During the development of the central nervous system (CNS), neuroepithelial cells in the ventricular zone are multipotent stem cells that can generate neurons and macroglia (astrocytes or oligodendrocytes). It is generally thought that these progenitor cells sequentially produce neurons followed by glia. The lineage relation between astrocytes and oligodendrocytes has long been under study, but remains controversial. In the 1980s, Martin Raff’s group [1] described a glial progenitor cell, termed an O-2A cell, that can differentiate into astrocytes or oligodendrocytes in vitro, depending on the culture conditions. In the presence of serum, O-2A cells differentiate into type II astrocytes. In culture medium without serum, they differentiate into oligodendrocytes. However, bi-potential glial progenitors were not found in vivo with retroviral lineage labeling approaches [2], and thus were regarded as an in vitro phenomenon until the Rao group reported the identification of embryonic-derived glial-restricted precursor cells that can differentiate into astrocytes and oligodendrocytes in vivo [3]. At about the same time came the identification and functional characterization of the Olig1 and Olig2 genes whose expression marks differentiating oligodendrocytes [4,5]. In the ventricular zone of the telencephalon, they also mark multipotent progenitors as well as oligodendrocyte progenitor cells (OPCs). In the developing spinal cord, analysis of the Olig genes demonstrated that OPCs and astrocytes originate from distinct domains of neural progenitor cells (NPCs) [4,5]. While OPCs are derived from the motor neuron precursor domain, astrocytes originate from other domains of NPCs and share a lineage with spinal cord interneurons. In support of this concept, mutation of the Olig2 gene eliminates the generation of both motoneurons and OPCs, but does not disrupt the development of interneurons and astrocytes [4,5]. These findings disputed the concept of a common lineage of astrocytes and oligodendrocytes at least in the developing spinal cord. Surprisingly, several years later, it was shown that conditional knockout of the Olig2 gene in embryonic cortex affects not only the development of oligodendrocytes but also that of cortical astrocytes [6], rekindling the possibility of a common lineage for cortical astrocytes and oligodendrocytes. Thus, despite intensive study of the embryonic origin of astrocytes and oligodendrocytes in the past several decades, it remains highly debatable whether astrocytes and oligodendrocytes are derived from the same progenitors in the developing CNS.
In this issue, Li et al. [7] took a big step forward in deciphering cortical gliogenesis with a combination of sophisticated molecular approaches. In the mouse cerebral cortex, radial glial cells (RGCs) give rise to different types of glutaminergic pyramidal neurons (PyNs) from E11.5 to E16.5 either directly or indirectly though intermediate progenitor cells (IPCs) [8]. After that, the remaining RGCs switch their properties to produce cortical oligodendrocytes, astrocytes, and olfactory bulb interneurons (OBiNs) [8]. To investigate the lineage relationship of RGCs at late prenatal stages, Li et al. [7] labeled the cortical RGCs at E15.5 by introducing the pCAG-Cre plasmid into the cortical ventricular zone (VZ) of IS reporter mice (Rosa-CAG-LSL-Frt-tdTomato-Frt-EGFP) by in-utero electroporation. As expected, at postnatal day 1 (P1), the tdTomato+ RGC progeny cells were detected in the cortical VZ, cortical subventricular zone (SVZ), cortical plate, and olfactory bulb. These P1 tdTomato+ cells were then purified using fluorescence-activated cell sorting (FACS), and analyzed by single-cell RNA sequencing (scRNA-Seq). Bio-informative analyses grouped these P1 cells (derived from E15.5 cortical RGCs) into 30 clusters, belonging to 8 cell types: ependymal cells, RGCs, apical multipotent intermediate progenitor cells (aMIPCs), basal multipotent IPCs (bMIPCs), PyN-lineage, oligodendrocyte-lineage, astrocyte-lineage, and OBiN-lineage. In parallel, scATAC-Seq on FACS-sorted GFP+ cells from E18.5 hGFAP-GFP transgenic cortex similarly identified 17 clusters grouped as RGCs, MIPCs, astrocyte-lineage, oligodendrocyte-lineage, OBiN-lineage, and PyN-lineage.
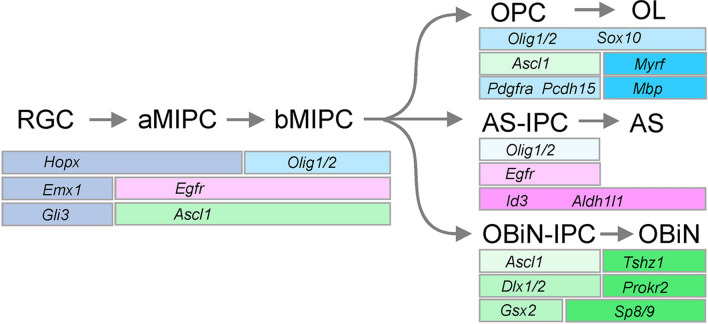
Based on a combination of scRNA-Seq, scATAC-Seq, and intersectional lineage analyses, they outline a blueprint of how cortical glial cells are sequentially generated from RGCs [7]. Before E16.5, cortical RGCs primarily give rise to PyNs. After that, the Gli3+/Hopx+/Emx1+ RGCs start to produce aMIPCs, whose end-feet are not attached to the pia surface (Fig. 1). The aMIPCs are marked by the expression of Egfr and Ascl1, but Mki67 is undetectable. aMIPCs rapidly become bMIPCs, which start to express Olig1/2 and become Mki67+. Subsequently, the bMIPCs undergo heterogeneous differentiation programs. A subset of bMIPCs downregulate the expression of Ascl1 and become Egfr+/Olig2+/Id3+ astrocyte-IPCs, which then differentiate into astrocytes that express Aldh1l1. Some of bMIPCs proceed to become OPCs; they down-regulate Egfr, maintain Olig1/2 and Ascl1, and start to express Sox10 and Pdgfra. Another group of bMIPCs lose expression of Egfr and Olig1/2, but gradually gain the expression of OBiN-IPC markers such as Gsx1/2 and Dlx1/2 before they eventually differentiate into Sp8/9+/Tshz1+/Prokr2+ OBiNs. Thus, the bMIPCs might be tri-potential neuroglial progenitor cells that can give rise to cortical astrocytes, oligodendrocytes, and OBiNs.
Fig. 1.
The combinatorial molecular code for neural and glial cells during late corticogenesis. Cortical RGCs at about E16.5 acquire the properties of aMIPCs with the up-regulation of Egfr and Ascl1 expression. aMIPCs start to express Olig1/2 and become bMIPCs. During subsequent development, bMIPCs that retain Olig1/2 and Ascl1 expression proceed to produce Sox10+/Pdgfra+/Pcdh15+ OPCs; those that maintain Egfr expression develop into Egfr+/Id3+ astrocyte-IPCs; and those that turn off the expression of both Egfr and Olig1/2 give rise to Gsx2+/Dlx1/2+ OBiN-IPCs. When OPCs differentiate into oligodendrocytes, they start to express Myrf and Mbp. Mature astrocytes are Egfr-/Aldh1l1+, and mature OBiNs express Sp8/9, Tshz1, and Prokr2. RGC, radial glial cell; IPC, intermediate progenitor cell; MIPC, multipotent intermediate progenitor cell; aMIPC, apical MIPC; bMIPC, basal MIPC; OPC, oligodendrocyte progenitor cell; OL, oligodendrocyte; AS, astrocyte; OBiN, olfactory bulb interneuron.
To trace the fate of Ascl1 + MIPCs, Li et al. [7] delivered pCAG-Cre plasmids into the cortical VZ of Ascl1Flpo/Flpo; IS embryos at E15.5 by in-utero electroporation. In this instance, the electroporated cortical RGCs and their progenies were tdTomato+. Once starting to express Ascl1, these cells and their progeny express EGFP. By P21, all cortical EGFP+ cells develop into oligodendrocyte-lineage cells or astrocytes. In the olfactory bulb, nearly all of the labelled cells are EGFP+, very few OBiNs being tdTomato+. These data confirmed that the Ascl1+ MIPCs are the major sources of cortical oligodendrocytes, astrocytes, and OBiNs. Using a similar approach, they further provided evidence that cortical Olig2+ MIPCs give birth to the vast majority of cortical oligodendrocytes and astrocytes, as well as OBiNs.
Taken together, Li et al. [7] clearly demonstrated a common lineage of cortical astrocytes and oligodendrocytes. In general, after the generation of PyNs, the cortical RGCs change their properties to aMIPCs around E16.5, and then quickly transform into proliferative bMIPCs. These tri-potential MIPCs are the common progenitor pool for cortical astrocytes and oligodendrocytes. Equally importantly, this study has also assigned a combinatorial molecular code for various populations of neural and glial progenitors (Fig 1); this code is a foundation for future studies on fate specification, differentiation, and migration. Of note, Egfr+-like MIPCs have also been described in monkey and human cortex [9,10], suggesting that the process of gliogenesis is conserved between rodents and primates.
In the embryonic spinal cord, OPCs and astrocytes are produced from distinct domains of NPCs. OPCs share a lineage with motor neurons, and astrocytes share lineages with spinal interneurons. Furthermore, during early neural development, neurogenesis occurs prior to gliogenesis in both brain and spinal cord. In the study by Li et al. [7], they focused on the late embryonic stage of cortical development and showed that OBiNs and both glial types are generated from common progenitors. They clearly demonstrated the heterogeneity of gliogenesis in different regions of the CNS and the uniqueness of OBiN neurogenesis. Crucially, they provided molecular evidence for the common lineage of cortical OPCs and astrocytes. This tri-potential progenitor pool appears to be preserved in postnatal development. Most recently, Zhang et al. [11] reported that tri-potential cortical Gsx2+ MIPCs, similar to the OBiN-IPCs described by this study, also give rise to OBiNs, astrocytes, and OPCs in P21 mouse brain. Even in adult brain, type B/C neural stem/progenitor cells in the SVZ retain the potential to generate OBiNs, astrocytes, and OPCs [12,13]. Thus, it would be interesting to see whether the molecular signatures of bMIPCs are preserved in postnatal and adult neural/progenitor cells. Intriguingly, in the ventral forebrain, NPCs similarly express Ascl1, Olig2, Gsx2, and Egfr, and produce OPCs, astrocytes and GABAergic inhibitory interneurons [14]. Is there a similar tri-potential progenitor population for the generation of these neurons and glia, and if so, do they share a lineage progression similar to bMIPCs and are they guided by a common regulatory network?
In conclusion, this study revealed the origin and developmental process of cortical oligodendrocytes, astrocytes, and OBiNs, and decoded their combinatorial molecular signatures, providing novel insights into cortical gliogenesis and neurogenesis.
Acknowledgements
This Editorial was supported by the Natural Science Foundation of Zhejiang Province (LQ19C090001) and the National Natural Science Foundation of China (31900703 and 31771621).
Conflict of interest
JLR is cofounder, stockholder, and currently on the scientific board of Neurona, a company studying the potential therapeutic use of interneuron transplantation.
Contributor Information
John L. Rubenstein, Email: john.rubenstein@ucsf.edu
Mengsheng Qiu, Email: m0qiu001@yahoo.com.
References
- 1.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 2.Costa MR, Bucholz O, Schroeder T, Gotz M. Late origin of glia-restricted progenitors in the developing mouse cerebral cortex. Cereb Cortex. 2009;19(Suppl 1):i135–143. doi: 10.1093/cercor/bhp046. [DOI] [PubMed] [Google Scholar]
- 3.Rao MS, Noble M, Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95:3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Q, Anderson DJ. The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell. 2002;109:61–73. doi: 10.1016/S0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- 5.Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH, et al. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/S0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Chen Y, Cai WH, Hurlock EC, Wu H, Kernie SG, et al. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- 7.Li X, Liu G, Yang Y, Li Z, Zhang Z, Xu Z, et al. Decoding cortical glial cell development. Neurosci Bull. 2020 doi: 10.1007/s12264-021-00640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci 2009, 32: 149–184. [DOI] [PMC free article] [PubMed]
- 9.Huang W, Bhaduri A, Velmeshev D, Wang S, Wang L, Rottkamp CA, et al. Origins and proliferative states of human oligodendrocyte precursor cells. Cell. 2020;182(594–608):e511. doi: 10.1016/j.cell.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rash BG, Duque A, Morozov YM, Arellano JI, Micali N, Rakic P. Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc Natl Acad Sci U S A 2019, 116: 7089–7094. [DOI] [PMC free article] [PubMed]
- 11.Zhang Y, Liu G, Guo T, Liang XG, Du H, Yang L, et al. Cortical neural stem cell lineage progression is regulated by extrinsic signaling molecule sonic hedgehog. Cell Rep. 2020;30(4490–4504):e4494. doi: 10.1016/j.celrep.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y, Yang Z. Adult neural stem cells: constant extension from embryonic ancestors. Neurosci Bull. 2019;35:1120–1122. doi: 10.1007/s12264-019-00396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowitch DH, Kriegstein AR. Developmental genetics of vertebrate glial-cell specification. Nature. 2010;468:214–222. doi: 10.1038/nature09611. [DOI] [PubMed] [Google Scholar]