Abstract
The orbitofrontal cortex (OFC) is involved in diverse brain functions via its extensive projections to multiple target regions. There is a growing understanding of the overall outputs of the OFC at the population level, but reports of the projection patterns of individual OFC neurons across different cortical layers remain rare. Here, by combining neuronal sparse and bright labeling with a whole-brain florescence imaging system (fMOST), we obtained an uninterrupted three-dimensional whole-brain dataset and achieved the full morphological reconstruction of 25 OFC pyramidal neurons. We compared the whole-brain projection targets of these individual OFC neurons in different cortical layers as well as in the same cortical layer. We found cortical layer-dependent projections characterized by divergent patterns for information delivery. Our study not only provides a structural basis for understanding the principles of laminar organizations in the OFC, but also provides clues for future functional and behavioral studies on OFC pyramidal neurons.
Electronic supplementary material
The online version of this article (10.1007/s12264-020-00616-1) contains supplementary material, which is available to authorized users.
Keywords: Orbitofrontal cortex, Whole-brain imaging, Morphological reconstruction, Output, Projection pattern
Introduction
Understanding the principle of how information is processed within neural networks is the central goal of neuroscience. The past decade has witnessed tremendous progress in elucidating the connections of neural networks at the mesoscale level, such as the Mouse Connectome Project and the Allen Mouse Brain Connectivity Atlas [1, 2]. These large projects greatly enhance our understanding of the brain-wide projections of diverse cell types at the population level, however, they fail to define the detailed projection pathways and corresponding targeted brain regions of specific single neurons. To address this problem, much more recent attention has been paid to the dissection of single neurons within neural networks [3–5]. The complete morphologies of > 1,000 projection neurons in the motor cortex (MO), thalamus (TH), subiculum, and hypothalamus (HY) have been reconstructed, and this facilitates our understanding not only of the principles of information processing but also of the classification of cell types [5]. As the basic unit of neural networks, single neurons receive multiple sources of input information through the synaptic connections formed at their numerous dendritic spines and broadcast the integrated information to diverse cortical and subcortical brain regions via long-range projecting axons. To dissect the specific roles of single neurons, a critical step is to obtain their detailed morphologies, including brain-wide axonal projections along with the corresponding somal locations and dendritic morphologies.
The orbitofrontal cortex (OFC; or orbital cortex), one of the main subregions of the prefrontal cortex besides its medial and lateral counterparts, has attracted much recent attention due to its crucial roles in diverse brain functions [6–9]. It has many advanced executive functions, including controlling and suppressing inappropriate behaviors, emotional responses, and decision-making [10–14]. OFC injury usually results in motivation imbalance and failure to predict behavioral consequences, leading to abnormal behaviors, such as obsessive-compulsive disorder or depression [15–19]. Moreover, functional imaging studies suggest that the medial OFC region is involved in pure emotional processing, especially negative emotions, while the lateral OFC region is especially involved in positive emotions [20, 21]. Recently, a study using optogenetic and in vivo electrophysiology demonstrated that the ventrolateral OFC participates in memory retrieval [22]. These functional differences strongly suggest the presence of anatomical differences, and extensive research has attempted to elucidate the functions of these subregions. These complex functions of emotional regulation are due to the extensive OFC neuron fiber connections with other parts of the brain [23–27]. In other words, the OFC is involved in these functions via its brain-wide long-range projections to form connections with multiple brain regions. To unveil the detailed anatomical structures beneath this neural circuitry, great efforts have been made during the last past decades, which fall into two main categories: sectioning-based three-dimensional (3D) reconstruction and population labeling-based whole-brain imaging. In the first category, several studies have used injections of anterograde or retrograde tracers to label a limited number or even individual neurons in the OFC, then brain samples were sectioned manually followed by reconstruction with the assistance of 3D reconstruction systems such as Neurolucida [6, 28–33]. In spite of substantial progress, these strategies suffer from several shortcomings: (i) manual sectioning usually results in fragmented axons and uncertain reconstructed projections; (ii) due to manual sectioning, the reconstruction of a whole brain sample is time-consuming and labor-intensive; (iii) direct comparisons between individual neurons in the same layer or across different layers within a whole-brain sample are far from achievable; and (iv) signals of fluorescent dyes are prone to fade out at distal axonal arborizations, leading to incomplete reconstructions. For population labeling-based whole-brain imaging, researchers applied fluorescent protein-expressing adeno-associated viral vectors (e.g., AAV-EGFP) along with uninterrupted whole-brain imaging systems (e.g., serial two-photon tomography) to obtain brain-wide projections of OFC populations, which provide guidelines for the overall projection patterns [1]. However, the axons and dendrites of adjacent neurons intermingle in these studies and fail to be separated easily due to dense labeling. Overall, few reports [4] so far have demonstrated the precise projection pathways of individual neurons in the OFC. Furthermore, compared with increasing comprehensive understanding of the projections of individual neurons in other cortical regions, such as primary and secondary MO and visual cortex (VIS) [3–5], knowledge about the projection patterns of individual OFC neurons with great completeness and accuracy are still lacking.
To address these issues, we sparsely and brightly labeled neurons in the OFC in mice and performed whole-brain imaging using the dual-color fluorescence micro-optical sectioning tomography (fMOST) system [34] at a voxel size of 0.3 × 0.3 × 1 µm3. Based on our uninterrupted 3D whole-brain dataset, we achieved complete morphological reconstructions of 8 neurons located in layer 5b (L5b) and 17 intratelencephalic (IT) neurons located in layers 2/3 (L2/3). With the aid of propidium iodide (PI) staining, we located and compared the brain-wide projection targets of these neurons in different cortical layers as well as in the same layer. Our study not only facilitates our understanding of the laminar organization of the OFC but also provides detailed anatomical clues for further functional and behavior studies related to OFC pyramidal neurons.
Materials and Methods
Animals and Virus Injection
Two-month-old male C57BL/6J mice and a special co-packaged AAV were used. The mice were from Hunan SJA Laboratory Animal Co., Ltd (Hunan, China) and housed under a 12-h light/dark cycle (19:00 dark and 07:00 light) in a strict specific pathogen-free environment. The special co-packaged AAV was produced as in our previous report (https://www.biorxiv.org/content/10.1101/705772v1) by BrainVTA Co., Ltd (Wuhan, China). All vectors involved in the virus production were provided by BrainVTA and extracted using a Qiagen EndoFree Plasmid Maxi Kit (cat. no.12362, Qiagen, Hilden, Germany) following the manufacturer’s instructions. Briefly, a Cre-expressing vector (AAV-CMV-Cre, 1.2 µg/µL) and a Cre-dependent vector (AAV-EF1α-double floxed-EYFP, 1.0 µg/µL, Addgene #20296, Cambridge, MA, USA) [35] were premixed at a ratio of 1:200,000 before transfection into HEK293T cells, followed by the standard processes of AAV production as in previous studies [36]. The serotype was AAV 2/9 and the titer of the co-packaged AAV was determined by quantitative real-time PCR and estimated to be 7.0 × 1012 viral genomes (vg)/mL.
For virus injection, the mice were deeply anaesthetized by 2% isoflurane (cat. no. R510-22-4, RWD Life Science, Shenzhen, China) in oxygen and then placed on a stereotaxic apparatus (World Precision Instruments, Florida, USA). A small hole was made in the right skull with a dental drill. The injection coordinates of the OFC were 2.9 mm anteroposterior, 1.8 mm mediolateral, and 2.5 mm dorsoventral from bregma according to the Paxinos Mouse Brain Atlas [37]. In the Allen Brain Atlas (http://mouse.brain-map.org/), the injection site of the OFC is also known as agranular insular area, dorsal part. All virus solutions with a volume of 100 nL were injected through a glass micropipette at 10 nL/min, the micropipette was left for an additional 10 min before removal. After virus injection, the mice were housed individually with careful care. All animal experiments were approved by the Institutional Animal Ethics Committee of Huazhong University of Science and Technology.
Tissue Preparation and Imaging
Three weeks after viral injection, the mice were transcardially perfused with 0.01 mol/L phosphate-buffered saline (PBS, cat. no. P3813, Sigma-Aldrich, St. Louis, MO, USA) followed by 4% paraformaldehyde (PFA, cat. no. 158127, Sigma-Aldrich, St. Louis, MO, USA) in 0.01 mol/L PBS. Each brain was extracted and post-fixed overnight at 4°C.
For the preparation of frozen sections and confocal imaging, brain samples were placed in 30% sucrose (cat. no. V900116, Sigma-Aldrich, St. Louis, MO, USA) for 48 h–72 h after overnight post-fixation. Frozen sections were cut at 50 μm on a freezing microtome (Leica CM1950, Nussloch, Germany). Sections with labeled cells were screened out under a slide-scanning microscope (Nikon Ni-E, Tokyo, Japan). All the representative brain slices were counterstained with 4,6-diamidino-2-phenylindole (DAPI, cat. no. C1002, Beyotime Biotechnology, Shanghai, China) (5 μg/mL) to determine the cortical and laminar borders. Images were acquired with a confocal laser-scanning microscope (LSM710; Carl Zeiss, Oberkochen, Germany). For the imaging of whole coronal sections, a 10× (NA 0.5) objective with a zoom of 1 was used for the injection site and a 20× (NA 0.8) objective with a zoom of 0.6 was used for long-range axonal projections. Regions of interests were all imaged in z-stacks (one-section spacing) with 10× or 20× for labeled somata and 40× (NA 1.4) oil for the fine structure of dendrites and axonal arborizations.
For resin embedding of whole-brain samples with Technovit 9100 Methyl Methacrylate (MMA, Electron Microscopy Sciences, Hatfield, PA, USA), the PFA post-fixed brains were first dehydrated in a graded series of ethanols and then immersed in xylene to make them transparent. Next, the samples were treated with a graded series of infiltration solutions. Finally, the samples were transferred into gelatin capsules and immersed in a polymerization solution. The details of the sample procedures were as in previous reports [38, 39]. Among the resin-embedded samples, we randomly selected one for whole-brain imaging. Because different brain subregions had different shrinkage ratios during resin embedding, it was very difficult to estimate the shrinkage factor.
The 3D whole-brain dataset was acquired with the fMOST [34] (Wuhan, China). The resin-embedded mouse brain was immersed in 0.05 mol/L Na2CO3 (cat. no. 10019260, Sinopharrm Chemical Reagent, Shanghai, China) and PI (cat. no. P1304MP, Thermo Fisher Scientific, Massachusetts, USA) solutions (2 μg/mL) during the whole-brain imaging process. The fMOST system performed multiple cycles of automatic sectioning with an axial step size of 1 μm followed by imaging at a voxel size of 0.3 × 0.3 × 1 µm3 with two channels (one for EYFP-labeled neurons and the other for PI-stained cytoarchitecture). Each coronal section was acquired with 16-bit and 8-bit depth for the EYFP and PI channels, respectively. After 10 days of uninterrupted sectioning and imaging, a 3D whole-brain dataset containing a total of 10,100 coronal plane images was obtained and uploaded to the data center for subsequent processing.
Image Processing
Generally, the whole-brain dataset was processed using Amira software (Visage Imaging Inc., San Diego, CA, USA) and the processing of all the fluorescent images used ImageJ (National Institutes of Health, Bethesda, USA), which included the spilt and merge channels, stacks Z project, 3D project, and adjust functions. The whole-brain dataset underwent several pre-processing procedures, including seamless stitch to obtain the entire coronal sections, ribbon stripping and matching the coronal sections of two channels with pixel adjustment, as previously reported [38]. The pre-processed images were stored in LZW compression TIFF format with 16-bit depth for the green channel and 8-bit depth for the PI channel. To generate a 3D view showing brain-wide long-range projections in the OFC brain sample (Fig. 2), we selected 9740 continuous coronal sections from the green-channel data set and resampled to 3 × 3 × 3 μm3 before loading them into Amira software. To generate the contour of the brain sample, coronal sections from the red channel were resampled to 25 × 25 × 100 μm3 for convenience. To demonstrate the raw signals in Fig. S2, coronal sections with maximum-intensity projections were processed with two or three-fold downsampling (0.6 × 0.6 or 1 × 1 μm2) due to the limit of image size. Fine structures including somata, axonal branches, and axonal arborizations were all selected from corresponding coronal sections at the original resolution (0.3 × 0.3 μm2). The projection strengths of axonal terminals of the 8 L5b neurons were calculated as in previous reports [4, 40].
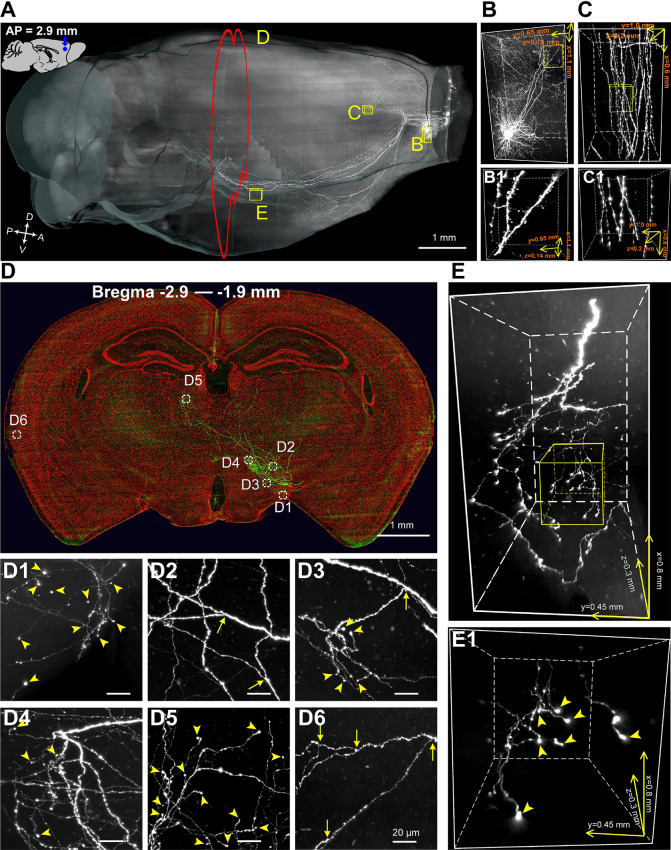
Fig. 2.
Whole-brain imaging of a sparsely-labeled mouse brain. A Sagittal view of a whole mouse brain reconstructed from 9,740 consecutive coronal sections (each 1 µm thick) with downsampling of 3 × 3 × 3 µm3. B, C 3D rendering of the yellow boxes in A. B Fine morphology of a single neuron in the injection site (volume: 1.10 × 0.65 × 0.14 mm3). C Projecting axons in the corpus callosum (cc, volume: 0.60 × 1.00 × 0.20 mm3). B1, C1 Enlargements of the yellow boxes in B and C showing dendritic spines of apical dendrites (B1) and individual axons and axonal en-passant boutons in the cc (C1). D Maximum-intensity projections of 1000 coronal sections (green, thickness: 1 µm each) 5 mm distant from the injection site (white coronal plane in A). PI-staining signals (5 µm thick) are shown in red. Note the composite image is downsampled to 0.9 × 0.9 × 1 µm3 for display. D1–D6 Enlargements of the white dashed boxes in D 400 × 400 µm2 in size. Yellow arrows, branch points; arrowheads, terminal boutons. E 3D rendering of a data block with a volume of 0.90 × 0.70 × 0.26 mm3 showing axonal arborizations in the lateral hypothalamus. E1 Enlargement of the yellow box in E; yellow arrowheads, terminal boutons.
Individual Neuron Reconstruction
We randomly selected 25 OFC neurons from the whole-brain dataset and two skilled technicians independently manually traced them using Amira software. We traced the long-range axonal projections of the labeled neurons in the retrograde direction, that is, from the terminal projection targets to the parent axon and finally to the soma. To reconstruct the 8 L5b neurons, tracing was initiated from various subcortical targets, such as the principal sensory nucleus of the trigeminal (PSV), pyramid (py), periaqueductal gray (PAG), and striatum-like amygdalar nuclei (sAMY). To reconstruct the 17 IT neurons, tracing was initiated from the ipsilateral/contralateral MO and caudatoputamen (CP). In each cycle of tracing, a data block with a fixed size (1000 × 1000 × 400 μm3) was imported into the Amira software. During the tracing process, disagreements were solved by flexibly adjusting the brightness of the raw data signal and the thickness of the data block. Neurons with uncertainties were excluded from the final results. The reconstruction of the dendritic morphology of all neurons was done in a manner similar to reconstruction by axonal tracing, but in a separate profile.
Localization of Long-range Axonal Projections
Abbreviations and definitions of the brain regions were as in the Allen Brain Atlas. To localize cell bodies and the destinations of axonal projections, each coronal section in the whole-brain dataset was registered to the Allen Brain Atlas with the assistance of PI signals. We were conservative with the localization of nuclei in the TH and sAMY. For instance, we found that the axons of OFC neurons were distributed among a collection of diverse subregions in the TH, including the mediodorsal, ventromedial, and centromedial nuclei and the parafascicular nucleus (PF). However, due to our inability to differentiate them precisely, we included the projections in several subregions of the TH as a whole. In contrast, only two subregions, the lateral habenula in the epithalamus and the medial geniculate complex (MG) in the geniculate group in the dorsal TH were extracted from the TH as exceptions. A similar case was found in the sAMY which included several subregions such as the medial amygdalar nucleus, anterior amygdalar area, and bed nucleus of the accessory olfactory tract, so the localization of projection targets that were found in these regions were also treated as a whole.
Results
Strategy for Sparse Neuronal Labeling with Strong Brightness in the OFC
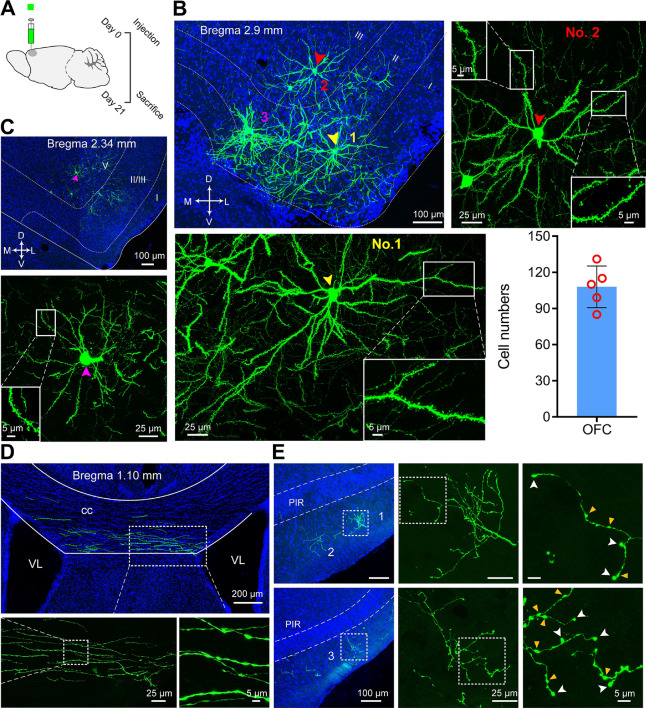
To achieve sparse and super-bright neuronal labeling in the OFC, we used the following criteria for assessment: (1) the labeled somata in the injection site were as sparse as possible and were well separated; (2) the fine structure of the labeled neurons, such as dendrites and dendritic spines, were visualized clearly and proximal dendrites belonging to different neurons did not intermingle; and (3) long-range projections, especially terminal axonal arborizations far from the injection site, were also labeled with high brightness. Our previous report (https://www.biorxiv.org/content/10.1101/705772v1) has shown that the special co-packaged AAV can achieve sparse and super-bright neuronal labeling in the MO. However, because different brain regions contain different types of cells, it was not clear that the co-packaged AAV (see Methods for details) displayed sparse and super-bright neuronal labeling in the OFC. Therefore, we first injected the co-packaged AAV into the OFC of 5 wild-type mice (Fig. 1A). Three weeks later, the mouse brains were sectioned at 50 μm on a freezing microtome and imaged with a confocal laser scanning microscope. The total numbers of labeled neurons at the injection sites in these brains were 85, 99, 110, 115, and 131 (114 ± 7; mean ± SEM). These labeled neurons were distributed from layer 2 (L2) to layer 6 (L6) and preferentially resided in the superficial layers (L2/3) within sections proximal to the injection site followed by a gradual alteration to deeper layers (L5/6) along the anterior-posterior axis (Fig. 1B, C). All of the labeled neurons were well-separated and exhibited extremely high brightness and fine structure such as dendritic spines and multiple local axons surrounding the dendrites of the labeled neurons were clearly visualized (Fig. 1B, C).
Fig. 1.
Strategy for sparse neuronal labeling with strong brightness. A Schematic of the experimental design and timeline from virus injection to data analysis. B Representative coronal section showing the fine structures of L2/3 pyramidal neurons. Upper left panel, low-magnification image showing the distributions of 3 L2/3 pyramidal neurons (labeled neurons 1–3). Lower left and upper right panels, high-magnification images showing the fine structures of neuron 1 (L2, lower left, yellow arrowhead) and neuron 2 (L3, upper right, red arrowhead). Insets are enlargements of white boxes showing different parts of dendrites of respective neurons. Lower right panel, quantification of labeled neurons in the injection site (n = 5 mice, each red circle represents one mouse). C A representative coronal section showing the fine structures of an L5 pyramidal neuron. Upper panel, low-magnification image indicating the somal location of the selected L5 pyramidal neuron. Lower panel, high-magnification image showing the fine structures of this neuron (purple arrowhead). Insets, enlarged view of region in white box showing basal dendrites. D A representative coronal section showing sparse main axons in the cc, originating from the injection site and projecting to the contralateral hemisphere. E Two sequential coronal sections showing long-range projections in the PIR. Distributions of 3 axon terminal structures are shown in low-magnification images (left panel); two of them marked by the dashed box are enlarged progressively from middle to right panels displaying two types of boutons: en passant (yellow arrowheads) and terminal boutons (white arrowheads). Dashed lines define the boundaries of cortical layers. I-V, cortical layers I to V; cc, corpus callosum; VL, lateral ventricle; PIR, piriform area.
We next examined the brightness of the long-range axonal projections of these labeled neurons far from the injection site. In general, these projections displayed two projection pathways: one projected to the contralateral hemisphere through the corpus callosum (cc) and the other projected subcortically (Figs. 1D and S1). We found projection targets of these neurons in diverse cortical regions, such as several ipsilateral and contralateral subdivisions of the OFC (lateral, medial, and ventrolateral), subdivisions of the medial prefrontal cortex (e.g., prelimbic area), several neocortical areas (e.g., motor, somatosensory, and retrosplenial areas), and several limbic cortical areas (e.g., piriform (PIR), ectorhinal, and perirhinal areas), as well as diverse subcortical regions, such as the CP, amygdala, TH, ventral tegmental area (VTA), substantia nigra, pars reticulata, and superior colliculus (SC) in the midbrain, in accordance with previous reports (Fig. S1) [6, 28–30, 33]. Furthermore, the fine structure of long-range projections, especially terminal axonal arborizations in several regions (e.g., PIR), finally ended with numerous brightly-labeled terminal boutons (Fig. 1E). These results indicated that the co-packaged AAV can label neurons in the OFC with not only excellent sparseness at the injection site but also great brightness of their local and long-range projections, and thus is suitable for single-neuron tracing across the whole brain.
Whole-brain Imaging with fMOST
Though the co-packaged AAV can achieve sparse and super-bright neuronal labeling in the OFC, it was still difficult to reconstruct the fragmented axons of individual neurons in different layers using a classical confocal microscope. To address this issue, we exploited fMOST [34], a whole-brain florescence imaging system, to systematically and automatically section and image a whole mouse brain and obtained continuous images, allowing the reliable and efficient reconstruction of an uninterrupted 3D whole-brain image.
After labeling the OFC region with the co-packaged AAV, the whole brain was embedded in resin and imaged by fMOST via two channels: one for EYFP-labeled neurons and the other for PI-stained cytoarchitecture. The whole-brain dataset was acquired at a voxel resolution of 0.3 × 0.3 × 1 µm3. The resin-embedding process required by fMOST system before imaging markedly reduced the fluorescence signal of EYFP. Fortunately, the addition of 0.05 mol/L Na2CO3 during the whole-brain imaging process recovered the EYFP fluorescence up to 90% and EYFP-labeled neurons were also well-preserved [38, 39, 41].
After 10 days of uninterrupted sectioning and imaging, a whole-brain dataset containing 10,100 frames of coronal sections was obtained (Fig. 2A). In this dataset were a total of 162 neurons distributed from L2 to L6, of which 123 were located in the superficial layers (L2 and L3) and 38 in the deeper layers (L5 and L6) (Table S1). Previous studies [42, 43] have shown that cortical pyramidal neurons and interneurons can be distinguished by several criteria, including somal size, cell body shape, and whether they contain evident apical and basal dendrites or their dendrites are covered with numerous spines. Based on these criteria, 142 of the labeled neurons were identified as cortical pyramidal neurons, and 20 were interneurons (Figs. 3, S3, and Table S1). We also found that the labeled interneurons were dim: their brightness was much lower than the labeled pyramidal neurons (Fig. S3). The brightness difference may be caused by two factors: (1) neurons usually absorb AAV particles through receptor-mediated endocytosis [44]. The size of the cell body and the surface area of the neurites (e.g., dendrites and dendritic spines) affect the number of AAV particles absorbed by each cell. Compared with pyramidal neurons, interneurons have smaller cell bodies and fewer dendrites and dendritic spines, possibly leading to less absorption of AAVs, and (2) the AAV might have greater tropism for pyramidal neurons than interneurons [44, 45]. Therefore, it is worth noting that the ratio of each neuron type labeled here may not reflect the actual ratio of cell types, and some cell types may be ignored due to the tropism of the AAV labeling system for specific cell types [44, 45].
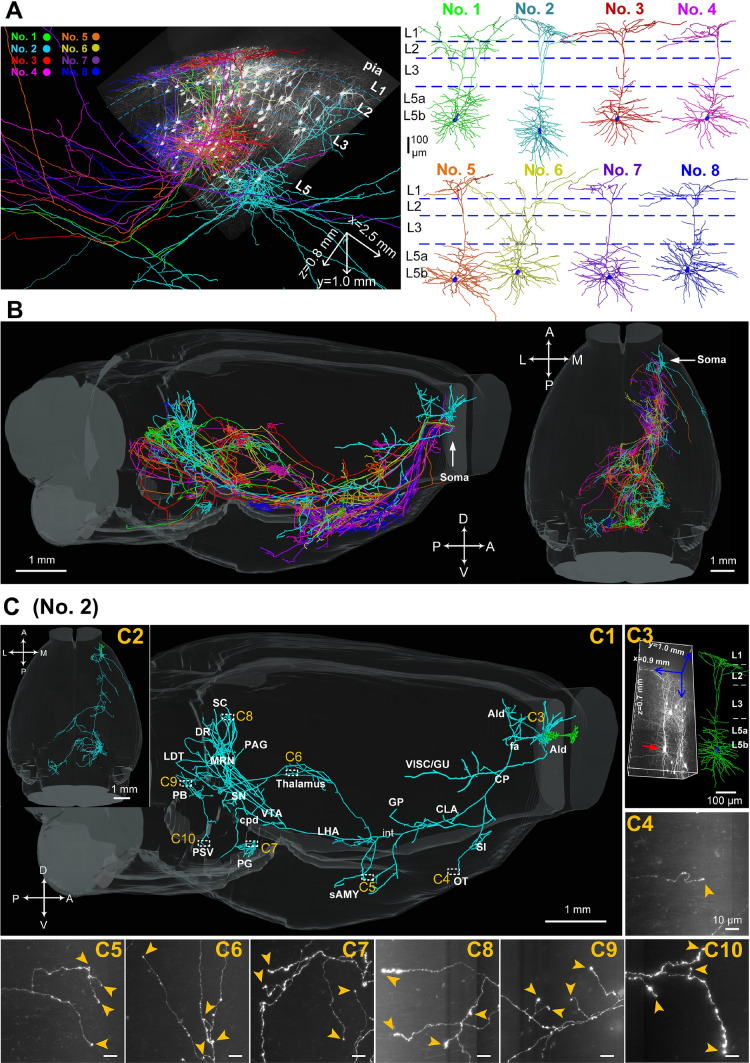
Fig. 3.
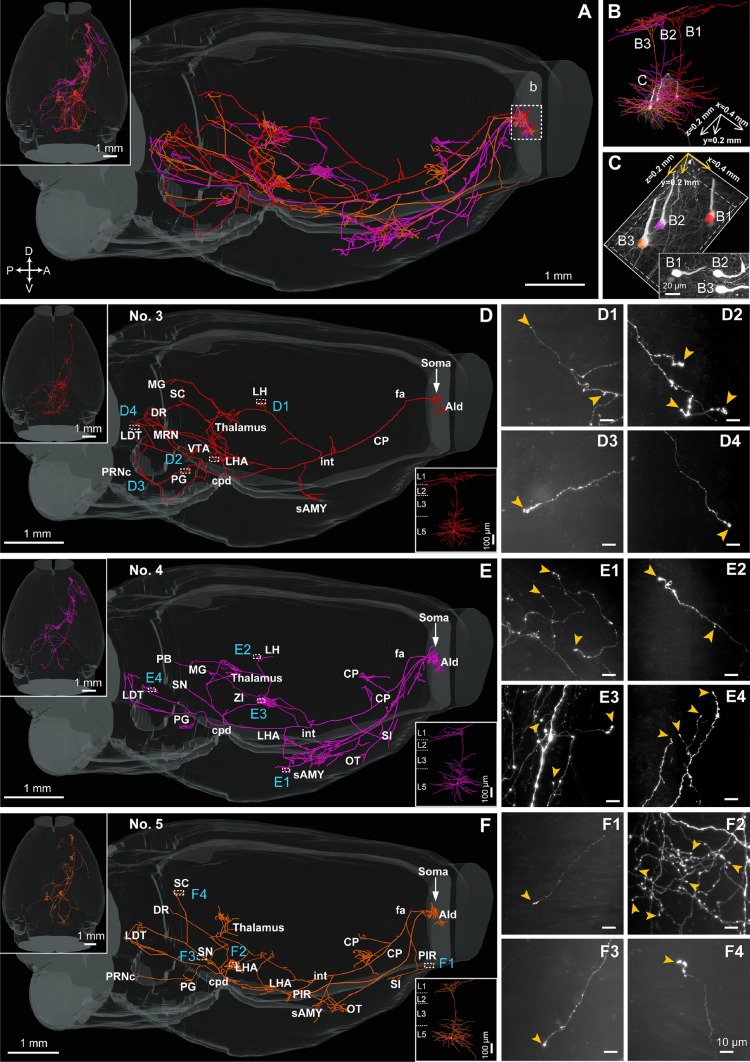
Reconstructions of the complete morphologies of L5b neurons in the OFC. A Left, overview of the cell body locations, dendrites, and axons of 8 L5b neurons (#1–8) from a data block with a volume of 2.5 × 1.0 × 0.8 mm3. Right, dendrites of the 8 L5b neurons displayed in different colors (blue dashed lines, approximate laminar borders). B Sagittal (left panel) and horizontal views (right panel) demonstrating the brain-wide axonal morphologies of the 8 L5b neurons (white arrows, locations of cell bodies). Axons of each neuron are listed in specific colors matching the dendrites in A. C Comprehensive brain-wide projection targets of a representative L5b neuron (#2) selected from B in sagittal (C1) and horizontal (C2) views (green, dendrites; cyan, axons). C3 Left panel, raw data with a volume of 0.9 × 1.0 × 0.7 mm3 containing neuron #2 (red arrow). Right panel, reconstructed dendritic morphology (green). Cell body is shown in blue. C4–C10 Enlargements of terminals in the OT (C4), sAMY (C5), thalamus (C6), PG (C7), SC (C8), PB (C9), and PSV (C10) (scale bars, 10 µm). All images are 200 µm thick maximum-intensity projections 300 × 300 µm2 in size (yellow arrowheads, terminal boutons). C4–C10 scale bar 10 µm. AId, agranular insular area, dorsal part; CLA, claustrum; CP, caudatoputamen; cpd, cerebral peduncle; DR, dorsal nucleus raphe; fa, corpus callosum, anterior forceps; GP, globus pallidus; GU, gustatory area; int, internal capsule; LDT, laterodorsal tegmental nucleus; LHA, lateral hypothalamic area; MRN, midbrain reticular nucleus; OT, olfactory tubercle; PAG, periaqueductal gray; PB, parabrachial nucleus; PG, pontine gray; PSV, principal sensory nucleus of the trigeminal; sAMY, striatum-like amygdalar nuclei; SC, superior colliculus; SI, substantia innominata; SN, substantia nigra; VISC, visceral area; VTA, ventral tegmental area.
In the whole-brain dataset, the dendrites of the individual neurons as well as the main axons emerged from the injection site (Fig. 2) and some passed through the cc and projected to the contralateral hemisphere (Fig. 2C). They were easily distinguished from each other in the 3D view due to the sparse and bright neuronal labeling. On the other hand, in the continuous whole-brain sectioning, the long-range axonal projections for several centimeters from the injection site exhibited tremendous complexity and great degree of completeness with several orders of branches (Figs. 2D–E and S2G–O). Overall, the whole-brain dataset containing sparsely and super-brightly labeled neurons and PI-stained cytoarchitecture made it feasible to reconstruct the complete morphology of individual neurons and locate their precise positions and projections.
Individual Neuron Reconstructions in the OFC
To reveal the detail projection pathways of individual neurons in the OFC, we randomly selected 25 pyramidal neurons from the 162 labelled neurons in the whole-brain dataset and manually reconstructed their morphologies. We had to do the reconstruction manually because there was no automated or semi-automatic software to help us at the time. Fortunately, more and more automatic or semi-automatic software, such as GTree [46] and the semi-automated reconstruction pipeline [5] are becoming available to improve the reconstruction efficiency. We determined the locations of the cell bodies and destinations of the axonal projections according to the Allen Brain Atlas with the assistance of PI signals (Fig. S4). Previous reports [5, 47, 48] have shown that pyramidal tract neurons, also known as subcortical projection neurons, project to the midbrain (MB), brainstem, and spinal cord. Their axons project to the ipsilateral cortex, striatum (STR), and TH but do not cross the cc. In contrast, IT neurons only project their axons within the telencephalon (neocortex, STR, and cortical structures). Based on these criteria, among the 25 pyramidal neurons only one (#1) sent its axons into the pyramid (py) and was regarded as a pyramidal tract neuron while all of the 8 L5b neurons (#1–8) projected to multiple subcortical regions, such as CP, TH, HY, MB, and medulla were considered to be corticofugal neurons. The other 17 neurons located in L2/3 only projected to the ipsilateral/contralateral cerebral cortex and STR, and were regarded as IT neurons.
Neurons in Layer 5b of the OFC
The 8 neurons in L5b displayed a broad projection and multi-source projection patterns which broadcast information from cell bodies to diverse subcortical brain regions, including the cortical plate, cortical subplate, STR, pallidum, TH, HY, MB, pons, and pyramidal tract, in accordance with the previous report [39] (Fig. 3A, B). Remarkably, a representative neuron (#2) had 20 projection regions with a broad coverage of cortical projection targets in ipsilateral visceral/gustatory areas as well as numerous subcortical targets, such as the olfactory tubercle (OT) and sAMY in the STR, TH, lateral hypothalamic area (LHA) in the HY, VTA, substantia nigra (SN, pars reticulata and pars compacta), midbrain reticular nucleus, PAG and SC in the MB, dorsal raphe, laterodorsal tegmental nucleus, parabrachial nucleus (PB), pontine gray (PG) and PSV in the pons (Fig. 3C), suggesting that individual OFC pyramidal neurons maintain connections with brain regions regulating diverse functions such as fear, anxiety, reward, punishment, and depression [19, 49–53].
The reconstruction results also showed that the 8 neurons (#1–#8) were convergent with their overall projection pathway but diverged in the number of termination regions and the projection strengths in specific regions (Figs. 4 and S5). First, the parent axons of all 8 neurons travelled in a similar path which descended through the cc, anterior forceps (fa) to the CP followed by the internal capsule (int) and cerebral peduncle (cpd) (cell body→fa→CP→int→cpd) (Fig. S7). Furthermore, the axonal collaterals projecting to the CP, OT, and substantia innominata were usually emitted from parent axons in the CP; the axonal collaterals projecting to the sAMY, diagonal band nucleus, TH, and HY were generally emitted from parent axons in the int, and the axonal collaterals with projections to diverse MB regions, including the VTA, SN, SC, PAG, dorsal raphe, and midbrain reticular nucleus and diverse regions in the pons including the PG, pontine reticular nucleus, caudal part (PRNc), laterodorsal tegmental nucleus, PB, and PSV were frequently emitted from parent axons in the cpd (Fig. S7A). However, the 8 corticofugal neurons differed in their terminations: five (#2, 4–7) terminated in the PG (Figs. 3C, 5E, F and S5 B, C); two extended beyond the PG and finally terminated in the py (#1) (Fig. S5A) and PRNc (#3) (Fig. 5D); and in contrast, only one neuron (#8) terminated in the PAG rather than the PG (Fig. S5D). None of the 8 reconstructed corticofugal neurons had projections in the nucleus accumbens. However, axon collaterals were found in both the ipsilateral and contralateral nucleus accumbens (Fig. S2 H); they may belong to labeled neurons other than the 8 reconstructed L5b neurons.
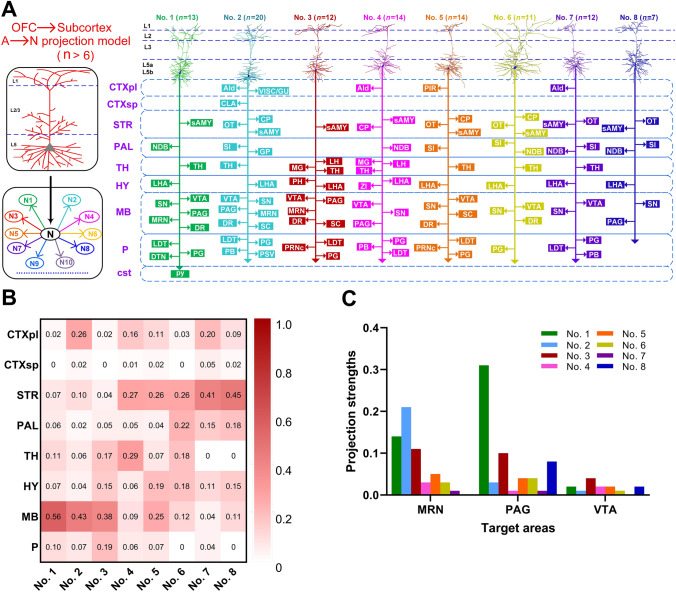
Fig. 4.
Projections of the 8 neurons in L5b of the OFC. A Projection patterns of the 8 reconstructed L5b neurons. Left panel, proposed projection patterns. Right panel, summary of the detailed projection targets. B Projection strengths in ipsilateral and contralateral target areas. C Projection strengths in the MB subnuclei. CTXpl, cortical plate; CTXsp, cortical subplate; STR, striatum; PAL, pallidum; TH, thalamus; HY, hypothalamus; MB, midbrain; P, pons; AId, agranular insular area, dorsal part; AI, agranular insular area; CLA, claustrum; CP, caudatoputamen; DR, dorsal nucleus raphe; DTN, dorsal tegmental nucleus; GP, globus pallidus; GU, gustatory; LDT, laterodorsal tegmental nucleus; LH, lateral habenula; LHA, lateral hypothalamic area; MG, medial geniculate complex; MO, motor cortex; MRN, midbrain reticular nucleus; NDB, diagonal band nucleus; OT, olfactory tubercle; PAG, periaqueductal gray; PB, parabrachial nucleus; PG, pontine gray; PH, posterior hypothalamic nucleus; PIR, piriform area; PRNc, pontine reticular nucleus, caudal part; PSV, principal sensory nucleus of the trigeminal; py, pyramid; sAMY, striatum-like amygdalar nuclei; SC, superior colliculus; SI, substantia innominata; SN, substantia nigra; SSs, supplemental somatosensory area; TC, temporal cortex; VISC, visceral area; VTA, ventral tegmental area; ZI, zona incerta.
Fig. 5.
Comparison of the brain-wide axonal projections of 3 L5b neurons. A Overview of the axonal morphologies of the 3 neurons (#3, red; #4, pink; and #5, orange; dashed white box indicates location of cell body). B Dendritic morphologies of the 3 neurons in A. C 3D view (volume: 400 × 200 × 200 µm3) showing the locations of the 3 neurons in B (inset, maximum-intensity projection of the data block). D–F Detailed annotations of the projected brain regions of these 3 neurons. D1–D4, E1–E4, and F1–F4 Raw data 300 × 300 × 200 µm3 size, emphasizing the terminal boutons in each region (white arrows, locations of the 3 neurons; yellow arrowheads, terminal boutons), and the scale bar of them are all 10 µm. AId, agranular insular area, dorsal part; CP, caudatoputamen; cpd, cerebral peduncle; DR, dorsal nucleus raphe; fa, corpus callosum, anterior forceps; int, internal capsule; LDT, laterodorsal tegmental nucleus; LH, lateral habenula; LHA, lateral hypothalamic area; MG, medial geniculate complex; MRN, midbrain reticular nucleus; OT, olfactory tubercle; PB, parabrachial nucleus; PG, pontine gray; PRNc, pontine reticular nucleus, caudal part; sAMY, striatum-like amygdalar nuclei; SC, superior colliculus; SI, substantia innominata; SN, substantia nigra; VTA, ventral tegmental area; ZI, zona incerta.
We then analyzed the projection strengths of the 8 neurons in L5b (Figs. 4B, C and S6). We quantified the projection strength as the ratio of the axonal length in the target area to the total length of the axons of each neuron because the axonal length per area has been shown to be directly related to the number of synapses [54, 55]. The projection strengths of all of 8 neurons varied widely in their terminal regions (Fig. 4B). For example, the projection strengths of these neurons in the MB ranged from 0.04 (#7) to 0.56 (#1), implying that the connection intensities of these pyramidal OFC neurons differ in the MB. Many subnuclei have different functions, so we further analyzed the projection strengths in the subnuclei (e.g., MB, Figs. 4C and S6). The projection strengths of these neurons in the MB subnuclei also varied greatly, ranging from 0.31 to 0 (Fig. 4C), implying that these neurons play different roles in different functions. It is worth noting that we only divided TH and STR into three subregions each: LHA, PH, and zona incerta and sAMY, CP, and OT, respectively. We did not subdivide them into smaller subnuclei because the resin embedding process resulted in a degree of deformation and shrinkage [38, 39, 41], which made it difficult to locate these small and complex subnuclei with ambiguous borders based on the Allen Brain Atlas using PI signals alone.
We also calculated the length and total number of branches of dendrites and axons of the 8 corticofugal neurons (Table 1). The results showed that the lengths of dendrites and axons of these neurons varied significantly, ranging from 7.71 mm (#7) to 11.16 mm (#2), and 76.45 mm (#8) to 146 mm (#2), respectively. There were also significant differences in the total number of dendritic and axonal branches, ranging from 124 (#4) to 265 (#2) and 304 (#6) to 1219 (#2), respectively. Since the dendritic and axonal morphologies of these neurons were extremely complex, it was very difficult to infer the axonal projections from the dendritic morphology or to find correlations between dendritic, somatic, and axonal morphologies.
Table 1.
Quantitative analysis of the dendrites and axons of the 8 L5b neurons.
| Neurons | Dendritic branches | Dendritic length (mm) | Axonal branches | Axonal length (mm) |
|---|---|---|---|---|
| No. 1 | 193 | 10.23 | 649 | 84.12 |
| No. 2 | 265 | 11.16 | 1219 | 146.00 |
| No. 3 | 152 | 7.35 | 304 | 79.58 |
| No. 4 | 124 | 7.28 | 633 | 111.00 |
| No. 5 | 157 | 8.58 | 929 | 98.68 |
| No. 6 | 131 | 9.18 | 727 | 112.30 |
| No. 7 | 141 | 7.71 | 686 | 106.23 |
| No. 8 | 172 | 11.12 | 894 | 76.45 |
Interestingly, we found that 3 neurons (#3, #4, and #5; Fig. 5), which were gathered in a microregion of L5b only 400 × 200 × 200 µm3 in size, differed in their termination regions (Fig. 5D–F) regardless of their adjacent somal location (two of them, #4 and #5 were < 20 µm apart; Fig. 4C). For instance, both of neurons #4 and #5 had axon collaterals in the CP, with strong projections (14% and 18%) terminating in the CP, while neuron #3 only had 4% projections to the CP (Fig. S6B). Furthermore, neuron #4 innervated the sAMY with an intense axonal projection, followed by neuron #5 with a medium projection and neuron #3 with almost no output to the sAMY. In addition, both neurons #3 and #4 had small projection strengths (< 3%) to the lateral habenula (LH) (Fig. S6B), a region implicated in various cognitive and emotional functions (e.g., reward processing) and various neuropsychiatric disorders (e.g., depression and schizophrenia) [53]. And the difference between the two LH-projecting neurons was that #3 projected to the ipsilateral hemisphere while #4 projected contralaterally (Fig. 5D, E), while another (#5) did not project to the LH (Fig. 5F). These results suggested the diversity of projections and potential existence of subtypes of corticofugal neurons in the OFC, in accordance with the expression characteristics of several selective Cre-recombinase driver lines constructed using molecular genetic techniques [56].
IT Neurons in Layer 2/3 of the OFC
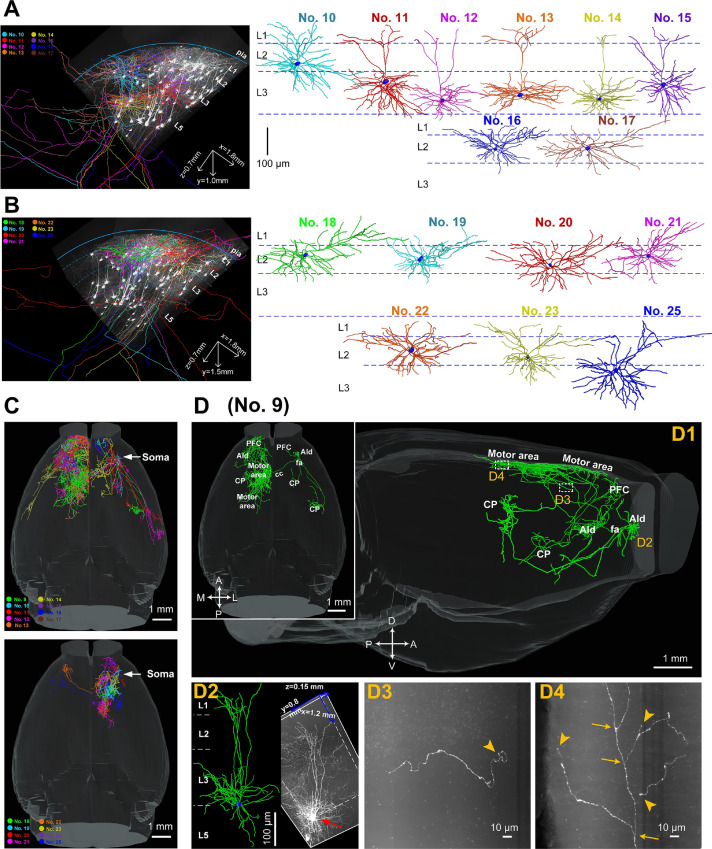
Among the 17 reconstructed L2/3 IT neurons, 8 were located in L3 (#9, 11–15, 24, 25) and the other 9 were located in L2 (#10, 16–23; Fig. 6A, B). These L2/3 IT neurons generally projected to the unilateral or bilateral STR or cerebral cortex, including prefrontal cortex, MO, somatosensory cortex (SS) and temporal cortex (Fig. S8). In contrast to the broad projection appearance of the 8 corticofugal neurons (#1–8), these L2/3 IT neurons displayed a cluster-like aggregation of axonal collaterals in each terminal region (Fig. 6C). The axonal morphologies of the L3 IT neurons differed greatly and exhibited depth-dependent projection patterns. Those located deeper in L3 (#9, 12–14, close to L5) usually exhibited more complicated axonal morphologies and had more projection targets than those located in the superficial or intermediate L3 (#15, 25, close to L2) (Figs. 6D and S8). For instance, the neuron located deep in layer 3 adjacent to layer 5 (#9) exhibited extremely complex axonal morphology and covered nearly the whole contralateral motor area with projections to both the ipsilateral and contralateral CP (Fig. 6D). In contrast, another neuron located in superficial L3 (#15) only contained very sparse axons projecting to the contralateral motor area (Fig. S8).
Fig. 6.
Reconstructions of the complete morphologies of L2/3 IT neurons in the OFC. A, B Left panels, two data blocks containing 15 IT neurons (#10–17, 18–23, 25). Note that #9 and #24 are located elsewhere. Right, reconstructed dendritic morphologies of the 15 IT neurons displayed in different colors (blue dashed lines, approximate laminar borders). C Sagittal (left panel) and horizontal views (right panel) demonstrating reconstructed axonal morphologies of 17 IT neurons (upper panel, #9–17; lower panel, #18–25; white arrows, locations of cell bodies). Axons of each neuron are shown in colors matching the dendrites in A and B. D Comprehensive brain-wide projection targets of a representative L3 IT neuron (#9, axon in green) selected from C in sagittal (D1) and horizontal views (inset in D1). D2 Left panel, raw data with a volume of 1.2 × 0.8 × 0.15 mm3 containing the selected neuron (red arrow); right panel, reconstructed dendritic morphology (green). Cell body is shown in blue. D3, D4 Enlargements of terminals in motor cortex. All images are 200 µm thick maximum projections with 300 × 300 µm2 in size (yellow arrowheads, terminal boutons; yellow arrows, branch points). AId, agranular insular area, dorsal part; cc, corpus callosum; CP, caudatoputamen; fa, corpus callosum, anterior forceps; PFC, prefrontal cortex.
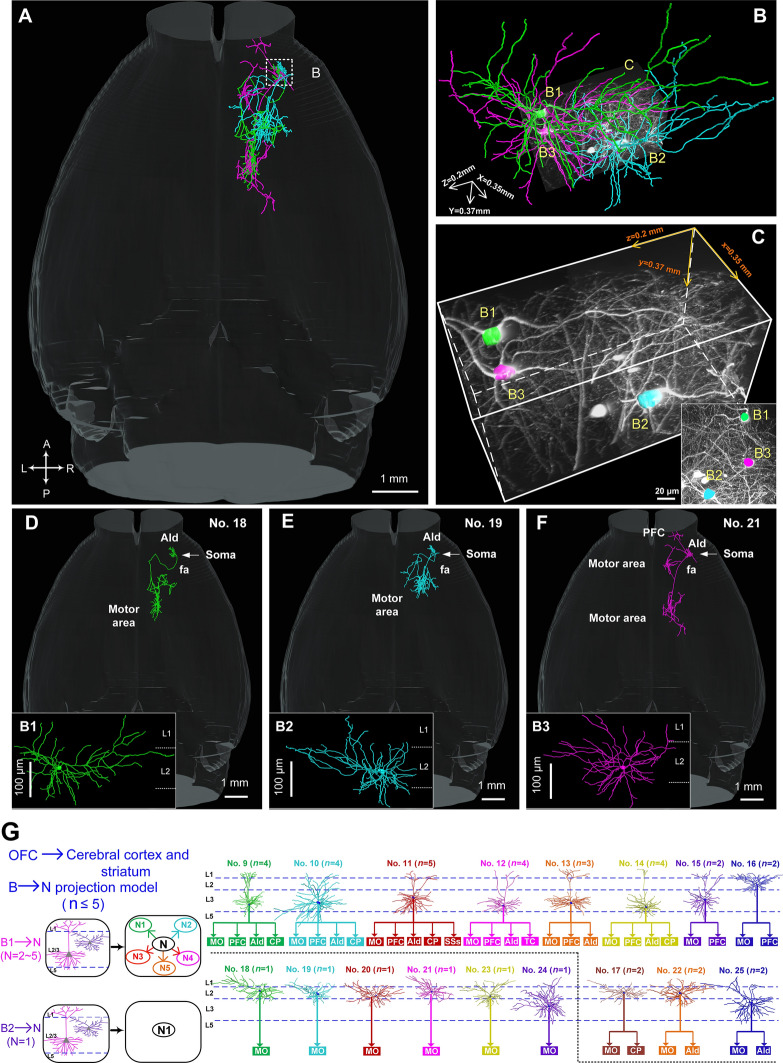
In contrast to the various axonal morphologies of the 8 IT neurons in L3, the 9 IT neurons in L2 usually exhibited a relatively simple projection pattern (Fig. S9). Six of them had a sole projection target, i.e., from cell body to the MO only via fa (Figs. 7 and S8). For example, the long-range projections of neurons #18, 19, and 21 (Fig. 7D–F) were located in a small volume of 350 × 370 × 200 µm3 (Fig. 7B, C) solely in the ipsilateral MO (Fig. 7D–F), which was significantly different from the projections of the corticofugal neurons (#3–5) (Fig. 5). The same phenomenon was also found in the other three neurons (#20, 23, 24; Fig. S8), suggesting that L2 pyramidal neurons have relatively homogenous projection patterns, consistent with the previously reported relatively homogenous physiological characteristics [57].
Fig. 7.
Reconstructions of the complete morphologies of L2/3 IT neurons in the OFC. A Overview of the axonal morphologies of 3 neurons (#18, green; #19, cyan, and #21, purple; dashed white box shown in B). B Dendritic morphologies of the 3 neurons in A. C 3D view (volume: 350 × 370 × 200 µm3) showing the locations of the 3 neurons in B (inset, maximum-intensity projection of the data block). D–F Detailed annotations of the projection regions of these 3 neurons. G Projection patterns of 17 L2/3 IT neurons in the OFC. Left panel, proposed projection patterns for L2/3 IT neurons; right panel, summary of the detailed projection targets of 17 reconstructed L2/3 IT neurons according to the Allen Brain Atlas with the assistance of PI signals.
Discussion
By combining sparse and bright neuronal labeling with fMOST, we obtained an uninterrupted 3D whole-brain dataset and achieved full morphological reconstructions of 25 OFC pyramidal neurons, including 8 corticofugal neurons in L5b and 17 IT neurons in L2/3. Our results provide information on the detailed morphologies and projection pathways of these neurons.
First, the L5b corticofugal neurons (#1–8) displayed a broad projection and exhibited multiple-source projection patterns. This pattern has also been frequently found in MO, SS, and VIS neurons [3–5, 58, 59], suggesting that corticofugal neurons are evolutionarily conserved in different neocortical areas. In contrast, the L2/3 IT neurons (#9–25) exhibited a simple pattern and projected unilaterally or bilaterally to the cerebral cortex or STR, appearing as clusters in each terminal region, similar to the neurons in primary and secondary motor areas [4, 5]. Meanwhile, the detailed projections of these OFC neurons were slightly different from those in other cortical regions. For instance, L5 neurons in the MO and SS have large proportions of projections in the corticospinal tract [58, 60], while the reconstructed L5 corticofugal neurons in the OFC had almost no projection in this tract. The results suggest that these neurons in OFC control fewer functions in motor behavior than neurons in the MO and SS. Different from the diverse projection patterns of the L2 IT neurons in primary and secondary motor areas and VIS [3–5], the reconstructed L2 IT neurons in the OFC preferentially tended to exhibit relatively simple projection patterns (Fig. 6). This result may imply a unique projection pattern of L2 IT neurons in the OFC, but further electrophysiological data is needed to validate this pattern. Furthermore, we found that the L2/3 IT neurons in the OFC had denser axonal collaterals in the contralateral MO than ipsilaterally, while the L2/3 neurons in secondary motor areas [4] had more projections in the ipsilateral than the contralateral MO. The results imply that these L2/3 neurons in the OFC and in secondary motor areas have different regulatory effects on the behavioral functions of the ipsilateral and contralateral hemispheres. Therefore, our results, along with previously reported single-neuron morphologies in the MO, SS, and VIS, facilitate our understanding of the evolution and development of the neocortex and the connections between different cortical regions.
Second, the reconstructed L5b corticofugal neurons all had complex dendritic and abundant dendritic spines, implying that they can receive several different synaptic inputs: (1) their apical branches in L2/3 and basal dendrites in deep layers receive strong intracortical feed-forward projections from L2/3 (direct inputs from TH) pyramidal neurons; (2) the larger extension of apical tufts with many branches can serve as numerous sites to receive extensive cortical feedback inputs from multiple areas of higher cortex; and (3) the dense basal dendritic branches in deep layers can receive strong inputs directly from TH. These results suggest that these L5b corticofugal neurons integrate these divergent, multi-source synaptic inputs and act as the main cortical output layer via broadcasting information to diverse subcortical regions [47, 61–64]. In contrast, the simple projection patterns of the reconstructed L2 IT neurons can be explained by their less developed basal dendrites receiving fewer TH inputs, different from the reconstructed L3 IT neurons whose basal dendrites possess more branches [47, 64]. Our results also show that these neurons, whether located in L5b or L2/3, had different projection patterns and projection strengths, and even the neurons located in a microregion (#3–5) showed significantly different projection patterns, suggesting the potential existence of subtypes of these OFC pyramidal neurons.
In summary, we manually reconstructed the complete morphologies of 8 corticofugal neurons and 17 IT neurons in the OFC based on our uninterrupted 3D whole-brain dataset. As far as we known, this is the first time the complete morphologies of individual neurons in OFC have been shown. Our results not only provide a structural basis to facilitate understanding of the principles of laminar organization in the OFC but also provide clues for future studies on the functional and behavioral actions of OFC pyramidal neurons.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Optical Bioimaging Core Facility of WNLO-HUST and the Analytical and Testing Center of HUST for support in data acquisition. This work was supported by the National Natural Science Foundation of China (61827825, 31770924, 31470056, and 31600692), the Science Fund for Creative Research Group of China (61721092), and the Director Fund of Wuhan National Laboratory for Optoelectronics.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Junjun Wang and Pei Sun have contributed equally to this work.
References
- 1.Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zingg B, Hintiryan H, Gou L, Song Monica Y, Bay M, Bienkowski Michael S, et al. Neural networks of the mouse neocortex. Cell. 2014;156:1096–1111. doi: 10.1016/j.cell.2014.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Y, Kebschull JM, Campbell RAA, Cowan D, Imhof F, Zador AM, et al. The logic of single-cell projections from visual cortex. Nature. 2018;556:51–56. doi: 10.1038/nature26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin HM, Kuang JX, Sun P, Li N, Lv X, Zhang YH. Reconstruction of intratelencephalic neurons in the mouse secondary motor cortex reveals the diverse projection patterns of single neurons. Front Neuroanat. 2018;12:86. doi: 10.3389/fnana.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winnubst J, Bas E, Ferreira TA, Wu Z, Economo MN, Edson P, et al. Reconstruction of 1,000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell 2019, 179: 268–281.e13. [DOI] [PMC free article] [PubMed]
- 6.Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51:495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Hoover WB, Vertes RP. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol. 2011;519:3766–3801. doi: 10.1002/cne.22733. [DOI] [PubMed] [Google Scholar]
- 9.Kuramoto E, Iwai H, Yamanaka A, Ohno S, Seki H, Tanaka YR, et al. Dorsal and ventral parts of thalamic nucleus submedius project to different areas of rat orbitofrontal cortex: A single neuron-tracing study using virus vectors. J Comp Neurol. 2017;525:3821–3839. doi: 10.1002/cne.24306. [DOI] [PubMed] [Google Scholar]
- 10.Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JFW, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 11.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Doherty J. Can’t learn without you: predictive value coding in orbitofrontal cortex requires the basolateral amygdala. Neuron. 2003;39:731–733. doi: 10.1016/s0896-6273(03)00525-7. [DOI] [PubMed] [Google Scholar]
- 13.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MFS. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 14.Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Poyraz AK. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Hou J, Wu W, Lin Y, Wang J, Zhou D, Guo J, et al. Localization of cerebral functional deficits in patients with obsessive–compulsive disorder: A resting-state fMRI study. J Affect Disord. 2012;138:313–321. doi: 10.1016/j.jad.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Payne ME, Steffens DC, Mcquoid DR, Lai TJ, Provenzale JM, et al. Subcortical lesion severity and orbitofrontal cortex volume in geriatric depression. Biol Psychiatry. 2003;54:529–533. doi: 10.1016/s0006-3223(03)00063-5. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Poh JS, Wen DJ, Broekman BFP, Chong YS, Yap F, et al. Functional and structural networks of lateral and medial orbitofrontal cortex as potential neural pathways for depression in childhood. Depress Anxiety. 2019;36:365–374. doi: 10.1002/da.22874. [DOI] [PubMed] [Google Scholar]
- 20.Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- 21.Northoff G, Richter A, Gessner M, Schlagenhauf F, Fell J, Baumgart F, et al. Functional dissociation between medial and lateral prefrontal cortical spatiotemporal activation in negative and positive emotions: A combined fMRI/MEG study. Cereb Cortex. 2000;10:93–107. doi: 10.1093/cercor/10.1.93. [DOI] [PubMed] [Google Scholar]
- 22.Qi X, Du ZJ, Zhu L, Liu X, Xu H, Zhou Z, et al. The glutamatergic postrhinal cortex-ventrolateral orbitofrontal cortex pathway regulates spatial memory retrieval. Neurosci Bull. 2019;35:447–460. doi: 10.1007/s12264-018-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 24.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Haber SN, Kunishio K, Mizobuchi M, Lyndbalta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkey. J Comp Neurol. 1993;337:1–31. doi: 10.1002/cne.903370102. [DOI] [PubMed] [Google Scholar]
- 27.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry—An update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 28.Bedwell SA, Billett EE, Crofts JJ, MacDonald DM, Tinsley CJ. The topology of connections between rat prefrontal and temporal cortices. Front Syst Neurosci. 2015;9:80. doi: 10.3389/fnsys.2015.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedwell SA, Billett EE, Crofts JJ, Tinsley CJ. The topology of connections between rat prefrontal, motor and sensory cortices. Front Syst Neurosci. 2014;8:177. doi: 10.3389/fnsys.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 31.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- 32.Goncalves L, Nogueira MI, Shammah-Lagnado SJ, Metzger M. Prefrontal afferents to the dorsal raphe nucleus in the rat. Brain Res Bull. 2009;78:240–247. doi: 10.1016/j.brainresbull.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Kondo H, Witter MP. Topographic organization of orbitofrontal projections to the parahippocampal region in rats. J Comp Neurol. 2014;522:772–793. doi: 10.1002/cne.23442. [DOI] [PubMed] [Google Scholar]
- 34.Yang T, Zheng T, Shang Z, Wang X, Lv X, Yuan J, et al. Rapid imaging of large tissues using high-resolution stage-scanning microscopy. Biomed Opt Express. 2015;6:1867–1875. doi: 10.1364/BOE.6.001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- 37.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Psychoneuroendocrinology 1997, 28.
- 38.Gong H, Xu D, Yuan J, Li X, Guo C, Peng J, et al. High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nat Commun. 2016;7:12142. doi: 10.1038/ncomms12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong H, Zhou Z, Zhu M, Lv X, Li A, Li S, et al. Chemical reactivation of quenched fluorescent protein molecules enables resin-embedded fluorescence microimaging. Nat Commun. 2014;5:3992. doi: 10.1038/ncomms4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Zhang Y, Wang X, Dai J, Hua R, Zeng S, et al. Anxiety-related cell-type-specific neural circuits in the anterior-dorsal bed nucleus of the stria terminalis. Sci Bull. 2020;65:1203–1216. doi: 10.1016/j.scib.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 41.Gang Y, Zhou H, Jia Y, Liu L, Liu X, Rao G, et al. Embedding and chemical reactivation of green fluorescent protein in the whole mouse brain for optical micro-imaging. Front Neurosci. 2017;11:121. doi: 10.3389/fnins.2017.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 43.Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- 44.Aschauer DF, Kreuz S, Rumpel S. Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One. 2013;8:e76310. doi: 10.1371/journal.pone.0076310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watakabe A, Ohtsuka M, Kinoshita M, Takaji M, Isa K, Mizukami H, et al. Comparative analyses of adeno-associated viral vector serotypes 1, 2, 5, 8 and 9 in marmoset, mouse and macaque cerebral cortex. Neurosci Res. 2015;93:144–157. doi: 10.1016/j.neures.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Quan T, Zhou H, Li J, Li S, Li A, Li Y, et al. NeuroGPS-Tree: automatic reconstruction of large-scale neuronal populations with dense neurites. Nat Methods. 2016;13:51–54. doi: 10.1038/nmeth.3662. [DOI] [PubMed] [Google Scholar]
- 47.Harris KD, Shepherd GM. The neocortical circuit: themes and variations. Nat Neurosci. 2015;18:170–181. doi: 10.1038/nn.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepherd GMG. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14:278–291. doi: 10.1038/nrn3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benkelfat C, Ellenbogen MA, Dean P, Palmour RM, Young SN. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch Gen Psychiatry. 1994;51:687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- 50.Benkelfat C, Nordahl TE, Semple WE, King AC, Murphy DL, Cohen RM. Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients treated with clomipramine. Arch Gen Psychiatry. 1987;47:840–848. doi: 10.1001/archpsyc.1990.01810210048007. [DOI] [PubMed] [Google Scholar]
- 51.Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- 52.Markowitsch HJ, Vandekerckhove MMP, Lanfermann H, Russ MO. Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex. 2003;39:643–665. doi: 10.1016/s0010-9452(08)70858-x. [DOI] [PubMed] [Google Scholar]
- 53.Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, et al. beta CaMKII in lateral habenula mediates core symptoms of depression. Science. 2013;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohno S, Kuramoto E, Furuta T, Hioki H, Tanaka YR, Fujiyama F, et al. A morphological analysis of thalamocortical axon fibers of rat posterior thalamic nuclei: A single neuron tracing study with viral vectors. Cereb Cortex. 2012;22:2840–2857. doi: 10.1093/cercor/bhr356. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Moreno J, Rollenhagen A, Arlandis J, Santuy A, Merchan-Perez A, DeFelipe J, et al. Quantitative 3D ultrastructure of thalamocortical synapses from the “lemniscal” ventral posteromedial nucleus in mouse barrel cortex. Cereb Cortex. 2018;28:3159–3175. doi: 10.1093/cercor/bhx187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Aerde KI, Feldmeyer D. Morphological and physiological characterization of pyramidal neuron subtypes in rat medial prefrontal cortex. Cereb Cortex. 2015;25:788–805. doi: 10.1093/cercor/bht278. [DOI] [PubMed] [Google Scholar]
- 58.Guo C, Peng J, Zhang Y, Li A, Li Y, Yuan J, et al. Single-axon level morphological analysis of corticofugal projection neurons in mouse barrel field. Sci Rep. 2017;7:2846. doi: 10.1038/s41598-017-03000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y, Jiang S, Xu Z, Gong H, Li A, Luo Q, et al. Pinpointing morphology and projection of excitatory neurons in mouse visual cortex. Front Neurosci. 2019;13:912. doi: 10.3389/fnins.2019.00912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci. 2012;32:5990–5999. doi: 10.1523/JNEUROSCI.5717-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kampa BM, Letzkus JJ, Stuart GJ. Cortical feed-forward networks for binding different streams of sensory information. Nat Neurosci. 2006;9:1472–1473. doi: 10.1038/nn1798. [DOI] [PubMed] [Google Scholar]
- 62.Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Constantinople CM, Bruno RM. Deep cortical layers are activated directly by thalamus. Science. 2013;340:1591–1594. doi: 10.1126/science.1236425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feldmeyer D. Excitatory neuronal connectivity in the barrel cortex. Front Neuroanat. 2012;6:24. doi: 10.3389/fnana.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.