Abstract
A novel approach targeting self-inducible surfactin synthesis under oxygen-limited conditions is presented. Because both the nitrate (NarGHI) and nitrite (NasDE) reductase are highly expressed during anaerobic growth of B. subtilis, the native promoter PsrfA of the surfactin operon in strain B. subtilis JABs24 was replaced by promoters PnarG and PnasD to induce surfactin synthesis anaerobically. Shake flask cultivations with varying oxygen availabilities indicated no significant differences in native PsrfA expression. As hypothesized, activity of PnarG and PnasD increased with lower oxygen levels and surfactin was not produced by PsrfA::PnarG as well as PsrfA::PnasD mutant strains under conditions with highest oxygen availability. PnarG showed expressions similar to PsrfA at lowest oxygen availability, while maximum value of PnasD was more than 5.5-fold higher. Although the promoter exchange PsrfA::PnarG resulted in a decreased surfactin titer at lowest oxygen availability, the strain carrying PsrfA::PnasD reached a 1.4-fold increased surfactin concentration with 696 mg/L and revealed an exceptional high overall YP/X of 1.007 g/g. This value also surpassed the YP/X of the reference strain JABs24 at highest and moderate oxygen availability. Bioreactor cultivations illustrated that significant cell lysis occurred when the process of “anaerobization” was performed too fast. However, processes with a constantly low agitation and aeration rate showed promising potential for process improvement, especially by employing the strain carrying PsrfA::PnasD promoter exchange. Additionally, replacement of other native promoters by nitrite reductase promoter PnasD represents a promising tool for anaerobic-inducible bioprocesses in Bacillus.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-021-01218-4.
Keywords: Bacillus subtilis, Lipopeptide biosurfactants, Surfactin, Microaerobic, Promoter exchange, Oxygen
Introduction
The cyclic lipopeptide surfactin synthesized by Bacillus subtilis displays promising characteristics in a variety of industrial sectors (Geissler et al. 2019a) due to its excellent surface-active properties and antimicrobial activities (Falardeau et al. 2013; Li et al. 2019). In addition, surfactin is a promising alternative to surfactants of petrochemical and oleochemical origin (Henkel et al. 2017). However, two bottlenecks regarding surfactin production must be mentioned that are often addressed in research. First, the excessive foaming during conventional aerobic processes, and second, the overall low titers of wild-type strains that are insufficient for industrial production.
Studies reported both on difficulties in operation due to uncontrolled foaming and on a great loss of cultivation medium at high agitation and aeration rates (Davis et al. 2001; Yao et al. 2015; Yeh et al. 2006). If product separation by foaming is integrated into the process as a first surfactin enrichment, cells are also lost for further surfactin production (Willenbacher et al. 2014). Issues with blocked exhaust air filters and hence increase in pressure can also occur. However, high agitation and aeration rates are indispensable when defined oxygen levels shall be maintained in commonly employed aerobic cultivations. Indeed, Yeh et al. (2006) reported that up to a certain high level of aeration and stirrer speed surfactin synthesis was improved in carrier-assisted cultivation due to improved oxygen transfer rate and mass transfer efficiency. In contrast to these circumstances, other studies described that an enhanced surfactin production rate was reached in oxygen-limited conditions (Davis et al. 1999; Kim et al. 1997). In this sense, the set-points for both aeration and agitation rate are crucial to have the optimal performance in a bioreactor cultivation with a defined strain. Interestingly, an anaerobic cultivation of B. subtilis as demonstrated by Willenbacher et al. (2015) resulted in a completely foam-free approach for surfactin formation. Nevertheless, anaerobic nitrate respiration reveals some restrictions. Both the overall low growth rates of B. subtilis producer strains and comparatively low surfactin titers make these processes inferior to aerobic counterparts. Still, promising and high values with regard to product per biomass yields were obtained (Geissler et al. 2019b; Willenbacher et al. 2015). Another aspect is the negative impact of nitrite as well as acetate on anaerobic cell growth. Especially, the latter one increased drastically throughout nitrate respiration of B. subtilis and as such is an interesting candidate for strain engineering (Hoffmann et al. 2020).
With respect to the overall low surfactin titers of wild-type strains, rational strain engineering is also often employed, as heterologous production of surfactin in other host strains is difficult to realize (Hu et al. 2019). In this field, substitution of the native promoter PsrfA, whose regulation is dependent on a complex quorum-sensing mechanism (Geissler et al. 2019a), constitutes one promising approach. The replacement of PsrfA by constitutively promoters was reported in several studies (Coutte et al. 2010a; Willenbacher et al. 2016). Results demonstrated that both gene expression during the time course of cultivation as well as the ability of the wild-type strain to produce surfactin influence final surfactin concentrations. In contrast to constitutive promoters, several studies reported on improved surfactin titers up to 17-fold employing IPTG-inducible promoters (Jiao et al. 2017; Sun et al. 2009; Wang et al. 2018). However, IPTG is rather expensive, which poses a bottleneck for large-scale production.
The current study aimed to address the afore mentioned aspects and examined an oxygen-dependent, self-inducible expression system employing the promoters of the nitrate reductase PnarG and the nitrite reductase PnasD. Hence, the presented method takes advantage of the ability of B. subtilis to grow anaerobically by nitrate respiration, the most effective life style to generate ATP after aerobic growth with oxygen as electron acceptor (Härtig and Jahn 2012). The adaptation of aerobic growing cells to anaerobic conditions is dependent on the interplay of three major regulators, which are ResDE, Fnr and Rex (a detailed overview is given by Härtig and Jahn (2012)). In a first step, nitrate is reduced to nitrite by the catabolic nitrate reductase NarGHI (Nakano et al. 1998). In a second step, nitrite is further reduced to ammonium by the nitrite reductase NasDE. Indeed, NasDE is also involved in aerobic assimilatory reduction of nitrate to nitrite. However, the expression of NasDE was reported to be significantly induced in the absence of oxygen emanating from a promoter located in between nasC and nasD (Nakano et al. 1998). Hence, both PnarG and PnasD are involved in anaerobic nitrate respiration, although also the availability of nitrogen sources plays an important role in this issue. In addition to these anaerobically inducible promoters, B. subtilis exhibits more regulatory networks that allow induction of gene expression under oxygen limitation. An overview of B. subtilis transcriptome under anaerobic growth was provided by Nicolas et al. (2012). Hence, further promising promoters for anaerobic induction of gene expression are PlctE, PalsS and Phmp.
We hypothesized that the presented self-inducible system poses an interesting novel cultivation strategy to synthesize surfactin under oxygen-limited conditions resulting in reduced foam formation. The proposed system is not only promising for foaming agents like surfactin, but also for other oxygen-sensitive target products. On top, preliminary investigations in bioreactor cultivations to establish a simple and robust cultivation strategy using a native B. subtilis surfactin producer strain and derivative strains with promoter exchanges are presented. Emerging limitations of bioreactor cultivations will be emphasized that display a promising starting position for further optimizations.
Materials and methods
Chemicals and materials
All chemicals (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) were of analytical grade. The reference standard for surfactin (≥ 98% purity) and glucose were from Sigma-Aldrich Laborchemikalien GmbH (Seelze, Germany).
Microorganisms and genetic engineering
All strains are listed in Table 1 including strain B. subtilis JABs24 (Geissler et al. 2019b) and derivatives thereof. Chemical competent E. coli BL21-Gold strains were used for plasmid propagation. Plasmids and primers (Eurofins Genomics GmbH, Ebersberg, Germany) used for strain construction are summarized in Additional file 1: Table S1 and Table S2. Transformants were selected on LB agar plates supplemented with either ampicillin (100 µg/mL), spectinomycin (125 µg/mL) or erythromycin (10 µg/mL for E. coli, 5 µg/mL for B. subtilis).
Table 1.
Overview of strains used in the current study
| Strain | Genotype or description | References |
|---|---|---|
| B. subtilis | ||
| JABs24 | trp+ sfp+ ΔmanPA | Geissler et al. (2019b) |
| MG1 | trp+ sfp+ ΔmanPA amyE::[PnarG-lacZ, spcR] | Hoffmann et al. (2020) |
| MG5 | trp+ sfp+ ΔmanPA amyE::[PnasD-lacZ, spcR] | Hoffmann et al. (2020) |
| KM1016 | trp+ sfp+ ΔmanPA amyE::[PsrfA-lacZ, spcR] | This study |
| MG11 | trp+ sfp+ ΔmanPA PsrfA::PnarG | This study |
| MG12 | trp+ sfp+ ΔmanPA PsrfA::PnarG amyE::[PnarG-lacZ, spcR] | This study |
| MG13 | trp+ sfp+ ΔmanPA PsrfA::PnasD | This study |
| MG14 | trp+ sfp+ ΔmanPA PsrfA::PnasD amyE::[PnasD-lacZ, spcR] | This study |
| E. coli | ||
| BL21-Gold (DE3) | F- ompT hsdS(rB- mB-) dcm+ Tetr gal λ(DE3) endA Hte | Agilent, Waldbronn, Germany |
For promoter exchange studies, strain JABs24 was used to replace the native promoter PsrfA markerless by either PnasD or PnarG similar to the protocol described by Vahidinasab et al. (2020). Briefly, generated fragments upstream(PsrfA)-PnarG-downstream(PsrfA) and upstream(PsrfA)-PnasD-downstream(PsrfA) were ligated into SmaI digested plasmid pJOE4786.1 resulting in plasmids pRIK2 for PsrfA::PnarG and pPB1 for PsrfA::PnasD. After confirmation by sequencing, fragments of interest were digested of pRIK2 and pPB1 by HindIII and were ligated into plasmid pJOE6743.1 resulting in plasmids for chromosomal promoter exchange, called pRIK4 (PsrfA::PnarG) and pPB2 (PsrfA::PnasD). These plasmids were isolated and transformed in natural competent B. subtilis JABs24 cells. A markerless promoter exchange of PsrfA was ensured by mannose counterselection as described by Wenzel and Altenbuchner (2015). Final validation of successful integration was performed by sequencing and resulted in strains MG11 for PsrfA::PnarG and MG13 for PsrfA::PnasD. Using plasmids pKAM446 (PsrfA-lacZ), pKAM452 (PnarG-lacZ) and pSHX2 (PnasD-lacZ) for construction of reporter strains and the protocol described in Hoffmann et al. (2020), the integration of promoter-lacZ fusion into amyE locus was performed for strains JABs24, MG11 and MG13. This resulted in strains KM1016 (amyE::[PsrfA-lacZ, spcR]), MG12 (PsrfA::PnarG; amyE::[PnarG-lacZ, spcR]) and MG14 (PsrfA::PnasD; amyE::[PnasD-lacZ, spcR]), respectively.
Cultivation medium and conditions
Main cultivations were performed in 500 mL baffled shake flasks with relative filling volumes (Rv) of 10% (= 0.1 mL/mL), 50% (= 0.5 mL/mL) and 100% (= 1 mL/mL) at 120 rpm. This approach results in different oxygen availabilities in shake flasks (Heyman et al. 2019; Schiefelbein et al. 2013). In any case, even at 100% Rv, overflowing during shaking did not occur as the shake flask neck posed a sufficient barrier. Cultivations in bioreactors were performed in custom-built bioreactors (ZETA GmbH, Graz/Lieboch, Austria) equipped with foam centrifuge as described in Hoffmann et al. (2020) at 37 °C. Due to the installation and feed-program of the bioreactors, batch and feed media were prepared as g/kg respectively mol/kg.
Preparation of LB medium for the first pre-culture and a modified mineral salt medium for all other shake flask and bioreactor cultivations was performed as described in Hoffmann et al. (2020). The concentrations of the buffer, MgSO4∙7H2O and trace elements were the same, while the glucose concentration was increased to 40 g/L for shake flask cultivations and 20 g/kg for the bioreactor batch medium. The initial nitrogen source was 0.1 mol/L (or mol/kg) NaNO3 and 0.1 mol/L (or mol/kg) (NH4)2SO4, if not indicated otherwise in the results part. For bioreactor cultivations, a feed solution was prepared containing 400 g/kg glucose as well as 20 mL TES/kg and 3.69 g/kg MgSO4∙7H2O.
Pre-cultures and the main culture were prepared as described in Hoffmann et al. (2020). The second pre-culture was inoculated for 12–16 h. Bioreactor cultivations had an initial batch volume of 20 kg. The agitation rate was fixed at 300 rpm throughout cultivation. A constant aeration rate of 1.2 L/min, equal to 0.06 vvm at T0 and the lowest possible aeration rate with the given technical equipment, was set without further pO2 regulation. pH was maintained at 7 by addition of 4 M NaOH and 4 M HNO3, the latter one serving as self-regulated nitrate-feed due to the basic pH shift caused by anaerobic nitrate respiration. The foam centrifuge run at 2790 rpm when activated by a sensor in the headspace of the bioreactor vessel. The feed was started when glucose became depleting with a set growth rate of 0.03 1/h and an initial feed addition of 0.04 kg/h. The feed was limited to 2 kg.
Sampling and sample analysis
After measuring the OD600 (Biochrom WPA CO8000, Biochrom Ltd., Cambridge, UK) of the samples, cell-free supernatants were stored at – 20 °C. Cell dry weight (CDW) was calculated by dividing the OD600 by the factor 3.762, which was established previously (Geissler et al. 2019b). Glucose and surfactin concentrations were determined using an HPTLC method as specified in Geissler et al. (2017, 2019b). Nitrate, nitrite and ammonium concentrations were analyzed with spectrophotometric assays (nitrate: Cat. No. 1.09713.0001, nitrite: Cat. No. 1.14776.0001, ammonium: Cat. No. 1.14752.0001, Merck KGaA, Darmstadt, Germany). Miller units (MU) as indicator for the promoter activity of the lacZ-fused promoters were determined by the β-galactosidase assay as described in Hoffmann et al. (2020).
Data analysis
Process parameters were calculated in the same approach, means Δt yields and overall yields, as explained in Hoffmann et al. (2020). For bioreactor cultivations, all concentrations were converted to absolute values by multiplying the respective value by the weight of the medium. This allowed to compensate for the dilution occurred due to the addition of feed, acid and base solutions. All experiments were performed in duplicates. Depending on the aim of the respective experiment, either strain JABs24, KM1016, MG1 or MG5 was used as reference strain.
Results
Influence of different oxygen availabilities on nitrogen metabolism
Varying filling volumes in shake flasks are associated with different oxygen availabilities for cells. In case of B. subtilis, altered patterns in nitrogen consumption were expected due to assimilatory and dissimilatory pathways. More precisely, low filling volumes result in highest oxygen availability (Heyman et al. 2019; Schiefelbein et al. 2013) and hence aerobic growth is predominant. In this sense, ammonium should be identified as preferred nitrogen source for biomass assimilation, when both ammonium and nitrate are present. High filling volumes result in low oxygen availability (Heyman et al. 2019; Schiefelbein et al. 2013) and nitrate consumption increases due to anaerobic nitrate respiration. To validate these theses, B. subtilis JABs24 was cultivated in 10%, 50% and 100% relative filling volume (Rv). Figure 1 displays the corresponding CDW and the concentrations of nitrate, nitrite and ammonium during the time course of cultivation. CDWmax was reached after 44 h, 48 h and 36 h with 5.85 ± 0.66 g/L, 5.12 ± 0.20 g/L and 1.21 ± 0.01 g/L at increasing cultivation volumes. Additional glucose measurements excluded carbon limitation (data not shown). Nitrate concentrations were relatively constant at 10% and 50% Rv, with a slight decrease detectable in the last-mentioned. On the contrary, nitrate limitation occurred in 100% Rv after 36 h. Hence, growth was limited by nitrate depletion. As expected, the ammonium concentration decreased during cultivation with 10% Rv. At 50% Rv, ammonium initially decreased slightly before a relatively constant concentration could be measured. A continuous increase in ammonium was detected in the cultivation using 100% Rv of the flask capacity. Nitrite, the intermediate of nitrate respiration, was not detected in cultivations using 10% Rv. Nitrite peaked to values of 4.11 ± 1.17 mmol/L and 4.95 ± 0.03 mmol/L after 20 h of cultivation in both 50% and 100% Rv, respectively. Those nitrite concentrations particularly affect a prolonged lag phase of cultivation of anaerobically growing B. subtilis cultures, while the values of maximum specific growth rates µmax remain unchanged (Hoffmann et al. 2020). However, concentrations further declined until CDWmax. Hence, the time course of nitrogen sources indicated that higher filling volumes resulted in a converged nitrogen consumption pattern. More precisely, nitrate respiration was preferred with increasing Rv and hence lower oxygen availabilities can be assumed.
Fig. 1.
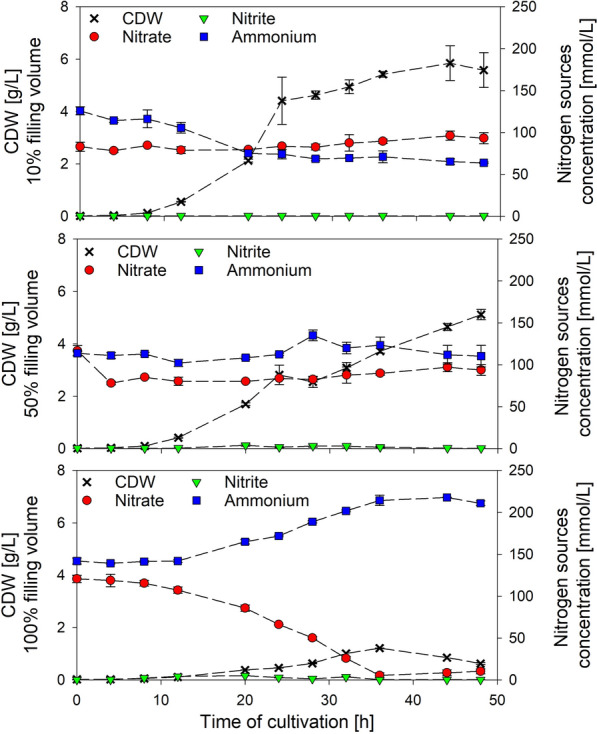
Cell dry weight (CDW) (g/L) and concentrations of nitrate, nitrite and ammonium (all [mmol/L]) of strain B. subtilis JABs24 cultivated in a mineral salt medium employing 40 g/L glucose, 0.1 mol/L NH4+ and 0.1 mol/L NO3− in baffled shake flasks with three different relative filling volumes of 10%, 50% and 100% representing different oxygen availabilities
Suitability of the promoters PnarG and PnasD for gene expression under anaerobic conditions
The nitrogen patterns of the preliminary cultivations have indicated that different oxygen availabilities are present in cultivations with 10%, 50% and 100% Rv. Further cultivations aimed at investigating the suitability and expression of PnarG and PnasD as anaerobically inducible promoters. Therefore, strains KM1016, MG1 and MG5 carrying PsrfA-lacZ, PnarG-lacZ and PnasD-lacZ-fusions, respectively, were cultivated in shake flasks with 10%, 50% and 100% Rv. Comparable growth curves were observed as described in Fig. 1. Figure 2 displays the promoter activities as determined by Miller units (MU) during cultivations.
Fig. 2.
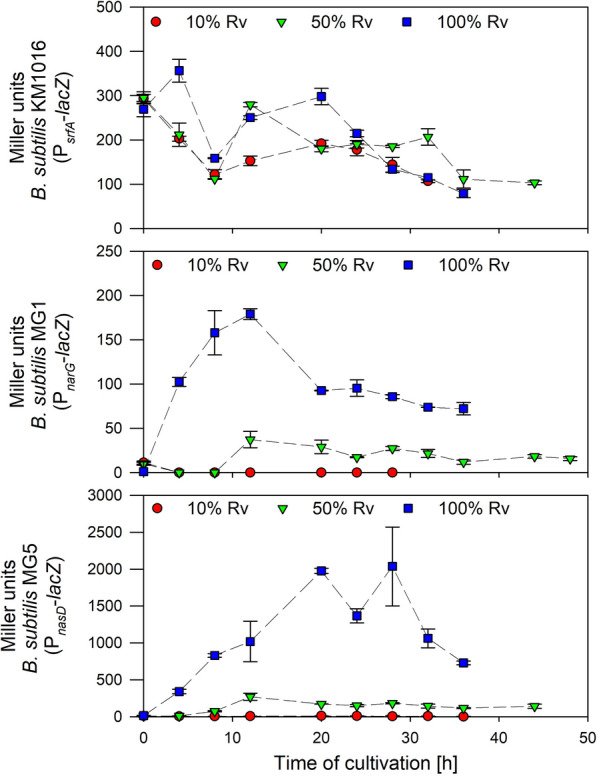
Time course of Miller units determined by the Miller Assay representing expression of promoters PsrfA, PnarG and PnasD. Data recorded until CDWmax of cultivation of strains B. subtilis KM1016 (PsrfA-lacZ), MG1 (PnarG-lacZ) and MG5 (PnasD-lacZ) in a mineral salt medium employing 40 g/L glucose, 0.1 mol/L NH4+ and 0.1 mol/L NO3− in baffled shake flasks with three different relative filling volumes (Rv) of 10%, 50% and 100% representing different oxygen availabilities
The PsrfA expression was comparatively congruent amongst the different Rv values tested. Altogether, a marginal tendency to higher mean MU with increasing Rv was noticed. In general, PsrfA expression dropped from ~ 300 MU to ~ 125 MU after 8 h of cultivation. Another slight increase in expression was followed by a subsequent decline to 70–100 MU at CDWmax. For both PnarG and PnasD expressions, MU increased with increasing Rv. At 10% Rv, LacZ activity was not detectable for transcriptional fusion with PnarG and expression did not surpass 10 MU for PnasD. At 50% Rv, both expression levels of PnarG and PnasD increased to a maximum after 12 h of cultivation with ~ 40 MU and ~ 270 MU, respectively, with an ensuing decrease to ~ 15 MU and ~ 140 MU until CDWmax. Highest expression levels were monitored at 100% Rv. For PnarG, expression increased to ~ 180 MU after 12 h of cultivation before a decline to ~ 80 MU was detectable. In contrast to that, maximum expression of PnasD was ~ 10-fold higher and reached ~ 2000 MU after 20 h of cultivation. Noticeably, comparable to PnarG expression, a reduction of MU values to ~ 800 MU could be observed at CDWmax. In this context, as already described by Hoffmann et al. (2020), a strong PnasD activity is detectable, although the overflow metabolite acetate increases with higher glucose amounts in anaerobic cultivations. In summary, while PsrfA expression was relatively constant amongst the different Rv tested, both PnarG and PnasD expressions were induced at higher Rv and hence lower oxygen availabilities. More specifically, PnasD revealed the overall highest expression values.
To apply PnarG and PnasD promoter systems for bioprocesses, more information about B. subtilis physiology is important. In brief, B. subtilis harbors two nitrate reductases with the genes nasBC and narGHI encoding for the assimilatory and dissimilatory nitrate reductase, respectively. The transcription of the latter one, narGHI, is thereby repressed in the presence of oxygen (Hoffmann et al. 1995; Nakano et al. 1996). On the contrary, nasDE encodes for both assimilatory and dissimilatory nitrite reductase and transcription is hence also feasible aerobically (Nakano et al. 1998). To demonstrate that the PnasD expression is dependent on the presence of ammonium or nitrate, respectively, B. subtilis MG5 was cultivated in media containing either NH4+ or NO3− in 10% Rv. As expected, mean MU for PnasD expression was below 10 MU in the medium containing only NH4+, whereas much higher MU values (continuously ~ 200 MU) were detected in NO3− supplemented medium (Additional file 1: Fig. S1). In addition, in media supplemented with either 0.005 or 0.01 mol/L NH4+, a time-delayed expression of PnasD was monitored (Additional file 1: Fig. S1) which was triggered by depletion of NH4+ (data not shown). Hence, it can be concluded that PnasD is a strong, anaerobically inducible promoter, but the effect of anaerobic induction is dependent on the availability of NH4+ during aerobic growth.
Investigation of strains B. subtilis MG12 and MG14 carrying promoter exchanges PsrfA::PnarG and PsrfA::PnasD
Growth behavior and surfactin synthesis
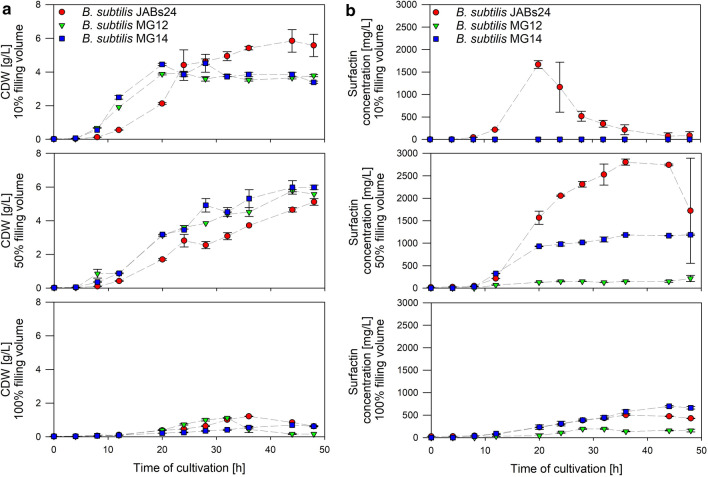
The promoter exchange strains MG12 (PsrfAA::PnarG; amyE::[PnarG-lacZ, spcR]) and MG14 (PsrfA::PnasD; amyE::[PnasD-lacZ, spcR]) were cultivated in shake flasks with 10%, 50% and 100% Rv. An overview of CDW and surfactin concentrations of these strains, as well as the reference strain JABs24, are displayed in Fig. 3a and b. In 10% Rv, strains MG12 and MG14 reached CDWmax after 24 h and 20 h with 3.93 ± 0.11 g/L and 4.45 ± 0.07 g/L, respectively. CDW remained constant until the end of cultivation. CDWmax for strain JABs24 was reached after 44 h with 5.85 ± 0.66 g/L. In 50% Rv, all strains reached CDWmax after 44 to 48 h with 5.12 ± 0.20 g/L, 5.78 ± 0.07 g/L and 5.98 ± 0.30 g/L for strains JABs24, MG12 and MG14, respectively. In contrast, CDWmax was much lower in cultivations with 100% Rv and did not surpass 1.21 ± 0.01 g/L, 1.12 ± 0.03 g/L and 0.69 ± 0.00 g/L for strains JABs24, MG12 and MG14 after 36 h, 32 h and 44 h, respectively, before the concentration of biomass dropped. In addition, cell agglutination was visible with progressive cultivation causing difficulties in OD600 measurements. However, strong induction of PnasD activity was detected, suggesting that cell agglutination does not counteract nasD expression.
Fig. 3.
Time course of cell dry weight (CDW) [g/L] (a) and surfactin concentration [mg/L] (b) of strains B. subtilis JABs24 (reference), MG12 (PnarG-lacZ; amyE::[PnarG-lacZ, spcR]) and MG14 (PsrfA::PnasD; amyE::[PnasD-lacZ, spcR]) cultivated in a mineral salt medium with 40 g/L glucose, 0.1 mol/L NH4+ and 0.1 mol/L NO3− employing three different relative filling volumes of 10%, 50 and 100% representing different oxygen availabilities
As expected, strains MG12 and MG14 did not synthesize surfactin at 10% Rv (Fig. 3b). For the reference strain JABs24, the maximum surfactin concentration was measured after 20 h of cultivation with 1668 ± 87 mg/L. During subsequent bacterial growth, the concentration of surfactin declined to 146 ± 118 mg/L at CDWmax. The surfactin concentration of the reference strain was even higher with 2806 ± 68 mg/L in 50% Rv. Under this condition, strain MG12 produced up to 215 ± 69 mg/L and strain MG14 up to 1189 ± 15 mg/L. In 100% Rv, lowest surfactin titer was detected for strain MG12 with 193 mg/L, surfactinmax of strain MG14 surpassed the titer of strain JABs24 by ~ 200 mg/L with 696 ± 19 mg/L. With respect to foam formation, this issue could be observed for strain JABs24 from the beginning in 10% and 50% Rv, while foam formation was time-delayed for strains MG12 and MG14. At 100% Rv, foam was not present in any cultivation due to the high fluid level and low turbulences.
The corresponding Miller units of strains MG12 and MG14 are displayed in Additional file 1: Fig. S2. In sum, MU values of strain MG12 were similar to strain MG1 (Fig. 2). For strain MG14, MU values in 10% and 50% Rv were also comparable to the cultivation of strain MG5 (Fig. 2). At 100% Rv, highest MU values for MG14 did not surpass ~ 1100 MU and remained relatively constant until CDWmax.
In all cultivations, glucose was not depleted. With respect to the nitrogen sources, displayed in Additional file 1: Fig. S3, the patterns were similar as illustrated in Fig. 1 with one exception. In 50% Rv, nitrate consumption and hence increase in ammonium were more conspicuous in strains MG12 and MG14 than for the reference strain. After ~ 32 h of cultivation, nitrate was almost depleted and until this time point ammonium increased.
Strain B. subtilis MG14 reaches remarkable YP/X values
All yields and growth rates of the cultivation in 10%, 50% and 100% Rv are displayed in Table 2. B. subtilis MG12 reached highest overall and maximum growth rates in all cultivations. In 10% and 50% Rv, JABs24 had lowest overall and maximum growth rates. Using 100% Rv, however, lowest growth rates were monitored for strain MG14. A trend amongst the strains for the YX/S was not identified. Highest Rv and hence lowest oxygen availability resulted in lowest YX/S. As no surfactin was produced in 10% Rv for strain MG12 and MG14, no data could be calculated for YP/X, YP/S and q. In accordance with surfactin concentrations, strain JABs24 was superior with respect to YP/X, YP/S and q in cultivations using 50% Rv. Noticeable, strain MG14 reached an overall YP/X of 1.007 ± 0.028 g/g in 100% Rv, representing an excellent result. In addition, the overall and maximum productivity q was the highest for strain MG14 in 100% Rv with 0.023 ± 0.001 g/(g h) and 0.209 ± 0.012 g/(g h), even surpassing the productivities of strain JABs24 in 10% and 50% Rv.
Table 2.
Comparison of calculated overall and ∆tmax yields and specific growth rates µ of shake flask cultivations with different relative filling volumes employing strains B. subtilis JABs24 (reference), MG12 (PsrfA::PnarG amyE::[PnarG-lacZ, spcR]) and MG14 (PsrfA::PnasD amyE::[PnasD-lacZ, spcR])
| Relative filling volume | B. subtilis strain | Overall yield to CDWmax | ||||
|---|---|---|---|---|---|---|
| YP/X [g/g] | YX/S [g/g] | YP/S [g/g] | q [g/(g h)] | µ [1/h] | ||
| 10% | JABs24 | 0.021 ± 0.001 | 0.216 ± 0.026 | 0.005 ± 0.001 | 0.001 ± 0.000 | 0.144 ± 0.013 |
| MG12 | 0.000 ± 0.000 | 0.175 ± 0.030 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.223 ± 0.001 | |
| MG14 | 0.000 ± 0.000 | 0.239 ± 0.055 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.185 ± 0.002 | |
| 50% | JABs24 | 0.346 ± 0.242 | 0.129 ± 0.000 | 0.044 ± 0.031 | 0.007 ± 0.005 | 0.116 ± 0.002 |
| MG12 | 0.026 ± 0.004 | 0.183 ± 0.012 | 0.005 ± 0.001 | 0.001 ± 0.000 | 0.130 ± 0.000 | |
| MG14 | 0.191 ± 0.009 | 0.181 ± 0.019 | 0.035 ± 0.002 | 0.004 ± 0.000 | 0.122 ± 0.005 | |
| 100% | JABs24 | 0.413 ± 0.002 | 0.090 ± 0.006 | 0.036 ± 0.002 | 0.011 ± 0.000 | 0.111 ± 0.001 |
| MG12 | 0.173 ± 0.015 | 0.120 ± 0.064 | 0.020 ± 0.010 | 0.005 ± 0.000 | 0.126 ± 0.003 | |
| MG14 | 1.007 ± 0.028 | 0.051 ± 0.006 | 0.053 ± 0.005 | 0.023 ± 0.001 | 0.079 ± 0.000 | |
| Relative filling volume | B. subtilis strain | ∆tmax | ||||
|---|---|---|---|---|---|---|
| YP/X [g/g] | YX/S [g/g] | YP/S [g/g] | q [g/(g h)] | µ [1/h] | ||
| 10% | JABs24 | 1.081 ± 0.039 | 0.535 ± 0.181 | 0.194 ± 0.001 | 0.141 ± 0.008 | 0.369 ± 0.022 |
| MG12 | 0.000 ± 0.000 | 0.289 ± 0.032 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.775 ± 0.006 | |
| MG14 | 0.000 ± 0.000 | 0.339 ± 0.093 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.527 ± 0.005 | |
| 50% | JABs24 | 1.273 ± 0.095 | 0.629 ± 0.156 | 0.217 ± 0.037 | 0.169 ± 0.002 | 0.343 ± 0.001 |
| MG12 | 0.080 ± 0.007 | 0.331 ± 0.059 | 0.304 ± 0.279 | 0.020 ± 0.002 | 0.833 ± 0.078 | |
| MG14 | 0.476 ± 0.005 | 0.451 ± 0.130 | 0.629 ± 0.554 | 0.119 ± 0.001 | 0.471 ± 0.003 | |
| 100% | JABs24 | 0.642 ± 0.015 | 0.208 ± 0.015 | 0.099 ± 0.017 | 0.090 ± 0.001 | 0.205 ± 0.007 |
| MG12 | 0.303 ± 0.030 | 0.291 ± 0.036 | 0.030 ± 0.012 | 0.076 ± 0.008 | 0.388 ± 0.015 | |
| MG14 | 1.061 ± 0.179 | 0.161 ± 0.089 | 0.243 ± 0.135 | 0.209 ± 0.012 | 0.151 ± 0.002 | |
Bioreactor cultivations
As a rule of thumb, filling volume in shake flasks should not exceed 10% (Rv = 0.1 mL/mL) of flask nominal volume to ensure sufficient oxygen supply. However, the previous shake flask cultivations have demonstrated that reference strain JABs24 reached highest surfactin titer at a 50% Rv, and even at 100% Rv both YP/X and q were promising. In the latter condition, B. subtilis MG14 with PsrfA::PnasD even surpassed the surfactin titers, YP/X and q of the reference strain. Consequently, the next step aimed at transferring these results to bioreactor scale.
Reference process and preliminary pO2 strategies
CDW, surfactin and pO2 levels of different processes employing B. subtilis JABs24 or MG1 are given in Fig. S4. A conventional batch process with foam centrifuge employing 20 g/kg glucose was performed as control with an initial agitation and aeration rate of 300 rpm and 2.0 L/min and a pO2 set-point of 20% (Additional file 1: Fig. S4a). CDWmax was reached after 24 h with 77.51 g prior to glucose depletion. Simultaneously, surfactinmax was reached with 57.43 g. Nitrate was almost constant throughout cultivation, while ammonium was reduced by ~ 50%. In addition, slight persistent foaming occurred which was accompanied by an increase in the pressure due to blocked exhaust air filters. In a subsequent fed-batch approach, the aeration rate was stopped after 21 h of cultivation and switched to N2 based ventilation (Additional file 1: Fig. S4b). At this time point, the pO2 was already at 20% for ~ 5 h. When the process air was stopped, the pO2 dropped to 0% within seconds. However, also the CDW dropped from 50.27 g to 3.67 g within 4.5 h. Surfactin reached 29.48 g prior to the change in pO2 and was reduced slightly when CDW dropped. However, cells restored the growth and after 41.5 h of cultivation, a biomass of 24.1 g was obtained with a slight increase in surfactin to 32 g. In a next approach, the pO2 was decreased stepwise to ideally avoid the observed drop of CDW by cell adaptation (Fig. S4c). Each pO2 level of 20%, 10%, 5% and 0% was kept for 3 h before aeration was switched to N2. In this approach, the CDW as well as surfactin still dropped, but to a much lower extent, from 80.74 g to 61.75 g, and 39.84 g to 34.80 g, respectively. Both CDW and surfactin increased afterwards and reached maximum values of 73.26 g and 41.91 g.
Comparison of strains B. subtilis KM1016 and MG14 with 1.2 L/min aeration rate employing a self-regulated HNO3 feed
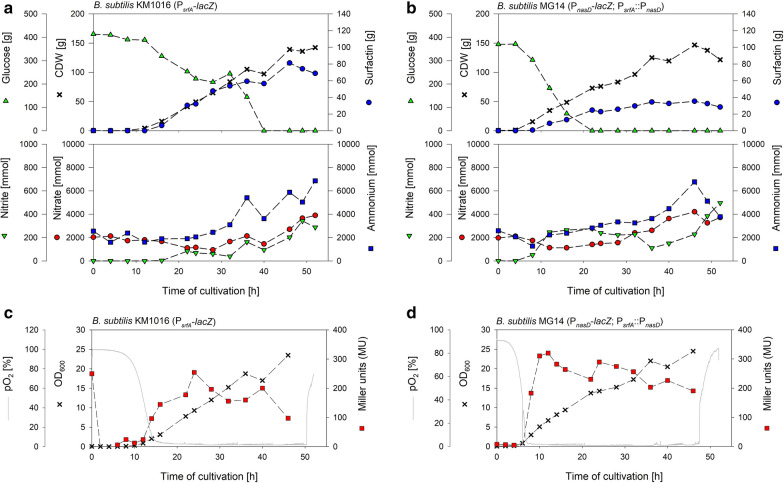
Due to the observed drops in CDW and the concomitant stagnating surfactin production, another approach was tested employing a constant aeration and agitation rate throughout the cultivation. This should result in a decline of available oxygen per cell and hence an adaptation of cells to microaerobic conditions. First, a fed-batch cultivation was performed with a constant aeration rate of 2 L/min employing the reporter strain MG1 to monitor PnarG expression, which is exclusively activated anaerobically. Cells grew without drop and reached a CDWmax of 196 g after 40 h. Surfactin values increased constantly during growth to a maximum of 104 g (Fig. S4d). Miller units revealed that only a small portion of cells adapted to anaerobic conditions. During Miller Assay, the yellow color as indicator to stop the reaction was visible in less than 3 min, but calculation revealed 5 MU, as the high OD600 of 30 is included in the calculation. Hence, it can be assumed that a high expression level of the promoter is present, but most cells did not grow by nitrate respiration. A cultivation with 1.2 L/min and strain MG1, which actually represents the lowest aeration rate that could be set with the given technical equipment, resulted in Miller units up to 35 for PnarG and was hence used as process to compare B. subtilis KM1016 and MG14 with PsrfA::PnasD. Figure 4 exemplarily illustrates the CDW, glucose, surfactin, nitrate, nitrite and ammonium values of a cultivation with B. subtilis KM1016 (Fig. 4a) and B. subtilis MG14 (Fig. 4b). In addition, OD600 in correlation to Miller units and pO2 are given in Fig. 4c and d for strains KM1016 and MG14, respectively. Comparable CDWmax values were reached for strain KM1016 and MG14 after 46 h with 140 g and 147 g, respectively, whereas the growth was terminated by glucose depletion. In contrast, different surfactinmax were reached at CDWmax with 81 g for KM1016 and 35 g for MG14. Strain KM1016 consumed a total of 5.6 mol nitrate until CDWmax. Nitrate addition by HNO3 was higher than consumption, resulting in a nitrate accumulation to 2.7 mol in the medium. Strain MG14 showed a similar behavior but consumed less nitrate. At CDWmax, total nitrate consumption was 4.5 mol and another 4.2 mol were detectable in the medium.
Fig. 4.
Exemplary cultivation results of fed-batch bioreactor cultivations employing strains B. subtilis KM1016 (PsrfA-lacZ) (a, c) and B. subtilis MG14 (PsrfA::PnasD; amyE::[PnasD-lacZ, spcR]) (b, d). Displayed are absolute values of cell dry weight (CDW), glucose, surfactin (all [g]), nitrate, nitrite and ammonium (all [mmol]) (a, b), as well as the time course of OD600, Miller units as determined by the Miller Assay to determine the expression of PsrfA and PnasD, and pO2 [%] (c, d)
The detection of ammonium showed an initial slight decrease before the amount increased steadily for both strains due to nitrate reduction and reached 5.8 mol and 6.7 mol for strains KM1016 and MG14, respectively. Nitrite concentrations increased until 22 h in both cultivations. After a subsequent decrease until ~ 30 h, nitrite again increased steadily and reached values of ~ 0.21 mol at CDWmax. The pO2 declined faster in the cultivation of strain MG14 which correlated to higher growth rates (Fig. 4d). For strain KM1016, a pO2 of ~ 14% was reached after ~ 15 h, and mean pO2 of ~ 1.8% was measured after 25 h of cultivation (Fig. 4c). On the contrary, the pO2 of strain MG14 fell to ~ 2% after 7 h of cultivation, and a mean pO2 of ~ 1.2% was reached after ~ 15 h of cultivation. For both strains, the pO2 increased drastically when cell growth was terminated. In terms of foaming, the foam centrifuge was activated right from the beginning for strain KM1016 and after ~ 6 h for strain MG14. With respect to the Miller units, PsrfA expression was detectable as soon as cells grew after a short lag-phase. A maximum of 250 MU was determined after 24 h of cultivation and values slightly decreased afterwards (Fig. 4c). For PnasD, induction levels were below 5 MU until 6 h of cultivation. In accordance with the pO2, induction increased to 180 MU after 8 h when the pO2 was < 2%. Highest expression was detected after 12 h with 320 MU before expression declined slightly (Fig. 4d).
Table 3 summarizes the main parameters and overall yields of the cultivations with strains KM1016 and MG14 employing an aeration rate of 1.2 L/min. For comparison, data of the reference process with B. subtilis JABs24 and the processes employing a constant aeration rate of 2 L/min with strain MG1 are included. Corresponding calculated Δtmax yields and growth rates are listed in Additional file 1: Table S3. For fed-batch processes and for better comparison to the reference batch cultivation, results are displayed as “after batch glucose consumption” calculations and “end of process” calculations. The fed-batch process employing the reference strain and 1.2 L/min reached only 82.14 g of surfactin, which resulted in a productivity q of 0.012 g/(g h). This value is similar to the 2 L/min fed-batch process with 0.013 g/(g h), where 104.57 g of surfactin were produced, but with biomass of 196.97 g. Comparing batch data, the reference batch process had a productivity q of 0.031 g/(g h). Here, the 2 L/min process was inferior with 0.022 g/(g h) and the 1.2 L/min process performed similar with 0.030 g/(g h). Comparing strains KM1016 and MG14, both processes reached comparable CDW of 147.52 g and 150.18 g, but the surfactin produced with strain MG14 with 31.56 g was ~ 2.5-fold lower. Hence, both productivities and yields were inferior for strain MG14 and solely the growth rates during the batch phase were superior.
Table 3.
Summary of main process data, calculated overall yields and specific growth rates of B. subtilis bioreactor cultivations
| Strategy | Reference pO2 set-point 20% | Fed-batch, constant aeration of 2 L/min | Fed-batch, constant aeration of 1.2 L/min |
Fed-batch, constant aeration of 1.2 L/min |
|||
|---|---|---|---|---|---|---|---|
| Evaluation until | End of batch | After batch glucose consumption | End of process | After batch glucose consumption | End of process | After batch glucose consumption | End of process |
| B. subtilis strain | JABs24 | MG1 | KM1016 | MG14 | |||
| Time of cultivation (h) | 24 | 22 | 40 | 26 | 46 | 19 | 46 |
| CDWmax (g) | 77.51 | 104.55 | 196.97 | 61.54 ± 9.21 | 147.52 ± 8.22 | 55.78 ± 13.24 | 150.18 ± 3.33 |
| Surfactinmax (g) | 57.43 | 51.65 | 104.57 | 48.47 ± 11.81 | 82.14 ± 0.91 | 15.49 ± 6.40 | 31.56 ± 3.74 |
| YP/X (g/g) | 0.741 | 0.494 | 0.531 | 0.776 ± 0.088 | 0.557 ± 0.024 | 0.266 ± 0.039 | 0.211 ± 0.030 |
| YX/S (g/g) | 0.145 | 0.249 | 0.195 | 0.161 ± 0.011 | 0.131 ± 0.005 | 0.134 ± 0.016 | 0.123 ± 0.003 |
| YP/S (g/g) | 0.108 | 0.124 | 0.103 | 0.127 ± 0.022 | 0.073 ± 0.001 | 0.036 ± 0.010 | 0.026 ± 0.004 |
| q (g/(g∙h)) | 0.031 | 0.022 | 0.013 | 0.030 ± 0.003 | 0.012 ± 0.001 | 0.014 ± 0.002 | 0.004 ± 0.001 |
| µ (1/h) | 0.222 | 0.272 | 0.165 | 0.204 ± 0.009 | 0.134 ± 0.005 | 0.271 ± 0.010 | 0.128 ± 0.003 |
Discussion
The current study aimed at introducing a promoter exchange strain that allows for self-inducible surfactin synthesis when oxygen-limiting, or even depleting, conditions occur and anaerobic nitrate respiration initiates. To mimic different oxygen availabilities in shake flask cultivations, either the filling volume or the agitation rate can be varied. This method was already used in other studies investigating surfactin synthesis in B. subtilis (Fahim et al. 2012; Jokari et al. 2013; Rangarajan et al. 2015; Rocha et al. 2020). In the current study, different filling volumes were employed. Studies of Schiefelbein et al. (2013) and Heyman et al. (2019), investigating either the kLa value or oxygen transfer rate OTR, have demonstrated that higher filling volumes result in lower oxygen availability. Results from this study supported the hypothesis when nitrogen sources were analyzed at different Rv resulting in either aerobic growth on ammonium as preferred nitrogen source, or anaerobic growth by nitrate respiration.
Experiments have demonstrated that B. subtilis MG14 with PsrfA::PnasD poses an interesting candidate for further bioreactor process developments targeting at self-inducible surfactin synthesis uncoupled from the native quorum-sensing system. In addition, this self-inducible system is advantageous compared to IPTG-induced promoters due to reduced production costs. Furthermore, foaming was also lowered, and time delayed in both shake flask and bioreactor cultivations. Interestingly, both strains MG12 (PsrfA::PnarG) and MG14 showed improved growth rates when no surfactin was produced in 10% Rv. At both 50% and 100% Rv, increased growth rates were obtained with decreasing surfactin titers. Hence, expression and presence of surfactin seems to result in reduced growth rates, which is in accordance with previous studies (Tsuge et al. 2001; Vahidinasab et al. 2020). Shake flask cultivations employing different Rv have been shown to be a suitable approach to investigate the activation of the promoters PnarG and PnasD. In the presented set-up, the reference strains MG1 and MG5 reached Miller units of up to 2000 for PnasD and 180 for PnarG, respectively. Hence, PnasD expressions were even slightly higher than reported by Hoffmann et al. (2020) when cultivating in anaerobic serum flasks. On the contrary, PnarG expression levels were reported to reach up to 440 MU (Hoffmann et al. 2020). Consequently, as PnarG is an exclusively anaerobically activated promoter, the combination of 100% Rv at 120 rpm probably did not result in full anaerobic induction. One possibility to further decrease the oxygen input would be to vary the agitation rate as performed by Fahim et al. (2012). However, cell agglutination already occurred at 120 rpm and is expected to appear even earlier at lower agitation rates. Hence, as nitrate was often observed as growth limiting factor, increasing the nitrate concentration to elongate the cultivation would not be possible under these circumstances. One option would be to perform further strain engineering and delete genes responsible for biofilm formation and thus for cell agglutination (Pedrido et al. 2013). Assuming microaerobic conditions at 100% Rv and 120 rpm, strain MG14 reached up to ~ 3-fold higher PnasD expression than PsrfA in the reference strain. On the contrary, the surfactin concentration was only 1.4-fold increased. Feedback mechanisms or nutrient limitations might result in this observed discrepancy, and further studies must investigate which optimizations have to be performed on a molecular level or in operational parameter to reach a 3-fold increase in surfactin titer to exploit the complete potential of PnasD.
As reported by Hoffmann et al. (2020), high ammonium and glucose concentrations reduced expression of PnarG, but such a reductive effect was not observed for PnasD. Still, fine-tuning of the medium is important to find a good balance between sufficient ammonium for aerobically growing cells, and cells performing nitrate respiration, hence providing ammonium. Particularly, the effect of different nitrate concentrations on the expression of both PnarG and PnasD has not been examined so far. This is also of utmost importance when employing a self-regulated HNO3-feed as pH regulator. Indeed, the amount of HNO3 added was higher than nitrate consumption due to the production of various metabolites that alkalize the medium on top to the pH shift caused by nitrate respiration, resulting in a continuous increase in nitrate. In addition, the amount of HNO3 needed is three-times higher than for e.g. H3PO4 (monovalent vs. trivalent acid). This results in further limitations as the bioreactor capacity is exhausted earlier. With the current set-up it cannot be examined if both oxygen-depleted and nitrate-depleted conditions reach even higher YP/X values as reported by Davis et al. (1999). For this purpose, mathematical modeling of the bioprocess combining a metabolic model of the nitrogen metabolism, oxygen availability and consumption, along with mechanisms of genetic regulation can be used to provide further insights into this complex interplay. Using this toolset, investigation and evaluation of a dual-limitation fed-batch process becomes feasible, which is a very demanding task for process control, as both limitations need to be tightly regulated (Noll and Henkel 2020).
With respect to bioreactor cultivations, the original intention was to perform so called switch-processes, with the first part aiming at biomass accumulation, and the second part resulting in surfactin production. However, a drastic decrease in biomass was observed when cells entered oxygen-limited or even depleted conditions too fast. It was recently reported that ~ 90% of cells died upon oxygen depletion, while the remaining cells maintain their viability (Arjes et al. 2020). Similar observations were made in the current employed processes with drop in CDW. Although cells restored their growth, the surfactin productivity of these cells was reduced. Consequently, when targeting at switch processes with different pO2-profiles, it is of utmost importance to relate oxygen availability to adaptation of cells to anaerobic conditions. For example, Hoffmann et al. (1995) reported on a lag-phase of 24 to 36 h when aerobically growing cells were transferred to anaerobic conditions. This would result in time-consuming processes and the increased growth rates observed in aerobic non-surfactin producing cells would probably not compensate for these adaptation times. In a further approach, a cultivation with a constant agitation rate of 300 rpm and aeration rate of 1.2 L/min was tested. This process set-up was identified as easily operable and issues due to foam formation were drastically reduced compared to conventional batch and fed-batch processes with varying agitation and aeration rates due to pO2 regulation. In addition, foaming occurred time-delayed in strain MG14 compared to the reference strain KM1016 which can be attributed in parts to the absence of surfactin during the first hours of cultivation. However, foam formation was yet present prior to the induction of surfactin production. In this sense, identification of other metabolites, such as proteins, in foam and deletion of corresponding genes might be a target in future works to further reduce the foam formation. Results of both surfactin titer and promoter activity of PnasD also clearly indicated that the employed set-up more likely correlated to shake flask cultivations with 50% Rv. Also based on the Miller units obtained, it can be assumed that most cells still grew aerobically. This leads to the open question how many cells have already adapted to anaerobic conditions and it is crucial to further characterize the cell differentiation patterns in aerobically-anaerobically growing cells. Hence, further bioreactor cultivations must be performed with even lower aeration rates, either by applying air with a lower oxygen concentration (currently 16%), pure oxygen or, of course, by adjusting the technical equipment to allow for aeration rates below 1.2 L/min. Ideally, applying pure oxygen at much lower aeration rates (< 0.2 L/min) would result in two advantages. First, even less foam formation is expected to occur, and second, the autoinduction of surfactin synthesis is expected to be much higher as the available amount of oxygen per cell decreases faster. Another issue faced was the drastic accumulation of nitrite in bioreactor cultivations, which was not observed in shake flasks. At these concentrations, nitrite is reported to reduce and even inhibit cell growth (Hoffmann et al. 2020). Prior to further bioreactor process optimization, studies must address this limitation and investigate the defect of nitrite reduction to ammonium. Nevertheless, even under the current bioreactor process conditions, strain MG14 with a YP/X of 0.211 g/g and productivity q of 0.004 g/(g h) was already superior to other non-conventional bioreactor processes reported by Chtioui et al. (2012) (Rotating disc bioreactor, YP/X = 0.068 g/g, q = 0.001 g/(g h)) Coutte et al. (2010b) (Membrane bioreactor, YP/X = 0.078 g/g, q = 0.002 g/(g h)). A comparative table was prepared previously in Hoffmann et al. (2020). A foam-free anaerobic cultivation was reported to reach a promising YP/X with 0.278 g/g (Willenbacher et al. 2015), but as summarized by Hoffmann et al. (2020), complete anaerobic processes are time-consuming due to low growth rates and the overall surfactin titers are by far not competitive to conventional aerobic batch processes. With further appropriate regulation of the nitrogen sources and aeration, processes employing strain MG14 are expected to become superior to the reference strain JABs24 as demonstrated in shake flask cultivations with a YP/X of 1.007 g/g and productivity q of 0.023 g/(g h). Reaching these yields in bioreactor cultivations, strain MG14 will also surpass reported yields of an IPTG-inducible promoter with YP/X of 0.92 and q of 0.025 g/(g h) (calculated based on data given in Jiao et al. (2017)), being additionally advantageous due to the proposed cheap, foam-reduced and self-inducible system.
To conclude, a self-inducible B. subtilis strain for surfactin synthesis induced upon oxygen-limitation has been developed. First, two interesting promoters, PnarG and PnasD that are involved in anaerobic nitrate respiration, were tested. PnarG has the advantage of being an exclusively anaerobically activated promoter, but surfactin titers and yields were shown to not be competitive to the reference strain B. subtilis JABs24. However, strain MG14 with PsrfA::PnasD surpassed the titer of strain JABs24 by 200 mg/L at low oxygen availability and reached an exceptionally high YP/X of 1.007 g/g. Strain B. subtilis MG14 poses an interesting candidate for further surfactin production process development. Additional process developments are crucial to elaborate the high potential of strain B. subtilis MG14 as self-inducible surfactin producer in reduced foaming or even foam-free environments.
Supplementary Information
Additional file 1. Information on plasmids and oligonucleotides used, main process data for bioreactor cultivations as well as the availability of nitrogen sources and their impact on target promoter expression during bioprocesses. Figure S1: Time course of CDW and PnasDexpression under different availability of nitrogen sources.Figure S2: Time course of PnarG and PnasD expression in MG12 and MG14 under different relative filling volumes. Figure S3: Availability of nitrate, nitrite and ammonium during cultivation of MG12, MG14 and JABs24 under different relative filling volumes. Figure S4: Strategies for oxygen-mediated bioreactor switch-processes using JABs24 and MG1.
Acknowledgements
The authors thank Eike Grunwaldt (Department of Bioprocess Engineering, University of Hohenheim) for technical assistance.
Authors’ contributions
MHo planned and executed the experiments, collected data, created the graphs and drafted the whole manuscript. AB and DSFC performed part of the experiments and collected and evaluated corresponding data. KR, PB, CT and PK were involved in strain engineering. MHe supported in interpretation of results. LL significantly contributed to conception and design of the study and interpretation of the experiments. RH substantially contributed to conception and design of the conducted experiments. All authors read and approved the final manuscript.
Funding
This study was financially supported by the German Research Foundation (DFG), Project number 365166982.
Availability of data and materials
All discussed data have been included into the manuscript or in the additional file. Please turn to the corresponding author for all other requests.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arjes HA, Lam V, Dunn CL, Kearns DB, Huang KC. Biosurfactant-mediated membrane depolarization maintains viability during oxygen depletion in Bacillus subtilis. Curr Biol. 2020;30:1–12. doi: 10.1016/j.cub.2020.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtioui O, Dimitrov K, Gancel F, Dhulster P, Nikov J. Rotating discs bioreactor, a new tool for lipopeptides production. Process Biochem. 2012;5:2020. doi: 10.1016/j.procbio.2012.07.013. [DOI] [Google Scholar]
- Coutte F, Leclère V, Béchet M, Guez J-S, Lecouturier D, Chollet-Imbert M, Kessler D, et al. Effect of pps disruption and constitutive expression of srfA on surfactin productivity, spreading and antagonistic properties of Bacillus subtilis 168 derivatives. J Appl Microbiol. 2010;109:480–491. doi: 10.1111/j.1365-2672.2010.04683.x. [DOI] [PubMed] [Google Scholar]
- Coutte F, Lecouturier D, Ait Yahia S, Leclère V, Béchet M, Jacques P, Kessler D. Production of surfactin and fengycin by Bacillus subtilis in a bubbleless membrane bioreactor. Appl Microbiol Biotechnol. 2010;87(2):499–507. doi: 10.1007/s00253-010-2504-8. [DOI] [PubMed] [Google Scholar]
- Davis DA, Lynch HC, Varley J. The production of Surfactin in batch culture by Bacillus subtilis ATCC 21332 is strongly influenced by the conditions of nitrogen metabolism. Enzyme Microbial Technol. 1999;25(3–5):322–329. doi: 10.1016/S0141-0229(99)00048-4. [DOI] [Google Scholar]
- Davis DA, Lynch HC, Varley J. The application of foaming for the recovery of Surfactin from B. subtilis ATCC 21332 cultures. Enzyme Microbial Technol. 2001;28(4–5):346–354. doi: 10.1016/S0141-0229(00)00327-6. [DOI] [PubMed] [Google Scholar]
- Fahim S, Dimitrov K, Gancel F, Vauchel P, Jacques P, Nikov I. Impact of energy supply and oxygen transfer on selective lipopeptide production by Bacillus subtilis BBG21. Biores Technol. 2012;126:1–6. doi: 10.1016/j.biortech.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Falardeau J, Wise C, Novitsky L, Avis TJ. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol. 2013;39(7):869–878. doi: 10.1007/s10886-013-0319-7. [DOI] [PubMed] [Google Scholar]
- Geissler M, Oellig C, Moss K, Schwack W, Henkel M, Hausmann R. High-performance thin-layer chromatography (HPTLC) for the simultaneous quantification of the cyclic lipopeptides Surfactin, Iturin A and Fengycin in culture samples of Bacillus species. J Chromatogr B. 2017;1044–1045:214–224. doi: 10.1016/j.jchromb.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Geissler M, Heravi KM, Henkel M, Hausmann R. Lipopeptide biosurfactants from Bacillus species. In: Hayes DG, Solaiman DKY, Ashby RD, editors. Biobased surfactants . Amsterdam: Elsevier; 2019. pp. 205–240. [Google Scholar]
- Geissler M, Kühle I, Morabbi Heravi K, Altenbuchner J, Henkel M, Hausmann R. Evaluation of surfactin synthesis in a genome reduced Bacillus subtilis strain. AMB Express. 2019;9(1):1–4. doi: 10.1186/s13568-019-0806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig E, Jahn D. Regulation of the anaerobic metabolism in Bacillus subtilis. Adv Microbial Physiol. 2012;61:195–216. doi: 10.1016/B978-0-12-394423-8.00005-6. [DOI] [PubMed] [Google Scholar]
- Henkel M, Geissler M, Weggenmann F, Hausmann R. Production of microbial biosurfactants: Status quo of rhamnolipid and surfactin towards large-scale production. Biotechnol J. 2017;12(7):1600561. doi: 10.1002/biot.201600561. [DOI] [PubMed] [Google Scholar]
- Heyman B, Lamm R, Tulke H, Regestein L, Büchs J. Shake flask methodology for assessing the influence of the maximum oxygen transfer capacity on 2,3-butanediol production. Microbial Cell Factory. 2019;18(1):78. doi: 10.1186/s12934-019-1126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann T, Troup B, Szabo A, Hungerer C, Jahn D. The anaerobic life of Bacillus subtilis: cloning of the genes encoding the respiratory nitrate reductase system. FEMS Microbiol Lett. 1995;131(2):219–225. doi: 10.1111/j.1574-6968.1995.tb07780.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Fernandez Cano Luna DS, Xiao S, Stegemüller L, Rief K, Morabbi Heravi K, Kessler D. Towards the anaerobic production of surfactin using Bacillus subtilis. Front Bioeng Biotechnol 2020;8:16. doi: 10.3389/fbioe.2020.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Liu Y, Li S. Rational strain improvement for surfactin production: enhancing the yield and generating novel structures. Microbial Cell Factory. 2019;18(1):42. doi: 10.1186/s12934-019-1089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Li X, Yu H, Yang H, Li X, Shen Z. In situ enhancement of surfactin biosynthesis in Bacillus subtilis using novel artificial inducible promoters: in situ enhancement of surfactin biosynthesis. Biotechnology Bioengineering. 2017;114(4):832–842. doi: 10.1002/bit.26197. [DOI] [PubMed] [Google Scholar]
- Jokari S, Rashedi H, Amoabediny Gh, Dilmaghani N, Mazaheri Assadi M. Optimization of surfactin production by Bacillus subtilis ATCC 6633 in a miniaturized bioreactor. Int J Environ Res. 2013;7(4):851–858. [Google Scholar]
- Kim H-S, Yoon B-D, Lee C-H, Suh H-H, Oh H-M, Katsuragi T, Kessler D. Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. J Ferment Bioeng. 1997;84(1):41–46. doi: 10.1016/S0922-338X(97)82784-5. [DOI] [Google Scholar]
- Li Y, Héloir M, Zhang X, Geissler M, Trouvelot S, Jacquens L. Surfactin and fengycin contribute to the protection of a Bacillus subtilis strain against grape downy mildew by both direct effect and defence stimulation. Mol Plant Pathol. 2019;20:1037–1050. doi: 10.1111/mpp.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Zuber P, Glaser P, Danchin A, Hulett FM. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J Bacteriol. 1996;178(13):3796–3802. doi: 10.1128/JB.178.13.3796-3802.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Hoffmann T, Zhu Y, Jahn D. Nitrogen and oxygen regulation of Bacillus subtilis nasDEF encoding NADH-dependent nitrite reductase by TnrA and ResDE. J Bacteriol. 1998;180:7. doi: 10.1128/jb.180.20.5344-5350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas P, Mäder U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Kessler D. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science. 2012;335(6072):1103–1106. doi: 10.1126/science.1206848. [DOI] [PubMed] [Google Scholar]
- Noll P, Henkel M. History and evolution of modeling in biotechnology: modeling & simulation, application and hardware performance. Comput Struct Biotechnol J. 2020;18:3309–3323. doi: 10.1016/j.csbj.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrido ME, de Oña P, Ramirez W, Leñini C, Goñi A, Grau R. Spo0A links de novo fatty acid synthesis to sporulation and biofilm development in Bacillus subtilis: Lipid synthesis and development. Mol Microbiol. 2013;87(2):348–367. doi: 10.1111/mmi.12102. [DOI] [PubMed] [Google Scholar]
- Rangarajan V, Dhanarajan G, Sen R. Bioprocess design for selective enhancement of fengycin production by a marine isolate Bacillus megaterium. Biochem Eng J. 2015;99:147–155. doi: 10.1016/j.bej.2015.03.016. [DOI] [Google Scholar]
- Rocha PM, dos Santos Mendes AC, de Oliveira Júnior SD, de Araújo Padilha CE, de Sá Leitão ALO, da Costa Nogueira C, Kessler D. Kinetic study and characterization of surfactin production by Bacillus subtilis UFPEDA 438 using sugarcane molasses as carbon source. Preparative Biochemistry & Biotechnology. 2020;1–9 [DOI] [PubMed]
- Schiefelbein S, Fröhlich A, John GT, Beutler F, Wittmann C, Becker J. Oxygen supply in disposable shake-flasks: prediction of oxygen transfer rate, oxygen saturation and maximum cell concentration during aerobic growth. Biotech Lett. 2013;35(8):1223–1230. doi: 10.1007/s10529-013-1203-9. [DOI] [PubMed] [Google Scholar]
- Sun H, Bie X, Lu F, Lu Y, Wu Y, Lu Z, Sun H, Bie X, Lu F, Lu Y, Wu Y, Lu Z. Enhancement of surfactin production of Bacillus subtilis fmbR by replacement of the native promoter with the Pspac promoter. Can J Microbiol. 2009;55:1003–1006. doi: 10.1139/W09-044. [DOI] [PubMed] [Google Scholar]
- Tsuge K, Ohata Y, Shoda M. Gene yerP, involved in surfactin self-resistance in Bacillus subtilis. Antimicrob Agents Chemother. 2001;45(12):3566–73. doi: 10.1128/AAC.45.12.3566-3573.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahidinasab M, Lilge L, Reinfurt A, Pfannstiel J, Henkel M, Morabbi Heravi K, Kessler D. Construction and description of a constitutive plipastatin mono-producing Bacillus subtilis. Microb Cell Fact. 2020;19(1):205. doi: 10.1186/s12934-020-01468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yu H, Wang M, Yang H, Shen Z. Enhanced biosynthesis and characterization of surfactin isoforms with engineered Bacillus subtilis through promoter replacement and Vitreoscilla hemoglobin co-expression. Process Biochem. 2018;70:36–44. doi: 10.1016/j.procbio.2018.04.003. [DOI] [Google Scholar]
- Wenzel M, Altenbuchner J. Development of a markerless gene deletion system for Bacillus subtilis based on the mannose phosphoenolpyruvate-dependent phosphotransferase system. Microbiology. 2015;161(10):1942–1949. doi: 10.1099/mic.0.000150. [DOI] [PubMed] [Google Scholar]
- Willenbacher J, Zwick M, Mohr T, Schmid F, Syldatk C, Hausmann R. Evaluation of different Bacillus strains in respect of their ability to produce Surfactin in a model fermentation process with integrated foam fractionation. Appl Microbiol Biotechnol. 2014;98(23):9623–9632. doi: 10.1007/s00253-014-6010-2. [DOI] [PubMed] [Google Scholar]
- Willenbacher J, Rau J-T, Rogalla J, Syldatk C, Hausmann R. Foam-free production of Surfactin via anaerobic fermentation of Bacillus subtilis DSM 10T. AMB Express. 2015;5(1):1–9. doi: 10.1186/s13568-014-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbacher J, Mohr T, Henkel M, Gebhard S, Mascher T, Syldatk C, Kessler D. Substitution of the native srfA promoter by constitutive Pveg in two B. subtilis strains and evaluation of the effect on Surfactin production. J Biotechnol. 2016;224:14–17. doi: 10.1016/j.jbiotec.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Yao S, Zhao S, Lu Z, Gao Y, Lv F, Bie X. Control of agitation and aeration rates in the production of surfactin in foam overflowing fed-batch culture with industrial fermentation. Revista Argentina de microbiologìa. 2015;47(4):344–349. doi: 10.1016/j.ram.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Yeh M-S, Wei Y-H, Chang J-S. Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Process Biochem. 2006;41(8):1799–1805. doi: 10.1016/j.procbio.2006.03.027. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Information on plasmids and oligonucleotides used, main process data for bioreactor cultivations as well as the availability of nitrogen sources and their impact on target promoter expression during bioprocesses. Figure S1: Time course of CDW and PnasDexpression under different availability of nitrogen sources.Figure S2: Time course of PnarG and PnasD expression in MG12 and MG14 under different relative filling volumes. Figure S3: Availability of nitrate, nitrite and ammonium during cultivation of MG12, MG14 and JABs24 under different relative filling volumes. Figure S4: Strategies for oxygen-mediated bioreactor switch-processes using JABs24 and MG1.
Data Availability Statement
All discussed data have been included into the manuscript or in the additional file. Please turn to the corresponding author for all other requests.