Abstract
Endovascular treatment of strokes caused by large vessel occlusion enables the histopathological investigation of the retrieved embolus, possibly providing a novel opportunity to contribute to the diagnostic workup of etiology and to define secondary prevention measures in strokes with uncertain genesis. We aimed to develop a classification rule based on pathophysiological considerations and adjustment to reference thrombi for distinction between cardiac and arteriosclerotic emboli and to validate this classification rule on a patient cohort. From 125 patients with stroke due to large vessel occlusion and thrombectomy, 82 patients with known etiology (55 cardioembolic and 27 arterioembolic strokes) were included. The corresponding emboli were histologically evaluated by two raters blinded to the etiology of stroke by means of a novel classification rule. Presumed etiology and classification results were compared. Agreement concerning cardiac emboli was 72.2% (95% CI: 58.4–83.5) for rater I and 78.2% (95% CI: 65.0–88.2) for rater II. Agreement concerning arteriosclerotic emboli was 70.4% (95% CI: 49.8–86.3) for rater I and 74.1% (95% CI: 53.7–88.9) for rater II. Overall agreement reached 71.6% (95% CI: 60.5–81.1) for rater I and 76.8% (95% CI: 66.2–85.4) for rater II. Within the limits of generally restricted accuracy of histological evaluations, the classification rule differentiates between cardiac and arteriosclerotic emboli of acute ischemic stroke patients. Further improvement is needed to provide valuable complementary data for stroke etiology workup.
Subject terms: Stroke, Microscopy
Introduction
Ischemic stroke is a major burden for healthcare. Increasing efforts have been made to improve secondary prevention, as 6.2% of minor strokes will suffer from a second possibly devastating stroke within one year, despite a continually applied treatment1. The recent advances in intraarterial neuroradiological intervention techniques as acute stroke treatment have enlarged the number of patients who have a good outcome from an otherwise devastating ischemic stroke caused by a larger artery occlusion2. The occlusion is commonly due to an embolus. The common site from which this occluding embolus originates is a stenosis of an internal carotid artery, the left atrial appendage, or the left ventricle. However, in a relevant portion of patients a so-called ESUS (“embolic stroke of unknown source”) is the result of a diagnostic workup, usually including cerebral CT or MRI, cerebrovascular imaging by CT or MRI angiography or ultrasonography, echocardiography and ECG monitoring3,4. These patients are dismissed with uncertainty regarding the optimal secondary prevention, as the undifferentiated approach of treating ESUS patients with novel oral anticoagulants has not been shown to be beneficial in randomized studies5,6. Thus, the diagnostic workup of stroke etiology remains an indispensable cornerstone in order to decrease the mortality and morbidity of patients with ESUS or competing stroke causes.
Recently, diagnostically relevant information has been introduced into this diagnostic field by examining the histology of emboli7–16. This approach uses thrombus material obtained from thrombectomies and thus takes advantage of the fact that in these patients, an embolic cause of stroke is very probable, and that the causing thrombus material is accessible. However, since experience in this field is limited, the optimal methodological strategy of examining this material is not known10.
The THROMBEX trial is a prospective, blinded trial performed in Lübeck and Gießen, Germany. The histological features of emboli extracted from acute ischemic stroke patients are examined and correlated with anamnestic, clinical, and imaging data as well as with periprocedural parameters.
As a first step, we attempted to provide a novel opportunity to contribute to the diagnostic workup of stroke etiology. This requires a differentiation between cardiac and arteriosclerotic emboli, which are the most frequent causes of proximal vessel occlusion of acute ischemic stroke patients. For this purpose, the following pathophysiological considerations were taken into consideration:
In the course of many cardiac diseases, the endocardium is frequently damaged. There is evidence that endothelial dysfunction is the primary underlying pathology that directly triggers clot formation with the side effect of atrial fibrillation and hemodynamic changes in the auricles17,18. Generally, separation thrombi originate in the area of endothelial damage19–24. Longer dwell times in the heart cavity caused by associated atrial fibrillation and reduced blood flow velocity enable increased clot organization with progressive cell death, further local thrombin activation, accumulation of fibrin and increased cross-linking. The resulting clots (described as “white clots” due to their low concentration of hemoglobin) contain dense fibrin nets, many and widely distributed platelets, as well as many shattered neutrophils and red cells19,20,22,24,25. Those separation thrombi become harder and more resistant before they are carried away by the blood flow causing a stroke.
Agglutinative thrombi do not exclusively result from hemodynamic impairment around reduced blood flow velocities. They also emerge in areas of high blood flow velocities as the tail part of a mixed-thrombus or from clotting processes in the slipstream of a stenosis. Usually, the head section of the mixed-thrombus is a separation thrombus firmly attached to a damaged arterial vessel wall20,26–28. They are torn away soon after formation by fast streaming blood and flow turbulences. The histological composition of agglutinative thrombi is smooth and loose with only little organized structure. In consequence, these “red clots” have a fine fibrin net, few and locally restricted platelets, and many intact neutrophil granulocytes and red cells. Due to these reasonable and profound results of prior investigations, we hypothesize that:
Cardiac emboli comply with separation thrombi with dense fibrin nets, many platelets, and small proportions of intact neutrophil granulocytes and red cells. These clots are dense, compact, rigid, stable and appear older.
Arteriosclerotic emboli usually result from agglutinative thrombi with only fine fibrin nets, few and locally restricted platelets, masses of red cells and high proportions of intact neutrophils and red cells. These clots are smooth, unstable, and their structure is poorly organized.
The goal of the present work is the development of a histological classification rule that is based on these hypotheses and its validation on a cohort of stroke patients.
Methods
The THROMBEX trail was approved by the local ethics committees in Lübeck (University of Lübeck, reference number: 11-257) and Gießen (Justus-Liebig-University Gießen, reference number: 281/13). All patients gave written informed consent prior to inclusion in the study. All experiments were performed in accordance with the Declaration of Helsinki.
Acquisition of thrombotic emboli and determination of stroke etiology
One thrombotic embolus was collected from each of 125 different acute ischemic stroke patients who underwent thrombectomy. In all cases, mechanical thrombectomy was performed by a standardized coaxial procedure with stent retriever systems (Trevo XP, 4 × 20 mm or 6 × 25 mm, Stryker, Fremont, USA).
105 emboli were removed from the anterior and 20 from the posterior circulation.
Following the ESUS criteria proposed by the Cryptogenic Stroke/ESUS International Working Group3,29 the underlying cause of stroke was evaluated independently by three stroke experts (board certified neurologists with at least one year experience in treating stroke patients) (Fig. 1). For this purpose, the detailed medical history and all diagnostic results had to be fully available. Instrumental investigations should at least include CT or MRI of the brain, 12-lead ECG, a 24-h Holter ECG, precordial or transesophageal echocardiography, and imaging of both the extracranial and intracranial arteries supplying the brain by catheter, MR, or CT angiography, or cervical and transcranial duplex sonography. Furthermore, patients who were younger than 55 years received a coagulation analysis and vasculitis serology. Only emboli from patients with cardioembolic or arterioembolic strokes unequivocally specified in the same way by all three stroke experts were included for the validation of the classification rule. Thus, 55 emboli of patients with a cardioembolic and 27 emboli of patients with an arterioembolic ischemic stroke were analyzed. Clinical characteristics of the two groups are listed in Table 1.
Figure 1.
Selection process of suitable thrombotic emboli for the validation of the classification rule. Stroke etiology was evaluated for 125 patients with acute ischemic stroke who underwent thrombectomy. The evaluation was independently performed by three stroke experts and followed the ESUS criteria of the Cryptogenic Stroke/ESUS International Working Group. ESUS: embolic stroke of undetermined source.
Table 1.
Baseline characteristics of patients included in the validation analysis.
| Baseline characteristics | Cardioembolic (E1) N = 55 | Arterioembolic (E2) N = 27 |
|---|---|---|
| Years of age [median (min; max)] | 72 (44; 88) | 67,5 (45; 81) |
| Female sex [%] | 50,9 | 48,1 |
| Vascular risk factors | ||
| Arterial hypertension [%] | 76,4 | 81,5 |
| Diabetes mellitus [%] | 25,5 | 29,6 |
| Coronary artery disease [%] | 25,5 | 22,2 |
| Atrial fibrillation [%] | 87,3 | 3,7 |
| Hyperlipoproteinemia [%] | 20,0 | 22,2 |
| History of smoking [%] | 16,4 | 51,9 |
| Physical state on admission [median (min; max)] | ||
| Body mass index [kg/m2] |
25,7 (19,1; 53,6) n = 52 |
27,7 (20,5; 36,1) n = 26 |
| Systolic blood pressure [mmHg] | 144 (97; 240) | 165 (119; 190) |
| Blood glucose [mg/dl] | 114 (68; 180) | 113,5 (97; 196) |
| NIHSS |
14 (4; 37) n = 53 |
14,5 (4; 37) |
| Laboratory data on admission [median (min; max)] | ||
| Platelet count [/nl] | 218 (98; 373) | 245 (142; 430) |
| Hematocrit [%] | 39 (21; 46) | 42 (33; 49) |
| Premedication | ||
| Antiplatelet therapy [%] | 49,1 | 29,6 |
| Anticoagulation [%] | 20,0 | 3,7 |
| Occlusion site | ||
| On the right: ICA, MCA, carotid T occlusion [%] | 47,3 | 48,1 |
| On the left: ICA, MCA, carotid T occlusion [%] | 38,2 | 44,4 |
| Basilar artery [%] | 14,5 | 7,4 |
| Thrombolytic therapy | ||
| None [%] | 21,8 | 22,2 |
| Bridging with Abciximab [%] | 29,1 | 25,9 |
| Systemic application of rt-PA [%] | 49,1 | 51,9 |
| Local application of rt-PA [%] | 10,9 | 7,4 |
| Course of thrombectomy [median (min; max)] | ||
| Duration: Symptom onset–recanalization [min] |
204 (87; 625) n = 47 |
229,5 (135; 575) n = 16 |
| Recanalization time [min] |
52 (9; 169) n = 53 |
48,5 (17; 153) |
For continuous variables, the median and the minimum and maximum values are indicated. Nominal variables are presented as percentage. In cases of missing data, the number of available data is specified. NIHSS: National Institutes of Health Stroke Scale. ICA: internal carotid artery; MCA: middle cerebral artery.
Acquisition of reference thrombi
11 clots of certain origin served as reference thrombi. Five clots were withdrawn out of left-sided heart cavities during cardiosurgical interventions. They were considered to be cardioembolic clots. Six clots represented arterioembolic clots as they were extracted from coronary arteries during cardiac catheter examinations.
Histological examination
All samples were formalin-fixed (4% buffered formalin, BÜFA, Hude, Germany) and paraffin embedded (FFPE, Paraffin, Leica Microsystems, Wetzlar, Germany).
2 µm thick slices were cut from the paraffin blocks by microtome and placed on Super Frost glass slides (Menzel, Braunschweig, Germany). Hematoxylin and eosin (HE) stains (Merck, Darmstadt, Germany) and Elastica-van Gieson (EvG) stains (Merck, Darmstadt and Waldeck/Chroma, Münster, both Germany) were carried out. Immunohistochemistry was performed using the PostBlock HRP-Polymer and ZytoChem Plus Kit (both Zytomed, Berlin, Germany). The CD61 (Zytomed, Berlin, Germany) antibody was diluted 1:250 and pretreated by steamer. Serial sections were stained and arranged on a single microscope slide. Thus, all clots were screened over a wide range before a representative section was selected for further evaluation. The histological cross sections were captured with 200-fold original magnification. Only fibrin net was pictured with 400-fold original magnification. Two raters using Olympus BX50 microscope or Carl Zeiss Axioskop 40 microscope both with fluorite objectives with plano-correction independently examined all hematoxylin and eosin (HE), Elastica-van Gieson (EvG) and CD61 stains. Both raters were blinded concerning the pathogenesis of the clots, their total number, the clinical diagnosis of the stroke patients and their demographics and treatment. For technical reasons, rater I could only evaluate 81 of the 82 emboli that were extracted from the stroke patients.
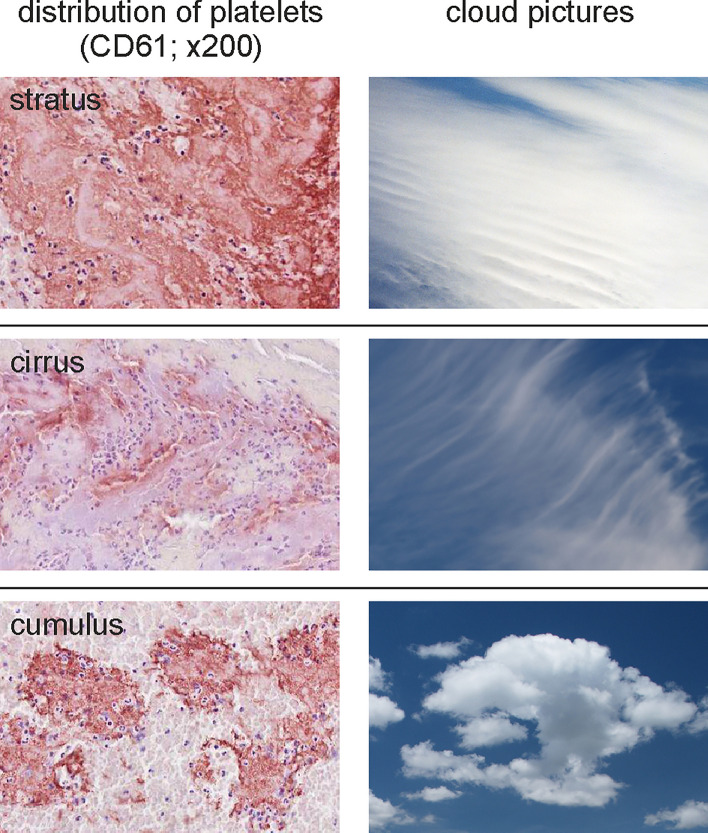
Upon microscopic evaluation of CD61 stains, the platelet distribution was interpreted and continuously assigned to the basic meteorological cloud shapes “stratus”, “cirrus”, and “cumulus” (Fig. 2) of the World Meteorological Organization30 according to their quantitative occurrence in percentage.
Figure 2.
Description of different platelet distributions used by the THROMBEX clot classification. In analogy to basic meteorological cloud shapes (right side) used by the World Meteorological Organization published in the International Cloud Atlas30 we described platelet distribution within clots (left side) as “stratus”, “cirrus”, and “cumulus”. Following the wording of the International Cloud Atlas, in a stratus distribution platelets occur as a merged layer with a uniform appearance or in the form of ragged patches. A cirrus distribution is characterized by detached clusters in the form of delicate filaments or narrow bands, having a hair-like appearance. Cumulus-like arranged platelets appear as detached and dense clusters with sharp outlines. They may be in the form of domes or towers. Sometimes they are ragged. (Prof. Stephan Borrmann of the Institute of Atmospheric Physics at the Johannes Gutenberg University of Mainz, Germany kindly provided the used cloud pictures.)
Reading the HE and EvG stains, histopathologists reviewed fibrin nets regarding its quality and assigned percentage values to each of the three features ”fine”, “coarse”, and “dense”. Finally, using the HE stains, neutrophils and red cells were examined assessing their integrity, dividing them up into wholesome or shattered. Continuous percentage scales were used to specify proportions of intact neutrophils and red cells to their total numbers.
According to the pathophysiological hypotheses, we formulated the following THROMBEX classification rule:
The histological structure of an embolus points to an arterioembolic formation process if two of the following three criteria are met:
Proportion of dense fibrin ≤ 30%.
Platelet distribution meets cumulus criterion ≥ 90%.
Proportion of intact neutrophils and red cells ≥ 80%.
Such emboli are defined as “ARTERIO “. Otherwise, histological classification points to a cardioembolic formation process (“CARDIO”).
In terms of an optimization task, the three cut-off values were determined by adjusting the classification rule until the maximum number of the 11 reference thrombi was correctly assigned to their assured pathophysiological formation process.
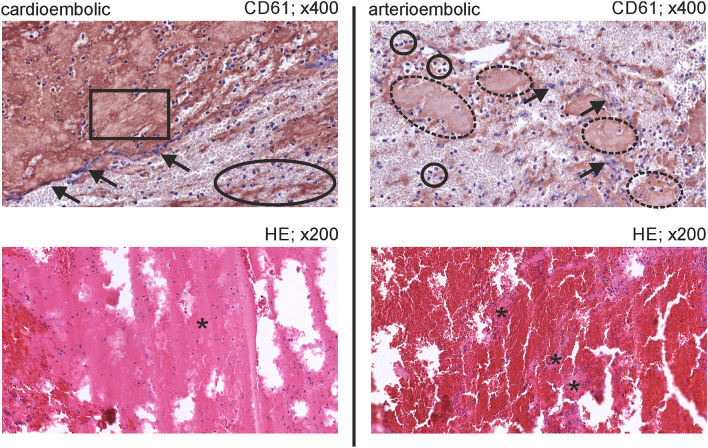
Figure 3 shows typical histological specimens of an arterioembolic and a cardioembolic clot that were correctly assigned by the classification rule.
Figure 3.
Histological presentation of typical cardioembolic and arterioembolic clots. For the two preparations immunohistochemical staining with CD61 (400-fold original magnification) and hematoxylin–eosin staining (200-fold original magnification) were prepared. The cardioembolic clot (left side) is characterized by a large number of platelets. Their distribution pattern reminds of stratus (rectangle) and cirrus (oval) cloud formations. Many disintegrated neutrophil granulocytes can be seen (arrows). Fibrin net is dense (star). This corresponds to a separation thrombus. In contrast, the arterioembolic clot (right side) is marked by a small amount of platelets arranged like cumulus clouds (dashed ovals). Apart from some disintegrated neutrophils (arrows), many intact neutrophils can be found (circles). Fibrin net is fine to coarse (stars). It is therefore an agglutinative thrombus that presumably arose from the tail part of a mixed-thrombus. Both emboli were extracted from patients with a definite stroke etiology.
Calculations and statistics
The proportions of concordant classifications were calculated, and exact 95% confidence intervals (CIs) are provided. Analyses were performed using R version 3.5.0 with the package epitools (version 0.5)31.
Results
Analysis of etiology of the 125 included stroke patients that underwent thrombectomy revealed predominant portions of cardioembolic (n = 55; 44%) and arterioembolic genesis (n = 27; 21.6%) as well as ESUS patients (n = 31; 24.8%). As expected, cases with competing causes (n = 9; 7.2%) or other specific causes (n = 3; 2.4%) were rare.
Only emboli of patients with cardioembolic or arterioembolic strokes were used to validate the classification rule. As expected, the distribution of their baseline characteristics (Table 1) showed that the incidence of cardiovascular risk factors (arterial hypertension, diabetes mellitus, hyperlipoproteinemia, increased body mass index, history of smoking) was rather associated with an arterioembolic genesis of stroke. In contrast, atrial fibrillation was far more frequent in the cardioembolic group (87.3% vs. 3.7%).
Occlusion of the basilar artery occurred almost twice as often in the group of cardioembolic strokes (14.5% vs. 7.4%). No striking difference was found concerning the affected side of the carotid flow area. Severity of stroke symptoms measured by the NIHSS was roughly equal in both groups. Patients with cardioembolic strokes received anticoagulants more often within their premedication. Local application of rt-PA was more frequently used in the course of acute treatment in the cardioembolic group (10.9% vs. 7.4%). No striking differences between the two groups were found regarding systemic thrombolysis or bridging with Abciximab. In the group of cardioembolic strokes, the mean duration between symptom onset and recanalization was shorter (204 vs. 229.5 min), whereas the mean recanalization time was prolonged (52 vs. 48.5 min).
To validate the classification rule agreement between stroke etiology defined by stroke experts and histological evaluation of the extracted emboli performed by two independent histopathologists (rater I and II) was determined. For both raters significant agreement was found (Table 2).
Table 2.
Fourfold table of the validation data.
| Rater I (N = 81)/Rater II (N = 82) | Histological classification | |
|---|---|---|
| CARDIO | ARTERIO | |
| Stroke etiology | ||
| Cardioembolic (E1) | 39/43 | 15/12 |
| Arterioembolic (E2) | 8/7 | 19/20 |
|
Agreement concerning E1 [%, (95% CI)], Rater I: N = 54; Rater II: N = 55 |
72,2 (58,4–83,5) / 78,2 (65,0–88,2) |
|
|
Agreement concerning E2 [%, (95% CI)], both raters: N = 27 |
70,4 (49,8–86,3) / 74,1 (53,7–88,9) |
|
|
Agreement concerning E1 + E2 [%, (95% CI)], Rater I: N = 81; Rater II: N = 82 |
71,6 (60,5–81,1) / 76,8 (66,2–85,4) |
|
Stroke etiology was independently determined by three stroke experts based on complete anamnestic information and diagnostic results concerning cause analysis of stroke. Histological classification was performed by two independent raters that were blinded for the defined etiology of the thrombotic emboli. Values for both raters are indicated before (rater I) and after (rater II) the slash. CI = confidence interval.
Only rater I did not obtain better than chance agreement in the allocation of arterioembolic clots. The histological evaluation of rater II delivered a somewhat higher total agreement (76.8%) than the evaluation of rater I (71.6%). Both raters achieved higher agreement for the classification of cardioembolic clots.
Discussion
Determination of stroke etiology and baseline characteristics of the included patients
Three independent stroke experts confirmed in our study the stroke etiology after examining medical history and diagnostic results of all necessary instrumental investigations. Unanimous decision of the three experts was required to specify the underlying etiology of stroke. This etiology was set to be the actual cause of stroke and served as reference to validate the developed classification rule.
The frequencies of the different stroke etiologies obtained (Fig. 1) are quite consistent with results found in the literature4,32. Explanations that are more detailed can be found in the discussion section in the Data Supplement.
The baseline characteristics of the included patients (Table 1) reflect some findings concerning already known predictors of different stroke subtypes. As expected, atrial fibrillation and therefore oral anticoagulation is far more frequent in the group of cardioembolic strokes. Atrial fibrillation is the most frequent cause of cardioembolic stroke33–36, or is at least a symptom of an endothelial dysfunction that has been recently found to be the main reason for thrombotic emboli17. By analogy, there are indications for the already known association between the common cardiovascular risk factors and arteriosclerosis leading to arterioembolic strokes37–39. In our patient cohort, basilar artery occlusion was caused more frequently by cardiac embolism. This finding is in line with the results of other work groups40–42.
In our study, the mean recanalization time was slightly increased in the cardioembolic group. This is in agreement with a previous study and may suggest a more difficult intervention in cardioembolic stroke43. Further considerations are made in the discussion section in the Data Supplement.
Probably, some of the above mentioned baseline characteristics affect the histological composition of the investigated emboli. In particular, medical treatment before symptom onset may play a decisive role.
Validation of the classification rule
According to the developed classification rule, cardiac emboli comply with separation thrombi with dense fibrin nets, a widespread distribution of platelets, and small proportions of intact neutrophils and red cells. They originate from platelet accumulation caused by an endothelial dysfunction of the heart. These clots are compact, rigid, stable and appear older. Arteriosclerotic emboli arise from agglutinative tail parts of mixed-thrombi or from clotting processes in the slipstream of a stenosis. They are torn away soon after formation by fast streaming blood and flow turbulences. Therefore, they are smooth and loose and their structure is poorly organized. These hypotheses have been developed using pathophysiological considerations and are reflected by the baseline characteristics of our subjects.
The classification rule is successfully validated. Only rater I barely misses significant agreement while assigning arterioembolic clots (Table 2). Therefore, the suggested classification rule is suitable to differentiate between certain cardiac and arteriosclerotic emboli with a prediction accuracy between 70 and 78%.
Both raters identify cardioembolic clots better than arterioembolic clots. This is probably due to the chosen cut-off values because they allocate a larger range for cardioembolic clots. Therefore, classification of cardiac emboli is straightforward.
The accuracy of histological evaluations is naturally limited by pathologist-related, study methodology-related, and specimen-related causes. Therefore, most kappa values concerning reproducibility of histological classifications are in the range of 0.41–0.6 (signifying moderate agreement)44–50. For the reliability of WHO and Gleason histologic grading systems in prostatic adenocarcinoma intraobserver reliabilities were 75.0% and 78.1%, respectively. Interobserver reliabilities were 60.4% and 70.8%, respectively51. This example shows that even common and useful histological classifications and grading systems are limited concerning their reliability. Considering this, the gained results of our classification rule are valuable keeping in mind that reproducibilities of different studies cannot be compared directly.
Comparison of the classification rule with results of other working groups
Brinjikji et al. performed a meta-analysis to identify a correlation between clot histology and stroke etiology10, identifying studies published between January 2005 and December 2015 that report findings related to histologic and/or imaging characteristics of thrombi in acute ischemic stroke secondary to large vessel occlusion. Finally, nine studies examining the association between the histology and etiology of emboli were selected, including in total 302 stroke patients. The two possible alternatives concerning stroke etiology were cardiac and arteriosclerotic embolism. In five studies, no correlation was found28,52–55. Two studies found that cardiac emboli were associated with an increased proportion of red cells and less fibrin56,57. In contrast, Niesten et al. found that arteriosclerotic emboli had a significantly higher proportion of red cells combined with cardiac emboli26. The research group of Boeckh-Behrens showed that cardiac emboli contained more white blood cells and were characterized by a higher level of formation and organization43. Finally, the meta-analysis drew the conclusion that due to insufficient and inconsistent data a correlation cannot be proven. However, it was stressed that most of the underlying histological investigations were done without immunohistochemical stains, therefore lacking important features. It is noteworthy that three25,26,56 of four studies using immunohistochemical stains found a correlation between histological features and the etiology of clots.
The classification rule presented fulfills the demand for additional immunohistochemical staining and is in line with the findings of the working groups of Niesten and Boeckh-Behrens26,43. Furthermore, it complies with some more recent studies proving that cardiac emboli were significantly correlated with higher proportions of fibrin, platelets, and white blood cells but with a lower proportion of red cells8,9,16. Appropriately, two other research groups found a significantly greater red cell proportion in arterioembolic clots than in cardioembolic clots, whereas fibrin proportion was significantly larger in cardioembolic clots that were furthermore characterized by a higher level of organization7,14. In a per-pass analysis of embolus composition Duffy et al. found that emboli retrieved in passes 1 and 2 from patients with arterioembolic strokes had a higher red cell composition, whereas the histological characteristics of the overall embolus load removed did not differ significantly among different etiologies11. An important limiting factor of their study was that more than one-third of all cases was classified as cryptogenic strokes impeding the correlation between histology of emboli and etiology of strokes.
Contrasting results showing higher red cell proportions13,15 and lower fibrin percentages in cardiac emboli13 were also obtained. Fitzgerald et al. found significantly higher proportions of platelets for large artery atherosclerosis etiology12. Table S1 in the Data Supplement gives an overview of the different studies.
In summary, most of the studies, in particular the both largest studies correspond with our developed classification rule. Further, the majority of studies based on additional immunohistochemical stains leads to concordant results. Most of the studies (4 of 5) contradicting our approach were performed with Asian patients.
All the previous studies are based on group comparison. Our classification rule was developed to enable inferences about stroke causes in individual patients. In particular, this promises huge benefit in strokes that cannot be assigned to a defined cause despite intensive diagnostic workup. Such cases are frequent and bear a high risk of recurrent strokes57.
The informative value of all relevant studies in this field is restricted by a single-center design, small numbers of arterioembolic clots, and an inherent selection bias: only those emboli that did not dissolve spontaneously or after rt-PA administration and that can successfully be retrieved via thrombectomy are available for these studies. This impedes the assessment of rt-PA susceptible and thrombectomy-resistant emboli. Furthermore, components of the retrieved embolus might not completely reflect those of the entire embolus. Emboli consist of complex, heterogeneous, and anisotropic materials that undergo a dynamic evolution. In particular medical treatment before symptom onset, the chosen acute therapy, and age of the emboli might alter its histological characteristics. Usually, the number of passes needed to retrieve the occluding embolus is neglected, although the per-pass composition of the clot is likely to change11. Finally, it remains uncertain whether the retrieved clot is captured from the exact occlusion site, or from a proximal or distal section.
Discrepancies between different studies may be associated with restrictions in the number of patients, ethnic affiliation of subjects, variations in procedural techniques of endovascular treatment and the lack of standardized methods concerning staining and evaluation of the histological preparations. A consensus statement from experts sought to solidify terminology and establish standardized analysis before conducting a multi-center trial.
Specific limitations of the present classification rule
In addition to the above-mentioned weaknesses of histological evaluations in general, some specific limitations should be mentioned concerning the present validation process:
The number of reference thrombi is low and finally they are not cerebral emboli. However, their origin is certain making them suitable for the adjustment of the cut-off values. The chosen method to evaluate the embolus includes manual segmentation and personal analysis, which might lead to an observer bias. Although stroke etiology was set unanimously by three independent stroke experts, it is not guaranteed that this cause of stroke is actually true.
Therefore, the present classification rule needs further improvement. In a first step, this could be achieved by more reference thrombi, further adjustment of the cut-off values and a more sophisticated definition of the classification rule.
It should be emphasized that the present study validates the classification rule for patients with a certain cardio- or arterioembolic stroke. Up to now, the accuracy of the present classification rule for embolic strokes of unknown source is not known. Therefore, a further prospective study is planned including patients whose stroke etiology is uncertain at the time of histological evaluation but will be defined later.
Conclusions
To our knowledge, this is the first attempt to develop a histological classification rule for cerebral emboli based on well-justified pathophysiological considerations and adjustment to reference thrombi before specimens are examined. The present classification rule enables the distinction between cardiac and arteriosclerotic emboli in histological samples of individual stroke patients that underwent thrombectomy by evaluating the fibrin network, distribution of platelets, and the proportion of intact neutrophils and red cells. The classification rule was successfully validated keeping in mind that the accuracy of histological evaluations is naturally restricted. Agreements between histological classification and stroke etiology defined by strokes experts are in the range of 70–78%. Advantages of this histological classification are the absence of harm for the patient and of hindrance for other diagnostic procedures and its easy and cheap implementation.
The present classification rule is supported by most of the relevant results gained by other research groups. Multi-center studies with larger patient numbers should confirm and improve the classification rule. Validation of the present classification rule was successful in cases of ensured cardio- or arterioembolic strokes. To what extent it might indicate the etiology of undetermined embolic strokes remains to be examined in a further prospective study. This is vitally important to improve secondary prevention of patients with embolic strokes of unknown source.
Supplementary Information
Acknowledgements
We are grateful to Ana Ivasiuc and Petra Dietz-Ruckstuhl for critical reading of the manuscript. Susanne Riebau supported us in recruiting patients. Lena Borchardt collected and stored the extracted emboli and sent them to the Research Centre Borstel. We would like to thank them both. Finally, the authors thank all the stroke patients who agreed to the investigation of their emboli and for their support to our scientific efforts. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
F.R. conceived and directed the THROMBEX study, recruited stroke patients, analyzed and interpreted the data, and wrote the manuscript. Together with F.R., N.K. and F.J. collected all baseline characteristics, evaluated the underlying cause of stroke, and assisted F.R. with data processing. A.K. performed the thrombectomy. J.R-I. and F.S. carried out all histological evaluations. I.R.K. led and performed the statistical evaluation. M.O. and G.R. supported F.R. regarding patient recruitment. G.R. gave crucial hints on planning the study.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87584-2.
References
- 1.Amarenco P, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N. Engl. J. Med. 2016;374:1533–1542. doi: 10.1056/NEJMoa1412981. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 4.Ntaios G, et al. Embolic strokes of undetermined source in the Athens stroke registry: a descriptive analysis. Stroke. 2015;46:176–181. doi: 10.1161/STROKEAHA.114.007240. [DOI] [PubMed] [Google Scholar]
- 5.Diener HC, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N. Engl. J. Med. 2019;380:1906–1917. doi: 10.1056/NEJMoa1813959. [DOI] [PubMed] [Google Scholar]
- 6.Hart RG, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N. Engl. J. Med. 2018;378:2191–2201. doi: 10.1056/NEJMoa1802686. [DOI] [PubMed] [Google Scholar]
- 7.Ahn SH, et al. Histologic features of acute thrombi retrieved from stroke patients during mechanical reperfusion therapy. Int. J. Stroke. 2016;11:1036–1044. doi: 10.1177/1747493016641965. [DOI] [PubMed] [Google Scholar]
- 8.Berndt M, et al. Thrombus permeability in admission computed tomographic imaging indicates stroke pathogenesis based on thrombus histology. Stroke. 2018;49:2674–2682. doi: 10.1161/STROKEAHA.118.021873. [DOI] [PubMed] [Google Scholar]
- 9.Boeckh-Behrens T, et al. Thrombus histology suggests cardioembolic cause in cryptogenic stroke. Stroke. 2016;47:1864–1871. doi: 10.1161/STROKEAHA.116.013105. [DOI] [PubMed] [Google Scholar]
- 10.Brinjikji W, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: a systematic review. J. Neurointerv. Surg. 2017;9:529–534. doi: 10.1136/neurintsurg-2016-012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy S, et al. Per-pass analysis of thrombus composition in patients with acute ischemic stroke undergoing mechanical thrombectomy. Stroke. 2019;50:1156–1163. doi: 10.1161/STROKEAHA.118.023419. [DOI] [PubMed] [Google Scholar]
- 12.Fitzgerald S, et al. Platelet-rich emboli in cerebral large vessel occlusion are associated with a large artery atherosclerosis source. Stroke. 2019;50:1907–1910. doi: 10.1161/STROKEAHA.118.024543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong L, et al. Bridging therapy versus direct mechanical thrombectomy in patients with acute ischemic stroke due to middle cerebral artery occlusion: a clinical-histological analysis of retrieved thrombi. Cell Transpl. 2019 doi: 10.1177/0963689718823206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maekawa K, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc. Dis. Extra. 2018;8:39–49. doi: 10.1159/000486042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin JW, Jeong HS, Kwon HJ, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS ONE. 2018 doi: 10.1371/journal.pone.0197492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sporns PB, et al. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke. 2017;48:2206–2210. doi: 10.1161/STROKEAHA.117.016590. [DOI] [PubMed] [Google Scholar]
- 17.Akoum N. New perspectives on atrial fibrillation and stroke. Heart. 2016;102:1788–1792. doi: 10.1136/heartjnl-2015-309066. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita T. Molecular basis of thromboembolism in association with atrial fibrillation. Circ. J. 2007;71:A40–A44. doi: 10.1253/circj.71.A40. [DOI] [PubMed] [Google Scholar]
- 19.Yuki I, et al. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR. 2012;33:643–648. doi: 10.3174/ajnr.A2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niesten JM, et al. DUtch acute Stroke Trial (DUST). Relationship between thrombus attenuation and different stroke subtypes. Neuroradiology. 2013;55:1071–1079. doi: 10.1007/s00234-013-1217-y. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Lip GY, Apostolakis S. Inflammatory biomarkers and atrial fibrillation: potential role of inflammatory pathways in the pathogenesis of atrial fibrillation-induced thromboembolism. Curr. Vasc. Pharmacol. 2015;13:192–201. doi: 10.2174/15701611113116660165. [DOI] [PubMed] [Google Scholar]
- 22.Gosk-Bierska I, Wasilewska M, Wysokiński W. Role of platelets in thromboembolism in patients with atrial fibrillation. Adv. Clin. Exp. Med. 2016;25:163–171. doi: 10.17219/acem/38544. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer A, Eigenthaler M, Bauersachs J. Platelet activation in heart failure. Clin. Lab. 2004;50:559–566. [PubMed] [Google Scholar]
- 24.Wysokinski WE, Owen WG, Fass DN, Patrzalek DD, Murphy L, McBane RD. Atrial fibrillation and thrombosis: immunohistochemical differences between in situ and embolized thrombi. J. Thromb. Haemost. 2004;2:1637–1644. doi: 10.1111/j.1538-7836.2004.00899.x. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, et al. Thrombus components in cardioembolic and atherothrombotic strokes. Thromb. Res. 2012;130:278–280. doi: 10.1016/j.thromres.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Niesten JM, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE. 2014;9:e88882. doi: 10.1371/journal.pone.0088882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvain J, et al. Composition of coronary thrombus in acute myocardial infarction. J. Am. Coll. Cardiol. 2011;57:1359–1367. doi: 10.1016/j.jacc.2010.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marder VJ, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 29.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017;48:867–872. doi: 10.1161/STROKEAHA.116.016414. [DOI] [PubMed] [Google Scholar]
- 30.Secretariat of the World Meteorological Organization, Geneva, Switzerland. World Meteorological Organization. International Cloud Atlas. ISBN 92-63-10407-7 (1975).
- 31.R Foundation for Statistical Computing, Vienna, Austria. R: a language and environment for statistical computing. https://www.R-project.org/. Accessed July 03th, 2020.
- 32.Perera KS, et al. Embolic strokes of undetermined source: prevalence and patient features in the ESUS Global Registry. Int. J. Stroke. 2016;11:526–533. doi: 10.1177/1747493016641967. [DOI] [PubMed] [Google Scholar]
- 33.Borowsky LH, Regan S, Chang Y, Ayres A, Greenberg SM, Singer DE. First diagnosis of atrial fibrillation at the time of stroke. Cerebrovasc. Dis. 2017;43:192–199. doi: 10.1159/000457809. [DOI] [PubMed] [Google Scholar]
- 34.Kamel H, Healey JS. Cardioembolic stroke. Circ. Res. 2017;120:514–526. doi: 10.1161/CIRCRESAHA.116.308407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Carroll CB, Barrett KM. Cardioembolic stroke. Continuum (Minneap Minn). 2017;23:111–132. doi: 10.1212/CON.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 36.Weir NU. An update on cardioembolic stroke. Postgrad. Med. J. 2008;84:133–142. doi: 10.1136/pgmj.2007.066563. [DOI] [PubMed] [Google Scholar]
- 37.Candido R, Bernardi S, Allen TJ. Linking diabetes and atherosclerosis. Expert. Rev. Endocrinol. Metab. 2009;4:603–624. doi: 10.1586/eem.09.46. [DOI] [PubMed] [Google Scholar]
- 38.Isomaa B, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 39.Mostaza JM, et al. Risk factors associated with the carotid intima-media thickness and plaques: ESPREDIA Study. Clin. Investig. Arterioscler. 2018;30:49–55. doi: 10.1016/j.arteri.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Caplan LR, et al. New England medical center posterior circulation registry. Ann. Neurol. 2004;56:389–398. doi: 10.1002/ana.20204. [DOI] [PubMed] [Google Scholar]
- 41.Nagel S. Stroke due to acute occlusion of the basilar artery: diagnosis and treatment. Med. Klin. Intensivmed. Notfmed. 2017;112:679–686. doi: 10.1007/s00063-017-0347-0. [DOI] [PubMed] [Google Scholar]
- 42.Uno J, et al. Mechanical thrombectomy for acute basilar artery occlusion in early therapeutic time window. Cerebrovasc. Dis. 2017;44:217–224. doi: 10.1159/000479939. [DOI] [PubMed] [Google Scholar]
- 43.Boeckh-Behrens T, et al. The impact of histological clot composition in embolic stroke. Clin. Neuroradiol. 2016;26:189–197. doi: 10.1007/s00062-014-0347-x. [DOI] [PubMed] [Google Scholar]
- 44.Allsbrook WC, Mangold KA, Johnson MH, Lane RB, Lane CG, Epstein JI. Interobserver reproducibility of Gleason grading of prostatic carcinoma: general pathologist. Hum. Pathol. 2001;32:81–88. doi: 10.1053/hupa.2001.21135. [DOI] [PubMed] [Google Scholar]
- 45.Thunnissen E, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod. Pathol. 2012;25:1574–1583. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartman DJ, Borczuk A, Dacic S, Krasinskas A. Reproducibility for histologic parameters in peritoneal mesothelioma. Hum Pathol. 2017;67:54–59. doi: 10.1016/j.humpath.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Tuna B, et al. Histologic grading of urothelial papillary neoplasms: impact of combined grading (two-numbered grading system) on reproducibility. Virchows Arch. 2011;458:659–664. doi: 10.1007/s00428-011-1072-3. [DOI] [PubMed] [Google Scholar]
- 48.Stoler MH, Schiffman M. Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500–1505. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 49.Boiesen P, et al. Histologic grading in breast cancer–reproducibility between seven pathologic departments. South Sweden Breast Cancer Group. Acta Oncol. 2000;39:41–45. doi: 10.1080/028418600430950. [DOI] [PubMed] [Google Scholar]
- 50.Santucci M, Biggeri A, Feller AC, Burg G. Accuracy, concordance, and reproducibility of histologic diagnosis in cutaneous T-cell lymphoma: an EORTC Cutaneous Lymphoma Project Group Study. European Organization for Research and Treatment of Cancer. Arch Dermatol. 2000;136:497–502. doi: 10.1001/archderm.136.4.497. [DOI] [PubMed] [Google Scholar]
- 51.Ozdamar SO, Sarikaya S, Yildiz L, Atilla MK, Kandemir B, Yildiz S. Intraobserver and interobserver reproducibility of WHO and Gleason histologic grading systems in prostatic adenocarcinomas. Int. Urol. Nephrol. 1996;28:73–77. doi: 10.1007/BF02550141. [DOI] [PubMed] [Google Scholar]
- 52.Liebeskind DS, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sallustio F, et al. Histological features of intracranial thrombo-emboli predict response to endovascular therapy for acute ischemic stroke. J. Neurol. Disorders Stroke. 2015;3:1105. [Google Scholar]
- 54.Simons N, Mitchell P, Dowling R, Gonzales M, Yan B. Thrombus composition in acute ischemic stroke: a histopathological study of thrombus extracted by endovascular retrieval. J. Neuroradiol. 2015;42:86–92. doi: 10.1016/j.neurad.2014.01.124. [DOI] [PubMed] [Google Scholar]
- 55.Singh P, Doostkam S, Reinhard M. Cerebrovascular occlusive disease histopathological analysis of thrombi retrieved by endovascular mechanical extraction during acute ischemic stroke. Int. J. Stroke. 2014;9:143. doi: 10.1111/j.1747-4949.2012.00916.x. [DOI] [Google Scholar]
- 56.Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS. Histologic analysis of retrieved clots in acute ischemic stroke: correlation with stroke etiology and gradient-echo MRI. AJNR. 2015;36:1756–1762. doi: 10.3174/ajnr.A4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molina-Seguin J, Vena AB, Colas-Campas L, Benalbdelhak I, Purroy F. A systematic review of the characteristics and prognosis of subjects who suffer an embolic stroke of undetermined source. Rev. Neurol. 2018;66:325–330. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.