Abstract
This study aimed to observe the safety and clinical efficacy of early application of ruxolitinib to prevent acute graft-versus-host disease (aGVHD) after alternative donor transplantation in acute leukemia. There were 57 patients undergoing allo-HSCT at the Affiliated Cancer Hospital of Zhengzhou University from July 2017 to October 2019. They were divided into control(16 patients) and ruxolitinib (41 patients) groups. For aGVHD prophylaxis, the control group received post-transplantation cyclophosphamide, antithymocyte globulin-Fresenius, cyclosporine A, and mycophenolate mofetil, while in the ruxolitinib group, ruxolitinib 5 mg/d in adults or 0.07–0.1 mg/(kg d) in children was administered from the day of neutrophil engraftment to 100 days post-transplantation based on control group. We found 55 patients had successful reconstitution of hematopoiesis; No significant difference was found in cGVHD, hemorrhagic cystitis, pulmonary infection, intestinal infection, Epstein-Barr virus infection, cytomegalovirus infection, relapse, death, and nonrelapse mortality. The incidences of aGVHD (50 vs. 22%, P = 0.046) and grade II–IV aGVHD (42.9 vs. 12.2%, P = 0.013) were significantly higher in the control group than in the ruxolitinib group. No significant differences were observed in overall survival (P = 0.514), disease-free survival (P = 0.691), and cumulative platelet transfusion within 100 days post-transplantation between two groups. This suggests early application of ruxolitinib can reduce the incidence and severity of aGVHD and patients are well tolerated.
Subject terms: Leukaemia, Acute lymphocytic leukaemia, Acute myeloid leukaemia
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is still a potentially curative approach for hematological diseases, especially with the continuous improvement in conditioning regimens and the emergence of new anti-graft-versus-host disease (GVHD) drugs. It has significantly improved the success rate of transplantation. Alternative donors remain an important source. Alternative donor-HSCT has achieved good results; the 4-year overall survival (OS) and disease-free survival (DFS) can be more than 80%1. However, transplant-related complications are still pivotal factors decreasing the success of transplantation. Especially acute GVHD (aGVHD)2,3is still a common serious complication of allo-HSCT, seriously affecting the survival and prognosis of patients. Many methods are available for preventing and treating GVHD, such as post-transplant cyclophosphamide(PT-Cy)4, antithymocyte globulin (ATG)5, calcineurin inhibitors (CNIs)6, monoclonal antibodies7, myeloid-derived suppressor cells8, and mesenchymal stem cells9. However, efficacy is still not satisfactory. aGVHD remains the main challenge of allo-HSCT. Therefore, new drugs and methods need to be continuously explored to reduce the incidence and severity of aGVHD.
Janus kinase (JAK) is an intracellular nonreceptor tyrosine kinase playing a key role in the development, proliferation, and cytokine signal transduction of various cells (including dendritic cells, macrophages, T cells, B cells, natural killer cells, and neutrophils)10. Activated JAKs are necessary for effector T-cell responses in different inflammatory diseases, and their blockade can potentially reduce acute GVHD11. Therefore, they are expected to become a new target for the prevention and treatment of GVHD. Ruxolitinib is a selective JAK 1/2 inhibitor, which mainly inhibits the activity of JAK by competitively blocking the binding site of ATP on the catalytic subunit of the JAK1/2 domain; it has the same effect on JAK1 and JAK2. Preclinical studies showed that ruxolitinib has good anti-GVHD effects; it not only has anti-GVHD activity but retains the GVL effect12. The Food and Drug Administration approved ruxolitinib for SR-aGVHD in adult and pediatric patients aged 12 years and older on May 24, 201913.
Many clinical studies were performed on the application of ruxolitinib in the treatment of GVHD, confirming that ruxolitinib was effective in steroid-refractory GVHD (SR-GVHD) with few side effects it didn’t increase the risk of recurrence of malignant tumors14–18. However, reports on the efficacy of ruxolitinib in preventing GVHD, appropriate dosage, patient tolerance, and impact on survival and prognosis were few. Therefore, the present study mainly retrospectively analyzed the clinical efficacy and safety of the early application of ruxolitinib to prevent aGVHD after transplantation, so as to provide new ideas and directions for the prevention of aGVHD.
Patients and methods
The study protocol was approved by the ethics committee of the Affiliated Cancer Hospital of Zhengzhou University. The study was performed in accordance with the Declaration of Helsinki, and all patients themselves or their guardians provided informed consent for their inclusion. The patients underwent allo-HSCT from July 2017 to October 2019 were included in this study. A total of 57 patients were screened (Table 1 and Fig. 1), we comprehensively evaluate the medical condition, the risk of GVHD, financial status and willingness of patients to choose to use ruxolitinib. 16 of whom agreed with common anti-GVHD therapy (control group). 41 of whom agreed with ruxolitinib and common anti-GVHD therapy (ruxolitinib group). Therefore, after allo-HSCT, 57 patients were assigned to control group (N = 16; common anti-GVHD therapy) or ruxolitinib group (N = 41; ruxolitinib and common anti-GVHD therapy).
Table 1.
Characteristics of all patients in the control and ruxolitinib group (N = 57).
| Control (n = 16) | Ruxolitinib (n = 41) | χ2 | P value | |
|---|---|---|---|---|
| Sex (n, %) | 0.299 | 0.585 | ||
| Male | 11 (68.8) | 25 (61.0) | ||
| Female | 5 (31.2) | 16 (39.0) | ||
| Primary disease (n, %) | 0.765 | 0.382 | ||
| ALL | 5 (31.2) | 18 (43.9) | ||
| AML | 11 (68.8) | 23 (56.1) | ||
| Gene mutation or fusion gene (n, %) | 1.435 | 0.231 | ||
| Yes | 10 (62.5) | 32 (78.0) | ||
| No | 6 (37.5) | 9 (22.0) | ||
| Abnormal chromosome (n, %) | 1.341 | 0.247 | ||
| Yes | 4 (25.0) | 17 (41.5) | ||
| No | 12 (75.0) | 24 (58.5) | ||
| Disease status at HSCT (n, %) | 1.93 | 0.381 | ||
| CR1 | 10 (62.5) | 32 (78.0) | ||
| CR2 and CR3 | 3 (18.75) | 3 (7.3) | ||
| NR | 3 (18.75) | 6 (14.7) | ||
| MRD (n, %) | 0.174 | 0.677 | ||
| Positive | 6 (37.5) | 13 (31.7) | ||
| Negative | 10 (62.5) | 28 (68.3) | ||
| Sex of donor–recipient (n, %) | 0.208 | 0.648 | ||
| Identical | 13 (81.3) | 31 (75.6) | ||
| Different | 3 (18.7) | 10 (24.4) | ||
| Donor–recipient ABO compatibility (n, %) | 2.557 | 0.110 | ||
| Compatible | 10 (62.5) | 16 (39.0) | ||
| Incompatible | 6 (37.5) | 25 (61.0) | ||
| Conditioning regimen (n, %) | 0.043 | 0.835 | ||
| TBI/Cy-based | 11 (68.7) | 27 (65.9) | ||
| Bu/Cy-based | 5 (31.3) | 14 (34.1) | ||
| Donor source (n, %) | 2.808 | 0.246 | ||
| Haploid donors | 10 (62.5) | 16 (39.0) | ||
| Matched unrelated donors | 5 (31.3) | 18 (43.9) | ||
| Mismatched unrelated donors | 1 (6.2) | 7(17.1) | ||
| Major gene mutation and/or fusion gene (n, %) | NA | NA | ||
| FLT3/ITD | 0 (0) | 9 (22.0) | ||
| TET2 | 6 (37.5) | 3(7.3) | ||
| BCR/ABL | 2 (12.5) | 5 (12.2) | ||
| CEBPA | 4 (25.0) | 5 (12.2) | ||
| Age | 14 (2–40) | 28 (1–56) | NA | NA |
| Age of donors | 37 (15–46) | 31 (12–60) | NA | NA |
| MNC × 108/kg | 10.6 (3.37–25.88) | 14.4 (3.11–32.8) | NA | NA |
| CD34 + × 108/kg | 6.45 (2.07–19.57) | 8.02 (2.13–18.69) | NA | NA |
| Time for engraftment of neutrophils (d) | 12 (10–28) | 13 (11–20) | NA | NA |
| Time for engraftment of PLT (d) | 13 (11–34) | 15 (12–34) | NA | NA |
ALL Acute lymphoblastic leukemia, AML acute myeloid leukemia, Bu busulfan, CR complete remission, Cy cyclophosphamide, HLA human leukocyte antigen, HSCT hematopoietic stem cell transplantation, MNC mononuclear cell, MRD minimal residual disease, NA not applicable, NR no remission, PLT platelet, TBI total-body irradiation.
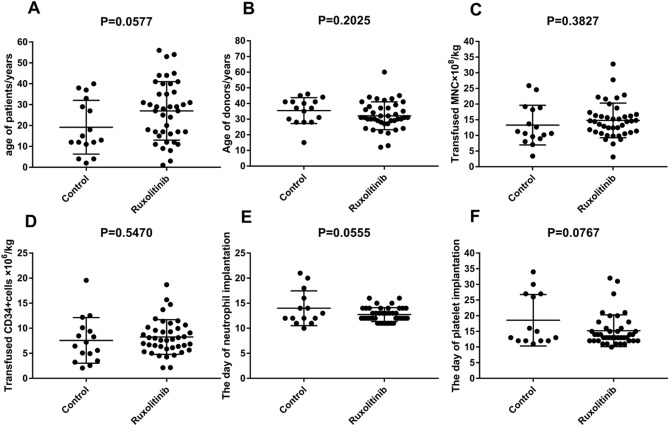
Figure 1.
Comparison of characteristics between the control and ruxolitinib groups. Age of patients (A), age of donors (B), transfused MNC × 108/kg (C), transfused CD34 + cells × 106/kg (D), day of neutrophil (E) and platelet (F) engraftment. (Graphpad prism 8.0.1 https://www.graphpad.com/scientific-software/prism/).
For aGVHD prophylaxis, the control group received 20 mg/kg PT-Cy for unrelated donor transplantation and 40 mg/kg for haploid transplantation, + 3 d and + 4 d; antithymocyte globulin-Fresenius (ATG-F), 3.0 mg/(kg ⋅ d), -1 d to -4 d for unrelated donor transplantation, and 5 mg/(kg ⋅ d), + 8 d for haploid transplantation; and cyclosporine A (CsA) and mycophenolate mofetil (MMF), + 5 d. The initial dose of CsA was 2 mg/kg.d for adults and 2.5 mg/kg.d for children. The dose was adjusted according to the concentration of CsA. For haploid transplant patients, the dose will be reduced after 6 months of transplantation and stop for 9–10 months. For unrelated donor transplantation, the dose will be reduced after 6 months of transplantation and stop for 6–8 months. The plasma concentration of CsA was assessed every 3 days and maintained within 150–250 ng/mL. The dosage of MMF usually was 500 mg twice a day, halved at 4 weeks after transplantation, and stopped at 6 weeks, the concentration of MMF was not assessed in all patients for some objective reasons. While in the ruxolitinib group, ruxolitinib 5 mg/d and 0.07–0.1 [mg/(kg ⋅ d)] was administered to adults and children, respectively, from the day of neutrophil engraftment to 100 days post-transplantation based on the control group. In the case of moderate or no aGVHD, ruxolitinib administration was discontinued directly or gradually reduced for severe aGVHD.
In this study, the conditioning regimens included busulfan (Bu)- and cyclophosphamide (CTX)-based regimens (Bu/Cy-based) and total-body irradiation (TBI) combined with CTX-based regimens (TBI/Cy-based) in patients. All patients were given symptomatic and comprehensive support treatment, including the prevention of infection and hemorrhagic cystitis, using granulocyte colony-stimulating factor (G-CSF), and infusion of blood products. In this study, there was no patient received letermovir during the transplant.
The first day when the neutrophil count was more than 0.5 × 109/L for three consecutive days was defined as the neutrophil engraftment time. The first day without platelet transfusion for 7 consecutive days and the platelet count greater than 20 × 109/L was defined as the platelet engraftment time 19. After hematopoietic reconstitution, bone marrow specimens were collected and assessed for engraftment by quantitative polymerase chain reaction (Q-PCR) assay or analysis of sex chromosomes. Disease relapse included the hematological and clinical recurrence of leukemia. Nonrelapse mortality was considered as death other than that due to disease relapse. OS was considered the time from the receipt of allo-HSCT to the end of the follow-up or death. DFS was considered from the receipt of allo-HSCT to relapse, death, or end of follow-up. Follow-up was performed via outpatient or inpatient visits, and the follow-up deadline was July 2020.
The classification data were represented as composition ratios. The count data were compared by using the chi-square or Fisher’s exact test, as appropriate. The data of the control and ruxolitinib groups were analyzed using the two-tailed t test. The impacts of factors on survival were compared using the log-rank test. Univariate analyses of DFS and OS were performed by the Kaplan–Meier method. The Cox regression model was used for multivariate survival analysis. All statistical analyses were performed using GraphPad Prism (version 8.0.1) and SPSS (version 21.0) software. All statistical tests were two tailed with statistical significance established at P < 0.05.
Results
Clinical characteristics
The clinical characteristics of patients are summarized in Table 1 and Fig. 1. In this study, all patients received peripheral blood stem cell transplantation in control and ruxolitinib group. Patients received transplantation at the same period, there is no difference in the period of transplantation between two groups. All patients undergo HLA high-resolution testing in both groups. All patients were evaluated for minimal residual disease (MRD) by flow cytometry, and the number of patients with MRD was 6 and 13 in the control and ruxolitinib groups before transplantation, respectively.
The median number of transfused mononuclear cells (MNCs) was 10.6 (3.27–25.88) × 108/kg and 14.4 (3.11–32.8) × 108/kg, and the median number of transfused CD34 + cells was 6.45 (2.07–19.57) × 106/kg and 8.02 (2.13–18.69) × 106/kg in the control and ruxolitinib groups, respectively. No significant differences were found in the basic clinical characteristics between the control and ruxolitinib groups (all P > 0.05, Table 1). By the end of follow-up, the rates of cessation of CsA in patients without relapse were 66.7(4/6)% and 72.7(16/22)% in control and ruxolitinib group, respectively.
Engraftment and complications
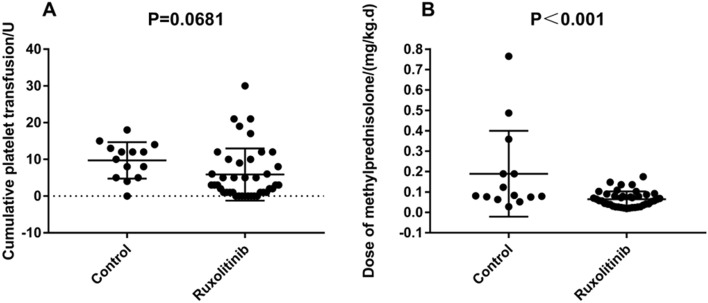
55 patients had successful reconstitution of hematopoiesis, while 2 patients experienced failure due to early graft rejection and serious infection in the control group. The hematopoietic reconstitution rate was 96.5%. The accumulation time of agranulocytosis did not exceed 7 days within 100 days after neutrophil engraftment in the control and ruxolitinib groups. The median time for neutrophil engraftment was day 12 (range, days 10–28) and day 13 (range, days 11–20), respectively, while the corresponding time for platelet engraftment was day 13 (range, days 11–34) and 15 (range, days 12–34) in the control and ruxolitinib groups, respectively. aGVHD20 and chronic GVHD (cGVHD)21 were diagnosed and graded by referring to the Seattle standard and the consensus of the National Institutes of Health22. No significant differences were observed in the cumulative incidences of cGVHD (P = 0.96), hemorrhagic cystitis (P = 0.937), pulmonary infection (P = 0.783), intestinal infection (P = 0.189), Epstein-Barr virus (EBV) infection (P = 0.983), and cytomegalovirus (CMV) infection (P = 0.967) between the control and ruxolitinib groups. GVHD mainly occurs in the liver, intestine and skin in both groups, there were 5, 5, 3 and 5, 3, 3 patients in the liver, intestine, and skin GVHD in the control and the ruxolitinib group, respectively. The cumulative incidences of aGVHD (50% vs 22% P = 0.046) , grade II-IV aGVHD (42.9% vs 12.2%, P = 0.013) and grade III-IV aGVHD (28.6% vs 7.3%, P = 0.039) were higher for the control than for the ruxolitinib group. The cumulative incidences of cGVHD and severe cGVHD were not significant difference between two groups, but the incidences of severe cGVHD was higher than for the control than for the ruxolitinib group (21.4% vs 12.2%). In addition, 3 and 5 patients were not sensitive to initial steroid therapy in control and ruxolitinib group, respectively. There was not significant difference (P = 0.398), and because of the application of ruxolitinib, the average dose of corticosteroids within 100 days for preventing engraftment syndrome and aGVHD after transplantation was significantly higher in the control group than in the ruxolitinib group (P < 0.001), while the cumulative platelet transfusion within 100 days between the control and ruxolitinib groups was not significantly different (P = 0.0681). (Table 2 and Fig. 2).
Table 2.
Complications and prognosis of all patients in the control and ruxolitinib groups (N = 55).
| Control (n = 14) | Ruxolitinib (n = 41) | χ2 | P value | |
|---|---|---|---|---|
| aGVHD (n, %) | 3.980 | 0.046 | ||
| Yes | 7 (50.0) | 9 (22.0) | ||
| No | 7 (50.0) | 32 (78.0) | ||
| Grade II–IV aGVHD (n, %) | 6.132 | 0.013 | ||
| Yes | 6 (42.9) | 5 (12.2) | ||
| No | 8 (57.1) | 36 (87.8) | ||
| Grade III-IV aGVHD (n, %) | 4.245 | 0.039 | ||
| Yes | 4 (28.6) | 3 (7.3) | ||
| No | 10 (71.4) | 38 (92.7) | ||
| cGVHD (n, %) | 0.002 | 0.960 | ||
| Yes | 4 (28.6) | 12 (29.3) | ||
| No | 10 (71.4) | 29 (70.7) | ||
| Severe cGVHD (n, %) | 0.716 | 0.398 | ||
| Yes | 3 (21.4) | 5 (12.2) | ||
| No | 11 (78.6) | 36 (87.8) | ||
| Hemorrhagic cystitis (n, %) | 0.006 | 0.937 | ||
| Yes | 7 (50.0) | 21 (51.2) | ||
| No | 7 (50.0) | 20 (48.8) | ||
| Pulmonary infection (n, %) | 0.076 | 0.783 | ||
| Yes | 9 (64.3) | 28 (68.3) | ||
| No | 5 (35.7) | 13 (31.7) | ||
| Intestinal infection (n, %) | 1.725 | 0.189 | ||
| Yes | 6 (42.9) | 10 (24.4) | ||
| No | 8 (57.1) | 31 (75.6) | ||
| EBV infection (n, %) | 0.000 | 0.983 | ||
| Yes | 1 (7.1) | 3 (7.3) | ||
| No | 13 (92.9) | 38 (92.7) | ||
| CMV infection (n, %) | 0.002 | 0.967 | ||
| Yes | 11 (78.6) | 32 (78.0) | ||
| No | 3 (21.4) | 9 (22.0) | ||
| Relapse (n, %) | 0.352 | 0.553 | ||
| Yes | 3 (21.4) | 6 (14.6) | ||
| No | 11 (78.6) | 35 (85.4) | ||
| Death (n, %) | 0.487 | 0.485 | ||
| Yes | 8 (57.1) | 19 (46.3) | ||
| No | 6 (42.9) | 22 (53.7) | ||
| Nonrelapse mortality (n, %) | 0.317 | 0.574 | ||
| Yes | 6 (42.9) | 16 (39.0) | ||
| No | 2 (14.3) | 3 (7.3) |
CMV Cytomegalovirus, EBV Epstein-Barr virus, GVHD graft-versus-host disease.
Figure 2.
Comparison of the cumulative platelet transfusion (A) and the average dose of corticosteroids (converted into methylprednisolone) (B) within 100 days after transplantation between the control and ruxolitinib groups. (Graphpad prism 8.0.1 https://www.graphpad.com/scientific-software/prism/).
Prognosis in the control and ruxolitinib groups
After a median follow-up of 9.5 (range 1.4–31.5) months, 3 and 6 patients experienced disease relapse in the control and ruxolitinib groups, respectively. The recurrence rate was not significantly different in the control and ruxolitinib group (21.4% vs 14.6%, P = 0.553). Further, 8 and 19 patients died in the control and ruxolitinib groups, respectively. Thus, the mortality rate (57.1% vs 46.3%, P = 0.485) and nonrelapse mortality (NRM) rate (42.9% vs 39%, P = 0.574) were not statistically significantly different in the control and ruxolitinib groups (Table 2). The main causes of non-relapse-related deaths of the patients were organ failure and severe pulmonary infection.
Survival analyses in 55 patients
The survival analysis showed no significant difference in OS (P = 0.514) and DFS (P = 0.691) between the control and ruxolitinib groups. The 2-year OS was (42.9 ± 13.2)% and (53.7 ± 7.8)%, and the 2-year DFS was (32.1 ± 13.6)% and (46.3 ± 7.8)%, respectively, in the control and ruxolitinib groups (Table 3 and Fig. 3 ). In addition, univariate and multivariate survival analyses were also performed on 55 patients, the results were showed in Table3 and Supplementary file.
Table 3.
Univariate survival analysis of OS and DFS after allo-HSCT (N = 55).
| Variables | No. of patients | OS | DFS | P | |
|---|---|---|---|---|---|
| Rate/% | P value | Rate/% | P value | ||
| Age | 0.719 | 0.549 | |||
| ≤ 25 | 27 | 14.007 ± 2.052 | 12.263 ± 2.044 | ||
| > 25 | 28 | 18.621 ± 2.625 | 16.713 ± 2.596 | ||
| Sex | 0.906 | 0.591 | |||
| Male | 34 | 17.679 ± 2.380 | 16.791 ± 2.388 | ||
| Female | 21 | 15.2 ± 2.418 | 12.248 ± 2.297 | ||
| Primary disease | 0.187 | 0.296 | |||
| ALL | 23 | 20.761 ± 2.804 | 18.291 ± 2.888 | ||
| AML | 32 | 12.988 ± 1.886 | 11.918 ± 1.829 | ||
| Gene mutation and/or fusion gene | 0.872 | 0.891 | |||
| Yes | 41 | 18.087 ± 2.164 | 15.929 ± 2.16 | ||
| No | 14 | 14.571 ± 2.985 | 13.98 ± 2.886 | ||
| Abnormal chromosome | 0.984 | 0.45 | |||
| Yes | 21 | 14.681 ± 2.369 | 11.695 ± 2.286 | ||
| No | 34 | 17.774 ± 2.366 | 16.922 ± 2.363 | ||
| Disease status at HSCT | 0.433 | 0.392 | |||
| CR1 | 41 | 18.756 ± 2.151 | 17.366 ± 2.163 | ||
| CR2 and CR3 | 6 | 8.767 ± 3.407 | 8.767 ± 3.407 | ||
| NR | 8 | 13.813 ± 3.613 | 10.044 ± 3.128 | ||
| MRD | 0.030 | 0.005 | |||
| Positive | 18 | 10.194 ± 2.318 | 8.069 ± 1.971 | ||
| Negative | 37 | 20.422 ± 2.215 | 18.957 ± 2.246 | ||
| Sex of donor–recipient | 0.585 | 0.649 | |||
| Identical | 42 | 17.467 ± 2.172 | 16.697 ± 2.177 | ||
| Different | 13 | 13.777 ± 2.284 | 9.885 ± 2.145 | ||
| Donor–recipient ABO compatibility | 0.277 | 0.209 | |||
| Compatible | 25 | 12.976 ± 2.148 | 11.272 ± 2.077 | ||
| Incompatible | 30 | 19.607 ± 2.492 | 17.694 ± 2.517 | ||
| Conditioning regimen | 0.406 | 0.872 | |||
| TBI/Cy-based | 36 | 14.072 ± 1.846 | 13.386 ± 1.849 | ||
| Bu/Cy-based | 19 | 19.942 ± 3.119 | 15.622 ± 3.074 | ||
| Donor source | 0.298 | 0.545 | |||
| Haploid donors | 25 | 13.048 ± 2.137 | 12.192 ± 2.11 | ||
| Unrelated donors | 30 | 19.547 ± 2.504 | 16.765 ± 2.515 | ||
| HLA matching | 0.541 | 0.707 | |||
| HLA-match donors | 23 | 16.048 ± 2.303 | 14.388 ± 2.286 | ||
| HLA-mismatched donors | 32 | 16.863 ± 2.443 | 15.1 ± 2.41 | ||
| GVHD prophylaxis | 0.514 | 0.691 | |||
| Control | 14 | 12.914 ± 2.813 | 12.3 ± 2.705 | ||
| Ruxolitinib | 41 | 18.641 ± 2.17 | 16.554 ± 2.178 | ||
| aGVHD | 0.003 | 0.008 | |||
| Yes | 16 | 8.675 ± 2.377 | 8.258 ± 2.225 | ||
| No | 39 | 21.008 ± 2.133 | 18.785 ± 2.207 | ||
| Grade II–IV aGVHD | 0.002 | 0.003 | |||
| Yes | 11 | 7.173 ± 2.575 | 6.718 ± 2.312 | ||
| No | 44 | 20.277 ± 2.040 | 18.307 ± 2.088 | ||
| cGVHD | 0.003 | < 0.001 | |||
| Yes | 16 | 26.9 ± 2.396 | 26.806 ± 2.448 | ||
| No | 39 | 11.967 ± 1.748 | 9.877 ± 1.608 | ||
| Hemorrhagic cystitis | 0.356 | 0.992 | |||
| Yes | 28 | 15.886 ± 2.569 | 15.789 ± 2.585 | ||
| No | 27 | 16.537 ± 2.134 | 13.521 ± 2.111 | ||
| Pulmonary infection | 0.105 | 0.445 | |||
| Yes | 37 | 15.7 ± 2.278 | 14.795 ± 2.262 | ||
| No | 18 | 18.533 ± 2.401 | 14.839 ± 2.573 | ||
| Intestinal infection | 0.703 | 0.41 | |||
| Yes | 16 | 13.125 ± 2.436 | 10.2 ± 2.046 | ||
| No | 39 | 18.503 ± 2.252 | 17.056 ± 2.258 | ||
| EBV infection | 0.282 | 0.205 | |||
| Yes | 4 | 13.25 ± 1.516 | 13.25 ± 1.516 | ||
| No | 51 | 17.286 ± 1.958 | 15.09 ± 1.926 | ||
| CMV infection | 0.279 | 0.193 | |||
| Yes | 43 | 15.986 ± 2.114 | 14.621 ± 2.09 | ||
| No | 12 | 19.1 ± 2.468 | 16.369 ± 2.598 | ||
Figure 3.
DFS (A) and OS (B) rates of 55 patients between the control and ruxolitinib groups. (Graphpad prism 8.0.1 https://www.graphpad.com/scientific-software/prism/).
Discussion
Acute GVHD is a serious complication of allo-HSCT and an important factor threatening the survival of patients. The incidence of aGVHD can also be as high as 30%–50% with a classic prophylaxis regimen23. The prevention and treatment of GVHD is still an important challenge of allo-HSCT. Ruxolitinib can impair the differentiation of CD4+ T cells into IFN-γ- and IL17A-producing cells, and both T-cell phenotypes are linked to GVHD. Thus, ruxolitinib may be a novel targeted drug in GVHD by inhibiting proinflammatory signaling that mediates tissue damage11. In recent years, ruxolitinib achieved significant efficacy as a very promising drug for the treatment of GVHD, with an overall response rate of up to 100%24. The 2-year OS was 61.2%25. In a retrospective multicenter survey from 19 centers, the overall response rate of ruxolitinib reached 81.5% and 85.4% in steroid-refractory aGVHD and cGVHD, respectively26. However, reports on the use of ruxolitinib in the prevention of GVHD are few27.
In the present study, the clinical efficacy, adverse effects, and incidences of other transplant-related complications in the control and ruxolitinib groups were compared. The incidences of aGVHD (22% vs 50%, P = 0.046), grade II–IV aGVHD (12.2% vs 42.9%, P = 0.013) and grade III–IV aGVHD (7.3% vs 28.6%, P = 0.039) were significantly lower in the ruxolitinib group than in the control group. Zhao et al.also suggested that ruxolitinib was applied at 5–10 mg twice daily until 2–3 months after transplantation, or reduced gradually with GVHD and discontinued by 6 months. The prophylactic application of ruxolitinib after allo-HSCT seemed to be safe and effective for preventing GVHD28. Additionally, the incidence of aGVHD after transplantation was significantly higher in the control group than in the ruxolitinib group (P < 0.001). This may also be related to the fact that the control group has more haploid and mismatched unrelated donors, although it is not statistically significant (P = 0.246). The major complication and side effects of ruxolitinib were CMV reactivation and cytopenia26. However, in this study, the cumulative platelet transfusion within 100 days after transplantation (P = 0.0681) and other transplant-related complications in the control and ruxolitinib groups were not significantly different. While, because of the application of ruxolitinib, the dose of steroids was significantly reduced. The accumulation time of agranulocytosis did not exceed 7 days within 100 days after neutrophil engraftment in the control and ruxolitinib groups. The amount of red blood cell transfusion within 100 days after transplantation was not statistically analyzed in the control and ruxolitinib groups, because patients might have hemorrhagic cystitis or gastrointestinal bleeding; this needs further exploration. These results indicated that the early application of ruxolitinib to prevent aGVHD reduced the incidence and severity of aGVHD. Also, patients had good tolerance, and the incidences of adverse effects of hematopoiesis and other transplant-related complications did not increase.
In addition, survival analysis was performed on 55 patients with hematopoietic reconstruction. OS (P = 0.514) and DFS (P = 0.691) were not significantly different in the control and ruxolitinib groups; the 2-year OS was (42.9 ± 13.2)% and (53.7 ± 7.8)%, and the 2-year DFS was (32.1 ± 13.6)% and (46.3 ± 7.8)%, respectively. The survival rate was lower than that in other reports, which might be related to the existence of poor prognosis gene mutations and abnormal chromosomes in patients. Poor gene mutations made patients insensitive to chemotherapy, leading to high-dose chemotherapy damages to the function of multiple organs, and various transplant-related complications such as infection and organ failure appeared in the early stage of transplantation, which affected the NRM, OS and DFS to a certain extent. However, ruxolitinib seemed to improve patients' OS and DFS to some extent. In this study, the main cause of NRM of the patients was non-GVHD-related death. The main causes of non-GVHD-related deaths of the patients were organ failure and severe pulmonary infection. There was no increase in mortality due to the application of ruxolitinib, which also shows the safety of ruxolitinib. This was similar to preventing GVHD in myelofibrosis after allo-HSCT29, indicating that the early application of ruxolitinib for preventing GVHD did not negatively influence the outcome after allo-HSCT. However, infection remained an important factor leading to nonrelapse mortality of patients, which needed attention. In addition, univariate and multivariate survival analyses were also performed on 55 patients, the results are consistent with previous research reports19,30–33. This further shows that the application of ruxolitinib does not affect the survival of patients.
Although ruxolitinib had a significant effect on the prevention and treatment of GVHD, some problems still needed to be resolved. First, the perfect time to apply ruxolitinib to prevent GVHD: are neutrophils implanted, or both neutrophils and platelets implanted? Second, the perfect time to discontinue ruxolitinib: can we quickly discontinue other immunosuppressants instead of stopping ruxolitinib to reduce the incidence of infection without significant cytopenia after engraftment? These issues need to be addressed to make better use of ruxolitinib.
In summary, the early application of ruxolitinib reduced the incidence and severity of aGVHD. The patients were well tolerated, and the incidence of other transplant-related complications did not increase and affect the survival and prognosis of patients. Of course, the sample size of our study is not yet sufficient, and the findings would be validated by a large mutli-center randomized clinical trials.
Supplementary Information
Acknowledgements
The authors would like to thank all patients for their cooperation and the Department of Hematology, Affiliated Cancer Hospital and Henan Cancer Hospital for providing administrative support and study patients.
Author contributions
B.Z., J.Z., and R.G. designed the study. B.Z. and L.C. collected data. B.Z., J.Z. drafted the manuscript. All authors participated in the revision of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jian Zhou, Zhenyu Ji and Yongping Song.
Contributor Information
Jian Zhou, Email: zhoujiandoctor@163.com.
Zhenyu Ji, Email: jizhenyu@zzu.edu.cn.
Yongping Song, Email: songyongping001@126.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-88080-3.
References
- 1.Dalle J, Balduzzi A, Bader P, Lankester A, Yaniv I, Wachowiak J, et al. Allogeneic Stem Cell Transplantation from HLA-mismatched donors for pediatric patients with acute lymphoblastic Leukemia treated according to the 2003 BFM and 2007 international BFM studies: impact of disease risk on outcomes. Biol. Blood Marrow Transpl. 2018;24(9):1848–1855. doi: 10.1016/j.bbmt.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Lazaryan A, Weisdorf DJ, DeFor T, Brunstein CG, MacMillan ML, Bejanyan N, et al. Risk factors for acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation with umbilical cord blood and matched sibling donors. Biol. Blood Marrow Transpl. 2016;22(1):134–140. doi: 10.1016/j.bbmt.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jagasia M, Arora M, Flowers M, Chao N, McCarthy P, Cutler C, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China—recommendations from the Chinese Society of Hematology. J. Hematol. Oncol. 2018;11(1):33. doi: 10.1186/s13045-018-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Wu D, Liu Q, Xu L, Liu K, Zhang X, et al. Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J. Hematol. Oncol. 2019;12(1):88. doi: 10.1186/s13045-019-0781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montoro J, Piñana J, Hernández-Boluda J, Hernani R, Lorenzo I, Pérez A et al.. Uniform graft-versus-host disease prophylaxis with posttransplant cyclophosphamide, sirolimus, and mycophenolate mofetil following hematopoietic stem cell transplantation from haploidentical, matched sibling and unrelated donors. Bone Marrow Transpl. 2020. [DOI] [PubMed]
- 7.Liu S, Zhang X, Xu L, Wang Y, Yan C, Chen H et al.. Prognostic factors and long-term follow-up of basiliximab for steroid-refractory acute graft-versus-host disease: updated experience from a large-scale study. Am. J. Hematol. 2020. [DOI] [PubMed]
- 8.Wang K, Lv M, Chang Y, Zhao X, Zhao X, Zhang Y, et al. Early myeloid-derived suppressor cells (HLA-DR−/lowCD33+CD16−) expanded by granulocyte colony-stimulating factor prevent acute graft-versus-host disease (GVHD) in humanized mouse and might contribute to lower GVHD in patients post allo-HSCT. J. Hematol. Oncol. 2019;12(1):31. doi: 10.1186/s13045-019-0710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Jin N, Wang F, Chen B. Mesenchymal stem cells: a promising way in therapies of graft-versus-host disease. Cancer Cell Int. 2020;20:114. doi: 10.1186/s12935-020-01193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quintás-Cardama A, Vaddi K, Liu P, Manshouri T, Li J, Scherle P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–3117. doi: 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spoerl S, Mathew N, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, et al. Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood. 2014;123(24):3832–3842. doi: 10.1182/blood-2013-12-543736. [DOI] [PubMed] [Google Scholar]
- 12.Carniti C, Gimondi S, Vendramin A, Recordati C, Confalonieri D, Bermema A, et al. Pharmacologic inhibition of JAK1/JAK2 signaling reduces experimental murine acute GVHD while preserving GVT effects. Clin. Cancer Res. 2015;21(16):3740–3749. doi: 10.1158/1078-0432.CCR-14-2758. [DOI] [PubMed] [Google Scholar]
- 13.Przepiorka D, Luo L, Subramaniam S, Qiu J, Gudi R, Cunningham L, et al. FDA approval summary: ruxolitinib for treatment of steroid-refractory acute graft-versus-host disease. Oncologist. 2020;25(2):e328–e334. doi: 10.1634/theoncologist.2019-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assouan D, Lebon D, Charbonnier A, Royer B, Marolleau J, Gruson B. Ruxolitinib as a promising treatment for corticosteroid-refractory graft-versus-host disease. Br. J. Haematol. 2018;181(5):687–689. doi: 10.1111/bjh.14679. [DOI] [PubMed] [Google Scholar]
- 15.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N. Engl. J. Med. 2020;382(19):1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 16.Abedin S, McKenna E, Chhabra S, Pasquini M, Shah N, Jerkins J, et al. Efficacy, toxicity, and infectious complications in ruxolitinib-treated patients with corticosteroid-refractory graft-versus-host disease after hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 2019;25(8):1689–1694. doi: 10.1016/j.bbmt.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Risitano A, Peffault DLR. Ruxolitinib for steroid-resistant acute GVHD. Blood. 2020;135(20):1721–1722. doi: 10.1182/blood.2020005364. [DOI] [PubMed] [Google Scholar]
- 18.Escamilla Gómez V, García-Gutiérrez V, López Corral L, García Cadenas I, Pérez Martínez A, Márquez Malaver F, et al. Ruxolitinib in refractory acute and chronic graft-versus-host disease: a multicenter survey study. Bone Marrow Transpl. 2020;55(3):641–648. doi: 10.1038/s41409-019-0731-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Zhou J, Yu F, Lv T, Fang B, Fan D, et al. Alternative donor peripheral blood stem cell transplantation for the treatment of high-risk refractory and/or relapsed childhood acute leukemia: a randomized trial. Exp. Hematol. Oncol. 2020;9:5. doi: 10.1186/s40164-020-00162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Przepiorka D, Weisdorf D, Martin P, Klingemann H, Beatty P, Hows J et al.. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995; 15(6): 825–8. [PubMed]
- 21.Shulman H, Sullivan K, Weiden P, McDonald G, Striker G, Sale G et al.. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am. J. Med. 1980; 69(2): 204–17. [DOI] [PubMed]
- 22.Martin P, Lee S, Przepiorka D, Horowitz M, Koreth J, Vogelsang G et al.. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. The 2014 Clinical Trial Design Working Group Report. Biol. Blood Marrow Transpl. 2015; 21(8): 1343–59. [DOI] [PMC free article] [PubMed]
- 23.Ruutu T, Gratwohl A, De Witte T, Afanasyev B, Apperley J, Bacigalupo A, et al. Prophylaxis and treatment of GVHD: EBMT-ELN working group recommendations for a standardized practice. Bone Marrow Transpl. 2014;49(2):168–173. doi: 10.1038/bmt.2013.107. [DOI] [PubMed] [Google Scholar]
- 24.Mori Y, Ikeda K, Inomata T, Yoshimoto G, Fujii N, Ago H, et al. Ruxolitinib treatment for GvHD in patients with myelofibrosis. Bone Marrow Transpl. 2016;51(12):1584–1587. doi: 10.1038/bmt.2016.256. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Wu H, Shi J, Luo Y, Li X, Lan J et al.. Ruxolitinib combined with etanercept induce a rapid response to corticosteroid-refractory severe acute graft vs host disease after allogeneic stem cell transplantation: Results of a multi-center prospective study. Am. J. Hematol. 2020. [DOI] [PubMed]
- 26.Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder S, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morozova E, Barabanshikova M, Moiseev I, Shakirova A, Barhatov I, Ushal I et al.. A prospective pilot study of graft-versus-host disease prophylaxis with post-transplantation cyclophosphamide and ruxolitinib in patients with myelofibrosis. Acta Haematol. Basel 2020: 1–8. [DOI] [PubMed]
- 28.Zhao Y, Shi J, Luo Y, Gao F, Tan Y, Lai X, et al. Calcineurin inhibitors replacement by ruxolitinib as graft-versus-host disease prophylaxis for patients after allogeneic stem cell transplantation. Biol. Blood Marrow Transpl. 2020;26(5):e128–e133. doi: 10.1016/j.bbmt.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Shahnaz Syed Abd Kadir S, Christopeit M, Wulf G, Wagner E, Bornhauser M, Schroeder T et al.. Impact of ruxolitinib pretreatment on outcomes after allogeneic stem cell transplantation in patients with myelofibrosis. Eur. J. Haematol. 2018; 101(3): 305–317. [DOI] [PubMed]
- 30.Yoshida N, Sakaguchi H, Yabe M, Hasegawa D, Hama A, Hasegawa D, et al. Clinical outcomes after allogeneic hematopoietic stem cell transplantation in children with juvenile myelomonocytic leukemia: a report from the Japan society for hematopoietic cell transplantation. Biol. Blood Marrow Transpl. 2020;26(5):902–910. doi: 10.1016/j.bbmt.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 31.Busca A, Passera R, Pini M, Zallio F, Dellacasa C, Audisio E, et al. The use of ATG abrogates the antileukemic effect of cytomegalovirus reactivation in patients with acute myeloid leukemia receiving grafts from unrelated donors. Am. J. Hematol. 2015;90(6):E117–E121. doi: 10.1002/ajh.23998. [DOI] [PubMed] [Google Scholar]
- 32.Kaito S, Najima Y, Harada K, Fukuda T, Noguchi Y, Ikegame K et al.. Allogeneic hematopoietic stem cell transplantation for adult patients with B-cell acute lymphoblastic leukemia harboring t(1;19)(q23;p13.3); comparison with normal karyotype. Bone Marrow Transpl. 2020; 55(7): 1337–1346. [DOI] [PubMed]
- 33.Gyurkocza B, Gutman J, Nemecek E, Bar M, Milano F, Ramakrishnan A, et al. Treosulfan, fludarabine, and 2-Gy total body irradiation followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome and acute myeloid leukemia. Biol. Blood Marrow Transpl. 2014;20(4):549–555. doi: 10.1016/j.bbmt.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.