, T1 represents seedlings sprayed with EBL of 0.5 × 10−7 mol L−1 concentration, and T2 represents seedlings sprayed with EBL of 0.5 × 10−4 mol L−1 concentration
BR, IAA, and GA3 biosynthesis-related gene expression
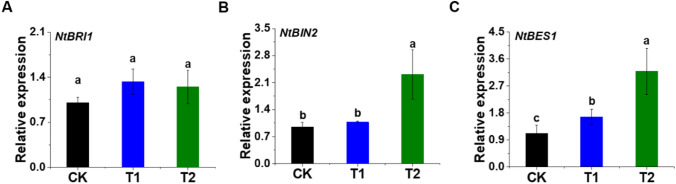
In this study, the BR synthesis- and signal transduction-related genes, IAA and GA3 biosynthesis-related genes are investigated in tobacco seedlings after exogenous EBL application (Figs. 4 and 5). As shown in Fig. 5A, the BR signalling genes, NTBRI1 expression is found non-significant while the transcript levels of NtBIN2 and NTBES1 are significantly increased in response to EBL application. NtBIN2 shows an approximately 1.4 fold change higher expression in T2 compared with control (Fig. 5B). Similarly, NtBES1 shows 0.5 and 1.8 fold change expression in T1 and T2, respectively, relative to control (Fig. 5C). The BR, IAA, and GA3 biosynthesis-related genes like NtDWF4, NtYUCCA6, and NtGA3ox-2, respectively, show a rise in their transcript levels in response to EBL application (Fig. 4D–F) while the response observed in the NtGA2ox-3 gene expression is opposite (Fig. 4G).
Fig. 5.
Differential regulation of BR signaling pathway-related genes under the exogenous EBL application. Expression levels of the corresponding genes-related to the BR signaling pathway are NtBRI1 (A), NtBIN2 (B), and NtBES1 (C). Values represent mean ± SD. Different letters indicate significant differences (LSD test; p < 0.05). CK represents control seedlings sprayed with ddH2O, T1 represents seedlings sprayed with EBL of 0.5 × 10−7 mol L−1 concentration, and T2 represents seedlings sprayed with EBL of 0.5 × 10−4 mol L−1 concentration
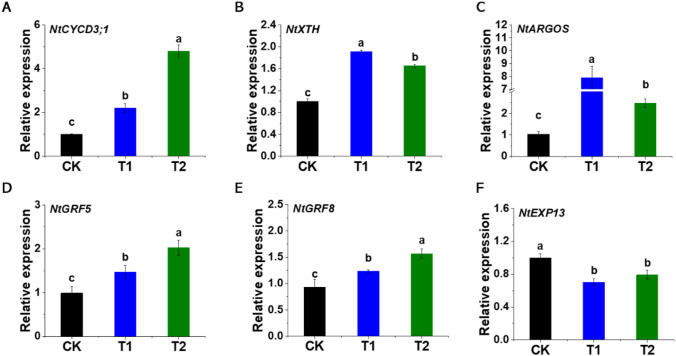
Cell division- and cell expansion-related gene expression
The cell area and cell numbers are increased in response to the EBL application. Therefore, to know the molecular mechanism related to enhancement in the cell area and number, we seek to analyze the expression levels of cell division- and cell expansion-related genes in response to exogenous EBL application. The expression of NtCYCD3;1, NtXTH, NtARGOS, NtGRF5, and NtGRF8 are significantly higher in EBL-treated than in control tobacco seedlings (Fig. 6A–E). The transcript levels of NtXTH and NtARGOS are more in T1 while the expression levels of NtCYCD3;1, NtGRF5, and NtGRF8 are higher in T2 treatment (Fig. 6A, D & E). NtXTH and NtARGOS gene expression are notably increased in T1 by about 0.9- and 6.6-time, respectively, while NtCYCD3;1, NtGRF5, and NtGRF8 gene expression are distinctly higher in T2 by approximately 3.8-, 1-, and 0.7-time, respectively. Overall, the results show that exogenous EBL application promoted the expression of cell division- and cell expansion-related genes.
Fig. 6.
EBL application significantly regulates the expression of cell growth-related genes. The differential expression of cell growth-related genes; NtCYCD3;1 (A), NtXTH (B), NtARGOS (C), NtGRF5 (D), NtGRF8 (E), and NtEXP13 (F) are presented relative to the control samples. Different letters above vertical bars indicate significant differences using the LSD test at p < 0.05. CK represents control seedlings sprayed with ddH2O, T1 represents seedlings sprayed with EBL of 0.5 × 10−7 mol L−1 concentration, and T2 represents seedlings sprayed with EBL of 0.5 × 10−4 mol L−1 concentration
Discussion
Brassinosteroids are the steroidal class hormones that play decisive roles in various biological processes during the plant life cycle (Saini et al. 2015; Planas-Riverola et al. 2019). Earlier studies confirm that BR regulates various developmental processes like germination (Steber and McCourt 2001), root growth and development (Müssig et al. 2003; Wei and Li 2016; Lv et al. 2018; Li et al. 2020), organ boundary formation in the shoot apical meristem (Gendron et al. 2012), root meristem development (Li et al. 2020), cell division and cell expansion (Nakaya et al. 2002; Zhiponova et al. 2013), stomatal opening (Inoue et al. 2017), and floral induction (Li et al. 2010).
EBL activates BR signaling pathway
Previously, it is well-documented that BR played crucial roles to improve plant growth and development in various plant species (Que et al. 2017; Zheng et al. 2019a, b; Trevisan et al. 2020) and it is important to know the BR perception and signalling in plants. BRI1, BIN2, and BES1 genes are the key components of the BR signalling pathway which regulated the bulk number of genes (Ye et al. 2011). The BR directly binds to its receptor BRI1 at the cell membrane through an extracellular domain (Wang et al. 2001). The BRI1 physically interacts with another receptor kinase BAK1 and activates their kinase activity via transphosphorylation (Chinchilla et al. 2009; Nam and Li, 2002). The formation of the BRI1-BAK1 complex initiates the BR signalling (Wang et al. 2014). The signal is then transduced from the cell membrane to the cytoplasm via the activation BRI1-BAK1 complex and indirectly regulates the activity of BIN2 which then directly regulates the phosphorylation of BZR1 and BES1 transcription factors and subsequently BR-responsive gene expression (Wang et al. 2006). Overexpressed SlBRI1 lines enhance the BR signalling thereby increase the germination rate, plant height, and flower size (Nie et al. 2017). BIN2, a BR signalling component that is involved in the regulation of pavement cells and leaf growth via its involvement in mediating the stabilization of microtubules (Liu et al. 2018a, b). Yin et al. (2002) show that the bes1-D bri1-119 double mutants possess long and bending petioles with curly leaves formation. Similarly, Saito et al. (2018) demonstrate that BES1 is involved in the regulation of xylem and phloem cell differentiation from vascular stem cells. The expression levels of these genes are significantly enhanced by the exogenous EBL application, proposing that they can elevate the endogenous levels of BR and signaling activity to promote tobacco leaf growth and development.
EBL enhances the endogenous BR, IAA, and GA3 hormone contents and its biosynthesis-related gene expression
The three main hormones auxin, brassinosteroids, and gibberellin play important roles in plant growth in general while specifically in cell elongation (Depuydt and Hardtke 2011). In Arabidopsis, the BR, auxin and gibberellin regulate the hypocotyl elongation. The cell elongation in the hypocotyl region is also regulated by the signalling network of BR, auxin and ethylene phytohormones which integrate the light and hormone signal via BZR-ARF-PIF/DELLA-ERF module (Liu et al. 2018a, b). The endogenous BR contents increase in response to the EBL application which is supported by the up-regulation of its biosynthesis-related gene NtDWF4. In this study, the EBL application enhances the IAA content as it is also witnessed by the up-regulation of NtYUCCA6, the biosynthesis gene of IAA. These results are in line with (Mao et al. 2017) in terms of the elevated IAA content due to the exogenous EBL application. Similarly, exogenous EBL greatly induces the expression of NtGA3ox-2 and ultimately enhances the GA3 contents and suppresses the expression of NtGA2ox-3 which is the inactivation gene of GA3 (He et al. 2019), thus enhances its synthesis and accumulation and inhibits its deactivation. The results of this study are supported by Tong et al. (2014) which demonstrate that BR modulates the GA metabolism by targeting D18/GA3ox-2 to promote cell expansion in the coleoptile elongation of rice. Similarly, Unterholzner et al. (2015) show that BR is a master regulator of GA biosynthesis. The expression of GA20ox1 in a BR signalling deficient mutant (bri1-301) restore GA biosynthesis and other developmental process. Other studies also find higher content of GA3 which is induced by brassinosteroid application (Que et al. 2018). The final size of the leaf is strongly controlled by cell division and cell elongation via independently different pathways by analyzing various hormone levels, transcriptomes, and metabolomes (Gonzalez et al. 2010). Similarly, the size and shape of the leaves are also dependent upon the Spatio-temporal regulation of cell division and expansion (Kalve et al. 2014). Hormones regulate the final size of the organ by crosstalk synergistically or antagonistically to stimulate cell proliferation (Wolters and Jürgens 2009). Previous studies have shown that exogenous application of EBL promoted the leaf area of Coleus (Swamy and Rao 2011), mung bean under nickel toxicity (Yusuf et al. 2014), and under normal and high-temperature conditions in Leymus chinensis (Niu et al. 2016). Yuan et al. (2012) have also shown a promotion effect of exogenous EBL application on a leaf under salt stress in cucumber. The results of this study show an increase in the epidermal cell numbers, area, and ultimately leaf area under exogenous EBL application as BR is required for cell elongation and cell division (Azpiroz et al. 1998; Nolan et al. 2020; Oh et al. 2020). Nakaya et al. (2002) also demonstrate that the BR application restores cell proliferation and cell expansion in brassinosteroid-related mutants. Auxin is involved in plant shaping architecture by regulating cell division and elongation (Jiang et al. 2020). GA enhances cell elongation in the leaf sheath of the dwarf mutant of rice (Matsukura et al. 1998) while it also enhances the coleoptile length and seedling leaf one length and area in Rht12 dwarf bread wheat lines (Chen et al. 2014). The results of this study in terms of higher endogenous gibberellin and auxin levels are congruent with (Talarek-Karwel et al. 2020). Similarly, Sun et al. (2020) results are also analogous to our study which have higher auxin and GA3 contents under foliar application of BR. BR and GA coordinate to regulate plant growth and development and controlling cell elongation through mediating crosstalk between them (Li and He 2013). Kozuka et al. (2010) also notice crosstalk between auxin and brassinosteroid to promote petiole elongation via cell elongation to shade stimulus in Arabidopsis. The growth of the apple tree is also regulated by the interactions of auxin, gibberellin, and brassinosteroid which further activate their signaling pathways and cell growth-related gene expression (Zheng et al. 2019a, b). In the light of the above reports, we speculate that exogenous application of EBL promotes the tobacco leaf area along with increment in cell numbers and cell area might be due to higher endogenous BR, IAA and GA3 contents and its biosynthesis related-genes and further regulate the cell growth-related genes expression.
EBL induces cell growth-related genes
Plant development is controlled by environmental and endogenous signals, BR and GA signaling pathways involve in cell elongation are supported by physiological and molecular mechanisms (Gallego-Bartolomé et al. 2012). Similarly, auxin and brassinosteroids are also involved in both cell expansion and proliferation which is confirmed at physiological levels and might be at molecular levels via the involvement of the upstream connection of calcium–calmodulin and phosphoinositide signaling in both hormones signaling pathways (Hardtke et al. 2007). Goda et al. (2002) provide a comprehensive view of cell elongation and cell wall organization-related genes induced by BR in Arabidopsis. Miyazawa et al. (2003) also speculate that BR is involved in the regulation of genes related to the cell cycle and its multiplication in tobacco BY-2 cells. Xu et al. (2020) demonstrate that brassinosteroid controls the expression of XTH19 and XTH23 via BES1, as it acts directly upstream of these genes and regulates the lateral root development in Arabidopsis under salt stress. Analogous to our results, Zheng et al. (2019a, b) also show that exogenous application of EBL significantly increases the expression levels of CYCD, XTH, and GRF genes in apple plants. ARGOS-LIKE (ARL) gene is involved in cell expansion during organogenesis in Arabidopsis by mediating BR-related cell expansion signals, as it acts downstream of BRI1 (Hu et al. 2006). In another study, the ARGOS gene is also involved in cell proliferation during organ growth which might induce auxin signaling pathway. Overexpression of ARGOS gene increases the organ size in Arabidopsis and also its ectopic expression induces higher expression of CYCD3;1 which show that the organ size increase is due to cell proliferation (Hu et al. 2003). It is well established that brassinosteroids and auxin are the key regulators in plant growth and induce morphogenesis (Zheng et al. 2019a, b). As it is mentioned above that CYCD3;1 and ARGOS genes are mediated by both auxin and BR signaling pathways, so in this study the exogenous application results in higher CYCD3;1 and ARGOS genes expression as well as higher IAA levels indicating crosstalk between them and helps in promoting leaf area. The transcript levels of cell growth-related genes (NtCYCD3;1, NtXTH, NtARGOS, NtGRF5, and NtGRF8) are increased in response to exogenous EBL application with different concentrations, thus imply that BR promoted the expression of these genes which results in an increment in cell numbers, cell area, and finally the leaf area is increased.
Conclusions
Our study provides a systematic analysis of the tobacco leaf growth at morphological, biochemical, anatomical, and molecular levels in response to exogenous application of EBL. Based on the current study reports (Fig. 7), it is concluded that (i) EBL application improves the tobacco seedlings growth by having a greater leaf area. (ii) EBL application promotes the leaf area by an increase in the epidermal cell numbers and cell area. (iii) The BR signaling pathway is activated and higher endogenous BR, IAA, and GA3 hormone contents are biosynthesized in response to the EBL application. (iv) Exogenous EBL application enhances the cell division and expansion via the up-regulation of cell growth-related genes. Overall, these results suggested that the exogenous EBL application promoted the tobacco leaf growth and its expansion by modulating cell division and expansion via higher endogenous BR, IAA, and GA3 hormones content and regulation of cell growth-related gene expression.
Fig. 7.
Promotion effect of the exogenous 2,4-epibrassinolide application on tobacco leaf growth and expansion. This schematic model shows the proposed mechanism of the involvement of EBL in growth and expansion from a morphological level to cellular and molecular levels
Supplementary Information
Below is the link to the electronic supplementary material.
Authors Contribution
XM conceived, designed, and supervised the experiment. JZ, YZ, and RK performed the experiments, finalized the figures and tables and their interpretation, and wrote the manuscript. XW and LZ contributed to qRT-PCR studies; NX and SD took part in anatomical studies. All authors read and approved the final manuscript. Juan Zhang, Yan Zhang, and Rayyan Khan contributed equally to this work.
Funding
This work was supported by the Central Public-Interest Scientific Institution Basal Research Fund (No. Y2019PT13) and the Agricultural Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (ASTIP-TRIC03).
Declaration
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juan Zhang, Yan Zhang and Rayyan Khan contributed equally to this work.
References
- Ahanger MA, Mir RA, Alyemeni MN, Ahmad P. Combined effects of brassinosteroid and kinetin mitigates salinity stress in tomato through the modulation of antioxidant and osmolyte metabolism. Plant Physiol Biochem. 2020;147:31–42. doi: 10.1016/j.plaphy.2019.12.007. [DOI] [PubMed] [Google Scholar]
- Ahmad P, Abd Allah EF, Alyemeni MN, Wijaya L, Alam P, Bhardwaj R, Siddique KHM. Exogenous application of calcium to 24-epibrassinosteroid pre-treated tomato seedlings mitigates NaCl toxicity by modifying ascorbate–glutathione cycle and secondary metabolites. Sci Rep. 2018;8:1–15. doi: 10.1038/s41598-018-31917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P, Ahanger MA, Egamberdieva D, Alam P, Alyemeni MN, Ashraf M. Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J Plant Growth Regul. 2018;37:309–322. doi: 10.1007/s00344-017-9730-6. [DOI] [Google Scholar]
- Alabadí D, Blázquez MA, Carbonell J, Ferrándiz C, Pérez-Amador MA. Instructive roles for hormones in plant development. Int J Dev Biol. 2009;53:1597–1608. doi: 10.1387/ijdb.072423da. [DOI] [PubMed] [Google Scholar]
- Alam P, Albalawi TH, Altalayan FH, Bakht MA, Ahanger MA, Raja V, Ashraf M, Ahmad P. 24-epibrassinolide (EBR) confers tolerance against NaCl stress in soybean plants by up-regulating antioxidant system, ascorbate-glutathione cycle, and glyoxalase system. Biomolecules. 2019;9:640. doi: 10.3390/biom9110640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou CM. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J Agron Crop Sci. 2011;197:177–185. doi: 10.1111/j.1439-037X.2010.00459.x. [DOI] [Google Scholar]
- Azpiroz R, Wu Y, Locascio JC, Feldmann KA. An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Ori N. Leaf development and morphogenesis. Development (Cambridge) 2014;141:4219–4230. doi: 10.1242/dev.106195. [DOI] [PubMed] [Google Scholar]
- Chen L, Hao L, Condon AG, Hu YG. Exogenous GA3 application can compensate the morphogenetic effects of the GA-responsive dwarfing gene Rht12 in bread wheat. PLOS ONE. 2014;9:e86431. doi: 10.1371/journal.pone.0086431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LG, Gao Z, Zhao Z, Liu X, Li Y, Zhang Y, Liu X, Sun Y, Tang W. BZR1 family transcription factors function redundantly and indispensably in BR signalling but exhibit BRI1-independent function in regulating anther development in arabidopsis. Mol Plant. 2019;12:1408–1415. doi: 10.1016/j.molp.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Cheon J, Fujioka S, Dilkes BP, Choe S. Brassinosteroids regulate plant growth through distinct signaling pathways in Selaginella and Arabidopsis. PLOS ONE. 2013;8:1–9. doi: 10.1371/journal.pone.0081938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. One for all: the receptor-associated kinase BAK1. Trends Plant Sci. 2009;14:535–541. doi: 10.1016/j.tplants.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse SD, Langford M, McMorris TC. A brassinosteroid-lnsensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996;111:671–678. doi: 10.1104/pp.111.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan J, Voronin N, Gong F, Sun TP, Hedden P, Fromm H, Aloni R. Leaf-induced gibberellin signalling is essential for internode elongation, cambial activity, and fiber differentiation in tobacco stems. Plant Cell. 2012;24:66–79. doi: 10.1105/tpc.111.093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis-Gomes M, Pinheiro DT, Bressan-Smith R, Campostrini E. Exogenous brassinosteroid application delays senescence and promotes hyponasty in Carica papaya L. leaves. Theor Exp Plant Physiol. 2018;30:193–201. doi: 10.1007/s40626-018-0114-5. [DOI] [Google Scholar]
- Deng XG, Zhu T, Zhang DW, Lin HH. The alternative respiratory pathway is involved in brassinosteroid-induced environmental stress tolerance in Nicotiana benthamiana. J Exp Bot. 2015;66:6219–6232. doi: 10.1093/jxb/erv328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng XG, Zhu T, Peng XJ, Xi DH, Guo H, Yin Y, Zhang DW, Lin HH. Role of brassinosteroid signaling in modulating Tobacco mosaic virus resistance in Nicotiana benthamiana. Sci Rep. 2016;6:1–14. doi: 10.1038/srep20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt S, Hardtke CS. Hormone signalling crosstalk in plant growth regulation. Curr Biol. 2011;21:R365–R373. doi: 10.1016/j.cub.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Ding J, Mao LJ, Wang ST, Yuan BF, Feng YQ. Determination of endogenous brassinosteroids in plant tissues using solid-phase extraction with double layered cartridge followed by high-performance liquid chromatography-tandem mass spectrometry. Phytochem Anal. 2013;24:386–394. doi: 10.1002/pca.2421. [DOI] [PubMed] [Google Scholar]
- Divi UK, Rahman T, Krishna P. Berseaarschs airnticolesteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010;10:1–14. doi: 10.1186/1471-2229-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev PA, Kamínek M. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr A. 2002;950:21–29. doi: 10.1016/S0021-9673(02)00024-9. [DOI] [PubMed] [Google Scholar]
- Gallego-Bartolomé J, Minguet EG, Grau-Enguix F, Abbas M, Locascio A, Thomas SG, Alabadí D, Blázquez MA. Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc Natl Acad Sci USA. 2012;109:13446–13451. doi: 10.1073/pnas.1119992109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Liang XG, Zhang L, Lin S, Zhao X, Zhou LL, Shen S, Zhou SL. Spraying exogenous 6-benzyladenine and brassinolide at tasseling increases maize yield by enhancing source and sink capacity. Field Crop Res. 2017;211:1–9. doi: 10.1016/j.fcr.2017.05.027. [DOI] [Google Scholar]
- Gendron JM, Liu JS, Fan M, Bai MY, Wenkel S, Springer PS, Barton MK, Wang ZY. Brassinosteroids regulate organ boundary formation in the shoot apical meristem of Arabidopsis. Proc Natl Acad Sci USA. 2012;109:21152–21157. doi: 10.1073/pnas.1210799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Shimada Y, Asami T, Fujioka S, Yoshida S. Microarray analysis of brassinosteroid-regulated genes in arabidopsis. Plant Physiol. 2002;130:1319–1334. doi: 10.1104/pp.011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, de Bodt S, Sulpice R, Jikumaru Y, Chae E, Dhondt S, van Daele T, de Milde L, Weigel D, Kamiya Y, Stitt M, Beemster GTS, Inzé D. Increased leaf size: different means to an end. Plant Physiol. 2010;153:1261–1279. doi: 10.1104/pp.110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Dorcey E, Osmont KS, Sibout R. Phytohormone collaboration: zooming in on auxin-brassinosteroid interactions. Trends Cell Biol. 2007;17:485–492. doi: 10.1016/j.tcb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- He H, Liang G, Lu S, Wang P, Liu T, Ma Z, Zuo C, Sun X, Chen B, Mao J. Genome-wide identification and expression analysis of GA2ox, GA3ox, and GA20ox are related to gibberellin oxidase genes in grape (Vitis vinifera L.) Genes. 2019;10:1–21. doi: 10.3390/genes10090680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Ashikari M, Kitano H, Matsuoka M. A Rice Brassinosteroid-deficient mutant, ebisu dwarf (d2), Is caused by a loss of function of a new member of cytochrome P450. Plant Cell. 2003;15:2900–2910. doi: 10.1105/tpc.014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell. 2005;17:2243–2254. doi: 10.1105/tpc.105.030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Xie Q, Chua NH. The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell. 2003;15:1951–1961. doi: 10.1105/tpc.013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Poh HM, Chua NH. The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. Plant J. 2006;47:1–9. doi: 10.1111/j.1365-313X.2006.02750.x. [DOI] [PubMed] [Google Scholar]
- Inoue SI, Iwashita N, Takahashi Y, Gotoh E, Okuma E, Hayashi M, Tabata R, Takemiya A, Murata Y, Doi M, Kinoshita T, Shimazaki KI. Brassinosteroid involvement in Arabidopsis thaliana stomatal opening. Plant Cell Physiol. 2017;58:1048–1058. doi: 10.1093/pcp/pcx049. [DOI] [PubMed] [Google Scholar]
- Jakubowska D, Janicka M. The role of brassinosteroids in the regulation of the plasma membrane H+-ATPase and NADPH oxidase under cadmium stress. Plant Sci. 2017;264:37–47. doi: 10.1016/j.plantsci.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Jan S, Alyemeni MN, Wijaya L, Alam P, Siddique KH, Ahmad P. Interactive effect of 24-epibrassinolide and silicon alleviates cadmium stress via the modulation of antioxidant defense and glyoxalase systems and macronutrient content in Pisum sativum L. seedlings. BMC Plant Biol. 2018;18:1–18. doi: 10.1186/s12870-018-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan S, Noman A, Kaya C, Ashraf M, Alyemeni MN, Ahmad P. 24-Epibrassinolide alleviates the injurious effects of Cr(VI) toxicity in tomato plants: insights into growth, physio-biochemical attributes, antioxidant activity and regulation of Ascorbate-glutathione and glyoxalase cycles. J Plant Growth Regul. 2020;39:1587–1604. doi: 10.1007/s00344-020-10169-2. [DOI] [Google Scholar]
- Jiang Z, Liu D, Wang T, Liang X, Cui Y, Liu Z, Li W. Concentration difference of auxin involved in stem development in soybean. J Integr Agric. 2020;19:953–964. doi: 10.1016/S2095-3119(19)62676-6. [DOI] [Google Scholar]
- Kalve S, Fotschki J, Beeckman T, Vissenberg K, Beemster GTS. Three-dimensional patterns of cell division and expansion throughout the development of Arabidopsis thaliana leaves. J Exp Bot. 2014;65:6385–6397. doi: 10.1093/jxb/eru358. [DOI] [PubMed] [Google Scholar]
- Kaur H, Sirhindi G, Bhardwaj R, Alyemeni MN, Siddique KHM, Ahmad P. 28-homobrassinolide regulates antioxidant enzyme activities and gene expression in response to salt- and temperature-induced oxidative stress in Brassica juncea. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-27032-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya C, Ashraf M, Wijaya L, Ahmad P. The putative role of endogenous nitric oxide in brassinosteroid-induced antioxidant defence system in pepper (Capsicum annuum L.) plants under water stress. Plant Physiol Biochem. 2019;143:119–128. doi: 10.1016/j.plaphy.2019.08.024. [DOI] [PubMed] [Google Scholar]
- Kaya C, Ashraf M, Alyemeni MN, Ahmad P. The role of nitrate reductase in brassinosteroid-induced endogenous nitric oxide generation to improve cadmium stress tolerance of pepper plants by upregulating the ascorbate-glutathione cycle. Ecotoxicol Environ Saf. 2020;196:110483. doi: 10.1016/j.ecoenv.2020.110483. [DOI] [PubMed] [Google Scholar]
- Kim TW, Wang ZY. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- Kim JH, Choi D, Kende H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 2003;36:94–104. doi: 10.1046/j.1365-313X.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- Kozuka T, Kobayashi J, Horiguchi G, Demura T, Sakakibara H, Tsukaya H, Nagatani A. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 2010;153:1608–1618. doi: 10.1104/pp.110.156802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Khurana A, Sharma AK. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J Exp Bot. 2014;65:4561–4575. doi: 10.1093/jxb/eru277. [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G. Brassinosteroids and gibberellins promote tobacco seed germination by distinct pathways. Planta. 2001;213:758–763. doi: 10.1007/s004250100542. [DOI] [PubMed] [Google Scholar]
- Li QF, He JX. Mechanisms of signaling crosstalk between brassinosteroids and gibberellins. Plant Signal Behav. 2013;8:e24686. doi: 10.4161/psb.24686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nam KH, Vafeados D, Chory J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 2001;127:14–22. doi: 10.1104/pp.127.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li Y, Chen S, An L. Involvement of brassinosteroid signals in the floral-induction network of Arabidopsis. J Exp Bot. 2010;61:4221–4230. doi: 10.1093/jxb/erq241. [DOI] [PubMed] [Google Scholar]
- Li W, Li X, Chao J, Zhang Z, Wang W, Guo Y. NAC family transcription factors in tobacco and their potential role in regulating leaf senescence. Front Plant Sci. 2018;871:1–15. doi: 10.3389/fpls.2018.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lei W, He R, Tang X, Han J, Zou L, Yin Y, Lin H, Zhang D. Brassinosteroids regulate root meristem development by mediating BIN2-UPB1 module in Arabidopsis. PLoS Genet. 2020;16:1–27. doi: 10.1371/journal.pgen.1008883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Li Y, Chen X, Li L, Liu K, Zhao H, Wang Y, Han S. ERF72 interacts with ARF6 and BZR1 to regulate hypocotyl elongation in Arabidopsis. J Exp Bot. 2018;69:3933–3947. doi: 10.1093/jxb/ery220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yang Q, Wang Y, Wang L, Fu Y, Wang X. Brassinosteroids regulate pavement cell growth by mediating BIN2-induced microtubule stabilization. J Exp Bot. 2018;69:1037–1049. doi: 10.1093/jxb/erx467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lv B, Tian H, Zhang F, Liu J, Lu S, Bai M, Li C, Ding Z. Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 2018;14:1–26. doi: 10.1371/journal.pgen.1007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Zhang D, Li K, Liu Z, Liu X, Song C, Li G, Zhao C, Ma J, Han M. Effect of exogenous Brassinolide (BR) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Plant Growth Regul. 2017;82:391–401. doi: 10.1007/s10725-017-0264-5. [DOI] [Google Scholar]
- Matsukura C, Itoh SI, Nemoto K, Tanimoto E, Yamaguchi J. Promotion of leaf sheath growth by gibberellic acid in a dwarf mutant of rice. Planta. 1998;205:145–152. doi: 10.1007/s004250050306. [DOI] [Google Scholar]
- Miyazawa Y, Nakajima N, Abe T, Sakai A, Fujioka S, Kawano S, Kuroiwa T, Yoshida S. Activation of cell proliferation by brassinolide application in tobacco BY-2 cells: effects of brassinolide on cell multiplication, cell-cycle-related gene expression, and organellar DNA contents. J Exp Bot. 2003;54:2669–2678. doi: 10.1093/jxb/erg312. [DOI] [PubMed] [Google Scholar]
- Müssig C, Shin GH, Altmann T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003;133:1261–1271. doi: 10.1104/pp.103.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Fujioka S, Sunohara H, Kamiya N, Hong Z, Inukai Y, Miura K, Takatsuto S, Yoshida S, Ueguchi-Tanaka M, Hasegawa Y, Kitano H, Matsuoka M. The role of OsBRI1 and its homologous genes, OsBRL1 and OsBRL3, in rice. Plant Physiol. 2006;140:580–590. doi: 10.1104/pp.105.072330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J. 2003;33:887–898. doi: 10.1046/j.1365-313X.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Tsukaya H, Murakami N, Kato M. Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol. 2002;43:239–244. doi: 10.1093/pcp/pcf024. [DOI] [PubMed] [Google Scholar]
- Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- Nie S, Huang S, Wang S, Cheng D, Liu J, Lv S, Li Q, Wang X. Enhancing brassinosteroid signaling via overexpression of tomato (Solanum lycopersicum) SlBRI1 improves major agronomic traits. Front Plant Sci. 2017;8:1–12. doi: 10.3389/fpls.2017.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu JH, Anjum SA, Wang R, Li JH, Liu MR, Song JX, Zohaib A, Lv J, Wang SG, Zong XF. Exogenous application of brassinolide can alter morphological and physiological traits of Leymus chinensis (Trin.) Tzvelev under room and high temperatures. Chil J Agric Res. 2016;76:27–33. doi: 10.4067/S0718-58392016000100004. [DOI] [Google Scholar]
- Nolan TM, Vukasinović N, Liu D, Russinova E, Yin Y. Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell. 2020;32:298–318. doi: 10.1105/tpc.19.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MH, Honey SH, Tax FE. The control of cell expansion, cell division, and vascular development by brassinosteroids: a historical perspective. Int J Mol Sci. 2020;21:1–15. doi: 10.3390/ijms21051743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Godza B, Watanabe B, Fujioka S, Hategan L, Ide K, Shibata K, Yokota T, Szekeres M, Mizutani M. CYP90A1/CPD, a brassinosteroid biosynthetic cytochrome P450 of Arabidopsis, catalyzes C-3 oxidation. J Biol Chem. 2012;287:31551–31560. doi: 10.1074/jbc.M112.392720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas-Riverola A, Gupta A, Betegoń-Putze I, Bosch N, Ibañes M, Cano-Delgado AI. Brassinosteroid signaling in plant development and adaptation to stress. Development (Cambridge) 2019;146:1–11. doi: 10.1242/dev.151894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS, Sussex IM. The developmental morphology and growth dynamics of the tobacco leaf. Planta. 1985;165:158–169. doi: 10.1007/BF00395038. [DOI] [PubMed] [Google Scholar]
- Que F, Wang GL, Xu ZS, Wang F, Xiong AS. Transcriptional regulation of brassinosteroid accumulation during carrot development and the potential role of brassinosteroids in petiole elongation. Front Plant Sci. 2017;8:1–13. doi: 10.3389/fpls.2017.01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que F, Khadr A, Wang GL, Li T, Wang YH, Xu ZS, Xiong AS. Exogenous brassinosteroids altered cell length, gibberellin content, and cellulose deposition in promoting carrot petiole elongation. Plant Sci. 2018;277:110–120. doi: 10.1016/j.plantsci.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Saini S, Sharma I, Pati PK. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signalling and crosstalks. Front Plant Sci. 2015;6:1–17. doi: 10.3389/fpls.2015.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Kondo Y, Fukuda H. BES1 and BZR1 redundantly promote phloem and xylem differentiation. Plant Cell Physiol. 2018;59:590–600. doi: 10.1093/pcp/pcy012. [DOI] [PubMed] [Google Scholar]
- Si J, Sun Y, Wang L, Qin Y, Wang C, Wang X. Functional analyses of Populus euphratica brassinosteroid biosynthesis enzyme genes DWF4 (PeDWF4) and CPD (PeCPD) in the regulation of growth and development of Arabidopsis thaliana. J Biosci. 2016;41:727–742. doi: 10.1007/s12038-016-9635-8. [DOI] [PubMed] [Google Scholar]
- Sirhindi G, Kumar S, Bhardwaj R, Kumar M. Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol Mol Biol Plants. 2009;15:335–341. doi: 10.1007/s12298-009-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares TFSN, dos Dias DCF, S, Oliveira AMS, Ribeiro DM, Dias LA dos S, Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol Environ Saf. 2020;193:110296. doi: 10.1016/j.ecoenv.2020.110296. [DOI] [PubMed] [Google Scholar]
- Soliman M, Elkelish A, Souad T, Alhaithloul H, Farooq M. Brassinosteroid seed priming with nitrogen supplementation improves salt tolerance in soybean. Physiol Mol Biol Plants. 2020;26:501–511. doi: 10.1007/s12298-020-00765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber CM, McCourt P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001;125:763–769. doi: 10.1104/pp.125.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Q, Zheng X, Tian Y, Wang C. Exogenous Brassinolide alleviates salt stress in Malus hupehensis Rehd. by regulating the transcription of NHX-Type Na+(K+)/H+ Antiporters. Front Plant Sci. 2020;11:1–13. doi: 10.3389/fpls.2020.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, He Y, Irfan AR, Liu X, Yu Q, Zhang Q, Yang D. Exogenous brassinolide enhances the growth and cold resistance of maize (Zea mays L.) seedlings under chilling stress. Agronomy. 2020;10:488. doi: 10.3390/agronomy10040488. [DOI] [Google Scholar]
- Swamy KN, Rao SSR. Effect of brassinosteroids on the performance of coleus (Coleus forskohlii) J Herbs Spices Med Plants. 2011;17:12–20. doi: 10.1080/10496475.2011.556985. [DOI] [Google Scholar]
- Talarek-Karwel M, Bajguz A, Piotrowska-Niczyporuk A. Hormonal response of Acutodesmus obliquus exposed to combined treatment with 24-epibrassinolide and lead. J Appl Phycol. 2020;32:2903–2914. doi: 10.1007/s10811-020-02191-4. [DOI] [Google Scholar]
- Tanabe S, Ashikari M, Fujioka S, Takatsuto S, Yoshida S, Yano M, Yoshimura A, Kitano H, Matsuoka M, Fujisawa Y, Kato H, Iwasaki Y. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell. 2005;17:776–790. doi: 10.1105/tpc.104.024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong H, Xiao Y, Liu D, Gao S, Liu L, Yin Y, Jin Y, Qian Q, Chu C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in ricec w open. Plant Cell. 2014;26:4376–4393. doi: 10.1105/tpc.114.132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan S, Forestan C, Brojanigo S, Quaggiotti S, Varotto S. Brassinosteroid application affects the growth and gravitropic response of maize by regulating gene expression in the roots, shoots and leaves. Plant Growth Regul. 2020;92:117–130. doi: 10.1007/s10725-020-00626-z. [DOI] [Google Scholar]
- Unterholzner SJ, Rozhon W, Papacek M, Ciomas J, Lange T, Kugler KG, Mayer KF, Sieberer T, Poppenberger B. Brassinosteroids are master regulators of gibberellin biosynthesis in Arabidopsis. Plant Cell. 2015;27:2261–2272. doi: 10.1105/tpc.15.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Wang Q, Chong K, Wang F, Wang L, Bai M, Jia C. The brassinosteroid signal transduction pathway. Cell Res. 2006;16:427–434. doi: 10.1038/sj.cr.7310054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZX, Wang J, Jiang J, Wang J, Chen L, Fan SL, Wu JW, Wang X. Structural insights into the negative regulation of BRI1 signaling by BRI1-interacting protein BKI1. Cell Res. 2014;24:1328–1341. doi: 10.1038/cr.2014.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Li J. Brassinosteroids regulate root growth, development, and symbiosis. Mol Plant. 2016;9:86–100. doi: 10.1016/j.molp.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Wolters H, Jürgens G. Survival of the flexible: hormonal growth control and adaptation in plant development. Nat Rev Genet. 2009;10:305–317. doi: 10.1038/nrg2558. [DOI] [PubMed] [Google Scholar]
- Xu P, Fang S, Chen H, Cai W. The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. 2020;104:59–75. doi: 10.1111/tpj.14905. [DOI] [PubMed] [Google Scholar]
- Ye H, Li L, Yin Y. Recent advances in the regulation of Brassinosteroid signalling and biosynthesis pathways. J Integr Plant Biol. 2011;53:455–468. doi: 10.1111/j.1744-7909.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/S0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Yuan L, Shu S, Sun J, Guo S, Tezuka T. Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system, and chloroplast ultrastructure in Cucumis sativus L. under Ca(NO3)2 stress. Photosynth Res. 2012;112:205–214. doi: 10.1007/s11120-012-9774-1. [DOI] [PubMed] [Google Scholar]
- Yusuf M, Fariduddin Q, Ahmad I, Ahmad A. Brassinosteroid-mediated evaluation of antioxidant system and nitrogen metabolism in two contrasting cultivars of Vigna radiata under different levels of nickel. Physiol Mol Biol Plants. 2014;20:449–460. doi: 10.1007/s12298-014-0259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Zhao C, Mao J, Song C, Ma J, Zhang D, Han M, An N. Genome-wide identification and expression analysis of brassinosteroid biosynthesis and metabolism genes regulating apple tree shoot and lateral root growth. J Plant Physiol. 2018;231:68–85. doi: 10.1016/j.jplph.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Zheng L, Gao C, Zhao C, Zhang L, Han M, An N, Ren X. Effects of Brassinosteroid associated with auxin and gibberellin on apple tree growth and gene expression patterns. Hortic Plant J. 2019;5:93–108. doi: 10.1016/j.hpj.2019.04.006. [DOI] [Google Scholar]
- Zheng M, Hu M, Yang H, Tang M, Zhang L, Liu H, Li X, Liu J, Sun X, Fan S, Zhang J, Terzaghi W, Pu H, Hua W. Three BnaIAA7 homologs are involved in auxin/brassinosteroid-mediated plant morphogenesis in rapeseed (Brassica napus L.) Plant Cell Rep. 2019;38:883–897. doi: 10.1007/s00299-019-02410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiponova MK, Vanhoutte I, Boudolf V, Betti C, Dhondt S, Coppens F, Mylle E, Maes S, González-García MP, Caño-Delgado AI, Inzé D, Beemster GTS, De Veylder L, Russinova E. Brassinosteroid production and signalling differentially control cell division and expansion in the leaf. New Phytol. 2013;197:490–502. doi: 10.1111/nph.12036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.